User login
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
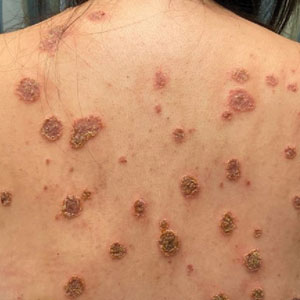
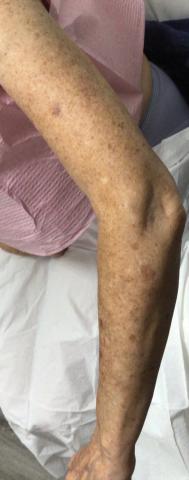
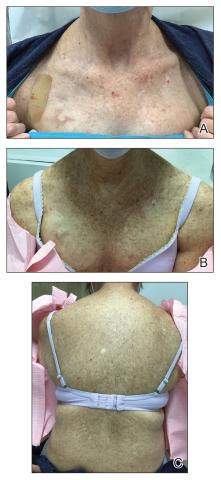
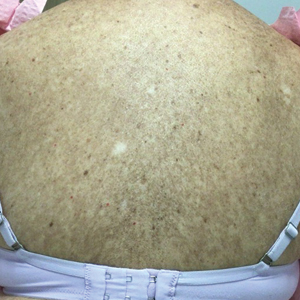
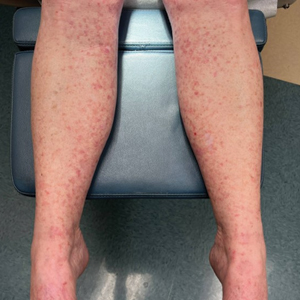
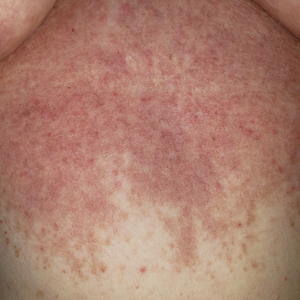
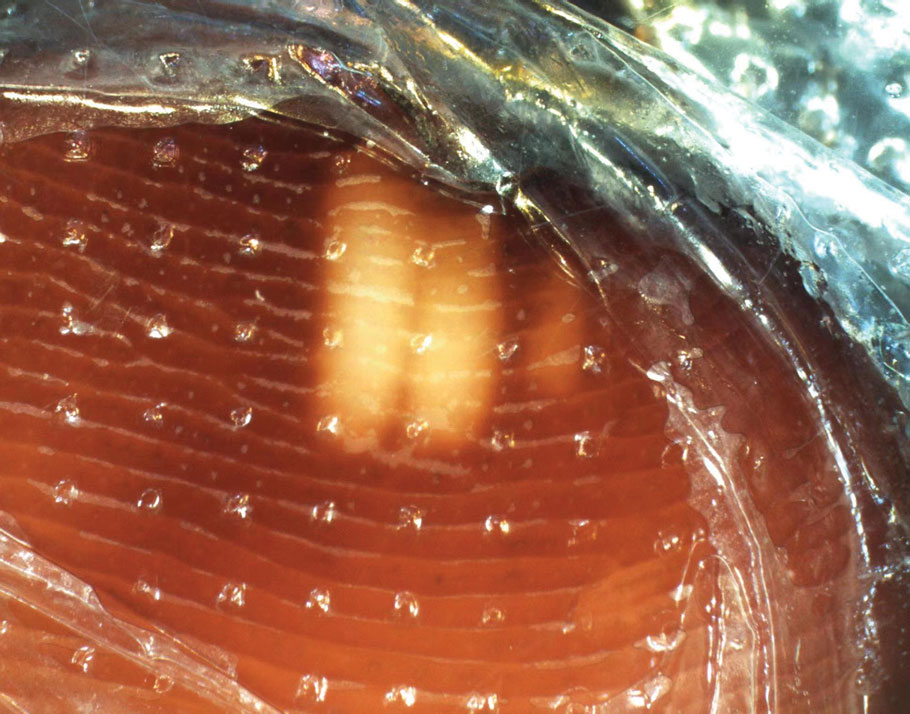
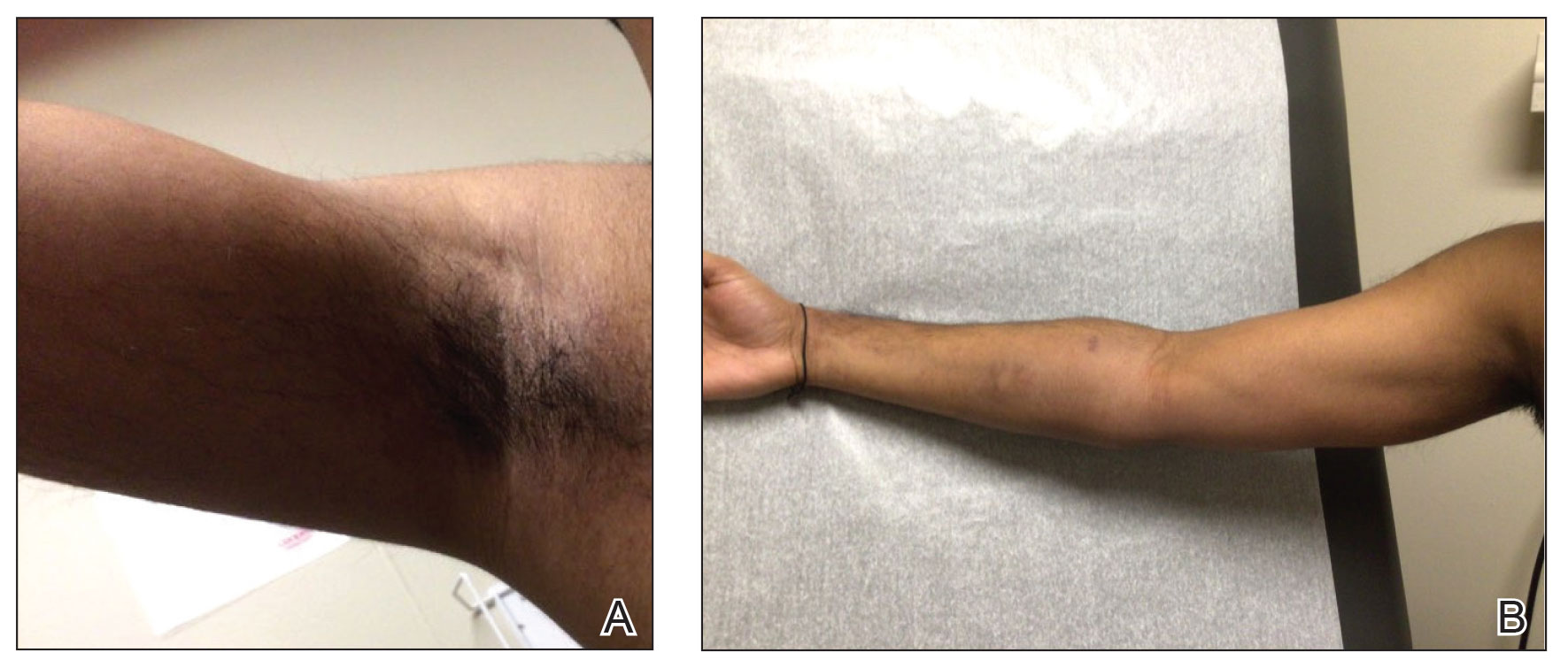
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.
Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
Practice Points
- Consider a rupioid id reaction when a patient presents with lesions featuring scale that is dirty appearing and resembles an oyster shell.
- Recognize that exuberant id reactions can manifest with peripheral eosinophilia; its presence should not lead you to automatically rule out an id reaction in favor of other eosinophilic eruptions.
- Focus on uncovering the source of an id reaction (eg, contactants, infections, bites); resolving the primary insult is essential for rapid clearance of even dramatic rupioid eruptions.
Millipede Burns: An Unusual Cause of Purplish Toes
To the Editor:
Millipedes do not have nearly as many feet as their name would suggest; most have fewer than 100.1 They are not actually insects; they are a wormlike arthropod in the Diplopoda class. Generally these harmless animals can be a welcome resident in gardens because they break down decaying plant material and rejuvenate the soil.1 However, they are less welcome in the home or underfoot because of what happens when these invertebrates are threatened or crushed.2
Millipedes, which typically have at least 30 pairs of legs, have 2 defense mechanisms: (1) body coiling to withstand external pressure, and (2) secretion of fluids with insecticidal properties from specialized glands distributed along their body.3 These secretions, which are used by the millipede to defend against predators, contain organic compounds including benzoquinone. When these secretions come into contact with skin, pigmentary changes resembling a burn or necrosis and irritation to the skin (pain, burning, itching) occur.4,5
Millipedes typically are found in tropical and temperate regions worldwide, such as the Amazon rainforest, Southeast Asia, tropical areas of Africa, forests, grasslands, and gardens in North America and Europe.6 They also are found in every US state as well as Puerto Rico.1 Millipedes are nocturnal, favor dark places, and can make their way into residential areas, including homes, basements, gardens, and yards.2,6 Although millipede burns commonly are reported in tropical regions, we present a case in China.6A 33-year-old woman presented with purplish-red discoloration on all 5 toes on the left foot. The patient recounted that she discovered a millipede in her shoe earlier in the day, removed it, and crushed it with her bare foot. That night, while taking a bath, she noticed that the toes had turned purplish-red (Figure 1). The patient brought the crushed millipede with her to the emergency department where she sought treatment. The dermatologist confirmed that it was a millipede; however, the team was unable to determine the specific species because it had been crushed (Figure 2).
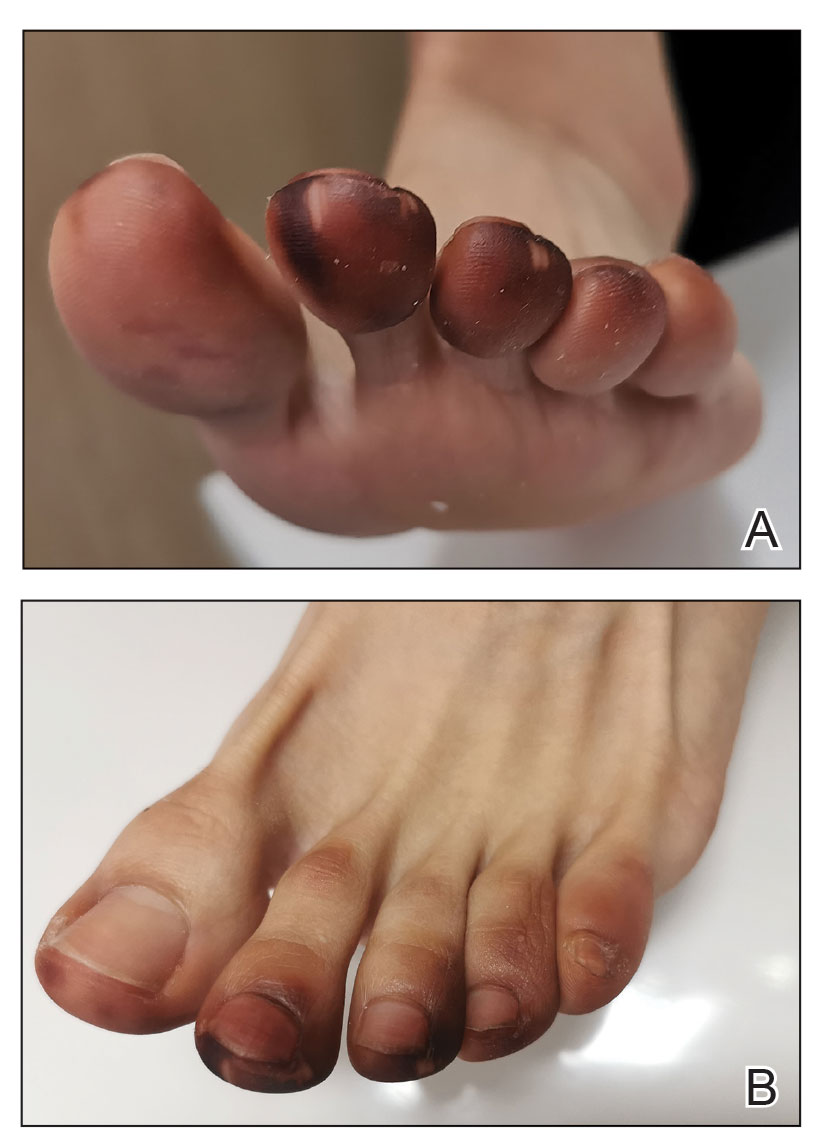

