User login
Stroke is the fifth leading cause of death in the United States. Approximately 795,000 strokes occur every year and about 130,000 patients die.1 The impact of stroke-related medical costs and disability is significant, making it a key target for treatment and prevention strategies.
Stroke is defined as an acute loss of neurologic function caused by damaged brain tissue. There are two primary types: ischemic and hemorrhagic. Ischemic strokes are by far the most common, accounting for 87% of all strokes.2 An ischemic stroke is caused by an arterial occlusion that restricts cerebral blood flow; a hemorrhagic stroke is caused by a rupture or leak in the cerebrovasculature. Treatment of an ischemic stroke focuses on thrombolysis and revascularization strategies to restore blood flow, whereas with hemorrhagic stroke, treatment focuses on controlling intracerebral bleeding, elevated intracranial pressure, and secondary brain injury. This article addresses a key factor in improved stroke outcomes—time to treatment—and the impact that a mobile stroke treatment unit (MSTU) can have on this factor.
DECLINING MORBIDITY AND MORTALITY RATES
Although the morbidity and mortality associated with stroke are high, the rates have been declining. From 2001 o 2011, the stroke mortality rate declined by 35%.2 The American Heart Association attributes the reduction to improvements in both prevention and treatment.
A significant portion of the decline has come from population-wide stroke prevention efforts. These include community efforts to control the major cardiovascular risk factors for stroke, including hypertension and hypercholesterolemia. Treating hypertension can reduce the incidence of stroke by up to 40%.3 In addition, community education efforts aimed at improving awareness of stroke symptoms and early detection have contributed to the declining rates, although, by some estimates, only about one-third of the population knows the major signs and symptoms of stroke.
Improved stroke treatments have also contributed to better outcomes, primarily through the more widespread use of thrombolytics. When first approved by the US Food and Drug Administration (FDA), thrombolytics were primarily the purview of cardiologists. However, as outcomes data accumulated, neurologists recognized the utility of thrombolytics in treating ischemic cerebrovascular disease and began investigating their use in clinical trials. Positive outcomes from those trials led to their FDA approval for stroke treatment and universal recognition as the primary therapy for acute stroke. More recent efforts have concentrated on early treatment by bringing the therapy to the patient as opposed to the traditional treatment algorithm of providing care in the emergency department. If therapy is instituted quickly enough, ischemic stroke symptoms can be reversed.
TIME TO TREATMENT
Therapeutic use of tissue plasminogen activators (tPA) has had a major impact on morbidity and mortality in patients with acute ischemic strokes. The efficacy of tPA as thrombolytic therapy in this patient population is well documented.4
Also well documented is the significant impact of time-to-tPA treatment on outcomes. If therapy is started within 3 to 4.5 hours of ischemic stroke onset, patients have improved functional outcomes 3 to 6 months after the incident (Figure 1). Between 31% and 50% of patients treated with tPA within 3 hours experienced improved recovery at 3 months compared with 20% to 38% of patients treated with placebo.5–9 Faster onset to treatment, measured in 15-minute increments, has been shown to significantly reduce in-hospital mortality, reduce intracranial hemorrhage, increase ability to walk at discharge, and increase number discharged to home.6 Even a 1-minute delay in time-to-tPA treatment has a substantial impact on rates of morbidity and mortality (Table 1).10 National and international guidelines recommend starting intravenous tPA within 1 hour of patient arrival in the emergency department and not longer than 4.5 hours since symptom onset, although some evidence indicates a 3-hour window.5,11,12
Although the evidence supports the benefit of rapid therapy for acute ischemic stroke, the national percentage of patients who actually receive tPA within the therapeutic window is small, by some estimates as low as 3% to 5%.13 For optimal stroke care, the rate should be 30% to 50%.
IMPROVING TREATMENT TIMES
Studies have found that the major reason patients do not receive tPA is that they do not reach the hospital quickly enough to be assessed and treated within the treatment window.14,15 In essence, neurologists have the technology to treat most patients, but are waiting for the patients to arrive. Many factors contribute to this delayed arrival time. On the patient level, the primary factors are related to failure to recognize stroke symptoms as well as failure to understand their seriousness.
From the healthcare provider’s perspective, a major barrier to reducing the time-to-treatment window is the need to accurately assess patients with acute ischemic stroke who are eligible for thrombolytic therapy. This is difficult to achieve in clinical practice because it requires neurologic imaging primarily with computed tomography (CT) or magnetic resonance imaging (MRI) and laboratory analyses so that hemorrhagic stroke and other contraindications to thrombolysis can be excluded. Traditionally, this type of analytic equipment had been available only in emergency departments, requiring patients to be brought to those facilities.
