User login
The Diagnosis: Leishmaniasis
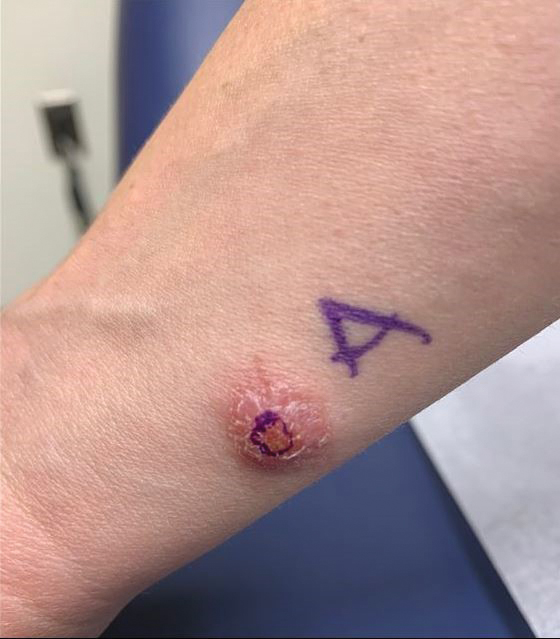
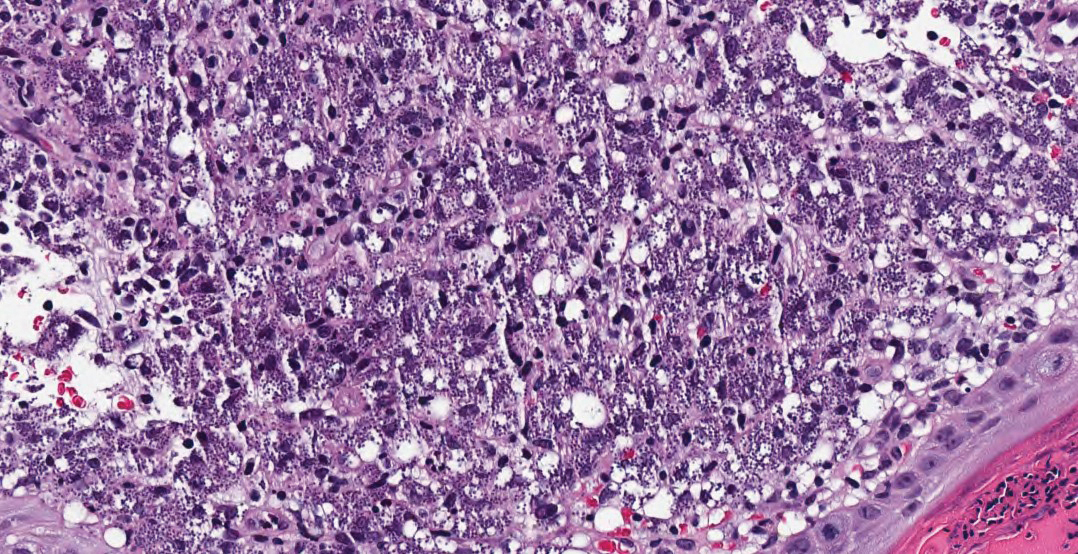
Hematoxylin and eosin staining of the tissue specimen revealed a dense histiocytic infiltrate with scattered lymphocytes and neutrophils. There were round to oval basophilic structures within the macrophages consistent with amastigotes. Giemsa staining was not necessary to visualize the organisms. The infiltrate abutted the overlying epidermis, which was acanthotic with pseudoepitheliomatous hyperplasia. There were collections of neutrophils, parakeratosis, and a serum crust overlying the epidermis (Figure). Clinical and histologic findings, as well as travel history, led to a diagnosis of cutaneous leishmaniasis (CL).
