User login
To the Editor:
Orificial tuberculosis (OT) constitutes 2% of cutaneous tuberculosis cases and 0.01% to 1% of all clinical presentations of tuberculosis.1 It is clinically classified as primary or secondary OT. In primary OT, the oral mucosa is the initial site of the infection without any internal organ involvement.2 This form is more prevalent among men and young adults.1,3 Secondary OT is the cutaneous tuberculosis type that occurs in patients with internal organ tuberculosis from autoinoculation of bacilli to the orificial area. It is more common and usually affects elderly patients.2-4 We present the development of primary OT in an immunocompetent woman.
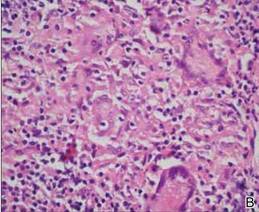
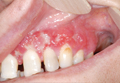
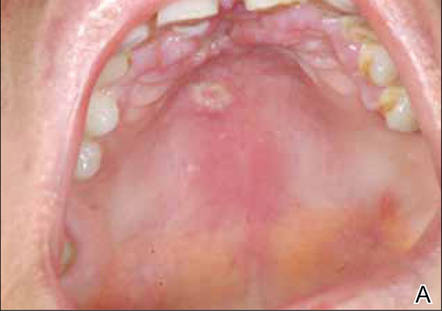
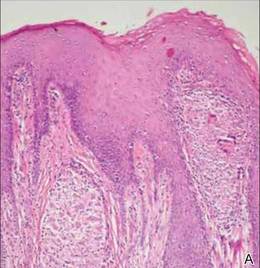
A 51-year-old woman was admitted with painful enlarging oral ulcers of 1 year’s duration. There was no history of tuberculosis infection, dental trauma, or smoking habit prior to the development of oral ulcers, and no family history of tuberculosis. On dermatological examination white-yellow indurated ulcers with 1×1.5-cm irregular margins located on the hard palate and gingiva were observed (Figures 1A and 1B). Oral hygiene was good. There was no regional lym-phadenopathy on palpation. Physical findings were normal. The histopathology of the biopsy from the gingival ulcer revealed noncaseating granulomatous inflammation in the dermis (Figure 2). Ziehl-Neelsen and periodic acid–Schiff stains were negative for acid-fast bacilli and fungi, respectively.
| 
| |

| 
| |
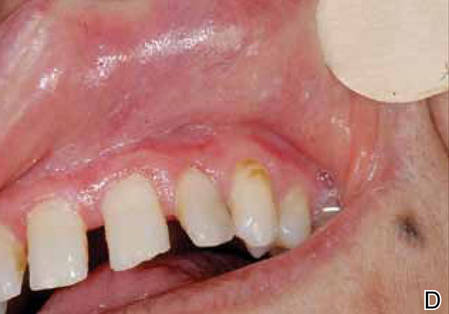
| Figure 1. White-yellow indurated ulcers with 1×1.5-cm irregular margins located on the hard palate (A) and gingiva (B). Resolution of lesions on the hard palate (C) and the gingiva (D) after 9 months of antituberculosis therapy. | ||
Laboratory results from blood chemistry, complete blood cell count, erythrocyte sedimentation rate, C-reactive protein level, and urine analysis, as well as titers of serum immunoglobulin, antineutrophil cytoplasmic antibodies, and antinuclear antibodies, were within reference range. Human immunodeficiency virus serology was negative. Chest radiography and ultrasonography of the abdomen revealed no abnormalities. A purified protein derivative (tuberculin) test showed an induration of 20 mm. Mycobacterium tuberculosis grew on the culture of the tissue specimen.
|
|
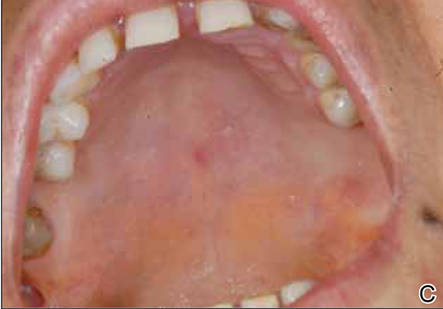
The patient was diagnosed with primary OT and treated with isoniazid (300 mg daily), rifampin (600 mg daily), ethambutol (1500 mg daily), and pyrazinamide (2000 mg daily). At 2 months of therapy the lesions started to heal and showed complete resolution at the end of 9 months of treatment (Figures 1C and 1D). There was no recurrence at 2-year follow-up.
