User login
In 2011, two papers were published that will shape the way we think about lung cancer screening for years to come.
See related patient information sheet
While the ability to screen for lung cancer is a major positive change, it also raises many thorny questions, such as who should be screened, how often should they be screened, and how should we respond when a nodule is detected.
To answer some of these questions, we will outline how Cleveland Clinic has structured its lung cancer screening program, and the rationale we used for making pragmatic patient-care decisions within this program. We will conclude with our thoughts about the potential evolution of lung cancer screening programs.
THE 40-YEAR QUEST FOR EFFECTIVE LUNG CANCER SCREENING
Lung cancer kills more people in the United States than the next four most lethal types of cancer combined.3 It is curable if found early in its course. Unfortunately, most people who develop lung cancer feel no symptoms when it is early in its course, and therefore it is too often diagnosed at a late stage. Treatment for late-stage lung cancer is effective, but it is rarely curative.
Screening refers to testing people at risk of developing a disease before its symptoms or signs have appeared. The goal of screening is to reduce the disease-specific mortality rate. For this to happen, the disease must be detectable in a preclinical form, and treatment must be more successful when applied early. Ideally, the screening test should pose little risk to the patient, be sensitive for detecting the disease early in its course, give few false-positive results, be acceptable to the patient, and be relatively inexpensive to the health system.
Over the past 4 decades, a large volume of research has been done in the hope of proving that conventional radiography or CT could be an effective screening test for lung cancer.4,5
Cohort studies (ie, in which all the patients were screened) of radiography or CT have shown a longer survival from the time of lung cancer diagnosis than would be expected without screening. These studies were not designed to prove a reduction in the lung cancer-specific mortality rate.
Controlled trials (in which half the patients received the screening and the other half did not) of chest radiography have been interpreted as not showing a reduction in lung cancer mortality rates, though debate about the interpretation of these trials persisted until this past year. Biases inherent in using duration of survival rather than the mortality rate as an end point have been suggested as the reason for the apparent benefit in survival without a reduction in the mortality rate.
Controlled trials of CT screening were started nearly a decade ago. Until 2011, the results of these trials were not mature enough to comment on.
THE PROSTATE, LUNG, COLORECTAL, AND OVARIAN TRIAL
The lung cancer screening portion of the PLCO trial aimed to determine the effect of screening chest radiography on lung cancer-specific mortality rates.1
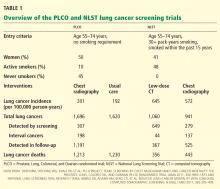
In this trial, 154,901 people were randomized to undergo either posteroanterior chest radiography every year for 4 years or usual care, ie, no lung cancer screening. Participants were men and women age 55 to 74 with no history of prostate, lung, colorectal, or ovarian cancer. They did not need to be a smoker to participate. Those who had never smoked and who were randomized to the screening group received only 3 years of testing. All were followed for 13 years or until the conclusion of the study (8 years after the final participant was enrolled). About half were women, and nearly two-thirds were age 55 through 64. Only 10% were current smokers, while a full 45% had never smoked.
Results. Adherence to screening in the screening group ranged from 79% to 86.6% over the years of screening, and 11% of the usual-care group was estimated to have undergone screening chest radiography.
Cumulative lung cancer incidence rates were 201 per 100,000 person-years in the screening group and 192 in the usual-care group.
In the screening group, there were a total of 1,696 lung cancers during the entire study. Of these, 307 (18%) were detected by screening, 198 (12%) were interval cancers (diagnosed during the screening period but not by the screening test), and the remainder were diagnosed after the screening period during the years of follow-up. In the screening group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also in the screening group, the cancers detected by screening were more likely to be stage I (50%) than those not detected by screening.
The cumulative number of deaths from lung cancer was slightly but not significantly lower in the screening group from years 4 through 11. However, by the end of follow-up, the number of lung cancer deaths was equal between the groups (1,213 in the screening group vs 1,230 in the usual-care group). The cumulative overall mortality rate was also similar between the groups. For the subgroup who would have qualified for the NLST (see below), the lung cancer mortality rate was statistically similar between the two groups.
Comments. The results of the PLCO screening trial will be interpreted as the final word in lung cancer screening with standard chest radiography. The conclusion is that annual screening with chest radiography does not reduce lung cancer mortality rates and thus should not be performed in this context.
THE NATIONAL LUNG SCREENING TRIAL
The NLST aimed to determine if screening with low-dose chest CT could reduce lung cancer mortality rates.2
This controlled trial enrolled 53,454 people, who were randomized to undergo either low-dose chest CT or posteroanterior chest radiography at baseline and then yearly for 2 years.
Participants were men and women age 55 to 74 with at least 30 pack-years of cigarette smoking. If they had quit smoking, they had to have quit within the past 15 years. All were followed until study conclusion (median 6.5 years, maximum 7.4). About 41% were women, and nearly three-quarters were age 55 through 64. More than 48% were current smokers, with the rest being former smokers.
Results. Adherence to screening was 95% in the CT group and 93% in the radiography group, with a 4.3% annual rate of CT outside the study during the screening phase.
Cumulative lung cancer incidence rates were 645 per 100,000 person-years in the CT group and 572 in the radiography group.
In the CT group there were a total of 1,060 lung cancers during the entire study. Of these, 649 (61%) were detected by screening, 44 (4%) were interval cancers, and the rest were diagnosed after the screening period during follow-up.
In the chest radiography group, there were a total of 941 lung cancers during the entire study. Of these, 279 (30%) were detected by screening, 137 (15%) were interval cancers, and the rest were diagnosed after the screening period. Within the CT group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also within the CT group, the cancers detected by screening were more likely to be stage I (63%) than those not detected by screening.
The cumulative number of deaths from lung cancer was 443 in the radiography group, but only 356 in the CT group—20.0% lower (P =.004). The cumulative overall mortality rate was 6.7% lower in the CT group (P = .02).
Comments. The results of the NLST provide the first evidence that lung cancer mortality rates can be reduced by screening. Though many questions remain, the conclusions of this study are that screening a well-defined high-risk group with low-dose CT reduces the rate of death from lung cancer.
REMAINING CHALLENGES
The NLST showed that lung cancer screening with low-dose CT can meet the most important criterion for a successful screening program, ie, a reduction in the disease-specific mortality rate. Many challenges remain in meeting the other criteria for a successful or ideal screening program (low risk, few false-positive results, acceptability to the patient, and affordability). The issues with low-dose CT-based screening that challenge these ideals are outlined in this section.
Lung nodules: Benign or malignant?
A meta-analysis of CT screening studies found that for every 1,000 people screened at baseline, 9 were found to have stage I non-small-cell lung cancer, 235 had false-positive nodules, and 4 underwent thoracotomy for benign lesions.6
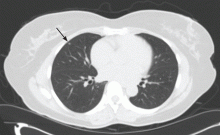
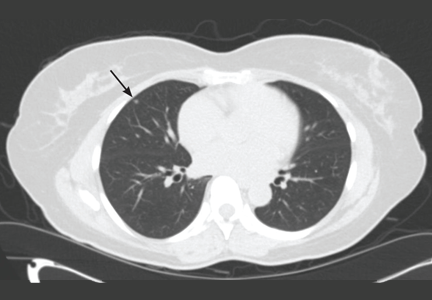
The NLST results were similar. In this trial, only nodules that were 4 mm or greater in diameter were reported. Using these criteria, over 27% of all study participants were found to have a lung nodule on CT at baseline and at year 1. The rate fell to nearly 17% at year 2, as nodules present from baseline were not reported. Of all the lung nodules detected, only 3.6% were ultimately proven to represent lung cancer.2
Many issues with small lung nodules need to be considered. The nodules are difficult to find, with highly variable reporting even by expert radiologists.7 They are difficult to measure accurately and thus are difficult to assess for growth.8 Adjunctive imaging and nonsurgical biopsy have a low yield for small nodules.9–11 Follow-up of these lung nodules includes additional imaging and nonsurgical and surgical biopsy procedures, adding expense to the program and risk to the patient. Finally, knowing that they have a lung nodule makes patients feel anxious and thus negatively affects their quality of life.12,13
Radiation exposure: How great is the risk?