Leishmaniases is a group of diseases caused by a parasitic infection with flagellated protozoa of the genus Leishmania. There are more than 20 different Leishmania species that are pathogenic to humans, all presenting with cutaneous findings. The presentation depends on the inoculating species and the host cellular immune response and includes cutaneous, mucosal, and visceral involvement. The disease is transmitted via the bite of an infected bloodsucking female sand fly.1 There are approximately 30 different species of sand flies that are proven to be vectors of the disease, with up to 40 more suspected of involvement in transmission, predominantly from the genera Phlebotomus (Old World) and Lutzomyia (New World).1,2 There are an estimated 1 to 2 million new cases of cutaneous leishmaniasis diagnosed annually in 70 endemic countries of the tropics, subtropics, and southern Europe.1,3,4
The differential diagnosis included cutaneous tuberculosis, which can have a similar progression and clinical appearance. Cutaneous tuberculosis starts as firm, reddish-brown, painless papules that slowly enlarge and ulcerate.5 It may be further differentiated on histopathology by the presence of tuberculoid granulomas, caseating necrosis, and acid-fast bacilli, which are easily detected in early lesions but are less prevalent after the granuloma develops.6 Sporotrichosis presents as a nodule, which may or may not ulcerate, on the extremities. However, the classic morphology is a sporotrichoid pattern, which describes the initial lesion plus subcutaneous nodular spread along the lymphatics.7 On histology, sporotrichosis has a characteristic “sporotrichoid asteroid” comprised of the yeast form surrounded by eosinophilic hyaline material in raylike processes that are found in the center of suppurative granulomas or foci.8
Atypical mycobacteria, principally Mycobacterium marinum (swimming pool granuloma) and Mycobacterium ulcerans (Buruli ulcer), are capable of causing cutaneous infections. They may be differentiated histologically by a neutrophilic infiltrate of poorly formed granulomas without caseation and extensive coagulative necrosis with little cellular infiltrate, respectively.6 Histoplasma capsulatum also infects histiocytes and may appear similar in size and shape; however, histoplasmosis is surrounded by a pseudocapsule and evenly spaced.8
Conversely, the histology of leishmaniasis lacks a pseudocapsule. The amastigotes may form the classic marquee sign by lining the periphery of the macrophage or they can be randomly spaced. Classically, the epidermis shows hyperkeratosis and acanthosis. Sometimes atrophy, ulceration, or intraepidermal abscesses also can be observed. Pseudoepitheliomatous hyperplasia can be seen in some long-standing lesions.1,4 Many of these findings were observed on hematoxylin and eosin staining from a punch biopsy obtained from the center of the lesion in our patient. For further delineation, a speciation kit was obtained from Walter Reed National Military Medical Center (Bethesda, Maryland). A second punch biopsy was obtained from the lesion edge, sectioned into 4 individual pieces, and placed in Schneider tissue culture medium. It was sent for tissue culture, polymerase chain reaction, and histology. Polymerase chain reaction analysis was positive for Leishmania, which was further identified as Leishmania tropica by tissue culture.
Leishmania tropica (Old World CL) commonly causes CL and is endemic to Central Asia, the Middle East, parts of North Africa, and Southeast Asia. Old and New World CL start as a small erythematous papule after a bite from an infected female sand fly. The papule develops into a nodule over weeks to months. The lesion may ulcerate and typically heals leaving an atrophic scar in months to years.1 Speciation of CL is important to guide therapy.
Leishmania mexicana, a New World species that commonly causes CL, classically is found in Central and South America, but there also have been documented cases in Texas. A 2008 case series identified 9 cases in northern Texas in residents without a travel history to endemic locations.9 Similarly, a cross-sectional study identified 41 locally endemic cases of CL over a 10-year period (2007-2017) in Texas; 22 of these cases had speciation by polymerase chain reaction, and all cases were attributed to L mexicana.10
In the United States, CL classically has been associated with travelers and military personnel returning from the Middle East; however, a growing body of literature suggests that it may be endemic to Texas, where it is now a reportable disease. Physicians should have an increased awareness of this entity and a high index of suspicion when treating patients with nonhealing cutaneous lesions.
- Bravo F. Protozoa and worms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1470-1502.
- Killick-Kendrick R. The biology and control of phlebotomine sandflies. Clin Dermatol. 1999;17:279-289.
- Reithinger R, Dujardin JC, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581-596.
- Patterson J. Protozoal infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:787-795.