Physical examination of the affected toes showed a clear boundary and iodinelike staining. The patient did not report pain. The stained skin had a normal temperature, pulse, texture, and sensation. Dermoscopy revealed multiple black-brown patches on the toes (Figure 3). The pigmented area gradually faded over a 1-month period. Superficial damage to the toenail revealed evidence of black-brown pigmentation on both the nail and the skin underneath. The diagnosis in the dermoscopy report suggested exogenous pigmentation of the toes. The patient was advised that no treatment was needed and that the condition would resolve on its own. At 1-month follow-up, the patient’s toes had returned to their normal color (Figure 4).
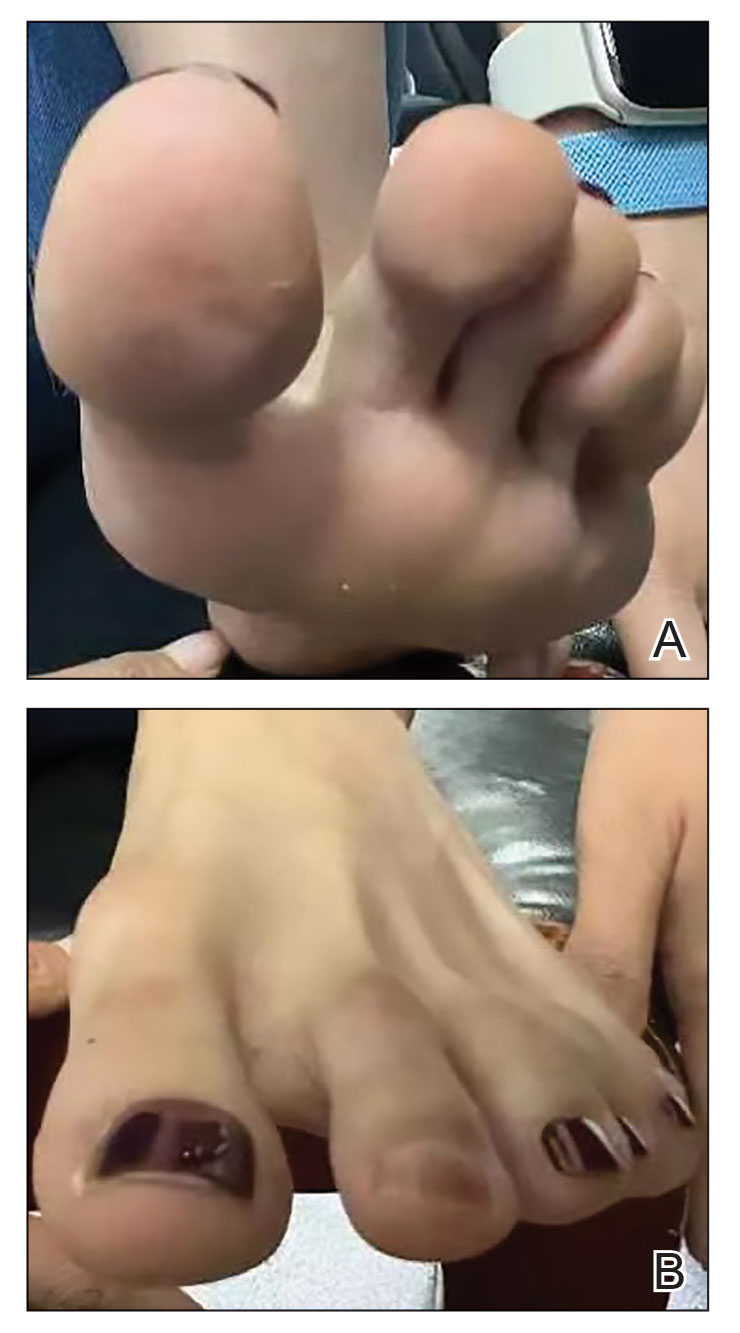
The feet are common sites of millipede burns; other exposed areas, such as the arms, face, and eyes, also are potential sites of involvement.5 The cutaneous pigmentary changes seen on our patient’s foot were a result of the millipede’s defense mechanism—secreted toxic chemicals that stained the foot. It is important to note that the pigmentation was not associated with the death of the millipede, as the millipede was still alive upon initial contact with the patient’s foot in her shoe.
When a patient presents with pigmentary changes, several conditions must be ruled out—notably acute arterial thrombosis. Patients with this condition will describe acute pain and weakness in the area of involvement. Physicians inspecting the area will note coldness and pallor in the affected limb as well as a diminished or absent pulse. In severe cases, the skin may exhibit a purplish-red appearance.5 Millipede burns also should be distinguished from bacterial endocarditis and cryoglobulinemia.7 All 3 conditions can manifest with redness, swelling, blisters, and purpuralike changes. Positive blood culture is an important diagnostic basis for bacterial endocarditis; in addition, routine blood tests will demonstrate a decrease in red blood cells and hemoglobin, and routine urinalysis may show proteinuria and microscopic hematuria. Patients with cryoglobulinemia will have a positive cryoglobulin assay, increased IgM, and often decreased complement.7 It also is worth noting that millipede burns might resemble child abuse in pediatric patients, necessitating further evaluation.5
It is unusual to see a millipede burn in nontropical regions. Therefore, the identification of our patient’s millipede burn was notable and serves as a reminder to keep this diagnosis in the differential when caring for patients with pigmentary changes. An accurate diagnosis hinges on being alert to a millipede exposure history and recognizing the clinical manifestations. For affected patients, it may be beneficial to recommend they advise friends and relatives to avoid skin contact with millipedes and most importantly to avoid stepping on them with bare feet.
Millipedes. National Wildlife Federation. Accessed October 15, 2025. https://www.nwf.org/Educational-Resources/Wildlife-Guide/Invertebrates/Millipedes
Pennini SN, Rebello PFB, Guerra MdGVB, et al. Millipede accident with unusual dermatological lesion. An Bras Dermatol. 2019;94:765-767. doi:10.1016/j.abd.2019.10.003
Lima CAJ, Cardoso JLC, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda Class (“millipedes“). An Bras Dermatol. 2010;85:391-392. doi:10.1590/s0365-05962910000300018
De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila). 2011;49:187-190. doi:10.3109/15563650.2011.560855
Lacy FA, Elston DM. What’s eating you? millipede burns. Cutis. 2019;103:195-196.
Neto ASH, Filho FB, Martins G. Skin lesions simulating blue toe syndrome caused by prolonged contact with a millipede. Rev Soc Bras Med Trop. 2014;47:257-258. doi:10.1590/0037-8682-0212-2013
Sampaio FMS, Valviesse VRGdA, Lyra-da-Silva JO, et al. Pain and hyperpigmentation of the toes: a quiz. hyperpigmentation of the toes caused by millipedes. Acta Derm Venereol. 2014;94:253-254. doi:10.2340/00015555-1645
To the Editor:
Millipedes do not have nearly as many feet as their name would suggest; most have fewer than 100.1 They are not actually insects; they are a wormlike arthropod in the Diplopoda class. Generally these harmless animals can be a welcome resident in gardens because they break down decaying plant material and rejuvenate the soil.1 However, they are less welcome in the home or underfoot because of what happens when these invertebrates are threatened or crushed.2
Millipedes, which typically have at least 30 pairs of legs, have 2 defense mechanisms: (1) body coiling to withstand external pressure, and (2) secretion of fluids with insecticidal properties from specialized glands distributed along their body.3 These secretions, which are used by the millipede to defend against predators, contain organic compounds including benzoquinone. When these secretions come into contact with skin, pigmentary changes resembling a burn or necrosis and irritation to the skin (pain, burning, itching) occur.4,5
Millipedes typically are found in tropical and temperate regions worldwide, such as the Amazon rainforest, Southeast Asia, tropical areas of Africa, forests, grasslands, and gardens in North America and Europe.6 They also are found in every US state as well as Puerto Rico.1 Millipedes are nocturnal, favor dark places, and can make their way into residential areas, including homes, basements, gardens, and yards.2,6 Although millipede burns commonly are reported in tropical regions, we present a case in China.6A 33-year-old woman presented with purplish-red discoloration on all 5 toes on the left foot. The patient recounted that she discovered a millipede in her shoe earlier in the day, removed it, and crushed it with her bare foot. That night, while taking a bath, she noticed that the toes had turned purplish-red (Figure 1). The patient brought the crushed millipede with her to the emergency department where she sought treatment. The dermatologist confirmed that it was a millipede; however, the team was unable to determine the specific species because it had been crushed (Figure 2).


Physical examination of the affected toes showed a clear boundary and iodinelike staining. The patient did not report pain. The stained skin had a normal temperature, pulse, texture, and sensation. Dermoscopy revealed multiple black-brown patches on the toes (Figure 3). The pigmented area gradually faded over a 1-month period. Superficial damage to the toenail revealed evidence of black-brown pigmentation on both the nail and the skin underneath. The diagnosis in the dermoscopy report suggested exogenous pigmentation of the toes. The patient was advised that no treatment was needed and that the condition would resolve on its own. At 1-month follow-up, the patient’s toes had returned to their normal color (Figure 4).


