User login
Primary Cutaneous Marginal Zone B-Cell Lymphoma Discovered During Mohs Surgery for Basal Cell Carcinoma
Primary Cutaneous Marginal Zone B-Cell Lymphoma Discovered During Mohs Surgery for Basal Cell Carcinoma
To the Editor:
Primary cutaneous B-cell lymphomas (pcBCLs) can clinically mimic basal cell carcinomas (BCCs); however, histopathologic examination typically demonstrates features of lymphoma without evidence of an epithelial tumor. We present the case of a patient who demonstrated histologic features of both pcBCL and BCC in the same lesion, which was discovered during Mohs micrographic surgery.
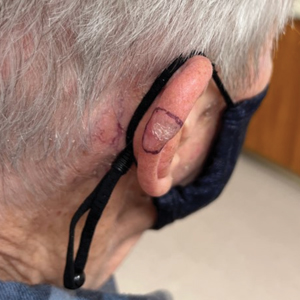
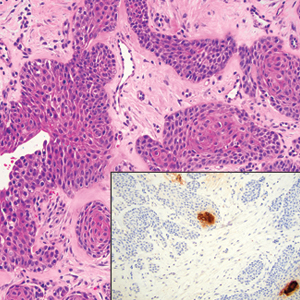
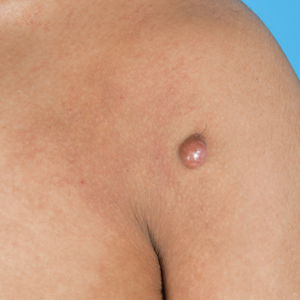
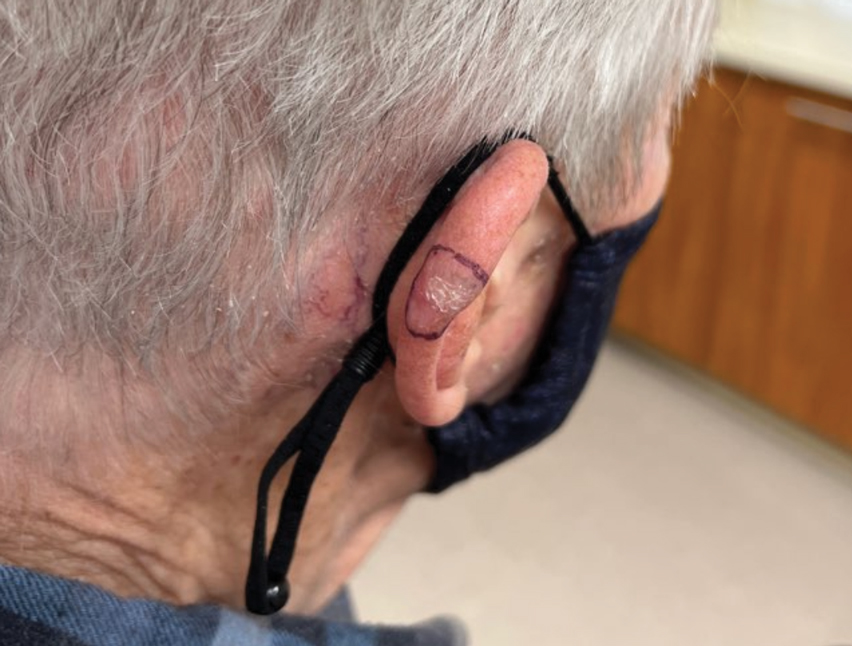
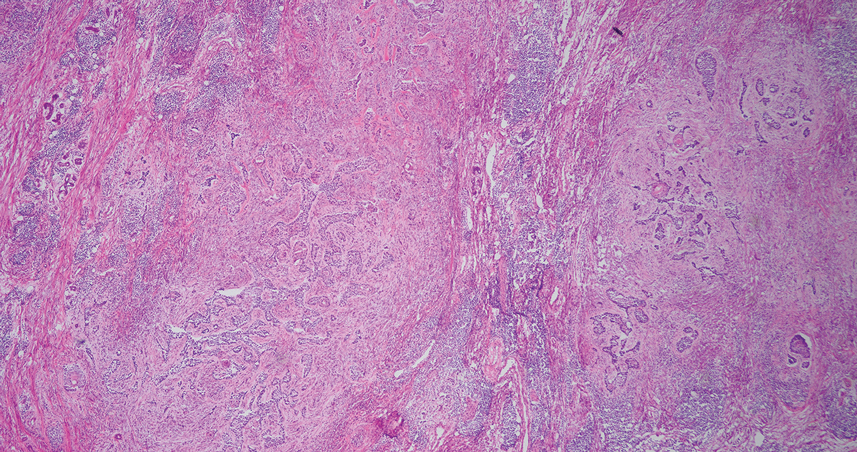
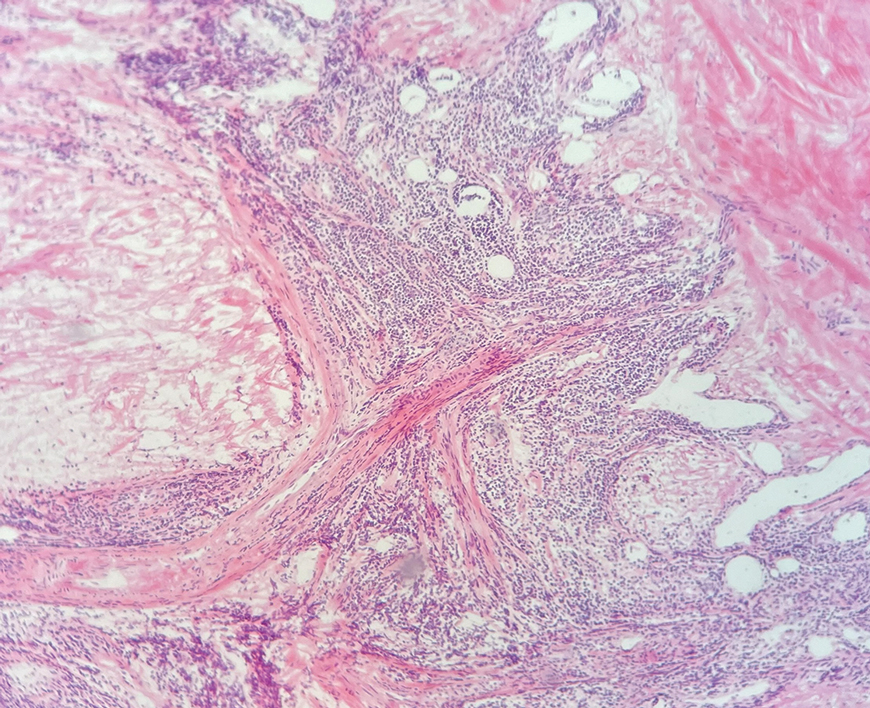
An 84-year-old man presented for Mohs surgery for a biopsy-proven nodular and infiltrative BCC on the right superior helix of the ear of 1 year’s duration. Physical examination of the ear revealed a 1.0×1.3–cm ulcerated indurated plaque with rolled borders and a central hyperkeratotic crust (Figure 1). Frozen sections from the first Mohs stage demonstrated residual superficial, infiltrative, and basosquamous BCC (Figure 2). In addition, there was a brisk inflammatory infiltrate throughout the deep margins. The second stage showed no residual BCC, but there still was a brisk atypical lymphocytic infiltrate, with some areas showing lymphocytes in a linear cordlike distribution (Figure 3). Permanent sections demonstrated infiltration of small to medium lymphoid cells. Immunohistochemistry stains were positive for CD20 and BCL2 and negative for CD5, CD10, BCL6, and CD43; a low Ki-67 proliferation fraction also was observed. B-cell clonality studies and polymerase chain reaction demonstrated rearrangements of the IgH and IgK genes, consistent with primary cutaneous marginal zone lymphoma (pcMZL). Positron emission tomography showed no spread of malignancy; therefore, medical oncology recommended observation and close monitoring.
Primary cutaneous B-cell lymphoma accounts for approximately 25% of all cutaneous lymphomas.1 Three main cutaneous subtypes exist: pcMZL; primary cutaneous follicular center lymphoma; and primary cutaneous diffuse large B-cell lymphoma, leg type. The second most common type of cutaneous lymphoma, pcMZL, accounts for 25% of cases of pcBCL.1 Primary cutaneous follicular center lymphoma makes up 60% of cutaneous lymphomas, and the remainder are primary cutaneous diffuse large B-cell lymphoma, leg type. All share a notable male predominance and onset most commonly in the sixth through eighth decades of life, although they also can occur in younger patients.1
Histologically, pcMZL has 2 distinct subtypes: one resembling mucosal-associated lymphoid tissue lymphomas and a more clinically aggressive subtype with heavy chain class switching, although intermediate forms also exist. Both are characterized by diffuse and/or nodular infiltrates in the subcutis and dermis with sparing of the epidermis. Often, these infiltrates are more prominent in the deeper sections examined, and occasionally they may be accompanied by germinal center follicles. Immunohistochemical stains are key in determining the pcBCL subtype. Primary cutaneous marginal zone lymphoma will most commonly show a BCL2+, BCL6–, CD20+, and CD10– immunophenotype, as in our case. If a majority of cells have undergone plasmacytoid differentiation, loss of CD20 can occur, but retention of other B-cell markers, such as CD79a and CD19, will be seen. Proliferation fraction via Ki-67 commonly is low, reflecting the indolence of this subtype of lymphoma.1
Monoclonal rearrangement of immunoglobulins also can occur, with IgH rearrangements detected in 60% to 80% of cases of pcMZL. Translocations are not a reliable method of diagnosis for pcMZL but can be present in a variable manner, with t(14;18), t(3;14), and t(11;18) reported in a subset of cases.2 Leukemic infiltrates encountered on frozen sections should prompt the Mohs surgeon to consider the possibility of a concomitant leukemia or lymphoma. In one study, 36% (20/55) of patients with chronic lymphocytic leukemia (CLL) were found to have predominantly leukemic B-cell infiltrates on frozen sections.3 Numerous reports also exist of asymptomatic patients being diagnosed with CLL due to leukemic infiltrates identified during Mohs surgery.4,5 Patients with systemic hematologic malignancies, including CLL and non-Hodgkin lymphoma, also are known to be at an increased risk for skin cancers, including keratinocyte cancers, melanoma, and Merkel cell carcinoma. This can be attributed partially to immunosuppression, a well-known risk factor for development of cutaneous malignancies.5 Padgett et al5 speculated that local immune suppression due to underlying pcBCL and reaction of lymphocytes to tumor antigens could have played a role in the development of BCC at this site. If a leukemic infiltrate is demonstrated, the surgeon should consider sending tissue for permanent section and immunostaining. This can be helpful to determine if it is a reactive or neoplastic process and aid in characterizing the leukemic infiltrate if it is suspected to be neoplastic in nature.
