User login
› Obtain a problem-focused history and physical, as well as chest radiography, to rule out active pulmonary tuberculosis (TB) before initiating treatment for latent tuberculosis infection (LTBI). B
› Prescribe isoniazid 5 mg/kg/d (10 mg/kg/d in children) up to a maximum dose of 300 mg/d for 9 months for most patients with LTBI. B
› Ensure that directly observed therapy is used for all patients with active TB, as well as for select high-risk cases of LTBI. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mitchell J, age 62, comes to see you because he’s had a cough with increasing dyspnea for a month. Mr. J has never smoked but has type 2 diabetes mellitus. He also tells you that over the past month, he’s had occasional night sweats and has lost 8 pounds, although he’s not changed his diet. During the past week, he’s noticed blood-tinged sputum. Physical examination reveals a thin, chronically ill appearing man with an oral temperature of 100.6°F and mild tachypnea. You order a complete blood count, chest x-ray, and metabolic profile, administer a tuberculin skin test (TST), and initiate levofloxacin 500 mg/d for a presumed bacterial pneumonia. His lab work reveals mild leukocytosis and hyperglycemia, and the chest x-ray shows a left upper lobe infiltrate. The TST reaction—4 mm 50 hours after placement—was negative.
Mr. J returns a week later and says he feels worse. Your examination reveals worsened tachypnea, with tachycardia and crackles over the left upper lung fields.
How would you proceed with his care?
More people die of tuberculosis (TB) each year than any other infectious disease except human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome. In 2013, an estimated 9 million people worldwide developed active TB and 1.5 million died of the disease.1 Many of these deaths could have been prevented if patients had received a diagnosis and treatment during the latent phase (when the patient was infected, but had no active disease), or as soon as the patient developed active disease. In this article we describe treatment for both latent and active TB.
Before treating latent TB infection, first rule out active TB
Patients with latent tuberculosis infection (LTBI) have a 5% to 10% lifetime risk of developing active TB disease.2 Treatment of LTBI can reduce this risk to 1% to 2%.3
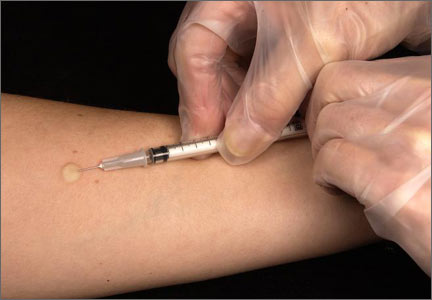
Although not the focus of this article, diagnosis of LTBI is made by using either a TST, in which the patient receives an intradermal injection of purified protein derivative and the size of the skin induration is measured 48 to 72 hours after administration, or an interferon-gamma release assay (IGRA), which requires a blood draw. After receiving a positive test result for LTBI, the next step is to rule out active TB.4 This is necessary because the primary treatment regimen for LTBI involves only one drug, whereas treating active TB with one drug is strongly associated with treatment failure and future resistance to that drug.5
To rule out active TB, perform a brief, problem-focused history and physical, and obtain a chest x-ray.4 Pertinent findings that suggest active disease include:
- any history of recent weight loss, unexplained fever, night sweats, cough or hemoptysis
- fever or any unexpected lung findings on physical exam
- any parenchymal infiltrates on chest x-ray. (Granulomas and scarring may be signs of previously healed TB infection, but do not indicate active TB.)
Any of these findings should prompt a further investigation to either confirm or definitively rule out active TB disease. In the absence of these findings, the physician may proceed with treatment for LTBI.
Latent TB infection treatment: Isoniazid alone, or another regimen?
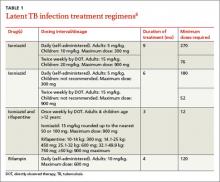
The current preferred regimen for most patients with LTBI is 9 months of isoniazid (INH) 5 mg/kg/d (10 mg/kg/d in children) up to a maximum of 300 mg/d. This regimen has been recommended by the Centers for Disease Control and Prevention (CDC), the American Thoracic Society, and the Infectious Diseases Society of America.3 However, there are 3 other CDC-recommended LTBI treatment regimens that include INH, INH plus rifapentine (RPT), or rifampin (RIF) for 6, 3, or 4 months, respectively (TABLE 1).6 These other regimens may be considered under certain circumstances. For example, INH and rifapentine might be used to treat an otherwise healthy patient who has had recent exposure to an individual with active, contagious TB.