The feet are common sites of millipede burns; other exposed areas, such as the arms, face, and eyes, also are potential sites of involvement.5 The cutaneous pigmentary changes seen on our patient’s foot were a result of the millipede’s defense mechanism—secreted toxic chemicals that stained the foot. It is important to note that the pigmentation was not associated with the death of the millipede, as the millipede was still alive upon initial contact with the patient’s foot in her shoe.
When a patient presents with pigmentary changes, several conditions must be ruled out—notably acute arterial thrombosis. Patients with this condition will describe acute pain and weakness in the area of involvement. Physicians inspecting the area will note coldness and pallor in the affected limb as well as a diminished or absent pulse. In severe cases, the skin may exhibit a purplish-red appearance.5 Millipede burns also should be distinguished from bacterial endocarditis and cryoglobulinemia.7 All 3 conditions can manifest with redness, swelling, blisters, and purpuralike changes. Positive blood culture is an important diagnostic basis for bacterial endocarditis; in addition, routine blood tests will demonstrate a decrease in red blood cells and hemoglobin, and routine urinalysis may show proteinuria and microscopic hematuria. Patients with cryoglobulinemia will have a positive cryoglobulin assay, increased IgM, and often decreased complement.7 It also is worth noting that millipede burns might resemble child abuse in pediatric patients, necessitating further evaluation.5
It is unusual to see a millipede burn in nontropical regions. Therefore, the identification of our patient’s millipede burn was notable and serves as a reminder to keep this diagnosis in the differential when caring for patients with pigmentary changes. An accurate diagnosis hinges on being alert to a millipede exposure history and recognizing the clinical manifestations. For affected patients, it may be beneficial to recommend they advise friends and relatives to avoid skin contact with millipedes and most importantly to avoid stepping on them with bare feet.
To the Editor:
Millipedes do not have nearly as many feet as their name would suggest; most have fewer than 100.1 They are not actually insects; they are a wormlike arthropod in the Diplopoda class. Generally these harmless animals can be a welcome resident in gardens because they break down decaying plant material and rejuvenate the soil.1 However, they are less welcome in the home or underfoot because of what happens when these invertebrates are threatened or crushed.2
Millipedes, which typically have at least 30 pairs of legs, have 2 defense mechanisms: (1) body coiling to withstand external pressure, and (2) secretion of fluids with insecticidal properties from specialized glands distributed along their body.3 These secretions, which are used by the millipede to defend against predators, contain organic compounds including benzoquinone. When these secretions come into contact with skin, pigmentary changes resembling a burn or necrosis and irritation to the skin (pain, burning, itching) occur.4,5
Millipedes typically are found in tropical and temperate regions worldwide, such as the Amazon rainforest, Southeast Asia, tropical areas of Africa, forests, grasslands, and gardens in North America and Europe.6 They also are found in every US state as well as Puerto Rico.1 Millipedes are nocturnal, favor dark places, and can make their way into residential areas, including homes, basements, gardens, and yards.2,6 Although millipede burns commonly are reported in tropical regions, we present a case in China.6A 33-year-old woman presented with purplish-red discoloration on all 5 toes on the left foot. The patient recounted that she discovered a millipede in her shoe earlier in the day, removed it, and crushed it with her bare foot. That night, while taking a bath, she noticed that the toes had turned purplish-red (Figure 1). The patient brought the crushed millipede with her to the emergency department where she sought treatment. The dermatologist confirmed that it was a millipede; however, the team was unable to determine the specific species because it had been crushed (Figure 2).


Physical examination of the affected toes showed a clear boundary and iodinelike staining. The patient did not report pain. The stained skin had a normal temperature, pulse, texture, and sensation. Dermoscopy revealed multiple black-brown patches on the toes (Figure 3). The pigmented area gradually faded over a 1-month period. Superficial damage to the toenail revealed evidence of black-brown pigmentation on both the nail and the skin underneath. The diagnosis in the dermoscopy report suggested exogenous pigmentation of the toes. The patient was advised that no treatment was needed and that the condition would resolve on its own. At 1-month follow-up, the patient’s toes had returned to their normal color (Figure 4).