There are numerous reports of pcBCL imitating the cutaneous findings of BCC clinically, but this is quite uncommon on histopathology. As in our case, findings of sheets of dense, monomorphic lymphocytes; inability to clear inflammation on deeper Mohs sections; presence of primordial follicles; and atypical cytology, including predominance of blastic forms, plasmacytoid cells, or cleaved lymphocytes, should give the clinician pause to consider further evaluation through permanent sections as well as genetic and immunoglobulin studies by a dermatopathologist. This case highlights the importance of further evaluation when an atypical finding is encountered during Mohs surgery.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161. doi:10.1016/j.hoc.2018.08.006
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651. doi:10.3389/fonc.2020.00651
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134. doi:10.1046/j.1524-4725.2003.29034.x
- Walters M, Chang C, Castillo JR. Diagnosis of chronic lymphocytic leukemia during Mohs micrographic surgery. JAAD Case Rep. 2023;33:1-3. doi:10.1016/j.jdcr.2022.12.012
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771. doi:10.1046/j.1524-4725.2003.29194.x
To the Editor:
Primary cutaneous B-cell lymphomas (pcBCLs) can clinically mimic basal cell carcinomas (BCCs); however, histopathologic examination typically demonstrates features of lymphoma without evidence of an epithelial tumor. We present the case of a patient who demonstrated histologic features of both pcBCL and BCC in the same lesion, which was discovered during Mohs micrographic surgery.
An 84-year-old man presented for Mohs surgery for a biopsy-proven nodular and infiltrative BCC on the right superior helix of the ear of 1 year’s duration. Physical examination of the ear revealed a 1.0×1.3–cm ulcerated indurated plaque with rolled borders and a central hyperkeratotic crust (Figure 1). Frozen sections from the first Mohs stage demonstrated residual superficial, infiltrative, and basosquamous BCC (Figure 2). In addition, there was a brisk inflammatory infiltrate throughout the deep margins. The second stage showed no residual BCC, but there still was a brisk atypical lymphocytic infiltrate, with some areas showing lymphocytes in a linear cordlike distribution (Figure 3). Permanent sections demonstrated infiltration of small to medium lymphoid cells. Immunohistochemistry stains were positive for CD20 and BCL2 and negative for CD5, CD10, BCL6, and CD43; a low Ki-67 proliferation fraction also was observed. B-cell clonality studies and polymerase chain reaction demonstrated rearrangements of the IgH and IgK genes, consistent with primary cutaneous marginal zone lymphoma (pcMZL). Positron emission tomography showed no spread of malignancy; therefore, medical oncology recommended observation and close monitoring.



Primary cutaneous B-cell lymphoma accounts for approximately 25% of all cutaneous lymphomas.1 Three main cutaneous subtypes exist: pcMZL; primary cutaneous follicular center lymphoma; and primary cutaneous diffuse large B-cell lymphoma, leg type. The second most common type of cutaneous lymphoma, pcMZL, accounts for 25% of cases of pcBCL.1 Primary cutaneous follicular center lymphoma makes up 60% of cutaneous lymphomas, and the remainder are primary cutaneous diffuse large B-cell lymphoma, leg type. All share a notable male predominance and onset most commonly in the sixth through eighth decades of life, although they also can occur in younger patients.1
Histologically, pcMZL has 2 distinct subtypes: one resembling mucosal-associated lymphoid tissue lymphomas and a more clinically aggressive subtype with heavy chain class switching, although intermediate forms also exist. Both are characterized by diffuse and/or nodular infiltrates in the subcutis and dermis with sparing of the epidermis. Often, these infiltrates are more prominent in the deeper sections examined, and occasionally they may be accompanied by germinal center follicles. Immunohistochemical stains are key in determining the pcBCL subtype. Primary cutaneous marginal zone lymphoma will most commonly show a BCL2+, BCL6–, CD20+, and CD10– immunophenotype, as in our case. If a majority of cells have undergone plasmacytoid differentiation, loss of CD20 can occur, but retention of other B-cell markers, such as CD79a and CD19, will be seen. Proliferation fraction via Ki-67 commonly is low, reflecting the indolence of this subtype of lymphoma.1
Monoclonal rearrangement of immunoglobulins also can occur, with IgH rearrangements detected in 60% to 80% of cases of pcMZL. Translocations are not a reliable method of diagnosis for pcMZL but can be present in a variable manner, with t(14;18), t(3;14), and t(11;18) reported in a subset of cases.2 Leukemic infiltrates encountered on frozen sections should prompt the Mohs surgeon to consider the possibility of a concomitant leukemia or lymphoma. In one study, 36% (20/55) of patients with chronic lymphocytic leukemia (CLL) were found to have predominantly leukemic B-cell infiltrates on frozen sections.3 Numerous reports also exist of asymptomatic patients being diagnosed with CLL due to leukemic infiltrates identified during Mohs surgery.4,5 Patients with systemic hematologic malignancies, including CLL and non-Hodgkin lymphoma, also are known to be at an increased risk for skin cancers, including keratinocyte cancers, melanoma, and Merkel cell carcinoma. This can be attributed partially to immunosuppression, a well-known risk factor for development of cutaneous malignancies.5 Padgett et al5 speculated that local immune suppression due to underlying pcBCL and reaction of lymphocytes to tumor antigens could have played a role in the development of BCC at this site. If a leukemic infiltrate is demonstrated, the surgeon should consider sending tissue for permanent section and immunostaining. This can be helpful to determine if it is a reactive or neoplastic process and aid in characterizing the leukemic infiltrate if it is suspected to be neoplastic in nature.
There are numerous reports of pcBCL imitating the cutaneous findings of BCC clinically, but this is quite uncommon on histopathology. As in our case, findings of sheets of dense, monomorphic lymphocytes; inability to clear inflammation on deeper Mohs sections; presence of primordial follicles; and atypical cytology, including predominance of blastic forms, plasmacytoid cells, or cleaved lymphocytes, should give the clinician pause to consider further evaluation through permanent sections as well as genetic and immunoglobulin studies by a dermatopathologist. This case highlights the importance of further evaluation when an atypical finding is encountered during Mohs surgery.
To the Editor:
Primary cutaneous B-cell lymphomas (pcBCLs) can clinically mimic basal cell carcinomas (BCCs); however, histopathologic examination typically demonstrates features of lymphoma without evidence of an epithelial tumor. We present the case of a patient who demonstrated histologic features of both pcBCL and BCC in the same lesion, which was discovered during Mohs micrographic surgery.
An 84-year-old man presented for Mohs surgery for a biopsy-proven nodular and infiltrative BCC on the right superior helix of the ear of 1 year’s duration. Physical examination of the ear revealed a 1.0×1.3–cm ulcerated indurated plaque with rolled borders and a central hyperkeratotic crust (Figure 1). Frozen sections from the first Mohs stage demonstrated residual superficial, infiltrative, and basosquamous BCC (Figure 2). In addition, there was a brisk inflammatory infiltrate throughout the deep margins. The second stage showed no residual BCC, but there still was a brisk atypical lymphocytic infiltrate, with some areas showing lymphocytes in a linear cordlike distribution (Figure 3). Permanent sections demonstrated infiltration of small to medium lymphoid cells. Immunohistochemistry stains were positive for CD20 and BCL2 and negative for CD5, CD10, BCL6, and CD43; a low Ki-67 proliferation fraction also was observed. B-cell clonality studies and polymerase chain reaction demonstrated rearrangements of the IgH and IgK genes, consistent with primary cutaneous marginal zone lymphoma (pcMZL). Positron emission tomography showed no spread of malignancy; therefore, medical oncology recommended observation and close monitoring.



Primary cutaneous B-cell lymphoma accounts for approximately 25% of all cutaneous lymphomas.1 Three main cutaneous subtypes exist: pcMZL; primary cutaneous follicular center lymphoma; and primary cutaneous diffuse large B-cell lymphoma, leg type. The second most common type of cutaneous lymphoma, pcMZL, accounts for 25% of cases of pcBCL.1 Primary cutaneous follicular center lymphoma makes up 60% of cutaneous lymphomas, and the remainder are primary cutaneous diffuse large B-cell lymphoma, leg type. All share a notable male predominance and onset most commonly in the sixth through eighth decades of life, although they also can occur in younger patients.1
Histologically, pcMZL has 2 distinct subtypes: one resembling mucosal-associated lymphoid tissue lymphomas and a more clinically aggressive subtype with heavy chain class switching, although intermediate forms also exist. Both are characterized by diffuse and/or nodular infiltrates in the subcutis and dermis with sparing of the epidermis. Often, these infiltrates are more prominent in the deeper sections examined, and occasionally they may be accompanied by germinal center follicles. Immunohistochemical stains are key in determining the pcBCL subtype. Primary cutaneous marginal zone lymphoma will most commonly show a BCL2+, BCL6–, CD20+, and CD10– immunophenotype, as in our case. If a majority of cells have undergone plasmacytoid differentiation, loss of CD20 can occur, but retention of other B-cell markers, such as CD79a and CD19, will be seen. Proliferation fraction via Ki-67 commonly is low, reflecting the indolence of this subtype of lymphoma.1
Monoclonal rearrangement of immunoglobulins also can occur, with IgH rearrangements detected in 60% to 80% of cases of pcMZL. Translocations are not a reliable method of diagnosis for pcMZL but can be present in a variable manner, with t(14;18), t(3;14), and t(11;18) reported in a subset of cases.2 Leukemic infiltrates encountered on frozen sections should prompt the Mohs surgeon to consider the possibility of a concomitant leukemia or lymphoma. In one study, 36% (20/55) of patients with chronic lymphocytic leukemia (CLL) were found to have predominantly leukemic B-cell infiltrates on frozen sections.3 Numerous reports also exist of asymptomatic patients being diagnosed with CLL due to leukemic infiltrates identified during Mohs surgery.4,5 Patients with systemic hematologic malignancies, including CLL and non-Hodgkin lymphoma, also are known to be at an increased risk for skin cancers, including keratinocyte cancers, melanoma, and Merkel cell carcinoma. This can be attributed partially to immunosuppression, a well-known risk factor for development of cutaneous malignancies.5 Padgett et al5 speculated that local immune suppression due to underlying pcBCL and reaction of lymphocytes to tumor antigens could have played a role in the development of BCC at this site. If a leukemic infiltrate is demonstrated, the surgeon should consider sending tissue for permanent section and immunostaining. This can be helpful to determine if it is a reactive or neoplastic process and aid in characterizing the leukemic infiltrate if it is suspected to be neoplastic in nature.
There are numerous reports of pcBCL imitating the cutaneous findings of BCC clinically, but this is quite uncommon on histopathology. As in our case, findings of sheets of dense, monomorphic lymphocytes; inability to clear inflammation on deeper Mohs sections; presence of primordial follicles; and atypical cytology, including predominance of blastic forms, plasmacytoid cells, or cleaved lymphocytes, should give the clinician pause to consider further evaluation through permanent sections as well as genetic and immunoglobulin studies by a dermatopathologist. This case highlights the importance of further evaluation when an atypical finding is encountered during Mohs surgery.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161. doi:10.1016/j.hoc.2018.08.006
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651. doi:10.3389/fonc.2020.00651
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134. doi:10.1046/j.1524-4725.2003.29034.x
- Walters M, Chang C, Castillo JR. Diagnosis of chronic lymphocytic leukemia during Mohs micrographic surgery. JAAD Case Rep. 2023;33:1-3. doi:10.1016/j.jdcr.2022.12.012
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771. doi:10.1046/j.1524-4725.2003.29194.x
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161. doi:10.1016/j.hoc.2018.08.006
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651. doi:10.3389/fonc.2020.00651
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134. doi:10.1046/j.1524-4725.2003.29034.x
- Walters M, Chang C, Castillo JR. Diagnosis of chronic lymphocytic leukemia during Mohs micrographic surgery. JAAD Case Rep. 2023;33:1-3. doi:10.1016/j.jdcr.2022.12.012
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771. doi:10.1046/j.1524-4725.2003.29194.x
Primary Cutaneous Marginal Zone B-Cell Lymphoma Discovered During Mohs Surgery for Basal Cell Carcinoma
Primary Cutaneous Marginal Zone B-Cell Lymphoma Discovered During Mohs Surgery for Basal Cell Carcinoma
Practice Points
- Collision tumors of cutaneous B-cell lymphoma and basal cell carcinoma occurring within the same lesion are uncommon findings during Mohs surgery.
