User login
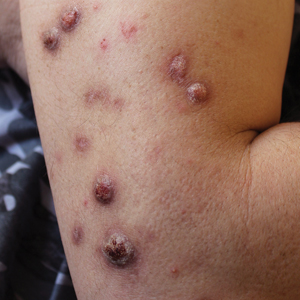
Prurigo nodularis (PN), a condition that historically has been a challenge to treat, now has a US Food and Drug Administration (FDA)–approved therapy—dupilumab—with other agents in the pipeline. As clinicians, we recognize PN as typically symmetric, keratotic, papular and nodular lesions presenting in older adults with chronic pruritus; patients with atopic dermatitis make up roughly half of patients with PN, but a workup for pruritus is indicated in other settings.1 In the United States, Black patients are 3.4-times more likely than White patients to have PN.2 The differential diagnosis includes conditions such nodular scabies, pemphigoid nodularis, acquired perforating disorders, and hypertrophic lichen planus, which also should be considered, especially in cases that are refractory to first-line therapies. Recent breakthroughs in therapy have come from substantial progress in our understanding of the pathogenesis of PN as driven by disorders of cytokine expression and/or neurocutaneous aberrations. We review progress in the treatment of PN over the last 3 years.
Treatment Guidelines
In 2020, an expert panel published consensus treatment guidelines for PN.1 The panel, which proposed a 4-tiered approach targeting both neural and immunologic mechanisms in the pathogenesis of PN, emphasized the importance of tailoring treatment to the individual patient. Topical therapies remained the mainstay of treatment, with agents such as topical capsaicin, ketamine, lidocaine, and amitriptyline targeting the neural component and topical corticosteroids, calcineurin inhibitors, and calcipotriol and intralesional corticosteroids targeting the immunologic component. Phototherapy, methotrexate, cyclosporine, antidepressants, and gabapentinoids used with varying degrees of success were noted to have acceptable tolerability.1
FDA-Approved Therapy
In September 2022, the FDA approved dupilumab for the treatment of PN. An antagonist of the IL-4 receptor, dupilumab was found to reduce both pruritus and skin lesions over a 24-week period in 2 phase 3 clinical trials.3 Results also demonstrated progressive improvements in measures assessing quality of life and pruritus over the study period, suggesting that continued treatment could lead to even further improvements in these measures. Adverse events were minimal and similar between the dupilumab- and placebo-treated groups.3
The FDA approval of dupilumab is a promising step in decreasing the disease burden of widespread or refractory PN, both for patients and the health care system. The treatment of patients with PN has been more challenging due to comorbidities, including mental health conditions, endocrine disorders, cardiovascular conditions, renal conditions, malignancy, and HIV.4,5 These comorbidities can complicate the use of traditional systemic and immunosuppressive agents. Dupilumab has virtually no contraindications and has demonstrated safety in almost all patient populations.6
Consistent insurance coverage for patients who respond to dupilumab remains to be determined. A review investigating the use of dupilumab in patients with atopic dermatitis at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) found that of 179 patients, 67 (37.4%) did not start dupilumab, mainly due to insurance denial (34/179 [19%]) or copay (20/179 [11%]). Medicare patients were less likely to receive treatment compared to those on private insurance or Medicaid.7 In a recent review of 701 patients with PN, the mean age was 64.8 years,5 highlighting the concern about obtaining insurance coverage for dupilumab in this population given the higher likelihood that these patients will be on Medicare. Prescribers should be aware that coverage denials are likely and should be prepared to advocate for their patients by citing recent studies to hopefully obtain coverage for dupilumab in the treatment of PN. Resources such as the Dupixent MyWay program (https://www.dupixent.com/support-savings/dupixent-my-way) can provide useful recommendations for pursuing insurance approval for this agent.
