User login
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
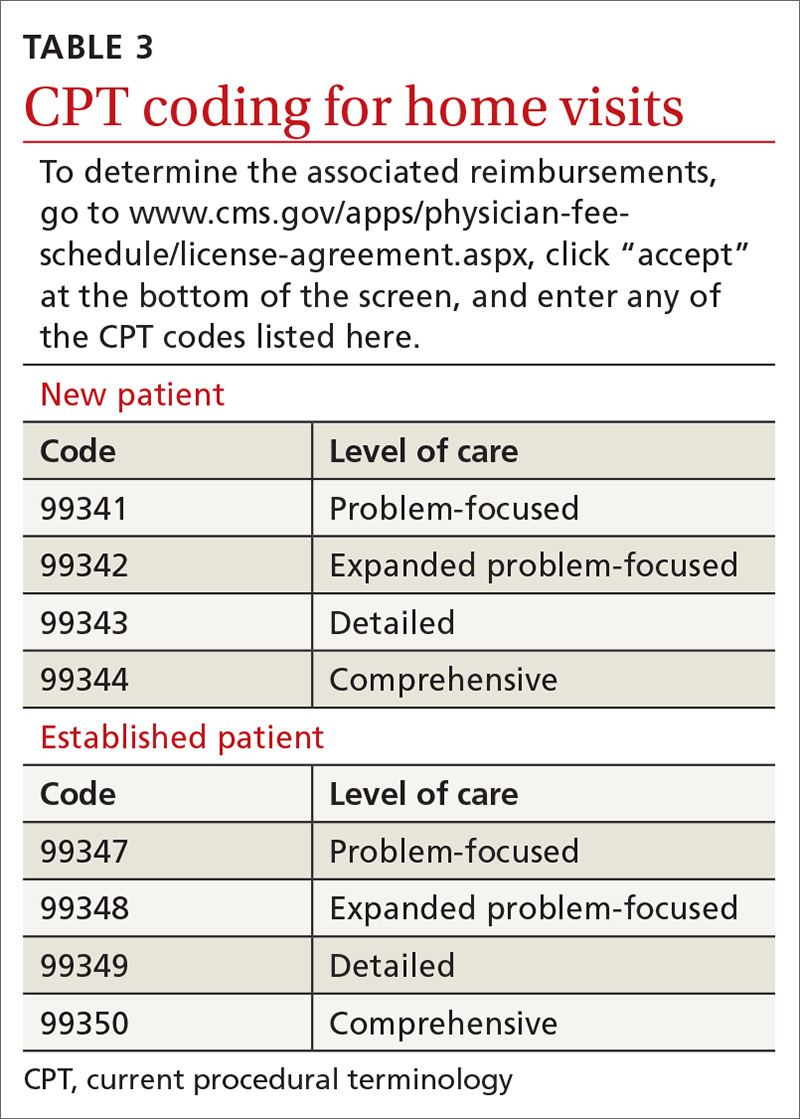
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tuberculosis testing: Which patients, which test?
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
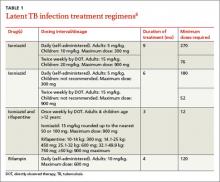
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
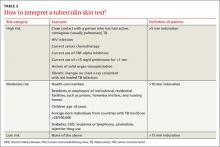
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
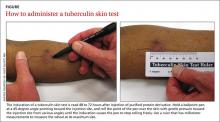
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
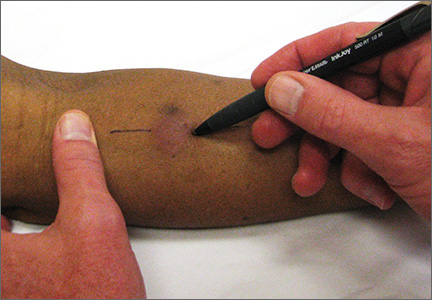
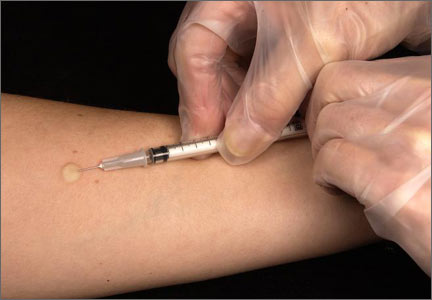
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
Tuberculosis: Which drug regimen and when
› Obtain a problem-focused history and physical, as well as chest radiography, to rule out active pulmonary tuberculosis (TB) before initiating treatment for latent tuberculosis infection (LTBI). B
› Prescribe isoniazid 5 mg/kg/d (10 mg/kg/d in children) up to a maximum dose of 300 mg/d for 9 months for most patients with LTBI. B
› Ensure that directly observed therapy is used for all patients with active TB, as well as for select high-risk cases of LTBI. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mitchell J, age 62, comes to see you because he’s had a cough with increasing dyspnea for a month. Mr. J has never smoked but has type 2 diabetes mellitus. He also tells you that over the past month, he’s had occasional night sweats and has lost 8 pounds, although he’s not changed his diet. During the past week, he’s noticed blood-tinged sputum. Physical examination reveals a thin, chronically ill appearing man with an oral temperature of 100.6°F and mild tachypnea. You order a complete blood count, chest x-ray, and metabolic profile, administer a tuberculin skin test (TST), and initiate levofloxacin 500 mg/d for a presumed bacterial pneumonia. His lab work reveals mild leukocytosis and hyperglycemia, and the chest x-ray shows a left upper lobe infiltrate. The TST reaction—4 mm 50 hours after placement—was negative.
