User login
Lung transplant: Candidates for referral and the waiting list
Lung transplant is the therapy of choice for a growing number of patients with end-stage lung diseases. Patients receiving a lung transplant are faced with many challenges including drug toxicities, infections, and the risk of rejection.1 Despite these challenges, lung transplant may significantly prolong survival and improve quality of life for many patients.
CANDIDATES FOR LUNG TRANSPLANT
Identifying patients who are appropriate candidates for lung transplant is important to achieving favorable transplant outcomes and to maximizing life expectancy for each patient. The most recent edition of International Society for Heart and Lung Transplant (ISHLT) Guidelines for the Selection of Lung Transplant Candidates is an excellent guide to help physicians identify when to refer potential patients and to how to identify patients who are the most likely to benefit from lung transplant.2
Adults with end-stage lung disease are generally candidates for lung transplant if they meet the following criteria:
- A greater than 50% risk of death from lung disease within 2 years if a lung transplant is not performed
- A greater than 80% likelihood of surviving at least 90 days after the lung transplant procedure
- A greater than 80% likelihood of a 5-year survival posttransplant if graft function is preserved.2
These can only be estimated by transplant programs and not by the referring team in most cases.
Once a patient is identified as a candidate for lung transplant, early referral of patients to a lung transplant program has several advantages and is essential for positive outcomes. Early patient referral allows for timely completion of the formal evaluation of candidacy, patient and family education, as well as the opportunity for the patient and family to raise funds or use other resources to overcome financial hurdles. Listing a patient on the transplant waitlist implies that the patient has a limited life expectancy without a lung transplant and that the risk-benefit ratio favors lung transplant since all other medical options have been exhausted.1
NONCANDIDATES FOR LUNG TRANSPLANT
There are very few absolute contraindications to lung transplant. Generally, most transplant centers in the United States agree that contraindications to lung transplant include conditions associated with increased risk of mortality, including:
- A recent history of a major malignancy. Patients with a 2-year, disease-free interval combined with a low predicted risk of recurrence may be considered in certain cases of localized, non-melanoma skin cancer. A 5-year, disease-free survival is strongly suggested in patients with a history of breast, bladder, or kidney cancer as well as in cases of sarcoma, melanoma, lymphoma and certain hematologic disorders.
- The presence of significant dysfunction of another major organ systems including the heart, liver, kidney, or brain unless a combined organ transplant can be considered and performed.
- Significant coronary heart disease not amenable to revascularization or intervention prior to or at the time of lung transplant.
- The presence of an acute medical condition including but not limited to sepsis and acute liver failure.
- Active Mycobacterium tuberculosis and other highly virulent or highly resistant microbes that are poorly controlled pretransplant.
- Severe obesity with a body mass index greater than 35.
- A history of nonadherence to medical therapy, psychiatric or psychological conditions that might lead to nonadherence, poor or limited social support system, and limited functional status not amenable to rehabilitation.
- Current substance abuse or dependence, including illicit substances, alcohol, and tobacco (nicotine-containing substances). Most centers require at least 6 months’ abstinence from illicit substances prior to being added to the lung transplant waitlist.2
CANDIDATE COMORBIDITIES
Age
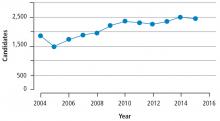
Many transplant centers in the US define the age cutoff for lung transplant at 65; however, some centers may consider candidates older than 65. Advanced age by itself should not be considered a contraindication to lung transplant. However, increased age is usually associated with other comorbid conditions that may increase perioperative and long-term morbidity and mortality. As mentioned previously, the number of older candidates for lung transplant has increased. In the US, 29% of the patients on the national waiting list in 2015 were over age 65.4
Past chest surgery
It is not uncommon for lung transplant candidates to have a history of chest surgery such as lung resection, pleurodesis, or coronary artery bypass grafting. The limited literature regarding the outcomes for these patients suggests they may experience higher rates of bleeding, re-exploration, and renal dysfunction.2 However, these patients should not be excluded from lung transplant and successful transplant outcomes have been achieved in this population by experienced centers.5 In candidates with a history of chronic obstructive pulmonary disease (COPD) and lung-volume reduction surgery (LVRS), early case series indicate that these patients did well after lung transplant.6 However, more recent data demonstrate that patients with prior LVRS who undergo lung transplant experience higher rates of bleeding, worse early graft dysfunction, and worse outcomes overall.7 As with lung transplant candidates with previous chest surgery, lung transplant candidates with previous LVRS are best served by experienced transplant centers.
Hepatitis and HIV
Patients with a history of infection with hepatitis B, hepatitis C, or human immunodeficiency virus (HIV) are candidates for lung transplant at centers experienced with lung transplant in patients with these infections. Most centers advocate that patients with a history of hepatitis B or C have viral infection levels that are controlled or reduced as low as possible and that there is no evidence of portal hypertension or severe cirrhosis.8,9 In the case of HIV, patients should have controlled disease with a negative or undetectable viral load and have no current acquired immunodeficiency defining illness.10 Patients colonized with particular species of Burkholderia cepacia or Mycobacterium abscessus subspecies can be considered for lung transplant only at centers with established preoperative and postoperative protocols for these infections due to the increased risk of perioperative mortality associated with these organisms.11,12
DISEASE-SPECIFIC INDICATIONS
Chronic obstructive pulmonary disease
COPD (both non- and alpha-1 antitrypsin deficiency) is the most common indication for lung transplant and accounts for almost 32% of lung transplants worldwide.13 Patients should be referred for lung transplant when medical therapies, surgical interventions (ie, LVRS) and pulmonary rehabilitation have been maximized. In COPD, the loss of lung function occurs over a long period of time but patients are often more limited by diminished quality of life as lung function slowly declines.
Patients with COPD should be referred for lung transplant if the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index is 5 to 6.2 The original BODE index developed by Celli et al,14 is a scoring system from 0 to 10 with a higher score indicating more severe disease and worse survival. A score of 5 to 6 indicates an estimated mortality of 60% at 4 yrs.2,14,15 Other considerations for referral for lung transplant include the presence of hypercapnia with partial pressure of carbon dioxide greater than 50 mm Hg or higher or hypoxemia with partial pressure of oxygen less than 60 mm Hg or a forced expiratory volume at 1 sec (FEV1) less than 25% predicted.
Patients with COPD should considered for listing for lung transplant if any one of the following criteria is met: BODE index of 7 or greater; FEV1 less than 15% to 20%; 3 of more severe exacerbations during the preceding year; 1 severe exacerbation with acute hypercapnic respiratory failure; or presence of moderate to severe pulmonary hypertension.2,16
Cystic fibrosis
In patients with cystic fibrosis, lung transplant should be considered in patients with an estimated 2-year survival of less than 50% and with a New York Heart Association (NYHA) Functional Classification III or IV. Referral for lung transplant is recommended for patients with a rapid decrease in FEV1 despite optimal therapy, female patients with declining weight and lung function, colonization or infection with nontuberculous mycobacterial disease, or cystic fibrosis-related diabetes. The development of pulmonary hypertension, reduction in walk distance, increasing antibiotic resistance, acute respiratory failure requiring noninvasive ventilation, worsening nutritional status, pneumothorax, and life-threatening hemoptysis despite embolization are all indications for referral for lung transplant.
Patients with cystic fibrosis with hypoxia or hypercapnia with declining lung function, needing long-term noninvasive ventilation, having more frequent exacerbations or exhibiting a decline in functional status should be listed for lung transplant.2,17–19
Restrictive lung disease
Patients with restrictive lung diseases, including interstitial pulmonary fibrosis (usual interstitial pneumonitis, nonspecific interstitial pneumonia), or interstitial lung disease, and hypersensitivity pneumonitis, should be referred for transplant evaluation at the time of diagnosis irrespective of lung function due to the unpredictable nature of these diseases.20 Some clinicians may advocate for a trial of medical therapy with antifibrotics, but this should be done in conjunction with transplant referral.
Patients should be listed for transplant if a 10% or greater decrease in FEV1 occurred in the past 6 months (of note, even a 5% decrease in FEV1 is associated with an overall poorer prognosis and warrants consideration of listing for transplant), if the diffusing capacity of the lung for carbon monoxide decreases 15% or greater during the 6-month follow-up, or if a decline of more than 50 meters is noted on the 6-minute walking test. A documented desaturation of less than 88% or a distance of less than 250 meters on the 6-minute walking test is another indication for listing. Any evidence of secondary pulmonary hypertension on right heart catheterization or on echocardiography or hospitalization for respiratory decline are also indications for listing.21 In cases of scleroderma-associated interstitial lung disease or mixed connective tissue interstitial lung disease, similar guidelines for referral and listing should be followed.2
Pulmonary arterial hypertension
Patients with pulmonary arterial hypertension should be referred for lung transplant if any 1 of the following conditions is present: rapidly progressive disease; NYHA Functional Classification III or IV symptoms during escalating therapy; use of parenteral pulmonary arterial hypertension therapy; or known or suspected pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis.2,22
Patients with pulmonary arterial hypertension should be listed for lung transplant if any of the following are present: NYHA Functional Classification III or IV symptoms despite combination therapy; right heart catheterization demonstrating a cardiac index less than 2 L/min/m2; mean right atrial pressure greater than 15 mm Hg; 6-minute walking test less than 350 meters; or development of pericardial effusion, hemoptysis, or signs of worsening right heart failure, including renal insufficiency, rising bilirubin or evidence of ascites.2,22
BRIDGE TO TRANSPLANT
Acute respiratory decompensation may occur in some candidates for lung transplant prior to listing for transplant or while on the transplant waitlist. In patients with failure of a single lung, a bridge to transplant may be necessary until a suitable organ is available. Mechanical ventilation and extracorporeal life support (ECLS) are 2 bridge strategies for lung transplant candidates. Mechanical ventilation is the most common lung transplant bridge strategy but it is less than ideal because it can lead to deconditioning and ventilator-associated infections that can negatively impact a patient’s suitability for transplant.
ECLS techniques that allow spontaneous breathing and potentially ambulation, known as awake or ambulatory ECLS, is a popular bridge therapy. Ambulatory ECLS is used as an alternative to mechanical ventilation to avoid the complications of mechanical ventilation and allow patients to avoid sedation and participate in rehabilitation.23 Irrespective of the therapy used as a bridge to transplant, patients considered for a bridge are optimally evaluated from a medical and psychosocial perspective prior to bridge therapy.
Both bridge therapies increase the risk of infection, bleeding, and neurologic events; thus, patients need to be assessed repeatedly for these risks to determine ongoing suitability for lung transplant. It is important to note that delayed referral of patients with advanced disease or patients in an acute exacerbation negatively impacts the evaluation for lung transplant, placement on the lung transplant waitlist, outcomes, and suitability for bridge transplant strategies.
CONCLUSION
To ensure good patient outcomes, the evaluation and selection of candidates for lung transplant requires communication between referring physicians and lung transplant centers. Physicians need basic knowledge of patient conditions appropriate for lung transplant and direct communication with lung transplant centers. The workup, required testing, and timing of listing for lung transplant varies among transplant centers across the country, making communication between the referring providers and transplant centers crucial to good patient care. An open, 2-way dialogue between referring providers and transplant centers facilitates listing patients for transplant in a timely manner, reduces delays, and improves outcomes.
- Kreider M, Hadjiliadis D, Kotloff R. Candidate selection, timing of listing, and choice of procedure for lung transplantation. Clin Chest Med 2011; 32:199–211.