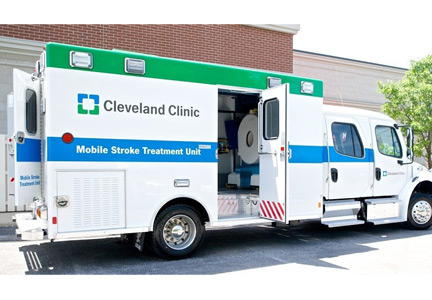
Recent innovation in this area led to the development of specialized ambulances equipped with a CT scanner, point-of-care laboratory equipment, and telemedicine connection along with the appropriate treatment options/medications and trained healthcare personnel to provide prehospital stroke treatment. These specially equipped ambulances are known as MSTUs or stroke emergency mobile (STEMO) units. Their development has dramatically altered the strategy from one of taking the patient to the treatment to taking the treatment to the patient.
MOBILE STROKE TREATMENT UNITS
Two technological innovations have been fundamental to the creation and success of MSTU: portable CT scanners and high-speed wireless data transmission.
CT scanners. A key element was the development of a portable diagnostic-quality head and neck CT scanner that can be fit inside a typical-sized ambulance. This 8-slice CT scanner is capable of creating the same scan types and quality found in radiology department CT scanners, including axial imaging, helical angiography, and perfusion imaging. The resolution and slice thickness (1.25 mm) of the images are of suitable quality to enable neurologists and neuroradiologists to exclude hemorrhage in acute stroke, to assess the degree of brain injury, and to identify the vascular lesion responsible for the ischemic deficit. These technologies also enable diagnostic differentiation between brain tissue that is irreversibly infarcted from that which is potentially salvageable, thereby allowing more accurate patient assessment. The imaging data currently obtainable by CT scanners fitted on ambulances is only likely to improve with future technological advances.
Wireless data transmission. Cellular wireless providers have developed the technology and equipment to provide high-speed wireless broadband capable of transmitting high-quality CT and MRI images. It also enables encrypted feed of video telemedicine, data transmission, and download of patient data. This allows the MSTU to electronically sit inside the firewalls of healthcare facilities, providing access to the patients’ electronic health records and to on-site stroke experts.
The successes have been impressive. Studies have found that the deployment of an MSTU significantly reduces the median time from 9-1-1 alarm to intravenous thrombolysis without increasing adverse events.16–19 These data are primarily from the PHANTOM-S study, a pilot program conducted in Germany.18,19 Results showed a significant reduction in alarm-to-treatment times, from 76 minutes in the hospital control group to 52 minutes in the MSTU group (Table 2).17,19 Further, among patients who suffered an ischemic stroke, the proportion who received tPA within 1 hour of symptom onset was sixfold higher after MSTU deployment (Table 3).18 In a separate European study, prehospital stroke assessment using an MSTU significantly reduced the median time from alarm-to-therapy decision: 35 minutes versus 76 in the hospital group.16
The prehospital cerebrovascular diagnostic workup provided by an MSTU also can improve the emergency management of other stroke types. By providing more diagnostic data and higher quality imaging, the units improve the accuracy of the diagnosis. In turn, this enables emergency personnel to provide accurate therapy and to transfer patients to hospitals with the appropriate level of stroke care, decreasing the need for additional intrahospital transfers.20
Overall, it has been shown that an MSTU equipped with the necessary imaging and laboratory testing equipment can provide appropriate, accurate, and safe ambulance-based prehospital tPA administration, reduce the time to tPA administration, and increase the number of patients who receive tPA administration. All of these factors combine to improve outcomes in patients with acute ischemic stroke.
CLEVELAND CLINIC EXPERIENCE
Cleveland Clinic has a tradition of providing high-quality and innovative stroke care. Recognizing the importance of an appropriately equipped MSTU in reducing the time to stroke treatment, especially tPA administration, Cleveland Clinic instituted a plan to develop an MSTU for the care of patients in the Cleveland area. The development required several planning, funding, and development phases.
Planning. Establishing relations with both city planners and area hospitals was central to planning the MSTU startup. An agreement with the city of Cleveland included creating an emergency medical system (EMS) triage algorithm for the 9-1-1 dispatch center. When a call is received, the dispatcher uses a stroke checklist to perform an initial screening. If a stroke is suspected, the MSTU is dispatched along with a Cleveland EMS or other first-responder unit.
As part of the agreement, Cleveland officials required that the MSTU treat all patients, regardless of their ability to pay. This requirement has been beneficial to the MSTU mission as it allows for treating more patients with tPA as quickly as possible without concern for health insurance, which maximizes the potential for neurologic recovery.
