User login
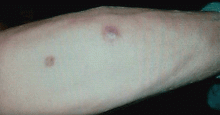
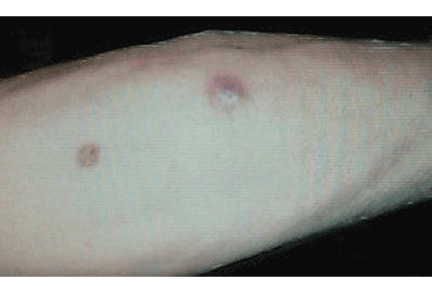
Q: What is the probable diagnosis?
- Kaposi sarcoma
- Majocchi (trichophytic) granuloma
- Staphylococcus aureus furunculosis
- Primary cutaneous mucormycosis
- Disseminated zoster
CAUSES AND RISK FACTORS
Mucormycosis is an invasive infection caused by fungi of a variety of genera (Rhizopus, Rhizomucor, Mucor, Absidia, Apophysomyces, Cunninghamella, Saksenaea, Conidiobolus, and Basidiobolus species) belonging to the class Zygomycetes in the order Mucorales. These ubiquitous environmental fungi have been found widely distributed in hospital sources, having been cultured from plaster casts,1 tongue depressors,2 cloth tapes for securing endotracheal tubes,3 peritoneal dialysis catheters,4 surgical wounds,5 contaminated dressings, needles, and intravenous catheters.6
These fungi rarely cause disease in healthy people, but patients with certain risk factors may develop disseminated disease, in which the death rate is high.6 Risk factors include diabetes mellitus, renal failure, trauma, burns, malnutrition, solid organ transplantation, hematologic malignancies, use of immunosuppressive drugs (eg, chemotherapeutic agents, corticosteroids, cyclosporine, methotrexate, infliximab [Remicade]7), and iron overload and iron-chelating therapy with deferoxamine. 8 Patients such as ours—an organ transplant recipient with concomitant diabetes mellitus—are highly susceptible to this infection.
DIAGNOSIS
The clinical presentation of mucormycosis can be rhino-orbitocerebral (most common),9 pulmonary, primary cutaneous, gastrointestinal, cardiac, or disseminated infection.6 Primary cutaneous mucormycosis occurs from fungal inoculation of the dermis. Small areas of trauma may be all that is needed for this inoculation to occur.
The initial lesion may be an erythematous patch, plaque, or nodule that may subsequently ulcerate and become gangrenous or necrotic.10
The differential diagnosis of new cutaneous nodules in an immunocompromised patient includes a wide variety of infections, such as ecthyma gangrenosum caused by Pseudomonas or Candida species, herpes simplex virus infection, cryptococcal infection, phaeohyphomycosis, and cutaneous aspergillosis.
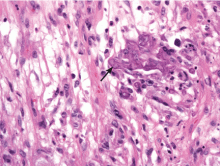
Because the morphology of cutaneous mucormycosis is not distinctive, and because we need to cast our net wide for the numerous, potentially lethal diagnostic possibilities in an immunosuppressed patient, it is crucial that we have a low threshold for prompt biopsy to establish the diagnosis and initiate definitive treatment. While skin biopsy for microscopy can suggest certain fungi, the exact diagnosis is confirmed by a fungal culture of a biopsy specimen.
TREATMENT
Localized soft tissue infection is more amenable to therapy and therefore carries a better prognosis than visceral or disseminated disease.
The best treatment outcomes of primary cutaneous mucormycosis are achieved with both complete excision and debridement of necrotic tissue and systemic antifungal therapy. Amphotericin B in conventional form (Fungizone) and liposomal form (AmBisome) and posaconazole (Noxafil)11–12 are effective.
Paradoxically, in contrast to deferoxamine, other iron chelators such as deferiprone (Ferriprox, not available in the United States) and deferasirox (Exjade), which do not supply iron to the fungus, had been shown to be effective against Zygomycetes in in vitro and animal models. The role of iron chelators as adjunctive therapy for mucormycosis needs further investigation.8
Primary cutaneous mucormycosis may become a disseminated infection. Therefore, one should have a high index of suspicion. When a transplant recipient develops a cutaneous plaque or nodule, biopsy should be performed promptly. Failure to do so can increase the risk of morbidity and death.
- Johnson AS, Ranson M, Scarffe JH, Morgenstern GR, Shaw AJ, Oppenheim BA. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect 1993; 25:293–296.
- Paydas S, Yavuz S, Disel U, et al. Mucormycosis of the tongue in a patient with acute lymphoblastic leukemia: a possible relation with use of a tongue depressor. Am J Med 2003; 114:618–620.
- Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest 1998; 114:340–342.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–217.
- Chew HH, Abuzeid A, Singh D, Tai CC. Surgical wound mucormycosis necessitating hand amputation: a case report. J Orthop Surg (Hong Kong) 2008; 16:267–269.
- Benbow EW, Stoddart RW. Systemic zygomycosis. Postgrad Med J 1986; 62:985–996.
- Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. J Clin Rheumatol 2007; 13:361–362.
- Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Chakrabarti A, Das A, Sharma A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 2001; 42:261–266.
- Nouri-Majalan N, Moghimi M. Skin mucormycosis presenting as an erythema-nodosum-like rash in a renal transplant recipient: a case report. J Med Case Reports 2008, 2:112.
- Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002; 46:1581–1582.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin Microbial Rev 2005; 18:556–569.
Q: What is the probable diagnosis?
- Kaposi sarcoma
- Majocchi (trichophytic) granuloma
- Staphylococcus aureus furunculosis
- Primary cutaneous mucormycosis
- Disseminated zoster
CAUSES AND RISK FACTORS
Mucormycosis is an invasive infection caused by fungi of a variety of genera (Rhizopus, Rhizomucor, Mucor, Absidia, Apophysomyces, Cunninghamella, Saksenaea, Conidiobolus, and Basidiobolus species) belonging to the class Zygomycetes in the order Mucorales. These ubiquitous environmental fungi have been found widely distributed in hospital sources, having been cultured from plaster casts,1 tongue depressors,2 cloth tapes for securing endotracheal tubes,3 peritoneal dialysis catheters,4 surgical wounds,5 contaminated dressings, needles, and intravenous catheters.6
These fungi rarely cause disease in healthy people, but patients with certain risk factors may develop disseminated disease, in which the death rate is high.6 Risk factors include diabetes mellitus, renal failure, trauma, burns, malnutrition, solid organ transplantation, hematologic malignancies, use of immunosuppressive drugs (eg, chemotherapeutic agents, corticosteroids, cyclosporine, methotrexate, infliximab [Remicade]7), and iron overload and iron-chelating therapy with deferoxamine. 8 Patients such as ours—an organ transplant recipient with concomitant diabetes mellitus—are highly susceptible to this infection.
DIAGNOSIS
The clinical presentation of mucormycosis can be rhino-orbitocerebral (most common),9 pulmonary, primary cutaneous, gastrointestinal, cardiac, or disseminated infection.6 Primary cutaneous mucormycosis occurs from fungal inoculation of the dermis. Small areas of trauma may be all that is needed for this inoculation to occur.
The initial lesion may be an erythematous patch, plaque, or nodule that may subsequently ulcerate and become gangrenous or necrotic.10
The differential diagnosis of new cutaneous nodules in an immunocompromised patient includes a wide variety of infections, such as ecthyma gangrenosum caused by Pseudomonas or Candida species, herpes simplex virus infection, cryptococcal infection, phaeohyphomycosis, and cutaneous aspergillosis.
Because the morphology of cutaneous mucormycosis is not distinctive, and because we need to cast our net wide for the numerous, potentially lethal diagnostic possibilities in an immunosuppressed patient, it is crucial that we have a low threshold for prompt biopsy to establish the diagnosis and initiate definitive treatment. While skin biopsy for microscopy can suggest certain fungi, the exact diagnosis is confirmed by a fungal culture of a biopsy specimen.
TREATMENT
Localized soft tissue infection is more amenable to therapy and therefore carries a better prognosis than visceral or disseminated disease.
The best treatment outcomes of primary cutaneous mucormycosis are achieved with both complete excision and debridement of necrotic tissue and systemic antifungal therapy. Amphotericin B in conventional form (Fungizone) and liposomal form (AmBisome) and posaconazole (Noxafil)11–12 are effective.
Paradoxically, in contrast to deferoxamine, other iron chelators such as deferiprone (Ferriprox, not available in the United States) and deferasirox (Exjade), which do not supply iron to the fungus, had been shown to be effective against Zygomycetes in in vitro and animal models. The role of iron chelators as adjunctive therapy for mucormycosis needs further investigation.8
Primary cutaneous mucormycosis may become a disseminated infection. Therefore, one should have a high index of suspicion. When a transplant recipient develops a cutaneous plaque or nodule, biopsy should be performed promptly. Failure to do so can increase the risk of morbidity and death.
Q: What is the probable diagnosis?
- Kaposi sarcoma
- Majocchi (trichophytic) granuloma
- Staphylococcus aureus furunculosis
- Primary cutaneous mucormycosis
- Disseminated zoster
CAUSES AND RISK FACTORS
Mucormycosis is an invasive infection caused by fungi of a variety of genera (Rhizopus, Rhizomucor, Mucor, Absidia, Apophysomyces, Cunninghamella, Saksenaea, Conidiobolus, and Basidiobolus species) belonging to the class Zygomycetes in the order Mucorales. These ubiquitous environmental fungi have been found widely distributed in hospital sources, having been cultured from plaster casts,1 tongue depressors,2 cloth tapes for securing endotracheal tubes,3 peritoneal dialysis catheters,4 surgical wounds,5 contaminated dressings, needles, and intravenous catheters.6
These fungi rarely cause disease in healthy people, but patients with certain risk factors may develop disseminated disease, in which the death rate is high.6 Risk factors include diabetes mellitus, renal failure, trauma, burns, malnutrition, solid organ transplantation, hematologic malignancies, use of immunosuppressive drugs (eg, chemotherapeutic agents, corticosteroids, cyclosporine, methotrexate, infliximab [Remicade]7), and iron overload and iron-chelating therapy with deferoxamine. 8 Patients such as ours—an organ transplant recipient with concomitant diabetes mellitus—are highly susceptible to this infection.