- Weill D, Benden C, Corris P, et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplant Council of the International Society of Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34:1–15.
- Tsuang WM. Contemporary issues in lung transplant allocation practices. Curr Transplant Rep 2017; 4:238–242.
- Valapour M, Skeans MA, Smith JM, et al. OPTN/SRTR 2015 annual data report: lung. Am J Transplant 2017; 17(suppl 1):357–424.
- Omara M, Okamoto T, Arafat A, Thuita L, Blackstone EH, McCurry KR. Lung transplantation in patients who have undergone prior cardiothoracic procedures. J Heart Lung Transplant 2016; 35:1462–1470.
- Senbaklavaci O, Wisser W, Ozpeker C, et al. Successful lung volume reduction surgery brings patients into better condition for later lung transplantation. Eur J Cardiothorac Surg 2002; 22:363–367.
- Shigemura N, Gilbert S, Bhama JK et al. Lung transplantation after lung volume reduction surgery. Transplantation 2013; 96:421–425.
- Sahi H, Zein NN, Mehta AC, Blazey HC, Meyer KH, Budev M. Outcomes after lung transplantation in patients with chronic hepatitis C virus infection. J Heart Lung Transplant 2007; 26:466–471.
- Kim EY, Ko HH, Yoshida EM. A concise review of hepatitis C in heart and lung transplantation. Can J Gastroenterol 2011; 25:445–448.
- Kern RM, Seethamraju H, Blanc PD, et al. The feasibility of lung transplantation in HIV-seropositive patients. Ann Am Thorac Soc 2014; 11:882–889.
- De Soyza A, Corris A, McDowell A, Archer L, et al. Burkholderia cepacia complex genomovars and pulmonary transplant outcomes in patients with cystic fibrosis. Lancet 2001; 358:1780–1781.
- De Soyza A, Meachery G, Hester HL, et al. Lung transplant for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single center experience. J Heart Lung Transplant 2010; 29:1395–1404.
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult and heart-lung transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34:1264–1277.
- Celli BR, Cote CG, Marin JM, et al. The body–mass index, airflow obstruction, dyspna and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Marchand E. The BODE index as a tool to predict survival in COPD lung transplant candidates. Eur Respir J 2010; 36:1494–1495.
- Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplant for COPD. Eur Respir J 2010; 36:74–80.
- Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 2004; 126:412–419.
- Mayer-Hamblett N, Rosenfield M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med 2002; 166:1550–1556.
- Liou TG, Adler FR, Cahill BC, et al. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA 2001; 286:2683–2689.
- Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidenced-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824.
- Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003; 168:538–542.
- Edelman, JD. Navigating the road to transplantation for pulmonary arterial hypertension. Advances in Pulmonary Hypertension 2016; 15:14–18.
- Strueber M. Bridges to lung transplant. Curr Opin Organ Transplant 2011; 16:458–461.
Lung transplant is the therapy of choice for a growing number of patients with end-stage lung diseases. Patients receiving a lung transplant are faced with many challenges including drug toxicities, infections, and the risk of rejection.1 Despite these challenges, lung transplant may significantly prolong survival and improve quality of life for many patients.
CANDIDATES FOR LUNG TRANSPLANT
Identifying patients who are appropriate candidates for lung transplant is important to achieving favorable transplant outcomes and to maximizing life expectancy for each patient. The most recent edition of International Society for Heart and Lung Transplant (ISHLT) Guidelines for the Selection of Lung Transplant Candidates is an excellent guide to help physicians identify when to refer potential patients and to how to identify patients who are the most likely to benefit from lung transplant.2
Adults with end-stage lung disease are generally candidates for lung transplant if they meet the following criteria:
- A greater than 50% risk of death from lung disease within 2 years if a lung transplant is not performed
- A greater than 80% likelihood of surviving at least 90 days after the lung transplant procedure
- A greater than 80% likelihood of a 5-year survival posttransplant if graft function is preserved.2
These can only be estimated by transplant programs and not by the referring team in most cases.
Once a patient is identified as a candidate for lung transplant, early referral of patients to a lung transplant program has several advantages and is essential for positive outcomes. Early patient referral allows for timely completion of the formal evaluation of candidacy, patient and family education, as well as the opportunity for the patient and family to raise funds or use other resources to overcome financial hurdles. Listing a patient on the transplant waitlist implies that the patient has a limited life expectancy without a lung transplant and that the risk-benefit ratio favors lung transplant since all other medical options have been exhausted.1
NONCANDIDATES FOR LUNG TRANSPLANT
There are very few absolute contraindications to lung transplant. Generally, most transplant centers in the United States agree that contraindications to lung transplant include conditions associated with increased risk of mortality, including:
- A recent history of a major malignancy. Patients with a 2-year, disease-free interval combined with a low predicted risk of recurrence may be considered in certain cases of localized, non-melanoma skin cancer. A 5-year, disease-free survival is strongly suggested in patients with a history of breast, bladder, or kidney cancer as well as in cases of sarcoma, melanoma, lymphoma and certain hematologic disorders.
- The presence of significant dysfunction of another major organ systems including the heart, liver, kidney, or brain unless a combined organ transplant can be considered and performed.
- Significant coronary heart disease not amenable to revascularization or intervention prior to or at the time of lung transplant.
- The presence of an acute medical condition including but not limited to sepsis and acute liver failure.
- Active Mycobacterium tuberculosis and other highly virulent or highly resistant microbes that are poorly controlled pretransplant.
- Severe obesity with a body mass index greater than 35.
- A history of nonadherence to medical therapy, psychiatric or psychological conditions that might lead to nonadherence, poor or limited social support system, and limited functional status not amenable to rehabilitation.
- Current substance abuse or dependence, including illicit substances, alcohol, and tobacco (nicotine-containing substances). Most centers require at least 6 months’ abstinence from illicit substances prior to being added to the lung transplant waitlist.2
CANDIDATE COMORBIDITIES
Age
Many transplant centers in the US define the age cutoff for lung transplant at 65; however, some centers may consider candidates older than 65. Advanced age by itself should not be considered a contraindication to lung transplant. However, increased age is usually associated with other comorbid conditions that may increase perioperative and long-term morbidity and mortality. As mentioned previously, the number of older candidates for lung transplant has increased. In the US, 29% of the patients on the national waiting list in 2015 were over age 65.4
Past chest surgery
It is not uncommon for lung transplant candidates to have a history of chest surgery such as lung resection, pleurodesis, or coronary artery bypass grafting. The limited literature regarding the outcomes for these patients suggests they may experience higher rates of bleeding, re-exploration, and renal dysfunction.2 However, these patients should not be excluded from lung transplant and successful transplant outcomes have been achieved in this population by experienced centers.5 In candidates with a history of chronic obstructive pulmonary disease (COPD) and lung-volume reduction surgery (LVRS), early case series indicate that these patients did well after lung transplant.6 However, more recent data demonstrate that patients with prior LVRS who undergo lung transplant experience higher rates of bleeding, worse early graft dysfunction, and worse outcomes overall.7 As with lung transplant candidates with previous chest surgery, lung transplant candidates with previous LVRS are best served by experienced transplant centers.
Hepatitis and HIV
Patients with a history of infection with hepatitis B, hepatitis C, or human immunodeficiency virus (HIV) are candidates for lung transplant at centers experienced with lung transplant in patients with these infections. Most centers advocate that patients with a history of hepatitis B or C have viral infection levels that are controlled or reduced as low as possible and that there is no evidence of portal hypertension or severe cirrhosis.8,9 In the case of HIV, patients should have controlled disease with a negative or undetectable viral load and have no current acquired immunodeficiency defining illness.10 Patients colonized with particular species of Burkholderia cepacia or Mycobacterium abscessus subspecies can be considered for lung transplant only at centers with established preoperative and postoperative protocols for these infections due to the increased risk of perioperative mortality associated with these organisms.11,12
DISEASE-SPECIFIC INDICATIONS
Chronic obstructive pulmonary disease
COPD (both non- and alpha-1 antitrypsin deficiency) is the most common indication for lung transplant and accounts for almost 32% of lung transplants worldwide.13 Patients should be referred for lung transplant when medical therapies, surgical interventions (ie, LVRS) and pulmonary rehabilitation have been maximized. In COPD, the loss of lung function occurs over a long period of time but patients are often more limited by diminished quality of life as lung function slowly declines.
Patients with COPD should be referred for lung transplant if the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index is 5 to 6.2 The original BODE index developed by Celli et al,14 is a scoring system from 0 to 10 with a higher score indicating more severe disease and worse survival. A score of 5 to 6 indicates an estimated mortality of 60% at 4 yrs.2,14,15 Other considerations for referral for lung transplant include the presence of hypercapnia with partial pressure of carbon dioxide greater than 50 mm Hg or higher or hypoxemia with partial pressure of oxygen less than 60 mm Hg or a forced expiratory volume at 1 sec (FEV1) less than 25% predicted.
Patients with COPD should considered for listing for lung transplant if any one of the following criteria is met: BODE index of 7 or greater; FEV1 less than 15% to 20%; 3 of more severe exacerbations during the preceding year; 1 severe exacerbation with acute hypercapnic respiratory failure; or presence of moderate to severe pulmonary hypertension.2,16
Cystic fibrosis
In patients with cystic fibrosis, lung transplant should be considered in patients with an estimated 2-year survival of less than 50% and with a New York Heart Association (NYHA) Functional Classification III or IV. Referral for lung transplant is recommended for patients with a rapid decrease in FEV1 despite optimal therapy, female patients with declining weight and lung function, colonization or infection with nontuberculous mycobacterial disease, or cystic fibrosis-related diabetes. The development of pulmonary hypertension, reduction in walk distance, increasing antibiotic resistance, acute respiratory failure requiring noninvasive ventilation, worsening nutritional status, pneumothorax, and life-threatening hemoptysis despite embolization are all indications for referral for lung transplant.
Patients with cystic fibrosis with hypoxia or hypercapnia with declining lung function, needing long-term noninvasive ventilation, having more frequent exacerbations or exhibiting a decline in functional status should be listed for lung transplant.2,17–19
Restrictive lung disease
Patients with restrictive lung diseases, including interstitial pulmonary fibrosis (usual interstitial pneumonitis, nonspecific interstitial pneumonia), or interstitial lung disease, and hypersensitivity pneumonitis, should be referred for transplant evaluation at the time of diagnosis irrespective of lung function due to the unpredictable nature of these diseases.20 Some clinicians may advocate for a trial of medical therapy with antifibrotics, but this should be done in conjunction with transplant referral.