There is a great deal of concern about radiation exposure from medical imaging, as many people receive a substantial amount of radiation each year from medical testing.14 A single low-dose scan with chest CT delivers a whole-body effective dose of about 1.5 mSv—less than one-fifth of the radiation dose of a typical diagnostic CT scan.
Many have tried to estimate the consequences of radiation exposure from low-dose CT screening. All estimates are extrapolations from unrelated radiation exposures. The increase in risk of death ranged from 0.01% to a few percent,15 and the increase in cancers was as high as 1.8% over a 25-year screening period.16 In general, the risks are felt to be very low but not negligible.
Cost-effectiveness is unknown
The cost-effectiveness of lung cancer screening is also unknown. Many highly variable estimates have been published.17–20 The studies have differed in the perspective taken, the costs of testing assumed, and the rounds of screening included. The most cost-effective estimates are in populations with the highest risk of cancer, in programs that achieve the greatest reduction in mortality rate, and in programs that lead to high rates of smoking cessation.
Screening in the real world as opposed to a clinical trial may involve different risks, benefits, and costs. Compliance with screening and with nodule management algorithms may be lower outside of a study. One study suggested that those at highest risk of developing lung cancer would be the least likely to enroll in a screening program and the least likely to accept curative-intent surgery for screening-detected cancer.21
We expect that the NLST data will be analyzed for cost-effectiveness. This should provide the most accurate estimates for the group that was studied.
WE SET OUT TO DESIGN A SCREENING PROGRAM
With the evidence supporting a reduction in the rate of lung cancer mortality, and knowing the remaining challenges, we set out to provide a lung cancer screening program within Cleveland Clinic. In the design of our program, we considered several questions, outlined below.
Who should be offered low-dose CT screening?
The results of the NLST led to a great deal of excitement about lung cancer screening in both the medical community and the general public. The positive side of this publicity is that lung cancer is receiving attention that may lead to support for further advances. The negative side is that many patients who may seek out lung cancer screening are not at high enough risk of lung cancer to clearly benefit from it.
In the NLST, a very high-risk cohort was studied, as defined by clinical variables (age 55 to 74, at least 30 pack-years of smoking, and if a former smoker, had quit within the past 15 years). In this high-risk group, 320 patients needed to be screened (with three yearly chest CT scans) for one life to be saved from lung cancer, and only 3.6% of all lung nodules found (4 mm or larger) were actually lung cancer. In a group at lower risk, the number that needed to be screened to save one life would be higher, and the percentage of lung nodules that truly were lung cancer would be lower. This would lead to higher risks and costs related to screening, without a proven benefit to members of the lower-risk group.
The risk of the NLST cohort developing lung cancer was approximately 0.6% per year. Lung cancer risk-prediction models have been developed and published. Up to 2011, the three most commonly used models had only moderate accuracy at predicting risk.22–25 In 2011 a risk model based on the PLCO cohort was developed and published.26 This model seemed to be more accurate but perhaps a bit harder to apply in practice.
We discussed whether using a validated risk predictor with a target of 0.6% per year (ie, the risk in the NLST trial) would be an adequate means of deciding on candidacy for lung cancer screening or if we should strictly adhere to the inclusion criteria of the NLST cohort. We feel that the NLST cohort is the only group with true evidence of benefit (a reduction in the lung cancer-specific mortality rate). Thus, for our program’s entry criteria, we decided to use the same clinical predictors used for entry in the NLST.
How will the right patients get scheduled for low-dose screening CT?
Patients who enter the lung cancer screening program from our health system will require a physician’s order.
We are fortunate to have an electronic medical record in place. We have created an order set within the electronic record for low-dose chest CT. The order will eventually be able to be entered as “CT lung screening w/o” (ie, without contrast).
For patients from outside of our health system who would like to enter the lung cancer screening program, the entry criteria will be the same (see above). We will ask for the name of the patient’s primary care practitioner. If the patient does not have one, a member of our Respiratory Institute will see and enroll the patient.
How often should patients be screened, and for how many years?
Unfortunately, questions about the frequency of screening and how many years it should continue remain unanswered.
In the NLST, a similar number of early-stage lung cancers were detected during each of the three screening rounds. In both the NLST and PLCO trials, differences in the mortality rate curves began to narrow during the observation period, when active screening was no longer occurring. Thus, it is possible that a longer duration of screening could lead to a further reduction in mortality rates. Others have questioned whether a similar benefit, with less cost and risk, could be obtained by screening every 2 years.
The large amount of data obtained from the NLST and other CT-based studies is being reviewed so that models can be developed to help answer these questions. For now, we suggest at least three yearly CT screenings, with the hope that we will have clearer answers to these questions over time.
How will low-dose CT be performed and interpreted?
The parameters for low-dose CT were very tightly controlled and monitored during the NLST. This quality-control effort, designed to improve consistency across sites and to minimize risk to patients, should be carried into lung cancer screening programs.
Our program will closely mimic the CT performance criteria used in the NLST (tube current-time product 40 mAs for all patients, field of view lungs only, lung kernel images 3 mm at 1.5-mm intervals, and soft-tissue kernel images 5 mm at 2.5-mm intervals).27 In the initial phase of the program, all screening scans will be performed at Cleveland Clinic’s main imaging facility.
Small lung nodules remain quite challenging to detect and measure. To minimize variability in scan interpretation, the NLST readers were all expertly trained radiologists. Despite this, much variability was noted in the number of nodules detected, their measured size, and the follow-up recommendations. All of the screening CT images for our program will be interpreted by board-certified radiologists with expertise in chest imaging.
Other screening studies have included novel imaging assessment in their testing algorithms, particularly volumetric analysis of lung nodules.28 These tools may prove to assist in nodule detection, measurement, and management over time. At this point, we do not think they have been studied and standardized enough to include them in a standard-of-care screening program. We hope that they will evolve to the point of clinical utility in the near future.
Lung cancer screening is not currently covered by most insurers, including Medicare, although one major insurer has recently started to cover it. We expect decisions on coverage from other insurers in the next 12 months. In the meantime, we offer a low-dose screening chest CT to our patients for $125, which includes the radiologist’s fee for interpreting the scan.
Smoking cessation
The NLST showed that low-dose CT screening can reduce lung cancer mortality rates by 20% in a high-risk group. A 50-year-old active smoker who quits smoking reduces his or her risk of dying of lung cancer by more than 50%.29 Entry into a lung cancer screening program provides an opportunity for education and assistance with tobacco dependency.
At Cleveland Clinic, we have an active Tobacco Treatment Center within our Wellness Institute. All lung cancer screening participants who are identified as active smokers will be given a program brochure and will be offered a consult in the program.
What do we identify as a lung nodule, and how should they be managed?
Studies of CT-based screening have highlighted the tremendous number of lung nodules that are identified and the low likelihood of malignancy in those that are less than 1 cm in diameter. Many screening studies define a positive result as a lung nodule above a particular size. The NLST used 4 mm or greater as the cutoff. The lower the cutoff, the greater the number of nodules found, and the lower the overall likelihood of malignancy in the nodules.
Studies in which annual CT screening was the intervention are able to use size criteria in part because the study design ensures another CT will be performed 12 months later. Current nodule management guidelines suggest 12-month CT follow-up of incidentally discovered lung nodules, 4 mm or smaller, in at-risk patients.30 In a screening program, particularly one for which the patient must pay, the 12-month screening CT cannot be guaranteed. This makes it more difficult to ignore the smallest nodules identified on CT screening. Given this, we will be reporting all lung nodules identified, regardless of size on the initial screening.
Most studies of CT screening have reported any new nodule identified in subsequent screening rounds regardless of size. Though it is intuitive that a new nodule would have a high likelihood of malignancy in a high-risk cohort, malignancy rates have been reported to be as low as 1% for new nodules. As with the initial round of screening, we will report all new lung nodules identified in subsequent screening rounds.
