User login
› Avoid adding niacin to statin therapy, as it does not appear to provide any added benefit and may increase the risk of stroke. B
› Continue statin therapy in a patient who has chronic kidney disease progressing to end-stage renal disease, but do not initiate it in patients on dialysis. B
› Do not add statins to the medication regimen of patients with heart failure; focus on optimizing therapies known to reduce mortality in this patient population instead. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Morbidity and mortality from atherosclerotic disease have decreased significantly in the last several decades, in large part because of advances in therapies targeting serum lipids—including statins.1 Since their introduction in 1987, HMG Coenzyme A inhibitors have been intensively studied, and their use has increased dramatically. A recent report from the National Center for Health Statistics reveals that in the years 1999 to 2002, 26% of men ages 65 to 74 years were taking statins; several years later (2005-2008), that number had soared to 50%. In the same time frame, statin use among women ages 65 to 74 went from 24% to 36%.2
Statins lower serum low-density lipoprotein cholesterol (LDL-C) and triglycerides, raise high-density lipoprotein cholesterol (HDL-C), and improve surrogate markers for cardiovascular events. Most importantly, statins reduce the risk for major cardiovascular events, such as myocardial infarction (MI) and death from cardiovascular disease (CVD), in select populations. Yet doubts about the benefits of statins, alone or in combination with other lipid-lowering agents, for certain patient populations remain.
Primary care physicians need to know when, or whether, to add a second lipid-lowering agent to the drug regimen of patients whose response to a statin is less than hoped for, and which patient populations and clinical indicators statins have not been found to help. You’ll find answers, the results of the latest studies, and details of the 2013 cholesterol guideline released last month by the American Heart Association/American College of Cardiology and in this evidence-based update.
A statin is not enough? Don't add these drugs
In patients with very elevated LDL-C or mixed dyslipidemias that fail to reach the desired lipid levels on statin monotherapy, other classes of lipid-modifying agents are often added in an attempt to improve clinical outcomes.3 Fibrates, extended release (niacin, and n-3 polyunsaturated fatty acids have been frequently used for this purpose. But it is only in the last several years that large, well-designed studies have looked closely at patient-oriented outcomes associated with statins in combination with other lipid-modifying drugs.4-9
Fenofibrate + a statin yields little benefit
The ACCORD lipid placebo-controlled trial studied fenofibrate as a simvastatin add-on in patients with diabetes.4 Its findings? While the lipid levels of patients receiving this drug combination improved significantly, the primary endpoint (MI, stroke, or death from cardiovascular causes) was no different from that of the controls, who were taking the statin alone.
Sub-group analysis suggested that the simvastatin-fenofibrate combination benefitted only one particular group: patients with high triglyceride levels (≥204 mg/dL) and low HDL-C (≤34 mg/dL). This finding prompted the US Food and Drug Administration to call for an additional clinical trial to evaluate the effectiveness of add-on therapy with fenofibrate in patients who meet this criteria.10 The status of such a study is uncertain.
Adding niacin to a statin does more harm than good
The HATS trial, published in 2001, found the addition of niacin to a statin regimen to be beneficial.7 But because of the wide confidence interval associated with the clinical endpoints and the small number of subjects (N=160) in that study, larger trials were needed to confirm the positive results. In fact, they found the opposite.
Niacin increases stroke risk. In both the AIM-HIGH5 (N=3414) and HPS2-THRIVE6 (N=25,673) trials, the addition of extended release niacin not only failed to reduce the risk of major cardiovascular events, it was shown to increase the risk of stroke.
n-3 polyunsaturated fatty acids don’t help much
Studies evaluating the addition of n-3 polyunsaturated fatty acids to statin therapy have had mixed results. The JELIS8 trial had more than 18,000 participants, 20% of whom had known coronary artery disease. All were taking statins and randomized to either open-label eicosapentaenoic acid (EPA) 600 mg 3 times daily or placebo. The primary endpoint, a composite of sudden cardiac death, fatal or nonfatal MI, unstable angina, angioplasty, and stenting or coronary artery bypass grafting, was lower in the intervention group: (2.8% vs 3.5%; number needed to treat [NNT]: 143).
It is important to note, however, that only one of the individual components of the primary endpoint—unstable angina—was significantly reduced by EPA (2.1% vs 1.6%; P=.014).8 In the Alpha Omega trial,9 various n-3 polyunsaturated fatty acids were tested in combination with statins. None was found to be superior to placebo in reducing cardiovascular outcomes.