Staffing and procedures. The MSTU staff is composed of a paramedic, a critical care nurse, a CT technologist, and an emergency medicine technician/EMS driver. They perform CT scans and point-of-care laboratory tests on patients who have stroke symptoms. The CT scans and laboratory results are wirelessly transmitted to Cleveland Clinic. A neurologist assesses the data, consults with the MSTU staff on history and neurologic examination, and diagnoses the patient remotely. Patients are then transported to the closest hospital with the resources to meet their clinical needs. If thrombolytic therapy is indicated, intravenous tPA is initiated immediately at the scene. If the patient has sustained a hemorrhagic stroke, reversal of anticoagulation therapy is initiated, if indicated.
Outcomes. The success rates also have been impressive, with dramatic reductions in time to treatment. On average, patients received tPA 40 minutes faster in the MSTU model than in the standard model of ambulance transport and in-hospital evaluation and treatment: 64 minutes versus 104 minutes. Further, more patients in the MSTU group received tPA: 26% versus 14%. Results also showed a 21-minute reduction in time-to-CT completion, an important aspect of providing more timely care.21–23 This CT scanner is also capable of CT angiography. This enables large-vessel occlusion strokes to be identified in the field. When these types of strokes are identified in the field, the patients are transported directly to a stroke center capable of endovascular therapy, even bypassing some primary stroke centers.
Using the MSTU to bring diagnostic and stroke care to the patient has shown that the time between the onset of stroke-like symptoms and the delivery of treatment can be reduced. Thus, an MSTU has the potential to minimize the mortality and long-term morbidity associated with strokes.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke— United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–e322.
- Howard G, Banach M, Cushman M, et al. Is blood pressure control for stroke prevention the correct goal? The lost opportunity of preventing hypertension. Stroke 2015; 46:1595–1600.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333:1581–1587.
- Lees KR, Bluhmki E, von Kummer R, et al; ECASS, ATLANTIS, NINDS, and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375:1695–1703.
- Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379:2364–2372.
- Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015; 313:1451–1462.
- Saver JL. Time is brain—quantified. Stroke 2006; 37:263–266.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317– 1329.
- Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011; 42:1952–1955.
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001; 56:1015–1020.
- Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001– 2004. Stroke 2009; 40:3845–3850.
- Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol 2012; 11:397–404.
- Ebinger M, Winter B, Wendt M, et al; STEMO Consortium. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA 2014; 311:1622–1631.
- Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis: a prehospital acute neurological treatment and optimization of medical care in stroke (PHANTOM-S) substudy. JAMA Neurol 2015; 72:25–30.
- Weber JE, Ebinger M, Rozansk M, et al; STEMO-Consortium. Prehospital thrombolysis in acute stroke: results of the PHANTOM-S pilot study. Neurology 2013; 80:163–168.
- Wendt M, Ebinger M, Kunz A, et al; STEMO Consortium. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the prehospital acute neurological therapy and optimization of medical care in stroke study. Stroke 2015; 46: 740–745.
- Cho S, Cerejo R, Taqui A, et al. Feasibility of telemedicine on a mobile stroke treatment unit. Stroke 2015; 46:ATP200.
- Sheikhi L, Itrat A, Cerejo R, et al. Does portable CT imaging in a mobile stroke treatment unit (MSTU) provide adequate quality for early critical decision making? Stroke 2015; 46:AWP31.
- Taqui A, Cerejo R, Itrat A, et al. Reduction in time to imaging and intravenous thrombolysis by in-field evaluation and treatment in a mobile stroke treatment unit. Stroke 2015; 46:A54.
Stroke is the fifth leading cause of death in the United States. Approximately 795,000 strokes occur every year and about 130,000 patients die.1 The impact of stroke-related medical costs and disability is significant, making it a key target for treatment and prevention strategies.
Stroke is defined as an acute loss of neurologic function caused by damaged brain tissue. There are two primary types: ischemic and hemorrhagic. Ischemic strokes are by far the most common, accounting for 87% of all strokes.2 An ischemic stroke is caused by an arterial occlusion that restricts cerebral blood flow; a hemorrhagic stroke is caused by a rupture or leak in the cerebrovasculature. Treatment of an ischemic stroke focuses on thrombolysis and revascularization strategies to restore blood flow, whereas with hemorrhagic stroke, treatment focuses on controlling intracerebral bleeding, elevated intracranial pressure, and secondary brain injury. This article addresses a key factor in improved stroke outcomes—time to treatment—and the impact that a mobile stroke treatment unit (MSTU) can have on this factor.
DECLINING MORBIDITY AND MORTALITY RATES
Although the morbidity and mortality associated with stroke are high, the rates have been declining. From 2001 o 2011, the stroke mortality rate declined by 35%.2 The American Heart Association attributes the reduction to improvements in both prevention and treatment.