- Sheets of atypical monomorphic lymphocytes on deeper Mohs sections should prompt the surgeon to consider further evaluation, including sending tissue for permanent sections.
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
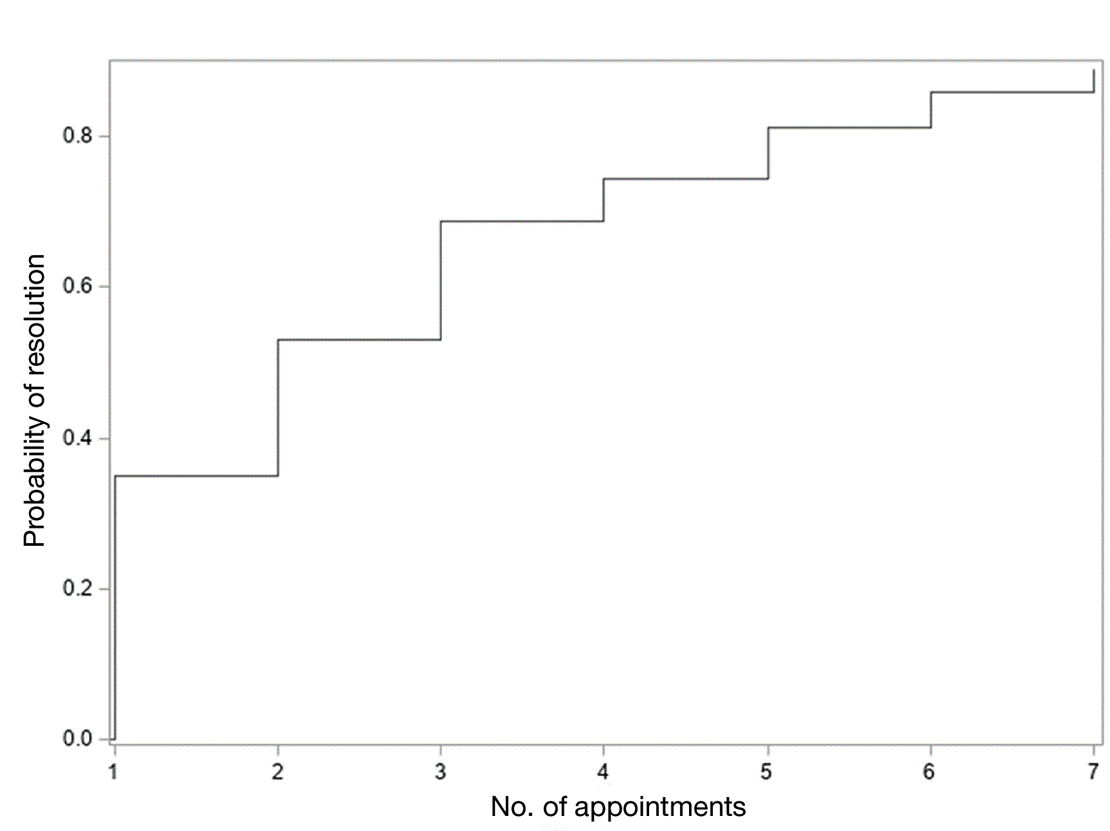
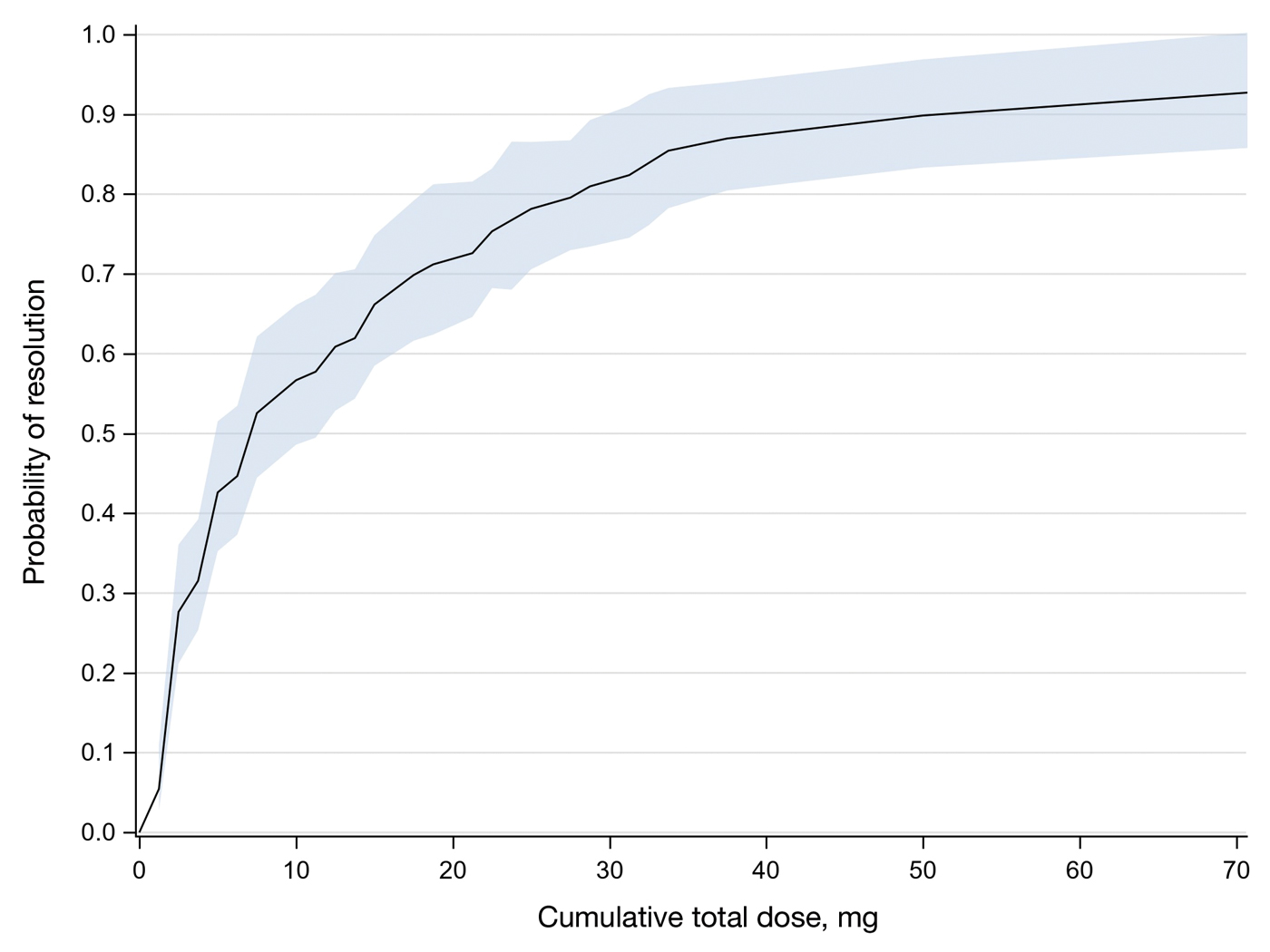
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).
Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
PRACTICE POINTS
- Intralesional methotrexate (IL-MTX) is an efficacious treatment option for cutaneous squamous cell carcinoma lesions in patients who are not good candidates for surgical excision.
- The starting concentration of the initial IL-MTX dose did not substantially impact outcomes; however, a 25 mg/mL concentration is standard for subsequent treatments to maintain efficacy.
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).
The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Practice Gap
Tattoos have become increasingly prevalent in Western culture, with approximately 1 in 4 Americans having at least 1 tattoo. Individuals invest money, time, and even pain in getting tattoos, many of which hold special personal, family, or religious significance.1 Various cutaneous pathologies may arise in areas of the skin with tattoos, including malignancies and inflammatory reactions to tattoo pigment, and in these cases, surgical management may be indicated.2,3
Nonmelanoma skin cancers (NMSCs) such as superficial basal cell carcinomas on broadly sun-damaged areas (eg, trunk, torso), squamous cell carcinomas, reactive keratoacanthomas, and reactive pseudoepitheliomatous squamous hyperplasia diagnosed as squamous cell carcinoma have been reported to occur in or near areas of the skin with tattoos.2 Mohs micrographic surgery (MMS) is the standard of care for removing NMSCs, particularly when they manifest in cosmetically sensitive areas.4 This treatment option allows for careful guided resection of tumors to minimize the risk for recurrence; it also preserves healthy tissue, which typically results in a smaller radial defect after the procedure is complete.
Chronic reactions to tattoo pigment may include granulomatous tattoo reactions and pseudolymphomas.3 Treatment options may include immunosuppressives such as intralesional triamcinolone as well as pigment destruction via lasers5; however, not all tattoos are responsive to these treatments. Surgical excision is an effective and definitive treatment in this context, as tattoo pigment resides in or above the mid dermis to a depth of approximately 400 μm. Intradermal excision effectively removes the antigenic pigment.5
In these clinical scenarios, patients may be hesitant to pursue surgical treatment due to concerns that it may alter tattoo appearance. Many clinicians and surgeons may consider definitive treatment and tattoo preservation to be mutually exclusive, but this is not always the case. We propose a technique that utilizes conservative thickness layers (CTL) to minimize disruption to the appearance of tattoos in MMS for treatment of cutaneous malignancies as well as intradermal excision of tattoo pigment in the setting of chronic inflammatory tattoo reactions.
The Technique
In the appropriate clinical context, CTL can effectively result in defects that heal well by secondary intention and minimize collateral tissue distortion.4 Lesions manifesting in or near tattooed skin often are responsive to treatment with CTL; furthermore, CTL may preserve some deeper tattoo pigment, resulting in only partial loss of the tattooed skin.
Conservative thickness layers are performed intradermally, similar to removing traditional layers in MMS. For treatment of NMSCs, a margin is scored around the lesion, and then the blade is passed carefully under the lesion nearly parallel to the skin through an intradermal plane. It is important to avoid entering the subcuticular fat (Figure 1). The tissue then is processed normally in the Mohs laboratory for complete circumferential margin evaluation. If necessary and possible, subsequent layers also can be performed in the intradermal plane. Once total circumferential margin control is obtained, the wound is allowed to granulate and heal by secondary intention. As these processes occur, we have found that wound contraction is less likely with the dermis intact, resulting in less impact on the overall appearance of the tattoo (Figures 1 and 2). For very thin lesions, resultant defects may retain some residual tattoo pigment. The residual scars also may be responsive to tattoo revision, although a period of monitoring for recurrence should be considered if there is concern that revising the tattoo could obscure early recurrent tumors. From our experience, utilizing CTL for NMSCs that arise within or near tattoos results in favorable preservation of the tattoo appearance and high patient satisfaction.