Patients should be listed for transplant if a 10% or greater decrease in FEV1 occurred in the past 6 months (of note, even a 5% decrease in FEV1 is associated with an overall poorer prognosis and warrants consideration of listing for transplant), if the diffusing capacity of the lung for carbon monoxide decreases 15% or greater during the 6-month follow-up, or if a decline of more than 50 meters is noted on the 6-minute walking test. A documented desaturation of less than 88% or a distance of less than 250 meters on the 6-minute walking test is another indication for listing. Any evidence of secondary pulmonary hypertension on right heart catheterization or on echocardiography or hospitalization for respiratory decline are also indications for listing.21 In cases of scleroderma-associated interstitial lung disease or mixed connective tissue interstitial lung disease, similar guidelines for referral and listing should be followed.2
Pulmonary arterial hypertension
Patients with pulmonary arterial hypertension should be referred for lung transplant if any 1 of the following conditions is present: rapidly progressive disease; NYHA Functional Classification III or IV symptoms during escalating therapy; use of parenteral pulmonary arterial hypertension therapy; or known or suspected pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis.2,22
Patients with pulmonary arterial hypertension should be listed for lung transplant if any of the following are present: NYHA Functional Classification III or IV symptoms despite combination therapy; right heart catheterization demonstrating a cardiac index less than 2 L/min/m2; mean right atrial pressure greater than 15 mm Hg; 6-minute walking test less than 350 meters; or development of pericardial effusion, hemoptysis, or signs of worsening right heart failure, including renal insufficiency, rising bilirubin or evidence of ascites.2,22
BRIDGE TO TRANSPLANT
Acute respiratory decompensation may occur in some candidates for lung transplant prior to listing for transplant or while on the transplant waitlist. In patients with failure of a single lung, a bridge to transplant may be necessary until a suitable organ is available. Mechanical ventilation and extracorporeal life support (ECLS) are 2 bridge strategies for lung transplant candidates. Mechanical ventilation is the most common lung transplant bridge strategy but it is less than ideal because it can lead to deconditioning and ventilator-associated infections that can negatively impact a patient’s suitability for transplant.
ECLS techniques that allow spontaneous breathing and potentially ambulation, known as awake or ambulatory ECLS, is a popular bridge therapy. Ambulatory ECLS is used as an alternative to mechanical ventilation to avoid the complications of mechanical ventilation and allow patients to avoid sedation and participate in rehabilitation.23 Irrespective of the therapy used as a bridge to transplant, patients considered for a bridge are optimally evaluated from a medical and psychosocial perspective prior to bridge therapy.
Both bridge therapies increase the risk of infection, bleeding, and neurologic events; thus, patients need to be assessed repeatedly for these risks to determine ongoing suitability for lung transplant. It is important to note that delayed referral of patients with advanced disease or patients in an acute exacerbation negatively impacts the evaluation for lung transplant, placement on the lung transplant waitlist, outcomes, and suitability for bridge transplant strategies.
CONCLUSION
To ensure good patient outcomes, the evaluation and selection of candidates for lung transplant requires communication between referring physicians and lung transplant centers. Physicians need basic knowledge of patient conditions appropriate for lung transplant and direct communication with lung transplant centers. The workup, required testing, and timing of listing for lung transplant varies among transplant centers across the country, making communication between the referring providers and transplant centers crucial to good patient care. An open, 2-way dialogue between referring providers and transplant centers facilitates listing patients for transplant in a timely manner, reduces delays, and improves outcomes.
Lung transplant is the therapy of choice for a growing number of patients with end-stage lung diseases. Patients receiving a lung transplant are faced with many challenges including drug toxicities, infections, and the risk of rejection.1 Despite these challenges, lung transplant may significantly prolong survival and improve quality of life for many patients.
CANDIDATES FOR LUNG TRANSPLANT
Identifying patients who are appropriate candidates for lung transplant is important to achieving favorable transplant outcomes and to maximizing life expectancy for each patient. The most recent edition of International Society for Heart and Lung Transplant (ISHLT) Guidelines for the Selection of Lung Transplant Candidates is an excellent guide to help physicians identify when to refer potential patients and to how to identify patients who are the most likely to benefit from lung transplant.2
Adults with end-stage lung disease are generally candidates for lung transplant if they meet the following criteria:
- A greater than 50% risk of death from lung disease within 2 years if a lung transplant is not performed
- A greater than 80% likelihood of surviving at least 90 days after the lung transplant procedure
- A greater than 80% likelihood of a 5-year survival posttransplant if graft function is preserved.2
These can only be estimated by transplant programs and not by the referring team in most cases.
Once a patient is identified as a candidate for lung transplant, early referral of patients to a lung transplant program has several advantages and is essential for positive outcomes. Early patient referral allows for timely completion of the formal evaluation of candidacy, patient and family education, as well as the opportunity for the patient and family to raise funds or use other resources to overcome financial hurdles. Listing a patient on the transplant waitlist implies that the patient has a limited life expectancy without a lung transplant and that the risk-benefit ratio favors lung transplant since all other medical options have been exhausted.1
NONCANDIDATES FOR LUNG TRANSPLANT
There are very few absolute contraindications to lung transplant. Generally, most transplant centers in the United States agree that contraindications to lung transplant include conditions associated with increased risk of mortality, including:
- A recent history of a major malignancy. Patients with a 2-year, disease-free interval combined with a low predicted risk of recurrence may be considered in certain cases of localized, non-melanoma skin cancer. A 5-year, disease-free survival is strongly suggested in patients with a history of breast, bladder, or kidney cancer as well as in cases of sarcoma, melanoma, lymphoma and certain hematologic disorders.
- The presence of significant dysfunction of another major organ systems including the heart, liver, kidney, or brain unless a combined organ transplant can be considered and performed.
- Significant coronary heart disease not amenable to revascularization or intervention prior to or at the time of lung transplant.
- The presence of an acute medical condition including but not limited to sepsis and acute liver failure.
- Active Mycobacterium tuberculosis and other highly virulent or highly resistant microbes that are poorly controlled pretransplant.
- Severe obesity with a body mass index greater than 35.
- A history of nonadherence to medical therapy, psychiatric or psychological conditions that might lead to nonadherence, poor or limited social support system, and limited functional status not amenable to rehabilitation.
- Current substance abuse or dependence, including illicit substances, alcohol, and tobacco (nicotine-containing substances). Most centers require at least 6 months’ abstinence from illicit substances prior to being added to the lung transplant waitlist.2
CANDIDATE COMORBIDITIES
Age
Many transplant centers in the US define the age cutoff for lung transplant at 65; however, some centers may consider candidates older than 65. Advanced age by itself should not be considered a contraindication to lung transplant. However, increased age is usually associated with other comorbid conditions that may increase perioperative and long-term morbidity and mortality. As mentioned previously, the number of older candidates for lung transplant has increased. In the US, 29% of the patients on the national waiting list in 2015 were over age 65.4
Past chest surgery
It is not uncommon for lung transplant candidates to have a history of chest surgery such as lung resection, pleurodesis, or coronary artery bypass grafting. The limited literature regarding the outcomes for these patients suggests they may experience higher rates of bleeding, re-exploration, and renal dysfunction.2 However, these patients should not be excluded from lung transplant and successful transplant outcomes have been achieved in this population by experienced centers.5 In candidates with a history of chronic obstructive pulmonary disease (COPD) and lung-volume reduction surgery (LVRS), early case series indicate that these patients did well after lung transplant.6 However, more recent data demonstrate that patients with prior LVRS who undergo lung transplant experience higher rates of bleeding, worse early graft dysfunction, and worse outcomes overall.7 As with lung transplant candidates with previous chest surgery, lung transplant candidates with previous LVRS are best served by experienced transplant centers.
Hepatitis and HIV
Patients with a history of infection with hepatitis B, hepatitis C, or human immunodeficiency virus (HIV) are candidates for lung transplant at centers experienced with lung transplant in patients with these infections. Most centers advocate that patients with a history of hepatitis B or C have viral infection levels that are controlled or reduced as low as possible and that there is no evidence of portal hypertension or severe cirrhosis.8,9 In the case of HIV, patients should have controlled disease with a negative or undetectable viral load and have no current acquired immunodeficiency defining illness.10 Patients colonized with particular species of Burkholderia cepacia or Mycobacterium abscessus subspecies can be considered for lung transplant only at centers with established preoperative and postoperative protocols for these infections due to the increased risk of perioperative mortality associated with these organisms.11,12
DISEASE-SPECIFIC INDICATIONS
Chronic obstructive pulmonary disease
COPD (both non- and alpha-1 antitrypsin deficiency) is the most common indication for lung transplant and accounts for almost 32% of lung transplants worldwide.13 Patients should be referred for lung transplant when medical therapies, surgical interventions (ie, LVRS) and pulmonary rehabilitation have been maximized. In COPD, the loss of lung function occurs over a long period of time but patients are often more limited by diminished quality of life as lung function slowly declines.
Patients with COPD should be referred for lung transplant if the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index is 5 to 6.2 The original BODE index developed by Celli et al,14 is a scoring system from 0 to 10 with a higher score indicating more severe disease and worse survival. A score of 5 to 6 indicates an estimated mortality of 60% at 4 yrs.2,14,15 Other considerations for referral for lung transplant include the presence of hypercapnia with partial pressure of carbon dioxide greater than 50 mm Hg or higher or hypoxemia with partial pressure of oxygen less than 60 mm Hg or a forced expiratory volume at 1 sec (FEV1) less than 25% predicted.
Patients with COPD should considered for listing for lung transplant if any one of the following criteria is met: BODE index of 7 or greater; FEV1 less than 15% to 20%; 3 of more severe exacerbations during the preceding year; 1 severe exacerbation with acute hypercapnic respiratory failure; or presence of moderate to severe pulmonary hypertension.2,16
Cystic fibrosis
In patients with cystic fibrosis, lung transplant should be considered in patients with an estimated 2-year survival of less than 50% and with a New York Heart Association (NYHA) Functional Classification III or IV. Referral for lung transplant is recommended for patients with a rapid decrease in FEV1 despite optimal therapy, female patients with declining weight and lung function, colonization or infection with nontuberculous mycobacterial disease, or cystic fibrosis-related diabetes. The development of pulmonary hypertension, reduction in walk distance, increasing antibiotic resistance, acute respiratory failure requiring noninvasive ventilation, worsening nutritional status, pneumothorax, and life-threatening hemoptysis despite embolization are all indications for referral for lung transplant.
Patients with cystic fibrosis with hypoxia or hypercapnia with declining lung function, needing long-term noninvasive ventilation, having more frequent exacerbations or exhibiting a decline in functional status should be listed for lung transplant.2,17–19
Restrictive lung disease
Patients with restrictive lung diseases, including interstitial pulmonary fibrosis (usual interstitial pneumonitis, nonspecific interstitial pneumonia), or interstitial lung disease, and hypersensitivity pneumonitis, should be referred for transplant evaluation at the time of diagnosis irrespective of lung function due to the unpredictable nature of these diseases.20 Some clinicians may advocate for a trial of medical therapy with antifibrotics, but this should be done in conjunction with transplant referral.
Patients should be listed for transplant if a 10% or greater decrease in FEV1 occurred in the past 6 months (of note, even a 5% decrease in FEV1 is associated with an overall poorer prognosis and warrants consideration of listing for transplant), if the diffusing capacity of the lung for carbon monoxide decreases 15% or greater during the 6-month follow-up, or if a decline of more than 50 meters is noted on the 6-minute walking test. A documented desaturation of less than 88% or a distance of less than 250 meters on the 6-minute walking test is another indication for listing. Any evidence of secondary pulmonary hypertension on right heart catheterization or on echocardiography or hospitalization for respiratory decline are also indications for listing.21 In cases of scleroderma-associated interstitial lung disease or mixed connective tissue interstitial lung disease, similar guidelines for referral and listing should be followed.2
Pulmonary arterial hypertension
Patients with pulmonary arterial hypertension should be referred for lung transplant if any 1 of the following conditions is present: rapidly progressive disease; NYHA Functional Classification III or IV symptoms during escalating therapy; use of parenteral pulmonary arterial hypertension therapy; or known or suspected pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis.2,22
Patients with pulmonary arterial hypertension should be listed for lung transplant if any of the following are present: NYHA Functional Classification III or IV symptoms despite combination therapy; right heart catheterization demonstrating a cardiac index less than 2 L/min/m2; mean right atrial pressure greater than 15 mm Hg; 6-minute walking test less than 350 meters; or development of pericardial effusion, hemoptysis, or signs of worsening right heart failure, including renal insufficiency, rising bilirubin or evidence of ascites.2,22
BRIDGE TO TRANSPLANT
Acute respiratory decompensation may occur in some candidates for lung transplant prior to listing for transplant or while on the transplant waitlist. In patients with failure of a single lung, a bridge to transplant may be necessary until a suitable organ is available. Mechanical ventilation and extracorporeal life support (ECLS) are 2 bridge strategies for lung transplant candidates. Mechanical ventilation is the most common lung transplant bridge strategy but it is less than ideal because it can lead to deconditioning and ventilator-associated infections that can negatively impact a patient’s suitability for transplant.
