User login
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
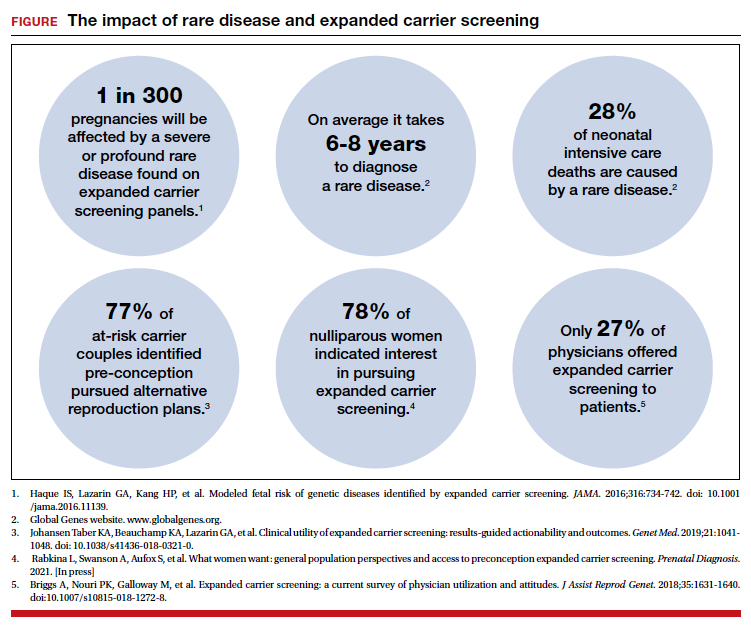
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.
Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.