The feet are common sites of millipede burns; other exposed areas, such as the arms, face, and eyes, also are potential sites of involvement.5 The cutaneous pigmentary changes seen on our patient’s foot were a result of the millipede’s defense mechanism—secreted toxic chemicals that stained the foot. It is important to note that the pigmentation was not associated with the death of the millipede, as the millipede was still alive upon initial contact with the patient’s foot in her shoe.
When a patient presents with pigmentary changes, several conditions must be ruled out—notably acute arterial thrombosis. Patients with this condition will describe acute pain and weakness in the area of involvement. Physicians inspecting the area will note coldness and pallor in the affected limb as well as a diminished or absent pulse. In severe cases, the skin may exhibit a purplish-red appearance.5 Millipede burns also should be distinguished from bacterial endocarditis and cryoglobulinemia.7 All 3 conditions can manifest with redness, swelling, blisters, and purpuralike changes. Positive blood culture is an important diagnostic basis for bacterial endocarditis; in addition, routine blood tests will demonstrate a decrease in red blood cells and hemoglobin, and routine urinalysis may show proteinuria and microscopic hematuria. Patients with cryoglobulinemia will have a positive cryoglobulin assay, increased IgM, and often decreased complement.7 It also is worth noting that millipede burns might resemble child abuse in pediatric patients, necessitating further evaluation.5
It is unusual to see a millipede burn in nontropical regions. Therefore, the identification of our patient’s millipede burn was notable and serves as a reminder to keep this diagnosis in the differential when caring for patients with pigmentary changes. An accurate diagnosis hinges on being alert to a millipede exposure history and recognizing the clinical manifestations. For affected patients, it may be beneficial to recommend they advise friends and relatives to avoid skin contact with millipedes and most importantly to avoid stepping on them with bare feet.
Millipedes. National Wildlife Federation. Accessed October 15, 2025. https://www.nwf.org/Educational-Resources/Wildlife-Guide/Invertebrates/Millipedes
Pennini SN, Rebello PFB, Guerra MdGVB, et al. Millipede accident with unusual dermatological lesion. An Bras Dermatol. 2019;94:765-767. doi:10.1016/j.abd.2019.10.003
Lima CAJ, Cardoso JLC, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda Class (“millipedes“). An Bras Dermatol. 2010;85:391-392. doi:10.1590/s0365-05962910000300018
De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila). 2011;49:187-190. doi:10.3109/15563650.2011.560855
Lacy FA, Elston DM. What’s eating you? millipede burns. Cutis. 2019;103:195-196.
Neto ASH, Filho FB, Martins G. Skin lesions simulating blue toe syndrome caused by prolonged contact with a millipede. Rev Soc Bras Med Trop. 2014;47:257-258. doi:10.1590/0037-8682-0212-2013
Sampaio FMS, Valviesse VRGdA, Lyra-da-Silva JO, et al. Pain and hyperpigmentation of the toes: a quiz. hyperpigmentation of the toes caused by millipedes. Acta Derm Venereol. 2014;94:253-254. doi:10.2340/00015555-1645
Millipedes. National Wildlife Federation. Accessed October 15, 2025. https://www.nwf.org/Educational-Resources/Wildlife-Guide/Invertebrates/Millipedes
Pennini SN, Rebello PFB, Guerra MdGVB, et al. Millipede accident with unusual dermatological lesion. An Bras Dermatol. 2019;94:765-767. doi:10.1016/j.abd.2019.10.003
Lima CAJ, Cardoso JLC, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda Class (“millipedes“). An Bras Dermatol. 2010;85:391-392. doi:10.1590/s0365-05962910000300018
De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila). 2011;49:187-190. doi:10.3109/15563650.2011.560855
Lacy FA, Elston DM. What’s eating you? millipede burns. Cutis. 2019;103:195-196.
Neto ASH, Filho FB, Martins G. Skin lesions simulating blue toe syndrome caused by prolonged contact with a millipede. Rev Soc Bras Med Trop. 2014;47:257-258. doi:10.1590/0037-8682-0212-2013
Sampaio FMS, Valviesse VRGdA, Lyra-da-Silva JO, et al. Pain and hyperpigmentation of the toes: a quiz. hyperpigmentation of the toes caused by millipedes. Acta Derm Venereol. 2014;94:253-254. doi:10.2340/00015555-1645
PRACTICE POINTS
- Millipede burns can resemble ischemia. The most common site of a millipede burn is the feet.
- Diagnosing a millipede burn hinges on obtaining a detailed history, viewing the site under a dermatoscope, and carefully assessing the temperature and pulse of the affected area.
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
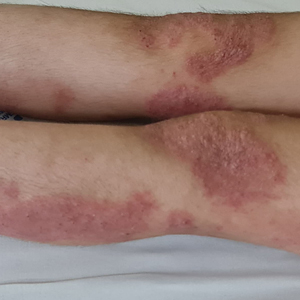
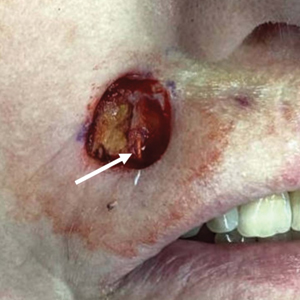
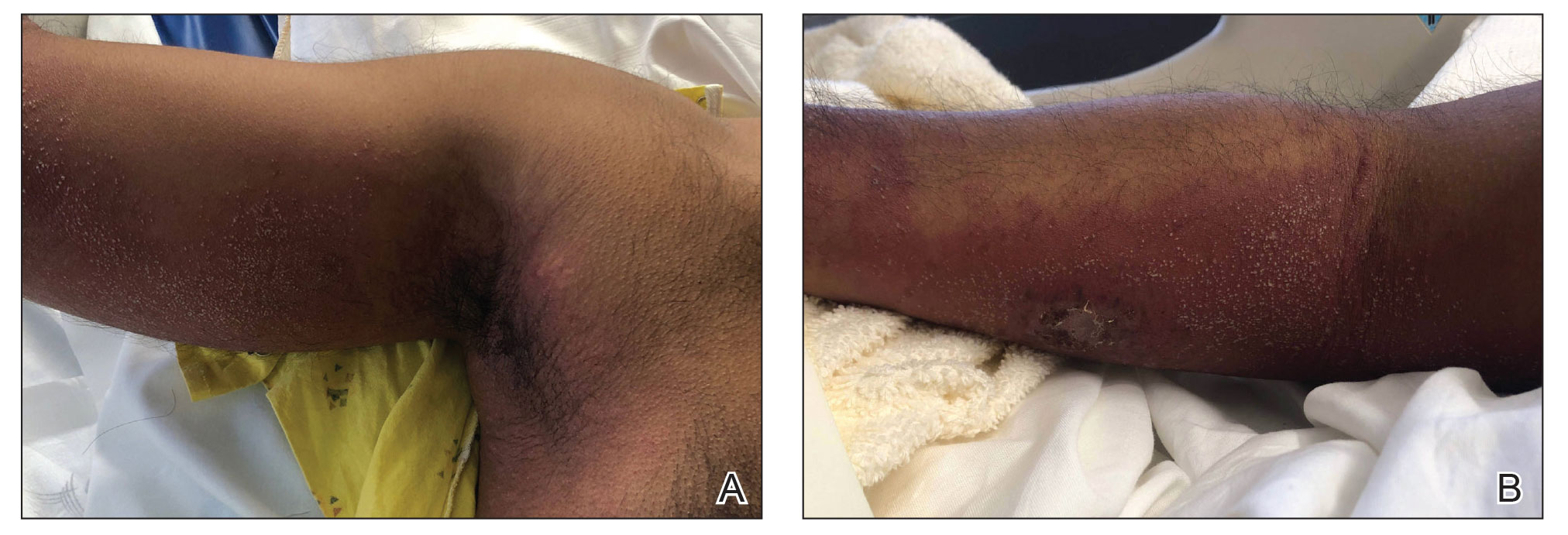
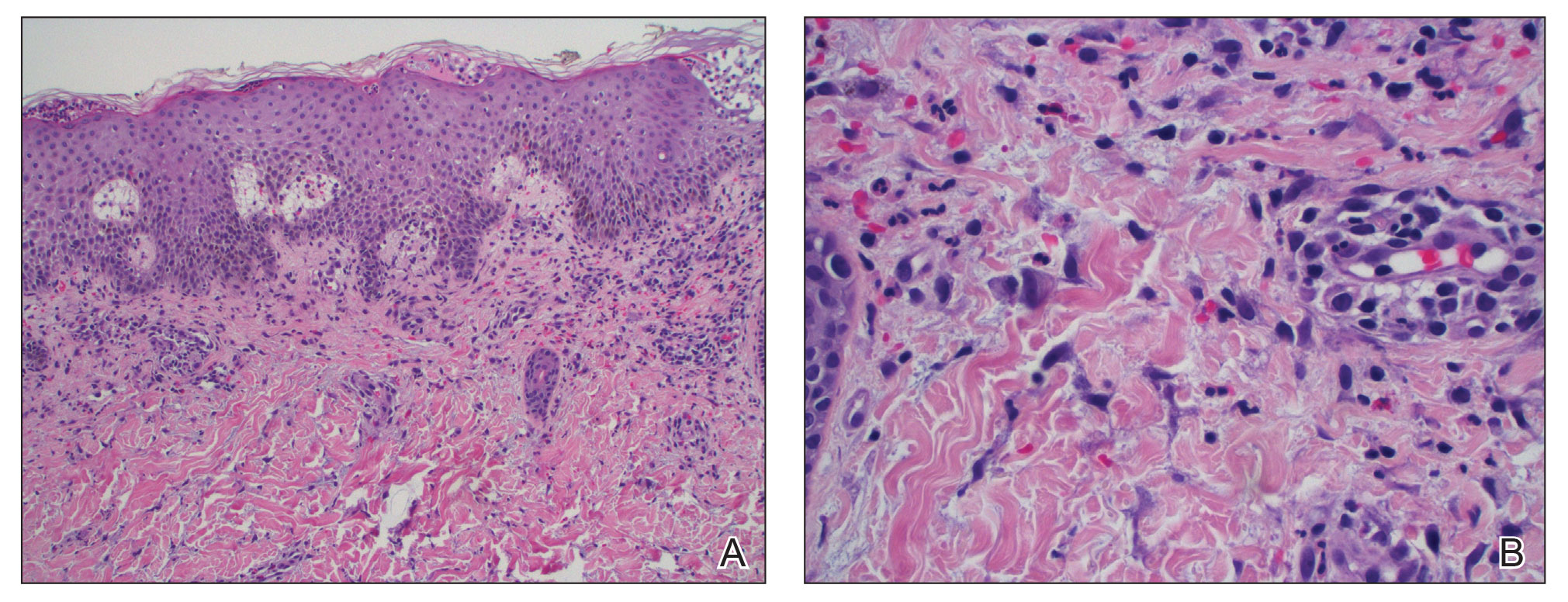
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.
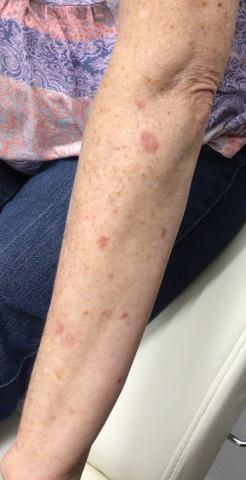
The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).
The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
PRACTICE POINTS
- Tapinarof cream 1% can be absorbed systemically and cause acute generalized exanthematous pustulosis (AGEP).
- Clinical configuration and histology can be useful to distinguish AGEP from mimickers.
- Topical application of drugs in general, particularly over large body surface areas, may lead to systemic drug eruptions.
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
To the Editor:
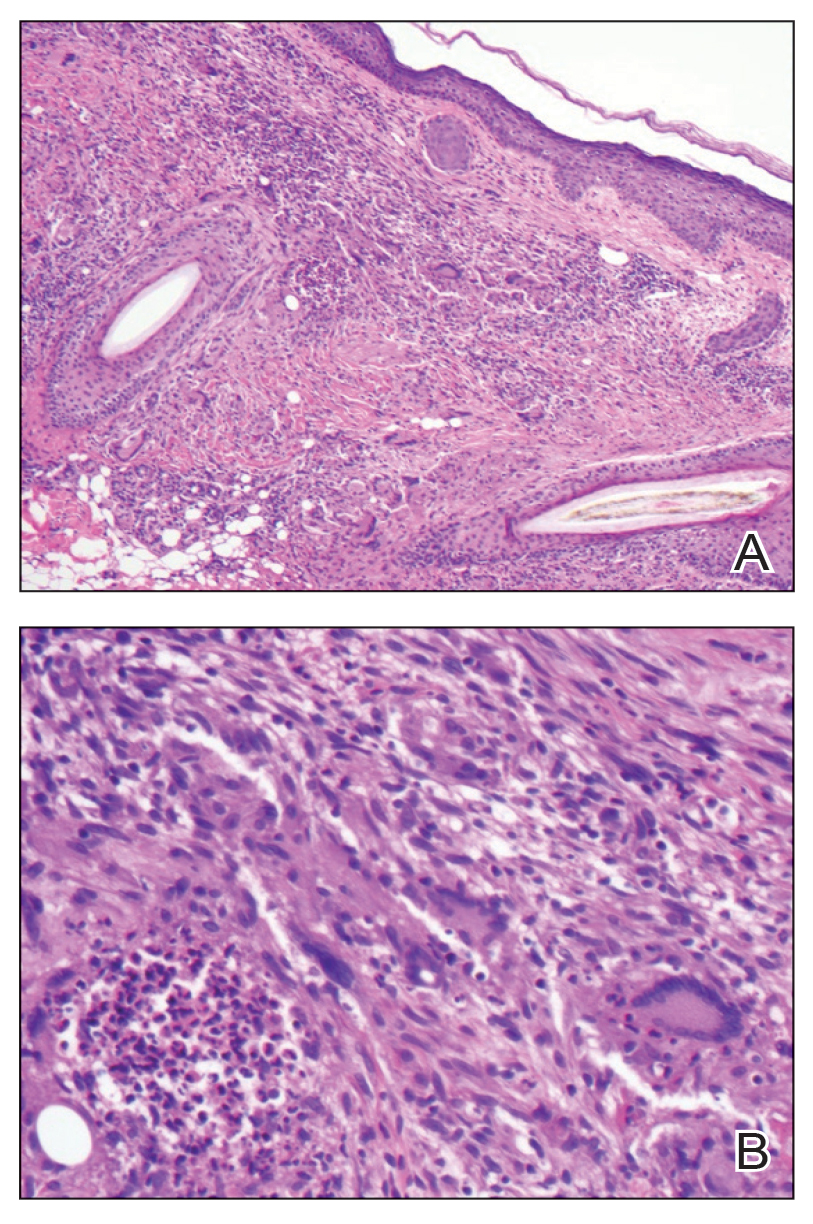
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
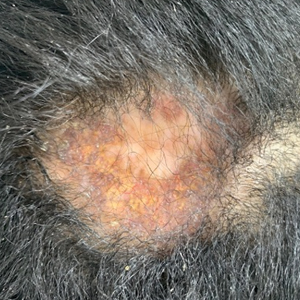
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.
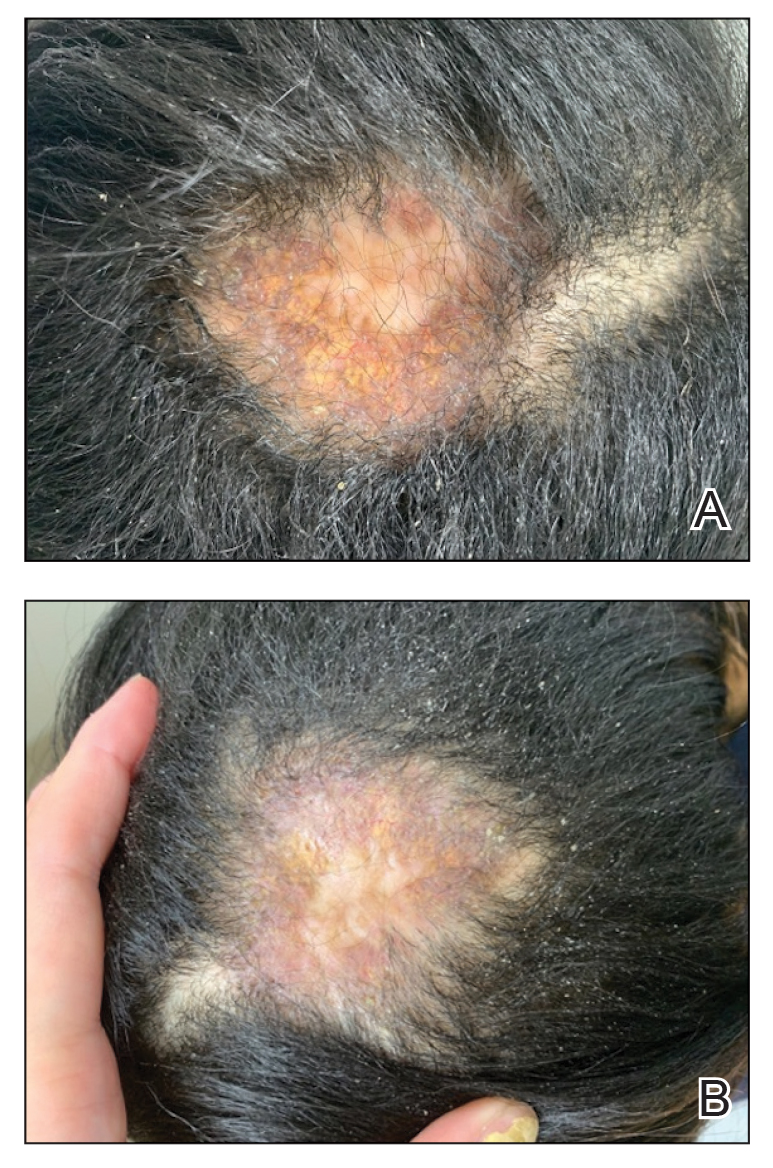
We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
PRACTICE POINTS
- In skin of color, necrobiotic xanthogranuloma can appear orange or brown compared to its yellow appearance in lighter skin types.
- When necrobiotic xanthogranuloma is suspected, a thorough malignancy workup should be conducted.
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
To the Editor:
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases and is characterized by age-related morphology and distribution of lesions. Although AD can manifest at any age, it often develops during childhood, with an estimated worldwide prevalence of 15% to 25% in children and 1% to 10% in adults.1 Clinical manifestation includes chronic or recurrent xerosis, pruritic eczematous lesions involving the flexural and extensor areas, and cutaneous infections. Immediate skin test reactivity and elevated total IgE levels can be found in up to 80% of patients.2
Although the pathogenesis of AD is complex, multifactorial, and not completely understood, some studies have highlighted the central role of a type 2 immune response, resulting in skin barrier dysfunction, cutaneous inflammation, and neuroimmune dysregulation.3,4 The primary goals of treatment are to mitigate these factors through improvement of symptoms and long-term disease control. Topical emollients are used to repair the epidermal barrier, and topical anti-inflammatory therapy with corticosteroids or calcineurin inhibitors might be applied during flares; however, systemic treatment is essential for patients with moderate to severe AD that is not controlled with topical treatment or phototherapy.5
Until recently, systemic immunosuppressant agents such as corticosteroids, cyclosporine, and methotrexate were the only systemic treatment options for severe AD; however, their effectiveness is limited and they may cause serious long-term adverse events, limiting their regular usage, especially in children.6
Therapies that target type 2 immune responses include anti–IL-4/IL-13, anti–IL-13, and anti–IL-31 biologics. Dupilumab is a fully human monoclonal antibody targeting the type 2 immune response. This biologic directly binds to IL-4Rα,which prevents signaling by both the IL-4 and IL-13 pathways. Dupilumab was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe AD, with demonstrated efficacy and a favorable safety profile.5
In addition to biologics, Janus kinase (JAK) inhibitors belong to the small-molecule class. These drugs block the JAK/STAT intracellular signaling pathway, leading to inhibition of downstream effects triggered by several cytokines related to AD pathogenesis. Upadacitinib is an oral JAK inhibitor that was approved by the FDA in 2022 for treatment of severe AD in adults and children aged 12 years and older. This drug promotes a selective and reversible JAK-1 inhibition and has demonstrated rapid onset of action and a sustained reduction in the signs and symptoms of AD.7 We report the case of a child with recalcitrant severe AD that showed significant clinical improvement following off-label treatment with upadacitinib after showing a poor clinical response to dupilumab.
A 9-year-old girl presented to our pediatrics department with progressive worsening of severe AD over the previous 2 years. The patient had been diagnosed with AD at 6 months old, at which time she was treated with several prescribed moisturizers, topical and systemic corticosteroids, and calcineurin inhibitors with no clinical improvement.
The patient initially presented to us for evaluation of severe pruritus and associated sleep loss at age 7 years; physical examination revealed severe xerosis and disseminated pruritic eczematous lesions. Her SCORAD (SCORing Atopic Dermatitis) score was 70 (range, 0-103), and laboratory testing showed a high eosinophil count (1.5×103/μL [range, 0-0.6×103], 13%) and IgE level (1686 κU/L [range, 0-90]); a skin prick test on the forearm was positive for Blomia tropicalis.
Following her presentation with severe AD at 7 years old, the patient was prescribed systemic treatments including methotrexate and cyclosporine. During treatment with these agents, she presented to our department with several bacterial skin infections that required oral and intravenous antibiotics for treatment. These agents ultimately were discontinued after 12 months due to the adverse effects and poor clinical improvement. At age 8 years, the patient received an initial 600-mg dose of dupilumab followed by 300 mg subcutaneously every 4 weeks for 6 months along with topical corticosteroids and emollients. During treatment with dupilumab, the patient showed no clinical improvement (SCORAD score, 62). Therefore, we decided to change the dose to 200 mg every 2 weeks. The patient still showed no improvement and presented at age 9 years with moderate conjunctivitis and oculocutaneous infection caused by herpes simplex virus, which required treatment with oral acyclovir (Figure 1).