ECLS techniques that allow spontaneous breathing and potentially ambulation, known as awake or ambulatory ECLS, is a popular bridge therapy. Ambulatory ECLS is used as an alternative to mechanical ventilation to avoid the complications of mechanical ventilation and allow patients to avoid sedation and participate in rehabilitation.23 Irrespective of the therapy used as a bridge to transplant, patients considered for a bridge are optimally evaluated from a medical and psychosocial perspective prior to bridge therapy.
Both bridge therapies increase the risk of infection, bleeding, and neurologic events; thus, patients need to be assessed repeatedly for these risks to determine ongoing suitability for lung transplant. It is important to note that delayed referral of patients with advanced disease or patients in an acute exacerbation negatively impacts the evaluation for lung transplant, placement on the lung transplant waitlist, outcomes, and suitability for bridge transplant strategies.
CONCLUSION
To ensure good patient outcomes, the evaluation and selection of candidates for lung transplant requires communication between referring physicians and lung transplant centers. Physicians need basic knowledge of patient conditions appropriate for lung transplant and direct communication with lung transplant centers. The workup, required testing, and timing of listing for lung transplant varies among transplant centers across the country, making communication between the referring providers and transplant centers crucial to good patient care. An open, 2-way dialogue between referring providers and transplant centers facilitates listing patients for transplant in a timely manner, reduces delays, and improves outcomes.
- Kreider M, Hadjiliadis D, Kotloff R. Candidate selection, timing of listing, and choice of procedure for lung transplantation. Clin Chest Med 2011; 32:199–211.
- Weill D, Benden C, Corris P, et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplant Council of the International Society of Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34:1–15.
- Tsuang WM. Contemporary issues in lung transplant allocation practices. Curr Transplant Rep 2017; 4:238–242.
- Valapour M, Skeans MA, Smith JM, et al. OPTN/SRTR 2015 annual data report: lung. Am J Transplant 2017; 17(suppl 1):357–424.
- Omara M, Okamoto T, Arafat A, Thuita L, Blackstone EH, McCurry KR. Lung transplantation in patients who have undergone prior cardiothoracic procedures. J Heart Lung Transplant 2016; 35:1462–1470.
- Senbaklavaci O, Wisser W, Ozpeker C, et al. Successful lung volume reduction surgery brings patients into better condition for later lung transplantation. Eur J Cardiothorac Surg 2002; 22:363–367.
- Shigemura N, Gilbert S, Bhama JK et al. Lung transplantation after lung volume reduction surgery. Transplantation 2013; 96:421–425.
- Sahi H, Zein NN, Mehta AC, Blazey HC, Meyer KH, Budev M. Outcomes after lung transplantation in patients with chronic hepatitis C virus infection. J Heart Lung Transplant 2007; 26:466–471.
- Kim EY, Ko HH, Yoshida EM. A concise review of hepatitis C in heart and lung transplantation. Can J Gastroenterol 2011; 25:445–448.
- Kern RM, Seethamraju H, Blanc PD, et al. The feasibility of lung transplantation in HIV-seropositive patients. Ann Am Thorac Soc 2014; 11:882–889.
- De Soyza A, Corris A, McDowell A, Archer L, et al. Burkholderia cepacia complex genomovars and pulmonary transplant outcomes in patients with cystic fibrosis. Lancet 2001; 358:1780–1781.
- De Soyza A, Meachery G, Hester HL, et al. Lung transplant for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single center experience. J Heart Lung Transplant 2010; 29:1395–1404.
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult and heart-lung transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34:1264–1277.
- Celli BR, Cote CG, Marin JM, et al. The body–mass index, airflow obstruction, dyspna and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Marchand E. The BODE index as a tool to predict survival in COPD lung transplant candidates. Eur Respir J 2010; 36:1494–1495.
- Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplant for COPD. Eur Respir J 2010; 36:74–80.
- Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 2004; 126:412–419.
- Mayer-Hamblett N, Rosenfield M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med 2002; 166:1550–1556.
- Liou TG, Adler FR, Cahill BC, et al. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA 2001; 286:2683–2689.
- Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidenced-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824.
- Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003; 168:538–542.
- Edelman, JD. Navigating the road to transplantation for pulmonary arterial hypertension. Advances in Pulmonary Hypertension 2016; 15:14–18.
- Strueber M. Bridges to lung transplant. Curr Opin Organ Transplant 2011; 16:458–461.
- Kreider M, Hadjiliadis D, Kotloff R. Candidate selection, timing of listing, and choice of procedure for lung transplantation. Clin Chest Med 2011; 32:199–211.
- Weill D, Benden C, Corris P, et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplant Council of the International Society of Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34:1–15.
- Tsuang WM. Contemporary issues in lung transplant allocation practices. Curr Transplant Rep 2017; 4:238–242.
- Valapour M, Skeans MA, Smith JM, et al. OPTN/SRTR 2015 annual data report: lung. Am J Transplant 2017; 17(suppl 1):357–424.
- Omara M, Okamoto T, Arafat A, Thuita L, Blackstone EH, McCurry KR. Lung transplantation in patients who have undergone prior cardiothoracic procedures. J Heart Lung Transplant 2016; 35:1462–1470.
- Senbaklavaci O, Wisser W, Ozpeker C, et al. Successful lung volume reduction surgery brings patients into better condition for later lung transplantation. Eur J Cardiothorac Surg 2002; 22:363–367.
- Shigemura N, Gilbert S, Bhama JK et al. Lung transplantation after lung volume reduction surgery. Transplantation 2013; 96:421–425.
- Sahi H, Zein NN, Mehta AC, Blazey HC, Meyer KH, Budev M. Outcomes after lung transplantation in patients with chronic hepatitis C virus infection. J Heart Lung Transplant 2007; 26:466–471.
- Kim EY, Ko HH, Yoshida EM. A concise review of hepatitis C in heart and lung transplantation. Can J Gastroenterol 2011; 25:445–448.
- Kern RM, Seethamraju H, Blanc PD, et al. The feasibility of lung transplantation in HIV-seropositive patients. Ann Am Thorac Soc 2014; 11:882–889.
- De Soyza A, Corris A, McDowell A, Archer L, et al. Burkholderia cepacia complex genomovars and pulmonary transplant outcomes in patients with cystic fibrosis. Lancet 2001; 358:1780–1781.
- De Soyza A, Meachery G, Hester HL, et al. Lung transplant for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single center experience. J Heart Lung Transplant 2010; 29:1395–1404.
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult and heart-lung transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34:1264–1277.
- Celli BR, Cote CG, Marin JM, et al. The body–mass index, airflow obstruction, dyspna and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Marchand E. The BODE index as a tool to predict survival in COPD lung transplant candidates. Eur Respir J 2010; 36:1494–1495.
- Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplant for COPD. Eur Respir J 2010; 36:74–80.
- Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 2004; 126:412–419.
- Mayer-Hamblett N, Rosenfield M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med 2002; 166:1550–1556.
- Liou TG, Adler FR, Cahill BC, et al. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA 2001; 286:2683–2689.
- Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidenced-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824.
- Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003; 168:538–542.
- Edelman, JD. Navigating the road to transplantation for pulmonary arterial hypertension. Advances in Pulmonary Hypertension 2016; 15:14–18.
- Strueber M. Bridges to lung transplant. Curr Opin Organ Transplant 2011; 16:458–461.
KEY POINTS
- Lung transplant is the therapy of choice for a growing number of patients with end-stage lung disease.
- There are very few absolute contraindications to lung transplant. Potential contraindications and comorbidities can be discussed with the transplant center and vetted prior to listing for lung transplant.
- The workup for a lung transplant varies among transplant centers across the country, thus good communication between referring providers and transplant centers is crucial to quality care.
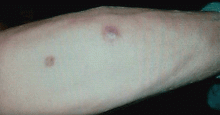
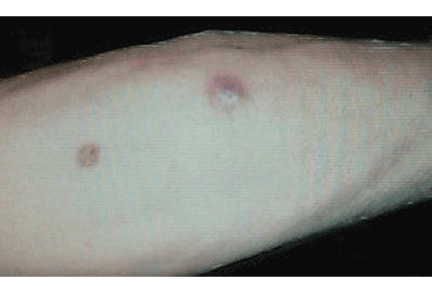
Ulcerative leg nodules in a transplant recipient
Q: What is the probable diagnosis?
- Kaposi sarcoma
- Majocchi (trichophytic) granuloma
- Staphylococcus aureus furunculosis
- Primary cutaneous mucormycosis
- Disseminated zoster
CAUSES AND RISK FACTORS
Mucormycosis is an invasive infection caused by fungi of a variety of genera (Rhizopus, Rhizomucor, Mucor, Absidia, Apophysomyces, Cunninghamella, Saksenaea, Conidiobolus, and Basidiobolus species) belonging to the class Zygomycetes in the order Mucorales. These ubiquitous environmental fungi have been found widely distributed in hospital sources, having been cultured from plaster casts,1 tongue depressors,2 cloth tapes for securing endotracheal tubes,3 peritoneal dialysis catheters,4 surgical wounds,5 contaminated dressings, needles, and intravenous catheters.6
These fungi rarely cause disease in healthy people, but patients with certain risk factors may develop disseminated disease, in which the death rate is high.6 Risk factors include diabetes mellitus, renal failure, trauma, burns, malnutrition, solid organ transplantation, hematologic malignancies, use of immunosuppressive drugs (eg, chemotherapeutic agents, corticosteroids, cyclosporine, methotrexate, infliximab [Remicade]7), and iron overload and iron-chelating therapy with deferoxamine. 8 Patients such as ours—an organ transplant recipient with concomitant diabetes mellitus—are highly susceptible to this infection.
DIAGNOSIS
The clinical presentation of mucormycosis can be rhino-orbitocerebral (most common),9 pulmonary, primary cutaneous, gastrointestinal, cardiac, or disseminated infection.6 Primary cutaneous mucormycosis occurs from fungal inoculation of the dermis. Small areas of trauma may be all that is needed for this inoculation to occur.
The initial lesion may be an erythematous patch, plaque, or nodule that may subsequently ulcerate and become gangrenous or necrotic.10
The differential diagnosis of new cutaneous nodules in an immunocompromised patient includes a wide variety of infections, such as ecthyma gangrenosum caused by Pseudomonas or Candida species, herpes simplex virus infection, cryptococcal infection, phaeohyphomycosis, and cutaneous aspergillosis.
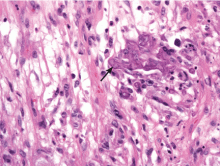
Because the morphology of cutaneous mucormycosis is not distinctive, and because we need to cast our net wide for the numerous, potentially lethal diagnostic possibilities in an immunosuppressed patient, it is crucial that we have a low threshold for prompt biopsy to establish the diagnosis and initiate definitive treatment. While skin biopsy for microscopy can suggest certain fungi, the exact diagnosis is confirmed by a fungal culture of a biopsy specimen.