Based on the evidence, the new cholesterol guideline does not support the routine use of these agents to reduce atherosclerotic CVD (See “The new cholesterol guideline: Beyond the headlines”.)11
Statins and kidney disease: Factors to consider
More than half of the deaths in patients with end-stage renal disease (ESRD) are from cardiovascular causes.12 The relationship between renal dysfunction and cardiovascular events is independent of other risk factors, including a history of CVD. Risk rises with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, with a sharp increase when the rate <45 mL/min/1.73m.2 Thus, strategies known to reduce major cardiovascular events in the general population, including statins, have the potential to offer substantial benefit for patients with chronic kidney disease (CKD).
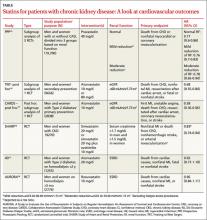
Statin use in patients with CKD has been evaluated in post-hoc and subgroup analyses of large clinical trials and, more recently, in RCTs targeting patients with both moderate and end-stage disease (TABLE).13-18
Three post-hoc analyses of large multicenter, double-blind RCTs13-15,19 compared patients with normal renal function with those with CKD. All 3 found that moderate or high-intensity statin therapy significantly reduced the incidence of the primary outcome—a composite of major cardiovascular events—compared with either placebo or a lower-intensity statin.
For patients with CKD, drug combo lowered the risk
The SHARP trial17 (N=9270) was the first large prospective, double-blind, multicenter RCT to compare the effect of a statin plus a second lipid-lowering drug (simvastatin plus ezetimibe) vs placebo in patients with CKD. A third of the participants were on dialysis at the start of the trial (ESRD was defined as starting long-term dialysis or requiring kidney transplantation).
Patients in the intervention group were significantly (17%) less likely to experience a major atherosclerotic event compared with those on placebo. This translated into an NNT of 47 over a period of 4.9 years. (Since no group received only simvastatin, it is not known what role ezetimibe had in the reduction of cardiovascular events.) Although no difference in outcomes was found when the results were stratified based on whether participants were on dialysis, this trial was not adequately powered for this subgroup analysis.17
Little benefit from statins in patients with end-stage disease
Two major prospective randomized, double-blinded, placebo-controlled, multicenter trials evaluating the effects of statin use on cardiovascular outcomes in ESRD patients on dialysis have been published.18,19 Both found a significant decline in LDL-C in patients receiving statin therapy. But neither found a significant difference in mortality rates in the statin vs placebo groups.
One group of researchers speculated that the lack of effect may be due to a difference in the pathogenesis of vascular events in patients with and without ESRD. Delayed use of statins until patients have ESRD will offer limited benefit, they concluded, and recommended against routine statin treatment in an attempt to reduce the incidence of CVD in this patient population.18 Based on these results, the new cholesterol guideline indicates that this group of patients may not benefit from statin therapy.
For patients with heart failure, statins offer limited benefit
More than half of the heart failure (HF) in the United States is caused by ischemic heart disease.20 Improvements in post-MI survival have increased the prevalence of chronic HF.
Statins have a well-established role in the prevention and treatment of atherosclerosis because of their ability to modify the natural course of the disease and reduce major adverse cardiovascular events. Thus, it seems reasonable to assume that, in patients who have or are at high risk for coronary heart disease, statins would help to prevent the occurrence or slow the progression of HF.
Early studies of statins either excluded patients with HF or enrolled so few HF patients that no conclusions could be reached regarding the safety or efficacy of statin use in this population.21-25 More recently, 2 large RCTs have studied the effect of statins in patients with HF. Both have found them to be ineffective.26,27
The CORONA trial enrolled elderly patients with HF of ischemic causes and ejection fraction ≤40% (≤35% in patients with New York Heart Association [NYHA] Class II), randomized to either rosuvastatin 10 mg/d or placebo.25 More than 40% of the participants had a history of MI, and more than 60% were NYHA Class III or IV. HF medications were well-managed; more than 90% of the patients were being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; 75%, with beta-blockers; and 39%, with aldosterone antagonists.
The researchers found no significant difference between the rosuvastatin and placebo groups in the primary outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke (11.4% in the rosuvastatin group vs 12.3% among those on placebo; 95% confidence interval, 0.83-1.02; P=.12). No difference was found in patients with a history of MI (13.9% placebo event rate vs 12.7% rosuvastatin arm; P=not significant [NS]). Neither death from worsening HF nor sudden death was reduced.26
There were fewer hospitalizations among those taking rosuvastatin, however (NNT=17 per year). And there was a trend towards a benefit among those with more advanced HF (NYHA III/IV), with primary outcome rates of 12.7% for those in the rosuvastatin group vs 14.2% in the placebo group. (P=NS). Rosuvastatin was safe for HF patients, as most types of adverse events were more common in the placebo group. Assessments of muscle toxicity were similar in both groups.