Investigation of Janus Kinase Inhibitors
Emerging data suggest that Janus kinase (JAK) inhibitors may be beneficial in the treatment of PN. Patients with refractory PN have been treated off label with the JAK inhibitor tofacitinib at a dosage of 5 mg twice daily with improvement in symptoms and minimal side effects.8,9 Similarly, a case report showed that off-label use of the JAK inhibitor baricitinib resulted in marked improvement in pruritus and clearance of lesions at a dosage of 4 mg daily, with reduction in pruritus seen as early as 1 week after treatment initiation.10 Although most patients are able to tolerate JAK inhibitors, known side effects include acne, viral infections, gastrointestinal tract upset, and the potential increased risk for malignancy.11 The use of topical JAK inhibitors such as ruxolitinib has not yet been studied in PN, though cost may limit use to localized disease.
Other New Therapies
Recent case reports and case series have found the vitamin A derivative alitretinoin to be an effective treatment for recalcitrant PN, typically at a dosage of 30 mg daily.12,13 Sustained remission was noted even after discontinuation of the medication.12 Alitretinoin, which has been demonstrated to be effective in treating dermatitis,14 was well tolerated. Similar to JAK inhibitors, there are minimal data investigating the use of topical retinoids in the treatment of localized PN.
Topical cannabinoids have shown benefit in the treatment of pruritus15 and may be beneficial for the treatment of PN, though there currently are limited data in the literature. With the use of both medical and legal recreational marijuana on the rise, there is an increased interest in cannabinoids, particularly as many patients consider these agents to be more “natural”—and therefore preferable—treatment options. As the use of cannabis derivatives become more commonplace in both traditional and complementary medicine, providers should be prepared to field questions from patients about their potential for PN.
Finally, the IL-31RA inhibitor nemolizumab also has shown promise in the treatment of PN. A recent study suggested that nemolizumab helps modulate inflammatory and neural signaling in PN.16 Nemolizumab has been granted breakthrough therapy designation for the treatment of pruritus in PN based on a phase 2 study that demonstrated improvement in pruritus and skin lesions in a group of 70 patients with moderate to severe PN.17 Nemolizumab, which is used to treat pruritus in atopic dermatitis, has minimal side effects including upper respiratory tract infections and peripheral edema.18
Final Thoughts
Prurigo nodularis historically has been considered difficult to treat, particularly in those with widespread lesions. Dupilumab—the first FDA-approved treatment of PN—is now an exciting option, not just for patients with underlying atopic dermatitis. Not all patients will respond to the medication, and the ease of obtaining insurance approval has yet to be established; therefore, having other treatment options will be imperative. In patients with recalcitrant disease, several other treatment options have shown promise in the treatment of PN; in particular, JAK inhibitors, alitretinoin, and nemolizumab should be considered in patients with widespread refractory PN who are willing to try alternative agents. Ongoing research should be focused on these medications as well as on the development of other novel treatments aimed at relieving affected patients.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus [published online July 15, 2020]. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Joel MZ, Hydol-Smith J, Kambala A, et al. Prevalence and comorbidity burden of prurigo nodularis in United States adults enrolled in the All of Us research program. J Am Acad Dermatol. 2023;89:1056-1058. doi:10.1016/j.jaad.2023.06.045
- Dupixent. Package insert. Regeneron Pharmaceuticals, Inc; 2017.
