User login
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
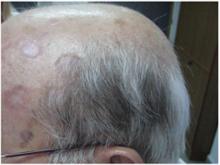
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
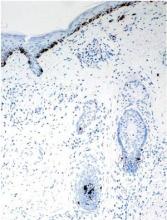
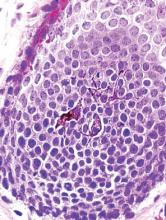
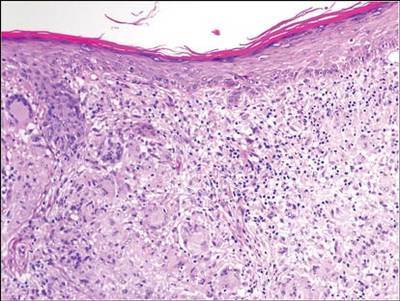
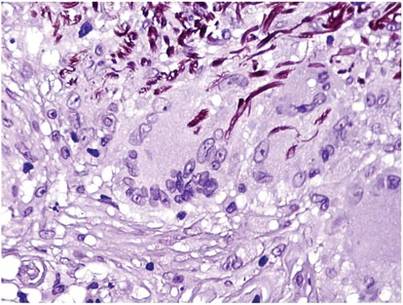
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.
| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.

| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.

| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
Practice Points
- Hair repigmentation can be a clinical clue to a subjacent inflammatory disease.
- Hair depigmentation associated with aging may be a reversible condition under proper stimulation.