User login
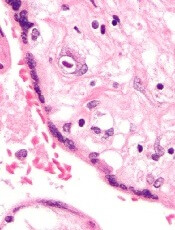
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to ATA230 for the treatment of congenital cytomegalovirus (CMV) infection.
ATA230 is an allogeneic T-cell immunotherapy targeting antigens expressed by CMV.
Rare pediatric disease designation is granted to drugs that show promise to treat orphan diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 and younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, a drug developer with rare pediatric disease designation that receives an approval of a new drug application is eligible for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
The company developing ATA230 is Atara Biotherapeutics, Inc. The company also received orphan designation for ATA230.
ATA230 trials
ATA230 has been investigated in phase 1 and 2 studies of immunocompromised patients with CMV viremia or disease who are refractory or resistant to antiviral drug treatment in the post-transplant setting.
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to ATA230 for the treatment of congenital cytomegalovirus (CMV) infection.
ATA230 is an allogeneic T-cell immunotherapy targeting antigens expressed by CMV.
Rare pediatric disease designation is granted to drugs that show promise to treat orphan diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 and younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, a drug developer with rare pediatric disease designation that receives an approval of a new drug application is eligible for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
The company developing ATA230 is Atara Biotherapeutics, Inc. The company also received orphan designation for ATA230.
ATA230 trials
ATA230 has been investigated in phase 1 and 2 studies of immunocompromised patients with CMV viremia or disease who are refractory or resistant to antiviral drug treatment in the post-transplant setting.
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to ATA230 for the treatment of congenital cytomegalovirus (CMV) infection.
ATA230 is an allogeneic T-cell immunotherapy targeting antigens expressed by CMV.
Rare pediatric disease designation is granted to drugs that show promise to treat orphan diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 and younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, a drug developer with rare pediatric disease designation that receives an approval of a new drug application is eligible for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
The company developing ATA230 is Atara Biotherapeutics, Inc. The company also received orphan designation for ATA230.
ATA230 trials
ATA230 has been investigated in phase 1 and 2 studies of immunocompromised patients with CMV viremia or disease who are refractory or resistant to antiviral drug treatment in the post-transplant setting.
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230. ![]()