- Ramos-e-Silva M, Ribeiro de Castro MC. Mycobacterial infections. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1296-1318.
- Patterson J. Bacterial and rickettsial infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:673-709.
- Elewski B, Hughey L, Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1329-1363.
- Patterson J. Mycoses and algal infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:721-755.
- Wright NA, Davis LE, Aftergut KS, et al. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas [published online February 4, 2008]. J Am Acad Dermatol. 2008;58:650-652. doi:10.1016/j .jaad.2007.11.008.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039. doi:10.1001/jamadermatol.2018.2133
The Diagnosis: Leishmaniasis
Hematoxylin and eosin staining of the tissue specimen revealed a dense histiocytic infiltrate with scattered lymphocytes and neutrophils. There were round to oval basophilic structures within the macrophages consistent with amastigotes. Giemsa staining was not necessary to visualize the organisms. The infiltrate abutted the overlying epidermis, which was acanthotic with pseudoepitheliomatous hyperplasia. There were collections of neutrophils, parakeratosis, and a serum crust overlying the epidermis (Figure). Clinical and histologic findings, as well as travel history, led to a diagnosis of cutaneous leishmaniasis (CL).

Leishmaniases is a group of diseases caused by a parasitic infection with flagellated protozoa of the genus Leishmania. There are more than 20 different Leishmania species that are pathogenic to humans, all presenting with cutaneous findings. The presentation depends on the inoculating species and the host cellular immune response and includes cutaneous, mucosal, and visceral involvement. The disease is transmitted via the bite of an infected bloodsucking female sand fly.1 There are approximately 30 different species of sand flies that are proven to be vectors of the disease, with up to 40 more suspected of involvement in transmission, predominantly from the genera Phlebotomus (Old World) and Lutzomyia (New World).1,2 There are an estimated 1 to 2 million new cases of cutaneous leishmaniasis diagnosed annually in 70 endemic countries of the tropics, subtropics, and southern Europe.1,3,4
The differential diagnosis included cutaneous tuberculosis, which can have a similar progression and clinical appearance. Cutaneous tuberculosis starts as firm, reddish-brown, painless papules that slowly enlarge and ulcerate.5 It may be further differentiated on histopathology by the presence of tuberculoid granulomas, caseating necrosis, and acid-fast bacilli, which are easily detected in early lesions but are less prevalent after the granuloma develops.6 Sporotrichosis presents as a nodule, which may or may not ulcerate, on the extremities. However, the classic morphology is a sporotrichoid pattern, which describes the initial lesion plus subcutaneous nodular spread along the lymphatics.7 On histology, sporotrichosis has a characteristic “sporotrichoid asteroid” comprised of the yeast form surrounded by eosinophilic hyaline material in raylike processes that are found in the center of suppurative granulomas or foci.8
Atypical mycobacteria, principally Mycobacterium marinum (swimming pool granuloma) and Mycobacterium ulcerans (Buruli ulcer), are capable of causing cutaneous infections. They may be differentiated histologically by a neutrophilic infiltrate of poorly formed granulomas without caseation and extensive coagulative necrosis with little cellular infiltrate, respectively.6 Histoplasma capsulatum also infects histiocytes and may appear similar in size and shape; however, histoplasmosis is surrounded by a pseudocapsule and evenly spaced.8
Conversely, the histology of leishmaniasis lacks a pseudocapsule. The amastigotes may form the classic marquee sign by lining the periphery of the macrophage or they can be randomly spaced. Classically, the epidermis shows hyperkeratosis and acanthosis. Sometimes atrophy, ulceration, or intraepidermal abscesses also can be observed. Pseudoepitheliomatous hyperplasia can be seen in some long-standing lesions.1,4 Many of these findings were observed on hematoxylin and eosin staining from a punch biopsy obtained from the center of the lesion in our patient. For further delineation, a speciation kit was obtained from Walter Reed National Military Medical Center (Bethesda, Maryland). A second punch biopsy was obtained from the lesion edge, sectioned into 4 individual pieces, and placed in Schneider tissue culture medium. It was sent for tissue culture, polymerase chain reaction, and histology. Polymerase chain reaction analysis was positive for Leishmania, which was further identified as Leishmania tropica by tissue culture.
