User login
Advances in radiotherapy over the past 50 years have dramatically improved outcomes in patients with malignancy. Five-year overall survival rates for Hodgkin lymphoma and non-Hodgkin lymphoma now stand at 80%, and breast cancer survival is 90%.1
Increased longevity, however, has come at the cost of late side effects such as radiation-induced heart disease (RIHD). Cardiac dysfunction due to radiation involves a spectrum of disease processes in patients who have undergone mediastinal, thoracic, or breast radiotherapy and may involve any cardiac structure, including the pericardium, myocardium, valves, conduction system, and coronary arteries.
Overall, compared with nonirradiated patients, patients who have undergone chest radiotherapy have a 2% higher absolute risk of cardiac morbidity and death at 5 years and a 23% increased absolute risk after 20 years.2
This article will review the pathophysiology and epidemiology of RIHD and will offer a practical approach to its diagnosis and management.
MOST DAMAGE IS ENDOTHELIAL
Cardiac myocytes are relatively resistant to radiation damage because of their postmitotic state. But endothelial cells remain sensitive to radiation, and the pathophysiology of most forms of RIHD appears to be associated with damage to endothelial cells. Conventional cardiac risk factors such as hyperlipidemia and smoking have been shown to compound and accelerate radiation-induced endothelial damage in animal models.3
Radiation is believed to result in transient increases in oxidative stress, resulting in formation of reactive oxygen species and a subsequent inflammatory response that includes activation of nuclear factor-kappa B. Upregulation of proinflammatory pathways results in increased expression of matrix metalloproteinases, adhesion molecules, and proinflammatory cytokines and downregulation of vasculoprotective nitric oxide.4 Indirect evidence for radiation-induced vascular inflammation comes from numerous studies that demonstrated increased levels of the proinflammatory cytokines interleukin 6, tumor necrosis factor alpha, and interferon gamma in Japanese atomic bomb survivors.5
RISK FACTORS
Risk factors for RIHD are summarized in Table 1.
The volume of heart irradiated is a major determinant of the development of RIHD.6 A retrospective study of 960 breast cancer patients in Stockholm between 1971 and 1976 found that those who had received the highest doses and volumes of cardiac radiation had a threefold higher risk of cardiac death. By comparison, those with lesser volumes of the heart exposed to radiation had no increase in risk of cardiac death compared with the general population.7
Younger age at the time of radiotherapy is associated with an increased risk of RIHD in breast cancer and lymphoma patients. A retrospective analysis of 635 patients under age 21 with Hodgkin lymphoma treated with radiotherapy showed a relative risk of fatal myocardial infarction of 41.5 compared with a general population matched for age, sex, and race.8
Conventional cardiac risk factors such as smoking, hypertension, diabetes, and hyperlipidemia further increase the risk of RIHD, and radiation increases the cardiotoxicity of chemotherapeutic agents such as anthracyclines.9
In general, high-risk patients are defined as those with at least one risk factor for RIHD who underwent anterior or left-sided chest irradiation (Table 1).10
CORONARY ARTERY DISEASE
Ischemic heart disease is the most common cause of cardiac death in patients who have undergone radiation therapy. Atherosclerotic lesions in RIHD are morphologically identical to those in nonirradiated vessels and are characterized by intimal proliferation, accumulation of lipid-rich macrophages, and plaque formation.11
A retrospective single-institution study of 415 patients with Hodgkin lymphoma who had undergone radiation therapy found the incidence of coronary artery disease 20 years later to be 10%. The mean time to development of coronary artery disease was 9 years, and all patients who developed it had at least one conventional cardiac risk factor.12
A meta-analysis of more than 20,000 breast cancer patients who received radiotherapy in 40 randomized controlled trials found an increase in the rate of non-breast-cancer deaths, primarily from vascular causes (annual event ratio 1.27, P < .0001).13
A randomized controlled trial comparing breast cancer patients who underwent preoperative or postoperative radiotherapy vs those who had surgery alone revealed a significantly higher death rate from coronary artery disease in the postradiotherapy group.7
The risk of radiation-induced coronary artery disease is proportional to both the dose and the duration of radiation therapy. A retrospective study of more than 2,000 women undergoing radiotherapy for breast cancer found that the relative risk of coronary artery disease increased linearly by about 7.4% per Gy of radiation to the heart, with no apparent ceiling.14
The distribution of atherosclerotic coronary arteries correlates well with the areas exposed to the highest doses of radiation. For instance, in left-sided breast cancer, the apex and anterior wall of the heart typically receive the highest doses of radiation; consequently, the left anterior descending and distal diagonal branches are most prominently involved.15 In patients with lymphoma who undergo radiotherapy to mediastinal nodes and in breast cancer patients receiving radiotherapy to the internal mammary chain, basal structures may be exposed as well. Ostial lesions can also be seen in these patients.16
The clinical presentation of coronary artery disease in radiotherapy recipients does not differ significantly from that in the general population. Ischemia may be silent, may lead to classic anginal symptoms, or may cause sudden cardiac death. The incidence of silent myocardial infarction has been reported to be higher after mediastinal radiotherapy than it is in the general population, possibly from damage to nerve endings within the radiation field.17
Management of radiation-associated coronary artery disease
Managing patients with radiation-associated coronary artery disease is challenging, but the therapeutic options remain the same as those in nonirradiated patients and include medical therapy, percutaneous coronary intervention, and coronary artery bypass grafting, depending on the site and extent of disease.18 Although results are conflicting, there does not seem to be a significant difference in the rates of stent restenosis between patients with a history of radiation therapy and the general population.
Percutaneous coronary intervention is generally preferred to coronary artery bypass grafting in these patients for several reasons. Radiation-induced fibrosis of surrounding structures generally makes surgical procedures more difficult,19 and inclusion of the internal mammary artery or internal thoracic artery in the radiation field may result in stenosis of these vessels, rendering them unsuitable for harvesting.20 Moreover, many patients with RIHD have concurrent radiation-induced lung damage, which increases the risk of perioperative pulmonary complications.21
If the coronary lesions are not amenable to percutaneous intervention, a careful valvular evaluation should be performed preoperatively in view of the frequency of radiation-associated valvular disease. In a study of 72 patients with RIHD undergoing coronary artery bypass grafting, 40% required valvular surgery at the time of surgery or shortly thereafter.22
Results of studies of coronary artery bypass graft outcomes in patients with a history of thoracic radiation therapy have been conflicting, but success seems to depend on the status of the internal mammary and internal thoracic arteries.23 Therefore, the patency of these vessels should be elucidated preoperatively by angiography and intraoperatively by visual inspection of the vessels for fibrosis.
A large single-institution study by Wu et al24 revealed higher short-term and long-term mortality rates in patients with RIHD undergoing cardiac surgery than in control patients without RIHD undergoing similar procedures.
VALVULAR DISEASE
Radiation therapy may directly affect heart valves, and both stenotic (Figure 1) and regurgitant lesions have been described. Pathologic findings include leaflet retraction, fibrotic thickening, and late calcification.25
The precise mechanism of radiation-induced valvular disease is unknown but is thought to be a change in the phenotype of valvular interstitial cells from a myofibroblast to an osteoblast-like cell. Radiation results in significant expression of osteogenic factors such as bone morphogenic protein 2, osteopontin, alkaline phosphatase, and runt-related transcription factor 2 by valvular interstitial cells.26
Valvular heart disease is evident in as many as 81% of patients with RIHD, with the aortic and mitral valves affected more commonly than the tricuspid and pulmonic valves.27 Why there are more left-sided valve lesions than pulmonic valve lesions, despite the pulmonic valve’s anterior position in the heart, is unknown but may be due to higher pressures across the left-sided heart valves.
Although valvular disease is common in patients with RIHD, clinically significant disease is not; more than 70% of patients with radiation-induced valvular disease have no symptoms. A study of 38 cases of radiation-induced valvular disease reported a mean time to development of asymptomatic valvular lesions of 11.5 years and an average time to symptomatic valvular dysfunction of 16.5 years, indicating that 5 years seems to be the interval required for progression from asymptomatic to symptomatic valvular RIHD.28
The thickness of the aortomitral curtain (the junction between the base of the anterior mitral leaflet and the aortic root) is an independent predictor of the long-term risk of death in patients with valvular RIHD.29
Management of radiation-induced valvular disease
Management of patients with valvular RIHD poses a major clinical conundrum because of the high rates of perioperative morbidity and death in patients with a history of chest radiotherapy. In one study,23 the long-term mortality rate was 45% in postradiotherapy patients undergoing single-valve surgery and 61% in those undergoing surgery on two or more valves, compared with 13% and 17% in patients with no history of chest radiotherapy.23
Furthermore, valve repair is an unattractive option in these patients because of high failure rates of mitral valve and tricuspid valve repair attributed to ongoing radiotherapy-induced valvular changes after repair.30
As a result, valve replacement is generally preferred in this group. Patients should be advised of the higher risk of perioperative and long-term morbidity and death associated with open heart surgery than in the general population, and that the risks are even higher with repeat open heart surgery.
This risk has implications for the choice of replacement valves in younger patients. Bioprosthetic valves, which deteriorate over time, may not be advisable. Transcatheter aortic valve replacement has been successful in radiation-induced valvular disease and may become the preferred method of aortic valve replacement.31
PERICARDIAL DISEASE
Pericardial disease is a frequent manifestation of RIHD and covers a spectrum of manifestations from acute pericarditis, pericardial effusion, and tamponade to constrictive pericarditis. In a necropsy study, 70% of patients with RIHD were found to have pericardial involvement.32
The mechanism is believed to be radiation-induced microvascular injury resulting in increased capillary permeability and the sometimes rapid development of a protein-rich exudate. Associated inflammation may cause acute pericarditis, which may eventually be complicated by chronic pericarditis. The parietal surface tends to be affected more severely than the epicardium.33
Perhaps as a result of recent advances such as lower radiation doses, equal weighting of the anterior and posterior fields, and subcarinal blocking, incidence rates of pericarditis as low as 2.5% have been reported.34
Pericardial RIHD may be divided into early acute pericarditis, delayed chronic pericardial effusion, and constrictive pericarditis.