A significant portion of the decline has come from population-wide stroke prevention efforts. These include community efforts to control the major cardiovascular risk factors for stroke, including hypertension and hypercholesterolemia. Treating hypertension can reduce the incidence of stroke by up to 40%.3 In addition, community education efforts aimed at improving awareness of stroke symptoms and early detection have contributed to the declining rates, although, by some estimates, only about one-third of the population knows the major signs and symptoms of stroke.
Improved stroke treatments have also contributed to better outcomes, primarily through the more widespread use of thrombolytics. When first approved by the US Food and Drug Administration (FDA), thrombolytics were primarily the purview of cardiologists. However, as outcomes data accumulated, neurologists recognized the utility of thrombolytics in treating ischemic cerebrovascular disease and began investigating their use in clinical trials. Positive outcomes from those trials led to their FDA approval for stroke treatment and universal recognition as the primary therapy for acute stroke. More recent efforts have concentrated on early treatment by bringing the therapy to the patient as opposed to the traditional treatment algorithm of providing care in the emergency department. If therapy is instituted quickly enough, ischemic stroke symptoms can be reversed.
TIME TO TREATMENT
Therapeutic use of tissue plasminogen activators (tPA) has had a major impact on morbidity and mortality in patients with acute ischemic strokes. The efficacy of tPA as thrombolytic therapy in this patient population is well documented.4
Also well documented is the significant impact of time-to-tPA treatment on outcomes. If therapy is started within 3 to 4.5 hours of ischemic stroke onset, patients have improved functional outcomes 3 to 6 months after the incident (Figure 1). Between 31% and 50% of patients treated with tPA within 3 hours experienced improved recovery at 3 months compared with 20% to 38% of patients treated with placebo.5–9 Faster onset to treatment, measured in 15-minute increments, has been shown to significantly reduce in-hospital mortality, reduce intracranial hemorrhage, increase ability to walk at discharge, and increase number discharged to home.6 Even a 1-minute delay in time-to-tPA treatment has a substantial impact on rates of morbidity and mortality (Table 1).10 National and international guidelines recommend starting intravenous tPA within 1 hour of patient arrival in the emergency department and not longer than 4.5 hours since symptom onset, although some evidence indicates a 3-hour window.5,11,12
Although the evidence supports the benefit of rapid therapy for acute ischemic stroke, the national percentage of patients who actually receive tPA within the therapeutic window is small, by some estimates as low as 3% to 5%.13 For optimal stroke care, the rate should be 30% to 50%.
IMPROVING TREATMENT TIMES
Studies have found that the major reason patients do not receive tPA is that they do not reach the hospital quickly enough to be assessed and treated within the treatment window.14,15 In essence, neurologists have the technology to treat most patients, but are waiting for the patients to arrive. Many factors contribute to this delayed arrival time. On the patient level, the primary factors are related to failure to recognize stroke symptoms as well as failure to understand their seriousness.
From the healthcare provider’s perspective, a major barrier to reducing the time-to-treatment window is the need to accurately assess patients with acute ischemic stroke who are eligible for thrombolytic therapy. This is difficult to achieve in clinical practice because it requires neurologic imaging primarily with computed tomography (CT) or magnetic resonance imaging (MRI) and laboratory analyses so that hemorrhagic stroke and other contraindications to thrombolysis can be excluded. Traditionally, this type of analytic equipment had been available only in emergency departments, requiring patients to be brought to those facilities.
Recent innovation in this area led to the development of specialized ambulances equipped with a CT scanner, point-of-care laboratory equipment, and telemedicine connection along with the appropriate treatment options/medications and trained healthcare personnel to provide prehospital stroke treatment. These specially equipped ambulances are known as MSTUs or stroke emergency mobile (STEMO) units. Their development has dramatically altered the strategy from one of taking the patient to the treatment to taking the treatment to the patient.
MOBILE STROKE TREATMENT UNITS
Two technological innovations have been fundamental to the creation and success of MSTU: portable CT scanners and high-speed wireless data transmission.
CT scanners. A key element was the development of a portable diagnostic-quality head and neck CT scanner that can be fit inside a typical-sized ambulance. This 8-slice CT scanner is capable of creating the same scan types and quality found in radiology department CT scanners, including axial imaging, helical angiography, and perfusion imaging. The resolution and slice thickness (1.25 mm) of the images are of suitable quality to enable neurologists and neuroradiologists to exclude hemorrhage in acute stroke, to assess the degree of brain injury, and to identify the vascular lesion responsible for the ischemic deficit. These technologies also enable diagnostic differentiation between brain tissue that is irreversibly infarcted from that which is potentially salvageable, thereby allowing more accurate patient assessment. The imaging data currently obtainable by CT scanners fitted on ambulances is only likely to improve with future technological advances.
Wireless data transmission. Cellular wireless providers have developed the technology and equipment to provide high-speed wireless broadband capable of transmitting high-quality CT and MRI images. It also enables encrypted feed of video telemedicine, data transmission, and download of patient data. This allows the MSTU to electronically sit inside the firewalls of healthcare facilities, providing access to the patients’ electronic health records and to on-site stroke experts.