The recommendations for the evaluation of lung nodules, both within the report and at the lung nodule clinic, are in keeping with currently available guidelines, such as those from the Fleischner Society30 and the American College of Chest Physicians.31 For incidentally discovered lung nodules in patients at high risk, the Fleischner Society recommendations are as follows30:
- For nodules 4 mm or smaller, follow-up in 12 months; if no growth, then no further follow-up
- For nodules 4 to 6 mm, follow-up at 6 to 12 months, then 18 to 24 months if no growth
- For nodules 6 to 8 mm, follow-up at 3 to 6 months, then 9 to 12 months, then 24 months if no growth
- For nodules 8 mm or larger, follow-up at 3, 9, and 24 months, or positron emission tomography, or biopsy, or both.
If the nodule is large enough or is deemed to be of high enough risk, adjuvant testing with diagnostic imaging, guided bronchoscopy, transthoracic needle aspiration, or minimally invasive resection will be offered. All patients with nodules believed to require biopsy will be discussed at our multidisciplinary lung cancer tumor board before biopsy.
How do we make practitioners and patients aware of the program and its indications, risks, and benefits?
Education will be the key to having lung cancer screening adopted as the standard of care, to lung cancer screening being provided within a well-designed and capable system, and to ensuring that patients have realistic expectations about screening. Articles such as this and grand rounds presentations within our health system will help provide education to our colleagues. Broader marketing campaigns will be considered in the future once demand and system capabilities are clearly identified. A patient information brochure will be provided at the time of the screening test (see the patient information sheet that accompanies this article).
How do we help to advance best practice?
As excited as we are that low-dose CT-based lung cancer screening has been proven to reduce lung cancer mortality rates, it is clear that there is a lot of room to improve the programs that are developed based on current data.
Advances in our ability to accurately predict an individual’s risk of developing lung cancer will allow us to offer screening to those it is most likely to benefit.
Advances in smoking cessation and chemoprevention will help to minimize the number of lung cancers that develop.
Advances in our ability to determine the nature of lung nodules will allow us to accelerate treatment of very early lung cancer while minimizing additional testing on benign nodules; advances in our ability to treat localized and advanced disease will improve the outcome for those identified as having lung cancer.
To help move the science of screening forward, we will develop a screening program registry that can be populated from the order set and the templated report. The registry can be used to ensure appropriate patient care, while studying relevant epidemiologic, quality, and cost-related questions.
We hope to assess novel imaging software capable of assisting with the detection and characterization of lung nodules.
We have an active biomarker development program to assess the ability of breath and blood-based biomarkers to identify those at risk of developing lung cancer; to assist with the management of screening-detected lung nodules; to assist with the diagnosis of early stage lung cancer; and to characterize the nature of the cancers identified. Accurate biomarkers could lead to further decreases in mortality rates while reducing the risks and costs of a screening program.
We have strong surgical, medical, and radiation oncology programs, actively pursuing advances in minimally invasive resection procedures and ablative and targeted therapies.
ENTERING A NEW ERA
We are entering a new era of lung cancer screening. The NLST has shown that lung cancer morality rates can be reduced through low-dose CT screening in a high-risk population. Many challenges remain, such as managing the nodules that are discovered, determining if the program is cost-effective, and minimizing radiation exposure. These need to be considered when designing a lung cancer screening program. Advances over time will help us optimize the programs that are developed.
- Oken MM, Hocking WG, Kvale PA, et al; PLCO Project Team. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011; 306:1865–1873.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Mazzone PJ, Mekhail T. Lung cancer screening. Curr Oncol Rep 2007; 9:265–274.
- Mazzone PJ. Lung cancer screening: an update, discussion, and look ahead. Curr Oncol Rep 2010; 12:226–234.
- Gopal M, Abdullah SE, Grady JJ, Goodwin JS. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010; 5:1233–1239.
- Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology 2008; 246:265–272.
- Singh S, Pinsky P, Fineberg NS, et al. Evaluation of reader variability in the interpretation of follow-up CT scans at lung cancer screening. Radiology 2011; 259:263–270.
- Lindell RM, Hartman TE, Swensen SJ, et al. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening chest CT in a high-risk population. AJR Am J Roentgenol 2005; 185:126–131.
- Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117:1049–1054.
- Kothary N, Lock L, Sze DY, Hofmann LV. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009; 10:360–363.
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010; 102:27–34.
- Lemonnier I, Baumann C, Jolly D, et al. Solitary pulmonary nodules: consequences for patient quality of life. Qual Life Res 2011; 20:101–109.
- Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009; 361:849–857.
- Buls N, de Mey J, Covens P, Stadnik T. Health screening with CT: prospective assessment of radiation dose and associated detriment. JBR-BTR 2005; 88:12–16.
- Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004; 231:440–445.
- Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 2003; 289:313–322.
- Wisnivesky JP, Mushlin AI, Sicherman N, Henschke C. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest 2003; 124:614–621.
- Manser R, Dalton A, Carter R, Byrnes G, Elwood M, Campbell DA. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer 2005; 48:171–185.
- McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011; 6:1841–1848.
- Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their nonsmoking counterparts. Thorax 2007; 62:126–130.
- Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003; 95:470–478.
- Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007; 99:715–726.
- Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008; 98:270–276.
- D’Amelio AM, Cassidy A, Asomaning K, et al. Comparison of discriminatory power and accuracy of three lung cancer risk models. Br J Cancer 2010; 103:423–429.
- Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst 2011; 103:1058–1068.
- National Lung Screening Trial Research Team; Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology 2011; 258:243–253.
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009; 361:2221–2229.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000; 321:323–329.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Gould MK, Fletcher J, Iannettoni MD, et al; American College of Chest Physicians. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132(suppl 3):108S–130S.
In 2011, two papers were published that will shape the way we think about lung cancer screening for years to come.
See related patient information sheet
While the ability to screen for lung cancer is a major positive change, it also raises many thorny questions, such as who should be screened, how often should they be screened, and how should we respond when a nodule is detected.
To answer some of these questions, we will outline how Cleveland Clinic has structured its lung cancer screening program, and the rationale we used for making pragmatic patient-care decisions within this program. We will conclude with our thoughts about the potential evolution of lung cancer screening programs.
THE 40-YEAR QUEST FOR EFFECTIVE LUNG CANCER SCREENING
Lung cancer kills more people in the United States than the next four most lethal types of cancer combined.3 It is curable if found early in its course. Unfortunately, most people who develop lung cancer feel no symptoms when it is early in its course, and therefore it is too often diagnosed at a late stage. Treatment for late-stage lung cancer is effective, but it is rarely curative.
Screening refers to testing people at risk of developing a disease before its symptoms or signs have appeared. The goal of screening is to reduce the disease-specific mortality rate. For this to happen, the disease must be detectable in a preclinical form, and treatment must be more successful when applied early. Ideally, the screening test should pose little risk to the patient, be sensitive for detecting the disease early in its course, give few false-positive results, be acceptable to the patient, and be relatively inexpensive to the health system.
Over the past 4 decades, a large volume of research has been done in the hope of proving that conventional radiography or CT could be an effective screening test for lung cancer.4,5
Cohort studies (ie, in which all the patients were screened) of radiography or CT have shown a longer survival from the time of lung cancer diagnosis than would be expected without screening. These studies were not designed to prove a reduction in the lung cancer-specific mortality rate.
Controlled trials (in which half the patients received the screening and the other half did not) of chest radiography have been interpreted as not showing a reduction in lung cancer mortality rates, though debate about the interpretation of these trials persisted until this past year. Biases inherent in using duration of survival rather than the mortality rate as an end point have been suggested as the reason for the apparent benefit in survival without a reduction in the mortality rate.
Controlled trials of CT screening were started nearly a decade ago. Until 2011, the results of these trials were not mature enough to comment on.