TREATMENT
Localized soft tissue infection is more amenable to therapy and therefore carries a better prognosis than visceral or disseminated disease.
The best treatment outcomes of primary cutaneous mucormycosis are achieved with both complete excision and debridement of necrotic tissue and systemic antifungal therapy. Amphotericin B in conventional form (Fungizone) and liposomal form (AmBisome) and posaconazole (Noxafil)11–12 are effective.
Paradoxically, in contrast to deferoxamine, other iron chelators such as deferiprone (Ferriprox, not available in the United States) and deferasirox (Exjade), which do not supply iron to the fungus, had been shown to be effective against Zygomycetes in in vitro and animal models. The role of iron chelators as adjunctive therapy for mucormycosis needs further investigation.8
Primary cutaneous mucormycosis may become a disseminated infection. Therefore, one should have a high index of suspicion. When a transplant recipient develops a cutaneous plaque or nodule, biopsy should be performed promptly. Failure to do so can increase the risk of morbidity and death.
- Johnson AS, Ranson M, Scarffe JH, Morgenstern GR, Shaw AJ, Oppenheim BA. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect 1993; 25:293–296.
- Paydas S, Yavuz S, Disel U, et al. Mucormycosis of the tongue in a patient with acute lymphoblastic leukemia: a possible relation with use of a tongue depressor. Am J Med 2003; 114:618–620.
- Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest 1998; 114:340–342.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–217.
- Chew HH, Abuzeid A, Singh D, Tai CC. Surgical wound mucormycosis necessitating hand amputation: a case report. J Orthop Surg (Hong Kong) 2008; 16:267–269.
- Benbow EW, Stoddart RW. Systemic zygomycosis. Postgrad Med J 1986; 62:985–996.
- Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. J Clin Rheumatol 2007; 13:361–362.
- Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Chakrabarti A, Das A, Sharma A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 2001; 42:261–266.
- Nouri-Majalan N, Moghimi M. Skin mucormycosis presenting as an erythema-nodosum-like rash in a renal transplant recipient: a case report. J Med Case Reports 2008, 2:112.
- Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002; 46:1581–1582.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin Microbial Rev 2005; 18:556–569.
Q: What is the probable diagnosis?
- Kaposi sarcoma
- Majocchi (trichophytic) granuloma
- Staphylococcus aureus furunculosis
- Primary cutaneous mucormycosis
- Disseminated zoster
CAUSES AND RISK FACTORS
Mucormycosis is an invasive infection caused by fungi of a variety of genera (Rhizopus, Rhizomucor, Mucor, Absidia, Apophysomyces, Cunninghamella, Saksenaea, Conidiobolus, and Basidiobolus species) belonging to the class Zygomycetes in the order Mucorales. These ubiquitous environmental fungi have been found widely distributed in hospital sources, having been cultured from plaster casts,1 tongue depressors,2 cloth tapes for securing endotracheal tubes,3 peritoneal dialysis catheters,4 surgical wounds,5 contaminated dressings, needles, and intravenous catheters.6
These fungi rarely cause disease in healthy people, but patients with certain risk factors may develop disseminated disease, in which the death rate is high.6 Risk factors include diabetes mellitus, renal failure, trauma, burns, malnutrition, solid organ transplantation, hematologic malignancies, use of immunosuppressive drugs (eg, chemotherapeutic agents, corticosteroids, cyclosporine, methotrexate, infliximab [Remicade]7), and iron overload and iron-chelating therapy with deferoxamine. 8 Patients such as ours—an organ transplant recipient with concomitant diabetes mellitus—are highly susceptible to this infection.
DIAGNOSIS
The clinical presentation of mucormycosis can be rhino-orbitocerebral (most common),9 pulmonary, primary cutaneous, gastrointestinal, cardiac, or disseminated infection.6 Primary cutaneous mucormycosis occurs from fungal inoculation of the dermis. Small areas of trauma may be all that is needed for this inoculation to occur.
The initial lesion may be an erythematous patch, plaque, or nodule that may subsequently ulcerate and become gangrenous or necrotic.10
The differential diagnosis of new cutaneous nodules in an immunocompromised patient includes a wide variety of infections, such as ecthyma gangrenosum caused by Pseudomonas or Candida species, herpes simplex virus infection, cryptococcal infection, phaeohyphomycosis, and cutaneous aspergillosis.
Because the morphology of cutaneous mucormycosis is not distinctive, and because we need to cast our net wide for the numerous, potentially lethal diagnostic possibilities in an immunosuppressed patient, it is crucial that we have a low threshold for prompt biopsy to establish the diagnosis and initiate definitive treatment. While skin biopsy for microscopy can suggest certain fungi, the exact diagnosis is confirmed by a fungal culture of a biopsy specimen.
TREATMENT
Localized soft tissue infection is more amenable to therapy and therefore carries a better prognosis than visceral or disseminated disease.
The best treatment outcomes of primary cutaneous mucormycosis are achieved with both complete excision and debridement of necrotic tissue and systemic antifungal therapy. Amphotericin B in conventional form (Fungizone) and liposomal form (AmBisome) and posaconazole (Noxafil)11–12 are effective.
Paradoxically, in contrast to deferoxamine, other iron chelators such as deferiprone (Ferriprox, not available in the United States) and deferasirox (Exjade), which do not supply iron to the fungus, had been shown to be effective against Zygomycetes in in vitro and animal models. The role of iron chelators as adjunctive therapy for mucormycosis needs further investigation.8
Primary cutaneous mucormycosis may become a disseminated infection. Therefore, one should have a high index of suspicion. When a transplant recipient develops a cutaneous plaque or nodule, biopsy should be performed promptly. Failure to do so can increase the risk of morbidity and death.
Q: What is the probable diagnosis?
- Kaposi sarcoma
- Majocchi (trichophytic) granuloma
- Staphylococcus aureus furunculosis
- Primary cutaneous mucormycosis
- Disseminated zoster
CAUSES AND RISK FACTORS
Mucormycosis is an invasive infection caused by fungi of a variety of genera (Rhizopus, Rhizomucor, Mucor, Absidia, Apophysomyces, Cunninghamella, Saksenaea, Conidiobolus, and Basidiobolus species) belonging to the class Zygomycetes in the order Mucorales. These ubiquitous environmental fungi have been found widely distributed in hospital sources, having been cultured from plaster casts,1 tongue depressors,2 cloth tapes for securing endotracheal tubes,3 peritoneal dialysis catheters,4 surgical wounds,5 contaminated dressings, needles, and intravenous catheters.6
These fungi rarely cause disease in healthy people, but patients with certain risk factors may develop disseminated disease, in which the death rate is high.6 Risk factors include diabetes mellitus, renal failure, trauma, burns, malnutrition, solid organ transplantation, hematologic malignancies, use of immunosuppressive drugs (eg, chemotherapeutic agents, corticosteroids, cyclosporine, methotrexate, infliximab [Remicade]7), and iron overload and iron-chelating therapy with deferoxamine. 8 Patients such as ours—an organ transplant recipient with concomitant diabetes mellitus—are highly susceptible to this infection.
DIAGNOSIS
The clinical presentation of mucormycosis can be rhino-orbitocerebral (most common),9 pulmonary, primary cutaneous, gastrointestinal, cardiac, or disseminated infection.6 Primary cutaneous mucormycosis occurs from fungal inoculation of the dermis. Small areas of trauma may be all that is needed for this inoculation to occur.
The initial lesion may be an erythematous patch, plaque, or nodule that may subsequently ulcerate and become gangrenous or necrotic.10
The differential diagnosis of new cutaneous nodules in an immunocompromised patient includes a wide variety of infections, such as ecthyma gangrenosum caused by Pseudomonas or Candida species, herpes simplex virus infection, cryptococcal infection, phaeohyphomycosis, and cutaneous aspergillosis.
Because the morphology of cutaneous mucormycosis is not distinctive, and because we need to cast our net wide for the numerous, potentially lethal diagnostic possibilities in an immunosuppressed patient, it is crucial that we have a low threshold for prompt biopsy to establish the diagnosis and initiate definitive treatment. While skin biopsy for microscopy can suggest certain fungi, the exact diagnosis is confirmed by a fungal culture of a biopsy specimen.
TREATMENT
Localized soft tissue infection is more amenable to therapy and therefore carries a better prognosis than visceral or disseminated disease.
The best treatment outcomes of primary cutaneous mucormycosis are achieved with both complete excision and debridement of necrotic tissue and systemic antifungal therapy. Amphotericin B in conventional form (Fungizone) and liposomal form (AmBisome) and posaconazole (Noxafil)11–12 are effective.
Paradoxically, in contrast to deferoxamine, other iron chelators such as deferiprone (Ferriprox, not available in the United States) and deferasirox (Exjade), which do not supply iron to the fungus, had been shown to be effective against Zygomycetes in in vitro and animal models. The role of iron chelators as adjunctive therapy for mucormycosis needs further investigation.8
Primary cutaneous mucormycosis may become a disseminated infection. Therefore, one should have a high index of suspicion. When a transplant recipient develops a cutaneous plaque or nodule, biopsy should be performed promptly. Failure to do so can increase the risk of morbidity and death.
- Johnson AS, Ranson M, Scarffe JH, Morgenstern GR, Shaw AJ, Oppenheim BA. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect 1993; 25:293–296.
- Paydas S, Yavuz S, Disel U, et al. Mucormycosis of the tongue in a patient with acute lymphoblastic leukemia: a possible relation with use of a tongue depressor. Am J Med 2003; 114:618–620.
- Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest 1998; 114:340–342.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–217.
- Chew HH, Abuzeid A, Singh D, Tai CC. Surgical wound mucormycosis necessitating hand amputation: a case report. J Orthop Surg (Hong Kong) 2008; 16:267–269.
- Benbow EW, Stoddart RW. Systemic zygomycosis. Postgrad Med J 1986; 62:985–996.
- Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. J Clin Rheumatol 2007; 13:361–362.
- Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Chakrabarti A, Das A, Sharma A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 2001; 42:261–266.
- Nouri-Majalan N, Moghimi M. Skin mucormycosis presenting as an erythema-nodosum-like rash in a renal transplant recipient: a case report. J Med Case Reports 2008, 2:112.
- Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002; 46:1581–1582.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin Microbial Rev 2005; 18:556–569.
- Johnson AS, Ranson M, Scarffe JH, Morgenstern GR, Shaw AJ, Oppenheim BA. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect 1993; 25:293–296.
- Paydas S, Yavuz S, Disel U, et al. Mucormycosis of the tongue in a patient with acute lymphoblastic leukemia: a possible relation with use of a tongue depressor. Am J Med 2003; 114:618–620.
- Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest 1998; 114:340–342.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–217.
- Chew HH, Abuzeid A, Singh D, Tai CC. Surgical wound mucormycosis necessitating hand amputation: a case report. J Orthop Surg (Hong Kong) 2008; 16:267–269.
- Benbow EW, Stoddart RW. Systemic zygomycosis. Postgrad Med J 1986; 62:985–996.
- Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. J Clin Rheumatol 2007; 13:361–362.
- Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Chakrabarti A, Das A, Sharma A, et al. Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 2001; 42:261–266.
- Nouri-Majalan N, Moghimi M. Skin mucormycosis presenting as an erythema-nodosum-like rash in a renal transplant recipient: a case report. J Med Case Reports 2008, 2:112.
- Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002; 46:1581–1582.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin Microbial Rev 2005; 18:556–569.