The successes have been impressive. Studies have found that the deployment of an MSTU significantly reduces the median time from 9-1-1 alarm to intravenous thrombolysis without increasing adverse events.16–19 These data are primarily from the PHANTOM-S study, a pilot program conducted in Germany.18,19 Results showed a significant reduction in alarm-to-treatment times, from 76 minutes in the hospital control group to 52 minutes in the MSTU group (Table 2).17,19 Further, among patients who suffered an ischemic stroke, the proportion who received tPA within 1 hour of symptom onset was sixfold higher after MSTU deployment (Table 3).18 In a separate European study, prehospital stroke assessment using an MSTU significantly reduced the median time from alarm-to-therapy decision: 35 minutes versus 76 in the hospital group.16
The prehospital cerebrovascular diagnostic workup provided by an MSTU also can improve the emergency management of other stroke types. By providing more diagnostic data and higher quality imaging, the units improve the accuracy of the diagnosis. In turn, this enables emergency personnel to provide accurate therapy and to transfer patients to hospitals with the appropriate level of stroke care, decreasing the need for additional intrahospital transfers.20
Overall, it has been shown that an MSTU equipped with the necessary imaging and laboratory testing equipment can provide appropriate, accurate, and safe ambulance-based prehospital tPA administration, reduce the time to tPA administration, and increase the number of patients who receive tPA administration. All of these factors combine to improve outcomes in patients with acute ischemic stroke.
CLEVELAND CLINIC EXPERIENCE
Cleveland Clinic has a tradition of providing high-quality and innovative stroke care. Recognizing the importance of an appropriately equipped MSTU in reducing the time to stroke treatment, especially tPA administration, Cleveland Clinic instituted a plan to develop an MSTU for the care of patients in the Cleveland area. The development required several planning, funding, and development phases.
Planning. Establishing relations with both city planners and area hospitals was central to planning the MSTU startup. An agreement with the city of Cleveland included creating an emergency medical system (EMS) triage algorithm for the 9-1-1 dispatch center. When a call is received, the dispatcher uses a stroke checklist to perform an initial screening. If a stroke is suspected, the MSTU is dispatched along with a Cleveland EMS or other first-responder unit.
As part of the agreement, Cleveland officials required that the MSTU treat all patients, regardless of their ability to pay. This requirement has been beneficial to the MSTU mission as it allows for treating more patients with tPA as quickly as possible without concern for health insurance, which maximizes the potential for neurologic recovery.
Staffing and procedures. The MSTU staff is composed of a paramedic, a critical care nurse, a CT technologist, and an emergency medicine technician/EMS driver. They perform CT scans and point-of-care laboratory tests on patients who have stroke symptoms. The CT scans and laboratory results are wirelessly transmitted to Cleveland Clinic. A neurologist assesses the data, consults with the MSTU staff on history and neurologic examination, and diagnoses the patient remotely. Patients are then transported to the closest hospital with the resources to meet their clinical needs. If thrombolytic therapy is indicated, intravenous tPA is initiated immediately at the scene. If the patient has sustained a hemorrhagic stroke, reversal of anticoagulation therapy is initiated, if indicated.
Outcomes. The success rates also have been impressive, with dramatic reductions in time to treatment. On average, patients received tPA 40 minutes faster in the MSTU model than in the standard model of ambulance transport and in-hospital evaluation and treatment: 64 minutes versus 104 minutes. Further, more patients in the MSTU group received tPA: 26% versus 14%. Results also showed a 21-minute reduction in time-to-CT completion, an important aspect of providing more timely care.21–23 This CT scanner is also capable of CT angiography. This enables large-vessel occlusion strokes to be identified in the field. When these types of strokes are identified in the field, the patients are transported directly to a stroke center capable of endovascular therapy, even bypassing some primary stroke centers.
Using the MSTU to bring diagnostic and stroke care to the patient has shown that the time between the onset of stroke-like symptoms and the delivery of treatment can be reduced. Thus, an MSTU has the potential to minimize the mortality and long-term morbidity associated with strokes.
Stroke is the fifth leading cause of death in the United States. Approximately 795,000 strokes occur every year and about 130,000 patients die.1 The impact of stroke-related medical costs and disability is significant, making it a key target for treatment and prevention strategies.