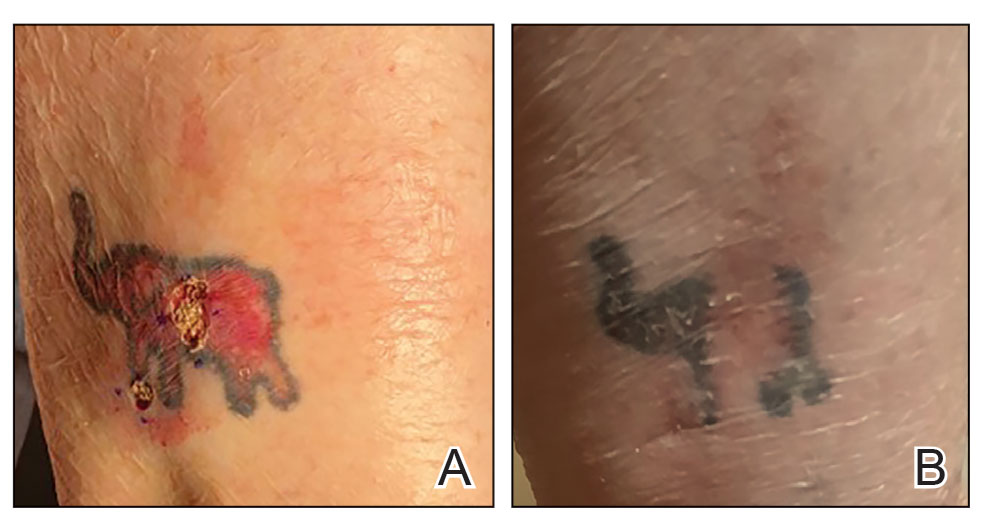
The procedure is performed similarly for removal of allergenic tattoo pigment, with careful excision to the mid dermis. Since the areas affected by the cutaneous reaction may be relatively large, surgical precision is required to maintain a uniform depth to remove the tattoo pigment and preserve the deep dermis (Figure 2). Once removed, the defect can be left to granulate and heal by secondary intention. If the patient wants to have the tattoo revised in the future, it would be prudent to utilize pigment that the patient has responded favorably to. In our experience, this approach is effective and yields high patient satisfaction and minimizes morbidity.
Practice Implications
Tattoos often hold special meaning for patients; therefore, treatment of pathologies arising in or near tattooed skin should emphasize maintaining the appearance of the tattoo while still being effective. Conservative thickness layers in MMS and intradermal excisions for allergic reactions to tattoo pigment are an effective treatment strategy that clinicians may consider.
One shortcoming of using CTL for MMS is the need for subsequent layers to clear the tumor; however, data suggest that first-stage cure rates are extremely high even with CTL for appropriately selected patients, with clearance of nearly 80% of tumors on the first stage. Tumors that may be most responsive to CTL include exophytic NMSCs and those arising in areas with a thicker dermis, including the back, legs, and scalp, although other locations including the face, hands, shins, ankles, and feet also may be well suited for CTL.4 Another shortcoming of CTL is that skin cancers arising in tattoos may not be considered appropriate for MMS based on the 2012
Conservative thickness layers in MMS and intradermal excisions of tattoo pigment are both effective techniques of minimizing disruption of tattoos while effectively treating patients.
- Roggenkamp H, Nicholls A, Pierre JM. Tattoos as a window to the psyche: how talking about skin art can inform psychiatric practice. World J Psychiatry. 2017;7:148-158. doi:10.5498/wjp.v7.i3.148
- Rubatto M, Gelato F, Mastorino L, et al. Nonmelanoma skin cancer arising on tattoos. Int J Dermatol. 2023;62:E155-E156. doi:10.1111/ijd.16381
- Atwater AR, Bembry R, Reeder M. Tattoo hypersensitivity reactions: inky business. Cutis. 2020;106:64-67. doi:10.12788/cutis.0028
- Tolkachjov SN, Cappel JA, Bryant EA, et al. Conservative thickness layers in Mohs micrographic surgery. Int J Dermatol. 2018;57:1128-1134. doi:10.1111/ijd.14043
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24. doi:10.4103/0974-2077.155068
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j.jaad.2012.06.009.
- Amthor Croley JA. Current controversies in mohs micrographic surgery. Cutis. 2019;104:E29-E31.
Practice Gap
Tattoos have become increasingly prevalent in Western culture, with approximately 1 in 4 Americans having at least 1 tattoo. Individuals invest money, time, and even pain in getting tattoos, many of which hold special personal, family, or religious significance.1 Various cutaneous pathologies may arise in areas of the skin with tattoos, including malignancies and inflammatory reactions to tattoo pigment, and in these cases, surgical management may be indicated.2,3
Nonmelanoma skin cancers (NMSCs) such as superficial basal cell carcinomas on broadly sun-damaged areas (eg, trunk, torso), squamous cell carcinomas, reactive keratoacanthomas, and reactive pseudoepitheliomatous squamous hyperplasia diagnosed as squamous cell carcinoma have been reported to occur in or near areas of the skin with tattoos.2 Mohs micrographic surgery (MMS) is the standard of care for removing NMSCs, particularly when they manifest in cosmetically sensitive areas.4 This treatment option allows for careful guided resection of tumors to minimize the risk for recurrence; it also preserves healthy tissue, which typically results in a smaller radial defect after the procedure is complete.
Chronic reactions to tattoo pigment may include granulomatous tattoo reactions and pseudolymphomas.3 Treatment options may include immunosuppressives such as intralesional triamcinolone as well as pigment destruction via lasers5; however, not all tattoos are responsive to these treatments. Surgical excision is an effective and definitive treatment in this context, as tattoo pigment resides in or above the mid dermis to a depth of approximately 400 μm. Intradermal excision effectively removes the antigenic pigment.5
In these clinical scenarios, patients may be hesitant to pursue surgical treatment due to concerns that it may alter tattoo appearance. Many clinicians and surgeons may consider definitive treatment and tattoo preservation to be mutually exclusive, but this is not always the case. We propose a technique that utilizes conservative thickness layers (CTL) to minimize disruption to the appearance of tattoos in MMS for treatment of cutaneous malignancies as well as intradermal excision of tattoo pigment in the setting of chronic inflammatory tattoo reactions.
The Technique
In the appropriate clinical context, CTL can effectively result in defects that heal well by secondary intention and minimize collateral tissue distortion.4 Lesions manifesting in or near tattooed skin often are responsive to treatment with CTL; furthermore, CTL may preserve some deeper tattoo pigment, resulting in only partial loss of the tattooed skin.
Conservative thickness layers are performed intradermally, similar to removing traditional layers in MMS. For treatment of NMSCs, a margin is scored around the lesion, and then the blade is passed carefully under the lesion nearly parallel to the skin through an intradermal plane. It is important to avoid entering the subcuticular fat (Figure 1). The tissue then is processed normally in the Mohs laboratory for complete circumferential margin evaluation. If necessary and possible, subsequent layers also can be performed in the intradermal plane. Once total circumferential margin control is obtained, the wound is allowed to granulate and heal by secondary intention. As these processes occur, we have found that wound contraction is less likely with the dermis intact, resulting in less impact on the overall appearance of the tattoo (Figures 1 and 2). For very thin lesions, resultant defects may retain some residual tattoo pigment. The residual scars also may be responsive to tattoo revision, although a period of monitoring for recurrence should be considered if there is concern that revising the tattoo could obscure early recurrent tumors. From our experience, utilizing CTL for NMSCs that arise within or near tattoos results in favorable preservation of the tattoo appearance and high patient satisfaction.


The procedure is performed similarly for removal of allergenic tattoo pigment, with careful excision to the mid dermis. Since the areas affected by the cutaneous reaction may be relatively large, surgical precision is required to maintain a uniform depth to remove the tattoo pigment and preserve the deep dermis (Figure 2). Once removed, the defect can be left to granulate and heal by secondary intention. If the patient wants to have the tattoo revised in the future, it would be prudent to utilize pigment that the patient has responded favorably to. In our experience, this approach is effective and yields high patient satisfaction and minimizes morbidity.
Practice Implications
Tattoos often hold special meaning for patients; therefore, treatment of pathologies arising in or near tattooed skin should emphasize maintaining the appearance of the tattoo while still being effective. Conservative thickness layers in MMS and intradermal excisions for allergic reactions to tattoo pigment are an effective treatment strategy that clinicians may consider.
One shortcoming of using CTL for MMS is the need for subsequent layers to clear the tumor; however, data suggest that first-stage cure rates are extremely high even with CTL for appropriately selected patients, with clearance of nearly 80% of tumors on the first stage. Tumors that may be most responsive to CTL include exophytic NMSCs and those arising in areas with a thicker dermis, including the back, legs, and scalp, although other locations including the face, hands, shins, ankles, and feet also may be well suited for CTL.4 Another shortcoming of CTL is that skin cancers arising in tattoos may not be considered appropriate for MMS based on the 2012
Conservative thickness layers in MMS and intradermal excisions of tattoo pigment are both effective techniques of minimizing disruption of tattoos while effectively treating patients.
Practice Gap
Tattoos have become increasingly prevalent in Western culture, with approximately 1 in 4 Americans having at least 1 tattoo. Individuals invest money, time, and even pain in getting tattoos, many of which hold special personal, family, or religious significance.1 Various cutaneous pathologies may arise in areas of the skin with tattoos, including malignancies and inflammatory reactions to tattoo pigment, and in these cases, surgical management may be indicated.2,3
Nonmelanoma skin cancers (NMSCs) such as superficial basal cell carcinomas on broadly sun-damaged areas (eg, trunk, torso), squamous cell carcinomas, reactive keratoacanthomas, and reactive pseudoepitheliomatous squamous hyperplasia diagnosed as squamous cell carcinoma have been reported to occur in or near areas of the skin with tattoos.2 Mohs micrographic surgery (MMS) is the standard of care for removing NMSCs, particularly when they manifest in cosmetically sensitive areas.4 This treatment option allows for careful guided resection of tumors to minimize the risk for recurrence; it also preserves healthy tissue, which typically results in a smaller radial defect after the procedure is complete.
Chronic reactions to tattoo pigment may include granulomatous tattoo reactions and pseudolymphomas.3 Treatment options may include immunosuppressives such as intralesional triamcinolone as well as pigment destruction via lasers5; however, not all tattoos are responsive to these treatments. Surgical excision is an effective and definitive treatment in this context, as tattoo pigment resides in or above the mid dermis to a depth of approximately 400 μm. Intradermal excision effectively removes the antigenic pigment.5
In these clinical scenarios, patients may be hesitant to pursue surgical treatment due to concerns that it may alter tattoo appearance. Many clinicians and surgeons may consider definitive treatment and tattoo preservation to be mutually exclusive, but this is not always the case. We propose a technique that utilizes conservative thickness layers (CTL) to minimize disruption to the appearance of tattoos in MMS for treatment of cutaneous malignancies as well as intradermal excision of tattoo pigment in the setting of chronic inflammatory tattoo reactions.
The Technique
In the appropriate clinical context, CTL can effectively result in defects that heal well by secondary intention and minimize collateral tissue distortion.4 Lesions manifesting in or near tattooed skin often are responsive to treatment with CTL; furthermore, CTL may preserve some deeper tattoo pigment, resulting in only partial loss of the tattooed skin.