Early acute pericarditis is rare and is thought to represent a reaction to tumor necrosis. It is defined as occurring during radiotherapy and occurs almost exclusively with high-dose radiotherapy for lymphoma. Due to the relatively benign course of acute pericarditis and fear of tumor recurrence, it is not an indication to withhold radiotherapy.35
Delayed chronic pericardial effusion occurs months to years after radiotherapy, is typically asymptomatic, and presents as an enlarged cardiac silhouette on chest imaging.35 Delayed pericardial effusion is followed with imaging. While in many cases it resolves within 2 years, it may also be long-standing. Pericardiocentesis or a pericardial window may be performed to treat symptomatic effusion or delayed effusion causing hemodynamic compromise.35–37 Hypothyroidism should be ruled out, as it can complicate mantle irradiation and result in chronic pericardial effusion.38
Constrictive pericarditis may occur as a late complication of radiotherapy and typically causes symptoms of congestive heart failure. Pericardial stripping in these patients is complicated by the possibility of coexisting RIHD of the valves, myocardium, or coronary arteries, as well as mediastinal fibrosis. A study of 163 patients who underwent pericardial stripping for chronic pericarditis found a 7-year overall survival rate of only 27%, far lower than the rate for those who had no history of radiation exposure.39 Therefore, these patients are often treated for symptom control with diuretics and a low-salt diet rather than with surgery.
MYOCARDIAL DISEASE
Microvascular injury in the myocardium results in chronic ischemia, which may lead to myocardial fibrosis, typically manifesting as diastolic dysfunction. Chest radiotherapy may result in both systolic and diastolic dysfunction, and dilated and restrictive cardiomyopathy are well-recognized complications.40
Historically, high radiation doses resulted in systolic dysfunction in more than half of patients who underwent thoracic radiotherapy.41 Now, however, fewer than 5% of patients develop reductions in left ventricular ejection fraction, and most cases of radiotherapy-induced cardiomyopathy have a restrictive pattern.42
In a single-institution study, diastolic dysfunction was reported in as many as 14% of patients who underwent thoracic radiotherapy for Hodgkin lymphoma.40 Systolic dysfunction is now seen almost exclusively in patients treated concurrently with cardiotoxic chemotherapeutic agents such as anthracyclines in addition to radiotherapy.43
In a childhood cancer survival series, the hazard ratio of congestive heart failure in patients who had undergone radiotherapy for Wilms tumor was 6.6—almost identical to the occurrence in sibling controls. By contrast, the hazard ratio increased to 18.3 in those who received doxorubicin in addition to radiotherapy.44
Treatment of radiation-induced cardiomyopathy
Treatment of radiation-induced cardiomyopathy is similar to that for other forms of cardiomyopathy, with an emphasis on symptom management.
Heart transplant may be an option for highly selected patients with end-stage heart failure secondary to RIHD. In one report, a series of four RIHD patients received a heart transplant, and all four survived past 48 months.45 However, data from the United Network of Organ Sharing revealed an increase in the all-cause mortality rate in patients undergoing heart transplant for RIHD compared with those undergoing transplant for cardiomyopathy due to other causes.46 This trend may be confounded by a higher prevalence of prior cardiac surgery in the RIHD group—itself an established risk factor for poor posttransplant outcomes.
CONDUCTION SYSTEM DISEASE
Life-threatening arrhythmias have been reported that are distinct from the common, asymptomatic repolarization abnormalities that occur during radiotherapy. Atrioventricular nodal bradycardia, all degrees of heart block, and sick sinus syndrome have all been reported after chest radiotherapy. As conduction abnormalities do not typically manifest until years after radiotherapy, it is difficult to establish causation and, consequently, to define incidence.
Right bundle branch block is the most common conduction abnormality because of the proximity of the right bundle to the endocardium on the right side.47
Chest radiotherapy is also associated with prolongation of the corrected QT interval (QTc). A study in patients with a history of thoracic radiotherapy found that the QTc characteristically increased with exercise, a poor prognostic indicator.48 In a study of 134 survivors of childhood cancer, 12.5% of those who had undergone radiotherapy had a resting QTc of 0.44 msec or more.49
Furthermore, a study of 69 breast cancer survivors found a higher incidence of conduction abnormalities at 6 months and 10 years after radiotherapy compared with baseline. The characteristic electrocardiographic changes at 6 months were T-wave changes. At 10 years, the T-wave abnormalities had resolved and were replaced by ST depression.50
As mentioned above, establishing radiotherapy as a cause for these conduction abnormalities is challenging, given the lag between radiation therapy and electrocardiographic changes. The following criteria have been proposed for establishing a link between atrioventricular blockade and prior radiation51:
- Total radiation dose to the heart > 40 Gy
- Delay of 10 years or more since therapy
- Abnormal interval electrocardiographic changes such as bundle branch block
- Prior pericardial involvement
- Associated cardiac or mediastinal lesions.
SCREENING GUIDELINES
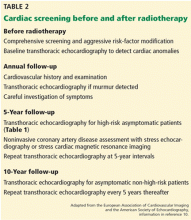
Consensus guidelines for identifying and monitoring RIHD have been published by the European Association of Cardiovascular Imaging and the American Society of Echocardiography (Table 2).10 The European Society of Medical Oncology has also issued guidelines for the prevention, diagnosis, and management of cardiovascular disease associated with cancer therapy.
Briefly, the guidelines call for aggressive cardiac risk-factor modification through weight loss, exercise, blood pressure control, and smoking cessation, in addition to early detection of RIHD. Cardiovascular screening for risk factors and a careful clinical examination should be performed in all patients. Baseline comprehensive transthoracic echocardiography is advocated in all patients before starting radiotherapy to detect cardiac anomalies. Beyond this, an annual history and physical examination, paying close attention to the signs and symptoms of cardiopulmonary disease, is essential. The development of new cardiopulmonary symptoms or a new physical finding such as a murmur should prompt evaluation with transthoracic echocardiography.
In patients without symptoms, screening transthoracic echocardiography at 10 years after the start of radiotherapy is recommended in light of the high probability of diagnosing cardiac disease at this juncture. In patients with no preexisting cardiac disease, surveillance transthoracic echocardiography should be at 5-year intervals thereafter.
In high-risk patients without symptoms (those who have undergone anterior or left-sided radiotherapy and have at least one risk factor for RIHD), initial screening transthoracic echocardiography is recommended 5 years after radiotherapy. These patients have a heightened risk of coronary events as described above and, consequently, are recommended to undergo noninvasive imaging 5 to 10 years after radiation exposure. If this initial examination is negative, stress testing should be repeated at 5-year intervals. Stress echocardiography and stress cardiac magnetic resonance imaging have higher specificity than stress electrocardiography and therefore are generally preferred. Stress scintigraphy should be used with caution, as it adds to the cumulative radiation exposure.
The role of magnetic resonance imaging and computed tomography depends on the results of initial transthoracic echocardiography and the clinical indication, in addition to the center’s expertise and facilities. However, there are currently no data advocating their use as screening tools, except for early detection of porcelain aorta in high-risk patients.10
MODERN RADIOTHERAPY TECHNIQUES
In recent years, there has been emphasis on exposing the patient to as little radiation as possible without compromising cure.52 The three major strategies employed to decrease cardiac exposure include reducing the radiation dose, reducing the radiation field and volume, and using newer planning and delivery techniques.
Reducing the radiation dose. It is well recognized that the mean dose of radiation to the heart is a significant predictor of cardiovascular disease, with one study demonstrating a linear increase in the risk of coronary artery disease with increasing mean heart radiation dose (excess relative risk per Gy 7.4%, 95% confidence interval 3.3%–14.8%).53
Reducing the radiation field and volume. Modern strategies and computed tomography-based radiotherapy planning have enabled a transition from older techniques such as extended-field radiation therapy, mantle-field radiation therapy, and involved-field radiation therapy to new techniques such as involved-node and involved-site radiation therapy.54 These have shown promise. For instance, a study in patients with early Hodgkin lymphoma found a mean heart dose of 27.5 Gy with mantle-field therapy compared with 7.7 Gy with involved-node therapy. This decrease in mean heart dose was associated with a reduction in the 25-year absolute excess cardiac risk from 9.1% to 1.4% and a reduction in cardiac mortality from 2.1% to 1%.55
Employing newer planning and delivery systems has also demonstrated some promise in reducing rates of cardiac morbidity and mortality. Extended-field radiation therapy, mantle-field radiotherapy, and involved-field radiation therapy were traditionally based on two-dimensional planning and often resulted in large volumes of myocardium being unnecessarily exposed to large doses of radiation because of the uncertainty in targeting. Involved-site and involved-node radiotherapy are based on computed tomography, resulting in more accurate targeting and sparing of normal tissue.
In addition, newer techniques such as intensity-modulated radiotherapy and proton beam therapy have resulted in further improvements in conformality compared with three-dimensional conformal radiotherapy.56,57 Respiratory motion management, including deep inspiration breath-holding and end-inspiration breath-holding, have decreased the radiation dose to the heart in patients undergoing mediastinal radiotherapy.58,59
TOWARD THE GOALS OF PREVENTION AND EARLIER DETECTION
As survival from breast cancer and lymphoma has increased, we continue to see legacy or latent effects of therapy, such as RIHD. Radiation therapy can affect any cardiac structure and is a major cause of morbidity and death in cancer survivors.
Modern radiation techniques use a variety of mechanisms to decrease the radiation dose to the heart. A large body of evidence emanating from an era of higher radiation doses and a lack of knowledge of the cardiac effects of radiation highlight the perilous cardiac consequences of chest radiation. With advances in radiotherapy and the development and widespread implementation of consensus guidelines, we envision earlier detection and less frequent occurrence of RIHD, although the latter trend could be blunted by increased cardiovascular risk factors within the population. Given the lag between irradiation and the cardiac consequences, it may be a number of years before any comparisons can be drawn.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011; 117:412–418.
- Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB. The synergism of x-irradiation and cholesterol-fat feeding on the development of coronary artery lesions. J Atheroscler Res 1964; 4:325–334.
- Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol 1999; 19:1387–1392.
- Hayashi T, Morishita Y, Kubo Y, et al. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med 2005; 118:83–86.
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010; 76(suppl 3):S77–S85.
- Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys 1992; 22:887–896.
- Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol 1993; 11:1208–1215.
- Meyer RM, Gospodarowicz MK, Connors JM, et al; NCIC Clinical Trials Group; Eastern Cooperative Oncology Group. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012; 366:399–408.
- Lancellotti P, Nkomo VT, Badano LP, et al; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2013; 26:1013–1032.
- Cheng RK, Lee MS, Seki A, et al. Radiation coronary arteritis refractory to surgical and percutaneous revascularization culminating in orthotopic heart transplantation. Cardiovasc Pathol 2013; 22:303–308.
- Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 2003; 290:2831–2837.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998.
- Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003; 55:914–920.
- Rademaker J, Schöder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol 2008; 191:32–37.
- Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J 1993; 69:496–500.
- Mousavi N, Nohria A. Radiation-induced cardiovascular disease. Curr Treat Options Cardiovasc Med 2013; 15:507–517.
- McEniery PT, Dorosti K, Schiavone WA, Pedrick TJ, Sheldon WC. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol 1987; 60:1020–1024.
- Renner SM, Massel D, Moon BC. Mediastinal irradiation: a risk factor for atherosclerosis of the internal thoracic arteries. Can J Cardiol 1999; 15:597–600.
- Chang AS, Smedira NG, Chang CL, et al. Cardiac surgery after mediastinal radiation: extent of exposure influences outcome. J Thorac Cardiovasc Surg 2007; 133:404–413.
- Handa N, McGregor CG, Danielson GK, et al. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg 1999; 117:1136–1142.
- Gharagozloo F, Clements IP, Mullany CJ. Use of the internal mammary artery for myocardial revascularization in a patient with radiation-induced coronary artery disease. Mayo Clin Proc 1992; 67:1081–1084.
- Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013; 127:1476–1485.
- Brand MD, Abadi CA, Aurigemma GP, Dauerman HL, Meyer TE. Radiation-associated valvular heart disease in Hodgkin’s disease is associated with characteristic thickening and fibrosis of the aortic-mitral curtain. J Heart Valve Dis 2001; 10:681–685.
- Nadlonek NA, Weyant MJ, Yu JA, et al. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg 2012; 144:1466–1470.
- Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation-induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg 2007; 55:53–56.
- Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-associated valvular disease. Chest 1991; 99:538–545.
- Desai MY, Wu W, Masri A, et al. Increased aorto-mitral curtain thickness independently predicts mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg 2014; 97:1348–1355.
- Crestanello JA, McGregor CG, Danielson GK, et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg 2004; 78:826–831.
- Latib A, Montorfano M, Figini F, et al. Percutaneous valve replacement in a young adult for radiation-induced aortic stenosis. J Cardiovasc Med (Hagerstown) 2012; 13:397–398.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996; 27:766–773.
- Carver JR, Shapiro CL, Ng A, et al; ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007; 25:3991–4008.
- Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s disease. An analysis of technique, tumor eradication, and complications. Cancer 1976; 37:2813–2825.
- Morton DL, Glancy DL, Joseph WL, Adkins PC. Management of patients with radiation-induced pericarditis with effusion: a note on the development of aortic regurgitation in two of them. Chest 1973; 64:291–297.
- Arsenian MA. Cardiovascular sequelae of therapeutic thoracic radiation. Prog Cardiovasc Dis 1991; 33:299–311.
- Imazio M, Brucato A, Mayosi BM, et al. Medical therapy of pericardial diseases: part II: Noninfectious pericarditis, pericardial effusion and constrictive pericarditis. J Cardiovasc Med (Hagerstown). 2010; 11:785–794.
- Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation 1993; 87:1435–1441.
- Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004; 43:1445–1452.
- Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005; 150:977–982.
- Burns RJ, Bar-Shlomo BZ, Druck MN, et al. Detection of radiation cardiomyopathy by gated radionuclide angiography. Am J Med 1983; 74:297–302.
- Constine LS, Schwartz RG, Savage DE, King V, Muhs A. Cardiac function, perfusion, and morbidity in irradiated long-term survivors of Hodgkin’s disease. Int J Radiat Oncol Biol Phys 1997; 39:897–906.
- Tolba KA, Deliargyris EN. Cardiotoxicity of cancer therapy. Cancer Invest 1999; 17:408–422.
- Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2011; 57:1210–1216.
- Handa N, McGregor CG, Daly RC, et al. Heart transplantation for radiation-associated end-stage heart failure. Transpl Int 2000; 13:162–165.
- DePasquale EC, Nasir K, Jacoby DL. Outcomes of adults with restrictive cardiomyopathy after heart transplantation. J Heart Lung Transplant 2012; 31:1269–1275.
- Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003; 13:346–356.
- Schwartz CL, Hobbie WL, Truesdell S, Constine LC, Clark EB. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol 1993; 11:1906–1910.
- Orzan F, Brusca A, Gaita F, Giustetto C, Figliomeni MC, Libero L. Associated cardiac lesions in patients with radiation-induced complete heart block. Int J Cardiol 1993; 39:151–156.
- Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol 1992; 70:73–77.
- Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998; 16:3493–3501.
- Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016; 374:833–842.
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 2016; 34:235–243.
- Maraldo MV, Ng AK. Minimizing cardiac risks with contemporary radiation therapy for Hodgkin lymphoma. J Clin Oncol 2016; 34:208–210.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012; 83:1232–1237.
- Maraldo MV, Specht L. A decade of comparative dose planning studies for early-stage Hodgkin lymphoma: what can we learn? Int J Radiat Oncol Biol Phys 2014; 90:1126–1135.
- Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for Stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a Phase II study. Int J Radiat Oncol Biol Phys 2012; 83:260–267.
- Petersen PM, Aznar MC, Berthelsen AK, et al. Prospective phase II trial of image-guided radiotherapy in Hodgkin lymphoma: benefit of deep inspiration breath-hold. Acta Oncol 2015; 54:60–66.
- Aznar MC, Maraldo MV, Schut DA, et al. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or both? Int J Radiat Oncol Biol Phys 2015; 92:169–174.
Advances in radiotherapy over the past 50 years have dramatically improved outcomes in patients with malignancy. Five-year overall survival rates for Hodgkin lymphoma and non-Hodgkin lymphoma now stand at 80%, and breast cancer survival is 90%.1
Increased longevity, however, has come at the cost of late side effects such as radiation-induced heart disease (RIHD). Cardiac dysfunction due to radiation involves a spectrum of disease processes in patients who have undergone mediastinal, thoracic, or breast radiotherapy and may involve any cardiac structure, including the pericardium, myocardium, valves, conduction system, and coronary arteries.
Overall, compared with nonirradiated patients, patients who have undergone chest radiotherapy have a 2% higher absolute risk of cardiac morbidity and death at 5 years and a 23% increased absolute risk after 20 years.2
This article will review the pathophysiology and epidemiology of RIHD and will offer a practical approach to its diagnosis and management.
MOST DAMAGE IS ENDOTHELIAL
Cardiac myocytes are relatively resistant to radiation damage because of their postmitotic state. But endothelial cells remain sensitive to radiation, and the pathophysiology of most forms of RIHD appears to be associated with damage to endothelial cells. Conventional cardiac risk factors such as hyperlipidemia and smoking have been shown to compound and accelerate radiation-induced endothelial damage in animal models.3
Radiation is believed to result in transient increases in oxidative stress, resulting in formation of reactive oxygen species and a subsequent inflammatory response that includes activation of nuclear factor-kappa B. Upregulation of proinflammatory pathways results in increased expression of matrix metalloproteinases, adhesion molecules, and proinflammatory cytokines and downregulation of vasculoprotective nitric oxide.4 Indirect evidence for radiation-induced vascular inflammation comes from numerous studies that demonstrated increased levels of the proinflammatory cytokines interleukin 6, tumor necrosis factor alpha, and interferon gamma in Japanese atomic bomb survivors.5
RISK FACTORS
Risk factors for RIHD are summarized in Table 1.
