User login
The Diagnosis: Prostate Cancer Metastases
Cutaneous involvement of visceral tumors can occur indirectly, with the tumor causing changes in the skin without the presence of tumor cells (ie, paraneoplastic processes), or directly, with the presence of tumor cells in the skin (ie, metastasis).1 Cutaneous metastases from solid primary tumors are uncommon. The incidence of metastasis to the skin from visceral malignancies ranges from 0.3% to 9.0%.2 The most common primary internal tumors to metastasize to the skin are those arising from the breasts, lungs, colon, ovaries, and head and neck.3 Although prostate cancer is the most common cancer in males (making up 28% of new cancer diagnoses), excluding basal and squamous cell skin cancers, it rarely metastasizes to the skin, representing less than 1% of all cutaneous metastasis.4,5
There are 4 proposed mechanisms for metastatic dissemination to the skin: (1) direct invasion from underlying neoplasm; (2) implantation from a surgical scar; (3) spread through the lymphatics; and/or (4) hematogenous spread. The most common sites of prostate cancer cutaneous metastasis are the inguinal area, penis, and lower abdomen,2 which is likely due to spread via the lymphatic drainage. Cutaneous metastasis to distant sites such as the scalp or chest, as in our patient, may be secondary to hematogenous spread.
Generally, cutaneous metastases from visceral malignancies manifest as urticaria or a nonspecific macular rash.2 However, prostate cancer cutaneous metastases commonly present as papules or subcutaneous nodules but also can have inflammatory, cicatricial, sclerodermoid, telangiectatic, or zosteriform morphologies.2,5,6 The lesions of prostate cancer cutaneous metastases are typically well-circumscribed, flesh-colored or pink to red, oval plaques or nodules ranging in size from several millimeters to a few centimeters.2 The cutaneous lesions generally are multiple and can be localized or diffuse in distribution. The clinical differential diagnosis is broad in patients undergoing systemic treatment of prostate cancer and often includes drug reaction, cutaneous lymphoma, atypical mycobacterial or deep fungal infection, or paraneoplastic dermatosis.
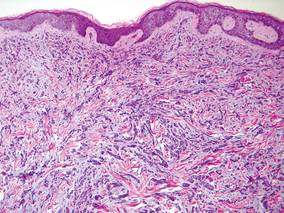
Laboratory studies often reveal an elevated serum prostate-specific antigen (PSA) level, a glycoprotein that functions in the liquefaction of seminal fluid made primarily by the prostate, often in benign hypertrophic and malignant prostatic processes.7 Definitive diagnosis of prostate cancer cutaneous metastasis can be established by histologic examination. Excisional or punch biopsy is preferred over superficial shave biopsy. Metastasis from prostate adenocarcinoma often reveals infiltrative growth of disorganized atypical epithelial cells along collagen bundles in the dermis and subcutis (Figure 1).2,8 The presence of lymphovascular invasion further increases the suspicion of metastasis. Metastatic lesions may resemble the primary lesion; however, they are often poorly differentiated, making histologic comparison difficult.
|
| Figure 1. Biopsy revealed cords of atypical epithelioid cells dissecting through dermal collagen bundles, accompanied by abundant mucin. Atypical cells displayed nuclear pleomorphism, and multiple mitotic figures were present (H&E, original magnification ×100). |
|
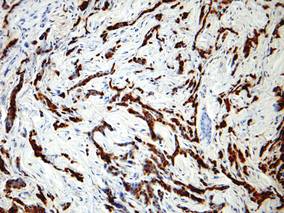
| Figure 2. Immunohistochemistry was strongly positive for prostate-specific antigen and focally positive for prostate-specific acid phosphatase (original magnification ×100). |
The diagnosis is confirmed immunohistochemically with PSA and/or prostate-specific acid phosphatase immunostaining (Figure 2), which together have nearly 100% specificity for prostate adenocarcinomas.9 Although rare, PSA and or prostate-specific acid phosphatase may be weakly positive in nephrogenic adenoma.10 Another biomarker, urinary prostate cancer antigen 3, is being evaluated to identify men with indolent prostate cancer.11 It is unknown how useful this marker will be in the immunohistochemical identification of prostate cancer cutaneous metastases.