The GISSI-HF study, another RCT of rosuvastatin vs placebo in HF patients, also showed a lack of benefit from statin treatment.27 Researchers enrolled more than 4500 patients with NYHA Class II to IV HF, from both ischemic and nonischemic causes.26 Similar to the findings in the CORONA trial, LDL-C was substantially reduced by rosuvastatin 10 mg/d (-32% vs no change for those in the placebo group), but this did not translate into clinically relevant endpoints. After 3.9 years of therapy, the primary endpoints of time to death and time to death or hospitalization for cardiovascular causes were not significantly reduced, nor were any of the secondary endpoints.26
The similar lack of benefit in these 2 trials is striking in view of the benefit of statins in patients with coronary heart disease but without HF. Given these findings, focusing on optimizing therapies known to reduce mortality in patients with HF rather than adding a statin in an attempt to alter the atherosclerotic process appears to be a better approach. Thus, the recently published cholesterol guideline does not advocate the initiation or continuation of statin therapy in patients with NYHA Class II-IV HF.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948-954.
2. US Department of Health and Human Services. Health, United States, 2010. Available at http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed July 22, 2011.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
5. AIM-HIGH Investigators; Boden WE, Probsfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. .N Engl J Med. 2011;365:2255-2267.
6. Niacin causes serious unexpected side-effects, but no worthwhile benefits for patients who are at increased risk of heart attacks and strokes [press release]. HPS2-THRIVE: University of Oxford; March 9, 2013. Available at: www.thrivestudy.org/press_release.htm. Accessed July 3, 2013.
7. Brown BG, Zhao X, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
8. Yokyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients. Lancet. 2007;369:1090-1098.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026.
10. The Endocrinologic and Metabolic Drugs Advisory Committee of the FDA, Center for Drug Evaluation and Research. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM261162.pdf. Published June 24, 2011. Accessed July 3, 2013.
11. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Associaton task force on practice guidelines . Circulation. 2013 Nov 12 [Epub ahead of print.]
12. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305.
13. Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563.
14. Shepherd J, Kastelein JJ, Bittner V, et al; TNT (Treating to New Targets) Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease. J Am Coll Cardiol. 2008;51:1448-1454.
15. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes. Am J Kidney Dis. 2009;54:810-819.
16. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181-2192.
17. Wanner C, Krane V, Màrz W, et al; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248.
18. Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407.
19. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685-696.
20. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1-e90.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001-1009.
22. Downs JR, Clearfield M, Weis S, et al; AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615-1622.
23. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
24. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383-1389.
25. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Lancet. 2002;360:1623-1630.
26. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
27. GISSI-HF Investigators; Tavozzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial). Lancet. 2008;372:1231-1239.
› Avoid adding niacin to statin therapy, as it does not appear to provide any added benefit and may increase the risk of stroke. B
› Continue statin therapy in a patient who has chronic kidney disease progressing to end-stage renal disease, but do not initiate it in patients on dialysis. B
› Do not add statins to the medication regimen of patients with heart failure; focus on optimizing therapies known to reduce mortality in this patient population instead. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Morbidity and mortality from atherosclerotic disease have decreased significantly in the last several decades, in large part because of advances in therapies targeting serum lipids—including statins.1 Since their introduction in 1987, HMG Coenzyme A inhibitors have been intensively studied, and their use has increased dramatically. A recent report from the National Center for Health Statistics reveals that in the years 1999 to 2002, 26% of men ages 65 to 74 years were taking statins; several years later (2005-2008), that number had soared to 50%. In the same time frame, statin use among women ages 65 to 74 went from 24% to 36%.2
Statins lower serum low-density lipoprotein cholesterol (LDL-C) and triglycerides, raise high-density lipoprotein cholesterol (HDL-C), and improve surrogate markers for cardiovascular events. Most importantly, statins reduce the risk for major cardiovascular events, such as myocardial infarction (MI) and death from cardiovascular disease (CVD), in select populations. Yet doubts about the benefits of statins, alone or in combination with other lipid-lowering agents, for certain patient populations remain.