The volume of heart irradiated is a major determinant of the development of RIHD.6 A retrospective study of 960 breast cancer patients in Stockholm between 1971 and 1976 found that those who had received the highest doses and volumes of cardiac radiation had a threefold higher risk of cardiac death. By comparison, those with lesser volumes of the heart exposed to radiation had no increase in risk of cardiac death compared with the general population.7
Younger age at the time of radiotherapy is associated with an increased risk of RIHD in breast cancer and lymphoma patients. A retrospective analysis of 635 patients under age 21 with Hodgkin lymphoma treated with radiotherapy showed a relative risk of fatal myocardial infarction of 41.5 compared with a general population matched for age, sex, and race.8
Conventional cardiac risk factors such as smoking, hypertension, diabetes, and hyperlipidemia further increase the risk of RIHD, and radiation increases the cardiotoxicity of chemotherapeutic agents such as anthracyclines.9
In general, high-risk patients are defined as those with at least one risk factor for RIHD who underwent anterior or left-sided chest irradiation (Table 1).10
CORONARY ARTERY DISEASE
Ischemic heart disease is the most common cause of cardiac death in patients who have undergone radiation therapy. Atherosclerotic lesions in RIHD are morphologically identical to those in nonirradiated vessels and are characterized by intimal proliferation, accumulation of lipid-rich macrophages, and plaque formation.11
A retrospective single-institution study of 415 patients with Hodgkin lymphoma who had undergone radiation therapy found the incidence of coronary artery disease 20 years later to be 10%. The mean time to development of coronary artery disease was 9 years, and all patients who developed it had at least one conventional cardiac risk factor.12
A meta-analysis of more than 20,000 breast cancer patients who received radiotherapy in 40 randomized controlled trials found an increase in the rate of non-breast-cancer deaths, primarily from vascular causes (annual event ratio 1.27, P < .0001).13
A randomized controlled trial comparing breast cancer patients who underwent preoperative or postoperative radiotherapy vs those who had surgery alone revealed a significantly higher death rate from coronary artery disease in the postradiotherapy group.7
The risk of radiation-induced coronary artery disease is proportional to both the dose and the duration of radiation therapy. A retrospective study of more than 2,000 women undergoing radiotherapy for breast cancer found that the relative risk of coronary artery disease increased linearly by about 7.4% per Gy of radiation to the heart, with no apparent ceiling.14
The distribution of atherosclerotic coronary arteries correlates well with the areas exposed to the highest doses of radiation. For instance, in left-sided breast cancer, the apex and anterior wall of the heart typically receive the highest doses of radiation; consequently, the left anterior descending and distal diagonal branches are most prominently involved.15 In patients with lymphoma who undergo radiotherapy to mediastinal nodes and in breast cancer patients receiving radiotherapy to the internal mammary chain, basal structures may be exposed as well. Ostial lesions can also be seen in these patients.16
The clinical presentation of coronary artery disease in radiotherapy recipients does not differ significantly from that in the general population. Ischemia may be silent, may lead to classic anginal symptoms, or may cause sudden cardiac death. The incidence of silent myocardial infarction has been reported to be higher after mediastinal radiotherapy than it is in the general population, possibly from damage to nerve endings within the radiation field.17
Management of radiation-associated coronary artery disease
Managing patients with radiation-associated coronary artery disease is challenging, but the therapeutic options remain the same as those in nonirradiated patients and include medical therapy, percutaneous coronary intervention, and coronary artery bypass grafting, depending on the site and extent of disease.18 Although results are conflicting, there does not seem to be a significant difference in the rates of stent restenosis between patients with a history of radiation therapy and the general population.
Percutaneous coronary intervention is generally preferred to coronary artery bypass grafting in these patients for several reasons. Radiation-induced fibrosis of surrounding structures generally makes surgical procedures more difficult,19 and inclusion of the internal mammary artery or internal thoracic artery in the radiation field may result in stenosis of these vessels, rendering them unsuitable for harvesting.20 Moreover, many patients with RIHD have concurrent radiation-induced lung damage, which increases the risk of perioperative pulmonary complications.21
If the coronary lesions are not amenable to percutaneous intervention, a careful valvular evaluation should be performed preoperatively in view of the frequency of radiation-associated valvular disease. In a study of 72 patients with RIHD undergoing coronary artery bypass grafting, 40% required valvular surgery at the time of surgery or shortly thereafter.22
Results of studies of coronary artery bypass graft outcomes in patients with a history of thoracic radiation therapy have been conflicting, but success seems to depend on the status of the internal mammary and internal thoracic arteries.23 Therefore, the patency of these vessels should be elucidated preoperatively by angiography and intraoperatively by visual inspection of the vessels for fibrosis.
A large single-institution study by Wu et al24 revealed higher short-term and long-term mortality rates in patients with RIHD undergoing cardiac surgery than in control patients without RIHD undergoing similar procedures.
VALVULAR DISEASE
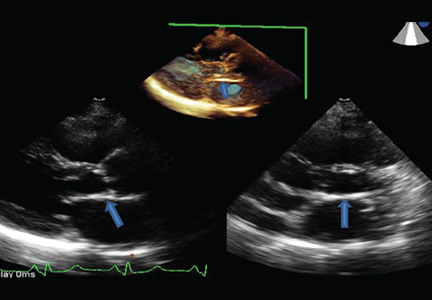
Radiation therapy may directly affect heart valves, and both stenotic (Figure 1) and regurgitant lesions have been described. Pathologic findings include leaflet retraction, fibrotic thickening, and late calcification.25
The precise mechanism of radiation-induced valvular disease is unknown but is thought to be a change in the phenotype of valvular interstitial cells from a myofibroblast to an osteoblast-like cell. Radiation results in significant expression of osteogenic factors such as bone morphogenic protein 2, osteopontin, alkaline phosphatase, and runt-related transcription factor 2 by valvular interstitial cells.26
Valvular heart disease is evident in as many as 81% of patients with RIHD, with the aortic and mitral valves affected more commonly than the tricuspid and pulmonic valves.27 Why there are more left-sided valve lesions than pulmonic valve lesions, despite the pulmonic valve’s anterior position in the heart, is unknown but may be due to higher pressures across the left-sided heart valves.
Although valvular disease is common in patients with RIHD, clinically significant disease is not; more than 70% of patients with radiation-induced valvular disease have no symptoms. A study of 38 cases of radiation-induced valvular disease reported a mean time to development of asymptomatic valvular lesions of 11.5 years and an average time to symptomatic valvular dysfunction of 16.5 years, indicating that 5 years seems to be the interval required for progression from asymptomatic to symptomatic valvular RIHD.28
The thickness of the aortomitral curtain (the junction between the base of the anterior mitral leaflet and the aortic root) is an independent predictor of the long-term risk of death in patients with valvular RIHD.29
Management of radiation-induced valvular disease
Management of patients with valvular RIHD poses a major clinical conundrum because of the high rates of perioperative morbidity and death in patients with a history of chest radiotherapy. In one study,23 the long-term mortality rate was 45% in postradiotherapy patients undergoing single-valve surgery and 61% in those undergoing surgery on two or more valves, compared with 13% and 17% in patients with no history of chest radiotherapy.23
Furthermore, valve repair is an unattractive option in these patients because of high failure rates of mitral valve and tricuspid valve repair attributed to ongoing radiotherapy-induced valvular changes after repair.30
As a result, valve replacement is generally preferred in this group. Patients should be advised of the higher risk of perioperative and long-term morbidity and death associated with open heart surgery than in the general population, and that the risks are even higher with repeat open heart surgery.
This risk has implications for the choice of replacement valves in younger patients. Bioprosthetic valves, which deteriorate over time, may not be advisable. Transcatheter aortic valve replacement has been successful in radiation-induced valvular disease and may become the preferred method of aortic valve replacement.31
PERICARDIAL DISEASE
Pericardial disease is a frequent manifestation of RIHD and covers a spectrum of manifestations from acute pericarditis, pericardial effusion, and tamponade to constrictive pericarditis. In a necropsy study, 70% of patients with RIHD were found to have pericardial involvement.32
The mechanism is believed to be radiation-induced microvascular injury resulting in increased capillary permeability and the sometimes rapid development of a protein-rich exudate. Associated inflammation may cause acute pericarditis, which may eventually be complicated by chronic pericarditis. The parietal surface tends to be affected more severely than the epicardium.33
Perhaps as a result of recent advances such as lower radiation doses, equal weighting of the anterior and posterior fields, and subcarinal blocking, incidence rates of pericarditis as low as 2.5% have been reported.34
Pericardial RIHD may be divided into early acute pericarditis, delayed chronic pericardial effusion, and constrictive pericarditis.
Early acute pericarditis is rare and is thought to represent a reaction to tumor necrosis. It is defined as occurring during radiotherapy and occurs almost exclusively with high-dose radiotherapy for lymphoma. Due to the relatively benign course of acute pericarditis and fear of tumor recurrence, it is not an indication to withhold radiotherapy.35
Delayed chronic pericardial effusion occurs months to years after radiotherapy, is typically asymptomatic, and presents as an enlarged cardiac silhouette on chest imaging.35 Delayed pericardial effusion is followed with imaging. While in many cases it resolves within 2 years, it may also be long-standing. Pericardiocentesis or a pericardial window may be performed to treat symptomatic effusion or delayed effusion causing hemodynamic compromise.35–37 Hypothyroidism should be ruled out, as it can complicate mantle irradiation and result in chronic pericardial effusion.38
Constrictive pericarditis may occur as a late complication of radiotherapy and typically causes symptoms of congestive heart failure. Pericardial stripping in these patients is complicated by the possibility of coexisting RIHD of the valves, myocardium, or coronary arteries, as well as mediastinal fibrosis. A study of 163 patients who underwent pericardial stripping for chronic pericarditis found a 7-year overall survival rate of only 27%, far lower than the rate for those who had no history of radiation exposure.39 Therefore, these patients are often treated for symptom control with diuretics and a low-salt diet rather than with surgery.
MYOCARDIAL DISEASE
Microvascular injury in the myocardium results in chronic ischemia, which may lead to myocardial fibrosis, typically manifesting as diastolic dysfunction. Chest radiotherapy may result in both systolic and diastolic dysfunction, and dilated and restrictive cardiomyopathy are well-recognized complications.40
Historically, high radiation doses resulted in systolic dysfunction in more than half of patients who underwent thoracic radiotherapy.41 Now, however, fewer than 5% of patients develop reductions in left ventricular ejection fraction, and most cases of radiotherapy-induced cardiomyopathy have a restrictive pattern.42
In a single-institution study, diastolic dysfunction was reported in as many as 14% of patients who underwent thoracic radiotherapy for Hodgkin lymphoma.40 Systolic dysfunction is now seen almost exclusively in patients treated concurrently with cardiotoxic chemotherapeutic agents such as anthracyclines in addition to radiotherapy.43
In a childhood cancer survival series, the hazard ratio of congestive heart failure in patients who had undergone radiotherapy for Wilms tumor was 6.6—almost identical to the occurrence in sibling controls. By contrast, the hazard ratio increased to 18.3 in those who received doxorubicin in addition to radiotherapy.44
Treatment of radiation-induced cardiomyopathy
Treatment of radiation-induced cardiomyopathy is similar to that for other forms of cardiomyopathy, with an emphasis on symptom management.