Mr. J returns a week later and says he feels worse. Your examination reveals worsened tachypnea, with tachycardia and crackles over the left upper lung fields.
How would you proceed with his care?
More people die of tuberculosis (TB) each year than any other infectious disease except human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome. In 2013, an estimated 9 million people worldwide developed active TB and 1.5 million died of the disease.1 Many of these deaths could have been prevented if patients had received a diagnosis and treatment during the latent phase (when the patient was infected, but had no active disease), or as soon as the patient developed active disease. In this article we describe treatment for both latent and active TB.
Before treating latent TB infection, first rule out active TB
Patients with latent tuberculosis infection (LTBI) have a 5% to 10% lifetime risk of developing active TB disease.2 Treatment of LTBI can reduce this risk to 1% to 2%.3
Although not the focus of this article, diagnosis of LTBI is made by using either a TST, in which the patient receives an intradermal injection of purified protein derivative and the size of the skin induration is measured 48 to 72 hours after administration, or an interferon-gamma release assay (IGRA), which requires a blood draw. After receiving a positive test result for LTBI, the next step is to rule out active TB.4 This is necessary because the primary treatment regimen for LTBI involves only one drug, whereas treating active TB with one drug is strongly associated with treatment failure and future resistance to that drug.5
To rule out active TB, perform a brief, problem-focused history and physical, and obtain a chest x-ray.4 Pertinent findings that suggest active disease include:
- any history of recent weight loss, unexplained fever, night sweats, cough or hemoptysis
- fever or any unexpected lung findings on physical exam
- any parenchymal infiltrates on chest x-ray. (Granulomas and scarring may be signs of previously healed TB infection, but do not indicate active TB.)
Any of these findings should prompt a further investigation to either confirm or definitively rule out active TB disease. In the absence of these findings, the physician may proceed with treatment for LTBI.
Latent TB infection treatment: Isoniazid alone, or another regimen?
The current preferred regimen for most patients with LTBI is 9 months of isoniazid (INH) 5 mg/kg/d (10 mg/kg/d in children) up to a maximum of 300 mg/d. This regimen has been recommended by the Centers for Disease Control and Prevention (CDC), the American Thoracic Society, and the Infectious Diseases Society of America.3 However, there are 3 other CDC-recommended LTBI treatment regimens that include INH, INH plus rifapentine (RPT), or rifampin (RIF) for 6, 3, or 4 months, respectively (TABLE 1).6 These other regimens may be considered under certain circumstances. For example, INH and rifapentine might be used to treat an otherwise healthy patient who has had recent exposure to an individual with active, contagious TB.
If the patient is pregnant. INH is a pregnancy category C drug. Treatment for LTBI during pregnancy is generally regarded as safe and should be strongly considered if the patient has risk factors for progression to active TB, such as a recent exposure to someone with active TB.7 In otherwise healthy patients, treatment for LTBI may be deferred until after delivery.
Take steps to avoid complications of drug therapy
Drug-induced hepatitis is the primary adverse effect of INH treatment. Risk increases with age, previous hepatic injury, or concomitant use of other hepatotoxic medications. The risk is very small (<0.1%) for healthy children but may be over 10% for adults with multiple risk factors.8 Hepatitis is generally preceded by asymptomatic elevation of liver function tests (LFTs), which is much more common than clinical hepatitis.
Baseline LFTs should be obtained in patients who:
- have underlying liver disease, such as hepatitis B or C9
- consume ≥2 alcoholic drinks daily or >5 drinks at a time on any occasion
- take other medications with potential hepatotoxicity, such as statins
- have HIV infection10
- are pregnant or postpartum.
If a patient being considered for INH treatment has not had serologic testing for HIV, hepatitis B, or hepatitis C, these tests should be done prior to initiating INH. LFTs should be monitored every 1 to 2 months during INH therapy for patients who have ≥1 of these conditions and normal baseline LFTs. If baseline transaminases are >3 times the upper limit of normal, treatment for LTBI should probably be withheld, though might be considered in those whose LFTs return to normal after withdrawal of a modifiable risk factor, such as alcohol or a statin medication.