Stroke is defined as an acute loss of neurologic function caused by damaged brain tissue. There are two primary types: ischemic and hemorrhagic. Ischemic strokes are by far the most common, accounting for 87% of all strokes.2 An ischemic stroke is caused by an arterial occlusion that restricts cerebral blood flow; a hemorrhagic stroke is caused by a rupture or leak in the cerebrovasculature. Treatment of an ischemic stroke focuses on thrombolysis and revascularization strategies to restore blood flow, whereas with hemorrhagic stroke, treatment focuses on controlling intracerebral bleeding, elevated intracranial pressure, and secondary brain injury. This article addresses a key factor in improved stroke outcomes—time to treatment—and the impact that a mobile stroke treatment unit (MSTU) can have on this factor.
DECLINING MORBIDITY AND MORTALITY RATES
Although the morbidity and mortality associated with stroke are high, the rates have been declining. From 2001 o 2011, the stroke mortality rate declined by 35%.2 The American Heart Association attributes the reduction to improvements in both prevention and treatment.
A significant portion of the decline has come from population-wide stroke prevention efforts. These include community efforts to control the major cardiovascular risk factors for stroke, including hypertension and hypercholesterolemia. Treating hypertension can reduce the incidence of stroke by up to 40%.3 In addition, community education efforts aimed at improving awareness of stroke symptoms and early detection have contributed to the declining rates, although, by some estimates, only about one-third of the population knows the major signs and symptoms of stroke.
Improved stroke treatments have also contributed to better outcomes, primarily through the more widespread use of thrombolytics. When first approved by the US Food and Drug Administration (FDA), thrombolytics were primarily the purview of cardiologists. However, as outcomes data accumulated, neurologists recognized the utility of thrombolytics in treating ischemic cerebrovascular disease and began investigating their use in clinical trials. Positive outcomes from those trials led to their FDA approval for stroke treatment and universal recognition as the primary therapy for acute stroke. More recent efforts have concentrated on early treatment by bringing the therapy to the patient as opposed to the traditional treatment algorithm of providing care in the emergency department. If therapy is instituted quickly enough, ischemic stroke symptoms can be reversed.
TIME TO TREATMENT
Therapeutic use of tissue plasminogen activators (tPA) has had a major impact on morbidity and mortality in patients with acute ischemic strokes. The efficacy of tPA as thrombolytic therapy in this patient population is well documented.4
Also well documented is the significant impact of time-to-tPA treatment on outcomes. If therapy is started within 3 to 4.5 hours of ischemic stroke onset, patients have improved functional outcomes 3 to 6 months after the incident (Figure 1). Between 31% and 50% of patients treated with tPA within 3 hours experienced improved recovery at 3 months compared with 20% to 38% of patients treated with placebo.5–9 Faster onset to treatment, measured in 15-minute increments, has been shown to significantly reduce in-hospital mortality, reduce intracranial hemorrhage, increase ability to walk at discharge, and increase number discharged to home.6 Even a 1-minute delay in time-to-tPA treatment has a substantial impact on rates of morbidity and mortality (Table 1).10 National and international guidelines recommend starting intravenous tPA within 1 hour of patient arrival in the emergency department and not longer than 4.5 hours since symptom onset, although some evidence indicates a 3-hour window.5,11,12
Although the evidence supports the benefit of rapid therapy for acute ischemic stroke, the national percentage of patients who actually receive tPA within the therapeutic window is small, by some estimates as low as 3% to 5%.13 For optimal stroke care, the rate should be 30% to 50%.
IMPROVING TREATMENT TIMES
Studies have found that the major reason patients do not receive tPA is that they do not reach the hospital quickly enough to be assessed and treated within the treatment window.14,15 In essence, neurologists have the technology to treat most patients, but are waiting for the patients to arrive. Many factors contribute to this delayed arrival time. On the patient level, the primary factors are related to failure to recognize stroke symptoms as well as failure to understand their seriousness.
From the healthcare provider’s perspective, a major barrier to reducing the time-to-treatment window is the need to accurately assess patients with acute ischemic stroke who are eligible for thrombolytic therapy. This is difficult to achieve in clinical practice because it requires neurologic imaging primarily with computed tomography (CT) or magnetic resonance imaging (MRI) and laboratory analyses so that hemorrhagic stroke and other contraindications to thrombolysis can be excluded. Traditionally, this type of analytic equipment had been available only in emergency departments, requiring patients to be brought to those facilities.
Recent innovation in this area led to the development of specialized ambulances equipped with a CT scanner, point-of-care laboratory equipment, and telemedicine connection along with the appropriate treatment options/medications and trained healthcare personnel to provide prehospital stroke treatment. These specially equipped ambulances are known as MSTUs or stroke emergency mobile (STEMO) units. Their development has dramatically altered the strategy from one of taking the patient to the treatment to taking the treatment to the patient.
MOBILE STROKE TREATMENT UNITS
Two technological innovations have been fundamental to the creation and success of MSTU: portable CT scanners and high-speed wireless data transmission.