Orificial tuberculosis is a rare form of cutaneous tuberculosis. Therefore, it is regarded as a “forgotten disease” in the literature.5 The pathogenesis of OT has not been clearly defined. The intact mucous membrane, thickened squamous epithelium, cleansing and antibacterial function of saliva, and presence of saprophytes act as protective mechanisms against penetration of mycobacteria. Some factors such as poor oral hygiene, smoking, local trauma, or presence of a dental cyst or an abscess may predispose the direct mucosal inoculation of the tuberculosis bacilli.1,2,6 Orificial tuberculosis may develop as an opportunistic infection in 1.33% of human immunodeficiency virus patients.7 However, our patient had no prior trauma or known predisposing factors.
The common presentation of OT is an ulcerative lesion with irregular and well-delineated margins with a yellowish granular base. A vesicle, papule, or nodule may precede the ulcers. The presence of yellow satellite nodules around the lesion is characteristic for OT.1,2,6 Although there were no satellite nodules around the ulcerations in our case, the lesions had a yellowish granular base and irregular, well-delineated margins.
The tongue, gingiva, lips, tonsils, and epiglottis are the most common sites of involvement.2,5,8 Hard palate involvement rarely has been reported, even in immunosuppressed patients.7 Enlarged painful cervical lymph nodes may accompany the disease. Most patients with OT have been reported to have active pulmonary tuberculosis at the time of diagnosis.6,8 Thus, internal organ involvement, particularly the pulmonary system, should be checked in patients with OT. In our patient, the hard palate was involved together with the gingiva despite the absence of immunosuppression. No internal organ involvement was found in the systemic evaluation.
Orificial tuberculosis is a form of cutaneous tuberculosis that is difficult to diagnose because of the varying nature of clinical features, failure of growth of M tuberculosis on culture, and rarity of the disease.1,2,6 As in our case, biopsies may not always exhibit a caseous necrosis, which is specific to tuberculosis.6,7 Thus, it may be difficult to distinguish oral cavity tuberculosis from conditions demonstrating oral ulcers such as bullous diseases, trauma, fungal diseases, syphilis, sarcoidosis, or squamous cell carcinoma by evaluating only signs and symptoms.6 Clinical suspicion is the first and foremost step in the diagnostic process of OT. In our patient, OT was suspected in the differential diagnosis because the resistant oral ulcerations showed the most common presentation of OT: irregular and well-delineated margins and a yellowish granular base. By considering tuberculosis within the differential diagnosis in our patient, microbiologic cultivation was performed from the oral mucosa and accurate diagnosis was established by determination of the pathogen’s growth in the culture.
Because of the increased incidence of tuberculosis and unusual manifestations, clinicians may easily overlook OT. It should be considered in the differential diagnosis of resistant nodules or ulcers of the oral cavity.
1. Kiliç A, Gül U, Gönül M, et al. Orificial tuberculosis of the lip: a case report and review of the literature. Int J Dermatol. 2009;48:178-180.
2. Ito FA, de Andrade CR, Vargas PA, et al. Primary tuberculosis of the oral cavity. Oral Dis. 2005;11:50-53.
3. Dixit R, Sharma S, Nuwal P. Tuberculosis of oral cavity. Indian J Tuberc. 2008;55:51-53.
4. Smolka W, Burger H, Iizuka T, et al. Primary tuberculosis of the oral cavity in an elderly nonimmunosuppressed patient: case report and review of the literature. Arch Otolaryngol Head Neck Surg. 2008;134:1107-1109.
5. Rodrigues G, Carnelio S, Valliathan M. Primary isolated gingival tuberculosis. Braz J Infect Dis. 2007;11:172-173.
6. Vilar FC, de Souza A, Moya MJ, et al. Atypical oral lesion in a patient with pulmonary tuberculosis. Int J Dermatol. 2009;48:910-912.
7. Kakisi OK, Kechagia AS, Kakisis IK, et al. Tuberculosis of the oral cavity: a systematic review. Eur J Oral Sci. 2010;118:103-109.