After beginning LTBI treatment, patients should be monitored regularly for signs and symptoms of hepatitis, including anorexia, nausea, abdominal pain, icterus, and dark urine, and LFTs performed if these develop. If during treatment transaminases increase to >3 times normal in a symptomatic patient (or >5 times normal in an asymptomatic patient), INH should be stopped and generally not resumed, even after LFTs return to normal. (Such patients would be considered to have partially treated LTBI, and their physicians should be alert to signs and symptoms of active TB, such as unexplained fever, weight loss, or blood-tinged sputum, during subsequent patient encounters.)
Peripheral neuropathy is a less common adverse effect of INH. It occurs in up to 2% of patients and is caused by interference with vitamin B6 (pyridoxine) metabolism. It can be prevented by supplementation with pyridoxine 25 to 50 mg/d. Vitamin B6, however, does not prevent INH-induced hepatotoxicity.
Noncompliance is a concern with INH therapy because treatment typically requires a 9-month course of daily medication.11 Patients for whom compliance is likely to be an issue might be considered for a 3-month, 12-dose course of once-weekly, directly-observed therapy (DOT) with INH and RPT administered by a public health agency. (See “Which patients with TB should receive directly observed therapy?” on page 32.12-14) A randomized, open-label trial involving nearly 8000 patients in 4 low-risk countries found this regimen was as effective as 9 months of self-administered INH.15 The CDC has published recommendations for using this regimen.16
Suspect active TB? Don’t wait for cultures to begin Tx
Unlike LTBI, for which the results of diagnostic testing are available within a few days, active TB is diagnosed by culture, which may take as long as 6 to 8 weeks. However, if you suspect your patient has active TB, do not delay treatment while waiting for culture results, or defer treatment for a patient who has a negative acid-fast bacilli (AFB) smear or rapid nucleic acid amplification test.17 These 2 tests, which are routinely performed during TB cultures, look for other evidence of the presence of TB bacilli; they are not as accurate as cultures, but results are available within days. Likewise, a negative TST or IGRA should not prevent empiric treatment for active TB. Treatment for active TB should be begun empirically based on risk factors and clinical presentation, and can be modified or stopped if cultures are negative, the patient fails to improve, or an alternative diagnosis is found to explain the patient’s symptoms.
Rapid testing for evidence of active TB disease—as well as resistance to medications commonly used to treat TB—can be performed using newer modalities such as MODS (Microscopic-Observation Drug-Susceptibility)18,19 or Xpert MTB/RIF20 testing. However, these tests are not available in many hospitals, and culture and drug sensitivity testing remain the gold standard.21
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.
If initial cultures are negative for Mycobacterium tuberculosis but the patient responds to treatment, he or she is considered to have “culture-negative TB,” and should generally be continued on INH and RIF for 2 more months after completion of the initial treatment phase (for a total of 4 months of INH and RIF).33
Remember to report. In the United States, active TB must be reported to your local health department, which can be invaluable in coordinating care and administering DOT.
Directly observed therapy (DOT) is preferred for certain high-risk patients with latent tuberculosis infection (LTBI), including those who are younger than 5 years of age, test positive for human immunodeficiency virus, are receiving immunosuppressive therapy, have chest radiography evidence of healed TB, have recently converted to active TB status while receiving serial TB testing, or have recently been exposed to active TB.12
Treatment for active TB should always be given by DOT.13 Because DOT is labor-intensive, twice-weekly dosing is usually preferred.14
CASE › In the hospital, Mr. J was placed in respiratory isolation, had prompt sputum cultures for TB, and was started on empiric treatment for active TB with INH, RIF, PZA, and EMB in standard doses. A search for other causes of nonresponsiveness to CAP showed no evidence of malignancy or HIV infection. He improved steadily and was discharged from the hospital after 2 weeks to complete 2 months of 4-drug therapy, with follow-up care coordinated by the local health department, including a home health nurse experienced in administering DOT. Cultures were positive for Mycobacterium tuberculosis sensitive to all drugs tested. After his initial 2 months of 4-drug therapy, he completed 4 months of additional treatment with INH and RIF, given by DOT, and recovered completely.
CORRESPONDENCE
Jeff Hall, MD, University of South Carolina Department of Family and Preventive Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected]
1. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed December 15, 2014.
2. Zumla AI, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;368:745-755.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 15, 2014.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77.
6. Centers for Disease Control and Prevention. Latent tuberculosis infection: A guide for primary health care providers. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/tb/publications/LTBI/default.htm. Accessed December 11, 2014.
7. Centers for Disease Control and Prevention. Fact sheet: tuberculosis and pregnancy. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/factsheets/specpop/pregnancy.htm. Accessed September 6, 2014.
8. Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374-1381.
9. Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054-1060.
10. Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
11. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401-409.
12. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060-2070.
13. Potter B, Rindfleisch K, Kraus CK. Management of active tuberculosis. Am Fam Physician. 2005;72:2225-2232.
14. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343.
15. Sterling TR, Villarina ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166.
16. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650-1653.