Heart transplant may be an option for highly selected patients with end-stage heart failure secondary to RIHD. In one report, a series of four RIHD patients received a heart transplant, and all four survived past 48 months.45 However, data from the United Network of Organ Sharing revealed an increase in the all-cause mortality rate in patients undergoing heart transplant for RIHD compared with those undergoing transplant for cardiomyopathy due to other causes.46 This trend may be confounded by a higher prevalence of prior cardiac surgery in the RIHD group—itself an established risk factor for poor posttransplant outcomes.
CONDUCTION SYSTEM DISEASE
Life-threatening arrhythmias have been reported that are distinct from the common, asymptomatic repolarization abnormalities that occur during radiotherapy. Atrioventricular nodal bradycardia, all degrees of heart block, and sick sinus syndrome have all been reported after chest radiotherapy. As conduction abnormalities do not typically manifest until years after radiotherapy, it is difficult to establish causation and, consequently, to define incidence.
Right bundle branch block is the most common conduction abnormality because of the proximity of the right bundle to the endocardium on the right side.47
Chest radiotherapy is also associated with prolongation of the corrected QT interval (QTc). A study in patients with a history of thoracic radiotherapy found that the QTc characteristically increased with exercise, a poor prognostic indicator.48 In a study of 134 survivors of childhood cancer, 12.5% of those who had undergone radiotherapy had a resting QTc of 0.44 msec or more.49
Furthermore, a study of 69 breast cancer survivors found a higher incidence of conduction abnormalities at 6 months and 10 years after radiotherapy compared with baseline. The characteristic electrocardiographic changes at 6 months were T-wave changes. At 10 years, the T-wave abnormalities had resolved and were replaced by ST depression.50
As mentioned above, establishing radiotherapy as a cause for these conduction abnormalities is challenging, given the lag between radiation therapy and electrocardiographic changes. The following criteria have been proposed for establishing a link between atrioventricular blockade and prior radiation51:
- Total radiation dose to the heart > 40 Gy
- Delay of 10 years or more since therapy
- Abnormal interval electrocardiographic changes such as bundle branch block
- Prior pericardial involvement
- Associated cardiac or mediastinal lesions.
SCREENING GUIDELINES
Consensus guidelines for identifying and monitoring RIHD have been published by the European Association of Cardiovascular Imaging and the American Society of Echocardiography (Table 2).10 The European Society of Medical Oncology has also issued guidelines for the prevention, diagnosis, and management of cardiovascular disease associated with cancer therapy.
Briefly, the guidelines call for aggressive cardiac risk-factor modification through weight loss, exercise, blood pressure control, and smoking cessation, in addition to early detection of RIHD. Cardiovascular screening for risk factors and a careful clinical examination should be performed in all patients. Baseline comprehensive transthoracic echocardiography is advocated in all patients before starting radiotherapy to detect cardiac anomalies. Beyond this, an annual history and physical examination, paying close attention to the signs and symptoms of cardiopulmonary disease, is essential. The development of new cardiopulmonary symptoms or a new physical finding such as a murmur should prompt evaluation with transthoracic echocardiography.
In patients without symptoms, screening transthoracic echocardiography at 10 years after the start of radiotherapy is recommended in light of the high probability of diagnosing cardiac disease at this juncture. In patients with no preexisting cardiac disease, surveillance transthoracic echocardiography should be at 5-year intervals thereafter.
In high-risk patients without symptoms (those who have undergone anterior or left-sided radiotherapy and have at least one risk factor for RIHD), initial screening transthoracic echocardiography is recommended 5 years after radiotherapy. These patients have a heightened risk of coronary events as described above and, consequently, are recommended to undergo noninvasive imaging 5 to 10 years after radiation exposure. If this initial examination is negative, stress testing should be repeated at 5-year intervals. Stress echocardiography and stress cardiac magnetic resonance imaging have higher specificity than stress electrocardiography and therefore are generally preferred. Stress scintigraphy should be used with caution, as it adds to the cumulative radiation exposure.
The role of magnetic resonance imaging and computed tomography depends on the results of initial transthoracic echocardiography and the clinical indication, in addition to the center’s expertise and facilities. However, there are currently no data advocating their use as screening tools, except for early detection of porcelain aorta in high-risk patients.10
MODERN RADIOTHERAPY TECHNIQUES
In recent years, there has been emphasis on exposing the patient to as little radiation as possible without compromising cure.52 The three major strategies employed to decrease cardiac exposure include reducing the radiation dose, reducing the radiation field and volume, and using newer planning and delivery techniques.
Reducing the radiation dose. It is well recognized that the mean dose of radiation to the heart is a significant predictor of cardiovascular disease, with one study demonstrating a linear increase in the risk of coronary artery disease with increasing mean heart radiation dose (excess relative risk per Gy 7.4%, 95% confidence interval 3.3%–14.8%).53
Reducing the radiation field and volume. Modern strategies and computed tomography-based radiotherapy planning have enabled a transition from older techniques such as extended-field radiation therapy, mantle-field radiation therapy, and involved-field radiation therapy to new techniques such as involved-node and involved-site radiation therapy.54 These have shown promise. For instance, a study in patients with early Hodgkin lymphoma found a mean heart dose of 27.5 Gy with mantle-field therapy compared with 7.7 Gy with involved-node therapy. This decrease in mean heart dose was associated with a reduction in the 25-year absolute excess cardiac risk from 9.1% to 1.4% and a reduction in cardiac mortality from 2.1% to 1%.55
Employing newer planning and delivery systems has also demonstrated some promise in reducing rates of cardiac morbidity and mortality. Extended-field radiation therapy, mantle-field radiotherapy, and involved-field radiation therapy were traditionally based on two-dimensional planning and often resulted in large volumes of myocardium being unnecessarily exposed to large doses of radiation because of the uncertainty in targeting. Involved-site and involved-node radiotherapy are based on computed tomography, resulting in more accurate targeting and sparing of normal tissue.
In addition, newer techniques such as intensity-modulated radiotherapy and proton beam therapy have resulted in further improvements in conformality compared with three-dimensional conformal radiotherapy.56,57 Respiratory motion management, including deep inspiration breath-holding and end-inspiration breath-holding, have decreased the radiation dose to the heart in patients undergoing mediastinal radiotherapy.58,59
TOWARD THE GOALS OF PREVENTION AND EARLIER DETECTION
As survival from breast cancer and lymphoma has increased, we continue to see legacy or latent effects of therapy, such as RIHD. Radiation therapy can affect any cardiac structure and is a major cause of morbidity and death in cancer survivors.
Modern radiation techniques use a variety of mechanisms to decrease the radiation dose to the heart. A large body of evidence emanating from an era of higher radiation doses and a lack of knowledge of the cardiac effects of radiation highlight the perilous cardiac consequences of chest radiation. With advances in radiotherapy and the development and widespread implementation of consensus guidelines, we envision earlier detection and less frequent occurrence of RIHD, although the latter trend could be blunted by increased cardiovascular risk factors within the population. Given the lag between irradiation and the cardiac consequences, it may be a number of years before any comparisons can be drawn.
Advances in radiotherapy over the past 50 years have dramatically improved outcomes in patients with malignancy. Five-year overall survival rates for Hodgkin lymphoma and non-Hodgkin lymphoma now stand at 80%, and breast cancer survival is 90%.1
Increased longevity, however, has come at the cost of late side effects such as radiation-induced heart disease (RIHD). Cardiac dysfunction due to radiation involves a spectrum of disease processes in patients who have undergone mediastinal, thoracic, or breast radiotherapy and may involve any cardiac structure, including the pericardium, myocardium, valves, conduction system, and coronary arteries.
Overall, compared with nonirradiated patients, patients who have undergone chest radiotherapy have a 2% higher absolute risk of cardiac morbidity and death at 5 years and a 23% increased absolute risk after 20 years.2
This article will review the pathophysiology and epidemiology of RIHD and will offer a practical approach to its diagnosis and management.
MOST DAMAGE IS ENDOTHELIAL
Cardiac myocytes are relatively resistant to radiation damage because of their postmitotic state. But endothelial cells remain sensitive to radiation, and the pathophysiology of most forms of RIHD appears to be associated with damage to endothelial cells. Conventional cardiac risk factors such as hyperlipidemia and smoking have been shown to compound and accelerate radiation-induced endothelial damage in animal models.3
Radiation is believed to result in transient increases in oxidative stress, resulting in formation of reactive oxygen species and a subsequent inflammatory response that includes activation of nuclear factor-kappa B. Upregulation of proinflammatory pathways results in increased expression of matrix metalloproteinases, adhesion molecules, and proinflammatory cytokines and downregulation of vasculoprotective nitric oxide.4 Indirect evidence for radiation-induced vascular inflammation comes from numerous studies that demonstrated increased levels of the proinflammatory cytokines interleukin 6, tumor necrosis factor alpha, and interferon gamma in Japanese atomic bomb survivors.5
RISK FACTORS
Risk factors for RIHD are summarized in Table 1.