Primary care physicians need to know when, or whether, to add a second lipid-lowering agent to the drug regimen of patients whose response to a statin is less than hoped for, and which patient populations and clinical indicators statins have not been found to help. You’ll find answers, the results of the latest studies, and details of the 2013 cholesterol guideline released last month by the American Heart Association/American College of Cardiology and in this evidence-based update.
A statin is not enough? Don't add these drugs
In patients with very elevated LDL-C or mixed dyslipidemias that fail to reach the desired lipid levels on statin monotherapy, other classes of lipid-modifying agents are often added in an attempt to improve clinical outcomes.3 Fibrates, extended release (niacin, and n-3 polyunsaturated fatty acids have been frequently used for this purpose. But it is only in the last several years that large, well-designed studies have looked closely at patient-oriented outcomes associated with statins in combination with other lipid-modifying drugs.4-9
Fenofibrate + a statin yields little benefit
The ACCORD lipid placebo-controlled trial studied fenofibrate as a simvastatin add-on in patients with diabetes.4 Its findings? While the lipid levels of patients receiving this drug combination improved significantly, the primary endpoint (MI, stroke, or death from cardiovascular causes) was no different from that of the controls, who were taking the statin alone.
Sub-group analysis suggested that the simvastatin-fenofibrate combination benefitted only one particular group: patients with high triglyceride levels (≥204 mg/dL) and low HDL-C (≤34 mg/dL). This finding prompted the US Food and Drug Administration to call for an additional clinical trial to evaluate the effectiveness of add-on therapy with fenofibrate in patients who meet this criteria.10 The status of such a study is uncertain.
Adding niacin to a statin does more harm than good
The HATS trial, published in 2001, found the addition of niacin to a statin regimen to be beneficial.7 But because of the wide confidence interval associated with the clinical endpoints and the small number of subjects (N=160) in that study, larger trials were needed to confirm the positive results. In fact, they found the opposite.
Niacin increases stroke risk. In both the AIM-HIGH5 (N=3414) and HPS2-THRIVE6 (N=25,673) trials, the addition of extended release niacin not only failed to reduce the risk of major cardiovascular events, it was shown to increase the risk of stroke.
n-3 polyunsaturated fatty acids don’t help much
Studies evaluating the addition of n-3 polyunsaturated fatty acids to statin therapy have had mixed results. The JELIS8 trial had more than 18,000 participants, 20% of whom had known coronary artery disease. All were taking statins and randomized to either open-label eicosapentaenoic acid (EPA) 600 mg 3 times daily or placebo. The primary endpoint, a composite of sudden cardiac death, fatal or nonfatal MI, unstable angina, angioplasty, and stenting or coronary artery bypass grafting, was lower in the intervention group: (2.8% vs 3.5%; number needed to treat [NNT]: 143).
It is important to note, however, that only one of the individual components of the primary endpoint—unstable angina—was significantly reduced by EPA (2.1% vs 1.6%; P=.014).8 In the Alpha Omega trial,9 various n-3 polyunsaturated fatty acids were tested in combination with statins. None was found to be superior to placebo in reducing cardiovascular outcomes.
Based on the evidence, the new cholesterol guideline does not support the routine use of these agents to reduce atherosclerotic CVD (See “The new cholesterol guideline: Beyond the headlines”.)11
Statins and kidney disease: Factors to consider
More than half of the deaths in patients with end-stage renal disease (ESRD) are from cardiovascular causes.12 The relationship between renal dysfunction and cardiovascular events is independent of other risk factors, including a history of CVD. Risk rises with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, with a sharp increase when the rate <45 mL/min/1.73m.2 Thus, strategies known to reduce major cardiovascular events in the general population, including statins, have the potential to offer substantial benefit for patients with chronic kidney disease (CKD).
Statin use in patients with CKD has been evaluated in post-hoc and subgroup analyses of large clinical trials and, more recently, in RCTs targeting patients with both moderate and end-stage disease (TABLE).13-18
Three post-hoc analyses of large multicenter, double-blind RCTs13-15,19 compared patients with normal renal function with those with CKD. All 3 found that moderate or high-intensity statin therapy significantly reduced the incidence of the primary outcome—a composite of major cardiovascular events—compared with either placebo or a lower-intensity statin.
For patients with CKD, drug combo lowered the risk
The SHARP trial17 (N=9270) was the first large prospective, double-blind, multicenter RCT to compare the effect of a statin plus a second lipid-lowering drug (simvastatin plus ezetimibe) vs placebo in patients with CKD. A third of the participants were on dialysis at the start of the trial (ESRD was defined as starting long-term dialysis or requiring kidney transplantation).