Conservative thickness layers are performed intradermally, similar to removing traditional layers in MMS. For treatment of NMSCs, a margin is scored around the lesion, and then the blade is passed carefully under the lesion nearly parallel to the skin through an intradermal plane. It is important to avoid entering the subcuticular fat (Figure 1). The tissue then is processed normally in the Mohs laboratory for complete circumferential margin evaluation. If necessary and possible, subsequent layers also can be performed in the intradermal plane. Once total circumferential margin control is obtained, the wound is allowed to granulate and heal by secondary intention. As these processes occur, we have found that wound contraction is less likely with the dermis intact, resulting in less impact on the overall appearance of the tattoo (Figures 1 and 2). For very thin lesions, resultant defects may retain some residual tattoo pigment. The residual scars also may be responsive to tattoo revision, although a period of monitoring for recurrence should be considered if there is concern that revising the tattoo could obscure early recurrent tumors. From our experience, utilizing CTL for NMSCs that arise within or near tattoos results in favorable preservation of the tattoo appearance and high patient satisfaction.


The procedure is performed similarly for removal of allergenic tattoo pigment, with careful excision to the mid dermis. Since the areas affected by the cutaneous reaction may be relatively large, surgical precision is required to maintain a uniform depth to remove the tattoo pigment and preserve the deep dermis (Figure 2). Once removed, the defect can be left to granulate and heal by secondary intention. If the patient wants to have the tattoo revised in the future, it would be prudent to utilize pigment that the patient has responded favorably to. In our experience, this approach is effective and yields high patient satisfaction and minimizes morbidity.
Practice Implications
Tattoos often hold special meaning for patients; therefore, treatment of pathologies arising in or near tattooed skin should emphasize maintaining the appearance of the tattoo while still being effective. Conservative thickness layers in MMS and intradermal excisions for allergic reactions to tattoo pigment are an effective treatment strategy that clinicians may consider.
One shortcoming of using CTL for MMS is the need for subsequent layers to clear the tumor; however, data suggest that first-stage cure rates are extremely high even with CTL for appropriately selected patients, with clearance of nearly 80% of tumors on the first stage. Tumors that may be most responsive to CTL include exophytic NMSCs and those arising in areas with a thicker dermis, including the back, legs, and scalp, although other locations including the face, hands, shins, ankles, and feet also may be well suited for CTL.4 Another shortcoming of CTL is that skin cancers arising in tattoos may not be considered appropriate for MMS based on the 2012
Conservative thickness layers in MMS and intradermal excisions of tattoo pigment are both effective techniques of minimizing disruption of tattoos while effectively treating patients.
- Roggenkamp H, Nicholls A, Pierre JM. Tattoos as a window to the psyche: how talking about skin art can inform psychiatric practice. World J Psychiatry. 2017;7:148-158. doi:10.5498/wjp.v7.i3.148
- Rubatto M, Gelato F, Mastorino L, et al. Nonmelanoma skin cancer arising on tattoos. Int J Dermatol. 2023;62:E155-E156. doi:10.1111/ijd.16381
- Atwater AR, Bembry R, Reeder M. Tattoo hypersensitivity reactions: inky business. Cutis. 2020;106:64-67. doi:10.12788/cutis.0028
- Tolkachjov SN, Cappel JA, Bryant EA, et al. Conservative thickness layers in Mohs micrographic surgery. Int J Dermatol. 2018;57:1128-1134. doi:10.1111/ijd.14043
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24. doi:10.4103/0974-2077.155068
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j.jaad.2012.06.009.
- Amthor Croley JA. Current controversies in mohs micrographic surgery. Cutis. 2019;104:E29-E31.
- Roggenkamp H, Nicholls A, Pierre JM. Tattoos as a window to the psyche: how talking about skin art can inform psychiatric practice. World J Psychiatry. 2017;7:148-158. doi:10.5498/wjp.v7.i3.148
- Rubatto M, Gelato F, Mastorino L, et al. Nonmelanoma skin cancer arising on tattoos. Int J Dermatol. 2023;62:E155-E156. doi:10.1111/ijd.16381
- Atwater AR, Bembry R, Reeder M. Tattoo hypersensitivity reactions: inky business. Cutis. 2020;106:64-67. doi:10.12788/cutis.0028
- Tolkachjov SN, Cappel JA, Bryant EA, et al. Conservative thickness layers in Mohs micrographic surgery. Int J Dermatol. 2018;57:1128-1134. doi:10.1111/ijd.14043
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24. doi:10.4103/0974-2077.155068
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j.jaad.2012.06.009.
- Amthor Croley JA. Current controversies in mohs micrographic surgery. Cutis. 2019;104:E29-E31.
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Nonhealing Friable Nodule on the Distal Edge of the Toe
Nonhealing Friable Nodule on the Distal Edge of the Toe
THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1
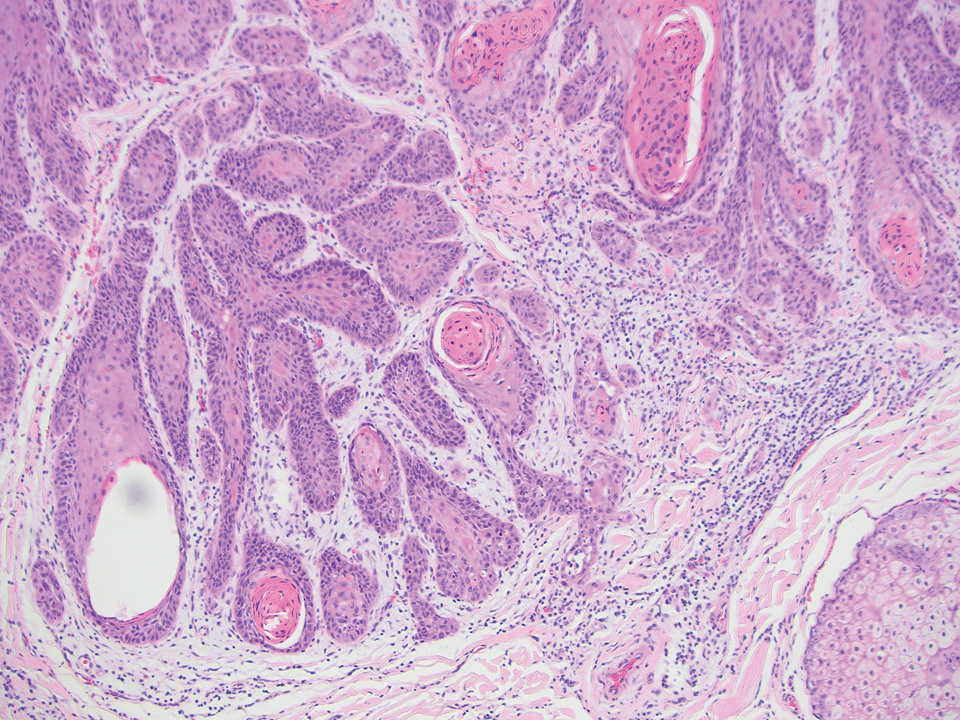
The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4
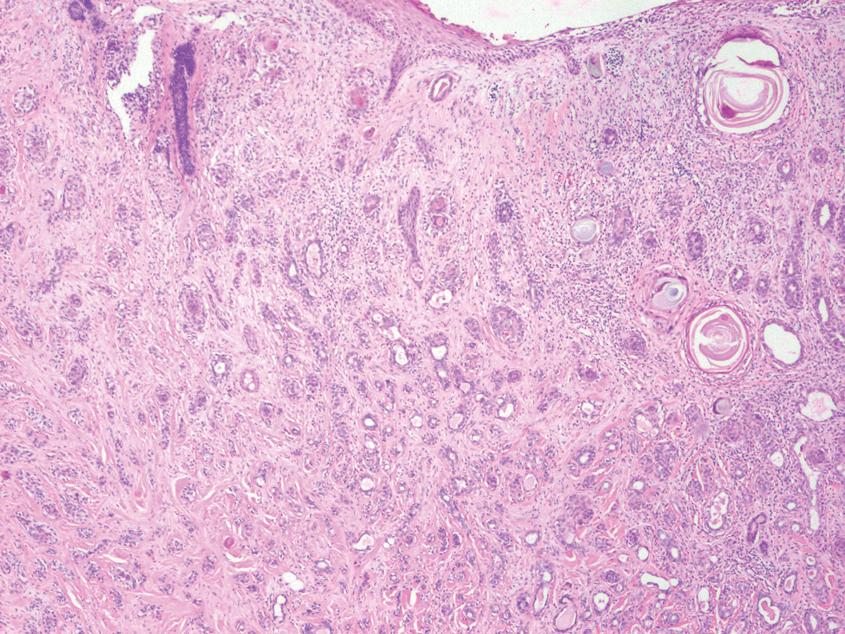
Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5
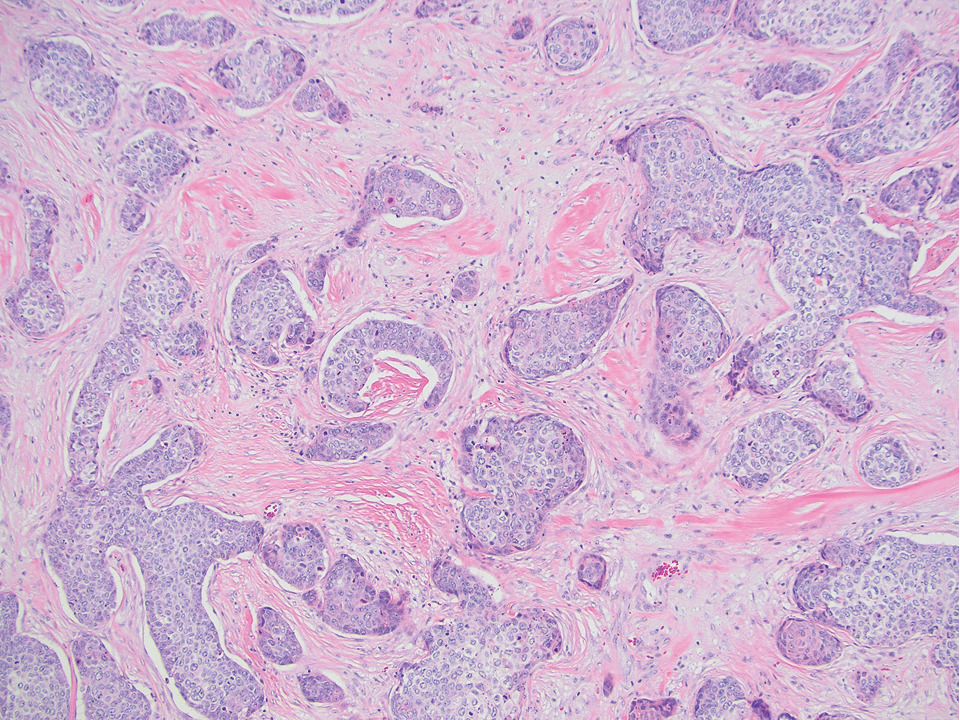
Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.