A large review of reported cases of cutaneous metastases from internal malignancies revealed that the majority of patients had known systemic disease but enjoyed a good performance status at the time of diagnosis.2 As such, skin metastases may serve as the initial indicator of visceral recurrence. Prostate cancer is second only to lung cancer as the deadliest cancer in males,4 and cutaneous metastasis garners a particularly poor prognosis. The mean survival time after diagnosis of cutaneous metastasis is 7 months.8 Therefore, treatment of cutaneous metastases is largely palliative, including local excision and intralesional chemotherapy.
1. Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009;59:73-98.
2. Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
3. Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
4. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
5. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-182.
6. Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600.
7. Tosoian J, Loeb S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. ScientificWorldJournal. 2010;10:1919-1931.
8. Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684.
9. Nadji M, Tabei SZ, Castro A, et al. Prostate-specific antigen: an immunohistochemical marker for prostatic neoplasms. Cancer. 1981;48:1229-1232.
10. Paner GP, Luthringer DJ, Amin MB. Best practice in diagnostic immunohistochemistry: prostate carcinoma and its mimics in needle core biopsies. Arch Pathol Lab Med. 2008;132:1388-1396.
11. Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669-3676.
The Diagnosis: Prostate Cancer Metastases
Cutaneous involvement of visceral tumors can occur indirectly, with the tumor causing changes in the skin without the presence of tumor cells (ie, paraneoplastic processes), or directly, with the presence of tumor cells in the skin (ie, metastasis).1 Cutaneous metastases from solid primary tumors are uncommon. The incidence of metastasis to the skin from visceral malignancies ranges from 0.3% to 9.0%.2 The most common primary internal tumors to metastasize to the skin are those arising from the breasts, lungs, colon, ovaries, and head and neck.3 Although prostate cancer is the most common cancer in males (making up 28% of new cancer diagnoses), excluding basal and squamous cell skin cancers, it rarely metastasizes to the skin, representing less than 1% of all cutaneous metastasis.4,5
There are 4 proposed mechanisms for metastatic dissemination to the skin: (1) direct invasion from underlying neoplasm; (2) implantation from a surgical scar; (3) spread through the lymphatics; and/or (4) hematogenous spread. The most common sites of prostate cancer cutaneous metastasis are the inguinal area, penis, and lower abdomen,2 which is likely due to spread via the lymphatic drainage. Cutaneous metastasis to distant sites such as the scalp or chest, as in our patient, may be secondary to hematogenous spread.
Generally, cutaneous metastases from visceral malignancies manifest as urticaria or a nonspecific macular rash.2 However, prostate cancer cutaneous metastases commonly present as papules or subcutaneous nodules but also can have inflammatory, cicatricial, sclerodermoid, telangiectatic, or zosteriform morphologies.2,5,6 The lesions of prostate cancer cutaneous metastases are typically well-circumscribed, flesh-colored or pink to red, oval plaques or nodules ranging in size from several millimeters to a few centimeters.2 The cutaneous lesions generally are multiple and can be localized or diffuse in distribution. The clinical differential diagnosis is broad in patients undergoing systemic treatment of prostate cancer and often includes drug reaction, cutaneous lymphoma, atypical mycobacterial or deep fungal infection, or paraneoplastic dermatosis.
Laboratory studies often reveal an elevated serum prostate-specific antigen (PSA) level, a glycoprotein that functions in the liquefaction of seminal fluid made primarily by the prostate, often in benign hypertrophic and malignant prostatic processes.7 Definitive diagnosis of prostate cancer cutaneous metastasis can be established by histologic examination. Excisional or punch biopsy is preferred over superficial shave biopsy. Metastasis from prostate adenocarcinoma often reveals infiltrative growth of disorganized atypical epithelial cells along collagen bundles in the dermis and subcutis (Figure 1).2,8 The presence of lymphovascular invasion further increases the suspicion of metastasis. Metastatic lesions may resemble the primary lesion; however, they are often poorly differentiated, making histologic comparison difficult.