Patients in the intervention group were significantly (17%) less likely to experience a major atherosclerotic event compared with those on placebo. This translated into an NNT of 47 over a period of 4.9 years. (Since no group received only simvastatin, it is not known what role ezetimibe had in the reduction of cardiovascular events.) Although no difference in outcomes was found when the results were stratified based on whether participants were on dialysis, this trial was not adequately powered for this subgroup analysis.17
Little benefit from statins in patients with end-stage disease
Two major prospective randomized, double-blinded, placebo-controlled, multicenter trials evaluating the effects of statin use on cardiovascular outcomes in ESRD patients on dialysis have been published.18,19 Both found a significant decline in LDL-C in patients receiving statin therapy. But neither found a significant difference in mortality rates in the statin vs placebo groups.
One group of researchers speculated that the lack of effect may be due to a difference in the pathogenesis of vascular events in patients with and without ESRD. Delayed use of statins until patients have ESRD will offer limited benefit, they concluded, and recommended against routine statin treatment in an attempt to reduce the incidence of CVD in this patient population.18 Based on these results, the new cholesterol guideline indicates that this group of patients may not benefit from statin therapy.
For patients with heart failure, statins offer limited benefit
More than half of the heart failure (HF) in the United States is caused by ischemic heart disease.20 Improvements in post-MI survival have increased the prevalence of chronic HF.
Statins have a well-established role in the prevention and treatment of atherosclerosis because of their ability to modify the natural course of the disease and reduce major adverse cardiovascular events. Thus, it seems reasonable to assume that, in patients who have or are at high risk for coronary heart disease, statins would help to prevent the occurrence or slow the progression of HF.
Early studies of statins either excluded patients with HF or enrolled so few HF patients that no conclusions could be reached regarding the safety or efficacy of statin use in this population.21-25 More recently, 2 large RCTs have studied the effect of statins in patients with HF. Both have found them to be ineffective.26,27
The CORONA trial enrolled elderly patients with HF of ischemic causes and ejection fraction ≤40% (≤35% in patients with New York Heart Association [NYHA] Class II), randomized to either rosuvastatin 10 mg/d or placebo.25 More than 40% of the participants had a history of MI, and more than 60% were NYHA Class III or IV. HF medications were well-managed; more than 90% of the patients were being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; 75%, with beta-blockers; and 39%, with aldosterone antagonists.
The researchers found no significant difference between the rosuvastatin and placebo groups in the primary outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke (11.4% in the rosuvastatin group vs 12.3% among those on placebo; 95% confidence interval, 0.83-1.02; P=.12). No difference was found in patients with a history of MI (13.9% placebo event rate vs 12.7% rosuvastatin arm; P=not significant [NS]). Neither death from worsening HF nor sudden death was reduced.26
There were fewer hospitalizations among those taking rosuvastatin, however (NNT=17 per year). And there was a trend towards a benefit among those with more advanced HF (NYHA III/IV), with primary outcome rates of 12.7% for those in the rosuvastatin group vs 14.2% in the placebo group. (P=NS). Rosuvastatin was safe for HF patients, as most types of adverse events were more common in the placebo group. Assessments of muscle toxicity were similar in both groups.
The GISSI-HF study, another RCT of rosuvastatin vs placebo in HF patients, also showed a lack of benefit from statin treatment.27 Researchers enrolled more than 4500 patients with NYHA Class II to IV HF, from both ischemic and nonischemic causes.26 Similar to the findings in the CORONA trial, LDL-C was substantially reduced by rosuvastatin 10 mg/d (-32% vs no change for those in the placebo group), but this did not translate into clinically relevant endpoints. After 3.9 years of therapy, the primary endpoints of time to death and time to death or hospitalization for cardiovascular causes were not significantly reduced, nor were any of the secondary endpoints.26
The similar lack of benefit in these 2 trials is striking in view of the benefit of statins in patients with coronary heart disease but without HF. Given these findings, focusing on optimizing therapies known to reduce mortality in patients with HF rather than adding a statin in an attempt to alter the atherosclerotic process appears to be a better approach. Thus, the recently published cholesterol guideline does not advocate the initiation or continuation of statin therapy in patients with NYHA Class II-IV HF.