THE PROSTATE, LUNG, COLORECTAL, AND OVARIAN TRIAL
The lung cancer screening portion of the PLCO trial aimed to determine the effect of screening chest radiography on lung cancer-specific mortality rates.1
In this trial, 154,901 people were randomized to undergo either posteroanterior chest radiography every year for 4 years or usual care, ie, no lung cancer screening. Participants were men and women age 55 to 74 with no history of prostate, lung, colorectal, or ovarian cancer. They did not need to be a smoker to participate. Those who had never smoked and who were randomized to the screening group received only 3 years of testing. All were followed for 13 years or until the conclusion of the study (8 years after the final participant was enrolled). About half were women, and nearly two-thirds were age 55 through 64. Only 10% were current smokers, while a full 45% had never smoked.
Results. Adherence to screening in the screening group ranged from 79% to 86.6% over the years of screening, and 11% of the usual-care group was estimated to have undergone screening chest radiography.
Cumulative lung cancer incidence rates were 201 per 100,000 person-years in the screening group and 192 in the usual-care group.
In the screening group, there were a total of 1,696 lung cancers during the entire study. Of these, 307 (18%) were detected by screening, 198 (12%) were interval cancers (diagnosed during the screening period but not by the screening test), and the remainder were diagnosed after the screening period during the years of follow-up. In the screening group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also in the screening group, the cancers detected by screening were more likely to be stage I (50%) than those not detected by screening.
The cumulative number of deaths from lung cancer was slightly but not significantly lower in the screening group from years 4 through 11. However, by the end of follow-up, the number of lung cancer deaths was equal between the groups (1,213 in the screening group vs 1,230 in the usual-care group). The cumulative overall mortality rate was also similar between the groups. For the subgroup who would have qualified for the NLST (see below), the lung cancer mortality rate was statistically similar between the two groups.
Comments. The results of the PLCO screening trial will be interpreted as the final word in lung cancer screening with standard chest radiography. The conclusion is that annual screening with chest radiography does not reduce lung cancer mortality rates and thus should not be performed in this context.
THE NATIONAL LUNG SCREENING TRIAL
The NLST aimed to determine if screening with low-dose chest CT could reduce lung cancer mortality rates.2
This controlled trial enrolled 53,454 people, who were randomized to undergo either low-dose chest CT or posteroanterior chest radiography at baseline and then yearly for 2 years.
Participants were men and women age 55 to 74 with at least 30 pack-years of cigarette smoking. If they had quit smoking, they had to have quit within the past 15 years. All were followed until study conclusion (median 6.5 years, maximum 7.4). About 41% were women, and nearly three-quarters were age 55 through 64. More than 48% were current smokers, with the rest being former smokers.
Results. Adherence to screening was 95% in the CT group and 93% in the radiography group, with a 4.3% annual rate of CT outside the study during the screening phase.
Cumulative lung cancer incidence rates were 645 per 100,000 person-years in the CT group and 572 in the radiography group.
In the CT group there were a total of 1,060 lung cancers during the entire study. Of these, 649 (61%) were detected by screening, 44 (4%) were interval cancers, and the rest were diagnosed after the screening period during follow-up.
In the chest radiography group, there were a total of 941 lung cancers during the entire study. Of these, 279 (30%) were detected by screening, 137 (15%) were interval cancers, and the rest were diagnosed after the screening period. Within the CT group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also within the CT group, the cancers detected by screening were more likely to be stage I (63%) than those not detected by screening.
The cumulative number of deaths from lung cancer was 443 in the radiography group, but only 356 in the CT group—20.0% lower (P =.004). The cumulative overall mortality rate was 6.7% lower in the CT group (P = .02).
Comments. The results of the NLST provide the first evidence that lung cancer mortality rates can be reduced by screening. Though many questions remain, the conclusions of this study are that screening a well-defined high-risk group with low-dose CT reduces the rate of death from lung cancer.
REMAINING CHALLENGES
The NLST showed that lung cancer screening with low-dose CT can meet the most important criterion for a successful screening program, ie, a reduction in the disease-specific mortality rate. Many challenges remain in meeting the other criteria for a successful or ideal screening program (low risk, few false-positive results, acceptability to the patient, and affordability). The issues with low-dose CT-based screening that challenge these ideals are outlined in this section.
Lung nodules: Benign or malignant?
A meta-analysis of CT screening studies found that for every 1,000 people screened at baseline, 9 were found to have stage I non-small-cell lung cancer, 235 had false-positive nodules, and 4 underwent thoracotomy for benign lesions.6
The NLST results were similar. In this trial, only nodules that were 4 mm or greater in diameter were reported. Using these criteria, over 27% of all study participants were found to have a lung nodule on CT at baseline and at year 1. The rate fell to nearly 17% at year 2, as nodules present from baseline were not reported. Of all the lung nodules detected, only 3.6% were ultimately proven to represent lung cancer.2
Many issues with small lung nodules need to be considered. The nodules are difficult to find, with highly variable reporting even by expert radiologists.7 They are difficult to measure accurately and thus are difficult to assess for growth.8 Adjunctive imaging and nonsurgical biopsy have a low yield for small nodules.9–11 Follow-up of these lung nodules includes additional imaging and nonsurgical and surgical biopsy procedures, adding expense to the program and risk to the patient. Finally, knowing that they have a lung nodule makes patients feel anxious and thus negatively affects their quality of life.12,13
Radiation exposure: How great is the risk?
There is a great deal of concern about radiation exposure from medical imaging, as many people receive a substantial amount of radiation each year from medical testing.14 A single low-dose scan with chest CT delivers a whole-body effective dose of about 1.5 mSv—less than one-fifth of the radiation dose of a typical diagnostic CT scan.
Many have tried to estimate the consequences of radiation exposure from low-dose CT screening. All estimates are extrapolations from unrelated radiation exposures. The increase in risk of death ranged from 0.01% to a few percent,15 and the increase in cancers was as high as 1.8% over a 25-year screening period.16 In general, the risks are felt to be very low but not negligible.
Cost-effectiveness is unknown
The cost-effectiveness of lung cancer screening is also unknown. Many highly variable estimates have been published.17–20 The studies have differed in the perspective taken, the costs of testing assumed, and the rounds of screening included. The most cost-effective estimates are in populations with the highest risk of cancer, in programs that achieve the greatest reduction in mortality rate, and in programs that lead to high rates of smoking cessation.
Screening in the real world as opposed to a clinical trial may involve different risks, benefits, and costs. Compliance with screening and with nodule management algorithms may be lower outside of a study. One study suggested that those at highest risk of developing lung cancer would be the least likely to enroll in a screening program and the least likely to accept curative-intent surgery for screening-detected cancer.21
We expect that the NLST data will be analyzed for cost-effectiveness. This should provide the most accurate estimates for the group that was studied.
WE SET OUT TO DESIGN A SCREENING PROGRAM
With the evidence supporting a reduction in the rate of lung cancer mortality, and knowing the remaining challenges, we set out to provide a lung cancer screening program within Cleveland Clinic. In the design of our program, we considered several questions, outlined below.
Who should be offered low-dose CT screening?
The results of the NLST led to a great deal of excitement about lung cancer screening in both the medical community and the general public. The positive side of this publicity is that lung cancer is receiving attention that may lead to support for further advances. The negative side is that many patients who may seek out lung cancer screening are not at high enough risk of lung cancer to clearly benefit from it.
In the NLST, a very high-risk cohort was studied, as defined by clinical variables (age 55 to 74, at least 30 pack-years of smoking, and if a former smoker, had quit within the past 15 years). In this high-risk group, 320 patients needed to be screened (with three yearly chest CT scans) for one life to be saved from lung cancer, and only 3.6% of all lung nodules found (4 mm or larger) were actually lung cancer. In a group at lower risk, the number that needed to be screened to save one life would be higher, and the percentage of lung nodules that truly were lung cancer would be lower. This would lead to higher risks and costs related to screening, without a proven benefit to members of the lower-risk group.
The risk of the NLST cohort developing lung cancer was approximately 0.6% per year. Lung cancer risk-prediction models have been developed and published. Up to 2011, the three most commonly used models had only moderate accuracy at predicting risk.22–25 In 2011 a risk model based on the PLCO cohort was developed and published.26 This model seemed to be more accurate but perhaps a bit harder to apply in practice.