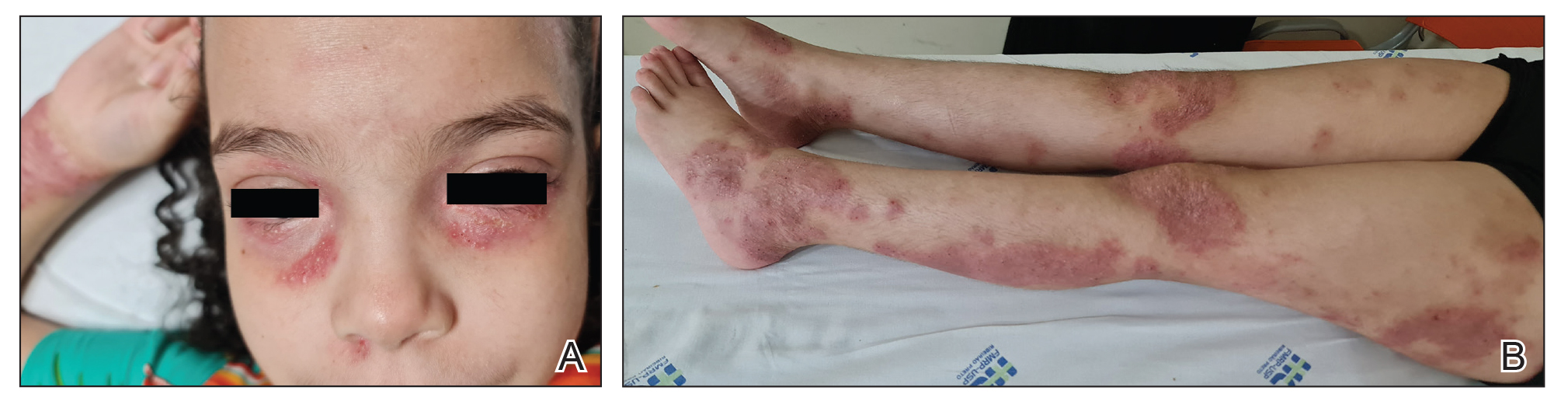
Considering the severe and refractory clinical course and the poor response to the recommended treatments for the patient’s age, oral upadacitinib was administered off label at a dose of 15 mg once daily after informed consent was obtained from her parents. She returned for follow-up once weekly for 1 month. Three days after starting treatment with upadacitinib, she showed considerable improvement in itch, and her SCORAD score decreased from 62 to 31 after 15 days. After 2 months of treatment, she reported no pruritus or sleep loss, and her SCORAD score was 4.5 (Figure 2). The results of a complete blood count, coagulation function test, and liver and kidney function tests were normal at 6-month and 12-month follow-up during upadacitinib therapy. No adverse effects were observed. The patient currently has completed 18 months of treatment, and the disease remains in complete remission.
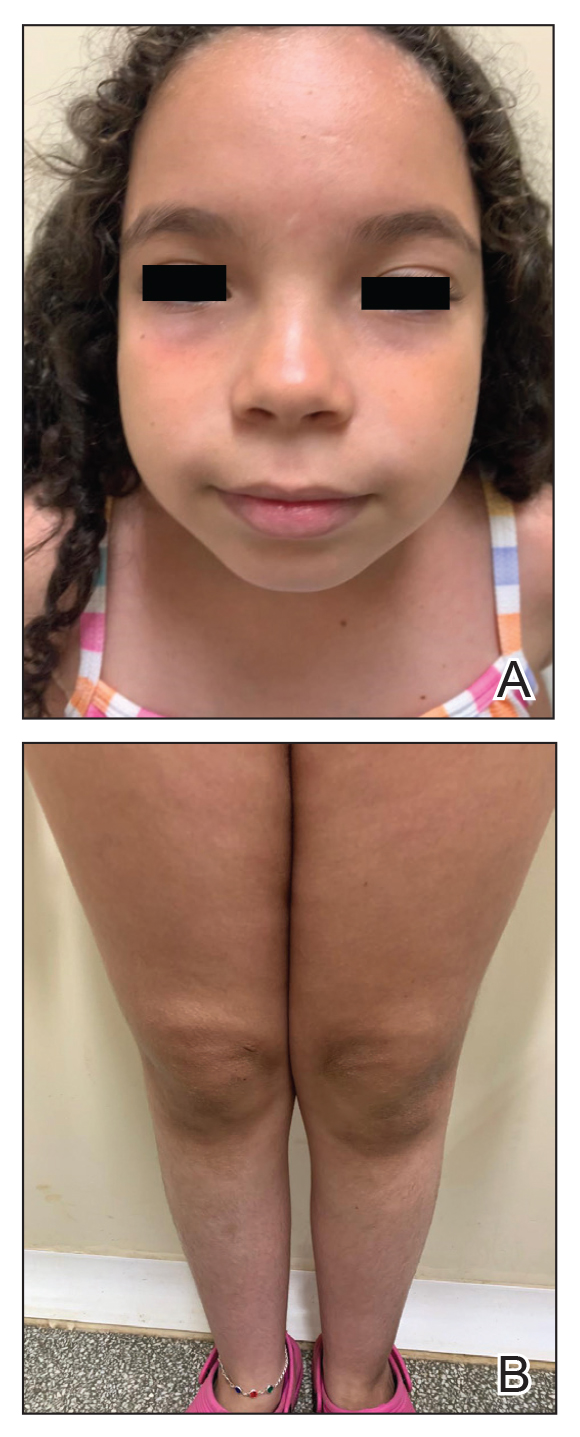
Atopic dermatitis is highly prevalent in children. According to the International Study of Asthma and Allergies in Childhood, the prevalence of eczema in 2009 was 8.2% among children aged 6 to 7 years and 5% among adolescents aged between 13 and 14 years in Brazil; severe AD was present in 1.5% of children in both age groups.8
The main systemic therapies currently available for patients with severe AD are immunosuppressants, biologics, and small-molecule drugs. The considerable adverse effects of immunosuppressants limit their application. Dupilumab is considered the first-line treatment for children with severe AD. Clinical trials and case reports have demonstrated that dupilumab is effective in patients with AD, promoting notable improvement of pruritic eczematous lesions and quality-of-life scores.9 Dupilumab has been approved by the FDA for children older than 6 months, and some studies have shown up to a 49% reduction of pruritus in this age group.9 The main reported adverse effects were mild conjunctivitis and oral herpes simplex virus infection.9,10
Upadacitinib is a reversible and selective JAK-1 inhibitor approved by the FDA for treatment of severe AD in patients aged 12 years and older. A multicenter, randomized, double-blind, placebo-controlled trial evaluated adolescents (12-17 years) and adults (18-75 years) with moderate to severe AD who were randomly assigned (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily for 16 weeks.11 A higher proportion of patients achieved an Eczema Area and Severity Index score of 75 at week 16 with both upadacitinib 15 mg daily (70%) and 30 mg daily (80%) compared to placebo. Improvements also were observed in both SCORAD and pruritus scores. The most commonly reported adverse events were acne, lipid profile abnormalities, and herpes zoster infection.11
Our patient was a child with severe refractory AD that demonstrated a poor treatment response to dupilumab. When switched to off-label upadacitinib, her disease was effectively controlled; the treatment also was well tolerated with no adverse effects. Reports of upadacitinib used to treat AD in patients younger than 12 years are limited in the literature. One case report described a 9-year-old child with concurrent alopecia areata and severe AD who was successfully treated off label with upadacitinib.12 A clinical trial also has evaluated the pharmacokinetics, safety, and tolerability of upadacitinib in children aged 2 to 12 years with severe AD (ClinicalTrials.gov Identifier: NCT03646604); although the trial was completed in 2024, at the time of this review (July 2025), the results have not been published.
Interestingly, there have been a few reports of adults with severe AD that failed to respond to treatment with immunosuppressants and dupilumab but showed notable clinical improvement when therapy was switched to upadacitinib,13,14 as we noticed with our patient. These findings suggest that the JAK-STAT intracellular signaling pathway plays an important role in the pathogenesis of AD.
Continued development of safe and efficient targeted treatment for children with severe AD is critical. Upadacitinib was a safe and effective option for treatment of refractory and severe AD in our patient; however, further studies are needed to confirm both the efficacy and safety of JAK inhibitors in this age group.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34 :2717-2744.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venererol. 1980;92:44-47.
- Nakahara T, Kido-Nakahara M, Tsuji G, et al. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130-139.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I—systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409-1431.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132:274-312.
- Rick JW, Lio P, Atluri S, et al. Atopic dermatitis: a guide to transitioning to janus kinase inhibitors. Dermatitis. 2023;34:297-300.
- Prado E, Pastorino AC, Harari DK, et al. Severe atopic dermatitis: a practical treatment guide from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq Asma Alerg Imunol. 2022;6:432-467.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908-919.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 ;397:2151-2168.
- Yu D, Ren Y. Upadacitinib for successful treatment of alopecia universalis in a child: a case report and literature review. Acta Derm Venererol. 2023;103:adv5578.
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35:E15346.
- Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. 2021;32:E85-E86.
To the Editor:
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases and is characterized by age-related morphology and distribution of lesions. Although AD can manifest at any age, it often develops during childhood, with an estimated worldwide prevalence of 15% to 25% in children and 1% to 10% in adults.1 Clinical manifestation includes chronic or recurrent xerosis, pruritic eczematous lesions involving the flexural and extensor areas, and cutaneous infections. Immediate skin test reactivity and elevated total IgE levels can be found in up to 80% of patients.2
Although the pathogenesis of AD is complex, multifactorial, and not completely understood, some studies have highlighted the central role of a type 2 immune response, resulting in skin barrier dysfunction, cutaneous inflammation, and neuroimmune dysregulation.3,4 The primary goals of treatment are to mitigate these factors through improvement of symptoms and long-term disease control. Topical emollients are used to repair the epidermal barrier, and topical anti-inflammatory therapy with corticosteroids or calcineurin inhibitors might be applied during flares; however, systemic treatment is essential for patients with moderate to severe AD that is not controlled with topical treatment or phototherapy.5
Until recently, systemic immunosuppressant agents such as corticosteroids, cyclosporine, and methotrexate were the only systemic treatment options for severe AD; however, their effectiveness is limited and they may cause serious long-term adverse events, limiting their regular usage, especially in children.6
Therapies that target type 2 immune responses include anti–IL-4/IL-13, anti–IL-13, and anti–IL-31 biologics. Dupilumab is a fully human monoclonal antibody targeting the type 2 immune response. This biologic directly binds to IL-4Rα,which prevents signaling by both the IL-4 and IL-13 pathways. Dupilumab was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe AD, with demonstrated efficacy and a favorable safety profile.5
In addition to biologics, Janus kinase (JAK) inhibitors belong to the small-molecule class. These drugs block the JAK/STAT intracellular signaling pathway, leading to inhibition of downstream effects triggered by several cytokines related to AD pathogenesis. Upadacitinib is an oral JAK inhibitor that was approved by the FDA in 2022 for treatment of severe AD in adults and children aged 12 years and older. This drug promotes a selective and reversible JAK-1 inhibition and has demonstrated rapid onset of action and a sustained reduction in the signs and symptoms of AD.7 We report the case of a child with recalcitrant severe AD that showed significant clinical improvement following off-label treatment with upadacitinib after showing a poor clinical response to dupilumab.
A 9-year-old girl presented to our pediatrics department with progressive worsening of severe AD over the previous 2 years. The patient had been diagnosed with AD at 6 months old, at which time she was treated with several prescribed moisturizers, topical and systemic corticosteroids, and calcineurin inhibitors with no clinical improvement.
The patient initially presented to us for evaluation of severe pruritus and associated sleep loss at age 7 years; physical examination revealed severe xerosis and disseminated pruritic eczematous lesions. Her SCORAD (SCORing Atopic Dermatitis) score was 70 (range, 0-103), and laboratory testing showed a high eosinophil count (1.5×103/μL [range, 0-0.6×103], 13%) and IgE level (1686 κU/L [range, 0-90]); a skin prick test on the forearm was positive for Blomia tropicalis.
Following her presentation with severe AD at 7 years old, the patient was prescribed systemic treatments including methotrexate and cyclosporine. During treatment with these agents, she presented to our department with several bacterial skin infections that required oral and intravenous antibiotics for treatment. These agents ultimately were discontinued after 12 months due to the adverse effects and poor clinical improvement. At age 8 years, the patient received an initial 600-mg dose of dupilumab followed by 300 mg subcutaneously every 4 weeks for 6 months along with topical corticosteroids and emollients. During treatment with dupilumab, the patient showed no clinical improvement (SCORAD score, 62). Therefore, we decided to change the dose to 200 mg every 2 weeks. The patient still showed no improvement and presented at age 9 years with moderate conjunctivitis and oculocutaneous infection caused by herpes simplex virus, which required treatment with oral acyclovir (Figure 1).