- Khosravi H, Zhang S, Anderson AM, et al. Dupilumab drug survival, treatment failures, and insurance approval at a tertiary care center in the United States. J Am Acad Dermatol. 2020;82:1023-1024. doi:10.1016/j.jaad.2019.12.034
- Liu T, Chu Y, Wang Y, et al. Successful treatment of prurigo nodularis with tofacitinib: the experience from a single center. Int J Dermatol. 2023;62:E293-E295. doi:10.1111/ijd.16568
- Molloy OE, Kearney N, Byrne N, et al. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45:918-920. doi:10.1111/ced.14320
- Yin M, Wu R, Chen J, et al. Successful treatment of refractory prurigo nodularis with baricitinib. Dermatol Ther. 2022;35:E15642. doi:10.1111/dth.15642
- Klein B, Treudler R, Simon JC. JAK-inhibitors in dermatology—small molecules, big impact? overview of the mechanism of action, previous study results and potential adverse effects. J Dtsch Dermatol Ges. 2022;20:19-24. doi:10.1111/ddg.14668
- Chung BY, Um JY, Kang SY, et al. Oral alitretinoin for patients with refractory prurigo. Medicina (Kaunas). 2020;56:599. doi:10.3390/medicina56110599
- Maqbool T, Kraft JN. Alitretinoin for prurigo nodularis. Clin Exp Dermatol. 2021;46:362-363. doi:10.1111/ced.14385
- Grahovac M, Molin S, Prinz JC, et al. Treatment of atopic eczema with oral alitretinoin. Br J Dermatol. 2010;162:217-218. doi:10.1111/j.1365-2133.2009.09522.x
- Avila C, Massick S, Kaffenberger BH, et al. Cannabinoids for the treatment of chronic pruritus: a review. J Am Acad Dermatol. 2020;82:1205-1212. doi:10.1016/j.jaad.2020.01.036
- Deng J, Liao V, Parthasarathy V, et al. Modulation of neuroimmune and epithelial dysregulation in patients with moderate to severe prurigo nodularis treated with nemolizumab. JAMA Dermatol. 2023;159:977-985. doi:10.1001/jamadermatol.2023.2609
- Park B. Nemolizumab gets breakthrough therapy status for prurigo nodularis. Medical Professionals Reference website. Published December 9, 2019. Accessed November 13, 2023. https://www.empr.com/home/news/nemolizumab-gets-breakthrough-therapy-status-for-prurigo-nodularis/
- Labib A, Vander Does A, Yosipovitch G. Nemolizumab for atopic dermatitis. Drugs Today (Barc). 2022;58:159-173. doi:10.1358/dot.2022.58.4.3378056
Prurigo nodularis (PN), a condition that historically has been a challenge to treat, now has a US Food and Drug Administration (FDA)–approved therapy—dupilumab—with other agents in the pipeline. As clinicians, we recognize PN as typically symmetric, keratotic, papular and nodular lesions presenting in older adults with chronic pruritus; patients with atopic dermatitis make up roughly half of patients with PN, but a workup for pruritus is indicated in other settings.1 In the United States, Black patients are 3.4-times more likely than White patients to have PN.2 The differential diagnosis includes conditions such nodular scabies, pemphigoid nodularis, acquired perforating disorders, and hypertrophic lichen planus, which also should be considered, especially in cases that are refractory to first-line therapies. Recent breakthroughs in therapy have come from substantial progress in our understanding of the pathogenesis of PN as driven by disorders of cytokine expression and/or neurocutaneous aberrations. We review progress in the treatment of PN over the last 3 years.
Treatment Guidelines
In 2020, an expert panel published consensus treatment guidelines for PN.1 The panel, which proposed a 4-tiered approach targeting both neural and immunologic mechanisms in the pathogenesis of PN, emphasized the importance of tailoring treatment to the individual patient. Topical therapies remained the mainstay of treatment, with agents such as topical capsaicin, ketamine, lidocaine, and amitriptyline targeting the neural component and topical corticosteroids, calcineurin inhibitors, and calcipotriol and intralesional corticosteroids targeting the immunologic component. Phototherapy, methotrexate, cyclosporine, antidepressants, and gabapentinoids used with varying degrees of success were noted to have acceptable tolerability.1
FDA-Approved Therapy
In September 2022, the FDA approved dupilumab for the treatment of PN. An antagonist of the IL-4 receptor, dupilumab was found to reduce both pruritus and skin lesions over a 24-week period in 2 phase 3 clinical trials.3 Results also demonstrated progressive improvements in measures assessing quality of life and pruritus over the study period, suggesting that continued treatment could lead to even further improvements in these measures. Adverse events were minimal and similar between the dupilumab- and placebo-treated groups.3
The FDA approval of dupilumab is a promising step in decreasing the disease burden of widespread or refractory PN, both for patients and the health care system. The treatment of patients with PN has been more challenging due to comorbidities, including mental health conditions, endocrine disorders, cardiovascular conditions, renal conditions, malignancy, and HIV.4,5 These comorbidities can complicate the use of traditional systemic and immunosuppressive agents. Dupilumab has virtually no contraindications and has demonstrated safety in almost all patient populations.6
Consistent insurance coverage for patients who respond to dupilumab remains to be determined. A review investigating the use of dupilumab in patients with atopic dermatitis at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) found that of 179 patients, 67 (37.4%) did not start dupilumab, mainly due to insurance denial (34/179 [19%]) or copay (20/179 [11%]). Medicare patients were less likely to receive treatment compared to those on private insurance or Medicaid.7 In a recent review of 701 patients with PN, the mean age was 64.8 years,5 highlighting the concern about obtaining insurance coverage for dupilumab in this population given the higher likelihood that these patients will be on Medicare. Prescribers should be aware that coverage denials are likely and should be prepared to advocate for their patients by citing recent studies to hopefully obtain coverage for dupilumab in the treatment of PN. Resources such as the Dupixent MyWay program (https://www.dupixent.com/support-savings/dupixent-my-way) can provide useful recommendations for pursuing insurance approval for this agent.
