User login
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
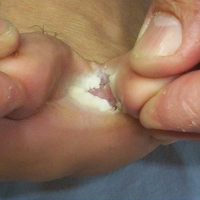
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
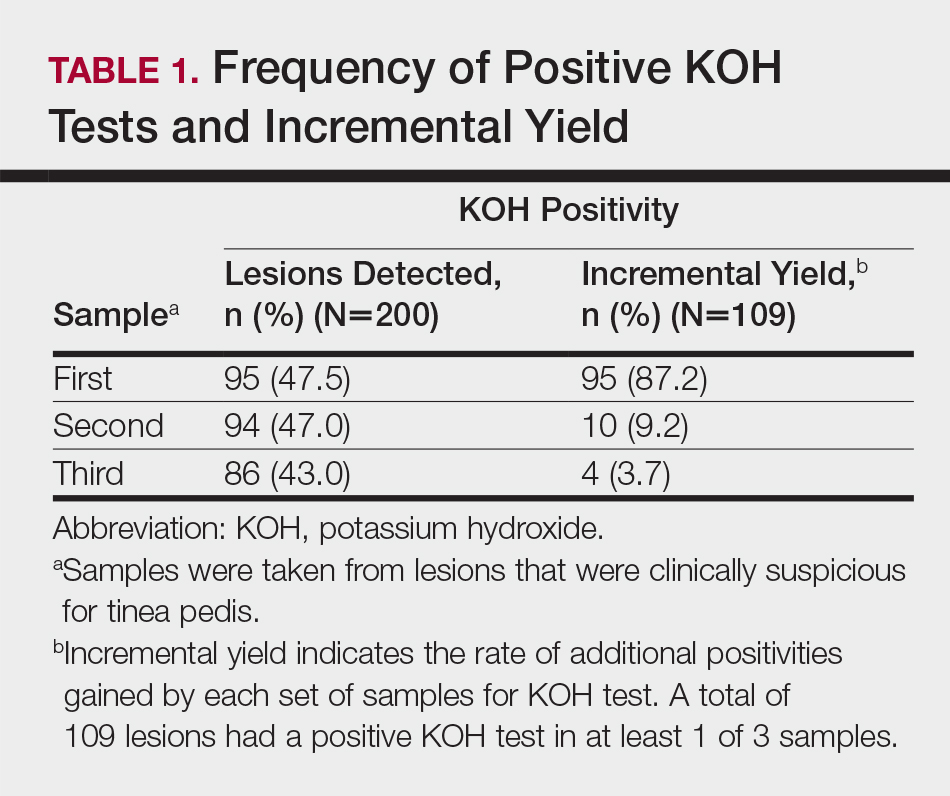
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).
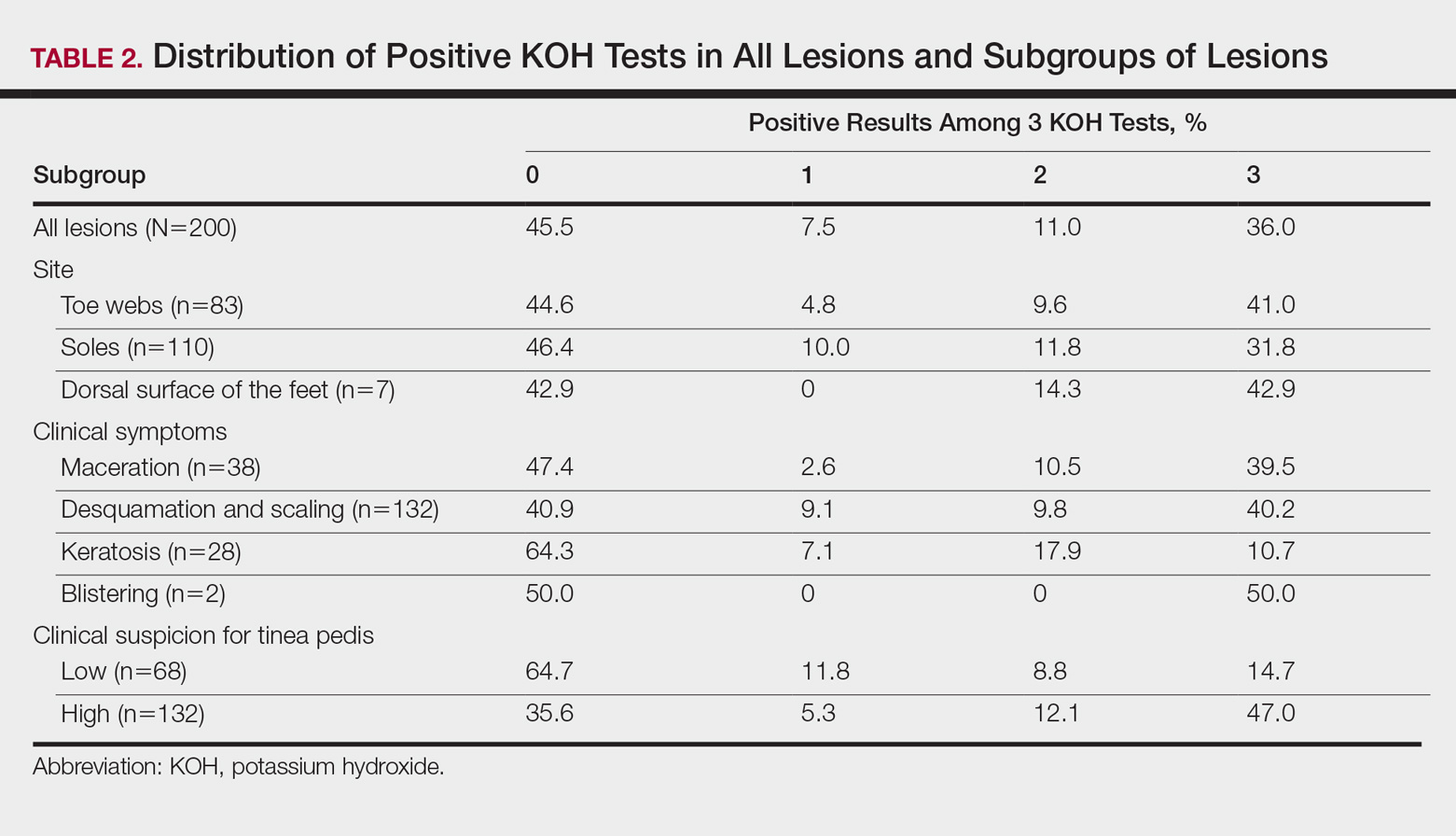
According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).
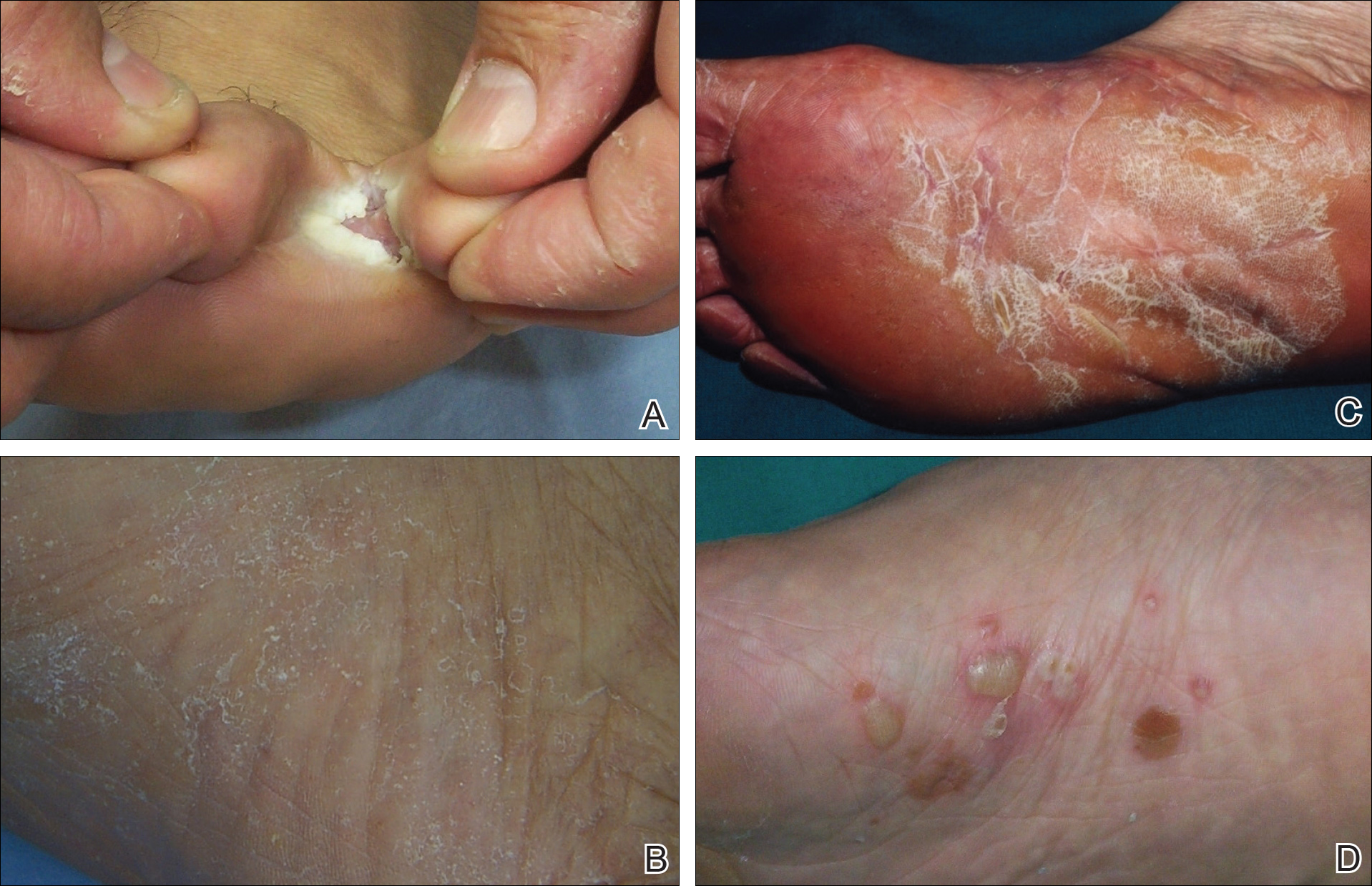
For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.
If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).

According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).

For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).

According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).

For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
Practice Points
- At least 2 samples should be taken for potassium hydroxide examination when tinea pedis is sus-pected clinically.
- The number of samples should be at least 3 if keratotic lesions are present.