17. Inge LD, Wilson JW. Update on the treatment of tuberculosis. Am Fam Physician. 2008;78:457-465.
18. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-1550.
19. Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688-698.
20. Boehme CC, Nabeta P, Hilleman D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-1015.
21. Arentz M, Sorensen B, Horne DJ, et al. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLoS One. 2013;8:e76533.
22. Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348-361.
23. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511-1519.
24. Centers for Disease Control and Prevention. Tuberculosis (TB). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/guidelines/default.htm. Accessed September 6, 2014.
25. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1. Accessed September 6, 2014.
26. International Union Against Tuberculosis and Lung Disease. Management of tuberculosis: A guide to the essentials of good clinical practice. 6th ed. 2010. International Union Against Tuberculosis and Lung Disease Web site. Available at: http://www.theunion.org/what-we-do/publications/technical/management-of-tuberculosis-a-guide-to-the-essentials-of-good-clinical-practice. Accessed September 6, 2014.
27. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity and acceptability. The report of the final results. Ann Intern Med. 1990;112:397-406.
28. Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29-36.
29. Chang KC, Leung CC, Grosset J, et al. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66:997-1007.
30. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
31. Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-579,ix-x.
32. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
33. Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867-870.
› Obtain a problem-focused history and physical, as well as chest radiography, to rule out active pulmonary tuberculosis (TB) before initiating treatment for latent tuberculosis infection (LTBI). B
› Prescribe isoniazid 5 mg/kg/d (10 mg/kg/d in children) up to a maximum dose of 300 mg/d for 9 months for most patients with LTBI. B
› Ensure that directly observed therapy is used for all patients with active TB, as well as for select high-risk cases of LTBI. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mitchell J, age 62, comes to see you because he’s had a cough with increasing dyspnea for a month. Mr. J has never smoked but has type 2 diabetes mellitus. He also tells you that over the past month, he’s had occasional night sweats and has lost 8 pounds, although he’s not changed his diet. During the past week, he’s noticed blood-tinged sputum. Physical examination reveals a thin, chronically ill appearing man with an oral temperature of 100.6°F and mild tachypnea. You order a complete blood count, chest x-ray, and metabolic profile, administer a tuberculin skin test (TST), and initiate levofloxacin 500 mg/d for a presumed bacterial pneumonia. His lab work reveals mild leukocytosis and hyperglycemia, and the chest x-ray shows a left upper lobe infiltrate. The TST reaction—4 mm 50 hours after placement—was negative.
Mr. J returns a week later and says he feels worse. Your examination reveals worsened tachypnea, with tachycardia and crackles over the left upper lung fields.
How would you proceed with his care?
More people die of tuberculosis (TB) each year than any other infectious disease except human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome. In 2013, an estimated 9 million people worldwide developed active TB and 1.5 million died of the disease.1 Many of these deaths could have been prevented if patients had received a diagnosis and treatment during the latent phase (when the patient was infected, but had no active disease), or as soon as the patient developed active disease. In this article we describe treatment for both latent and active TB.
Before treating latent TB infection, first rule out active TB
Patients with latent tuberculosis infection (LTBI) have a 5% to 10% lifetime risk of developing active TB disease.2 Treatment of LTBI can reduce this risk to 1% to 2%.3
Although not the focus of this article, diagnosis of LTBI is made by using either a TST, in which the patient receives an intradermal injection of purified protein derivative and the size of the skin induration is measured 48 to 72 hours after administration, or an interferon-gamma release assay (IGRA), which requires a blood draw. After receiving a positive test result for LTBI, the next step is to rule out active TB.4 This is necessary because the primary treatment regimen for LTBI involves only one drug, whereas treating active TB with one drug is strongly associated with treatment failure and future resistance to that drug.5
To rule out active TB, perform a brief, problem-focused history and physical, and obtain a chest x-ray.4 Pertinent findings that suggest active disease include:
- any history of recent weight loss, unexplained fever, night sweats, cough or hemoptysis
- fever or any unexpected lung findings on physical exam
- any parenchymal infiltrates on chest x-ray. (Granulomas and scarring may be signs of previously healed TB infection, but do not indicate active TB.)
Any of these findings should prompt a further investigation to either confirm or definitively rule out active TB disease. In the absence of these findings, the physician may proceed with treatment for LTBI.
Latent TB infection treatment: Isoniazid alone, or another regimen?
The current preferred regimen for most patients with LTBI is 9 months of isoniazid (INH) 5 mg/kg/d (10 mg/kg/d in children) up to a maximum of 300 mg/d. This regimen has been recommended by the Centers for Disease Control and Prevention (CDC), the American Thoracic Society, and the Infectious Diseases Society of America.3 However, there are 3 other CDC-recommended LTBI treatment regimens that include INH, INH plus rifapentine (RPT), or rifampin (RIF) for 6, 3, or 4 months, respectively (TABLE 1).6 These other regimens may be considered under certain circumstances. For example, INH and rifapentine might be used to treat an otherwise healthy patient who has had recent exposure to an individual with active, contagious TB.