Investigation of Janus Kinase Inhibitors
Emerging data suggest that Janus kinase (JAK) inhibitors may be beneficial in the treatment of PN. Patients with refractory PN have been treated off label with the JAK inhibitor tofacitinib at a dosage of 5 mg twice daily with improvement in symptoms and minimal side effects.8,9 Similarly, a case report showed that off-label use of the JAK inhibitor baricitinib resulted in marked improvement in pruritus and clearance of lesions at a dosage of 4 mg daily, with reduction in pruritus seen as early as 1 week after treatment initiation.10 Although most patients are able to tolerate JAK inhibitors, known side effects include acne, viral infections, gastrointestinal tract upset, and the potential increased risk for malignancy.11 The use of topical JAK inhibitors such as ruxolitinib has not yet been studied in PN, though cost may limit use to localized disease.
Other New Therapies
Recent case reports and case series have found the vitamin A derivative alitretinoin to be an effective treatment for recalcitrant PN, typically at a dosage of 30 mg daily.12,13 Sustained remission was noted even after discontinuation of the medication.12 Alitretinoin, which has been demonstrated to be effective in treating dermatitis,14 was well tolerated. Similar to JAK inhibitors, there are minimal data investigating the use of topical retinoids in the treatment of localized PN.
Topical cannabinoids have shown benefit in the treatment of pruritus15 and may be beneficial for the treatment of PN, though there currently are limited data in the literature. With the use of both medical and legal recreational marijuana on the rise, there is an increased interest in cannabinoids, particularly as many patients consider these agents to be more “natural”—and therefore preferable—treatment options. As the use of cannabis derivatives become more commonplace in both traditional and complementary medicine, providers should be prepared to field questions from patients about their potential for PN.
Finally, the IL-31RA inhibitor nemolizumab also has shown promise in the treatment of PN. A recent study suggested that nemolizumab helps modulate inflammatory and neural signaling in PN.16 Nemolizumab has been granted breakthrough therapy designation for the treatment of pruritus in PN based on a phase 2 study that demonstrated improvement in pruritus and skin lesions in a group of 70 patients with moderate to severe PN.17 Nemolizumab, which is used to treat pruritus in atopic dermatitis, has minimal side effects including upper respiratory tract infections and peripheral edema.18
Final Thoughts
Prurigo nodularis historically has been considered difficult to treat, particularly in those with widespread lesions. Dupilumab—the first FDA-approved treatment of PN—is now an exciting option, not just for patients with underlying atopic dermatitis. Not all patients will respond to the medication, and the ease of obtaining insurance approval has yet to be established; therefore, having other treatment options will be imperative. In patients with recalcitrant disease, several other treatment options have shown promise in the treatment of PN; in particular, JAK inhibitors, alitretinoin, and nemolizumab should be considered in patients with widespread refractory PN who are willing to try alternative agents. Ongoing research should be focused on these medications as well as on the development of other novel treatments aimed at relieving affected patients.