If the patient is pregnant. INH is a pregnancy category C drug. Treatment for LTBI during pregnancy is generally regarded as safe and should be strongly considered if the patient has risk factors for progression to active TB, such as a recent exposure to someone with active TB.7 In otherwise healthy patients, treatment for LTBI may be deferred until after delivery.
Take steps to avoid complications of drug therapy
Drug-induced hepatitis is the primary adverse effect of INH treatment. Risk increases with age, previous hepatic injury, or concomitant use of other hepatotoxic medications. The risk is very small (<0.1%) for healthy children but may be over 10% for adults with multiple risk factors.8 Hepatitis is generally preceded by asymptomatic elevation of liver function tests (LFTs), which is much more common than clinical hepatitis.
Baseline LFTs should be obtained in patients who:
- have underlying liver disease, such as hepatitis B or C9
- consume ≥2 alcoholic drinks daily or >5 drinks at a time on any occasion
- take other medications with potential hepatotoxicity, such as statins
- have HIV infection10
- are pregnant or postpartum.
If a patient being considered for INH treatment has not had serologic testing for HIV, hepatitis B, or hepatitis C, these tests should be done prior to initiating INH. LFTs should be monitored every 1 to 2 months during INH therapy for patients who have ≥1 of these conditions and normal baseline LFTs. If baseline transaminases are >3 times the upper limit of normal, treatment for LTBI should probably be withheld, though might be considered in those whose LFTs return to normal after withdrawal of a modifiable risk factor, such as alcohol or a statin medication.
After beginning LTBI treatment, patients should be monitored regularly for signs and symptoms of hepatitis, including anorexia, nausea, abdominal pain, icterus, and dark urine, and LFTs performed if these develop. If during treatment transaminases increase to >3 times normal in a symptomatic patient (or >5 times normal in an asymptomatic patient), INH should be stopped and generally not resumed, even after LFTs return to normal. (Such patients would be considered to have partially treated LTBI, and their physicians should be alert to signs and symptoms of active TB, such as unexplained fever, weight loss, or blood-tinged sputum, during subsequent patient encounters.)
Peripheral neuropathy is a less common adverse effect of INH. It occurs in up to 2% of patients and is caused by interference with vitamin B6 (pyridoxine) metabolism. It can be prevented by supplementation with pyridoxine 25 to 50 mg/d. Vitamin B6, however, does not prevent INH-induced hepatotoxicity.
Noncompliance is a concern with INH therapy because treatment typically requires a 9-month course of daily medication.11 Patients for whom compliance is likely to be an issue might be considered for a 3-month, 12-dose course of once-weekly, directly-observed therapy (DOT) with INH and RPT administered by a public health agency. (See “Which patients with TB should receive directly observed therapy?” on page 32.12-14) A randomized, open-label trial involving nearly 8000 patients in 4 low-risk countries found this regimen was as effective as 9 months of self-administered INH.15 The CDC has published recommendations for using this regimen.16
Suspect active TB? Don’t wait for cultures to begin Tx
Unlike LTBI, for which the results of diagnostic testing are available within a few days, active TB is diagnosed by culture, which may take as long as 6 to 8 weeks. However, if you suspect your patient has active TB, do not delay treatment while waiting for culture results, or defer treatment for a patient who has a negative acid-fast bacilli (AFB) smear or rapid nucleic acid amplification test.17 These 2 tests, which are routinely performed during TB cultures, look for other evidence of the presence of TB bacilli; they are not as accurate as cultures, but results are available within days. Likewise, a negative TST or IGRA should not prevent empiric treatment for active TB. Treatment for active TB should be begun empirically based on risk factors and clinical presentation, and can be modified or stopped if cultures are negative, the patient fails to improve, or an alternative diagnosis is found to explain the patient’s symptoms.
Rapid testing for evidence of active TB disease—as well as resistance to medications commonly used to treat TB—can be performed using newer modalities such as MODS (Microscopic-Observation Drug-Susceptibility)18,19 or Xpert MTB/RIF20 testing. However, these tests are not available in many hospitals, and culture and drug sensitivity testing remain the gold standard.21
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
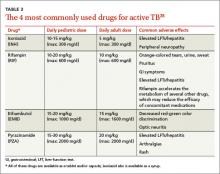
Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.