|
| Figure 1. Biopsy revealed cords of atypical epithelioid cells dissecting through dermal collagen bundles, accompanied by abundant mucin. Atypical cells displayed nuclear pleomorphism, and multiple mitotic figures were present (H&E, original magnification ×100). |

|
| Figure 2. Immunohistochemistry was strongly positive for prostate-specific antigen and focally positive for prostate-specific acid phosphatase (original magnification ×100). |
The diagnosis is confirmed immunohistochemically with PSA and/or prostate-specific acid phosphatase immunostaining (Figure 2), which together have nearly 100% specificity for prostate adenocarcinomas.9 Although rare, PSA and or prostate-specific acid phosphatase may be weakly positive in nephrogenic adenoma.10 Another biomarker, urinary prostate cancer antigen 3, is being evaluated to identify men with indolent prostate cancer.11 It is unknown how useful this marker will be in the immunohistochemical identification of prostate cancer cutaneous metastases.
A large review of reported cases of cutaneous metastases from internal malignancies revealed that the majority of patients had known systemic disease but enjoyed a good performance status at the time of diagnosis.2 As such, skin metastases may serve as the initial indicator of visceral recurrence. Prostate cancer is second only to lung cancer as the deadliest cancer in males,4 and cutaneous metastasis garners a particularly poor prognosis. The mean survival time after diagnosis of cutaneous metastasis is 7 months.8 Therefore, treatment of cutaneous metastases is largely palliative, including local excision and intralesional chemotherapy.
The Diagnosis: Prostate Cancer Metastases
Cutaneous involvement of visceral tumors can occur indirectly, with the tumor causing changes in the skin without the presence of tumor cells (ie, paraneoplastic processes), or directly, with the presence of tumor cells in the skin (ie, metastasis).1 Cutaneous metastases from solid primary tumors are uncommon. The incidence of metastasis to the skin from visceral malignancies ranges from 0.3% to 9.0%.2 The most common primary internal tumors to metastasize to the skin are those arising from the breasts, lungs, colon, ovaries, and head and neck.3 Although prostate cancer is the most common cancer in males (making up 28% of new cancer diagnoses), excluding basal and squamous cell skin cancers, it rarely metastasizes to the skin, representing less than 1% of all cutaneous metastasis.4,5
There are 4 proposed mechanisms for metastatic dissemination to the skin: (1) direct invasion from underlying neoplasm; (2) implantation from a surgical scar; (3) spread through the lymphatics; and/or (4) hematogenous spread. The most common sites of prostate cancer cutaneous metastasis are the inguinal area, penis, and lower abdomen,2 which is likely due to spread via the lymphatic drainage. Cutaneous metastasis to distant sites such as the scalp or chest, as in our patient, may be secondary to hematogenous spread.
Generally, cutaneous metastases from visceral malignancies manifest as urticaria or a nonspecific macular rash.2 However, prostate cancer cutaneous metastases commonly present as papules or subcutaneous nodules but also can have inflammatory, cicatricial, sclerodermoid, telangiectatic, or zosteriform morphologies.2,5,6 The lesions of prostate cancer cutaneous metastases are typically well-circumscribed, flesh-colored or pink to red, oval plaques or nodules ranging in size from several millimeters to a few centimeters.2 The cutaneous lesions generally are multiple and can be localized or diffuse in distribution. The clinical differential diagnosis is broad in patients undergoing systemic treatment of prostate cancer and often includes drug reaction, cutaneous lymphoma, atypical mycobacterial or deep fungal infection, or paraneoplastic dermatosis.
