User login
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.
However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
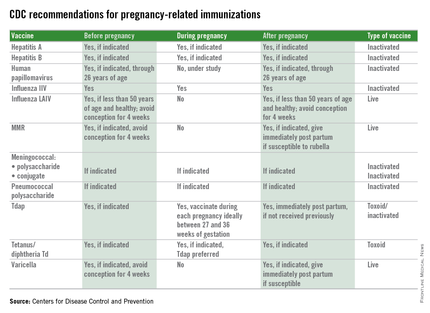
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].