If the patient is pregnant. INH is a pregnancy category C drug. Treatment for LTBI during pregnancy is generally regarded as safe and should be strongly considered if the patient has risk factors for progression to active TB, such as a recent exposure to someone with active TB.7 In otherwise healthy patients, treatment for LTBI may be deferred until after delivery.
Take steps to avoid complications of drug therapy
Drug-induced hepatitis is the primary adverse effect of INH treatment. Risk increases with age, previous hepatic injury, or concomitant use of other hepatotoxic medications. The risk is very small (<0.1%) for healthy children but may be over 10% for adults with multiple risk factors.8 Hepatitis is generally preceded by asymptomatic elevation of liver function tests (LFTs), which is much more common than clinical hepatitis.
Baseline LFTs should be obtained in patients who:
- have underlying liver disease, such as hepatitis B or C9
- consume ≥2 alcoholic drinks daily or >5 drinks at a time on any occasion
- take other medications with potential hepatotoxicity, such as statins
- have HIV infection10
- are pregnant or postpartum.
If a patient being considered for INH treatment has not had serologic testing for HIV, hepatitis B, or hepatitis C, these tests should be done prior to initiating INH. LFTs should be monitored every 1 to 2 months during INH therapy for patients who have ≥1 of these conditions and normal baseline LFTs. If baseline transaminases are >3 times the upper limit of normal, treatment for LTBI should probably be withheld, though might be considered in those whose LFTs return to normal after withdrawal of a modifiable risk factor, such as alcohol or a statin medication.
After beginning LTBI treatment, patients should be monitored regularly for signs and symptoms of hepatitis, including anorexia, nausea, abdominal pain, icterus, and dark urine, and LFTs performed if these develop. If during treatment transaminases increase to >3 times normal in a symptomatic patient (or >5 times normal in an asymptomatic patient), INH should be stopped and generally not resumed, even after LFTs return to normal. (Such patients would be considered to have partially treated LTBI, and their physicians should be alert to signs and symptoms of active TB, such as unexplained fever, weight loss, or blood-tinged sputum, during subsequent patient encounters.)
Peripheral neuropathy is a less common adverse effect of INH. It occurs in up to 2% of patients and is caused by interference with vitamin B6 (pyridoxine) metabolism. It can be prevented by supplementation with pyridoxine 25 to 50 mg/d. Vitamin B6, however, does not prevent INH-induced hepatotoxicity.
Noncompliance is a concern with INH therapy because treatment typically requires a 9-month course of daily medication.11 Patients for whom compliance is likely to be an issue might be considered for a 3-month, 12-dose course of once-weekly, directly-observed therapy (DOT) with INH and RPT administered by a public health agency. (See “Which patients with TB should receive directly observed therapy?” on page 32.12-14) A randomized, open-label trial involving nearly 8000 patients in 4 low-risk countries found this regimen was as effective as 9 months of self-administered INH.15 The CDC has published recommendations for using this regimen.16
Suspect active TB? Don’t wait for cultures to begin Tx
Unlike LTBI, for which the results of diagnostic testing are available within a few days, active TB is diagnosed by culture, which may take as long as 6 to 8 weeks. However, if you suspect your patient has active TB, do not delay treatment while waiting for culture results, or defer treatment for a patient who has a negative acid-fast bacilli (AFB) smear or rapid nucleic acid amplification test.17 These 2 tests, which are routinely performed during TB cultures, look for other evidence of the presence of TB bacilli; they are not as accurate as cultures, but results are available within days. Likewise, a negative TST or IGRA should not prevent empiric treatment for active TB. Treatment for active TB should be begun empirically based on risk factors and clinical presentation, and can be modified or stopped if cultures are negative, the patient fails to improve, or an alternative diagnosis is found to explain the patient’s symptoms.
Rapid testing for evidence of active TB disease—as well as resistance to medications commonly used to treat TB—can be performed using newer modalities such as MODS (Microscopic-Observation Drug-Susceptibility)18,19 or Xpert MTB/RIF20 testing. However, these tests are not available in many hospitals, and culture and drug sensitivity testing remain the gold standard.21
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.
If initial cultures are negative for Mycobacterium tuberculosis but the patient responds to treatment, he or she is considered to have “culture-negative TB,” and should generally be continued on INH and RIF for 2 more months after completion of the initial treatment phase (for a total of 4 months of INH and RIF).33
Remember to report. In the United States, active TB must be reported to your local health department, which can be invaluable in coordinating care and administering DOT.