If initial cultures are negative for Mycobacterium tuberculosis but the patient responds to treatment, he or she is considered to have “culture-negative TB,” and should generally be continued on INH and RIF for 2 more months after completion of the initial treatment phase (for a total of 4 months of INH and RIF).33
Remember to report. In the United States, active TB must be reported to your local health department, which can be invaluable in coordinating care and administering DOT.
Directly observed therapy (DOT) is preferred for certain high-risk patients with latent tuberculosis infection (LTBI), including those who are younger than 5 years of age, test positive for human immunodeficiency virus, are receiving immunosuppressive therapy, have chest radiography evidence of healed TB, have recently converted to active TB status while receiving serial TB testing, or have recently been exposed to active TB.12
Treatment for active TB should always be given by DOT.13 Because DOT is labor-intensive, twice-weekly dosing is usually preferred.14
CASE › In the hospital, Mr. J was placed in respiratory isolation, had prompt sputum cultures for TB, and was started on empiric treatment for active TB with INH, RIF, PZA, and EMB in standard doses. A search for other causes of nonresponsiveness to CAP showed no evidence of malignancy or HIV infection. He improved steadily and was discharged from the hospital after 2 weeks to complete 2 months of 4-drug therapy, with follow-up care coordinated by the local health department, including a home health nurse experienced in administering DOT. Cultures were positive for Mycobacterium tuberculosis sensitive to all drugs tested. After his initial 2 months of 4-drug therapy, he completed 4 months of additional treatment with INH and RIF, given by DOT, and recovered completely.
CORRESPONDENCE
Jeff Hall, MD, University of South Carolina Department of Family and Preventive Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected]
1. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed December 15, 2014.
2. Zumla AI, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;368:745-755.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 15, 2014.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77.
6. Centers for Disease Control and Prevention. Latent tuberculosis infection: A guide for primary health care providers. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/tb/publications/LTBI/default.htm. Accessed December 11, 2014.
7. Centers for Disease Control and Prevention. Fact sheet: tuberculosis and pregnancy. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/factsheets/specpop/pregnancy.htm. Accessed September 6, 2014.
8. Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374-1381.
9. Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054-1060.
10. Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
11. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401-409.
12. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060-2070.
13. Potter B, Rindfleisch K, Kraus CK. Management of active tuberculosis. Am Fam Physician. 2005;72:2225-2232.
14. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343.
15. Sterling TR, Villarina ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166.
16. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650-1653.
17. Inge LD, Wilson JW. Update on the treatment of tuberculosis. Am Fam Physician. 2008;78:457-465.
18. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-1550.
19. Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688-698.
20. Boehme CC, Nabeta P, Hilleman D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-1015.
21. Arentz M, Sorensen B, Horne DJ, et al. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLoS One. 2013;8:e76533.
22. Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348-361.
23. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511-1519.
24. Centers for Disease Control and Prevention. Tuberculosis (TB). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/guidelines/default.htm. Accessed September 6, 2014.
25. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1. Accessed September 6, 2014.
26. International Union Against Tuberculosis and Lung Disease. Management of tuberculosis: A guide to the essentials of good clinical practice. 6th ed. 2010. International Union Against Tuberculosis and Lung Disease Web site. Available at: http://www.theunion.org/what-we-do/publications/technical/management-of-tuberculosis-a-guide-to-the-essentials-of-good-clinical-practice. Accessed September 6, 2014.
27. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity and acceptability. The report of the final results. Ann Intern Med. 1990;112:397-406.
28. Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29-36.
29. Chang KC, Leung CC, Grosset J, et al. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66:997-1007.
30. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
31. Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-579,ix-x.
32. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
33. Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867-870.
› Obtain a problem-focused history and physical, as well as chest radiography, to rule out active pulmonary tuberculosis (TB) before initiating treatment for latent tuberculosis infection (LTBI). B
› Prescribe isoniazid 5 mg/kg/d (10 mg/kg/d in children) up to a maximum dose of 300 mg/d for 9 months for most patients with LTBI. B
› Ensure that directly observed therapy is used for all patients with active TB, as well as for select high-risk cases of LTBI. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mitchell J, age 62, comes to see you because he’s had a cough with increasing dyspnea for a month. Mr. J has never smoked but has type 2 diabetes mellitus. He also tells you that over the past month, he’s had occasional night sweats and has lost 8 pounds, although he’s not changed his diet. During the past week, he’s noticed blood-tinged sputum. Physical examination reveals a thin, chronically ill appearing man with an oral temperature of 100.6°F and mild tachypnea. You order a complete blood count, chest x-ray, and metabolic profile, administer a tuberculin skin test (TST), and initiate levofloxacin 500 mg/d for a presumed bacterial pneumonia. His lab work reveals mild leukocytosis and hyperglycemia, and the chest x-ray shows a left upper lobe infiltrate. The TST reaction—4 mm 50 hours after placement—was negative.