We discussed whether using a validated risk predictor with a target of 0.6% per year (ie, the risk in the NLST trial) would be an adequate means of deciding on candidacy for lung cancer screening or if we should strictly adhere to the inclusion criteria of the NLST cohort. We feel that the NLST cohort is the only group with true evidence of benefit (a reduction in the lung cancer-specific mortality rate). Thus, for our program’s entry criteria, we decided to use the same clinical predictors used for entry in the NLST.
How will the right patients get scheduled for low-dose screening CT?
Patients who enter the lung cancer screening program from our health system will require a physician’s order.
We are fortunate to have an electronic medical record in place. We have created an order set within the electronic record for low-dose chest CT. The order will eventually be able to be entered as “CT lung screening w/o” (ie, without contrast).
For patients from outside of our health system who would like to enter the lung cancer screening program, the entry criteria will be the same (see above). We will ask for the name of the patient’s primary care practitioner. If the patient does not have one, a member of our Respiratory Institute will see and enroll the patient.
How often should patients be screened, and for how many years?
Unfortunately, questions about the frequency of screening and how many years it should continue remain unanswered.
In the NLST, a similar number of early-stage lung cancers were detected during each of the three screening rounds. In both the NLST and PLCO trials, differences in the mortality rate curves began to narrow during the observation period, when active screening was no longer occurring. Thus, it is possible that a longer duration of screening could lead to a further reduction in mortality rates. Others have questioned whether a similar benefit, with less cost and risk, could be obtained by screening every 2 years.
The large amount of data obtained from the NLST and other CT-based studies is being reviewed so that models can be developed to help answer these questions. For now, we suggest at least three yearly CT screenings, with the hope that we will have clearer answers to these questions over time.
How will low-dose CT be performed and interpreted?
The parameters for low-dose CT were very tightly controlled and monitored during the NLST. This quality-control effort, designed to improve consistency across sites and to minimize risk to patients, should be carried into lung cancer screening programs.
Our program will closely mimic the CT performance criteria used in the NLST (tube current-time product 40 mAs for all patients, field of view lungs only, lung kernel images 3 mm at 1.5-mm intervals, and soft-tissue kernel images 5 mm at 2.5-mm intervals).27 In the initial phase of the program, all screening scans will be performed at Cleveland Clinic’s main imaging facility.
Small lung nodules remain quite challenging to detect and measure. To minimize variability in scan interpretation, the NLST readers were all expertly trained radiologists. Despite this, much variability was noted in the number of nodules detected, their measured size, and the follow-up recommendations. All of the screening CT images for our program will be interpreted by board-certified radiologists with expertise in chest imaging.
Other screening studies have included novel imaging assessment in their testing algorithms, particularly volumetric analysis of lung nodules.28 These tools may prove to assist in nodule detection, measurement, and management over time. At this point, we do not think they have been studied and standardized enough to include them in a standard-of-care screening program. We hope that they will evolve to the point of clinical utility in the near future.
Lung cancer screening is not currently covered by most insurers, including Medicare, although one major insurer has recently started to cover it. We expect decisions on coverage from other insurers in the next 12 months. In the meantime, we offer a low-dose screening chest CT to our patients for $125, which includes the radiologist’s fee for interpreting the scan.
Smoking cessation
The NLST showed that low-dose CT screening can reduce lung cancer mortality rates by 20% in a high-risk group. A 50-year-old active smoker who quits smoking reduces his or her risk of dying of lung cancer by more than 50%.29 Entry into a lung cancer screening program provides an opportunity for education and assistance with tobacco dependency.
At Cleveland Clinic, we have an active Tobacco Treatment Center within our Wellness Institute. All lung cancer screening participants who are identified as active smokers will be given a program brochure and will be offered a consult in the program.
What do we identify as a lung nodule, and how should they be managed?
Studies of CT-based screening have highlighted the tremendous number of lung nodules that are identified and the low likelihood of malignancy in those that are less than 1 cm in diameter. Many screening studies define a positive result as a lung nodule above a particular size. The NLST used 4 mm or greater as the cutoff. The lower the cutoff, the greater the number of nodules found, and the lower the overall likelihood of malignancy in the nodules.
Studies in which annual CT screening was the intervention are able to use size criteria in part because the study design ensures another CT will be performed 12 months later. Current nodule management guidelines suggest 12-month CT follow-up of incidentally discovered lung nodules, 4 mm or smaller, in at-risk patients.30 In a screening program, particularly one for which the patient must pay, the 12-month screening CT cannot be guaranteed. This makes it more difficult to ignore the smallest nodules identified on CT screening. Given this, we will be reporting all lung nodules identified, regardless of size on the initial screening.
Most studies of CT screening have reported any new nodule identified in subsequent screening rounds regardless of size. Though it is intuitive that a new nodule would have a high likelihood of malignancy in a high-risk cohort, malignancy rates have been reported to be as low as 1% for new nodules. As with the initial round of screening, we will report all new lung nodules identified in subsequent screening rounds.
The recommendations for the evaluation of lung nodules, both within the report and at the lung nodule clinic, are in keeping with currently available guidelines, such as those from the Fleischner Society30 and the American College of Chest Physicians.31 For incidentally discovered lung nodules in patients at high risk, the Fleischner Society recommendations are as follows30:
- For nodules 4 mm or smaller, follow-up in 12 months; if no growth, then no further follow-up
- For nodules 4 to 6 mm, follow-up at 6 to 12 months, then 18 to 24 months if no growth
- For nodules 6 to 8 mm, follow-up at 3 to 6 months, then 9 to 12 months, then 24 months if no growth
- For nodules 8 mm or larger, follow-up at 3, 9, and 24 months, or positron emission tomography, or biopsy, or both.
If the nodule is large enough or is deemed to be of high enough risk, adjuvant testing with diagnostic imaging, guided bronchoscopy, transthoracic needle aspiration, or minimally invasive resection will be offered. All patients with nodules believed to require biopsy will be discussed at our multidisciplinary lung cancer tumor board before biopsy.
How do we make practitioners and patients aware of the program and its indications, risks, and benefits?
Education will be the key to having lung cancer screening adopted as the standard of care, to lung cancer screening being provided within a well-designed and capable system, and to ensuring that patients have realistic expectations about screening. Articles such as this and grand rounds presentations within our health system will help provide education to our colleagues. Broader marketing campaigns will be considered in the future once demand and system capabilities are clearly identified. A patient information brochure will be provided at the time of the screening test (see the patient information sheet that accompanies this article).
How do we help to advance best practice?
As excited as we are that low-dose CT-based lung cancer screening has been proven to reduce lung cancer mortality rates, it is clear that there is a lot of room to improve the programs that are developed based on current data.
Advances in our ability to accurately predict an individual’s risk of developing lung cancer will allow us to offer screening to those it is most likely to benefit.
Advances in smoking cessation and chemoprevention will help to minimize the number of lung cancers that develop.
Advances in our ability to determine the nature of lung nodules will allow us to accelerate treatment of very early lung cancer while minimizing additional testing on benign nodules; advances in our ability to treat localized and advanced disease will improve the outcome for those identified as having lung cancer.
To help move the science of screening forward, we will develop a screening program registry that can be populated from the order set and the templated report. The registry can be used to ensure appropriate patient care, while studying relevant epidemiologic, quality, and cost-related questions.
We hope to assess novel imaging software capable of assisting with the detection and characterization of lung nodules.
We have an active biomarker development program to assess the ability of breath and blood-based biomarkers to identify those at risk of developing lung cancer; to assist with the management of screening-detected lung nodules; to assist with the diagnosis of early stage lung cancer; and to characterize the nature of the cancers identified. Accurate biomarkers could lead to further decreases in mortality rates while reducing the risks and costs of a screening program.
We have strong surgical, medical, and radiation oncology programs, actively pursuing advances in minimally invasive resection procedures and ablative and targeted therapies.
ENTERING A NEW ERA
We are entering a new era of lung cancer screening. The NLST has shown that lung cancer morality rates can be reduced through low-dose CT screening in a high-risk population. Many challenges remain, such as managing the nodules that are discovered, determining if the program is cost-effective, and minimizing radiation exposure. These need to be considered when designing a lung cancer screening program. Advances over time will help us optimize the programs that are developed.