CT scanners. A key element was the development of a portable diagnostic-quality head and neck CT scanner that can be fit inside a typical-sized ambulance. This 8-slice CT scanner is capable of creating the same scan types and quality found in radiology department CT scanners, including axial imaging, helical angiography, and perfusion imaging. The resolution and slice thickness (1.25 mm) of the images are of suitable quality to enable neurologists and neuroradiologists to exclude hemorrhage in acute stroke, to assess the degree of brain injury, and to identify the vascular lesion responsible for the ischemic deficit. These technologies also enable diagnostic differentiation between brain tissue that is irreversibly infarcted from that which is potentially salvageable, thereby allowing more accurate patient assessment. The imaging data currently obtainable by CT scanners fitted on ambulances is only likely to improve with future technological advances.
Wireless data transmission. Cellular wireless providers have developed the technology and equipment to provide high-speed wireless broadband capable of transmitting high-quality CT and MRI images. It also enables encrypted feed of video telemedicine, data transmission, and download of patient data. This allows the MSTU to electronically sit inside the firewalls of healthcare facilities, providing access to the patients’ electronic health records and to on-site stroke experts.
The successes have been impressive. Studies have found that the deployment of an MSTU significantly reduces the median time from 9-1-1 alarm to intravenous thrombolysis without increasing adverse events.16–19 These data are primarily from the PHANTOM-S study, a pilot program conducted in Germany.18,19 Results showed a significant reduction in alarm-to-treatment times, from 76 minutes in the hospital control group to 52 minutes in the MSTU group (Table 2).17,19 Further, among patients who suffered an ischemic stroke, the proportion who received tPA within 1 hour of symptom onset was sixfold higher after MSTU deployment (Table 3).18 In a separate European study, prehospital stroke assessment using an MSTU significantly reduced the median time from alarm-to-therapy decision: 35 minutes versus 76 in the hospital group.16
The prehospital cerebrovascular diagnostic workup provided by an MSTU also can improve the emergency management of other stroke types. By providing more diagnostic data and higher quality imaging, the units improve the accuracy of the diagnosis. In turn, this enables emergency personnel to provide accurate therapy and to transfer patients to hospitals with the appropriate level of stroke care, decreasing the need for additional intrahospital transfers.20
Overall, it has been shown that an MSTU equipped with the necessary imaging and laboratory testing equipment can provide appropriate, accurate, and safe ambulance-based prehospital tPA administration, reduce the time to tPA administration, and increase the number of patients who receive tPA administration. All of these factors combine to improve outcomes in patients with acute ischemic stroke.
CLEVELAND CLINIC EXPERIENCE
Cleveland Clinic has a tradition of providing high-quality and innovative stroke care. Recognizing the importance of an appropriately equipped MSTU in reducing the time to stroke treatment, especially tPA administration, Cleveland Clinic instituted a plan to develop an MSTU for the care of patients in the Cleveland area. The development required several planning, funding, and development phases.
Planning. Establishing relations with both city planners and area hospitals was central to planning the MSTU startup. An agreement with the city of Cleveland included creating an emergency medical system (EMS) triage algorithm for the 9-1-1 dispatch center. When a call is received, the dispatcher uses a stroke checklist to perform an initial screening. If a stroke is suspected, the MSTU is dispatched along with a Cleveland EMS or other first-responder unit.
As part of the agreement, Cleveland officials required that the MSTU treat all patients, regardless of their ability to pay. This requirement has been beneficial to the MSTU mission as it allows for treating more patients with tPA as quickly as possible without concern for health insurance, which maximizes the potential for neurologic recovery.
Staffing and procedures. The MSTU staff is composed of a paramedic, a critical care nurse, a CT technologist, and an emergency medicine technician/EMS driver. They perform CT scans and point-of-care laboratory tests on patients who have stroke symptoms. The CT scans and laboratory results are wirelessly transmitted to Cleveland Clinic. A neurologist assesses the data, consults with the MSTU staff on history and neurologic examination, and diagnoses the patient remotely. Patients are then transported to the closest hospital with the resources to meet their clinical needs. If thrombolytic therapy is indicated, intravenous tPA is initiated immediately at the scene. If the patient has sustained a hemorrhagic stroke, reversal of anticoagulation therapy is initiated, if indicated.
Outcomes. The success rates also have been impressive, with dramatic reductions in time to treatment. On average, patients received tPA 40 minutes faster in the MSTU model than in the standard model of ambulance transport and in-hospital evaluation and treatment: 64 minutes versus 104 minutes. Further, more patients in the MSTU group received tPA: 26% versus 14%. Results also showed a 21-minute reduction in time-to-CT completion, an important aspect of providing more timely care.21–23 This CT scanner is also capable of CT angiography. This enables large-vessel occlusion strokes to be identified in the field. When these types of strokes are identified in the field, the patients are transported directly to a stroke center capable of endovascular therapy, even bypassing some primary stroke centers.