Directly observed therapy (DOT) is preferred for certain high-risk patients with latent tuberculosis infection (LTBI), including those who are younger than 5 years of age, test positive for human immunodeficiency virus, are receiving immunosuppressive therapy, have chest radiography evidence of healed TB, have recently converted to active TB status while receiving serial TB testing, or have recently been exposed to active TB.12
Treatment for active TB should always be given by DOT.13 Because DOT is labor-intensive, twice-weekly dosing is usually preferred.14
CASE › In the hospital, Mr. J was placed in respiratory isolation, had prompt sputum cultures for TB, and was started on empiric treatment for active TB with INH, RIF, PZA, and EMB in standard doses. A search for other causes of nonresponsiveness to CAP showed no evidence of malignancy or HIV infection. He improved steadily and was discharged from the hospital after 2 weeks to complete 2 months of 4-drug therapy, with follow-up care coordinated by the local health department, including a home health nurse experienced in administering DOT. Cultures were positive for Mycobacterium tuberculosis sensitive to all drugs tested. After his initial 2 months of 4-drug therapy, he completed 4 months of additional treatment with INH and RIF, given by DOT, and recovered completely.
CORRESPONDENCE
Jeff Hall, MD, University of South Carolina Department of Family and Preventive Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected]
› Obtain a problem-focused history and physical, as well as chest radiography, to rule out active pulmonary tuberculosis (TB) before initiating treatment for latent tuberculosis infection (LTBI). B
› Prescribe isoniazid 5 mg/kg/d (10 mg/kg/d in children) up to a maximum dose of 300 mg/d for 9 months for most patients with LTBI. B
› Ensure that directly observed therapy is used for all patients with active TB, as well as for select high-risk cases of LTBI. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Mitchell J, age 62, comes to see you because he’s had a cough with increasing dyspnea for a month. Mr. J has never smoked but has type 2 diabetes mellitus. He also tells you that over the past month, he’s had occasional night sweats and has lost 8 pounds, although he’s not changed his diet. During the past week, he’s noticed blood-tinged sputum. Physical examination reveals a thin, chronically ill appearing man with an oral temperature of 100.6°F and mild tachypnea. You order a complete blood count, chest x-ray, and metabolic profile, administer a tuberculin skin test (TST), and initiate levofloxacin 500 mg/d for a presumed bacterial pneumonia. His lab work reveals mild leukocytosis and hyperglycemia, and the chest x-ray shows a left upper lobe infiltrate. The TST reaction—4 mm 50 hours after placement—was negative.
Mr. J returns a week later and says he feels worse. Your examination reveals worsened tachypnea, with tachycardia and crackles over the left upper lung fields.
How would you proceed with his care?
More people die of tuberculosis (TB) each year than any other infectious disease except human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome. In 2013, an estimated 9 million people worldwide developed active TB and 1.5 million died of the disease.1 Many of these deaths could have been prevented if patients had received a diagnosis and treatment during the latent phase (when the patient was infected, but had no active disease), or as soon as the patient developed active disease. In this article we describe treatment for both latent and active TB.
Before treating latent TB infection, first rule out active TB
Patients with latent tuberculosis infection (LTBI) have a 5% to 10% lifetime risk of developing active TB disease.2 Treatment of LTBI can reduce this risk to 1% to 2%.3
Although not the focus of this article, diagnosis of LTBI is made by using either a TST, in which the patient receives an intradermal injection of purified protein derivative and the size of the skin induration is measured 48 to 72 hours after administration, or an interferon-gamma release assay (IGRA), which requires a blood draw. After receiving a positive test result for LTBI, the next step is to rule out active TB.4 This is necessary because the primary treatment regimen for LTBI involves only one drug, whereas treating active TB with one drug is strongly associated with treatment failure and future resistance to that drug.5
To rule out active TB, perform a brief, problem-focused history and physical, and obtain a chest x-ray.4 Pertinent findings that suggest active disease include:
- any history of recent weight loss, unexplained fever, night sweats, cough or hemoptysis
- fever or any unexpected lung findings on physical exam
- any parenchymal infiltrates on chest x-ray. (Granulomas and scarring may be signs of previously healed TB infection, but do not indicate active TB.)
Any of these findings should prompt a further investigation to either confirm or definitively rule out active TB disease. In the absence of these findings, the physician may proceed with treatment for LTBI.
Latent TB infection treatment: Isoniazid alone, or another regimen?
The current preferred regimen for most patients with LTBI is 9 months of isoniazid (INH) 5 mg/kg/d (10 mg/kg/d in children) up to a maximum of 300 mg/d. This regimen has been recommended by the Centers for Disease Control and Prevention (CDC), the American Thoracic Society, and the Infectious Diseases Society of America.3 However, there are 3 other CDC-recommended LTBI treatment regimens that include INH, INH plus rifapentine (RPT), or rifampin (RIF) for 6, 3, or 4 months, respectively (TABLE 1).6 These other regimens may be considered under certain circumstances. For example, INH and rifapentine might be used to treat an otherwise healthy patient who has had recent exposure to an individual with active, contagious TB.
If the patient is pregnant. INH is a pregnancy category C drug. Treatment for LTBI during pregnancy is generally regarded as safe and should be strongly considered if the patient has risk factors for progression to active TB, such as a recent exposure to someone with active TB.7 In otherwise healthy patients, treatment for LTBI may be deferred until after delivery.