In 2011, two papers were published that will shape the way we think about lung cancer screening for years to come.
See related patient information sheet
While the ability to screen for lung cancer is a major positive change, it also raises many thorny questions, such as who should be screened, how often should they be screened, and how should we respond when a nodule is detected.
To answer some of these questions, we will outline how Cleveland Clinic has structured its lung cancer screening program, and the rationale we used for making pragmatic patient-care decisions within this program. We will conclude with our thoughts about the potential evolution of lung cancer screening programs.
THE 40-YEAR QUEST FOR EFFECTIVE LUNG CANCER SCREENING
Lung cancer kills more people in the United States than the next four most lethal types of cancer combined.3 It is curable if found early in its course. Unfortunately, most people who develop lung cancer feel no symptoms when it is early in its course, and therefore it is too often diagnosed at a late stage. Treatment for late-stage lung cancer is effective, but it is rarely curative.
Screening refers to testing people at risk of developing a disease before its symptoms or signs have appeared. The goal of screening is to reduce the disease-specific mortality rate. For this to happen, the disease must be detectable in a preclinical form, and treatment must be more successful when applied early. Ideally, the screening test should pose little risk to the patient, be sensitive for detecting the disease early in its course, give few false-positive results, be acceptable to the patient, and be relatively inexpensive to the health system.
Over the past 4 decades, a large volume of research has been done in the hope of proving that conventional radiography or CT could be an effective screening test for lung cancer.4,5
Cohort studies (ie, in which all the patients were screened) of radiography or CT have shown a longer survival from the time of lung cancer diagnosis than would be expected without screening. These studies were not designed to prove a reduction in the lung cancer-specific mortality rate.
Controlled trials (in which half the patients received the screening and the other half did not) of chest radiography have been interpreted as not showing a reduction in lung cancer mortality rates, though debate about the interpretation of these trials persisted until this past year. Biases inherent in using duration of survival rather than the mortality rate as an end point have been suggested as the reason for the apparent benefit in survival without a reduction in the mortality rate.
Controlled trials of CT screening were started nearly a decade ago. Until 2011, the results of these trials were not mature enough to comment on.
THE PROSTATE, LUNG, COLORECTAL, AND OVARIAN TRIAL
The lung cancer screening portion of the PLCO trial aimed to determine the effect of screening chest radiography on lung cancer-specific mortality rates.1
In this trial, 154,901 people were randomized to undergo either posteroanterior chest radiography every year for 4 years or usual care, ie, no lung cancer screening. Participants were men and women age 55 to 74 with no history of prostate, lung, colorectal, or ovarian cancer. They did not need to be a smoker to participate. Those who had never smoked and who were randomized to the screening group received only 3 years of testing. All were followed for 13 years or until the conclusion of the study (8 years after the final participant was enrolled). About half were women, and nearly two-thirds were age 55 through 64. Only 10% were current smokers, while a full 45% had never smoked.
Results. Adherence to screening in the screening group ranged from 79% to 86.6% over the years of screening, and 11% of the usual-care group was estimated to have undergone screening chest radiography.
Cumulative lung cancer incidence rates were 201 per 100,000 person-years in the screening group and 192 in the usual-care group.
In the screening group, there were a total of 1,696 lung cancers during the entire study. Of these, 307 (18%) were detected by screening, 198 (12%) were interval cancers (diagnosed during the screening period but not by the screening test), and the remainder were diagnosed after the screening period during the years of follow-up. In the screening group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also in the screening group, the cancers detected by screening were more likely to be stage I (50%) than those not detected by screening.
The cumulative number of deaths from lung cancer was slightly but not significantly lower in the screening group from years 4 through 11. However, by the end of follow-up, the number of lung cancer deaths was equal between the groups (1,213 in the screening group vs 1,230 in the usual-care group). The cumulative overall mortality rate was also similar between the groups. For the subgroup who would have qualified for the NLST (see below), the lung cancer mortality rate was statistically similar between the two groups.
Comments. The results of the PLCO screening trial will be interpreted as the final word in lung cancer screening with standard chest radiography. The conclusion is that annual screening with chest radiography does not reduce lung cancer mortality rates and thus should not be performed in this context.
THE NATIONAL LUNG SCREENING TRIAL
The NLST aimed to determine if screening with low-dose chest CT could reduce lung cancer mortality rates.2
This controlled trial enrolled 53,454 people, who were randomized to undergo either low-dose chest CT or posteroanterior chest radiography at baseline and then yearly for 2 years.
Participants were men and women age 55 to 74 with at least 30 pack-years of cigarette smoking. If they had quit smoking, they had to have quit within the past 15 years. All were followed until study conclusion (median 6.5 years, maximum 7.4). About 41% were women, and nearly three-quarters were age 55 through 64. More than 48% were current smokers, with the rest being former smokers.
Results. Adherence to screening was 95% in the CT group and 93% in the radiography group, with a 4.3% annual rate of CT outside the study during the screening phase.
Cumulative lung cancer incidence rates were 645 per 100,000 person-years in the CT group and 572 in the radiography group.
In the CT group there were a total of 1,060 lung cancers during the entire study. Of these, 649 (61%) were detected by screening, 44 (4%) were interval cancers, and the rest were diagnosed after the screening period during follow-up.
In the chest radiography group, there were a total of 941 lung cancers during the entire study. Of these, 279 (30%) were detected by screening, 137 (15%) were interval cancers, and the rest were diagnosed after the screening period. Within the CT group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also within the CT group, the cancers detected by screening were more likely to be stage I (63%) than those not detected by screening.
The cumulative number of deaths from lung cancer was 443 in the radiography group, but only 356 in the CT group—20.0% lower (P =.004). The cumulative overall mortality rate was 6.7% lower in the CT group (P = .02).
Comments. The results of the NLST provide the first evidence that lung cancer mortality rates can be reduced by screening. Though many questions remain, the conclusions of this study are that screening a well-defined high-risk group with low-dose CT reduces the rate of death from lung cancer.
REMAINING CHALLENGES
The NLST showed that lung cancer screening with low-dose CT can meet the most important criterion for a successful screening program, ie, a reduction in the disease-specific mortality rate. Many challenges remain in meeting the other criteria for a successful or ideal screening program (low risk, few false-positive results, acceptability to the patient, and affordability). The issues with low-dose CT-based screening that challenge these ideals are outlined in this section.
Lung nodules: Benign or malignant?
A meta-analysis of CT screening studies found that for every 1,000 people screened at baseline, 9 were found to have stage I non-small-cell lung cancer, 235 had false-positive nodules, and 4 underwent thoracotomy for benign lesions.6
The NLST results were similar. In this trial, only nodules that were 4 mm or greater in diameter were reported. Using these criteria, over 27% of all study participants were found to have a lung nodule on CT at baseline and at year 1. The rate fell to nearly 17% at year 2, as nodules present from baseline were not reported. Of all the lung nodules detected, only 3.6% were ultimately proven to represent lung cancer.2
Many issues with small lung nodules need to be considered. The nodules are difficult to find, with highly variable reporting even by expert radiologists.7 They are difficult to measure accurately and thus are difficult to assess for growth.8 Adjunctive imaging and nonsurgical biopsy have a low yield for small nodules.9–11 Follow-up of these lung nodules includes additional imaging and nonsurgical and surgical biopsy procedures, adding expense to the program and risk to the patient. Finally, knowing that they have a lung nodule makes patients feel anxious and thus negatively affects their quality of life.12,13
Radiation exposure: How great is the risk?
There is a great deal of concern about radiation exposure from medical imaging, as many people receive a substantial amount of radiation each year from medical testing.14 A single low-dose scan with chest CT delivers a whole-body effective dose of about 1.5 mSv—less than one-fifth of the radiation dose of a typical diagnostic CT scan.