› Avoid adding niacin to statin therapy, as it does not appear to provide any added benefit and may increase the risk of stroke. B
› Continue statin therapy in a patient who has chronic kidney disease progressing to end-stage renal disease, but do not initiate it in patients on dialysis. B
› Do not add statins to the medication regimen of patients with heart failure; focus on optimizing therapies known to reduce mortality in this patient population instead. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Morbidity and mortality from atherosclerotic disease have decreased significantly in the last several decades, in large part because of advances in therapies targeting serum lipids—including statins.1 Since their introduction in 1987, HMG Coenzyme A inhibitors have been intensively studied, and their use has increased dramatically. A recent report from the National Center for Health Statistics reveals that in the years 1999 to 2002, 26% of men ages 65 to 74 years were taking statins; several years later (2005-2008), that number had soared to 50%. In the same time frame, statin use among women ages 65 to 74 went from 24% to 36%.2
Statins lower serum low-density lipoprotein cholesterol (LDL-C) and triglycerides, raise high-density lipoprotein cholesterol (HDL-C), and improve surrogate markers for cardiovascular events. Most importantly, statins reduce the risk for major cardiovascular events, such as myocardial infarction (MI) and death from cardiovascular disease (CVD), in select populations. Yet doubts about the benefits of statins, alone or in combination with other lipid-lowering agents, for certain patient populations remain.
Primary care physicians need to know when, or whether, to add a second lipid-lowering agent to the drug regimen of patients whose response to a statin is less than hoped for, and which patient populations and clinical indicators statins have not been found to help. You’ll find answers, the results of the latest studies, and details of the 2013 cholesterol guideline released last month by the American Heart Association/American College of Cardiology and in this evidence-based update.
A statin is not enough? Don't add these drugs
In patients with very elevated LDL-C or mixed dyslipidemias that fail to reach the desired lipid levels on statin monotherapy, other classes of lipid-modifying agents are often added in an attempt to improve clinical outcomes.3 Fibrates, extended release (niacin, and n-3 polyunsaturated fatty acids have been frequently used for this purpose. But it is only in the last several years that large, well-designed studies have looked closely at patient-oriented outcomes associated with statins in combination with other lipid-modifying drugs.4-9
Fenofibrate + a statin yields little benefit
The ACCORD lipid placebo-controlled trial studied fenofibrate as a simvastatin add-on in patients with diabetes.4 Its findings? While the lipid levels of patients receiving this drug combination improved significantly, the primary endpoint (MI, stroke, or death from cardiovascular causes) was no different from that of the controls, who were taking the statin alone.
Sub-group analysis suggested that the simvastatin-fenofibrate combination benefitted only one particular group: patients with high triglyceride levels (≥204 mg/dL) and low HDL-C (≤34 mg/dL). This finding prompted the US Food and Drug Administration to call for an additional clinical trial to evaluate the effectiveness of add-on therapy with fenofibrate in patients who meet this criteria.10 The status of such a study is uncertain.
Adding niacin to a statin does more harm than good
The HATS trial, published in 2001, found the addition of niacin to a statin regimen to be beneficial.7 But because of the wide confidence interval associated with the clinical endpoints and the small number of subjects (N=160) in that study, larger trials were needed to confirm the positive results. In fact, they found the opposite.
Niacin increases stroke risk. In both the AIM-HIGH5 (N=3414) and HPS2-THRIVE6 (N=25,673) trials, the addition of extended release niacin not only failed to reduce the risk of major cardiovascular events, it was shown to increase the risk of stroke.
n-3 polyunsaturated fatty acids don’t help much
Studies evaluating the addition of n-3 polyunsaturated fatty acids to statin therapy have had mixed results. The JELIS8 trial had more than 18,000 participants, 20% of whom had known coronary artery disease. All were taking statins and randomized to either open-label eicosapentaenoic acid (EPA) 600 mg 3 times daily or placebo. The primary endpoint, a composite of sudden cardiac death, fatal or nonfatal MI, unstable angina, angioplasty, and stenting or coronary artery bypass grafting, was lower in the intervention group: (2.8% vs 3.5%; number needed to treat [NNT]: 143).
It is important to note, however, that only one of the individual components of the primary endpoint—unstable angina—was significantly reduced by EPA (2.1% vs 1.6%; P=.014).8 In the Alpha Omega trial,9 various n-3 polyunsaturated fatty acids were tested in combination with statins. None was found to be superior to placebo in reducing cardiovascular outcomes.