Take steps to avoid complications of drug therapy
Drug-induced hepatitis is the primary adverse effect of INH treatment. Risk increases with age, previous hepatic injury, or concomitant use of other hepatotoxic medications. The risk is very small (<0.1%) for healthy children but may be over 10% for adults with multiple risk factors.8 Hepatitis is generally preceded by asymptomatic elevation of liver function tests (LFTs), which is much more common than clinical hepatitis.
Baseline LFTs should be obtained in patients who:
- have underlying liver disease, such as hepatitis B or C9
- consume ≥2 alcoholic drinks daily or >5 drinks at a time on any occasion
- take other medications with potential hepatotoxicity, such as statins
- have HIV infection10
- are pregnant or postpartum.
If a patient being considered for INH treatment has not had serologic testing for HIV, hepatitis B, or hepatitis C, these tests should be done prior to initiating INH. LFTs should be monitored every 1 to 2 months during INH therapy for patients who have ≥1 of these conditions and normal baseline LFTs. If baseline transaminases are >3 times the upper limit of normal, treatment for LTBI should probably be withheld, though might be considered in those whose LFTs return to normal after withdrawal of a modifiable risk factor, such as alcohol or a statin medication.
After beginning LTBI treatment, patients should be monitored regularly for signs and symptoms of hepatitis, including anorexia, nausea, abdominal pain, icterus, and dark urine, and LFTs performed if these develop. If during treatment transaminases increase to >3 times normal in a symptomatic patient (or >5 times normal in an asymptomatic patient), INH should be stopped and generally not resumed, even after LFTs return to normal. (Such patients would be considered to have partially treated LTBI, and their physicians should be alert to signs and symptoms of active TB, such as unexplained fever, weight loss, or blood-tinged sputum, during subsequent patient encounters.)
Peripheral neuropathy is a less common adverse effect of INH. It occurs in up to 2% of patients and is caused by interference with vitamin B6 (pyridoxine) metabolism. It can be prevented by supplementation with pyridoxine 25 to 50 mg/d. Vitamin B6, however, does not prevent INH-induced hepatotoxicity.
Noncompliance is a concern with INH therapy because treatment typically requires a 9-month course of daily medication.11 Patients for whom compliance is likely to be an issue might be considered for a 3-month, 12-dose course of once-weekly, directly-observed therapy (DOT) with INH and RPT administered by a public health agency. (See “Which patients with TB should receive directly observed therapy?” on page 32.12-14) A randomized, open-label trial involving nearly 8000 patients in 4 low-risk countries found this regimen was as effective as 9 months of self-administered INH.15 The CDC has published recommendations for using this regimen.16
Suspect active TB? Don’t wait for cultures to begin Tx
Unlike LTBI, for which the results of diagnostic testing are available within a few days, active TB is diagnosed by culture, which may take as long as 6 to 8 weeks. However, if you suspect your patient has active TB, do not delay treatment while waiting for culture results, or defer treatment for a patient who has a negative acid-fast bacilli (AFB) smear or rapid nucleic acid amplification test.17 These 2 tests, which are routinely performed during TB cultures, look for other evidence of the presence of TB bacilli; they are not as accurate as cultures, but results are available within days. Likewise, a negative TST or IGRA should not prevent empiric treatment for active TB. Treatment for active TB should be begun empirically based on risk factors and clinical presentation, and can be modified or stopped if cultures are negative, the patient fails to improve, or an alternative diagnosis is found to explain the patient’s symptoms.
Rapid testing for evidence of active TB disease—as well as resistance to medications commonly used to treat TB—can be performed using newer modalities such as MODS (Microscopic-Observation Drug-Susceptibility)18,19 or Xpert MTB/RIF20 testing. However, these tests are not available in many hospitals, and culture and drug sensitivity testing remain the gold standard.21
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.
If initial cultures are negative for Mycobacterium tuberculosis but the patient responds to treatment, he or she is considered to have “culture-negative TB,” and should generally be continued on INH and RIF for 2 more months after completion of the initial treatment phase (for a total of 4 months of INH and RIF).33
Remember to report. In the United States, active TB must be reported to your local health department, which can be invaluable in coordinating care and administering DOT.
Directly observed therapy (DOT) is preferred for certain high-risk patients with latent tuberculosis infection (LTBI), including those who are younger than 5 years of age, test positive for human immunodeficiency virus, are receiving immunosuppressive therapy, have chest radiography evidence of healed TB, have recently converted to active TB status while receiving serial TB testing, or have recently been exposed to active TB.12
Treatment for active TB should always be given by DOT.13 Because DOT is labor-intensive, twice-weekly dosing is usually preferred.14
CASE › In the hospital, Mr. J was placed in respiratory isolation, had prompt sputum cultures for TB, and was started on empiric treatment for active TB with INH, RIF, PZA, and EMB in standard doses. A search for other causes of nonresponsiveness to CAP showed no evidence of malignancy or HIV infection. He improved steadily and was discharged from the hospital after 2 weeks to complete 2 months of 4-drug therapy, with follow-up care coordinated by the local health department, including a home health nurse experienced in administering DOT. Cultures were positive for Mycobacterium tuberculosis sensitive to all drugs tested. After his initial 2 months of 4-drug therapy, he completed 4 months of additional treatment with INH and RIF, given by DOT, and recovered completely.