Mr. J returns a week later and says he feels worse. Your examination reveals worsened tachypnea, with tachycardia and crackles over the left upper lung fields.
How would you proceed with his care?
More people die of tuberculosis (TB) each year than any other infectious disease except human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome. In 2013, an estimated 9 million people worldwide developed active TB and 1.5 million died of the disease.1 Many of these deaths could have been prevented if patients had received a diagnosis and treatment during the latent phase (when the patient was infected, but had no active disease), or as soon as the patient developed active disease. In this article we describe treatment for both latent and active TB.
Before treating latent TB infection, first rule out active TB
Patients with latent tuberculosis infection (LTBI) have a 5% to 10% lifetime risk of developing active TB disease.2 Treatment of LTBI can reduce this risk to 1% to 2%.3
Although not the focus of this article, diagnosis of LTBI is made by using either a TST, in which the patient receives an intradermal injection of purified protein derivative and the size of the skin induration is measured 48 to 72 hours after administration, or an interferon-gamma release assay (IGRA), which requires a blood draw. After receiving a positive test result for LTBI, the next step is to rule out active TB.4 This is necessary because the primary treatment regimen for LTBI involves only one drug, whereas treating active TB with one drug is strongly associated with treatment failure and future resistance to that drug.5
To rule out active TB, perform a brief, problem-focused history and physical, and obtain a chest x-ray.4 Pertinent findings that suggest active disease include:
- any history of recent weight loss, unexplained fever, night sweats, cough or hemoptysis
- fever or any unexpected lung findings on physical exam
- any parenchymal infiltrates on chest x-ray. (Granulomas and scarring may be signs of previously healed TB infection, but do not indicate active TB.)
Any of these findings should prompt a further investigation to either confirm or definitively rule out active TB disease. In the absence of these findings, the physician may proceed with treatment for LTBI.
Latent TB infection treatment: Isoniazid alone, or another regimen?
The current preferred regimen for most patients with LTBI is 9 months of isoniazid (INH) 5 mg/kg/d (10 mg/kg/d in children) up to a maximum of 300 mg/d. This regimen has been recommended by the Centers for Disease Control and Prevention (CDC), the American Thoracic Society, and the Infectious Diseases Society of America.3 However, there are 3 other CDC-recommended LTBI treatment regimens that include INH, INH plus rifapentine (RPT), or rifampin (RIF) for 6, 3, or 4 months, respectively (TABLE 1).6 These other regimens may be considered under certain circumstances. For example, INH and rifapentine might be used to treat an otherwise healthy patient who has had recent exposure to an individual with active, contagious TB.
If the patient is pregnant. INH is a pregnancy category C drug. Treatment for LTBI during pregnancy is generally regarded as safe and should be strongly considered if the patient has risk factors for progression to active TB, such as a recent exposure to someone with active TB.7 In otherwise healthy patients, treatment for LTBI may be deferred until after delivery.
Take steps to avoid complications of drug therapy
Drug-induced hepatitis is the primary adverse effect of INH treatment. Risk increases with age, previous hepatic injury, or concomitant use of other hepatotoxic medications. The risk is very small (<0.1%) for healthy children but may be over 10% for adults with multiple risk factors.8 Hepatitis is generally preceded by asymptomatic elevation of liver function tests (LFTs), which is much more common than clinical hepatitis.
Baseline LFTs should be obtained in patients who:
- have underlying liver disease, such as hepatitis B or C9
- consume ≥2 alcoholic drinks daily or >5 drinks at a time on any occasion
- take other medications with potential hepatotoxicity, such as statins
- have HIV infection10
- are pregnant or postpartum.
If a patient being considered for INH treatment has not had serologic testing for HIV, hepatitis B, or hepatitis C, these tests should be done prior to initiating INH. LFTs should be monitored every 1 to 2 months during INH therapy for patients who have ≥1 of these conditions and normal baseline LFTs. If baseline transaminases are >3 times the upper limit of normal, treatment for LTBI should probably be withheld, though might be considered in those whose LFTs return to normal after withdrawal of a modifiable risk factor, such as alcohol or a statin medication.
