User login
The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).

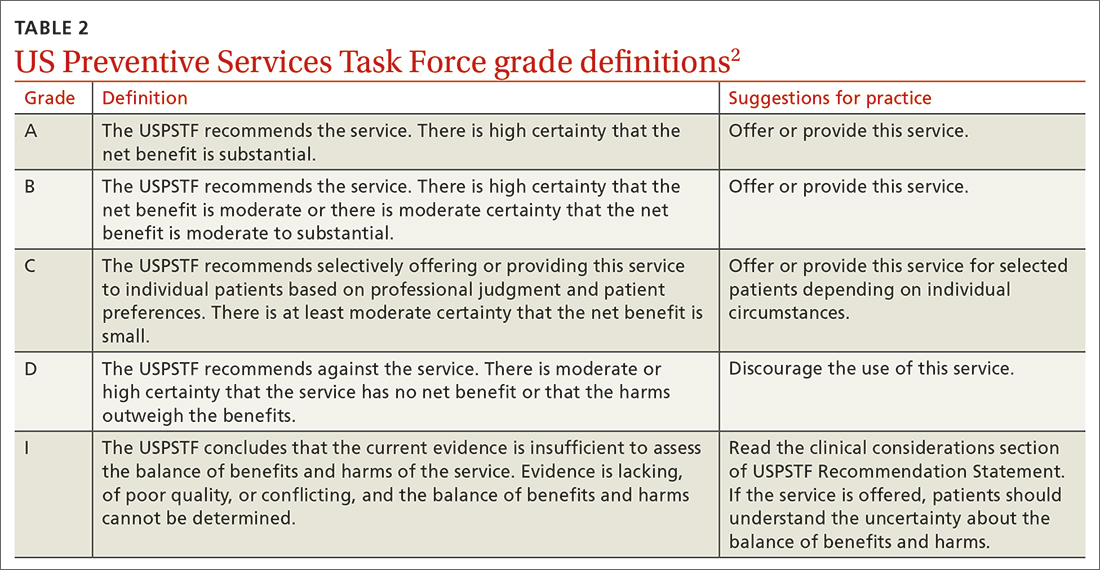
A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)
What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...
The recommendation pertains to the initiation of aspirin, not the continuation or discontinuation for those who have been using aspirin without complications. The USPSTF suggests that the dose of aspirin, if used, should be 81 mg and that it should not be continued past age 75 years. A more detailed discussion of this recommendation and some of its clinical considerations is contained in a recent Practice Alert.5
“D” is for “don’t”(with a few caveats)
Avoiding unnecessary or harmful testing and treatments is just as important as offering preventive services of proven benefit. Those practices listed in TABLE 11 with a “D” recommendation should be avoided in practice.
However, it is worth mentioning that, while postmenopausal hormone replacement therapy should not be prescribed for the prevention of chronic conditions, this does not mean it should not be used to alleviate postmenopausal vasomotor symptoms—albeit for a limited period of time.
Also, it is important to appreciate the difference between screening and diagnostic tests. When the USPSTF recommends for or against screening, they are referring to the practice in asymptomatic people. The recommendation does not pertain to diagnostic testing to confirm or rule out a condition in a person with symptoms suggestive of a condition. Thus, the recommendation against screening adults for chronic obstructive pulmonary disease applies only to those without symptoms.
Be selective with services graded “C” or “I”
The USPSTF recommendations that require the most clinical judgment and are the most difficult to implement are those with a “C.” Few individuals will benefit from these interventions, and those most likely to benefit usually are described in the clinical considerations that accompany the recommendation. These interventions are time consuming and may be subject to insurance co-pays and deductibles. All 3 “C” recommendations made in 2022 (see TABLE 11) pertained to the prevention of CVD, still the leading cause of death in the United States.
Continue it: As "I" statement is not the same...
An “I” statement is not the same as a recommendation against the service—but if the service is offered, both the physician and the patient should understand the uncertainty involved. The services the USPSTF has determined lack sufficient evidence of benefits and/or harms are often recommended by other organizations—and in fact, the use of the “I” statement distinguishes the USPSTF from other clinical guideline groups.
If good evidence does not exist, the USPSTF will not make a recommendation. This is the main reason that, when the USPSTF reevaluates a topic (about every 6 to 7 years), they seldom make significant changes to their previous recommendations. Good evidence tends to survive the test of time.
However, adherence to this standard can cause the USPSTF to lag behind other guideline producers for some commonly used interventions. This delay can be considered a detriment if the intervention eventually proves to be effective, but it is a benefit if the intervention proves to be nonbeneficial or even harmful.
Putting recommendations into best practice
Given the time constraints in primary care practice, the most efficient way of providing high-quality, clinical preventive services is by implementing USPSTF “A” and “B” recommendations, being very selective about who receives an intervention with a “C” recommendation or “I” statement, and avoiding interventions with a “D” recommendation.
BREAKING NEWS
At press time, the USPSTF issued a draft recommendation statement that women begin receiving biennial mammograms starting at age 40 years (through age 74 years). For more, see: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#fullrecommendation start
1. USPSTF. Recommendation topics. Accessed April 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Grade definitions. Updated October 2018. Accessed April 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
3. Campos-Outcalt D. Whom to screen for anxiety and depression: updated USPSTF recommendations. J Fam Pract. 2022;71:423-425. doi: 10.12788/jfp.0519
4. USPSTF. Aspirin use to prevent cardiovascular disease: USPSTF recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
5. Campos-Outcalt D. USPSTF updates recommendations on aspirin and CVD. J Fam Pract. 2022;71:262-264. doi: 10.12788/jfp.0452
The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).