The volume of heart irradiated is a major determinant of the development of RIHD.6 A retrospective study of 960 breast cancer patients in Stockholm between 1971 and 1976 found that those who had received the highest doses and volumes of cardiac radiation had a threefold higher risk of cardiac death. By comparison, those with lesser volumes of the heart exposed to radiation had no increase in risk of cardiac death compared with the general population.7
Younger age at the time of radiotherapy is associated with an increased risk of RIHD in breast cancer and lymphoma patients. A retrospective analysis of 635 patients under age 21 with Hodgkin lymphoma treated with radiotherapy showed a relative risk of fatal myocardial infarction of 41.5 compared with a general population matched for age, sex, and race.8
Conventional cardiac risk factors such as smoking, hypertension, diabetes, and hyperlipidemia further increase the risk of RIHD, and radiation increases the cardiotoxicity of chemotherapeutic agents such as anthracyclines.9
In general, high-risk patients are defined as those with at least one risk factor for RIHD who underwent anterior or left-sided chest irradiation (Table 1).10
CORONARY ARTERY DISEASE
Ischemic heart disease is the most common cause of cardiac death in patients who have undergone radiation therapy. Atherosclerotic lesions in RIHD are morphologically identical to those in nonirradiated vessels and are characterized by intimal proliferation, accumulation of lipid-rich macrophages, and plaque formation.11
A retrospective single-institution study of 415 patients with Hodgkin lymphoma who had undergone radiation therapy found the incidence of coronary artery disease 20 years later to be 10%. The mean time to development of coronary artery disease was 9 years, and all patients who developed it had at least one conventional cardiac risk factor.12
A meta-analysis of more than 20,000 breast cancer patients who received radiotherapy in 40 randomized controlled trials found an increase in the rate of non-breast-cancer deaths, primarily from vascular causes (annual event ratio 1.27, P < .0001).13
A randomized controlled trial comparing breast cancer patients who underwent preoperative or postoperative radiotherapy vs those who had surgery alone revealed a significantly higher death rate from coronary artery disease in the postradiotherapy group.7
The risk of radiation-induced coronary artery disease is proportional to both the dose and the duration of radiation therapy. A retrospective study of more than 2,000 women undergoing radiotherapy for breast cancer found that the relative risk of coronary artery disease increased linearly by about 7.4% per Gy of radiation to the heart, with no apparent ceiling.14
The distribution of atherosclerotic coronary arteries correlates well with the areas exposed to the highest doses of radiation. For instance, in left-sided breast cancer, the apex and anterior wall of the heart typically receive the highest doses of radiation; consequently, the left anterior descending and distal diagonal branches are most prominently involved.15 In patients with lymphoma who undergo radiotherapy to mediastinal nodes and in breast cancer patients receiving radiotherapy to the internal mammary chain, basal structures may be exposed as well. Ostial lesions can also be seen in these patients.16
The clinical presentation of coronary artery disease in radiotherapy recipients does not differ significantly from that in the general population. Ischemia may be silent, may lead to classic anginal symptoms, or may cause sudden cardiac death. The incidence of silent myocardial infarction has been reported to be higher after mediastinal radiotherapy than it is in the general population, possibly from damage to nerve endings within the radiation field.17
Management of radiation-associated coronary artery disease
Managing patients with radiation-associated coronary artery disease is challenging, but the therapeutic options remain the same as those in nonirradiated patients and include medical therapy, percutaneous coronary intervention, and coronary artery bypass grafting, depending on the site and extent of disease.18 Although results are conflicting, there does not seem to be a significant difference in the rates of stent restenosis between patients with a history of radiation therapy and the general population.
Percutaneous coronary intervention is generally preferred to coronary artery bypass grafting in these patients for several reasons. Radiation-induced fibrosis of surrounding structures generally makes surgical procedures more difficult,19 and inclusion of the internal mammary artery or internal thoracic artery in the radiation field may result in stenosis of these vessels, rendering them unsuitable for harvesting.20 Moreover, many patients with RIHD have concurrent radiation-induced lung damage, which increases the risk of perioperative pulmonary complications.21
If the coronary lesions are not amenable to percutaneous intervention, a careful valvular evaluation should be performed preoperatively in view of the frequency of radiation-associated valvular disease. In a study of 72 patients with RIHD undergoing coronary artery bypass grafting, 40% required valvular surgery at the time of surgery or shortly thereafter.22
Results of studies of coronary artery bypass graft outcomes in patients with a history of thoracic radiation therapy have been conflicting, but success seems to depend on the status of the internal mammary and internal thoracic arteries.23 Therefore, the patency of these vessels should be elucidated preoperatively by angiography and intraoperatively by visual inspection of the vessels for fibrosis.
A large single-institution study by Wu et al24 revealed higher short-term and long-term mortality rates in patients with RIHD undergoing cardiac surgery than in control patients without RIHD undergoing similar procedures.
VALVULAR DISEASE
Radiation therapy may directly affect heart valves, and both stenotic (Figure 1) and regurgitant lesions have been described. Pathologic findings include leaflet retraction, fibrotic thickening, and late calcification.25
The precise mechanism of radiation-induced valvular disease is unknown but is thought to be a change in the phenotype of valvular interstitial cells from a myofibroblast to an osteoblast-like cell. Radiation results in significant expression of osteogenic factors such as bone morphogenic protein 2, osteopontin, alkaline phosphatase, and runt-related transcription factor 2 by valvular interstitial cells.26
Valvular heart disease is evident in as many as 81% of patients with RIHD, with the aortic and mitral valves affected more commonly than the tricuspid and pulmonic valves.27 Why there are more left-sided valve lesions than pulmonic valve lesions, despite the pulmonic valve’s anterior position in the heart, is unknown but may be due to higher pressures across the left-sided heart valves.
Although valvular disease is common in patients with RIHD, clinically significant disease is not; more than 70% of patients with radiation-induced valvular disease have no symptoms. A study of 38 cases of radiation-induced valvular disease reported a mean time to development of asymptomatic valvular lesions of 11.5 years and an average time to symptomatic valvular dysfunction of 16.5 years, indicating that 5 years seems to be the interval required for progression from asymptomatic to symptomatic valvular RIHD.28
The thickness of the aortomitral curtain (the junction between the base of the anterior mitral leaflet and the aortic root) is an independent predictor of the long-term risk of death in patients with valvular RIHD.29
Management of radiation-induced valvular disease
Management of patients with valvular RIHD poses a major clinical conundrum because of the high rates of perioperative morbidity and death in patients with a history of chest radiotherapy. In one study,23 the long-term mortality rate was 45% in postradiotherapy patients undergoing single-valve surgery and 61% in those undergoing surgery on two or more valves, compared with 13% and 17% in patients with no history of chest radiotherapy.23
Furthermore, valve repair is an unattractive option in these patients because of high failure rates of mitral valve and tricuspid valve repair attributed to ongoing radiotherapy-induced valvular changes after repair.30
As a result, valve replacement is generally preferred in this group. Patients should be advised of the higher risk of perioperative and long-term morbidity and death associated with open heart surgery than in the general population, and that the risks are even higher with repeat open heart surgery.
This risk has implications for the choice of replacement valves in younger patients. Bioprosthetic valves, which deteriorate over time, may not be advisable. Transcatheter aortic valve replacement has been successful in radiation-induced valvular disease and may become the preferred method of aortic valve replacement.31
PERICARDIAL DISEASE
Pericardial disease is a frequent manifestation of RIHD and covers a spectrum of manifestations from acute pericarditis, pericardial effusion, and tamponade to constrictive pericarditis. In a necropsy study, 70% of patients with RIHD were found to have pericardial involvement.32
The mechanism is believed to be radiation-induced microvascular injury resulting in increased capillary permeability and the sometimes rapid development of a protein-rich exudate. Associated inflammation may cause acute pericarditis, which may eventually be complicated by chronic pericarditis. The parietal surface tends to be affected more severely than the epicardium.33
Perhaps as a result of recent advances such as lower radiation doses, equal weighting of the anterior and posterior fields, and subcarinal blocking, incidence rates of pericarditis as low as 2.5% have been reported.34
Pericardial RIHD may be divided into early acute pericarditis, delayed chronic pericardial effusion, and constrictive pericarditis.
Early acute pericarditis is rare and is thought to represent a reaction to tumor necrosis. It is defined as occurring during radiotherapy and occurs almost exclusively with high-dose radiotherapy for lymphoma. Due to the relatively benign course of acute pericarditis and fear of tumor recurrence, it is not an indication to withhold radiotherapy.35
Delayed chronic pericardial effusion occurs months to years after radiotherapy, is typically asymptomatic, and presents as an enlarged cardiac silhouette on chest imaging.35 Delayed pericardial effusion is followed with imaging. While in many cases it resolves within 2 years, it may also be long-standing. Pericardiocentesis or a pericardial window may be performed to treat symptomatic effusion or delayed effusion causing hemodynamic compromise.35–37 Hypothyroidism should be ruled out, as it can complicate mantle irradiation and result in chronic pericardial effusion.38
Constrictive pericarditis may occur as a late complication of radiotherapy and typically causes symptoms of congestive heart failure. Pericardial stripping in these patients is complicated by the possibility of coexisting RIHD of the valves, myocardium, or coronary arteries, as well as mediastinal fibrosis. A study of 163 patients who underwent pericardial stripping for chronic pericarditis found a 7-year overall survival rate of only 27%, far lower than the rate for those who had no history of radiation exposure.39 Therefore, these patients are often treated for symptom control with diuretics and a low-salt diet rather than with surgery.
MYOCARDIAL DISEASE
Microvascular injury in the myocardium results in chronic ischemia, which may lead to myocardial fibrosis, typically manifesting as diastolic dysfunction. Chest radiotherapy may result in both systolic and diastolic dysfunction, and dilated and restrictive cardiomyopathy are well-recognized complications.40
Historically, high radiation doses resulted in systolic dysfunction in more than half of patients who underwent thoracic radiotherapy.41 Now, however, fewer than 5% of patients develop reductions in left ventricular ejection fraction, and most cases of radiotherapy-induced cardiomyopathy have a restrictive pattern.42
In a single-institution study, diastolic dysfunction was reported in as many as 14% of patients who underwent thoracic radiotherapy for Hodgkin lymphoma.40 Systolic dysfunction is now seen almost exclusively in patients treated concurrently with cardiotoxic chemotherapeutic agents such as anthracyclines in addition to radiotherapy.43
In a childhood cancer survival series, the hazard ratio of congestive heart failure in patients who had undergone radiotherapy for Wilms tumor was 6.6—almost identical to the occurrence in sibling controls. By contrast, the hazard ratio increased to 18.3 in those who received doxorubicin in addition to radiotherapy.44
Treatment of radiation-induced cardiomyopathy
Treatment of radiation-induced cardiomyopathy is similar to that for other forms of cardiomyopathy, with an emphasis on symptom management.