Laboratory studies often reveal an elevated serum prostate-specific antigen (PSA) level, a glycoprotein that functions in the liquefaction of seminal fluid made primarily by the prostate, often in benign hypertrophic and malignant prostatic processes.7 Definitive diagnosis of prostate cancer cutaneous metastasis can be established by histologic examination. Excisional or punch biopsy is preferred over superficial shave biopsy. Metastasis from prostate adenocarcinoma often reveals infiltrative growth of disorganized atypical epithelial cells along collagen bundles in the dermis and subcutis (Figure 1).2,8 The presence of lymphovascular invasion further increases the suspicion of metastasis. Metastatic lesions may resemble the primary lesion; however, they are often poorly differentiated, making histologic comparison difficult.

|
| Figure 1. Biopsy revealed cords of atypical epithelioid cells dissecting through dermal collagen bundles, accompanied by abundant mucin. Atypical cells displayed nuclear pleomorphism, and multiple mitotic figures were present (H&E, original magnification ×100). |

|
| Figure 2. Immunohistochemistry was strongly positive for prostate-specific antigen and focally positive for prostate-specific acid phosphatase (original magnification ×100). |
The diagnosis is confirmed immunohistochemically with PSA and/or prostate-specific acid phosphatase immunostaining (Figure 2), which together have nearly 100% specificity for prostate adenocarcinomas.9 Although rare, PSA and or prostate-specific acid phosphatase may be weakly positive in nephrogenic adenoma.10 Another biomarker, urinary prostate cancer antigen 3, is being evaluated to identify men with indolent prostate cancer.11 It is unknown how useful this marker will be in the immunohistochemical identification of prostate cancer cutaneous metastases.
A large review of reported cases of cutaneous metastases from internal malignancies revealed that the majority of patients had known systemic disease but enjoyed a good performance status at the time of diagnosis.2 As such, skin metastases may serve as the initial indicator of visceral recurrence. Prostate cancer is second only to lung cancer as the deadliest cancer in males,4 and cutaneous metastasis garners a particularly poor prognosis. The mean survival time after diagnosis of cutaneous metastasis is 7 months.8 Therefore, treatment of cutaneous metastases is largely palliative, including local excision and intralesional chemotherapy.
1. Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009;59:73-98.
2. Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
3. Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
4. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
5. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-182.
6. Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600.
7. Tosoian J, Loeb S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. ScientificWorldJournal. 2010;10:1919-1931.
8. Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684.
9. Nadji M, Tabei SZ, Castro A, et al. Prostate-specific antigen: an immunohistochemical marker for prostatic neoplasms. Cancer. 1981;48:1229-1232.
10. Paner GP, Luthringer DJ, Amin MB. Best practice in diagnostic immunohistochemistry: prostate carcinoma and its mimics in needle core biopsies. Arch Pathol Lab Med. 2008;132:1388-1396.
11. Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669-3676.
1. Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009;59:73-98.
2. Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
3. Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
4. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
5. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-182.
6. Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600.
7. Tosoian J, Loeb S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. ScientificWorldJournal. 2010;10:1919-1931.
8. Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684.
9. Nadji M, Tabei SZ, Castro A, et al. Prostate-specific antigen: an immunohistochemical marker for prostatic neoplasms. Cancer. 1981;48:1229-1232.
10. Paner GP, Luthringer DJ, Amin MB. Best practice in diagnostic immunohistochemistry: prostate carcinoma and its mimics in needle core biopsies. Arch Pathol Lab Med. 2008;132:1388-1396.
11. Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669-3676.