Prurigo nodularis (PN), a condition that historically has been a challenge to treat, now has a US Food and Drug Administration (FDA)–approved therapy—dupilumab—with other agents in the pipeline. As clinicians, we recognize PN as typically symmetric, keratotic, papular and nodular lesions presenting in older adults with chronic pruritus; patients with atopic dermatitis make up roughly half of patients with PN, but a workup for pruritus is indicated in other settings.1 In the United States, Black patients are 3.4-times more likely than White patients to have PN.2 The differential diagnosis includes conditions such nodular scabies, pemphigoid nodularis, acquired perforating disorders, and hypertrophic lichen planus, which also should be considered, especially in cases that are refractory to first-line therapies. Recent breakthroughs in therapy have come from substantial progress in our understanding of the pathogenesis of PN as driven by disorders of cytokine expression and/or neurocutaneous aberrations. We review progress in the treatment of PN over the last 3 years.
Treatment Guidelines
In 2020, an expert panel published consensus treatment guidelines for PN.1 The panel, which proposed a 4-tiered approach targeting both neural and immunologic mechanisms in the pathogenesis of PN, emphasized the importance of tailoring treatment to the individual patient. Topical therapies remained the mainstay of treatment, with agents such as topical capsaicin, ketamine, lidocaine, and amitriptyline targeting the neural component and topical corticosteroids, calcineurin inhibitors, and calcipotriol and intralesional corticosteroids targeting the immunologic component. Phototherapy, methotrexate, cyclosporine, antidepressants, and gabapentinoids used with varying degrees of success were noted to have acceptable tolerability.1
FDA-Approved Therapy
In September 2022, the FDA approved dupilumab for the treatment of PN. An antagonist of the IL-4 receptor, dupilumab was found to reduce both pruritus and skin lesions over a 24-week period in 2 phase 3 clinical trials.3 Results also demonstrated progressive improvements in measures assessing quality of life and pruritus over the study period, suggesting that continued treatment could lead to even further improvements in these measures. Adverse events were minimal and similar between the dupilumab- and placebo-treated groups.3
The FDA approval of dupilumab is a promising step in decreasing the disease burden of widespread or refractory PN, both for patients and the health care system. The treatment of patients with PN has been more challenging due to comorbidities, including mental health conditions, endocrine disorders, cardiovascular conditions, renal conditions, malignancy, and HIV.4,5 These comorbidities can complicate the use of traditional systemic and immunosuppressive agents. Dupilumab has virtually no contraindications and has demonstrated safety in almost all patient populations.6
Consistent insurance coverage for patients who respond to dupilumab remains to be determined. A review investigating the use of dupilumab in patients with atopic dermatitis at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) found that of 179 patients, 67 (37.4%) did not start dupilumab, mainly due to insurance denial (34/179 [19%]) or copay (20/179 [11%]). Medicare patients were less likely to receive treatment compared to those on private insurance or Medicaid.7 In a recent review of 701 patients with PN, the mean age was 64.8 years,5 highlighting the concern about obtaining insurance coverage for dupilumab in this population given the higher likelihood that these patients will be on Medicare. Prescribers should be aware that coverage denials are likely and should be prepared to advocate for their patients by citing recent studies to hopefully obtain coverage for dupilumab in the treatment of PN. Resources such as the Dupixent MyWay program (https://www.dupixent.com/support-savings/dupixent-my-way) can provide useful recommendations for pursuing insurance approval for this agent.
Investigation of Janus Kinase Inhibitors
Emerging data suggest that Janus kinase (JAK) inhibitors may be beneficial in the treatment of PN. Patients with refractory PN have been treated off label with the JAK inhibitor tofacitinib at a dosage of 5 mg twice daily with improvement in symptoms and minimal side effects.8,9 Similarly, a case report showed that off-label use of the JAK inhibitor baricitinib resulted in marked improvement in pruritus and clearance of lesions at a dosage of 4 mg daily, with reduction in pruritus seen as early as 1 week after treatment initiation.10 Although most patients are able to tolerate JAK inhibitors, known side effects include acne, viral infections, gastrointestinal tract upset, and the potential increased risk for malignancy.11 The use of topical JAK inhibitors such as ruxolitinib has not yet been studied in PN, though cost may limit use to localized disease.