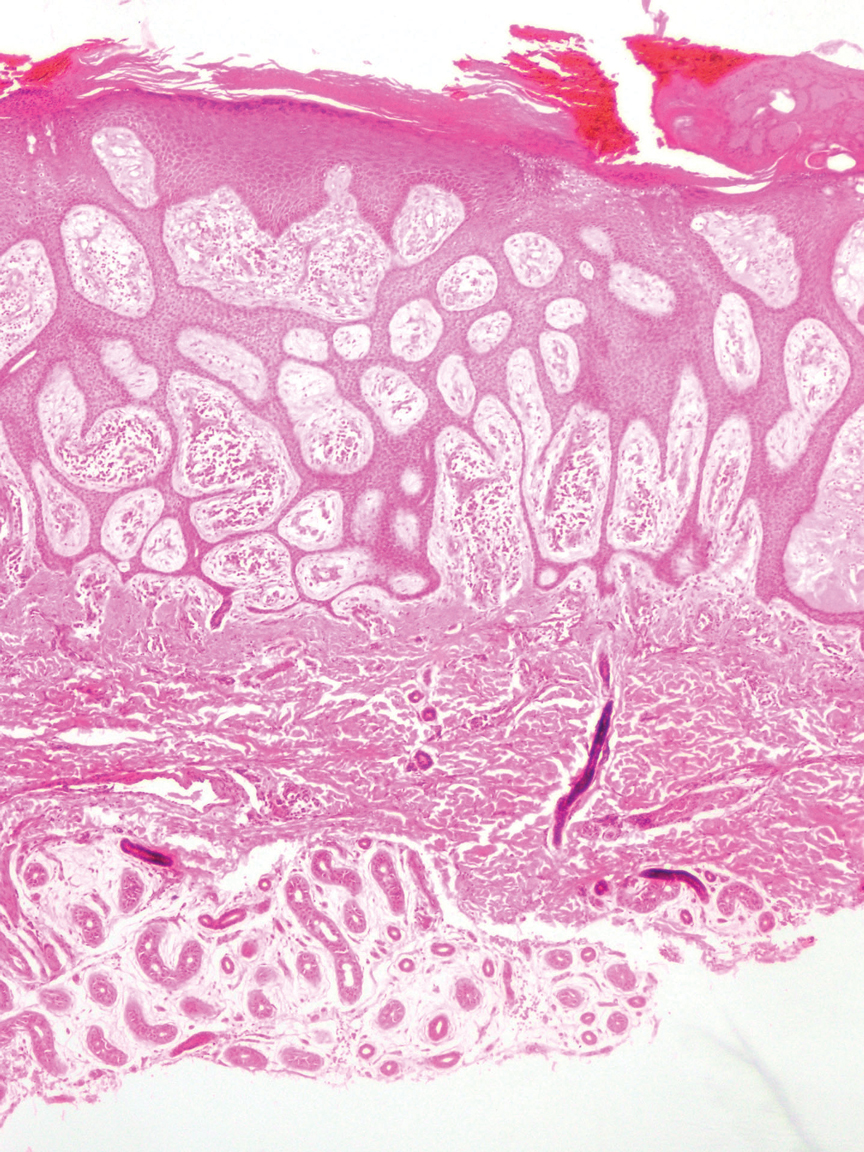
- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1

The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4

Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5

Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.

THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1

The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4

Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5

Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.

- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
Nonhealing Friable Nodule on the Distal Edge of the Toe
Nonhealing Friable Nodule on the Distal Edge of the Toe
A 37-year-old woman with no notable medical history presented to the dermatology clinic with a nonhealing wound on the left fifth toe of 10 month’s duration. The patient reported that the wound developed after burning the toe on an indoor space heater. Physical examination revealed a friable pink papule with a hemorrhagic crust. A biopsy of the lesion was performed.
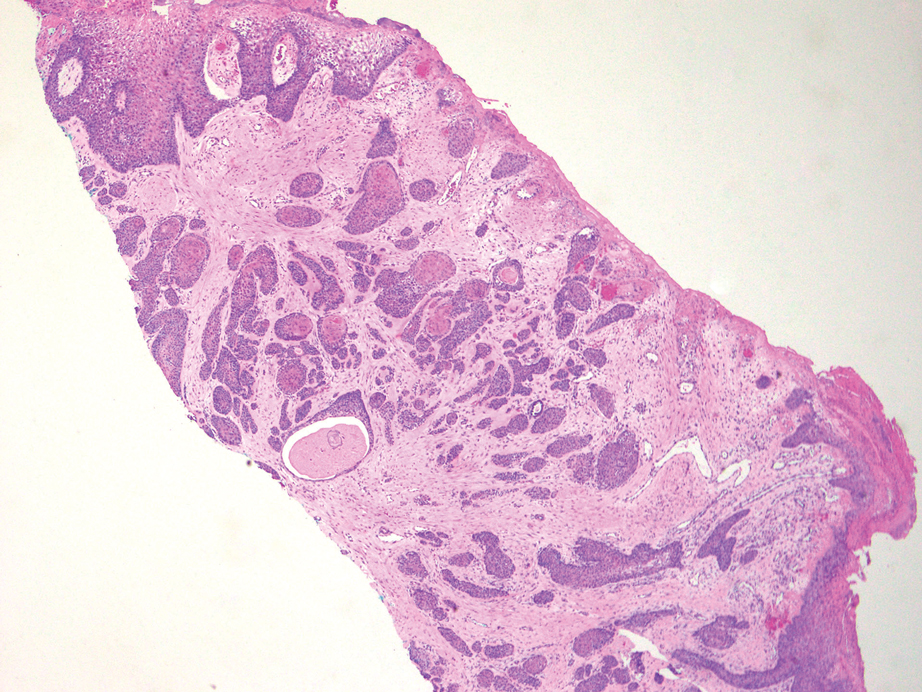
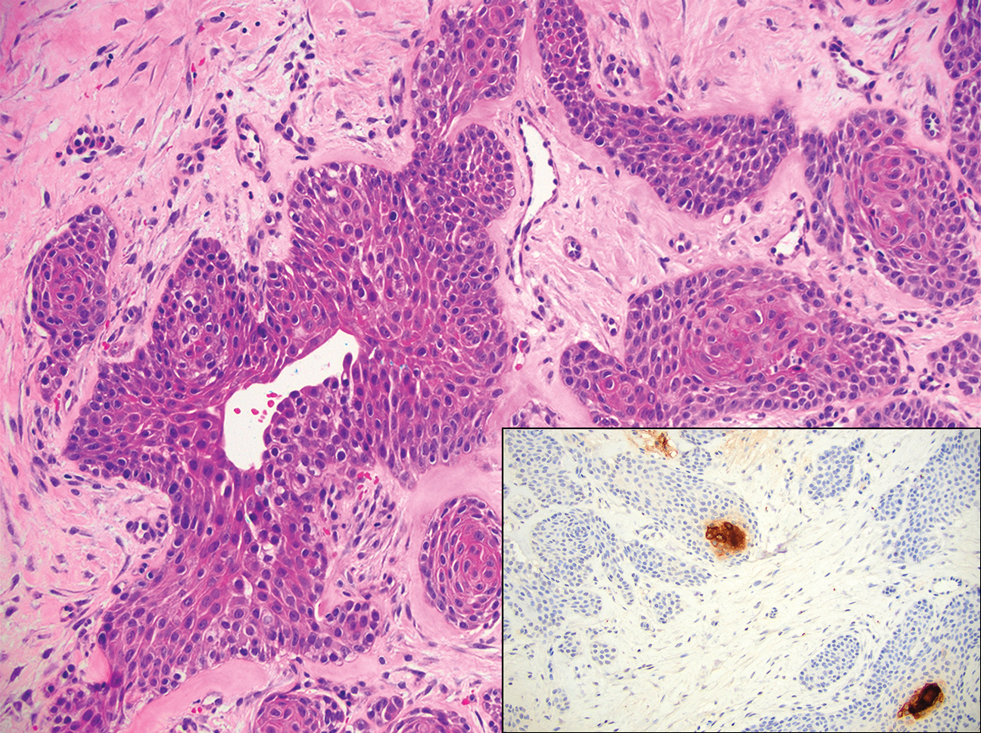
Survival Outcomes of Skin Adnexal Tumors: A National Cancer Database Analysis
Purpose
Skin adnexal tumors (SAT) include a group of benign and malignant appendageal tumors that arise from hair follicles, sebaceous glands, or sweat glands. They typically appear as small, painless bumps or nodules on the skin, and are more common in men compared to women. The 5-year overall SAT survival rate ranges from 74-90%. To better understand the differences in survival outcomes based on subtypes of SAT, the National Cancer Database (NCDB) was analyzed.
Methods
A retrospective cohort study of 11,627 patients with histologically confirmed SAT between 2004 and 2021 was conducted across 1,500 Commission on Cancer facilities located in the US and Puerto Rico. Demographic factors such as sex, age, and race were analyzed using Pearson Chi-squared tests, and survival outcomes were analyzed by Kaplan- Meier survival analysis. P value < 0.05 was considered statistically significant.
Results
Most patients with SAT were male (57.3%). The average age at diagnosis was 65.9 (SD=14.4, range 0-90). Of the patient sample, 87.2% were White, 7.6% Black, 2.5% Asian, and 2.7% other. Several subtypes disproportionately affected Black individuals, including apocrine adenocarcinoma (15.7%) and hidradenocarcinoma (13.6%). The estimated 5-year survival of SAT was 74.9% with an overall survival of 135.8 months (SE=1.1). Sebaceous carcinoma (which accounts for 41.8% of all cases) had the lowest average survival time of 119.6 months (SE=1.8), while digital papillary adenocarcinoma had the highest survival at around 183.5 months (SE=4.6).
Conclusions
This study supports a higher frequency of SAT among men. While White patients were more likely to get SAT overall, including the most common sebaceous carcinoma, Black race were associated with higher frequency of rarer subtypes. The average age of diagnosis of SAT mimics other non-melanoma skin cancers, but has a lower overall survival rate. Future studies should consider other risk factors that may be impacting the differences in survival outcomes to guide treatment and address health disparities among the various subtypes.
Purpose
Skin adnexal tumors (SAT) include a group of benign and malignant appendageal tumors that arise from hair follicles, sebaceous glands, or sweat glands. They typically appear as small, painless bumps or nodules on the skin, and are more common in men compared to women. The 5-year overall SAT survival rate ranges from 74-90%. To better understand the differences in survival outcomes based on subtypes of SAT, the National Cancer Database (NCDB) was analyzed.
Methods
A retrospective cohort study of 11,627 patients with histologically confirmed SAT between 2004 and 2021 was conducted across 1,500 Commission on Cancer facilities located in the US and Puerto Rico. Demographic factors such as sex, age, and race were analyzed using Pearson Chi-squared tests, and survival outcomes were analyzed by Kaplan- Meier survival analysis. P value < 0.05 was considered statistically significant.
Results
Most patients with SAT were male (57.3%). The average age at diagnosis was 65.9 (SD=14.4, range 0-90). Of the patient sample, 87.2% were White, 7.6% Black, 2.5% Asian, and 2.7% other. Several subtypes disproportionately affected Black individuals, including apocrine adenocarcinoma (15.7%) and hidradenocarcinoma (13.6%). The estimated 5-year survival of SAT was 74.9% with an overall survival of 135.8 months (SE=1.1). Sebaceous carcinoma (which accounts for 41.8% of all cases) had the lowest average survival time of 119.6 months (SE=1.8), while digital papillary adenocarcinoma had the highest survival at around 183.5 months (SE=4.6).
Conclusions
This study supports a higher frequency of SAT among men. While White patients were more likely to get SAT overall, including the most common sebaceous carcinoma, Black race were associated with higher frequency of rarer subtypes. The average age of diagnosis of SAT mimics other non-melanoma skin cancers, but has a lower overall survival rate. Future studies should consider other risk factors that may be impacting the differences in survival outcomes to guide treatment and address health disparities among the various subtypes.