Heart transplant may be an option for highly selected patients with end-stage heart failure secondary to RIHD. In one report, a series of four RIHD patients received a heart transplant, and all four survived past 48 months.45 However, data from the United Network of Organ Sharing revealed an increase in the all-cause mortality rate in patients undergoing heart transplant for RIHD compared with those undergoing transplant for cardiomyopathy due to other causes.46 This trend may be confounded by a higher prevalence of prior cardiac surgery in the RIHD group—itself an established risk factor for poor posttransplant outcomes.
CONDUCTION SYSTEM DISEASE
Life-threatening arrhythmias have been reported that are distinct from the common, asymptomatic repolarization abnormalities that occur during radiotherapy. Atrioventricular nodal bradycardia, all degrees of heart block, and sick sinus syndrome have all been reported after chest radiotherapy. As conduction abnormalities do not typically manifest until years after radiotherapy, it is difficult to establish causation and, consequently, to define incidence.
Right bundle branch block is the most common conduction abnormality because of the proximity of the right bundle to the endocardium on the right side.47
Chest radiotherapy is also associated with prolongation of the corrected QT interval (QTc). A study in patients with a history of thoracic radiotherapy found that the QTc characteristically increased with exercise, a poor prognostic indicator.48 In a study of 134 survivors of childhood cancer, 12.5% of those who had undergone radiotherapy had a resting QTc of 0.44 msec or more.49
Furthermore, a study of 69 breast cancer survivors found a higher incidence of conduction abnormalities at 6 months and 10 years after radiotherapy compared with baseline. The characteristic electrocardiographic changes at 6 months were T-wave changes. At 10 years, the T-wave abnormalities had resolved and were replaced by ST depression.50
As mentioned above, establishing radiotherapy as a cause for these conduction abnormalities is challenging, given the lag between radiation therapy and electrocardiographic changes. The following criteria have been proposed for establishing a link between atrioventricular blockade and prior radiation51:
- Total radiation dose to the heart > 40 Gy
- Delay of 10 years or more since therapy
- Abnormal interval electrocardiographic changes such as bundle branch block
- Prior pericardial involvement
- Associated cardiac or mediastinal lesions.
SCREENING GUIDELINES
Consensus guidelines for identifying and monitoring RIHD have been published by the European Association of Cardiovascular Imaging and the American Society of Echocardiography (Table 2).10 The European Society of Medical Oncology has also issued guidelines for the prevention, diagnosis, and management of cardiovascular disease associated with cancer therapy.
Briefly, the guidelines call for aggressive cardiac risk-factor modification through weight loss, exercise, blood pressure control, and smoking cessation, in addition to early detection of RIHD. Cardiovascular screening for risk factors and a careful clinical examination should be performed in all patients. Baseline comprehensive transthoracic echocardiography is advocated in all patients before starting radiotherapy to detect cardiac anomalies. Beyond this, an annual history and physical examination, paying close attention to the signs and symptoms of cardiopulmonary disease, is essential. The development of new cardiopulmonary symptoms or a new physical finding such as a murmur should prompt evaluation with transthoracic echocardiography.
In patients without symptoms, screening transthoracic echocardiography at 10 years after the start of radiotherapy is recommended in light of the high probability of diagnosing cardiac disease at this juncture. In patients with no preexisting cardiac disease, surveillance transthoracic echocardiography should be at 5-year intervals thereafter.
In high-risk patients without symptoms (those who have undergone anterior or left-sided radiotherapy and have at least one risk factor for RIHD), initial screening transthoracic echocardiography is recommended 5 years after radiotherapy. These patients have a heightened risk of coronary events as described above and, consequently, are recommended to undergo noninvasive imaging 5 to 10 years after radiation exposure. If this initial examination is negative, stress testing should be repeated at 5-year intervals. Stress echocardiography and stress cardiac magnetic resonance imaging have higher specificity than stress electrocardiography and therefore are generally preferred. Stress scintigraphy should be used with caution, as it adds to the cumulative radiation exposure.
The role of magnetic resonance imaging and computed tomography depends on the results of initial transthoracic echocardiography and the clinical indication, in addition to the center’s expertise and facilities. However, there are currently no data advocating their use as screening tools, except for early detection of porcelain aorta in high-risk patients.10
MODERN RADIOTHERAPY TECHNIQUES
In recent years, there has been emphasis on exposing the patient to as little radiation as possible without compromising cure.52 The three major strategies employed to decrease cardiac exposure include reducing the radiation dose, reducing the radiation field and volume, and using newer planning and delivery techniques.
Reducing the radiation dose. It is well recognized that the mean dose of radiation to the heart is a significant predictor of cardiovascular disease, with one study demonstrating a linear increase in the risk of coronary artery disease with increasing mean heart radiation dose (excess relative risk per Gy 7.4%, 95% confidence interval 3.3%–14.8%).53
Reducing the radiation field and volume. Modern strategies and computed tomography-based radiotherapy planning have enabled a transition from older techniques such as extended-field radiation therapy, mantle-field radiation therapy, and involved-field radiation therapy to new techniques such as involved-node and involved-site radiation therapy.54 These have shown promise. For instance, a study in patients with early Hodgkin lymphoma found a mean heart dose of 27.5 Gy with mantle-field therapy compared with 7.7 Gy with involved-node therapy. This decrease in mean heart dose was associated with a reduction in the 25-year absolute excess cardiac risk from 9.1% to 1.4% and a reduction in cardiac mortality from 2.1% to 1%.55
Employing newer planning and delivery systems has also demonstrated some promise in reducing rates of cardiac morbidity and mortality. Extended-field radiation therapy, mantle-field radiotherapy, and involved-field radiation therapy were traditionally based on two-dimensional planning and often resulted in large volumes of myocardium being unnecessarily exposed to large doses of radiation because of the uncertainty in targeting. Involved-site and involved-node radiotherapy are based on computed tomography, resulting in more accurate targeting and sparing of normal tissue.
In addition, newer techniques such as intensity-modulated radiotherapy and proton beam therapy have resulted in further improvements in conformality compared with three-dimensional conformal radiotherapy.56,57 Respiratory motion management, including deep inspiration breath-holding and end-inspiration breath-holding, have decreased the radiation dose to the heart in patients undergoing mediastinal radiotherapy.58,59
TOWARD THE GOALS OF PREVENTION AND EARLIER DETECTION
As survival from breast cancer and lymphoma has increased, we continue to see legacy or latent effects of therapy, such as RIHD. Radiation therapy can affect any cardiac structure and is a major cause of morbidity and death in cancer survivors.
Modern radiation techniques use a variety of mechanisms to decrease the radiation dose to the heart. A large body of evidence emanating from an era of higher radiation doses and a lack of knowledge of the cardiac effects of radiation highlight the perilous cardiac consequences of chest radiation. With advances in radiotherapy and the development and widespread implementation of consensus guidelines, we envision earlier detection and less frequent occurrence of RIHD, although the latter trend could be blunted by increased cardiovascular risk factors within the population. Given the lag between irradiation and the cardiac consequences, it may be a number of years before any comparisons can be drawn.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011; 117:412–418.
- Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB. The synergism of x-irradiation and cholesterol-fat feeding on the development of coronary artery lesions. J Atheroscler Res 1964; 4:325–334.
- Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol 1999; 19:1387–1392.
- Hayashi T, Morishita Y, Kubo Y, et al. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med 2005; 118:83–86.
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010; 76(suppl 3):S77–S85.
- Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys 1992; 22:887–896.
- Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol 1993; 11:1208–1215.
- Meyer RM, Gospodarowicz MK, Connors JM, et al; NCIC Clinical Trials Group; Eastern Cooperative Oncology Group. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012; 366:399–408.
- Lancellotti P, Nkomo VT, Badano LP, et al; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2013; 26:1013–1032.
- Cheng RK, Lee MS, Seki A, et al. Radiation coronary arteritis refractory to surgical and percutaneous revascularization culminating in orthotopic heart transplantation. Cardiovasc Pathol 2013; 22:303–308.
- Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 2003; 290:2831–2837.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998.
- Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003; 55:914–920.
- Rademaker J, Schöder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol 2008; 191:32–37.
- Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J 1993; 69:496–500.
- Mousavi N, Nohria A. Radiation-induced cardiovascular disease. Curr Treat Options Cardiovasc Med 2013; 15:507–517.
- McEniery PT, Dorosti K, Schiavone WA, Pedrick TJ, Sheldon WC. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol 1987; 60:1020–1024.
- Renner SM, Massel D, Moon BC. Mediastinal irradiation: a risk factor for atherosclerosis of the internal thoracic arteries. Can J Cardiol 1999; 15:597–600.
- Chang AS, Smedira NG, Chang CL, et al. Cardiac surgery after mediastinal radiation: extent of exposure influences outcome. J Thorac Cardiovasc Surg 2007; 133:404–413.
- Handa N, McGregor CG, Danielson GK, et al. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg 1999; 117:1136–1142.
- Gharagozloo F, Clements IP, Mullany CJ. Use of the internal mammary artery for myocardial revascularization in a patient with radiation-induced coronary artery disease. Mayo Clin Proc 1992; 67:1081–1084.
- Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013; 127:1476–1485.
- Brand MD, Abadi CA, Aurigemma GP, Dauerman HL, Meyer TE. Radiation-associated valvular heart disease in Hodgkin’s disease is associated with characteristic thickening and fibrosis of the aortic-mitral curtain. J Heart Valve Dis 2001; 10:681–685.
- Nadlonek NA, Weyant MJ, Yu JA, et al. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg 2012; 144:1466–1470.
- Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation-induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg 2007; 55:53–56.
- Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-associated valvular disease. Chest 1991; 99:538–545.
- Desai MY, Wu W, Masri A, et al. Increased aorto-mitral curtain thickness independently predicts mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg 2014; 97:1348–1355.
- Crestanello JA, McGregor CG, Danielson GK, et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg 2004; 78:826–831.
- Latib A, Montorfano M, Figini F, et al. Percutaneous valve replacement in a young adult for radiation-induced aortic stenosis. J Cardiovasc Med (Hagerstown) 2012; 13:397–398.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996; 27:766–773.