Leishmania tropica (Old World CL) commonly causes CL and is endemic to Central Asia, the Middle East, parts of North Africa, and Southeast Asia. Old and New World CL start as a small erythematous papule after a bite from an infected female sand fly. The papule develops into a nodule over weeks to months. The lesion may ulcerate and typically heals leaving an atrophic scar in months to years.1 Speciation of CL is important to guide therapy.
Leishmania mexicana, a New World species that commonly causes CL, classically is found in Central and South America, but there also have been documented cases in Texas. A 2008 case series identified 9 cases in northern Texas in residents without a travel history to endemic locations.9 Similarly, a cross-sectional study identified 41 locally endemic cases of CL over a 10-year period (2007-2017) in Texas; 22 of these cases had speciation by polymerase chain reaction, and all cases were attributed to L mexicana.10
In the United States, CL classically has been associated with travelers and military personnel returning from the Middle East; however, a growing body of literature suggests that it may be endemic to Texas, where it is now a reportable disease. Physicians should have an increased awareness of this entity and a high index of suspicion when treating patients with nonhealing cutaneous lesions.
The Diagnosis: Leishmaniasis
Hematoxylin and eosin staining of the tissue specimen revealed a dense histiocytic infiltrate with scattered lymphocytes and neutrophils. There were round to oval basophilic structures within the macrophages consistent with amastigotes. Giemsa staining was not necessary to visualize the organisms. The infiltrate abutted the overlying epidermis, which was acanthotic with pseudoepitheliomatous hyperplasia. There were collections of neutrophils, parakeratosis, and a serum crust overlying the epidermis (Figure). Clinical and histologic findings, as well as travel history, led to a diagnosis of cutaneous leishmaniasis (CL).

Leishmaniases is a group of diseases caused by a parasitic infection with flagellated protozoa of the genus Leishmania. There are more than 20 different Leishmania species that are pathogenic to humans, all presenting with cutaneous findings. The presentation depends on the inoculating species and the host cellular immune response and includes cutaneous, mucosal, and visceral involvement. The disease is transmitted via the bite of an infected bloodsucking female sand fly.1 There are approximately 30 different species of sand flies that are proven to be vectors of the disease, with up to 40 more suspected of involvement in transmission, predominantly from the genera Phlebotomus (Old World) and Lutzomyia (New World).1,2 There are an estimated 1 to 2 million new cases of cutaneous leishmaniasis diagnosed annually in 70 endemic countries of the tropics, subtropics, and southern Europe.1,3,4
The differential diagnosis included cutaneous tuberculosis, which can have a similar progression and clinical appearance. Cutaneous tuberculosis starts as firm, reddish-brown, painless papules that slowly enlarge and ulcerate.5 It may be further differentiated on histopathology by the presence of tuberculoid granulomas, caseating necrosis, and acid-fast bacilli, which are easily detected in early lesions but are less prevalent after the granuloma develops.6 Sporotrichosis presents as a nodule, which may or may not ulcerate, on the extremities. However, the classic morphology is a sporotrichoid pattern, which describes the initial lesion plus subcutaneous nodular spread along the lymphatics.7 On histology, sporotrichosis has a characteristic “sporotrichoid asteroid” comprised of the yeast form surrounded by eosinophilic hyaline material in raylike processes that are found in the center of suppurative granulomas or foci.8
Atypical mycobacteria, principally Mycobacterium marinum (swimming pool granuloma) and Mycobacterium ulcerans (Buruli ulcer), are capable of causing cutaneous infections. They may be differentiated histologically by a neutrophilic infiltrate of poorly formed granulomas without caseation and extensive coagulative necrosis with little cellular infiltrate, respectively.6 Histoplasma capsulatum also infects histiocytes and may appear similar in size and shape; however, histoplasmosis is surrounded by a pseudocapsule and evenly spaced.8
Conversely, the histology of leishmaniasis lacks a pseudocapsule. The amastigotes may form the classic marquee sign by lining the periphery of the macrophage or they can be randomly spaced. Classically, the epidermis shows hyperkeratosis and acanthosis. Sometimes atrophy, ulceration, or intraepidermal abscesses also can be observed. Pseudoepitheliomatous hyperplasia can be seen in some long-standing lesions.1,4 Many of these findings were observed on hematoxylin and eosin staining from a punch biopsy obtained from the center of the lesion in our patient. For further delineation, a speciation kit was obtained from Walter Reed National Military Medical Center (Bethesda, Maryland). A second punch biopsy was obtained from the lesion edge, sectioned into 4 individual pieces, and placed in Schneider tissue culture medium. It was sent for tissue culture, polymerase chain reaction, and histology. Polymerase chain reaction analysis was positive for Leishmania, which was further identified as Leishmania tropica by tissue culture.