Considering the severe and refractory clinical course and the poor response to the recommended treatments for the patient’s age, oral upadacitinib was administered off label at a dose of 15 mg once daily after informed consent was obtained from her parents. She returned for follow-up once weekly for 1 month. Three days after starting treatment with upadacitinib, she showed considerable improvement in itch, and her SCORAD score decreased from 62 to 31 after 15 days. After 2 months of treatment, she reported no pruritus or sleep loss, and her SCORAD score was 4.5 (Figure 2). The results of a complete blood count, coagulation function test, and liver and kidney function tests were normal at 6-month and 12-month follow-up during upadacitinib therapy. No adverse effects were observed. The patient currently has completed 18 months of treatment, and the disease remains in complete remission.

Atopic dermatitis is highly prevalent in children. According to the International Study of Asthma and Allergies in Childhood, the prevalence of eczema in 2009 was 8.2% among children aged 6 to 7 years and 5% among adolescents aged between 13 and 14 years in Brazil; severe AD was present in 1.5% of children in both age groups.8
The main systemic therapies currently available for patients with severe AD are immunosuppressants, biologics, and small-molecule drugs. The considerable adverse effects of immunosuppressants limit their application. Dupilumab is considered the first-line treatment for children with severe AD. Clinical trials and case reports have demonstrated that dupilumab is effective in patients with AD, promoting notable improvement of pruritic eczematous lesions and quality-of-life scores.9 Dupilumab has been approved by the FDA for children older than 6 months, and some studies have shown up to a 49% reduction of pruritus in this age group.9 The main reported adverse effects were mild conjunctivitis and oral herpes simplex virus infection.9,10
Upadacitinib is a reversible and selective JAK-1 inhibitor approved by the FDA for treatment of severe AD in patients aged 12 years and older. A multicenter, randomized, double-blind, placebo-controlled trial evaluated adolescents (12-17 years) and adults (18-75 years) with moderate to severe AD who were randomly assigned (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily for 16 weeks.11 A higher proportion of patients achieved an Eczema Area and Severity Index score of 75 at week 16 with both upadacitinib 15 mg daily (70%) and 30 mg daily (80%) compared to placebo. Improvements also were observed in both SCORAD and pruritus scores. The most commonly reported adverse events were acne, lipid profile abnormalities, and herpes zoster infection.11
Our patient was a child with severe refractory AD that demonstrated a poor treatment response to dupilumab. When switched to off-label upadacitinib, her disease was effectively controlled; the treatment also was well tolerated with no adverse effects. Reports of upadacitinib used to treat AD in patients younger than 12 years are limited in the literature. One case report described a 9-year-old child with concurrent alopecia areata and severe AD who was successfully treated off label with upadacitinib.12 A clinical trial also has evaluated the pharmacokinetics, safety, and tolerability of upadacitinib in children aged 2 to 12 years with severe AD (ClinicalTrials.gov Identifier: NCT03646604); although the trial was completed in 2024, at the time of this review (July 2025), the results have not been published.
Interestingly, there have been a few reports of adults with severe AD that failed to respond to treatment with immunosuppressants and dupilumab but showed notable clinical improvement when therapy was switched to upadacitinib,13,14 as we noticed with our patient. These findings suggest that the JAK-STAT intracellular signaling pathway plays an important role in the pathogenesis of AD.
Continued development of safe and efficient targeted treatment for children with severe AD is critical. Upadacitinib was a safe and effective option for treatment of refractory and severe AD in our patient; however, further studies are needed to confirm both the efficacy and safety of JAK inhibitors in this age group.
To the Editor:
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases and is characterized by age-related morphology and distribution of lesions. Although AD can manifest at any age, it often develops during childhood, with an estimated worldwide prevalence of 15% to 25% in children and 1% to 10% in adults.1 Clinical manifestation includes chronic or recurrent xerosis, pruritic eczematous lesions involving the flexural and extensor areas, and cutaneous infections. Immediate skin test reactivity and elevated total IgE levels can be found in up to 80% of patients.2
Although the pathogenesis of AD is complex, multifactorial, and not completely understood, some studies have highlighted the central role of a type 2 immune response, resulting in skin barrier dysfunction, cutaneous inflammation, and neuroimmune dysregulation.3,4 The primary goals of treatment are to mitigate these factors through improvement of symptoms and long-term disease control. Topical emollients are used to repair the epidermal barrier, and topical anti-inflammatory therapy with corticosteroids or calcineurin inhibitors might be applied during flares; however, systemic treatment is essential for patients with moderate to severe AD that is not controlled with topical treatment or phototherapy.5
Until recently, systemic immunosuppressant agents such as corticosteroids, cyclosporine, and methotrexate were the only systemic treatment options for severe AD; however, their effectiveness is limited and they may cause serious long-term adverse events, limiting their regular usage, especially in children.6
Therapies that target type 2 immune responses include anti–IL-4/IL-13, anti–IL-13, and anti–IL-31 biologics. Dupilumab is a fully human monoclonal antibody targeting the type 2 immune response. This biologic directly binds to IL-4Rα,which prevents signaling by both the IL-4 and IL-13 pathways. Dupilumab was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe AD, with demonstrated efficacy and a favorable safety profile.5
In addition to biologics, Janus kinase (JAK) inhibitors belong to the small-molecule class. These drugs block the JAK/STAT intracellular signaling pathway, leading to inhibition of downstream effects triggered by several cytokines related to AD pathogenesis. Upadacitinib is an oral JAK inhibitor that was approved by the FDA in 2022 for treatment of severe AD in adults and children aged 12 years and older. This drug promotes a selective and reversible JAK-1 inhibition and has demonstrated rapid onset of action and a sustained reduction in the signs and symptoms of AD.7 We report the case of a child with recalcitrant severe AD that showed significant clinical improvement following off-label treatment with upadacitinib after showing a poor clinical response to dupilumab.
A 9-year-old girl presented to our pediatrics department with progressive worsening of severe AD over the previous 2 years. The patient had been diagnosed with AD at 6 months old, at which time she was treated with several prescribed moisturizers, topical and systemic corticosteroids, and calcineurin inhibitors with no clinical improvement.
The patient initially presented to us for evaluation of severe pruritus and associated sleep loss at age 7 years; physical examination revealed severe xerosis and disseminated pruritic eczematous lesions. Her SCORAD (SCORing Atopic Dermatitis) score was 70 (range, 0-103), and laboratory testing showed a high eosinophil count (1.5×103/μL [range, 0-0.6×103], 13%) and IgE level (1686 κU/L [range, 0-90]); a skin prick test on the forearm was positive for Blomia tropicalis.
Following her presentation with severe AD at 7 years old, the patient was prescribed systemic treatments including methotrexate and cyclosporine. During treatment with these agents, she presented to our department with several bacterial skin infections that required oral and intravenous antibiotics for treatment. These agents ultimately were discontinued after 12 months due to the adverse effects and poor clinical improvement. At age 8 years, the patient received an initial 600-mg dose of dupilumab followed by 300 mg subcutaneously every 4 weeks for 6 months along with topical corticosteroids and emollients. During treatment with dupilumab, the patient showed no clinical improvement (SCORAD score, 62). Therefore, we decided to change the dose to 200 mg every 2 weeks. The patient still showed no improvement and presented at age 9 years with moderate conjunctivitis and oculocutaneous infection caused by herpes simplex virus, which required treatment with oral acyclovir (Figure 1).