A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)

What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...
The recommendation pertains to the initiation of aspirin, not the continuation or discontinuation for those who have been using aspirin without complications. The USPSTF suggests that the dose of aspirin, if used, should be 81 mg and that it should not be continued past age 75 years. A more detailed discussion of this recommendation and some of its clinical considerations is contained in a recent Practice Alert.5
“D” is for “don’t”(with a few caveats)
Avoiding unnecessary or harmful testing and treatments is just as important as offering preventive services of proven benefit. Those practices listed in TABLE 11 with a “D” recommendation should be avoided in practice.
However, it is worth mentioning that, while postmenopausal hormone replacement therapy should not be prescribed for the prevention of chronic conditions, this does not mean it should not be used to alleviate postmenopausal vasomotor symptoms—albeit for a limited period of time.
Also, it is important to appreciate the difference between screening and diagnostic tests. When the USPSTF recommends for or against screening, they are referring to the practice in asymptomatic people. The recommendation does not pertain to diagnostic testing to confirm or rule out a condition in a person with symptoms suggestive of a condition. Thus, the recommendation against screening adults for chronic obstructive pulmonary disease applies only to those without symptoms.
Be selective with services graded “C” or “I”
The USPSTF recommendations that require the most clinical judgment and are the most difficult to implement are those with a “C.” Few individuals will benefit from these interventions, and those most likely to benefit usually are described in the clinical considerations that accompany the recommendation. These interventions are time consuming and may be subject to insurance co-pays and deductibles. All 3 “C” recommendations made in 2022 (see TABLE 11) pertained to the prevention of CVD, still the leading cause of death in the United States.
Continue it: As "I" statement is not the same...
An “I” statement is not the same as a recommendation against the service—but if the service is offered, both the physician and the patient should understand the uncertainty involved. The services the USPSTF has determined lack sufficient evidence of benefits and/or harms are often recommended by other organizations—and in fact, the use of the “I” statement distinguishes the USPSTF from other clinical guideline groups.
If good evidence does not exist, the USPSTF will not make a recommendation. This is the main reason that, when the USPSTF reevaluates a topic (about every 6 to 7 years), they seldom make significant changes to their previous recommendations. Good evidence tends to survive the test of time.
However, adherence to this standard can cause the USPSTF to lag behind other guideline producers for some commonly used interventions. This delay can be considered a detriment if the intervention eventually proves to be effective, but it is a benefit if the intervention proves to be nonbeneficial or even harmful.
Putting recommendations into best practice
Given the time constraints in primary care practice, the most efficient way of providing high-quality, clinical preventive services is by implementing USPSTF “A” and “B” recommendations, being very selective about who receives an intervention with a “C” recommendation or “I” statement, and avoiding interventions with a “D” recommendation.
BREAKING NEWS
At press time, the USPSTF issued a draft recommendation statement that women begin receiving biennial mammograms starting at age 40 years (through age 74 years). For more, see: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#fullrecommendation start
The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).