Using the MSTU to bring diagnostic and stroke care to the patient has shown that the time between the onset of stroke-like symptoms and the delivery of treatment can be reduced. Thus, an MSTU has the potential to minimize the mortality and long-term morbidity associated with strokes.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke— United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–e322.
- Howard G, Banach M, Cushman M, et al. Is blood pressure control for stroke prevention the correct goal? The lost opportunity of preventing hypertension. Stroke 2015; 46:1595–1600.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333:1581–1587.
- Lees KR, Bluhmki E, von Kummer R, et al; ECASS, ATLANTIS, NINDS, and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375:1695–1703.
- Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379:2364–2372.
- Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015; 313:1451–1462.
- Saver JL. Time is brain—quantified. Stroke 2006; 37:263–266.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317– 1329.
- Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011; 42:1952–1955.
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001; 56:1015–1020.
- Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001– 2004. Stroke 2009; 40:3845–3850.
- Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol 2012; 11:397–404.
- Ebinger M, Winter B, Wendt M, et al; STEMO Consortium. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA 2014; 311:1622–1631.
- Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis: a prehospital acute neurological treatment and optimization of medical care in stroke (PHANTOM-S) substudy. JAMA Neurol 2015; 72:25–30.
- Weber JE, Ebinger M, Rozansk M, et al; STEMO-Consortium. Prehospital thrombolysis in acute stroke: results of the PHANTOM-S pilot study. Neurology 2013; 80:163–168.
- Wendt M, Ebinger M, Kunz A, et al; STEMO Consortium. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the prehospital acute neurological therapy and optimization of medical care in stroke study. Stroke 2015; 46: 740–745.
- Cho S, Cerejo R, Taqui A, et al. Feasibility of telemedicine on a mobile stroke treatment unit. Stroke 2015; 46:ATP200.
- Sheikhi L, Itrat A, Cerejo R, et al. Does portable CT imaging in a mobile stroke treatment unit (MSTU) provide adequate quality for early critical decision making? Stroke 2015; 46:AWP31.
- Taqui A, Cerejo R, Itrat A, et al. Reduction in time to imaging and intravenous thrombolysis by in-field evaluation and treatment in a mobile stroke treatment unit. Stroke 2015; 46:A54.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke— United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–e322.
- Howard G, Banach M, Cushman M, et al. Is blood pressure control for stroke prevention the correct goal? The lost opportunity of preventing hypertension. Stroke 2015; 46:1595–1600.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333:1581–1587.
- Lees KR, Bluhmki E, von Kummer R, et al; ECASS, ATLANTIS, NINDS, and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375:1695–1703.
- Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379:2364–2372.
- Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015; 313:1451–1462.
- Saver JL. Time is brain—quantified. Stroke 2006; 37:263–266.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317– 1329.
- Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011; 42:1952–1955.
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001; 56:1015–1020.
- Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001– 2004. Stroke 2009; 40:3845–3850.
- Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol 2012; 11:397–404.
- Ebinger M, Winter B, Wendt M, et al; STEMO Consortium. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA 2014; 311:1622–1631.
- Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis: a prehospital acute neurological treatment and optimization of medical care in stroke (PHANTOM-S) substudy. JAMA Neurol 2015; 72:25–30.
- Weber JE, Ebinger M, Rozansk M, et al; STEMO-Consortium. Prehospital thrombolysis in acute stroke: results of the PHANTOM-S pilot study. Neurology 2013; 80:163–168.
- Wendt M, Ebinger M, Kunz A, et al; STEMO Consortium. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the prehospital acute neurological therapy and optimization of medical care in stroke study. Stroke 2015; 46: 740–745.
- Cho S, Cerejo R, Taqui A, et al. Feasibility of telemedicine on a mobile stroke treatment unit. Stroke 2015; 46:ATP200.
- Sheikhi L, Itrat A, Cerejo R, et al. Does portable CT imaging in a mobile stroke treatment unit (MSTU) provide adequate quality for early critical decision making? Stroke 2015; 46:AWP31.
- Taqui A, Cerejo R, Itrat A, et al. Reduction in time to imaging and intravenous thrombolysis by in-field evaluation and treatment in a mobile stroke treatment unit. Stroke 2015; 46:A54.
KEY POINTS
- Therapeutic use of tissue plasminogen activators (tPA) has had a major impact on morbidity and mortality rates in patients with acute ischemic strokes.
- Even a 1-minute delay in time-to-tPA treatment affects morbidity and mortality rates.
- The major reason patients do not receive tPA is that they do not reach the hospital quickly enough to be assessed and treated within the treatment window.
- Portable computed tomography and high-speed wireless data transmission are fundamental to the success of mobile stroke treatment units.