Leishmania tropica (Old World CL) commonly causes CL and is endemic to Central Asia, the Middle East, parts of North Africa, and Southeast Asia. Old and New World CL start as a small erythematous papule after a bite from an infected female sand fly. The papule develops into a nodule over weeks to months. The lesion may ulcerate and typically heals leaving an atrophic scar in months to years.1 Speciation of CL is important to guide therapy.
Leishmania mexicana, a New World species that commonly causes CL, classically is found in Central and South America, but there also have been documented cases in Texas. A 2008 case series identified 9 cases in northern Texas in residents without a travel history to endemic locations.9 Similarly, a cross-sectional study identified 41 locally endemic cases of CL over a 10-year period (2007-2017) in Texas; 22 of these cases had speciation by polymerase chain reaction, and all cases were attributed to L mexicana.10
In the United States, CL classically has been associated with travelers and military personnel returning from the Middle East; however, a growing body of literature suggests that it may be endemic to Texas, where it is now a reportable disease. Physicians should have an increased awareness of this entity and a high index of suspicion when treating patients with nonhealing cutaneous lesions.
- Bravo F. Protozoa and worms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1470-1502.
- Killick-Kendrick R. The biology and control of phlebotomine sandflies. Clin Dermatol. 1999;17:279-289.
- Reithinger R, Dujardin JC, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581-596.
- Patterson J. Protozoal infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:787-795.
- Ramos-e-Silva M, Ribeiro de Castro MC. Mycobacterial infections. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1296-1318.
- Patterson J. Bacterial and rickettsial infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:673-709.
- Elewski B, Hughey L, Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1329-1363.
- Patterson J. Mycoses and algal infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:721-755.
- Wright NA, Davis LE, Aftergut KS, et al. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas [published online February 4, 2008]. J Am Acad Dermatol. 2008;58:650-652. doi:10.1016/j .jaad.2007.11.008.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039. doi:10.1001/jamadermatol.2018.2133
- Bravo F. Protozoa and worms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1470-1502.
- Killick-Kendrick R. The biology and control of phlebotomine sandflies. Clin Dermatol. 1999;17:279-289.
- Reithinger R, Dujardin JC, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581-596.
- Patterson J. Protozoal infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:787-795.
- Ramos-e-Silva M, Ribeiro de Castro MC. Mycobacterial infections. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1296-1318.
- Patterson J. Bacterial and rickettsial infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:673-709.
- Elewski B, Hughey L, Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. WB Saunders Co; 2018:1329-1363.
- Patterson J. Mycoses and algal infections. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:721-755.
- Wright NA, Davis LE, Aftergut KS, et al. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas [published online February 4, 2008]. J Am Acad Dermatol. 2008;58:650-652. doi:10.1016/j .jaad.2007.11.008.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039. doi:10.1001/jamadermatol.2018.2133
A 36-year-old active-duty male service member with no notable medical history presented to the dermatology clinic with an asymptomatic nodule on the right forearm that he initially noticed approximately 1 year prior while deployed in Syria and thought that it was a mosquito bite; it continued to enlarge slowly since that time. He attempted self-extraction but was only able to express a small amount of clear fluid. No other therapies had been used. He denied any other symptoms on a review of systems and was not taking any medications. Physical examination revealed a 1.5-cm, erythematous, nonulcerated, pink nodule on the right distal volar forearm without other cutaneous findings. A 4-mm punch biopsy was performed.