DIAGNOSIS
The clinical presentation of mucormycosis can be rhino-orbitocerebral (most common),9 pulmonary, primary cutaneous, gastrointestinal, cardiac, or disseminated infection.6 Primary cutaneous mucormycosis occurs from fungal inoculation of the dermis. Small areas of trauma may be all that is needed for this inoculation to occur.
The initial lesion may be an erythematous patch, plaque, or nodule that may subsequently ulcerate and become gangrenous or necrotic.10
The differential diagnosis of new cutaneous nodules in an immunocompromised patient includes a wide variety of infections, such as ecthyma gangrenosum caused by Pseudomonas or Candida species, herpes simplex virus infection, cryptococcal infection, phaeohyphomycosis, and cutaneous aspergillosis.
Because the morphology of cutaneous mucormycosis is not distinctive, and because we need to cast our net wide for the numerous, potentially lethal diagnostic possibilities in an immunosuppressed patient, it is crucial that we have a low threshold for prompt biopsy to establish the diagnosis and initiate definitive treatment. While skin biopsy for microscopy can suggest certain fungi, the exact diagnosis is confirmed by a fungal culture of a biopsy specimen.
TREATMENT
Localized soft tissue infection is more amenable to therapy and therefore carries a better prognosis than visceral or disseminated disease.
The best treatment outcomes of primary cutaneous mucormycosis are achieved with both complete excision and debridement of necrotic tissue and systemic antifungal therapy. Amphotericin B in conventional form (Fungizone) and liposomal form (AmBisome) and posaconazole (Noxafil)11–12 are effective.
Paradoxically, in contrast to deferoxamine, other iron chelators such as deferiprone (Ferriprox, not available in the United States) and deferasirox (Exjade), which do not supply iron to the fungus, had been shown to be effective against Zygomycetes in in vitro and animal models. The role of iron chelators as adjunctive therapy for mucormycosis needs further investigation.8
Primary cutaneous mucormycosis may become a disseminated infection. Therefore, one should have a high index of suspicion. When a transplant recipient develops a cutaneous plaque or nodule, biopsy should be performed promptly. Failure to do so can increase the risk of morbidity and death.
- Johnson AS, Ranson M, Scarffe JH, Morgenstern GR, Shaw AJ, Oppenheim BA. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect 1993; 25:293–296.
- Paydas S, Yavuz S, Disel U, et al. Mucormycosis of the tongue in a patient with acute lymphoblastic leukemia: a possible relation with use of a tongue depressor. Am J Med 2003; 114:618–620.
- Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest 1998; 114:340–342.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–217.
- Chew HH, Abuzeid A, Singh D, Tai CC. Surgical wound mucormycosis necessitating hand amputation: a case report. J Orthop Surg (Hong Kong) 2008; 16:267–269.
- Benbow EW, Stoddart RW. Systemic zygomycosis. Postgrad Med J 1986; 62:985–996.
- Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. J Clin Rheumatol 2007; 13:361–362.
- Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Chakrabarti A, Das A, Sharma A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 2001; 42:261–266.
- Nouri-Majalan N, Moghimi M. Skin mucormycosis presenting as an erythema-nodosum-like rash in a renal transplant recipient: a case report. J Med Case Reports 2008, 2:112.
- Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002; 46:1581–1582.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin Microbial Rev 2005; 18:556–569.
- Johnson AS, Ranson M, Scarffe JH, Morgenstern GR, Shaw AJ, Oppenheim BA. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect 1993; 25:293–296.
- Paydas S, Yavuz S, Disel U, et al. Mucormycosis of the tongue in a patient with acute lymphoblastic leukemia: a possible relation with use of a tongue depressor. Am J Med 2003; 114:618–620.
- Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest 1998; 114:340–342.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–217.
- Chew HH, Abuzeid A, Singh D, Tai CC. Surgical wound mucormycosis necessitating hand amputation: a case report. J Orthop Surg (Hong Kong) 2008; 16:267–269.
- Benbow EW, Stoddart RW. Systemic zygomycosis. Postgrad Med J 1986; 62:985–996.
- Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. J Clin Rheumatol 2007; 13:361–362.
- Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Chakrabarti A, Das A, Sharma A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 2001; 42:261–266.
- Nouri-Majalan N, Moghimi M. Skin mucormycosis presenting as an erythema-nodosum-like rash in a renal transplant recipient: a case report. J Med Case Reports 2008, 2:112.
- Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002; 46:1581–1582.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin Microbial Rev 2005; 18:556–569.