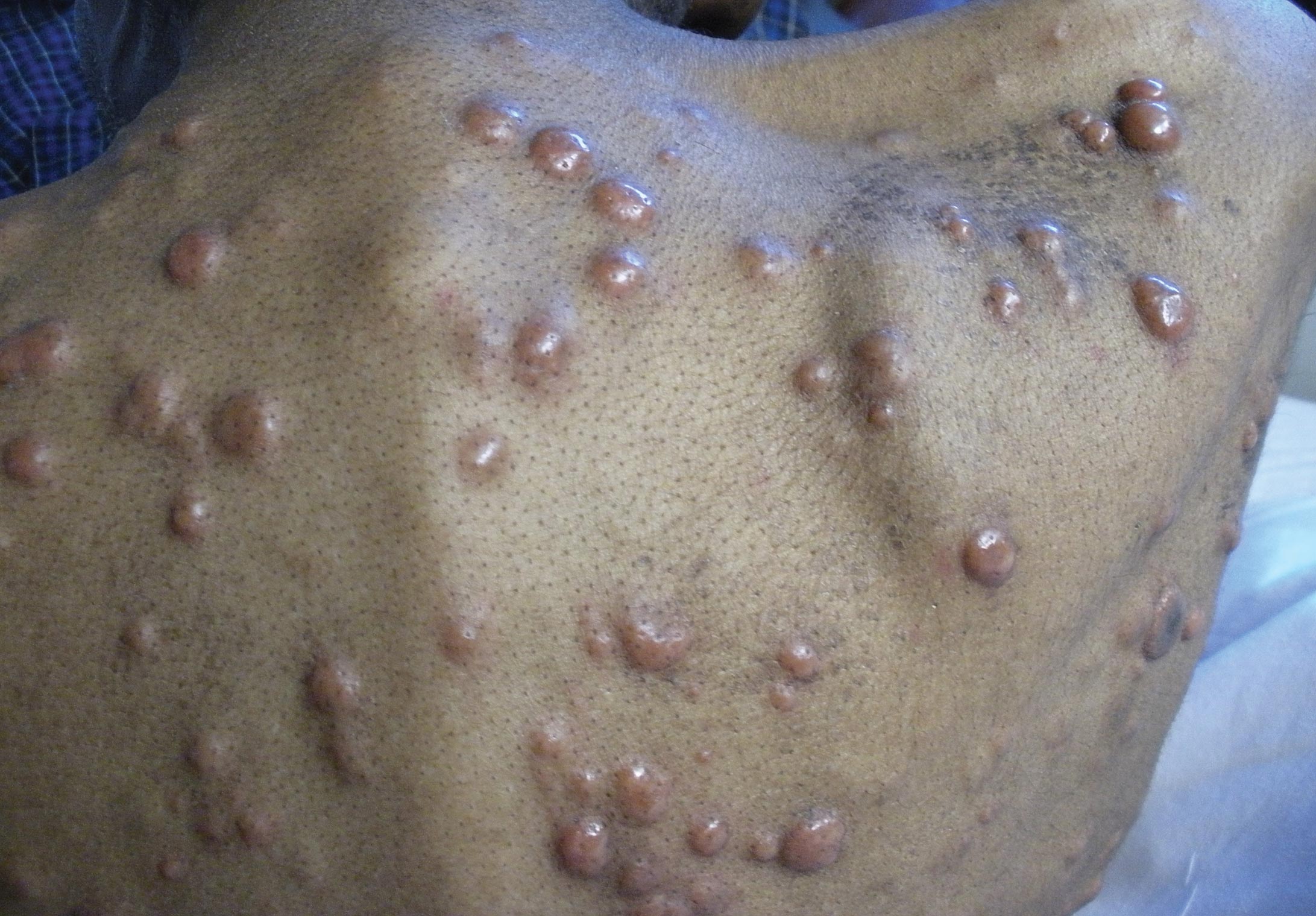
A 57-year-old cachectic man with a history of metastatic, hormone-refractory adenocarcinoma of the prostate presented with multiple tender subcutaneous nodules on the back and shoulders that developed over the course of 12 months. During that time, he was treated with cyclophosphamide, leuprolide acetate, bicalutamide, zoledronic acid, and filgrastim. A punch biopsy specimen obtained from the left shoulder revealed cords of atypical epithelioid cells dissecting through dermal collagen bundles, accompanied by abundant mucin. The atypical cells displayed nuclear pleomorphism, and multiple mitotic figures were observed.