CORRESPONDENCE
Jeff Hall, MD, University of South Carolina Department of Family and Preventive Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected]
1. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed December 15, 2014.
2. Zumla AI, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;368:745-755.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 15, 2014.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77.
6. Centers for Disease Control and Prevention. Latent tuberculosis infection: A guide for primary health care providers. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/tb/publications/LTBI/default.htm. Accessed December 11, 2014.
7. Centers for Disease Control and Prevention. Fact sheet: tuberculosis and pregnancy. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/factsheets/specpop/pregnancy.htm. Accessed September 6, 2014.
8. Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374-1381.
9. Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054-1060.
10. Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
11. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401-409.
12. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060-2070.
13. Potter B, Rindfleisch K, Kraus CK. Management of active tuberculosis. Am Fam Physician. 2005;72:2225-2232.
14. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343.
15. Sterling TR, Villarina ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166.
16. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650-1653.
17. Inge LD, Wilson JW. Update on the treatment of tuberculosis. Am Fam Physician. 2008;78:457-465.
18. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-1550.
19. Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688-698.
20. Boehme CC, Nabeta P, Hilleman D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-1015.
21. Arentz M, Sorensen B, Horne DJ, et al. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLoS One. 2013;8:e76533.
22. Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348-361.
23. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511-1519.
24. Centers for Disease Control and Prevention. Tuberculosis (TB). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/guidelines/default.htm. Accessed September 6, 2014.
25. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1. Accessed September 6, 2014.
26. International Union Against Tuberculosis and Lung Disease. Management of tuberculosis: A guide to the essentials of good clinical practice. 6th ed. 2010. International Union Against Tuberculosis and Lung Disease Web site. Available at: http://www.theunion.org/what-we-do/publications/technical/management-of-tuberculosis-a-guide-to-the-essentials-of-good-clinical-practice. Accessed September 6, 2014.
27. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity and acceptability. The report of the final results. Ann Intern Med. 1990;112:397-406.
28. Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29-36.
29. Chang KC, Leung CC, Grosset J, et al. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66:997-1007.
30. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
31. Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-579,ix-x.
32. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
33. Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867-870.
1. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed December 15, 2014.
2. Zumla AI, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;368:745-755.
3. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 15, 2014.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77.
6. Centers for Disease Control and Prevention. Latent tuberculosis infection: A guide for primary health care providers. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/tb/publications/LTBI/default.htm. Accessed December 11, 2014.
7. Centers for Disease Control and Prevention. Fact sheet: tuberculosis and pregnancy. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/factsheets/specpop/pregnancy.htm. Accessed September 6, 2014.
8. Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374-1381.
9. Bliven EE, Podewils LJ. The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. Int J Tuberc Lung Dis. 2009;13:1054-1060.
10. Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
11. Horsburgh CR Jr, Goldberg S, Bethel J, et al; Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401-409.
12. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060-2070.
13. Potter B, Rindfleisch K, Kraus CK. Management of active tuberculosis. Am Fam Physician. 2005;72:2225-2232.
14. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343.
15. Sterling TR, Villarina ME, Borisov AS, et al; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166.
16. Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650-1653.
17. Inge LD, Wilson JW. Update on the treatment of tuberculosis. Am Fam Physician. 2008;78:457-465.
18. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-1550.
19. Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688-698.
20. Boehme CC, Nabeta P, Hilleman D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-1015.
21. Arentz M, Sorensen B, Horne DJ, et al. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLoS One. 2013;8:e76533.
22. Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348-361.
23. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511-1519.
24. Centers for Disease Control and Prevention. Tuberculosis (TB). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/TB/publications/guidelines/default.htm. Accessed September 6, 2014.
25. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. World Health Organization Web site. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1. Accessed September 6, 2014.
26. International Union Against Tuberculosis and Lung Disease. Management of tuberculosis: A guide to the essentials of good clinical practice. 6th ed. 2010. International Union Against Tuberculosis and Lung Disease Web site. Available at: http://www.theunion.org/what-we-do/publications/technical/management-of-tuberculosis-a-guide-to-the-essentials-of-good-clinical-practice. Accessed September 6, 2014.
27. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity and acceptability. The report of the final results. Ann Intern Med. 1990;112:397-406.
28. Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29-36.
29. Chang KC, Leung CC, Grosset J, et al. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66:997-1007.
30. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
31. Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-579,ix-x.
32. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
33. Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867-870.