Considering the severe and refractory clinical course and the poor response to the recommended treatments for the patient’s age, oral upadacitinib was administered off label at a dose of 15 mg once daily after informed consent was obtained from her parents. She returned for follow-up once weekly for 1 month. Three days after starting treatment with upadacitinib, she showed considerable improvement in itch, and her SCORAD score decreased from 62 to 31 after 15 days. After 2 months of treatment, she reported no pruritus or sleep loss, and her SCORAD score was 4.5 (Figure 2). The results of a complete blood count, coagulation function test, and liver and kidney function tests were normal at 6-month and 12-month follow-up during upadacitinib therapy. No adverse effects were observed. The patient currently has completed 18 months of treatment, and the disease remains in complete remission.

Atopic dermatitis is highly prevalent in children. According to the International Study of Asthma and Allergies in Childhood, the prevalence of eczema in 2009 was 8.2% among children aged 6 to 7 years and 5% among adolescents aged between 13 and 14 years in Brazil; severe AD was present in 1.5% of children in both age groups.8
The main systemic therapies currently available for patients with severe AD are immunosuppressants, biologics, and small-molecule drugs. The considerable adverse effects of immunosuppressants limit their application. Dupilumab is considered the first-line treatment for children with severe AD. Clinical trials and case reports have demonstrated that dupilumab is effective in patients with AD, promoting notable improvement of pruritic eczematous lesions and quality-of-life scores.9 Dupilumab has been approved by the FDA for children older than 6 months, and some studies have shown up to a 49% reduction of pruritus in this age group.9 The main reported adverse effects were mild conjunctivitis and oral herpes simplex virus infection.9,10
Upadacitinib is a reversible and selective JAK-1 inhibitor approved by the FDA for treatment of severe AD in patients aged 12 years and older. A multicenter, randomized, double-blind, placebo-controlled trial evaluated adolescents (12-17 years) and adults (18-75 years) with moderate to severe AD who were randomly assigned (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily for 16 weeks.11 A higher proportion of patients achieved an Eczema Area and Severity Index score of 75 at week 16 with both upadacitinib 15 mg daily (70%) and 30 mg daily (80%) compared to placebo. Improvements also were observed in both SCORAD and pruritus scores. The most commonly reported adverse events were acne, lipid profile abnormalities, and herpes zoster infection.11
Our patient was a child with severe refractory AD that demonstrated a poor treatment response to dupilumab. When switched to off-label upadacitinib, her disease was effectively controlled; the treatment also was well tolerated with no adverse effects. Reports of upadacitinib used to treat AD in patients younger than 12 years are limited in the literature. One case report described a 9-year-old child with concurrent alopecia areata and severe AD who was successfully treated off label with upadacitinib.12 A clinical trial also has evaluated the pharmacokinetics, safety, and tolerability of upadacitinib in children aged 2 to 12 years with severe AD (ClinicalTrials.gov Identifier: NCT03646604); although the trial was completed in 2024, at the time of this review (July 2025), the results have not been published.
Interestingly, there have been a few reports of adults with severe AD that failed to respond to treatment with immunosuppressants and dupilumab but showed notable clinical improvement when therapy was switched to upadacitinib,13,14 as we noticed with our patient. These findings suggest that the JAK-STAT intracellular signaling pathway plays an important role in the pathogenesis of AD.
Continued development of safe and efficient targeted treatment for children with severe AD is critical. Upadacitinib was a safe and effective option for treatment of refractory and severe AD in our patient; however, further studies are needed to confirm both the efficacy and safety of JAK inhibitors in this age group.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34 :2717-2744.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venererol. 1980;92:44-47.
- Nakahara T, Kido-Nakahara M, Tsuji G, et al. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130-139.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I—systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409-1431.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132:274-312.
- Rick JW, Lio P, Atluri S, et al. Atopic dermatitis: a guide to transitioning to janus kinase inhibitors. Dermatitis. 2023;34:297-300.
- Prado E, Pastorino AC, Harari DK, et al. Severe atopic dermatitis: a practical treatment guide from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq Asma Alerg Imunol. 2022;6:432-467.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908-919.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 ;397:2151-2168.
- Yu D, Ren Y. Upadacitinib for successful treatment of alopecia universalis in a child: a case report and literature review. Acta Derm Venererol. 2023;103:adv5578.
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35:E15346.
- Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. 2021;32:E85-E86.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34 :2717-2744.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venererol. 1980;92:44-47.
- Nakahara T, Kido-Nakahara M, Tsuji G, et al. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130-139.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I—systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409-1431.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132:274-312.
- Rick JW, Lio P, Atluri S, et al. Atopic dermatitis: a guide to transitioning to janus kinase inhibitors. Dermatitis. 2023;34:297-300.
- Prado E, Pastorino AC, Harari DK, et al. Severe atopic dermatitis: a practical treatment guide from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq Asma Alerg Imunol. 2022;6:432-467.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908-919.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 ;397:2151-2168.
- Yu D, Ren Y. Upadacitinib for successful treatment of alopecia universalis in a child: a case report and literature review. Acta Derm Venererol. 2023;103:adv5578.
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35:E15346.
- Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. 2021;32:E85-E86.
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
PRACTICE POINTS
- Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases in pediatric patients.
- Dupilumab is the first-line treatment for severe AD in children and is approved for use in patients aged 6 months and older. Janus kinase inhibitors are approved only for patients aged 12 years and older.
- Upadacitinib may be a safe treatment option for severe AD in children, even those younger than 12 years.
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
PRACTICE POINTS
- Drug-induced hyperpigmentation is a common cause of acquired hyperpigmentation and should be evaluated after metabolic or endocrine causes are ruled out.
- Belumosudil for chronic graft-vs-host disease can induce rapid-onset diffuse bronzing hyperpigmentation, even in the absence of other systemic or laboratory abnormalities.
- Treatment entails discontinuation of the offending agent and limitation of exacerbating factors such as sun exposure.
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.
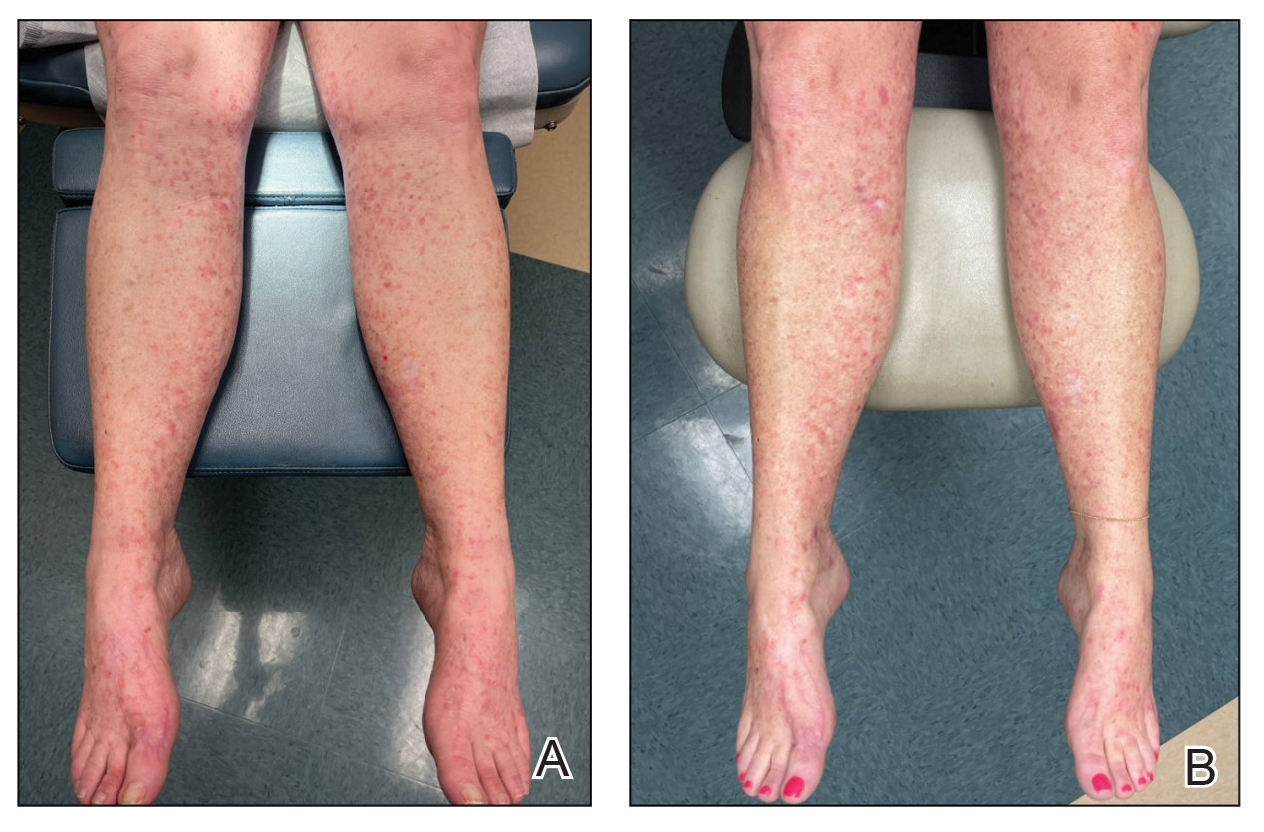
For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
PRACTICE POINTS
- There are numerous field-directed therapies for actinic keratoses (AKs); however, efficacy and tolerability vary among the available treatments.
- Diclofenac gel 1% is an affordable option that could potentially increase accessibility and decrease cost of field therapy for the treatment of AKs, while maintaining therapeutic efficacy.
Low-Dose Oral Naltrexone for Darier Disease
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.
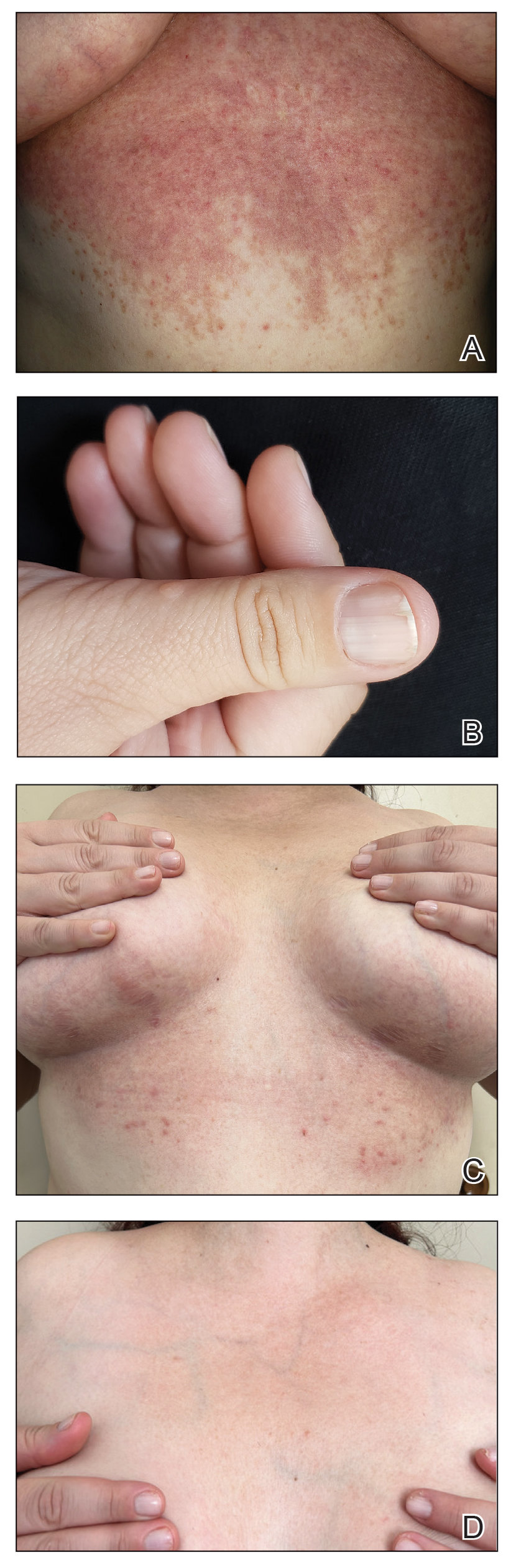
Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2
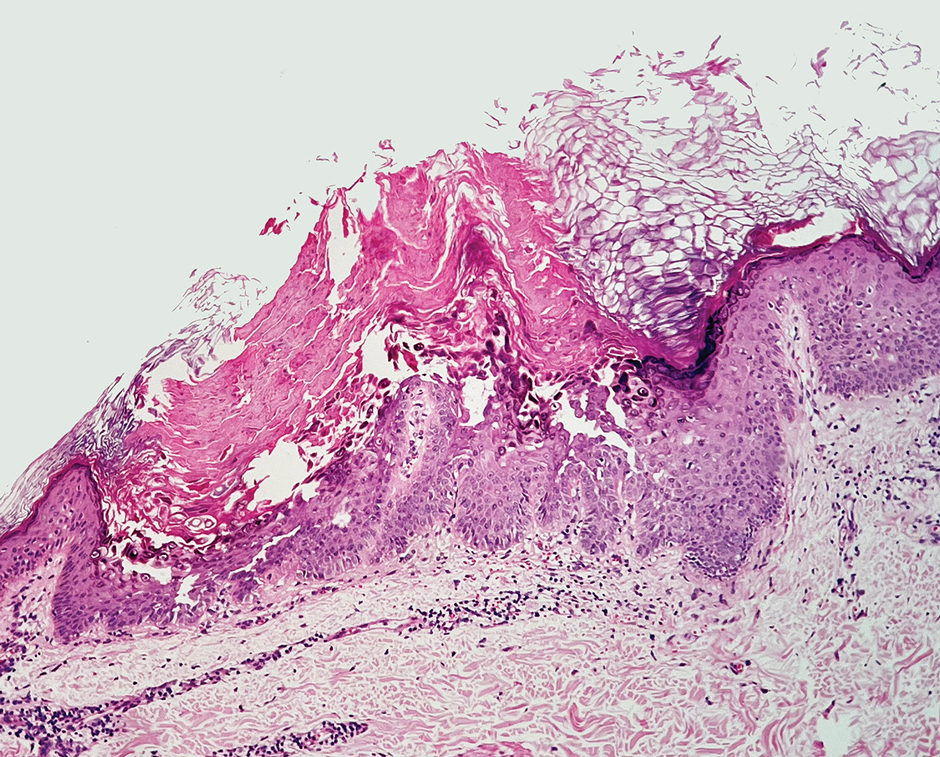
Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.

Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2

Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.

Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2

Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
Practice Points
- Consider low-dose naltrexone as a potential treatment option for patients with Darier disease, as it regulates opioid receptors and has shown benefits in enhancing epidermal differentiation, wound healing, and anti-inflammatory effects.
- Further research is needed to validate the efficacy and safety of low-dose naltrexone in treating Darier disease considering its observed clinical improvement in this single patient case.
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.
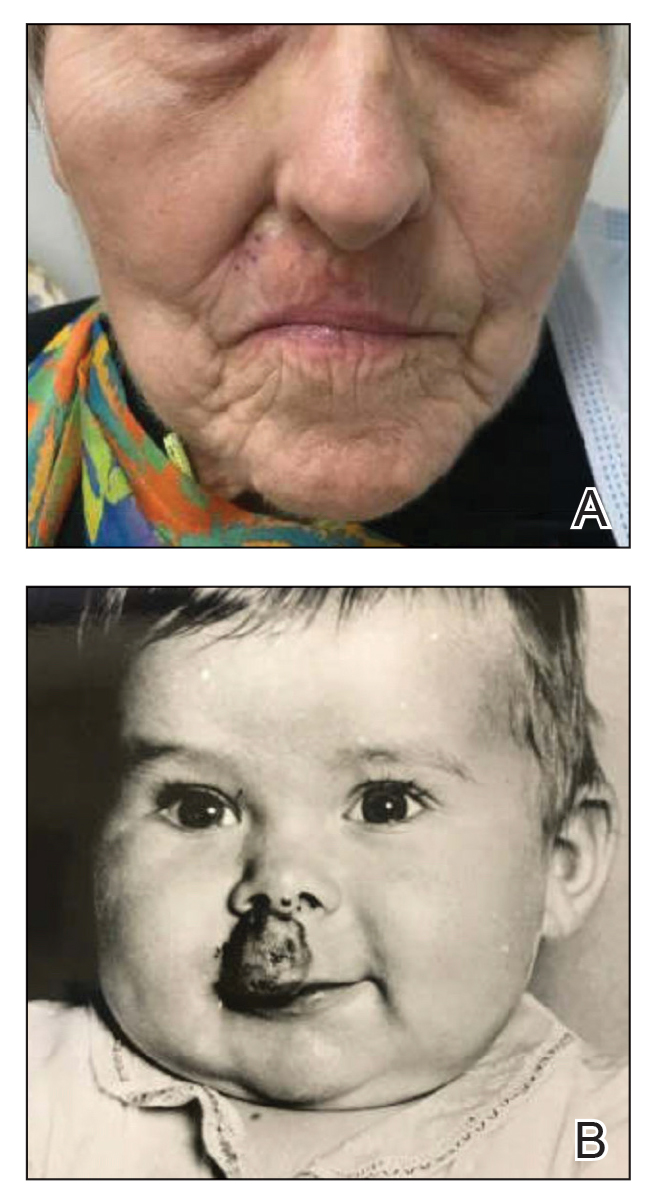
Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.
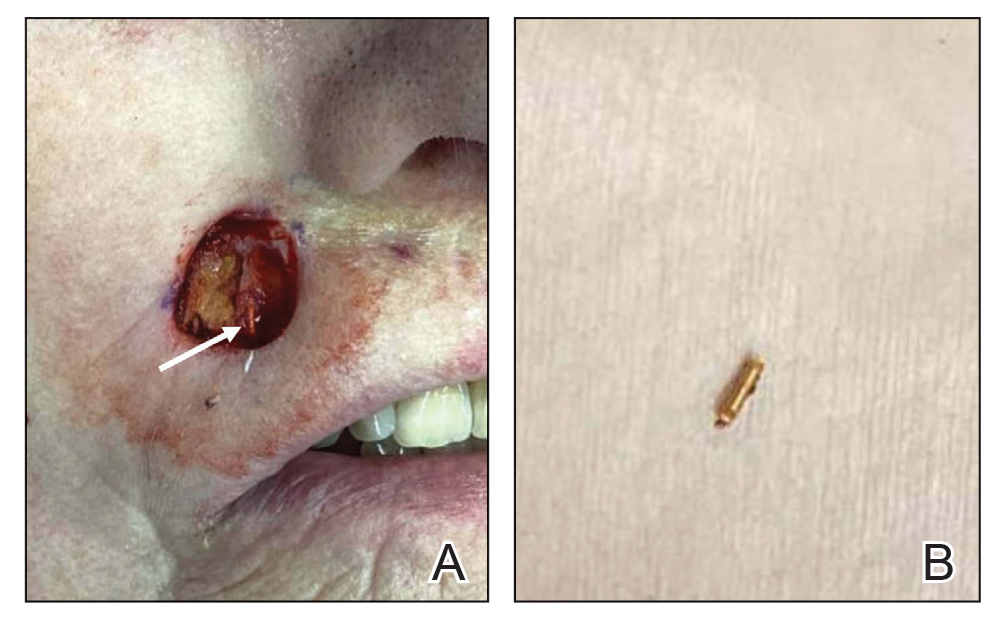
Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
PRACTICE POINTS
- Historical use of ionizing radiation to treat skin disease is a risk factor for basal cell carcinoma (BCC).
- Mohs micrographic surgery is the treatment of choice for BCC in high-risk areas such as the nose, eyelids, and lips, where tissue conservation and complete margin control are essential.
- Elderly patients should be screened for less common sources of radiation exposure for better risk stratification and to determine appropriate intervals for follow-up with a dermatologist.
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.
Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.

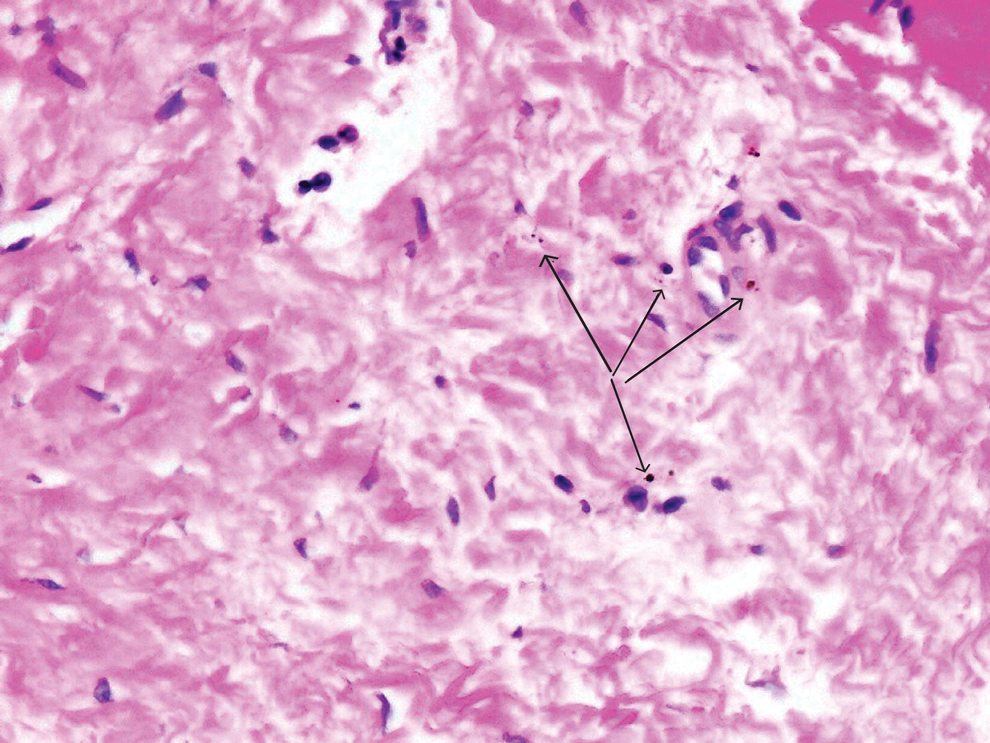
Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.

Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.



Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.

Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.



Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
PRACTICE POINTS
- Dermatologists should be aware that uveitis can develop as a delayed hypersensitivity reaction to tattoo ink, particularly in patients with blue ink tattoos.
- It is important to rule out systemic conditions such as sarcoidosis in patients presenting with uveitis and a history of tattoos.
- In a patient with progressive ocular symptoms, carbon dioxide laser ablation may be an effective treatment option if other potential rheumatologic conditions and sarcoidosis have been ruled out and other therapies have not resulted in improvement of symptoms.
- Continuous monitoring of ocular symptoms and intraocular pressure is vital to prevent complications such as glaucoma and potential blindness.