Purpose
Skin adnexal tumors (SAT) include a group of benign and malignant appendageal tumors that arise from hair follicles, sebaceous glands, or sweat glands. They typically appear as small, painless bumps or nodules on the skin, and are more common in men compared to women. The 5-year overall SAT survival rate ranges from 74-90%. To better understand the differences in survival outcomes based on subtypes of SAT, the National Cancer Database (NCDB) was analyzed.
Methods
A retrospective cohort study of 11,627 patients with histologically confirmed SAT between 2004 and 2021 was conducted across 1,500 Commission on Cancer facilities located in the US and Puerto Rico. Demographic factors such as sex, age, and race were analyzed using Pearson Chi-squared tests, and survival outcomes were analyzed by Kaplan- Meier survival analysis. P value < 0.05 was considered statistically significant.
Results
Most patients with SAT were male (57.3%). The average age at diagnosis was 65.9 (SD=14.4, range 0-90). Of the patient sample, 87.2% were White, 7.6% Black, 2.5% Asian, and 2.7% other. Several subtypes disproportionately affected Black individuals, including apocrine adenocarcinoma (15.7%) and hidradenocarcinoma (13.6%). The estimated 5-year survival of SAT was 74.9% with an overall survival of 135.8 months (SE=1.1). Sebaceous carcinoma (which accounts for 41.8% of all cases) had the lowest average survival time of 119.6 months (SE=1.8), while digital papillary adenocarcinoma had the highest survival at around 183.5 months (SE=4.6).
Conclusions
This study supports a higher frequency of SAT among men. While White patients were more likely to get SAT overall, including the most common sebaceous carcinoma, Black race were associated with higher frequency of rarer subtypes. The average age of diagnosis of SAT mimics other non-melanoma skin cancers, but has a lower overall survival rate. Future studies should consider other risk factors that may be impacting the differences in survival outcomes to guide treatment and address health disparities among the various subtypes.
Reddish Nodule on the Left Shoulder
Reddish Nodule on the Left Shoulder
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.
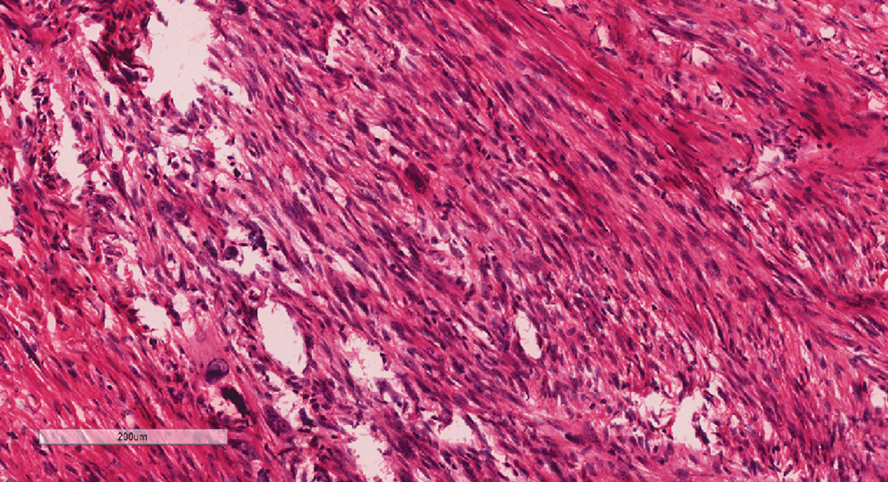
The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.

The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.

The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
Reddish Nodule on the Left Shoulder
Reddish Nodule on the Left Shoulder