Does noninvasive positive pressure ventilation have a role in managing hypercapnic respiratory failure due to an acute exacerbation of COPD?
Yes. In selected patients with hypercapnic respiratory failure due to an acute exacerbation of chronic obstructive pulmonary disease (COPD), noninvasive positive pressure ventilation (NIPPV) is an effective adjunct to usual medical therapy. In controlled trials, it reduced the need for endotracheal intubation, the length of hospital stay, and the risk of death.
Acute COPD exacerbations are responsible for more than 500,000 hospitalizations yearly in the United States, and 6% to 34% of patients die.1
Many patients need invasive ventilatory assistance via an endotracheal tube, but such therapy puts the patient at risk of ventilator-associated pneumonia, pneumothorax, and tracheal stenosis.
WHAT IS NONINVASIVE POSITIVE PRESSURE VENTILATION?
WHY IS IT BENEFICIAL?
Several mechanisms may explain why noninvasive positive pressure ventilation is beneficial in acute exacerbations of COPD.
Patients with decompensated respiratory failure lack sufficient alveolar ventilation, owing to abnormal respiratory mechanics and inspiratory muscle fatigue.10 For these patients, breathing faster does not fully compensate. Noninvasive positive pressure ventilation partially counteracts these factors by providing a larger tidal volume with the same inspiratory effort.10,11
Additionally, this treatment can decrease the work of breathing by partially overcoming auto-PEEP (positive end-expiratory pressure) in certain situations.2 Auto-PEEP is pressure greater than the atmospheric pressure remaining in the alveoli at the end of exhalation.12 This condition is related to limited expiratory flow and is common in those with severe COPD. Noninvasive positive pressure ventilation decreases the pressure difference between the atmosphere and the alveoli, thereby reducing the inspiratory force needed for initiation of inspiratory effort, which may reduce the work of breathing. However, caution should be used when using this therapy in tachypneic patients, in whom NIPPV may not fully overcome the auto-PEEP.
WHAT STUDIES SHOWED
Several randomized trials have shown NIPPV to be beneficial in acute hypercapnic COPD exacerbations. A recent meta-analysis of eight studies13 showed that, compared with usual care alone, this therapy was associated with:
- A lower mortality rate (relative risk 0.41; 95% confidence interval [CI] 0.26–0.64)
- Less need for endotracheal intubation (relative risk 0.42; 95% CI 0.31–0.59)
- A lower rate of treatment failure (relative risk 0.51; 95% CI 0.38–0.67)
- Greater improvements in the 1-hour post-treatment pH and PaCO2 levels
- A lower respiratory rate
- A shorter length of stay in the hospital.
WHICH PATIENTS SHOULD RECEIVE IT?
NIPPV is not suitable for all patients with hypercapnic respiratory failure. It should not be substituted for endotracheal intubation and mechanical ventilation if they are indicated, eg, in patients who are medically unstable because of hypotension, sepsis, hypoxia, or other life-threatening systemic illness. In addition, those who cannot protect the airway, who have had a worsening in mental status, or who have excessive secretions should not undergo NIPPV because they have a high risk of aspiration. Factors that predict that this therapy will fail include an Acute Physiology and Chronic Health Evaluation (APACHE) score of 29 or higher, a respiratory rate of 30 or higher, and a pH lower than 7.25 after 2 hours of this therapy.15
GENERAL WARD OR INTENSIVE CARE UNIT?
Mild to moderate COPD exacerbations (in which the pH is 7.30 or higher) can be effectively treated with NIPPV in a general ward if the staff has appropriate expertise.5,18 Keeping the patient in a general ward reduces cost and provides a favorable outcome in selected patients.5,19 However, if the patient’s hemodynamic or mental status deteriorates or if gas exchange, pH, respiratory rate, or dyspnea fail to improve, he or she should be transferred to an intensive care unit and endotracheal intubation should be considered.18 The use of NIPPV in general wards should always be approached with caution and should never be attempted without adequate patient supervision and an experienced respiratory therapy team.
TAKE-HOME MESSAGE
NIPPV has been shown to be an effective adjunct in the treatment of acute hypercapnic respiratory failure secondary to a COPD exacerbation, reducing the need for endotracheal intubation, the length of hospital stay, and the mortality rate. On the basis of controlled trials, NIPPV is now considered the ventilatory therapy of choice in selected patients with this condition. However, it should not be used as a substitute for intubation and mechanical ventilation if these are needed or if the patient is at risk of aspiration.
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and P for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154:959–967. (Erratum in: Am J Respir Crit Care Med 1997; 155:386).
- Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med 2001; 163:540–577.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995; 151:1799–1806.
- Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000; 355:1931–1935.
- Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest 1995; 107:761–768.
- Masip J, Betbesé AJ, Páez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet 2000; 356:2126–2132.
- Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998; 339:429–435.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA 2002; 288:932–935.
- Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990; 323:1523–1530.
- Mughal MM, Culver DA, Minai OA, Arroliga AC. Auto-positive end-expiratory pressure: mechanisms and treatment. Cleve Clin J Med 2005; 72:801–809.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185.
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax 2002; 57:192–211.
- Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 2005; 25:348–355.
- American Respiratory Care Foundation Consensus Conference. Non-invasive positive pressure ventilation. Respir Care 1997; 42:364–369.
- Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest 1998; 114:1636–1642.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Francaise, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Plant PK, Owen JL, Parrott S, Elliott MW. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ 2003; 326:956.
Yes. In selected patients with hypercapnic respiratory failure due to an acute exacerbation of chronic obstructive pulmonary disease (COPD), noninvasive positive pressure ventilation (NIPPV) is an effective adjunct to usual medical therapy. In controlled trials, it reduced the need for endotracheal intubation, the length of hospital stay, and the risk of death.
Acute COPD exacerbations are responsible for more than 500,000 hospitalizations yearly in the United States, and 6% to 34% of patients die.1
Many patients need invasive ventilatory assistance via an endotracheal tube, but such therapy puts the patient at risk of ventilator-associated pneumonia, pneumothorax, and tracheal stenosis.
WHAT IS NONINVASIVE POSITIVE PRESSURE VENTILATION?
WHY IS IT BENEFICIAL?
Several mechanisms may explain why noninvasive positive pressure ventilation is beneficial in acute exacerbations of COPD.
Patients with decompensated respiratory failure lack sufficient alveolar ventilation, owing to abnormal respiratory mechanics and inspiratory muscle fatigue.10 For these patients, breathing faster does not fully compensate. Noninvasive positive pressure ventilation partially counteracts these factors by providing a larger tidal volume with the same inspiratory effort.10,11
Additionally, this treatment can decrease the work of breathing by partially overcoming auto-PEEP (positive end-expiratory pressure) in certain situations.2 Auto-PEEP is pressure greater than the atmospheric pressure remaining in the alveoli at the end of exhalation.12 This condition is related to limited expiratory flow and is common in those with severe COPD. Noninvasive positive pressure ventilation decreases the pressure difference between the atmosphere and the alveoli, thereby reducing the inspiratory force needed for initiation of inspiratory effort, which may reduce the work of breathing. However, caution should be used when using this therapy in tachypneic patients, in whom NIPPV may not fully overcome the auto-PEEP.
WHAT STUDIES SHOWED
Several randomized trials have shown NIPPV to be beneficial in acute hypercapnic COPD exacerbations. A recent meta-analysis of eight studies13 showed that, compared with usual care alone, this therapy was associated with:
- A lower mortality rate (relative risk 0.41; 95% confidence interval [CI] 0.26–0.64)
- Less need for endotracheal intubation (relative risk 0.42; 95% CI 0.31–0.59)
- A lower rate of treatment failure (relative risk 0.51; 95% CI 0.38–0.67)
- Greater improvements in the 1-hour post-treatment pH and PaCO2 levels
- A lower respiratory rate
- A shorter length of stay in the hospital.
WHICH PATIENTS SHOULD RECEIVE IT?
NIPPV is not suitable for all patients with hypercapnic respiratory failure. It should not be substituted for endotracheal intubation and mechanical ventilation if they are indicated, eg, in patients who are medically unstable because of hypotension, sepsis, hypoxia, or other life-threatening systemic illness. In addition, those who cannot protect the airway, who have had a worsening in mental status, or who have excessive secretions should not undergo NIPPV because they have a high risk of aspiration. Factors that predict that this therapy will fail include an Acute Physiology and Chronic Health Evaluation (APACHE) score of 29 or higher, a respiratory rate of 30 or higher, and a pH lower than 7.25 after 2 hours of this therapy.15
GENERAL WARD OR INTENSIVE CARE UNIT?
Mild to moderate COPD exacerbations (in which the pH is 7.30 or higher) can be effectively treated with NIPPV in a general ward if the staff has appropriate expertise.5,18 Keeping the patient in a general ward reduces cost and provides a favorable outcome in selected patients.5,19 However, if the patient’s hemodynamic or mental status deteriorates or if gas exchange, pH, respiratory rate, or dyspnea fail to improve, he or she should be transferred to an intensive care unit and endotracheal intubation should be considered.18 The use of NIPPV in general wards should always be approached with caution and should never be attempted without adequate patient supervision and an experienced respiratory therapy team.
TAKE-HOME MESSAGE
NIPPV has been shown to be an effective adjunct in the treatment of acute hypercapnic respiratory failure secondary to a COPD exacerbation, reducing the need for endotracheal intubation, the length of hospital stay, and the mortality rate. On the basis of controlled trials, NIPPV is now considered the ventilatory therapy of choice in selected patients with this condition. However, it should not be used as a substitute for intubation and mechanical ventilation if these are needed or if the patient is at risk of aspiration.
Yes. In selected patients with hypercapnic respiratory failure due to an acute exacerbation of chronic obstructive pulmonary disease (COPD), noninvasive positive pressure ventilation (NIPPV) is an effective adjunct to usual medical therapy. In controlled trials, it reduced the need for endotracheal intubation, the length of hospital stay, and the risk of death.
Acute COPD exacerbations are responsible for more than 500,000 hospitalizations yearly in the United States, and 6% to 34% of patients die.1
Many patients need invasive ventilatory assistance via an endotracheal tube, but such therapy puts the patient at risk of ventilator-associated pneumonia, pneumothorax, and tracheal stenosis.
WHAT IS NONINVASIVE POSITIVE PRESSURE VENTILATION?
WHY IS IT BENEFICIAL?
Several mechanisms may explain why noninvasive positive pressure ventilation is beneficial in acute exacerbations of COPD.
Patients with decompensated respiratory failure lack sufficient alveolar ventilation, owing to abnormal respiratory mechanics and inspiratory muscle fatigue.10 For these patients, breathing faster does not fully compensate. Noninvasive positive pressure ventilation partially counteracts these factors by providing a larger tidal volume with the same inspiratory effort.10,11
Additionally, this treatment can decrease the work of breathing by partially overcoming auto-PEEP (positive end-expiratory pressure) in certain situations.2 Auto-PEEP is pressure greater than the atmospheric pressure remaining in the alveoli at the end of exhalation.12 This condition is related to limited expiratory flow and is common in those with severe COPD. Noninvasive positive pressure ventilation decreases the pressure difference between the atmosphere and the alveoli, thereby reducing the inspiratory force needed for initiation of inspiratory effort, which may reduce the work of breathing. However, caution should be used when using this therapy in tachypneic patients, in whom NIPPV may not fully overcome the auto-PEEP.
