User login
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
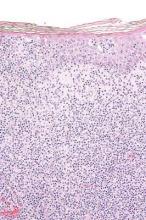
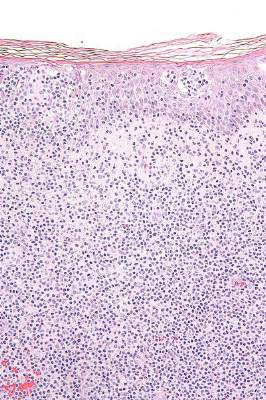
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
FROM BLOOD
Key clinical point: Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL).
Major finding: In total, 11 of 12 patients had significant improvement: 2 had resolution of all evidence of disease, and 9 experienced improvement greater than or equal to 50%.
Data source: The open-label, phase I trial evaluated 12 patients with early stage CTCL.
Disclosures: Dr. Rook and one coauthor have patents pending on HTS in cutaneous lymphoma. His coauthor is employed by Adaptive Biotechnologies.