Based on the evidence, the new cholesterol guideline does not support the routine use of these agents to reduce atherosclerotic CVD (See “The new cholesterol guideline: Beyond the headlines”.)11
Statins and kidney disease: Factors to consider
More than half of the deaths in patients with end-stage renal disease (ESRD) are from cardiovascular causes.12 The relationship between renal dysfunction and cardiovascular events is independent of other risk factors, including a history of CVD. Risk rises with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, with a sharp increase when the rate <45 mL/min/1.73m.2 Thus, strategies known to reduce major cardiovascular events in the general population, including statins, have the potential to offer substantial benefit for patients with chronic kidney disease (CKD).
Statin use in patients with CKD has been evaluated in post-hoc and subgroup analyses of large clinical trials and, more recently, in RCTs targeting patients with both moderate and end-stage disease (TABLE).13-18
Three post-hoc analyses of large multicenter, double-blind RCTs13-15,19 compared patients with normal renal function with those with CKD. All 3 found that moderate or high-intensity statin therapy significantly reduced the incidence of the primary outcome—a composite of major cardiovascular events—compared with either placebo or a lower-intensity statin.
For patients with CKD, drug combo lowered the risk
The SHARP trial17 (N=9270) was the first large prospective, double-blind, multicenter RCT to compare the effect of a statin plus a second lipid-lowering drug (simvastatin plus ezetimibe) vs placebo in patients with CKD. A third of the participants were on dialysis at the start of the trial (ESRD was defined as starting long-term dialysis or requiring kidney transplantation).
Patients in the intervention group were significantly (17%) less likely to experience a major atherosclerotic event compared with those on placebo. This translated into an NNT of 47 over a period of 4.9 years. (Since no group received only simvastatin, it is not known what role ezetimibe had in the reduction of cardiovascular events.) Although no difference in outcomes was found when the results were stratified based on whether participants were on dialysis, this trial was not adequately powered for this subgroup analysis.17
Little benefit from statins in patients with end-stage disease
Two major prospective randomized, double-blinded, placebo-controlled, multicenter trials evaluating the effects of statin use on cardiovascular outcomes in ESRD patients on dialysis have been published.18,19 Both found a significant decline in LDL-C in patients receiving statin therapy. But neither found a significant difference in mortality rates in the statin vs placebo groups.
One group of researchers speculated that the lack of effect may be due to a difference in the pathogenesis of vascular events in patients with and without ESRD. Delayed use of statins until patients have ESRD will offer limited benefit, they concluded, and recommended against routine statin treatment in an attempt to reduce the incidence of CVD in this patient population.18 Based on these results, the new cholesterol guideline indicates that this group of patients may not benefit from statin therapy.
For patients with heart failure, statins offer limited benefit
More than half of the heart failure (HF) in the United States is caused by ischemic heart disease.20 Improvements in post-MI survival have increased the prevalence of chronic HF.
Statins have a well-established role in the prevention and treatment of atherosclerosis because of their ability to modify the natural course of the disease and reduce major adverse cardiovascular events. Thus, it seems reasonable to assume that, in patients who have or are at high risk for coronary heart disease, statins would help to prevent the occurrence or slow the progression of HF.
Early studies of statins either excluded patients with HF or enrolled so few HF patients that no conclusions could be reached regarding the safety or efficacy of statin use in this population.21-25 More recently, 2 large RCTs have studied the effect of statins in patients with HF. Both have found them to be ineffective.26,27
The CORONA trial enrolled elderly patients with HF of ischemic causes and ejection fraction ≤40% (≤35% in patients with New York Heart Association [NYHA] Class II), randomized to either rosuvastatin 10 mg/d or placebo.25 More than 40% of the participants had a history of MI, and more than 60% were NYHA Class III or IV. HF medications were well-managed; more than 90% of the patients were being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; 75%, with beta-blockers; and 39%, with aldosterone antagonists.
The researchers found no significant difference between the rosuvastatin and placebo groups in the primary outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke (11.4% in the rosuvastatin group vs 12.3% among those on placebo; 95% confidence interval, 0.83-1.02; P=.12). No difference was found in patients with a history of MI (13.9% placebo event rate vs 12.7% rosuvastatin arm; P=not significant [NS]). Neither death from worsening HF nor sudden death was reduced.26
There were fewer hospitalizations among those taking rosuvastatin, however (NNT=17 per year). And there was a trend towards a benefit among those with more advanced HF (NYHA III/IV), with primary outcome rates of 12.7% for those in the rosuvastatin group vs 14.2% in the placebo group. (P=NS). Rosuvastatin was safe for HF patients, as most types of adverse events were more common in the placebo group. Assessments of muscle toxicity were similar in both groups.