After beginning LTBI treatment, patients should be monitored regularly for signs and symptoms of hepatitis, including anorexia, nausea, abdominal pain, icterus, and dark urine, and LFTs performed if these develop. If during treatment transaminases increase to >3 times normal in a symptomatic patient (or >5 times normal in an asymptomatic patient), INH should be stopped and generally not resumed, even after LFTs return to normal. (Such patients would be considered to have partially treated LTBI, and their physicians should be alert to signs and symptoms of active TB, such as unexplained fever, weight loss, or blood-tinged sputum, during subsequent patient encounters.)
Peripheral neuropathy is a less common adverse effect of INH. It occurs in up to 2% of patients and is caused by interference with vitamin B6 (pyridoxine) metabolism. It can be prevented by supplementation with pyridoxine 25 to 50 mg/d. Vitamin B6, however, does not prevent INH-induced hepatotoxicity.
Noncompliance is a concern with INH therapy because treatment typically requires a 9-month course of daily medication.11 Patients for whom compliance is likely to be an issue might be considered for a 3-month, 12-dose course of once-weekly, directly-observed therapy (DOT) with INH and RPT administered by a public health agency. (See “Which patients with TB should receive directly observed therapy?” on page 32.12-14) A randomized, open-label trial involving nearly 8000 patients in 4 low-risk countries found this regimen was as effective as 9 months of self-administered INH.15 The CDC has published recommendations for using this regimen.16
Suspect active TB? Don’t wait for cultures to begin Tx
Unlike LTBI, for which the results of diagnostic testing are available within a few days, active TB is diagnosed by culture, which may take as long as 6 to 8 weeks. However, if you suspect your patient has active TB, do not delay treatment while waiting for culture results, or defer treatment for a patient who has a negative acid-fast bacilli (AFB) smear or rapid nucleic acid amplification test.17 These 2 tests, which are routinely performed during TB cultures, look for other evidence of the presence of TB bacilli; they are not as accurate as cultures, but results are available within days. Likewise, a negative TST or IGRA should not prevent empiric treatment for active TB. Treatment for active TB should be begun empirically based on risk factors and clinical presentation, and can be modified or stopped if cultures are negative, the patient fails to improve, or an alternative diagnosis is found to explain the patient’s symptoms.
Rapid testing for evidence of active TB disease—as well as resistance to medications commonly used to treat TB—can be performed using newer modalities such as MODS (Microscopic-Observation Drug-Susceptibility)18,19 or Xpert MTB/RIF20 testing. However, these tests are not available in many hospitals, and culture and drug sensitivity testing remain the gold standard.21
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.
If initial cultures are negative for Mycobacterium tuberculosis but the patient responds to treatment, he or she is considered to have “culture-negative TB,” and should generally be continued on INH and RIF for 2 more months after completion of the initial treatment phase (for a total of 4 months of INH and RIF).33
Remember to report. In the United States, active TB must be reported to your local health department, which can be invaluable in coordinating care and administering DOT.
Directly observed therapy (DOT) is preferred for certain high-risk patients with latent tuberculosis infection (LTBI), including those who are younger than 5 years of age, test positive for human immunodeficiency virus, are receiving immunosuppressive therapy, have chest radiography evidence of healed TB, have recently converted to active TB status while receiving serial TB testing, or have recently been exposed to active TB.12
Treatment for active TB should always be given by DOT.13 Because DOT is labor-intensive, twice-weekly dosing is usually preferred.14
CASE › In the hospital, Mr. J was placed in respiratory isolation, had prompt sputum cultures for TB, and was started on empiric treatment for active TB with INH, RIF, PZA, and EMB in standard doses. A search for other causes of nonresponsiveness to CAP showed no evidence of malignancy or HIV infection. He improved steadily and was discharged from the hospital after 2 weeks to complete 2 months of 4-drug therapy, with follow-up care coordinated by the local health department, including a home health nurse experienced in administering DOT. Cultures were positive for Mycobacterium tuberculosis sensitive to all drugs tested. After his initial 2 months of 4-drug therapy, he completed 4 months of additional treatment with INH and RIF, given by DOT, and recovered completely.