Other New Therapies
Recent case reports and case series have found the vitamin A derivative alitretinoin to be an effective treatment for recalcitrant PN, typically at a dosage of 30 mg daily.12,13 Sustained remission was noted even after discontinuation of the medication.12 Alitretinoin, which has been demonstrated to be effective in treating dermatitis,14 was well tolerated. Similar to JAK inhibitors, there are minimal data investigating the use of topical retinoids in the treatment of localized PN.
Topical cannabinoids have shown benefit in the treatment of pruritus15 and may be beneficial for the treatment of PN, though there currently are limited data in the literature. With the use of both medical and legal recreational marijuana on the rise, there is an increased interest in cannabinoids, particularly as many patients consider these agents to be more “natural”—and therefore preferable—treatment options. As the use of cannabis derivatives become more commonplace in both traditional and complementary medicine, providers should be prepared to field questions from patients about their potential for PN.
Finally, the IL-31RA inhibitor nemolizumab also has shown promise in the treatment of PN. A recent study suggested that nemolizumab helps modulate inflammatory and neural signaling in PN.16 Nemolizumab has been granted breakthrough therapy designation for the treatment of pruritus in PN based on a phase 2 study that demonstrated improvement in pruritus and skin lesions in a group of 70 patients with moderate to severe PN.17 Nemolizumab, which is used to treat pruritus in atopic dermatitis, has minimal side effects including upper respiratory tract infections and peripheral edema.18
Final Thoughts
Prurigo nodularis historically has been considered difficult to treat, particularly in those with widespread lesions. Dupilumab—the first FDA-approved treatment of PN—is now an exciting option, not just for patients with underlying atopic dermatitis. Not all patients will respond to the medication, and the ease of obtaining insurance approval has yet to be established; therefore, having other treatment options will be imperative. In patients with recalcitrant disease, several other treatment options have shown promise in the treatment of PN; in particular, JAK inhibitors, alitretinoin, and nemolizumab should be considered in patients with widespread refractory PN who are willing to try alternative agents. Ongoing research should be focused on these medications as well as on the development of other novel treatments aimed at relieving affected patients.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus [published online July 15, 2020]. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Joel MZ, Hydol-Smith J, Kambala A, et al. Prevalence and comorbidity burden of prurigo nodularis in United States adults enrolled in the All of Us research program. J Am Acad Dermatol. 2023;89:1056-1058. doi:10.1016/j.jaad.2023.06.045
- Dupixent. Package insert. Regeneron Pharmaceuticals, Inc; 2017.
- Khosravi H, Zhang S, Anderson AM, et al. Dupilumab drug survival, treatment failures, and insurance approval at a tertiary care center in the United States. J Am Acad Dermatol. 2020;82:1023-1024. doi:10.1016/j.jaad.2019.12.034
- Liu T, Chu Y, Wang Y, et al. Successful treatment of prurigo nodularis with tofacitinib: the experience from a single center. Int J Dermatol. 2023;62:E293-E295. doi:10.1111/ijd.16568
- Molloy OE, Kearney N, Byrne N, et al. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45:918-920. doi:10.1111/ced.14320
- Yin M, Wu R, Chen J, et al. Successful treatment of refractory prurigo nodularis with baricitinib. Dermatol Ther. 2022;35:E15642. doi:10.1111/dth.15642
- Klein B, Treudler R, Simon JC. JAK-inhibitors in dermatology—small molecules, big impact? overview of the mechanism of action, previous study results and potential adverse effects. J Dtsch Dermatol Ges. 2022;20:19-24. doi:10.1111/ddg.14668