Many have tried to estimate the consequences of radiation exposure from low-dose CT screening. All estimates are extrapolations from unrelated radiation exposures. The increase in risk of death ranged from 0.01% to a few percent,15 and the increase in cancers was as high as 1.8% over a 25-year screening period.16 In general, the risks are felt to be very low but not negligible.
Cost-effectiveness is unknown
The cost-effectiveness of lung cancer screening is also unknown. Many highly variable estimates have been published.17–20 The studies have differed in the perspective taken, the costs of testing assumed, and the rounds of screening included. The most cost-effective estimates are in populations with the highest risk of cancer, in programs that achieve the greatest reduction in mortality rate, and in programs that lead to high rates of smoking cessation.
Screening in the real world as opposed to a clinical trial may involve different risks, benefits, and costs. Compliance with screening and with nodule management algorithms may be lower outside of a study. One study suggested that those at highest risk of developing lung cancer would be the least likely to enroll in a screening program and the least likely to accept curative-intent surgery for screening-detected cancer.21
We expect that the NLST data will be analyzed for cost-effectiveness. This should provide the most accurate estimates for the group that was studied.
WE SET OUT TO DESIGN A SCREENING PROGRAM
With the evidence supporting a reduction in the rate of lung cancer mortality, and knowing the remaining challenges, we set out to provide a lung cancer screening program within Cleveland Clinic. In the design of our program, we considered several questions, outlined below.
Who should be offered low-dose CT screening?
The results of the NLST led to a great deal of excitement about lung cancer screening in both the medical community and the general public. The positive side of this publicity is that lung cancer is receiving attention that may lead to support for further advances. The negative side is that many patients who may seek out lung cancer screening are not at high enough risk of lung cancer to clearly benefit from it.
In the NLST, a very high-risk cohort was studied, as defined by clinical variables (age 55 to 74, at least 30 pack-years of smoking, and if a former smoker, had quit within the past 15 years). In this high-risk group, 320 patients needed to be screened (with three yearly chest CT scans) for one life to be saved from lung cancer, and only 3.6% of all lung nodules found (4 mm or larger) were actually lung cancer. In a group at lower risk, the number that needed to be screened to save one life would be higher, and the percentage of lung nodules that truly were lung cancer would be lower. This would lead to higher risks and costs related to screening, without a proven benefit to members of the lower-risk group.
The risk of the NLST cohort developing lung cancer was approximately 0.6% per year. Lung cancer risk-prediction models have been developed and published. Up to 2011, the three most commonly used models had only moderate accuracy at predicting risk.22–25 In 2011 a risk model based on the PLCO cohort was developed and published.26 This model seemed to be more accurate but perhaps a bit harder to apply in practice.
We discussed whether using a validated risk predictor with a target of 0.6% per year (ie, the risk in the NLST trial) would be an adequate means of deciding on candidacy for lung cancer screening or if we should strictly adhere to the inclusion criteria of the NLST cohort. We feel that the NLST cohort is the only group with true evidence of benefit (a reduction in the lung cancer-specific mortality rate). Thus, for our program’s entry criteria, we decided to use the same clinical predictors used for entry in the NLST.
How will the right patients get scheduled for low-dose screening CT?
Patients who enter the lung cancer screening program from our health system will require a physician’s order.
We are fortunate to have an electronic medical record in place. We have created an order set within the electronic record for low-dose chest CT. The order will eventually be able to be entered as “CT lung screening w/o” (ie, without contrast).
For patients from outside of our health system who would like to enter the lung cancer screening program, the entry criteria will be the same (see above). We will ask for the name of the patient’s primary care practitioner. If the patient does not have one, a member of our Respiratory Institute will see and enroll the patient.
How often should patients be screened, and for how many years?
Unfortunately, questions about the frequency of screening and how many years it should continue remain unanswered.
In the NLST, a similar number of early-stage lung cancers were detected during each of the three screening rounds. In both the NLST and PLCO trials, differences in the mortality rate curves began to narrow during the observation period, when active screening was no longer occurring. Thus, it is possible that a longer duration of screening could lead to a further reduction in mortality rates. Others have questioned whether a similar benefit, with less cost and risk, could be obtained by screening every 2 years.
The large amount of data obtained from the NLST and other CT-based studies is being reviewed so that models can be developed to help answer these questions. For now, we suggest at least three yearly CT screenings, with the hope that we will have clearer answers to these questions over time.
How will low-dose CT be performed and interpreted?
The parameters for low-dose CT were very tightly controlled and monitored during the NLST. This quality-control effort, designed to improve consistency across sites and to minimize risk to patients, should be carried into lung cancer screening programs.
Our program will closely mimic the CT performance criteria used in the NLST (tube current-time product 40 mAs for all patients, field of view lungs only, lung kernel images 3 mm at 1.5-mm intervals, and soft-tissue kernel images 5 mm at 2.5-mm intervals).27 In the initial phase of the program, all screening scans will be performed at Cleveland Clinic’s main imaging facility.
Small lung nodules remain quite challenging to detect and measure. To minimize variability in scan interpretation, the NLST readers were all expertly trained radiologists. Despite this, much variability was noted in the number of nodules detected, their measured size, and the follow-up recommendations. All of the screening CT images for our program will be interpreted by board-certified radiologists with expertise in chest imaging.
Other screening studies have included novel imaging assessment in their testing algorithms, particularly volumetric analysis of lung nodules.28 These tools may prove to assist in nodule detection, measurement, and management over time. At this point, we do not think they have been studied and standardized enough to include them in a standard-of-care screening program. We hope that they will evolve to the point of clinical utility in the near future.
Lung cancer screening is not currently covered by most insurers, including Medicare, although one major insurer has recently started to cover it. We expect decisions on coverage from other insurers in the next 12 months. In the meantime, we offer a low-dose screening chest CT to our patients for $125, which includes the radiologist’s fee for interpreting the scan.
Smoking cessation
The NLST showed that low-dose CT screening can reduce lung cancer mortality rates by 20% in a high-risk group. A 50-year-old active smoker who quits smoking reduces his or her risk of dying of lung cancer by more than 50%.29 Entry into a lung cancer screening program provides an opportunity for education and assistance with tobacco dependency.
At Cleveland Clinic, we have an active Tobacco Treatment Center within our Wellness Institute. All lung cancer screening participants who are identified as active smokers will be given a program brochure and will be offered a consult in the program.
What do we identify as a lung nodule, and how should they be managed?
Studies of CT-based screening have highlighted the tremendous number of lung nodules that are identified and the low likelihood of malignancy in those that are less than 1 cm in diameter. Many screening studies define a positive result as a lung nodule above a particular size. The NLST used 4 mm or greater as the cutoff. The lower the cutoff, the greater the number of nodules found, and the lower the overall likelihood of malignancy in the nodules.
Studies in which annual CT screening was the intervention are able to use size criteria in part because the study design ensures another CT will be performed 12 months later. Current nodule management guidelines suggest 12-month CT follow-up of incidentally discovered lung nodules, 4 mm or smaller, in at-risk patients.30 In a screening program, particularly one for which the patient must pay, the 12-month screening CT cannot be guaranteed. This makes it more difficult to ignore the smallest nodules identified on CT screening. Given this, we will be reporting all lung nodules identified, regardless of size on the initial screening.
Most studies of CT screening have reported any new nodule identified in subsequent screening rounds regardless of size. Though it is intuitive that a new nodule would have a high likelihood of malignancy in a high-risk cohort, malignancy rates have been reported to be as low as 1% for new nodules. As with the initial round of screening, we will report all new lung nodules identified in subsequent screening rounds.
The recommendations for the evaluation of lung nodules, both within the report and at the lung nodule clinic, are in keeping with currently available guidelines, such as those from the Fleischner Society30 and the American College of Chest Physicians.31 For incidentally discovered lung nodules in patients at high risk, the Fleischner Society recommendations are as follows30:
- For nodules 4 mm or smaller, follow-up in 12 months; if no growth, then no further follow-up
- For nodules 4 to 6 mm, follow-up at 6 to 12 months, then 18 to 24 months if no growth
- For nodules 6 to 8 mm, follow-up at 3 to 6 months, then 9 to 12 months, then 24 months if no growth
- For nodules 8 mm or larger, follow-up at 3, 9, and 24 months, or positron emission tomography, or biopsy, or both.