A 20-year-old man presented to the dermatology clinic for evaluation of a slow-growing nodule on the left shoulder of 1 year’s duration. The patient reported a history of eczema since childhood, which had been treated by an external physician with cyclosporine and methotrexate; however, exact treatment records were unavailable as the patient had been treated at another institution. The eczema had been well controlled over the past year on topical steroids alone. The nodule was asymptomatic, and the patient denied any history of trauma or acne at the affected site. He also denied any family history of similar nodules or other notable skin findings. Physical examination revealed a well circumscribed, 15×12-mm, firm, flesh-colored to reddish nodule on the left shoulder with a slightly whitish center. An excisional biopsy was performed.
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12
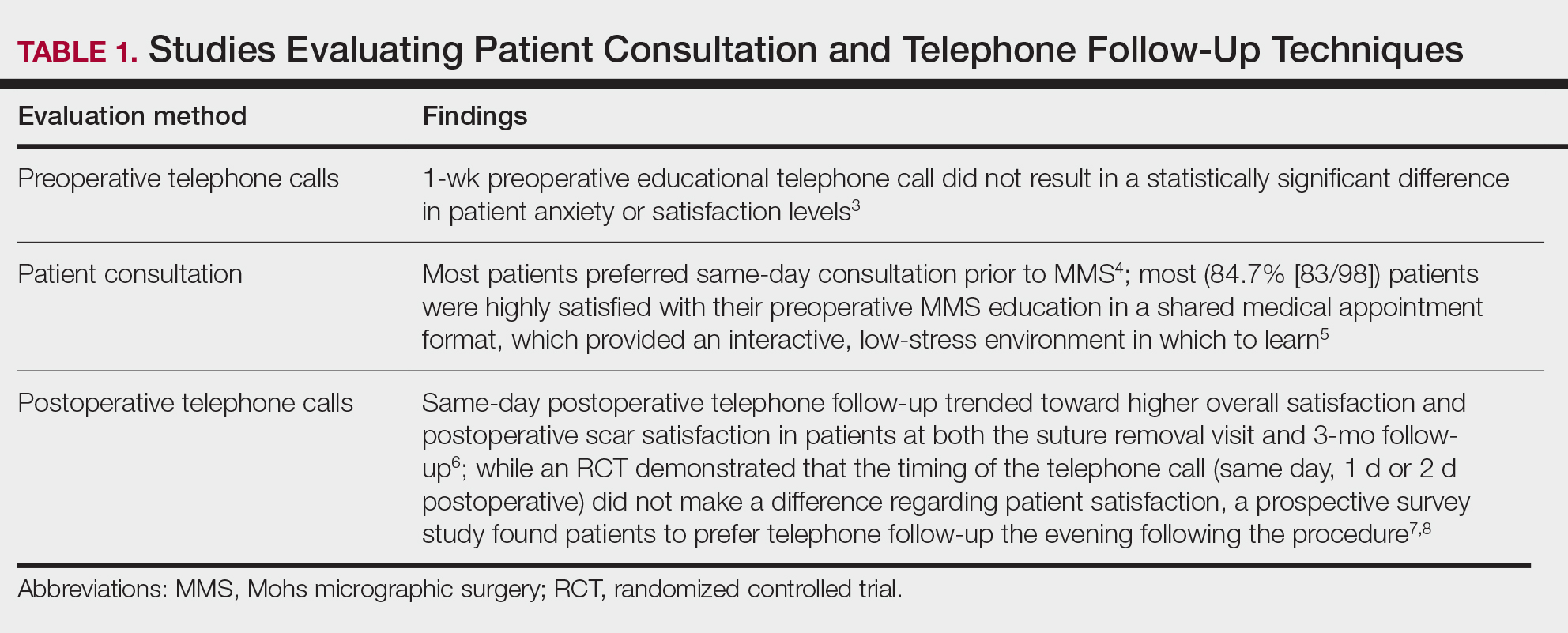
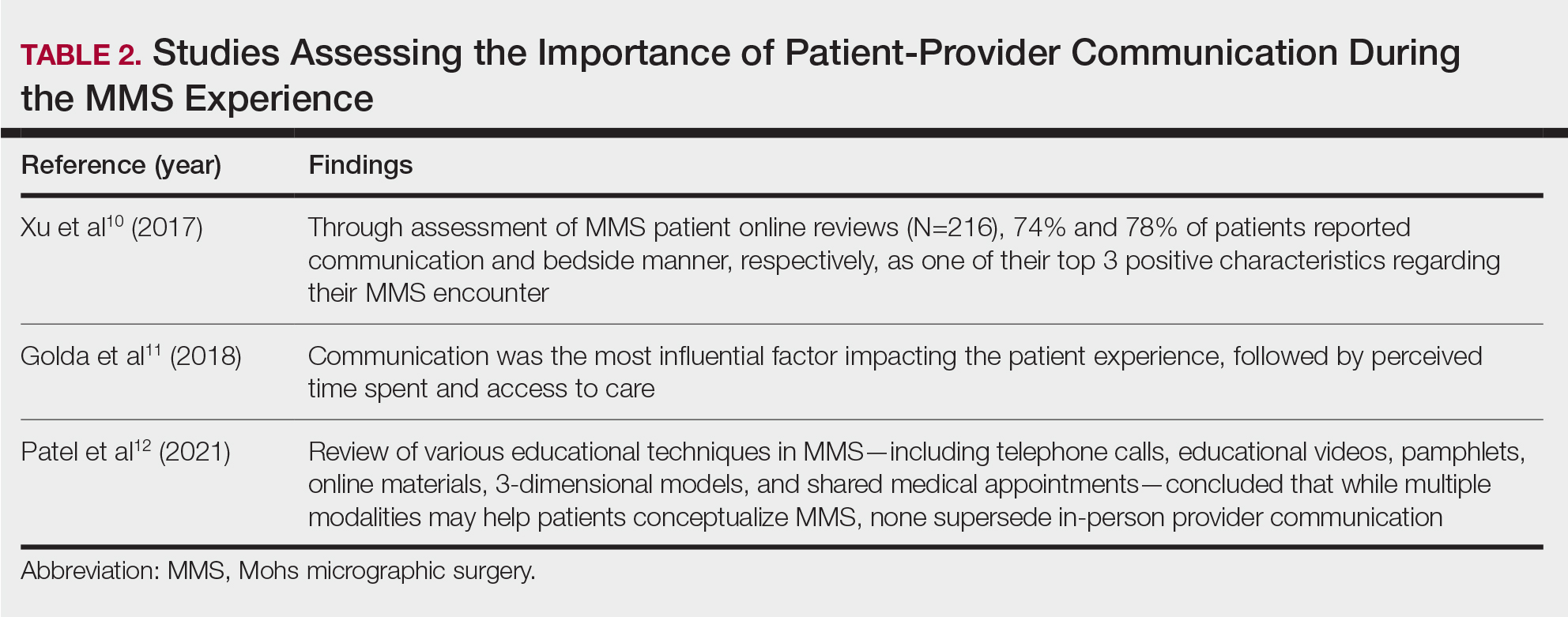
Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18
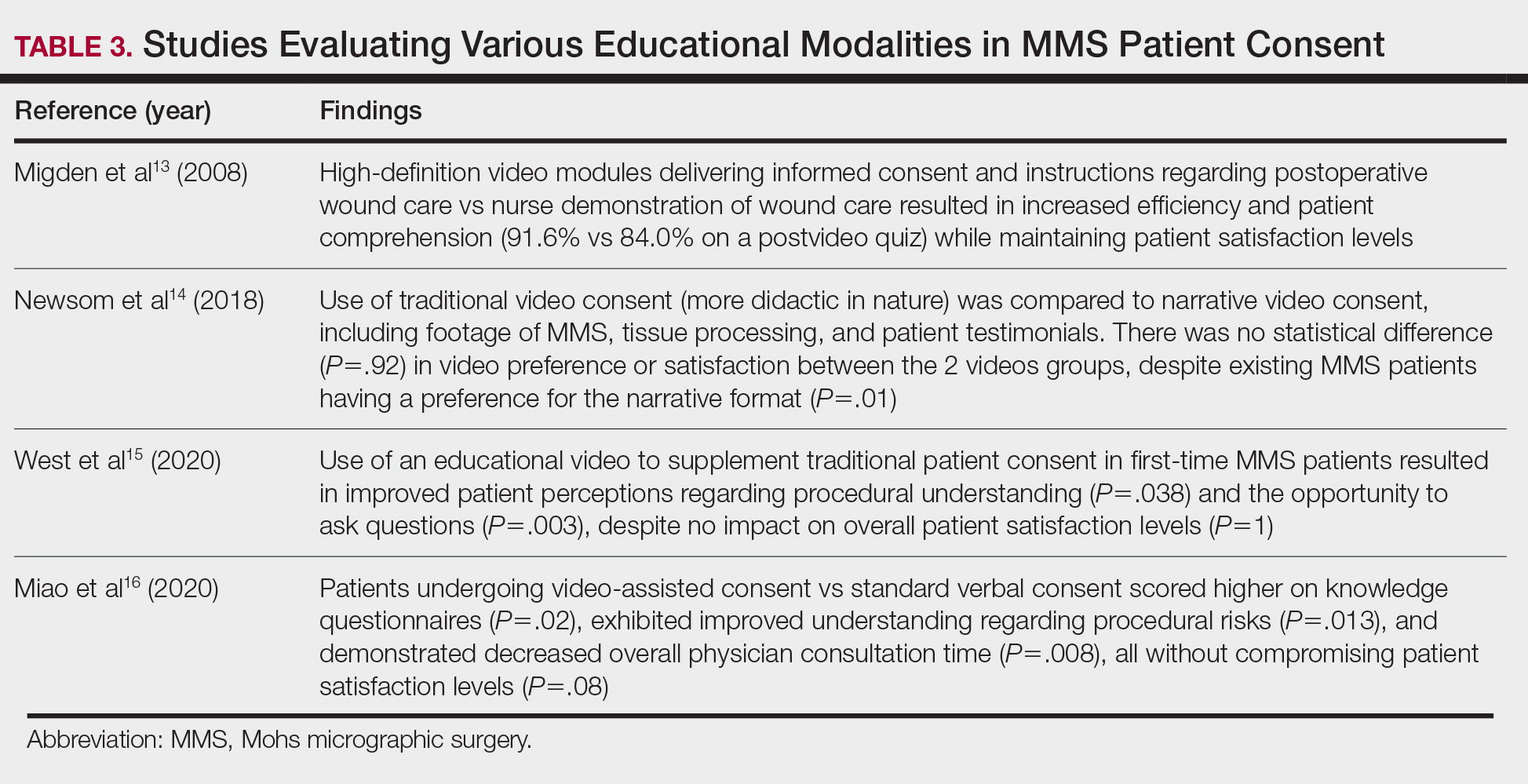
Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24
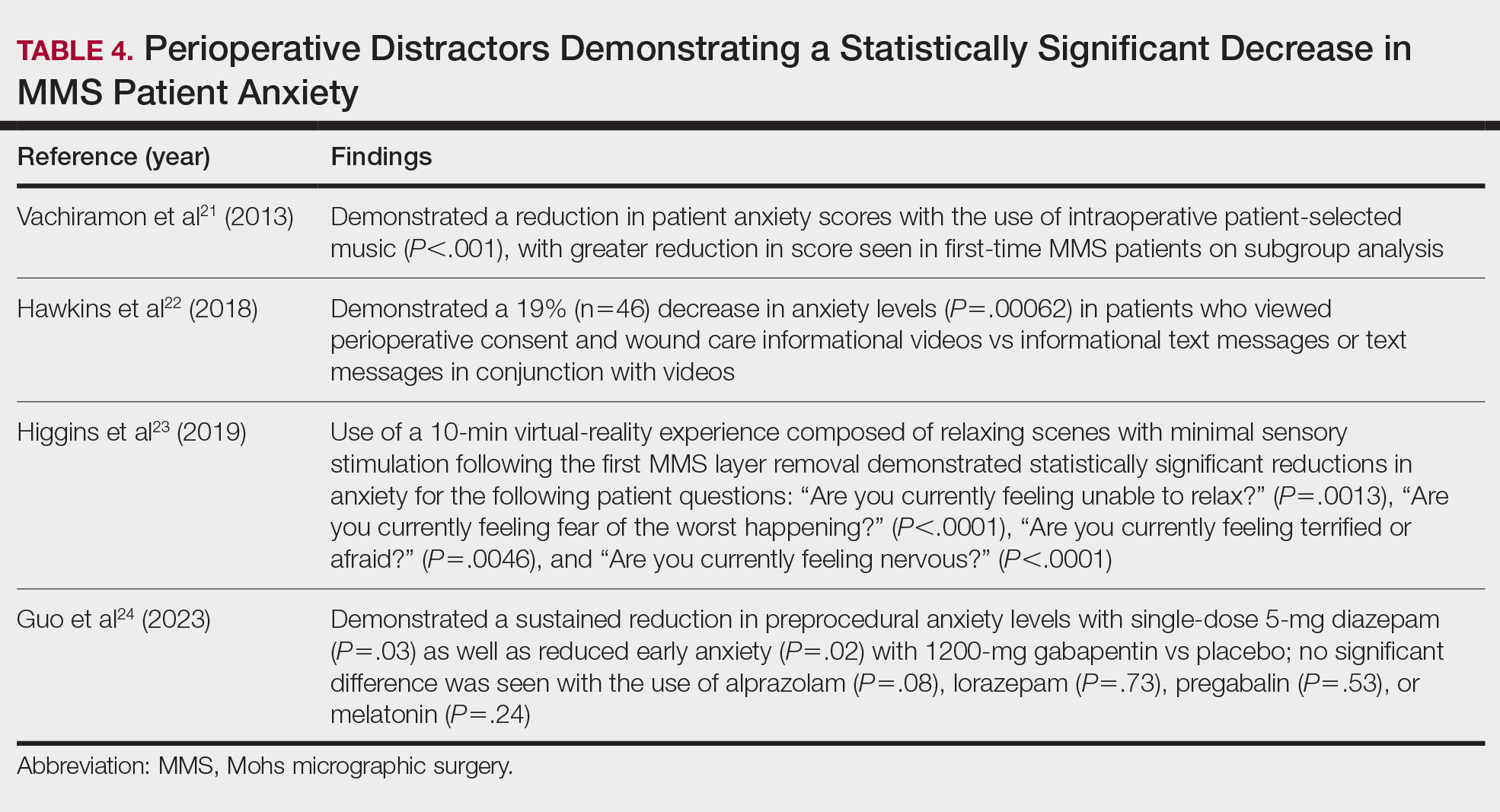
Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32
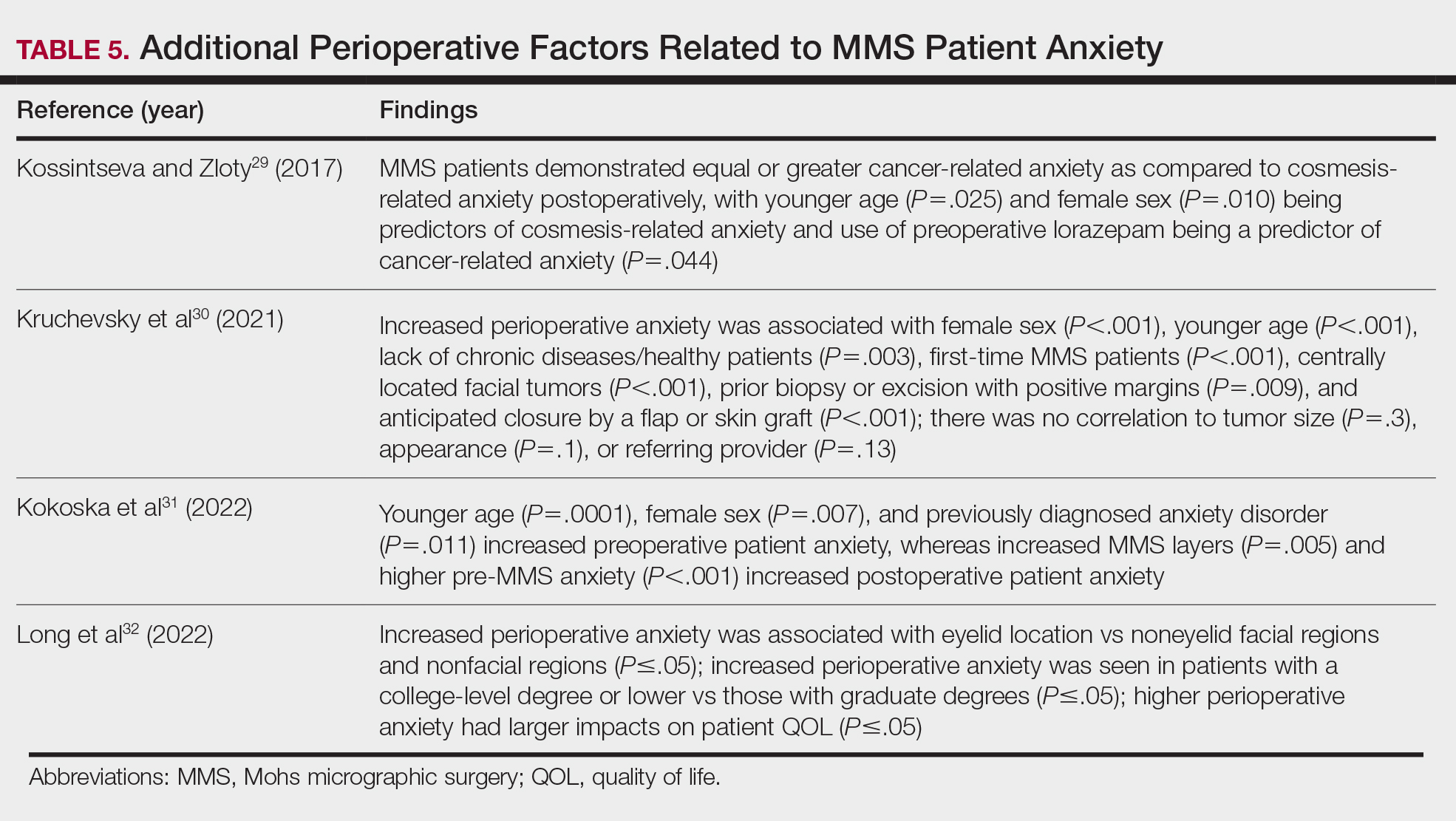
Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
PRACTICE POINTS
- When patients are treated with Mohs micrographic surgery (MMS), thorough in-person dialogue augmented by pre- and same-day telephone follow-ups can help them feel heard and better supported, even though follow-up calls alone may not drive satisfaction scores.
- Increased awareness and implementation of the various factors influencing patient satisfaction and quality of life in MMS can enhance clinical practice and improve patient experiences, with potential impacts on compliance, psychosocial well-being, medical outcomes, and physician reimbursement.
- Patient satisfaction and procedural understanding can be improved with video and visual-based education. Anxiety-reducing methods help lower perioperative stress.
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.
For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
PRACTICE POINTS
- There are numerous field-directed therapies for actinic keratoses (AKs); however, efficacy and tolerability vary among the available treatments.
- Diclofenac gel 1% is an affordable option that could potentially increase accessibility and decrease cost of field therapy for the treatment of AKs, while maintaining therapeutic efficacy.