The GISSI-HF study, another RCT of rosuvastatin vs placebo in HF patients, also showed a lack of benefit from statin treatment.27 Researchers enrolled more than 4500 patients with NYHA Class II to IV HF, from both ischemic and nonischemic causes.26 Similar to the findings in the CORONA trial, LDL-C was substantially reduced by rosuvastatin 10 mg/d (-32% vs no change for those in the placebo group), but this did not translate into clinically relevant endpoints. After 3.9 years of therapy, the primary endpoints of time to death and time to death or hospitalization for cardiovascular causes were not significantly reduced, nor were any of the secondary endpoints.26
The similar lack of benefit in these 2 trials is striking in view of the benefit of statins in patients with coronary heart disease but without HF. Given these findings, focusing on optimizing therapies known to reduce mortality in patients with HF rather than adding a statin in an attempt to alter the atherosclerotic process appears to be a better approach. Thus, the recently published cholesterol guideline does not advocate the initiation or continuation of statin therapy in patients with NYHA Class II-IV HF.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948-954.
2. US Department of Health and Human Services. Health, United States, 2010. Available at http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed July 22, 2011.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
5. AIM-HIGH Investigators; Boden WE, Probsfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. .N Engl J Med. 2011;365:2255-2267.
6. Niacin causes serious unexpected side-effects, but no worthwhile benefits for patients who are at increased risk of heart attacks and strokes [press release]. HPS2-THRIVE: University of Oxford; March 9, 2013. Available at: www.thrivestudy.org/press_release.htm. Accessed July 3, 2013.
7. Brown BG, Zhao X, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
8. Yokyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients. Lancet. 2007;369:1090-1098.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026.
10. The Endocrinologic and Metabolic Drugs Advisory Committee of the FDA, Center for Drug Evaluation and Research. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM261162.pdf. Published June 24, 2011. Accessed July 3, 2013.
11. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Associaton task force on practice guidelines . Circulation. 2013 Nov 12 [Epub ahead of print.]
12. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305.
13. Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563.
14. Shepherd J, Kastelein JJ, Bittner V, et al; TNT (Treating to New Targets) Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease. J Am Coll Cardiol. 2008;51:1448-1454.
15. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes. Am J Kidney Dis. 2009;54:810-819.
16. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181-2192.
17. Wanner C, Krane V, Màrz W, et al; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248.
18. Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407.
19. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685-696.
20. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1-e90.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001-1009.
22. Downs JR, Clearfield M, Weis S, et al; AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615-1622.
23. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
24. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383-1389.
25. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Lancet. 2002;360:1623-1630.
26. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
27. GISSI-HF Investigators; Tavozzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial). Lancet. 2008;372:1231-1239.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948-954.
2. US Department of Health and Human Services. Health, United States, 2010. Available at http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed July 22, 2011.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
5. AIM-HIGH Investigators; Boden WE, Probsfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. .N Engl J Med. 2011;365:2255-2267.
6. Niacin causes serious unexpected side-effects, but no worthwhile benefits for patients who are at increased risk of heart attacks and strokes [press release]. HPS2-THRIVE: University of Oxford; March 9, 2013. Available at: www.thrivestudy.org/press_release.htm. Accessed July 3, 2013.
7. Brown BG, Zhao X, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
8. Yokyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients. Lancet. 2007;369:1090-1098.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026.
10. The Endocrinologic and Metabolic Drugs Advisory Committee of the FDA, Center for Drug Evaluation and Research. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM261162.pdf. Published June 24, 2011. Accessed July 3, 2013.
11. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Associaton task force on practice guidelines . Circulation. 2013 Nov 12 [Epub ahead of print.]
12. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305.
13. Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563.
14. Shepherd J, Kastelein JJ, Bittner V, et al; TNT (Treating to New Targets) Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease. J Am Coll Cardiol. 2008;51:1448-1454.
15. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes. Am J Kidney Dis. 2009;54:810-819.
16. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181-2192.
17. Wanner C, Krane V, Màrz W, et al; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248.
18. Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407.
19. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685-696.
20. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1-e90.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001-1009.
22. Downs JR, Clearfield M, Weis S, et al; AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615-1622.
23. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
24. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383-1389.
25. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Lancet. 2002;360:1623-1630.
26. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
27. GISSI-HF Investigators; Tavozzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial). Lancet. 2008;372:1231-1239.