WHAT STUDIES SHOWED
Several randomized trials have shown NIPPV to be beneficial in acute hypercapnic COPD exacerbations. A recent meta-analysis of eight studies13 showed that, compared with usual care alone, this therapy was associated with:
- A lower mortality rate (relative risk 0.41; 95% confidence interval [CI] 0.26–0.64)
- Less need for endotracheal intubation (relative risk 0.42; 95% CI 0.31–0.59)
- A lower rate of treatment failure (relative risk 0.51; 95% CI 0.38–0.67)
- Greater improvements in the 1-hour post-treatment pH and PaCO2 levels
- A lower respiratory rate
- A shorter length of stay in the hospital.
WHICH PATIENTS SHOULD RECEIVE IT?
NIPPV is not suitable for all patients with hypercapnic respiratory failure. It should not be substituted for endotracheal intubation and mechanical ventilation if they are indicated, eg, in patients who are medically unstable because of hypotension, sepsis, hypoxia, or other life-threatening systemic illness. In addition, those who cannot protect the airway, who have had a worsening in mental status, or who have excessive secretions should not undergo NIPPV because they have a high risk of aspiration. Factors that predict that this therapy will fail include an Acute Physiology and Chronic Health Evaluation (APACHE) score of 29 or higher, a respiratory rate of 30 or higher, and a pH lower than 7.25 after 2 hours of this therapy.15
GENERAL WARD OR INTENSIVE CARE UNIT?
Mild to moderate COPD exacerbations (in which the pH is 7.30 or higher) can be effectively treated with NIPPV in a general ward if the staff has appropriate expertise.5,18 Keeping the patient in a general ward reduces cost and provides a favorable outcome in selected patients.5,19 However, if the patient’s hemodynamic or mental status deteriorates or if gas exchange, pH, respiratory rate, or dyspnea fail to improve, he or she should be transferred to an intensive care unit and endotracheal intubation should be considered.18 The use of NIPPV in general wards should always be approached with caution and should never be attempted without adequate patient supervision and an experienced respiratory therapy team.
TAKE-HOME MESSAGE
NIPPV has been shown to be an effective adjunct in the treatment of acute hypercapnic respiratory failure secondary to a COPD exacerbation, reducing the need for endotracheal intubation, the length of hospital stay, and the mortality rate. On the basis of controlled trials, NIPPV is now considered the ventilatory therapy of choice in selected patients with this condition. However, it should not be used as a substitute for intubation and mechanical ventilation if these are needed or if the patient is at risk of aspiration.
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and P for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154:959–967. (Erratum in: Am J Respir Crit Care Med 1997; 155:386).
- Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med 2001; 163:540–577.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995; 151:1799–1806.
- Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000; 355:1931–1935.
- Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest 1995; 107:761–768.
- Masip J, Betbesé AJ, Páez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet 2000; 356:2126–2132.
- Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998; 339:429–435.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA 2002; 288:932–935.
- Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990; 323:1523–1530.
- Mughal MM, Culver DA, Minai OA, Arroliga AC. Auto-positive end-expiratory pressure: mechanisms and treatment. Cleve Clin J Med 2005; 72:801–809.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185.
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax 2002; 57:192–211.
- Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 2005; 25:348–355.
- American Respiratory Care Foundation Consensus Conference. Non-invasive positive pressure ventilation. Respir Care 1997; 42:364–369.
- Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest 1998; 114:1636–1642.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Francaise, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Plant PK, Owen JL, Parrott S, Elliott MW. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ 2003; 326:956.
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and P for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154:959–967. (Erratum in: Am J Respir Crit Care Med 1997; 155:386).
- Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med 2001; 163:540–577.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995; 151:1799–1806.
- Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000; 355:1931–1935.
- Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest 1995; 107:761–768.
- Masip J, Betbesé AJ, Páez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet 2000; 356:2126–2132.
- Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998; 339:429–435.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA 2002; 288:932–935.
- Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990; 323:1523–1530.
- Mughal MM, Culver DA, Minai OA, Arroliga AC. Auto-positive end-expiratory pressure: mechanisms and treatment. Cleve Clin J Med 2005; 72:801–809.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185.
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax 2002; 57:192–211.
- Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 2005; 25:348–355.
- American Respiratory Care Foundation Consensus Conference. Non-invasive positive pressure ventilation. Respir Care 1997; 42:364–369.
- Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest 1998; 114:1636–1642.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Francaise, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Plant PK, Owen JL, Parrott S, Elliott MW. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ 2003; 326:956.
Accuracy of the physical examination in evaluating pleural effusion
In detecting and evaluating pleural effusion, technology has not replaced clinical skills. Yet, despite centuries of lore, data are limited on the role of the physical examination and on its accuracy compared with other noninvasive tests such as conventional chest radiography or ultrasonography.
The following is an overview of the value of the clinical history and physical examination in detecting pleural effusion and a brief review of the available information regarding its accuracy compared with other diagnostic methods.
POTENTIAL CAUSES ARE MANY
The pleurae consist of two membranes that protect the lungs, allow them to move, contribute to their shape, and prevent the alveoli at the pleural surface from becoming overdistended. Between the visceral pleura (covering the lung) and the parietal pleura (covering the diaphragm and the chest wall) is the pleural space.
In healthy adults, the pleural space contains an estimated 5 to 10 mL of pleural fluid (0.1 mg/kg body weight).1 Pleural effusion is an accumulation of an abnormal amount of fluid in the pleural space.
Although the potential causes are many, the most common are congestive heart failure, pneumonia (40% of patients hospitalized with pneumonia have pleural effusion),2,3 cancer, and pulmonary embolism.4
Because many diseases affecting different organs can cause a pleural effusion, we cannot overemphasize the importance of a thorough history and physical examination to uncover clues that will help identify its cause and narrow the diagnostic workup. For example, significant weight loss and cachexia could be due to cancer, and joint, skin, or eye symptoms could be due to a connective tissue disorder.
A thorough review of the patient’s medications is mandatory, since several medications (eg, amiodarone [Cordarone], methotrexate [Rheumatrex, Trexall], and nitrofurantoin [Macrobid]) can be associated with exudative effusions. In addition, the patient’s occupational history must be ascertained, since exposure to asbestos can raise the suspicion of a malignant disease of the pleura such as mesothelioma.
SYMPTOMS ARE NEITHER SENSITIVE NOR SPECIFIC
The symptoms of pleural effusion are neither sensitive nor specific, and many patients have manifestations of the underlying process but not of the effusion itself. The most common symptoms directly related to effusion are cough, dyspnea, and pleuritic chest pain.5
Cough. Many patients with a pleural effusion have a dry, nonproductive cough, a consequence of inflammation of the pleurae or compression of the bronchial walls. Although this symptom is rarely helpful in diagnosing a pleural effusion, if accompanied by purulent sputum it suggests pneumonia, and if complicated by hemoptysis it suggests cancer or pulmonary embolism.
Dyspnea is a consequence of a combination of a restrictive lung defect, a ventilation-perfusion mismatch, and a decrease in cardiac output. Although large pleural effusions reduce lung volume and are generally associated with dyspnea, the symptoms may be out of proportion to the size of the effusion, and patients with small to moderate effusions may also have shortness of breath if their baseline lung function is poor.2
Chest pain accompanying a pleural effusion suggests inflammation of the parietal pleura,6 but could be due to cancer in the chest wall and ribs—or to a benign disease of the thoracic wall such as rib fracture or costo-chondritis.
Pain of pleural origin can remain localized to the adjacent area of the chest, but sometimes it is referred to other areas. If the diaphragmatic pleura is involved, the pain is in many cases referred to the ipsilateral shoulder.5 Pain may also be referred to the abdomen.
Pleuritic chest pain is described as being worse with deep inspiration or when lying down. It is common in patients with pulmonary embolism, parapneumonic effusion, or viral pleurisy, but it can also occur in patients with pneumothorax or pericarditis. A dull, aching chest pain may be due to an underlying pleural malignancy.7
PHYSICAL EXAMINATION: LONG TRADITION, FEW DATA
Our knowledge of the role of physical examination in detecting pleural effusion is still based mostly on expert opinion and on small case series.8,9
Patterson et al11 prospectively compared physical examination (including auscultation, percussion, and tactile fremitus) with bedside ultrasonography and found that physical examination had a lower sensitivity (53% vs 80%, respectively) but a similar specificity (71%).
Bigger effusions are easier to detect
The physical findings are related to the volume of fluid in the pleural effusion and its effects on the chest wall, diaphragm, and lungs. Physical findings are generally normal if less than 300 mL of fluid is present, whereas large effusions (> 1,500 mL) can be associated with significant asymmetry of chest expansion and bulging of intercostal spaces.
Inspection
Although inspection of the chest is not very helpful in detecting a pleural effusion, it can provide other relevant information such as the respiratory rate and the breathing position adopted by the patient (patients with a large pleural effusion may have orthopnea); it can also reveal abnormalities in the shape of the thorax such as the increased anteroposterior diameter (“barrel shape”) seen in patients with chronic obstructive pulmonary disease.18
In addition, by inspection we can assess the expansion of the thorax. The utility of inspecting chest expansion to detect lung restriction was first noted by Laennec19 in 1821. A simple method of evaluating chest expansion is to place a measuring tape around the chest at the level of the nipples to compare the circumference at end-inspiration and at end-expiration.20 In the absence of emphysema, the difference should be at least 2 inches. An expansion of 1.5 inches or less is considered abnormal.21 More relevant to pleural effusion than the amount of overall chest expansion is whether the expansion is symmetrical, which we can assess by palpation.
Palpation
Signs of pleural effusion that can be detected by palpation include asymmetric chest expansion and asymmetric tactile fremitus.
Other signs. Palpation of the chest can also help in detecting underlying disease of the thorax sometimes associated with pleurisy or pleural effusions. Chest wall tumors or skin abscesses may be related to underlying empyema, localized tenderness may be associated with rib fractures or costochondritis, and crepitus may be due to subcutaneous emphysema.
Percussion
The chest can be percussed directly with the tips of the fingers of one hand or indirectly by placing a third finger against the surface to be percussed. There are two main techniques used to detect pleural effusions: comparative percussion and auscultatory percussion.
Since other conditions such as consolidation of the lung and atelectasis can also be associated with dullness to percussion, some authors advocate percussion in the lateral supine position to detect a shift in the dullness that would indicate movement of fluid in the chest.25
The sensitivity of comparative chest percussion and its accuracy related to the size of the effusion are unknown. Kalantri et al10 found that dullness to percussion had a positive predictive value of only 55% but a negative predictive value of 97%, suggesting that the absence of the sign is very helpful in ruling out an effusion.
According to classic textbook descriptions,26 percussive sounds penetrate a maximum of 6 cm (2 cm of body wall thickness and 4 cm of lung), and at least 500 mL of fluid must be present in order to be able to detect an effusion by physical examination.2,8 Most of these descriptions are based on original studies done in cadavers more than 100 years ago.
The auscultatory percussion technique was first described by Laennec and used to delineate the size of several organs by placing the stethoscope directly above the structure to be outlined, followed by percussion from the periphery towards the organ of interest. The original technique was subsequently modified for the examination of the chest by Guarino.27
Although auscultatory percussion was used initially to try to detect lung lesions, masses and consolidations, Guarino and Guarino12 found this technique to be highly effective in detecting pleural effusion. In a prospective blinded study in 118 patients, this method was highly (95%) sensitive and 100% specific in detecting pleural effusion, even in patients with obesity, pneumonia, or other pleural abnormalities. Of note, their findings suggested that auscultatory percussion can detect as little as 50 mL of pleural fluid.