8. Eng HL, Lu SY, Yang CH, et al. Oral tuberculosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:415-420.
To the Editor:
Orificial tuberculosis (OT) constitutes 2% of cutaneous tuberculosis cases and 0.01% to 1% of all clinical presentations of tuberculosis.1 It is clinically classified as primary or secondary OT. In primary OT, the oral mucosa is the initial site of the infection without any internal organ involvement.2 This form is more prevalent among men and young adults.1,3 Secondary OT is the cutaneous tuberculosis type that occurs in patients with internal organ tuberculosis from autoinoculation of bacilli to the orificial area. It is more common and usually affects elderly patients.2-4 We present the development of primary OT in an immunocompetent woman.
A 51-year-old woman was admitted with painful enlarging oral ulcers of 1 year’s duration. There was no history of tuberculosis infection, dental trauma, or smoking habit prior to the development of oral ulcers, and no family history of tuberculosis. On dermatological examination white-yellow indurated ulcers with 1×1.5-cm irregular margins located on the hard palate and gingiva were observed (Figures 1A and 1B). Oral hygiene was good. There was no regional lym-phadenopathy on palpation. Physical findings were normal. The histopathology of the biopsy from the gingival ulcer revealed noncaseating granulomatous inflammation in the dermis (Figure 2). Ziehl-Neelsen and periodic acid–Schiff stains were negative for acid-fast bacilli and fungi, respectively.

| 
| |

| 
| |
| Figure 1. White-yellow indurated ulcers with 1×1.5-cm irregular margins located on the hard palate (A) and gingiva (B). Resolution of lesions on the hard palate (C) and the gingiva (D) after 9 months of antituberculosis therapy. | ||
Laboratory results from blood chemistry, complete blood cell count, erythrocyte sedimentation rate, C-reactive protein level, and urine analysis, as well as titers of serum immunoglobulin, antineutrophil cytoplasmic antibodies, and antinuclear antibodies, were within reference range. Human immunodeficiency virus serology was negative. Chest radiography and ultrasonography of the abdomen revealed no abnormalities. A purified protein derivative (tuberculin) test showed an induration of 20 mm. Mycobacterium tuberculosis grew on the culture of the tissue specimen.

|
|
The patient was diagnosed with primary OT and treated with isoniazid (300 mg daily), rifampin (600 mg daily), ethambutol (1500 mg daily), and pyrazinamide (2000 mg daily). At 2 months of therapy the lesions started to heal and showed complete resolution at the end of 9 months of treatment (Figures 1C and 1D). There was no recurrence at 2-year follow-up.
Orificial tuberculosis is a rare form of cutaneous tuberculosis. Therefore, it is regarded as a “forgotten disease” in the literature.5 The pathogenesis of OT has not been clearly defined. The intact mucous membrane, thickened squamous epithelium, cleansing and antibacterial function of saliva, and presence of saprophytes act as protective mechanisms against penetration of mycobacteria. Some factors such as poor oral hygiene, smoking, local trauma, or presence of a dental cyst or an abscess may predispose the direct mucosal inoculation of the tuberculosis bacilli.1,2,6 Orificial tuberculosis may develop as an opportunistic infection in 1.33% of human immunodeficiency virus patients.7 However, our patient had no prior trauma or known predisposing factors.
The common presentation of OT is an ulcerative lesion with irregular and well-delineated margins with a yellowish granular base. A vesicle, papule, or nodule may precede the ulcers. The presence of yellow satellite nodules around the lesion is characteristic for OT.1,2,6 Although there were no satellite nodules around the ulcerations in our case, the lesions had a yellowish granular base and irregular, well-delineated margins.
The tongue, gingiva, lips, tonsils, and epiglottis are the most common sites of involvement.2,5,8 Hard palate involvement rarely has been reported, even in immunosuppressed patients.7 Enlarged painful cervical lymph nodes may accompany the disease. Most patients with OT have been reported to have active pulmonary tuberculosis at the time of diagnosis.6,8 Thus, internal organ involvement, particularly the pulmonary system, should be checked in patients with OT. In our patient, the hard palate was involved together with the gingiva despite the absence of immunosuppression. No internal organ involvement was found in the systemic evaluation.