- Chung BY, Um JY, Kang SY, et al. Oral alitretinoin for patients with refractory prurigo. Medicina (Kaunas). 2020;56:599. doi:10.3390/medicina56110599
- Maqbool T, Kraft JN. Alitretinoin for prurigo nodularis. Clin Exp Dermatol. 2021;46:362-363. doi:10.1111/ced.14385
- Grahovac M, Molin S, Prinz JC, et al. Treatment of atopic eczema with oral alitretinoin. Br J Dermatol. 2010;162:217-218. doi:10.1111/j.1365-2133.2009.09522.x
- Avila C, Massick S, Kaffenberger BH, et al. Cannabinoids for the treatment of chronic pruritus: a review. J Am Acad Dermatol. 2020;82:1205-1212. doi:10.1016/j.jaad.2020.01.036
- Deng J, Liao V, Parthasarathy V, et al. Modulation of neuroimmune and epithelial dysregulation in patients with moderate to severe prurigo nodularis treated with nemolizumab. JAMA Dermatol. 2023;159:977-985. doi:10.1001/jamadermatol.2023.2609
- Park B. Nemolizumab gets breakthrough therapy status for prurigo nodularis. Medical Professionals Reference website. Published December 9, 2019. Accessed November 13, 2023. https://www.empr.com/home/news/nemolizumab-gets-breakthrough-therapy-status-for-prurigo-nodularis/
- Labib A, Vander Does A, Yosipovitch G. Nemolizumab for atopic dermatitis. Drugs Today (Barc). 2022;58:159-173. doi:10.1358/dot.2022.58.4.3378056
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus [published online July 15, 2020]. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Joel MZ, Hydol-Smith J, Kambala A, et al. Prevalence and comorbidity burden of prurigo nodularis in United States adults enrolled in the All of Us research program. J Am Acad Dermatol. 2023;89:1056-1058. doi:10.1016/j.jaad.2023.06.045
- Dupixent. Package insert. Regeneron Pharmaceuticals, Inc; 2017.
- Khosravi H, Zhang S, Anderson AM, et al. Dupilumab drug survival, treatment failures, and insurance approval at a tertiary care center in the United States. J Am Acad Dermatol. 2020;82:1023-1024. doi:10.1016/j.jaad.2019.12.034
- Liu T, Chu Y, Wang Y, et al. Successful treatment of prurigo nodularis with tofacitinib: the experience from a single center. Int J Dermatol. 2023;62:E293-E295. doi:10.1111/ijd.16568
- Molloy OE, Kearney N, Byrne N, et al. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45:918-920. doi:10.1111/ced.14320
- Yin M, Wu R, Chen J, et al. Successful treatment of refractory prurigo nodularis with baricitinib. Dermatol Ther. 2022;35:E15642. doi:10.1111/dth.15642
- Klein B, Treudler R, Simon JC. JAK-inhibitors in dermatology—small molecules, big impact? overview of the mechanism of action, previous study results and potential adverse effects. J Dtsch Dermatol Ges. 2022;20:19-24. doi:10.1111/ddg.14668
- Chung BY, Um JY, Kang SY, et al. Oral alitretinoin for patients with refractory prurigo. Medicina (Kaunas). 2020;56:599. doi:10.3390/medicina56110599
- Maqbool T, Kraft JN. Alitretinoin for prurigo nodularis. Clin Exp Dermatol. 2021;46:362-363. doi:10.1111/ced.14385
- Grahovac M, Molin S, Prinz JC, et al. Treatment of atopic eczema with oral alitretinoin. Br J Dermatol. 2010;162:217-218. doi:10.1111/j.1365-2133.2009.09522.x
- Avila C, Massick S, Kaffenberger BH, et al. Cannabinoids for the treatment of chronic pruritus: a review. J Am Acad Dermatol. 2020;82:1205-1212. doi:10.1016/j.jaad.2020.01.036
- Deng J, Liao V, Parthasarathy V, et al. Modulation of neuroimmune and epithelial dysregulation in patients with moderate to severe prurigo nodularis treated with nemolizumab. JAMA Dermatol. 2023;159:977-985. doi:10.1001/jamadermatol.2023.2609
- Park B. Nemolizumab gets breakthrough therapy status for prurigo nodularis. Medical Professionals Reference website. Published December 9, 2019. Accessed November 13, 2023. https://www.empr.com/home/news/nemolizumab-gets-breakthrough-therapy-status-for-prurigo-nodularis/
- Labib A, Vander Does A, Yosipovitch G. Nemolizumab for atopic dermatitis. Drugs Today (Barc). 2022;58:159-173. doi:10.1358/dot.2022.58.4.3378056