If the nodule is large enough or is deemed to be of high enough risk, adjuvant testing with diagnostic imaging, guided bronchoscopy, transthoracic needle aspiration, or minimally invasive resection will be offered. All patients with nodules believed to require biopsy will be discussed at our multidisciplinary lung cancer tumor board before biopsy.
How do we make practitioners and patients aware of the program and its indications, risks, and benefits?
Education will be the key to having lung cancer screening adopted as the standard of care, to lung cancer screening being provided within a well-designed and capable system, and to ensuring that patients have realistic expectations about screening. Articles such as this and grand rounds presentations within our health system will help provide education to our colleagues. Broader marketing campaigns will be considered in the future once demand and system capabilities are clearly identified. A patient information brochure will be provided at the time of the screening test (see the patient information sheet that accompanies this article).
How do we help to advance best practice?
As excited as we are that low-dose CT-based lung cancer screening has been proven to reduce lung cancer mortality rates, it is clear that there is a lot of room to improve the programs that are developed based on current data.
Advances in our ability to accurately predict an individual’s risk of developing lung cancer will allow us to offer screening to those it is most likely to benefit.
Advances in smoking cessation and chemoprevention will help to minimize the number of lung cancers that develop.
Advances in our ability to determine the nature of lung nodules will allow us to accelerate treatment of very early lung cancer while minimizing additional testing on benign nodules; advances in our ability to treat localized and advanced disease will improve the outcome for those identified as having lung cancer.
To help move the science of screening forward, we will develop a screening program registry that can be populated from the order set and the templated report. The registry can be used to ensure appropriate patient care, while studying relevant epidemiologic, quality, and cost-related questions.
We hope to assess novel imaging software capable of assisting with the detection and characterization of lung nodules.
We have an active biomarker development program to assess the ability of breath and blood-based biomarkers to identify those at risk of developing lung cancer; to assist with the management of screening-detected lung nodules; to assist with the diagnosis of early stage lung cancer; and to characterize the nature of the cancers identified. Accurate biomarkers could lead to further decreases in mortality rates while reducing the risks and costs of a screening program.
We have strong surgical, medical, and radiation oncology programs, actively pursuing advances in minimally invasive resection procedures and ablative and targeted therapies.
ENTERING A NEW ERA
We are entering a new era of lung cancer screening. The NLST has shown that lung cancer morality rates can be reduced through low-dose CT screening in a high-risk population. Many challenges remain, such as managing the nodules that are discovered, determining if the program is cost-effective, and minimizing radiation exposure. These need to be considered when designing a lung cancer screening program. Advances over time will help us optimize the programs that are developed.
- Oken MM, Hocking WG, Kvale PA, et al; PLCO Project Team. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011; 306:1865–1873.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Mazzone PJ, Mekhail T. Lung cancer screening. Curr Oncol Rep 2007; 9:265–274.
- Mazzone PJ. Lung cancer screening: an update, discussion, and look ahead. Curr Oncol Rep 2010; 12:226–234.
- Gopal M, Abdullah SE, Grady JJ, Goodwin JS. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010; 5:1233–1239.
- Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology 2008; 246:265–272.
- Singh S, Pinsky P, Fineberg NS, et al. Evaluation of reader variability in the interpretation of follow-up CT scans at lung cancer screening. Radiology 2011; 259:263–270.
- Lindell RM, Hartman TE, Swensen SJ, et al. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening chest CT in a high-risk population. AJR Am J Roentgenol 2005; 185:126–131.
- Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117:1049–1054.
- Kothary N, Lock L, Sze DY, Hofmann LV. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009; 10:360–363.
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010; 102:27–34.
- Lemonnier I, Baumann C, Jolly D, et al. Solitary pulmonary nodules: consequences for patient quality of life. Qual Life Res 2011; 20:101–109.
- Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009; 361:849–857.
- Buls N, de Mey J, Covens P, Stadnik T. Health screening with CT: prospective assessment of radiation dose and associated detriment. JBR-BTR 2005; 88:12–16.
- Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004; 231:440–445.
- Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 2003; 289:313–322.
- Wisnivesky JP, Mushlin AI, Sicherman N, Henschke C. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest 2003; 124:614–621.
- Manser R, Dalton A, Carter R, Byrnes G, Elwood M, Campbell DA. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer 2005; 48:171–185.
- McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011; 6:1841–1848.
- Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their nonsmoking counterparts. Thorax 2007; 62:126–130.
- Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003; 95:470–478.
- Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007; 99:715–726.
- Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008; 98:270–276.
- D’Amelio AM, Cassidy A, Asomaning K, et al. Comparison of discriminatory power and accuracy of three lung cancer risk models. Br J Cancer 2010; 103:423–429.
- Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst 2011; 103:1058–1068.
- National Lung Screening Trial Research Team; Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology 2011; 258:243–253.
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009; 361:2221–2229.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000; 321:323–329.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Gould MK, Fletcher J, Iannettoni MD, et al; American College of Chest Physicians. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132(suppl 3):108S–130S.
- Oken MM, Hocking WG, Kvale PA, et al; PLCO Project Team. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011; 306:1865–1873.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Mazzone PJ, Mekhail T. Lung cancer screening. Curr Oncol Rep 2007; 9:265–274.
- Mazzone PJ. Lung cancer screening: an update, discussion, and look ahead. Curr Oncol Rep 2010; 12:226–234.
- Gopal M, Abdullah SE, Grady JJ, Goodwin JS. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010; 5:1233–1239.
- Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology 2008; 246:265–272.
- Singh S, Pinsky P, Fineberg NS, et al. Evaluation of reader variability in the interpretation of follow-up CT scans at lung cancer screening. Radiology 2011; 259:263–270.
- Lindell RM, Hartman TE, Swensen SJ, et al. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening chest CT in a high-risk population. AJR Am J Roentgenol 2005; 185:126–131.
- Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117:1049–1054.
- Kothary N, Lock L, Sze DY, Hofmann LV. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009; 10:360–363.
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010; 102:27–34.
- Lemonnier I, Baumann C, Jolly D, et al. Solitary pulmonary nodules: consequences for patient quality of life. Qual Life Res 2011; 20:101–109.
- Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009; 361:849–857.
- Buls N, de Mey J, Covens P, Stadnik T. Health screening with CT: prospective assessment of radiation dose and associated detriment. JBR-BTR 2005; 88:12–16.
- Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004; 231:440–445.
- Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 2003; 289:313–322.
- Wisnivesky JP, Mushlin AI, Sicherman N, Henschke C. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest 2003; 124:614–621.
- Manser R, Dalton A, Carter R, Byrnes G, Elwood M, Campbell DA. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer 2005; 48:171–185.
- McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011; 6:1841–1848.
- Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their nonsmoking counterparts. Thorax 2007; 62:126–130.
- Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003; 95:470–478.
- Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007; 99:715–726.
- Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008; 98:270–276.
- D’Amelio AM, Cassidy A, Asomaning K, et al. Comparison of discriminatory power and accuracy of three lung cancer risk models. Br J Cancer 2010; 103:423–429.
- Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst 2011; 103:1058–1068.
- National Lung Screening Trial Research Team; Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology 2011; 258:243–253.
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009; 361:2221–2229.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000; 321:323–329.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Gould MK, Fletcher J, Iannettoni MD, et al; American College of Chest Physicians. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132(suppl 3):108S–130S.
KEY POINTS
- The NLST documented a 20% reduction in the rate of death from lung cancer with low-dose CT screening compared with chest radiography screening (number needed to treat = 320). This was in a population at high risk (age 55–74 with a smoking history of at least 30 pack-years, at least some of it within the past 15 years).
- CT screening detects many lung nodules, of which only a few (3.6% in the NLST) prove to be cancer.
- In view of the positive results of the NLST, Cleveland Clinic has begun a lung cancer screening program, using the same entry criteria as those in the NLST.
- Of possibly greater impact than detecting lung cancer will be the opportunity to promote smoking cessation.