- Carver JR, Shapiro CL, Ng A, et al; ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007; 25:3991–4008.
- Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s disease. An analysis of technique, tumor eradication, and complications. Cancer 1976; 37:2813–2825.
- Morton DL, Glancy DL, Joseph WL, Adkins PC. Management of patients with radiation-induced pericarditis with effusion: a note on the development of aortic regurgitation in two of them. Chest 1973; 64:291–297.
- Arsenian MA. Cardiovascular sequelae of therapeutic thoracic radiation. Prog Cardiovasc Dis 1991; 33:299–311.
- Imazio M, Brucato A, Mayosi BM, et al. Medical therapy of pericardial diseases: part II: Noninfectious pericarditis, pericardial effusion and constrictive pericarditis. J Cardiovasc Med (Hagerstown). 2010; 11:785–794.
- Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation 1993; 87:1435–1441.
- Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004; 43:1445–1452.
- Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005; 150:977–982.
- Burns RJ, Bar-Shlomo BZ, Druck MN, et al. Detection of radiation cardiomyopathy by gated radionuclide angiography. Am J Med 1983; 74:297–302.
- Constine LS, Schwartz RG, Savage DE, King V, Muhs A. Cardiac function, perfusion, and morbidity in irradiated long-term survivors of Hodgkin’s disease. Int J Radiat Oncol Biol Phys 1997; 39:897–906.
- Tolba KA, Deliargyris EN. Cardiotoxicity of cancer therapy. Cancer Invest 1999; 17:408–422.
- Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2011; 57:1210–1216.
- Handa N, McGregor CG, Daly RC, et al. Heart transplantation for radiation-associated end-stage heart failure. Transpl Int 2000; 13:162–165.
- DePasquale EC, Nasir K, Jacoby DL. Outcomes of adults with restrictive cardiomyopathy after heart transplantation. J Heart Lung Transplant 2012; 31:1269–1275.
- Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003; 13:346–356.
- Schwartz CL, Hobbie WL, Truesdell S, Constine LC, Clark EB. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol 1993; 11:1906–1910.
- Orzan F, Brusca A, Gaita F, Giustetto C, Figliomeni MC, Libero L. Associated cardiac lesions in patients with radiation-induced complete heart block. Int J Cardiol 1993; 39:151–156.
- Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol 1992; 70:73–77.
- Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998; 16:3493–3501.
- Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016; 374:833–842.
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 2016; 34:235–243.
- Maraldo MV, Ng AK. Minimizing cardiac risks with contemporary radiation therapy for Hodgkin lymphoma. J Clin Oncol 2016; 34:208–210.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012; 83:1232–1237.
- Maraldo MV, Specht L. A decade of comparative dose planning studies for early-stage Hodgkin lymphoma: what can we learn? Int J Radiat Oncol Biol Phys 2014; 90:1126–1135.
- Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for Stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a Phase II study. Int J Radiat Oncol Biol Phys 2012; 83:260–267.
- Petersen PM, Aznar MC, Berthelsen AK, et al. Prospective phase II trial of image-guided radiotherapy in Hodgkin lymphoma: benefit of deep inspiration breath-hold. Acta Oncol 2015; 54:60–66.
- Aznar MC, Maraldo MV, Schut DA, et al. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or both? Int J Radiat Oncol Biol Phys 2015; 92:169–174.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011; 117:412–418.
- Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB. The synergism of x-irradiation and cholesterol-fat feeding on the development of coronary artery lesions. J Atheroscler Res 1964; 4:325–334.
- Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol 1999; 19:1387–1392.
- Hayashi T, Morishita Y, Kubo Y, et al. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med 2005; 118:83–86.
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010; 76(suppl 3):S77–S85.
- Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys 1992; 22:887–896.
- Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol 1993; 11:1208–1215.
- Meyer RM, Gospodarowicz MK, Connors JM, et al; NCIC Clinical Trials Group; Eastern Cooperative Oncology Group. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012; 366:399–408.
- Lancellotti P, Nkomo VT, Badano LP, et al; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2013; 26:1013–1032.
- Cheng RK, Lee MS, Seki A, et al. Radiation coronary arteritis refractory to surgical and percutaneous revascularization culminating in orthotopic heart transplantation. Cardiovasc Pathol 2013; 22:303–308.
- Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 2003; 290:2831–2837.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998.
- Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003; 55:914–920.
- Rademaker J, Schöder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol 2008; 191:32–37.
- Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J 1993; 69:496–500.
- Mousavi N, Nohria A. Radiation-induced cardiovascular disease. Curr Treat Options Cardiovasc Med 2013; 15:507–517.
- McEniery PT, Dorosti K, Schiavone WA, Pedrick TJ, Sheldon WC. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol 1987; 60:1020–1024.
- Renner SM, Massel D, Moon BC. Mediastinal irradiation: a risk factor for atherosclerosis of the internal thoracic arteries. Can J Cardiol 1999; 15:597–600.
- Chang AS, Smedira NG, Chang CL, et al. Cardiac surgery after mediastinal radiation: extent of exposure influences outcome. J Thorac Cardiovasc Surg 2007; 133:404–413.
- Handa N, McGregor CG, Danielson GK, et al. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg 1999; 117:1136–1142.
- Gharagozloo F, Clements IP, Mullany CJ. Use of the internal mammary artery for myocardial revascularization in a patient with radiation-induced coronary artery disease. Mayo Clin Proc 1992; 67:1081–1084.
- Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013; 127:1476–1485.
- Brand MD, Abadi CA, Aurigemma GP, Dauerman HL, Meyer TE. Radiation-associated valvular heart disease in Hodgkin’s disease is associated with characteristic thickening and fibrosis of the aortic-mitral curtain. J Heart Valve Dis 2001; 10:681–685.
- Nadlonek NA, Weyant MJ, Yu JA, et al. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg 2012; 144:1466–1470.
- Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation-induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg 2007; 55:53–56.
- Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-associated valvular disease. Chest 1991; 99:538–545.
- Desai MY, Wu W, Masri A, et al. Increased aorto-mitral curtain thickness independently predicts mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg 2014; 97:1348–1355.
- Crestanello JA, McGregor CG, Danielson GK, et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg 2004; 78:826–831.
- Latib A, Montorfano M, Figini F, et al. Percutaneous valve replacement in a young adult for radiation-induced aortic stenosis. J Cardiovasc Med (Hagerstown) 2012; 13:397–398.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996; 27:766–773.
- Carver JR, Shapiro CL, Ng A, et al; ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007; 25:3991–4008.
- Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s disease. An analysis of technique, tumor eradication, and complications. Cancer 1976; 37:2813–2825.
- Morton DL, Glancy DL, Joseph WL, Adkins PC. Management of patients with radiation-induced pericarditis with effusion: a note on the development of aortic regurgitation in two of them. Chest 1973; 64:291–297.
- Arsenian MA. Cardiovascular sequelae of therapeutic thoracic radiation. Prog Cardiovasc Dis 1991; 33:299–311.
- Imazio M, Brucato A, Mayosi BM, et al. Medical therapy of pericardial diseases: part II: Noninfectious pericarditis, pericardial effusion and constrictive pericarditis. J Cardiovasc Med (Hagerstown). 2010; 11:785–794.
- Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation 1993; 87:1435–1441.
- Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004; 43:1445–1452.
- Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005; 150:977–982.
- Burns RJ, Bar-Shlomo BZ, Druck MN, et al. Detection of radiation cardiomyopathy by gated radionuclide angiography. Am J Med 1983; 74:297–302.
- Constine LS, Schwartz RG, Savage DE, King V, Muhs A. Cardiac function, perfusion, and morbidity in irradiated long-term survivors of Hodgkin’s disease. Int J Radiat Oncol Biol Phys 1997; 39:897–906.
- Tolba KA, Deliargyris EN. Cardiotoxicity of cancer therapy. Cancer Invest 1999; 17:408–422.
- Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2011; 57:1210–1216.
- Handa N, McGregor CG, Daly RC, et al. Heart transplantation for radiation-associated end-stage heart failure. Transpl Int 2000; 13:162–165.
- DePasquale EC, Nasir K, Jacoby DL. Outcomes of adults with restrictive cardiomyopathy after heart transplantation. J Heart Lung Transplant 2012; 31:1269–1275.
- Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003; 13:346–356.
- Schwartz CL, Hobbie WL, Truesdell S, Constine LC, Clark EB. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol 1993; 11:1906–1910.
- Orzan F, Brusca A, Gaita F, Giustetto C, Figliomeni MC, Libero L. Associated cardiac lesions in patients with radiation-induced complete heart block. Int J Cardiol 1993; 39:151–156.
- Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol 1992; 70:73–77.
- Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998; 16:3493–3501.
- Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016; 374:833–842.
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 2016; 34:235–243.
- Maraldo MV, Ng AK. Minimizing cardiac risks with contemporary radiation therapy for Hodgkin lymphoma. J Clin Oncol 2016; 34:208–210.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012; 83:1232–1237.
- Maraldo MV, Specht L. A decade of comparative dose planning studies for early-stage Hodgkin lymphoma: what can we learn? Int J Radiat Oncol Biol Phys 2014; 90:1126–1135.
- Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for Stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a Phase II study. Int J Radiat Oncol Biol Phys 2012; 83:260–267.
- Petersen PM, Aznar MC, Berthelsen AK, et al. Prospective phase II trial of image-guided radiotherapy in Hodgkin lymphoma: benefit of deep inspiration breath-hold. Acta Oncol 2015; 54:60–66.
- Aznar MC, Maraldo MV, Schut DA, et al. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or both? Int J Radiat Oncol Biol Phys 2015; 92:169–174.
KEY POINTS
- Ischemic heart disease is the most common cause of cardiac death after radiotherapy. Valvular, pericardial, myocardial, and conduction system disease are also common.
- Surgery may not be an attractive option because of radiation-induced fibrosis of surrounding structures. Consequently, conservative interventions are preferred.
- The incidence of RIHD is expected to decline, as lower doses of radiation are being used in radiotherapy than in the past.