Orificial tuberculosis is a form of cutaneous tuberculosis that is difficult to diagnose because of the varying nature of clinical features, failure of growth of M tuberculosis on culture, and rarity of the disease.1,2,6 As in our case, biopsies may not always exhibit a caseous necrosis, which is specific to tuberculosis.6,7 Thus, it may be difficult to distinguish oral cavity tuberculosis from conditions demonstrating oral ulcers such as bullous diseases, trauma, fungal diseases, syphilis, sarcoidosis, or squamous cell carcinoma by evaluating only signs and symptoms.6 Clinical suspicion is the first and foremost step in the diagnostic process of OT. In our patient, OT was suspected in the differential diagnosis because the resistant oral ulcerations showed the most common presentation of OT: irregular and well-delineated margins and a yellowish granular base. By considering tuberculosis within the differential diagnosis in our patient, microbiologic cultivation was performed from the oral mucosa and accurate diagnosis was established by determination of the pathogen’s growth in the culture.
Because of the increased incidence of tuberculosis and unusual manifestations, clinicians may easily overlook OT. It should be considered in the differential diagnosis of resistant nodules or ulcers of the oral cavity.
To the Editor:
Orificial tuberculosis (OT) constitutes 2% of cutaneous tuberculosis cases and 0.01% to 1% of all clinical presentations of tuberculosis.1 It is clinically classified as primary or secondary OT. In primary OT, the oral mucosa is the initial site of the infection without any internal organ involvement.2 This form is more prevalent among men and young adults.1,3 Secondary OT is the cutaneous tuberculosis type that occurs in patients with internal organ tuberculosis from autoinoculation of bacilli to the orificial area. It is more common and usually affects elderly patients.2-4 We present the development of primary OT in an immunocompetent woman.
A 51-year-old woman was admitted with painful enlarging oral ulcers of 1 year’s duration. There was no history of tuberculosis infection, dental trauma, or smoking habit prior to the development of oral ulcers, and no family history of tuberculosis. On dermatological examination white-yellow indurated ulcers with 1×1.5-cm irregular margins located on the hard palate and gingiva were observed (Figures 1A and 1B). Oral hygiene was good. There was no regional lym-phadenopathy on palpation. Physical findings were normal. The histopathology of the biopsy from the gingival ulcer revealed noncaseating granulomatous inflammation in the dermis (Figure 2). Ziehl-Neelsen and periodic acid–Schiff stains were negative for acid-fast bacilli and fungi, respectively.

| 
| |

| 
| |
| Figure 1. White-yellow indurated ulcers with 1×1.5-cm irregular margins located on the hard palate (A) and gingiva (B). Resolution of lesions on the hard palate (C) and the gingiva (D) after 9 months of antituberculosis therapy. | ||
Laboratory results from blood chemistry, complete blood cell count, erythrocyte sedimentation rate, C-reactive protein level, and urine analysis, as well as titers of serum immunoglobulin, antineutrophil cytoplasmic antibodies, and antinuclear antibodies, were within reference range. Human immunodeficiency virus serology was negative. Chest radiography and ultrasonography of the abdomen revealed no abnormalities. A purified protein derivative (tuberculin) test showed an induration of 20 mm. Mycobacterium tuberculosis grew on the culture of the tissue specimen.

|
|
The patient was diagnosed with primary OT and treated with isoniazid (300 mg daily), rifampin (600 mg daily), ethambutol (1500 mg daily), and pyrazinamide (2000 mg daily). At 2 months of therapy the lesions started to heal and showed complete resolution at the end of 9 months of treatment (Figures 1C and 1D). There was no recurrence at 2-year follow-up.
Orificial tuberculosis is a rare form of cutaneous tuberculosis. Therefore, it is regarded as a “forgotten disease” in the literature.5 The pathogenesis of OT has not been clearly defined. The intact mucous membrane, thickened squamous epithelium, cleansing and antibacterial function of saliva, and presence of saprophytes act as protective mechanisms against penetration of mycobacteria. Some factors such as poor oral hygiene, smoking, local trauma, or presence of a dental cyst or an abscess may predispose the direct mucosal inoculation of the tuberculosis bacilli.1,2,6 Orificial tuberculosis may develop as an opportunistic infection in 1.33% of human immunodeficiency virus patients.7 However, our patient had no prior trauma or known predisposing factors.
The common presentation of OT is an ulcerative lesion with irregular and well-delineated margins with a yellowish granular base. A vesicle, papule, or nodule may precede the ulcers. The presence of yellow satellite nodules around the lesion is characteristic for OT.1,2,6 Although there were no satellite nodules around the ulcerations in our case, the lesions had a yellowish granular base and irregular, well-delineated margins.