CORRESPONDENCE
Jeff Hall, MD, University of South Carolina Department of Family and Preventive Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected]
› Obtain a problem-focused history and physical, as well as chest radiography, to rule out active pulmonary tuberculosis (TB) before initiating treatment for latent tuberculosis infection (LTBI). B
› Prescribe isoniazid 5 mg/kg/d (10 mg/kg/d in children) up to a maximum dose of 300 mg/d for 9 months for most patients with LTBI. B
› Ensure that directly observed therapy is used for all patients with active TB, as well as for select high-risk cases of LTBI. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mitchell J, age 62, comes to see you because he’s had a cough with increasing dyspnea for a month. Mr. J has never smoked but has type 2 diabetes mellitus. He also tells you that over the past month, he’s had occasional night sweats and has lost 8 pounds, although he’s not changed his diet. During the past week, he’s noticed blood-tinged sputum. Physical examination reveals a thin, chronically ill appearing man with an oral temperature of 100.6°F and mild tachypnea. You order a complete blood count, chest x-ray, and metabolic profile, administer a tuberculin skin test (TST), and initiate levofloxacin 500 mg/d for a presumed bacterial pneumonia. His lab work reveals mild leukocytosis and hyperglycemia, and the chest x-ray shows a left upper lobe infiltrate. The TST reaction—4 mm 50 hours after placement—was negative.
Mr. J returns a week later and says he feels worse. Your examination reveals worsened tachypnea, with tachycardia and crackles over the left upper lung fields.
How would you proceed with his care?
More people die of tuberculosis (TB) each year than any other infectious disease except human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome. In 2013, an estimated 9 million people worldwide developed active TB and 1.5 million died of the disease.1 Many of these deaths could have been prevented if patients had received a diagnosis and treatment during the latent phase (when the patient was infected, but had no active disease), or as soon as the patient developed active disease. In this article we describe treatment for both latent and active TB.
Before treating latent TB infection, first rule out active TB
Patients with latent tuberculosis infection (LTBI) have a 5% to 10% lifetime risk of developing active TB disease.2 Treatment of LTBI can reduce this risk to 1% to 2%.3
Although not the focus of this article, diagnosis of LTBI is made by using either a TST, in which the patient receives an intradermal injection of purified protein derivative and the size of the skin induration is measured 48 to 72 hours after administration, or an interferon-gamma release assay (IGRA), which requires a blood draw. After receiving a positive test result for LTBI, the next step is to rule out active TB.4 This is necessary because the primary treatment regimen for LTBI involves only one drug, whereas treating active TB with one drug is strongly associated with treatment failure and future resistance to that drug.5
To rule out active TB, perform a brief, problem-focused history and physical, and obtain a chest x-ray.4 Pertinent findings that suggest active disease include:
- any history of recent weight loss, unexplained fever, night sweats, cough or hemoptysis
- fever or any unexpected lung findings on physical exam
- any parenchymal infiltrates on chest x-ray. (Granulomas and scarring may be signs of previously healed TB infection, but do not indicate active TB.)
Any of these findings should prompt a further investigation to either confirm or definitively rule out active TB disease. In the absence of these findings, the physician may proceed with treatment for LTBI.
Latent TB infection treatment: Isoniazid alone, or another regimen?
The current preferred regimen for most patients with LTBI is 9 months of isoniazid (INH) 5 mg/kg/d (10 mg/kg/d in children) up to a maximum of 300 mg/d. This regimen has been recommended by the Centers for Disease Control and Prevention (CDC), the American Thoracic Society, and the Infectious Diseases Society of America.3 However, there are 3 other CDC-recommended LTBI treatment regimens that include INH, INH plus rifapentine (RPT), or rifampin (RIF) for 6, 3, or 4 months, respectively (TABLE 1).6 These other regimens may be considered under certain circumstances. For example, INH and rifapentine might be used to treat an otherwise healthy patient who has had recent exposure to an individual with active, contagious TB.
If the patient is pregnant. INH is a pregnancy category C drug. Treatment for LTBI during pregnancy is generally regarded as safe and should be strongly considered if the patient has risk factors for progression to active TB, such as a recent exposure to someone with active TB.7 In otherwise healthy patients, treatment for LTBI may be deferred until after delivery.
Take steps to avoid complications of drug therapy
Drug-induced hepatitis is the primary adverse effect of INH treatment. Risk increases with age, previous hepatic injury, or concomitant use of other hepatotoxic medications. The risk is very small (<0.1%) for healthy children but may be over 10% for adults with multiple risk factors.8 Hepatitis is generally preceded by asymptomatic elevation of liver function tests (LFTs), which is much more common than clinical hepatitis.
Baseline LFTs should be obtained in patients who:
- have underlying liver disease, such as hepatitis B or C9
- consume ≥2 alcoholic drinks daily or >5 drinks at a time on any occasion
- take other medications with potential hepatotoxicity, such as statins
- have HIV infection10
- are pregnant or postpartum.