A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)

What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...
The recommendation pertains to the initiation of aspirin, not the continuation or discontinuation for those who have been using aspirin without complications. The USPSTF suggests that the dose of aspirin, if used, should be 81 mg and that it should not be continued past age 75 years. A more detailed discussion of this recommendation and some of its clinical considerations is contained in a recent Practice Alert.5
“D” is for “don’t”(with a few caveats)
Avoiding unnecessary or harmful testing and treatments is just as important as offering preventive services of proven benefit. Those practices listed in TABLE 11 with a “D” recommendation should be avoided in practice.
However, it is worth mentioning that, while postmenopausal hormone replacement therapy should not be prescribed for the prevention of chronic conditions, this does not mean it should not be used to alleviate postmenopausal vasomotor symptoms—albeit for a limited period of time.
Also, it is important to appreciate the difference between screening and diagnostic tests. When the USPSTF recommends for or against screening, they are referring to the practice in asymptomatic people. The recommendation does not pertain to diagnostic testing to confirm or rule out a condition in a person with symptoms suggestive of a condition. Thus, the recommendation against screening adults for chronic obstructive pulmonary disease applies only to those without symptoms.
Be selective with services graded “C” or “I”
The USPSTF recommendations that require the most clinical judgment and are the most difficult to implement are those with a “C.” Few individuals will benefit from these interventions, and those most likely to benefit usually are described in the clinical considerations that accompany the recommendation. These interventions are time consuming and may be subject to insurance co-pays and deductibles. All 3 “C” recommendations made in 2022 (see TABLE 11) pertained to the prevention of CVD, still the leading cause of death in the United States.
Continue it: As "I" statement is not the same...
An “I” statement is not the same as a recommendation against the service—but if the service is offered, both the physician and the patient should understand the uncertainty involved. The services the USPSTF has determined lack sufficient evidence of benefits and/or harms are often recommended by other organizations—and in fact, the use of the “I” statement distinguishes the USPSTF from other clinical guideline groups.
If good evidence does not exist, the USPSTF will not make a recommendation. This is the main reason that, when the USPSTF reevaluates a topic (about every 6 to 7 years), they seldom make significant changes to their previous recommendations. Good evidence tends to survive the test of time.
However, adherence to this standard can cause the USPSTF to lag behind other guideline producers for some commonly used interventions. This delay can be considered a detriment if the intervention eventually proves to be effective, but it is a benefit if the intervention proves to be nonbeneficial or even harmful.
Putting recommendations into best practice
Given the time constraints in primary care practice, the most efficient way of providing high-quality, clinical preventive services is by implementing USPSTF “A” and “B” recommendations, being very selective about who receives an intervention with a “C” recommendation or “I” statement, and avoiding interventions with a “D” recommendation.
BREAKING NEWS
At press time, the USPSTF issued a draft recommendation statement that women begin receiving biennial mammograms starting at age 40 years (through age 74 years). For more, see: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#fullrecommendation start
1. USPSTF. Recommendation topics. Accessed April 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Grade definitions. Updated October 2018. Accessed April 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
3. Campos-Outcalt D. Whom to screen for anxiety and depression: updated USPSTF recommendations. J Fam Pract. 2022;71:423-425. doi: 10.12788/jfp.0519
4. USPSTF. Aspirin use to prevent cardiovascular disease: USPSTF recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
5. Campos-Outcalt D. USPSTF updates recommendations on aspirin and CVD. J Fam Pract. 2022;71:262-264. doi: 10.12788/jfp.0452
1. USPSTF. Recommendation topics. Accessed April 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Grade definitions. Updated October 2018. Accessed April 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
3. Campos-Outcalt D. Whom to screen for anxiety and depression: updated USPSTF recommendations. J Fam Pract. 2022;71:423-425. doi: 10.12788/jfp.0519
4. USPSTF. Aspirin use to prevent cardiovascular disease: USPSTF recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
5. Campos-Outcalt D. USPSTF updates recommendations on aspirin and CVD. J Fam Pract. 2022;71:262-264. doi: 10.12788/jfp.0452