The tongue, gingiva, lips, tonsils, and epiglottis are the most common sites of involvement.2,5,8 Hard palate involvement rarely has been reported, even in immunosuppressed patients.7 Enlarged painful cervical lymph nodes may accompany the disease. Most patients with OT have been reported to have active pulmonary tuberculosis at the time of diagnosis.6,8 Thus, internal organ involvement, particularly the pulmonary system, should be checked in patients with OT. In our patient, the hard palate was involved together with the gingiva despite the absence of immunosuppression. No internal organ involvement was found in the systemic evaluation.
Orificial tuberculosis is a form of cutaneous tuberculosis that is difficult to diagnose because of the varying nature of clinical features, failure of growth of M tuberculosis on culture, and rarity of the disease.1,2,6 As in our case, biopsies may not always exhibit a caseous necrosis, which is specific to tuberculosis.6,7 Thus, it may be difficult to distinguish oral cavity tuberculosis from conditions demonstrating oral ulcers such as bullous diseases, trauma, fungal diseases, syphilis, sarcoidosis, or squamous cell carcinoma by evaluating only signs and symptoms.6 Clinical suspicion is the first and foremost step in the diagnostic process of OT. In our patient, OT was suspected in the differential diagnosis because the resistant oral ulcerations showed the most common presentation of OT: irregular and well-delineated margins and a yellowish granular base. By considering tuberculosis within the differential diagnosis in our patient, microbiologic cultivation was performed from the oral mucosa and accurate diagnosis was established by determination of the pathogen’s growth in the culture.
Because of the increased incidence of tuberculosis and unusual manifestations, clinicians may easily overlook OT. It should be considered in the differential diagnosis of resistant nodules or ulcers of the oral cavity.
1. Kiliç A, Gül U, Gönül M, et al. Orificial tuberculosis of the lip: a case report and review of the literature. Int J Dermatol. 2009;48:178-180.
2. Ito FA, de Andrade CR, Vargas PA, et al. Primary tuberculosis of the oral cavity. Oral Dis. 2005;11:50-53.
3. Dixit R, Sharma S, Nuwal P. Tuberculosis of oral cavity. Indian J Tuberc. 2008;55:51-53.
4. Smolka W, Burger H, Iizuka T, et al. Primary tuberculosis of the oral cavity in an elderly nonimmunosuppressed patient: case report and review of the literature. Arch Otolaryngol Head Neck Surg. 2008;134:1107-1109.
5. Rodrigues G, Carnelio S, Valliathan M. Primary isolated gingival tuberculosis. Braz J Infect Dis. 2007;11:172-173.
6. Vilar FC, de Souza A, Moya MJ, et al. Atypical oral lesion in a patient with pulmonary tuberculosis. Int J Dermatol. 2009;48:910-912.
7. Kakisi OK, Kechagia AS, Kakisis IK, et al. Tuberculosis of the oral cavity: a systematic review. Eur J Oral Sci. 2010;118:103-109.
8. Eng HL, Lu SY, Yang CH, et al. Oral tuberculosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:415-420.
1. Kiliç A, Gül U, Gönül M, et al. Orificial tuberculosis of the lip: a case report and review of the literature. Int J Dermatol. 2009;48:178-180.
2. Ito FA, de Andrade CR, Vargas PA, et al. Primary tuberculosis of the oral cavity. Oral Dis. 2005;11:50-53.
3. Dixit R, Sharma S, Nuwal P. Tuberculosis of oral cavity. Indian J Tuberc. 2008;55:51-53.
4. Smolka W, Burger H, Iizuka T, et al. Primary tuberculosis of the oral cavity in an elderly nonimmunosuppressed patient: case report and review of the literature. Arch Otolaryngol Head Neck Surg. 2008;134:1107-1109.
5. Rodrigues G, Carnelio S, Valliathan M. Primary isolated gingival tuberculosis. Braz J Infect Dis. 2007;11:172-173.
6. Vilar FC, de Souza A, Moya MJ, et al. Atypical oral lesion in a patient with pulmonary tuberculosis. Int J Dermatol. 2009;48:910-912.
7. Kakisi OK, Kechagia AS, Kakisis IK, et al. Tuberculosis of the oral cavity: a systematic review. Eur J Oral Sci. 2010;118:103-109.
8. Eng HL, Lu SY, Yang CH, et al. Oral tuberculosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:415-420.