If a patient being considered for INH treatment has not had serologic testing for HIV, hepatitis B, or hepatitis C, these tests should be done prior to initiating INH. LFTs should be monitored every 1 to 2 months during INH therapy for patients who have ≥1 of these conditions and normal baseline LFTs. If baseline transaminases are >3 times the upper limit of normal, treatment for LTBI should probably be withheld, though might be considered in those whose LFTs return to normal after withdrawal of a modifiable risk factor, such as alcohol or a statin medication.
After beginning LTBI treatment, patients should be monitored regularly for signs and symptoms of hepatitis, including anorexia, nausea, abdominal pain, icterus, and dark urine, and LFTs performed if these develop. If during treatment transaminases increase to >3 times normal in a symptomatic patient (or >5 times normal in an asymptomatic patient), INH should be stopped and generally not resumed, even after LFTs return to normal. (Such patients would be considered to have partially treated LTBI, and their physicians should be alert to signs and symptoms of active TB, such as unexplained fever, weight loss, or blood-tinged sputum, during subsequent patient encounters.)
Peripheral neuropathy is a less common adverse effect of INH. It occurs in up to 2% of patients and is caused by interference with vitamin B6 (pyridoxine) metabolism. It can be prevented by supplementation with pyridoxine 25 to 50 mg/d. Vitamin B6, however, does not prevent INH-induced hepatotoxicity.
Noncompliance is a concern with INH therapy because treatment typically requires a 9-month course of daily medication.11 Patients for whom compliance is likely to be an issue might be considered for a 3-month, 12-dose course of once-weekly, directly-observed therapy (DOT) with INH and RPT administered by a public health agency. (See “Which patients with TB should receive directly observed therapy?” on page 32.12-14) A randomized, open-label trial involving nearly 8000 patients in 4 low-risk countries found this regimen was as effective as 9 months of self-administered INH.15 The CDC has published recommendations for using this regimen.16
Suspect active TB? Don’t wait for cultures to begin Tx
Unlike LTBI, for which the results of diagnostic testing are available within a few days, active TB is diagnosed by culture, which may take as long as 6 to 8 weeks. However, if you suspect your patient has active TB, do not delay treatment while waiting for culture results, or defer treatment for a patient who has a negative acid-fast bacilli (AFB) smear or rapid nucleic acid amplification test.17 These 2 tests, which are routinely performed during TB cultures, look for other evidence of the presence of TB bacilli; they are not as accurate as cultures, but results are available within days. Likewise, a negative TST or IGRA should not prevent empiric treatment for active TB. Treatment for active TB should be begun empirically based on risk factors and clinical presentation, and can be modified or stopped if cultures are negative, the patient fails to improve, or an alternative diagnosis is found to explain the patient’s symptoms.
Rapid testing for evidence of active TB disease—as well as resistance to medications commonly used to treat TB—can be performed using newer modalities such as MODS (Microscopic-Observation Drug-Susceptibility)18,19 or Xpert MTB/RIF20 testing. However, these tests are not available in many hospitals, and culture and drug sensitivity testing remain the gold standard.21
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.
If initial cultures are negative for Mycobacterium tuberculosis but the patient responds to treatment, he or she is considered to have “culture-negative TB,” and should generally be continued on INH and RIF for 2 more months after completion of the initial treatment phase (for a total of 4 months of INH and RIF).33
Remember to report. In the United States, active TB must be reported to your local health department, which can be invaluable in coordinating care and administering DOT.
Directly observed therapy (DOT) is preferred for certain high-risk patients with latent tuberculosis infection (LTBI), including those who are younger than 5 years of age, test positive for human immunodeficiency virus, are receiving immunosuppressive therapy, have chest radiography evidence of healed TB, have recently converted to active TB status while receiving serial TB testing, or have recently been exposed to active TB.12
Treatment for active TB should always be given by DOT.13 Because DOT is labor-intensive, twice-weekly dosing is usually preferred.14
CASE › In the hospital, Mr. J was placed in respiratory isolation, had prompt sputum cultures for TB, and was started on empiric treatment for active TB with INH, RIF, PZA, and EMB in standard doses. A search for other causes of nonresponsiveness to CAP showed no evidence of malignancy or HIV infection. He improved steadily and was discharged from the hospital after 2 weeks to complete 2 months of 4-drug therapy, with follow-up care coordinated by the local health department, including a home health nurse experienced in administering DOT. Cultures were positive for Mycobacterium tuberculosis sensitive to all drugs tested. After his initial 2 months of 4-drug therapy, he completed 4 months of additional treatment with INH and RIF, given by DOT, and recovered completely.
CORRESPONDENCE
Jeff Hall, MD, University of South Carolina Department of Family and Preventive Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected]
1. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed December 15, 2014.
2. Zumla AI, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;368:745-755.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 15, 2014.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77.
6. Centers for Disease Control and Prevention. Latent tuberculosis infection: A guide for primary health care providers. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/tb/publications/LTBI/default.htm. Accessed December 11, 2014.
7. Centers for Disease Control and Prevention. Fact sheet: tuberculosis and pregnancy. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/factsheets/specpop/pregnancy.htm. Accessed September 6, 2014.
8. Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374-1381.
9. Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054-1060.
10. Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
11. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401-409.
12. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060-2070.
13. Potter B, Rindfleisch K, Kraus CK. Management of active tuberculosis. Am Fam Physician. 2005;72:2225-2232.
14. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343.
15. Sterling TR, Villarina ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166.
16. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650-1653.
17. Inge LD, Wilson JW. Update on the treatment of tuberculosis. Am Fam Physician. 2008;78:457-465.
18. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-1550.
19. Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688-698.
20. Boehme CC, Nabeta P, Hilleman D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-1015.
21. Arentz M, Sorensen B, Horne DJ, et al. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLoS One. 2013;8:e76533.
22. Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348-361.
23. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511-1519.
24. Centers for Disease Control and Prevention. Tuberculosis (TB). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/guidelines/default.htm. Accessed September 6, 2014.
25. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1. Accessed September 6, 2014.
26. International Union Against Tuberculosis and Lung Disease. Management of tuberculosis: A guide to the essentials of good clinical practice. 6th ed. 2010. International Union Against Tuberculosis and Lung Disease Web site. Available at: http://www.theunion.org/what-we-do/publications/technical/management-of-tuberculosis-a-guide-to-the-essentials-of-good-clinical-practice. Accessed September 6, 2014.
27. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity and acceptability. The report of the final results. Ann Intern Med. 1990;112:397-406.
28. Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29-36.
29. Chang KC, Leung CC, Grosset J, et al. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66:997-1007.
30. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
31. Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-579,ix-x.
32. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
33. Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867-870.
1. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed December 15, 2014.
2. Zumla AI, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;368:745-755.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 15, 2014.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77.
6. Centers for Disease Control and Prevention. Latent tuberculosis infection: A guide for primary health care providers. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/tb/publications/LTBI/default.htm. Accessed December 11, 2014.
7. Centers for Disease Control and Prevention. Fact sheet: tuberculosis and pregnancy. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/factsheets/specpop/pregnancy.htm. Accessed September 6, 2014.
8. Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374-1381.
9. Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054-1060.
10. Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
11. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401-409.
12. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060-2070.
13. Potter B, Rindfleisch K, Kraus CK. Management of active tuberculosis. Am Fam Physician. 2005;72:2225-2232.
14. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343.
15. Sterling TR, Villarina ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166.
16. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650-1653.
17. Inge LD, Wilson JW. Update on the treatment of tuberculosis. Am Fam Physician. 2008;78:457-465.
18. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-1550.
19. Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688-698.
20. Boehme CC, Nabeta P, Hilleman D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-1015.
21. Arentz M, Sorensen B, Horne DJ, et al. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLoS One. 2013;8:e76533.
22. Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348-361.
23. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511-1519.
24. Centers for Disease Control and Prevention. Tuberculosis (TB). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/guidelines/default.htm. Accessed September 6, 2014.
25. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1. Accessed September 6, 2014.
26. International Union Against Tuberculosis and Lung Disease. Management of tuberculosis: A guide to the essentials of good clinical practice. 6th ed. 2010. International Union Against Tuberculosis and Lung Disease Web site. Available at: http://www.theunion.org/what-we-do/publications/technical/management-of-tuberculosis-a-guide-to-the-essentials-of-good-clinical-practice. Accessed September 6, 2014.
27. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity and acceptability. The report of the final results. Ann Intern Med. 1990;112:397-406.
28. Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29-36.
29. Chang KC, Leung CC, Grosset J, et al. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66:997-1007.
30. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
31. Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-579,ix-x.
32. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
33. Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867-870.