Bohadana et al13 compared auscultatory and conventional percussion with chest radiographic findings in 281 patients. They found that auscultatory percussion was 100% sensitive for detecting large pleural effusions.
However, when Bourke et al14 compared conventional and auscultatory percussion in 21 patients with abnormal radiographs, both methods had low sensitivity (15.4% vs 19.2%) but high specificity (97.3% vs 85.1%, respectively). It is important to mention that in this series only a few patients had a pleural effusion.
McDermott et al16 compared conventional and auscultatory percussion in detecting pleural effusion in 14 hospitalized patients, using ultrasonography instead of chest radiography as the gold standard for comparison. The findings on auscultatory percussion correlated better with the findings on ultrasonography than did those on conventional percussion. The authors gave no information about sensitivity or specificity.
Kalantri et al10 found that auscultatory percussion had a sensitivity of 58% and a specificity of 85%.
Auscultation
Originally described by Laennec (who invented the stethoscope),19 auscultation is perhaps the physical examination technique most used to detect pleural effusion.
Lichtenstein et al15 performed a study of auscultation in critically ill patients and found it to have a very low sensitivity (42%) but a higher specificity (90%), with an overall diagnostic accuracy of 60%. Of note, compared with chest radiography, auscultatory findings had similar sensitivity but higher accuracy.
Absent or diminished breath sounds strongly suggest an effusion.19
Egophonism. Laennec also described egophonism as a pathognomonic sign associated with a moderate degree of effusion. The word egophony comes from the Greek “ego,” which means goat; it is used to describe the change in the pronounced sound of E to A. The mechanism responsible for finding this sign in massive pleural effusions is probably upward displacement and compression or consolidation of the lung at the top of the effusion.
However, if the effusion is small, the consolidation will not be large enough to produce this sign. Similarly, other lung conditions associated with large consolidations may produce egophonism without a pleural effusion. Little is known about the predictive value of this sign, and significant interob-server variability needs to be taken into account.28
Pleural rub. Pleural effusions that result from any disease that causes direct inflammation of the pleurae can be associated with a pleural rub. This sound, classically described as rubbing of unoiled leather, is pathognomonic of pleural disease but not of pleural effusion. In fact, a pleural rub will disappear once an effusion develops. Little is known about the accuracy of this finding; in the study by Kalantri et al it had a very low sensitivity (5%) but a very high specificity (99%).10 The differential diagnosis includes pleuritis, pneumonia, mesothelioma, and tumors that metastasize to the pleura.
- Noppen M, De Waele M, Li R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162:1023–1026.
- Bouros D. Pleural Disease. New York: Marcel Dekker, 2004.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3:75–80.
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346:1971–1977.
- Light RW. Pleural Diseases, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest 1995; 107:1598–1603.
- Leopold SS, Hopkins HU. Leopold’s Principles and Methods of Physical Diagnosis, 3d ed. Philadelphia: W.B. Saunders, 1965.
- Norris GW, Landis HRM, Montgomery CM, Krumbhaar EB. Diseases of the Chest and the Principles of Physical Diagnosis, 4th ed, rev. Philadelphia, W.B. Saunders, 1929.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Patterson LA, Costantino TG, Satz WA. Diagnosing pleural effusion: a prospective comparison of physical examination with bedside ultra-sonography [abstract]. Ann Emerg Med 2004; 44:S112.
- Guarino JR, Guarino JC. Auscultatory percussion: a simple method to detect pleural effusion. J Gen Intern Med 1994; 9:71–74.
- Bohadana AB, Coimbra FT, Santiago JR. Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion. Respiration 1986; 50:218–225.
- Bourke S, Nunes D, Stafford F, Hurley G, Graham I. Percussion of the chest re-visited: a comparison of the diagnostic value of auscultatory and conventional chest percussion. Ir J Med Sci 1989; 158:82–84.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100:9–15.
- McDermott TD, McCarthy M, Chestnut T, Schumann L. A comparison of conventional percussion and auscultation percussion in the detection of pleural effusions of hospitalized patients. J Am Acad Nurse Pract 1997; 9:483–486.
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441.
- Pierce JA, Ebert RV. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am J Med 1958; 25:13–22.
- Laennec RTH. A Treatise On the Disease[s] of the Chest. New York: Library of the New York Academy of Medicine and Hafner Publishing Company, 1962.
- Bockenhauer SE, Chen H, Julliard KN, Weedon J. Measuring thoracic excursion: reliability of the cloth tape measure technique. J Am Osteopath Assoc 2007; 107:191–196.
- Fries JF. The reactive enthesopathies. Dis Mon 1985; 31 1:1–46.
- McGee SR. Evidence-based physical diagnosis. Philadelphia: W.B. Saunders, 2001.
- Auenbrugger L, Forbes J. On percussion of the chest: being a translation of Auenbrugger’s original treatise entitled “Inventum novum ex percussione thoracis humani, ut signo abstrusos interni pectoris morbos detegendi” (Vienna, 1761). Baltimore: The Johns Hopkins Press, 1936.
- Auenbrugger L, Neuburger M. Inventum Novum. London: Reprinted for Dawsons of Pall Mall, 1966.
- Gilbert VE. Shifting percussion dullness of the chest: a sign of pleural effusion. South Med J 1997; 90:1255–1256.
- Weil A. Handbuch und Atlas der topographischen Percussion nebst einer Darstellung der Lehre vom Percussionsschall. Leipzig: Vogel, 1880.
- Guarino JR. Auscultatory percussion, a new aid in the examination of the chest. J Kansas Med Soc 1974; 75:193–194.
- Sapira JD. About egophony. Chest 1995; 108:865–867.
In detecting and evaluating pleural effusion, technology has not replaced clinical skills. Yet, despite centuries of lore, data are limited on the role of the physical examination and on its accuracy compared with other noninvasive tests such as conventional chest radiography or ultrasonography.
The following is an overview of the value of the clinical history and physical examination in detecting pleural effusion and a brief review of the available information regarding its accuracy compared with other diagnostic methods.
POTENTIAL CAUSES ARE MANY
The pleurae consist of two membranes that protect the lungs, allow them to move, contribute to their shape, and prevent the alveoli at the pleural surface from becoming overdistended. Between the visceral pleura (covering the lung) and the parietal pleura (covering the diaphragm and the chest wall) is the pleural space.
In healthy adults, the pleural space contains an estimated 5 to 10 mL of pleural fluid (0.1 mg/kg body weight).1 Pleural effusion is an accumulation of an abnormal amount of fluid in the pleural space.
Although the potential causes are many, the most common are congestive heart failure, pneumonia (40% of patients hospitalized with pneumonia have pleural effusion),2,3 cancer, and pulmonary embolism.4
Because many diseases affecting different organs can cause a pleural effusion, we cannot overemphasize the importance of a thorough history and physical examination to uncover clues that will help identify its cause and narrow the diagnostic workup. For example, significant weight loss and cachexia could be due to cancer, and joint, skin, or eye symptoms could be due to a connective tissue disorder.
A thorough review of the patient’s medications is mandatory, since several medications (eg, amiodarone [Cordarone], methotrexate [Rheumatrex, Trexall], and nitrofurantoin [Macrobid]) can be associated with exudative effusions. In addition, the patient’s occupational history must be ascertained, since exposure to asbestos can raise the suspicion of a malignant disease of the pleura such as mesothelioma.
SYMPTOMS ARE NEITHER SENSITIVE NOR SPECIFIC
The symptoms of pleural effusion are neither sensitive nor specific, and many patients have manifestations of the underlying process but not of the effusion itself. The most common symptoms directly related to effusion are cough, dyspnea, and pleuritic chest pain.5
Cough. Many patients with a pleural effusion have a dry, nonproductive cough, a consequence of inflammation of the pleurae or compression of the bronchial walls. Although this symptom is rarely helpful in diagnosing a pleural effusion, if accompanied by purulent sputum it suggests pneumonia, and if complicated by hemoptysis it suggests cancer or pulmonary embolism.
Dyspnea is a consequence of a combination of a restrictive lung defect, a ventilation-perfusion mismatch, and a decrease in cardiac output. Although large pleural effusions reduce lung volume and are generally associated with dyspnea, the symptoms may be out of proportion to the size of the effusion, and patients with small to moderate effusions may also have shortness of breath if their baseline lung function is poor.2
Chest pain accompanying a pleural effusion suggests inflammation of the parietal pleura,6 but could be due to cancer in the chest wall and ribs—or to a benign disease of the thoracic wall such as rib fracture or costo-chondritis.
Pain of pleural origin can remain localized to the adjacent area of the chest, but sometimes it is referred to other areas. If the diaphragmatic pleura is involved, the pain is in many cases referred to the ipsilateral shoulder.5 Pain may also be referred to the abdomen.
Pleuritic chest pain is described as being worse with deep inspiration or when lying down. It is common in patients with pulmonary embolism, parapneumonic effusion, or viral pleurisy, but it can also occur in patients with pneumothorax or pericarditis. A dull, aching chest pain may be due to an underlying pleural malignancy.7
PHYSICAL EXAMINATION: LONG TRADITION, FEW DATA
Our knowledge of the role of physical examination in detecting pleural effusion is still based mostly on expert opinion and on small case series.8,9
Patterson et al11 prospectively compared physical examination (including auscultation, percussion, and tactile fremitus) with bedside ultrasonography and found that physical examination had a lower sensitivity (53% vs 80%, respectively) but a similar specificity (71%).
Bigger effusions are easier to detect
The physical findings are related to the volume of fluid in the pleural effusion and its effects on the chest wall, diaphragm, and lungs. Physical findings are generally normal if less than 300 mL of fluid is present, whereas large effusions (> 1,500 mL) can be associated with significant asymmetry of chest expansion and bulging of intercostal spaces.
Inspection
Although inspection of the chest is not very helpful in detecting a pleural effusion, it can provide other relevant information such as the respiratory rate and the breathing position adopted by the patient (patients with a large pleural effusion may have orthopnea); it can also reveal abnormalities in the shape of the thorax such as the increased anteroposterior diameter (“barrel shape”) seen in patients with chronic obstructive pulmonary disease.18
In addition, by inspection we can assess the expansion of the thorax. The utility of inspecting chest expansion to detect lung restriction was first noted by Laennec19 in 1821. A simple method of evaluating chest expansion is to place a measuring tape around the chest at the level of the nipples to compare the circumference at end-inspiration and at end-expiration.20 In the absence of emphysema, the difference should be at least 2 inches. An expansion of 1.5 inches or less is considered abnormal.21 More relevant to pleural effusion than the amount of overall chest expansion is whether the expansion is symmetrical, which we can assess by palpation.
Palpation
Signs of pleural effusion that can be detected by palpation include asymmetric chest expansion and asymmetric tactile fremitus.
Other signs. Palpation of the chest can also help in detecting underlying disease of the thorax sometimes associated with pleurisy or pleural effusions. Chest wall tumors or skin abscesses may be related to underlying empyema, localized tenderness may be associated with rib fractures or costochondritis, and crepitus may be due to subcutaneous emphysema.
Percussion
The chest can be percussed directly with the tips of the fingers of one hand or indirectly by placing a third finger against the surface to be percussed. There are two main techniques used to detect pleural effusions: comparative percussion and auscultatory percussion.
Since other conditions such as consolidation of the lung and atelectasis can also be associated with dullness to percussion, some authors advocate percussion in the lateral supine position to detect a shift in the dullness that would indicate movement of fluid in the chest.25
The sensitivity of comparative chest percussion and its accuracy related to the size of the effusion are unknown. Kalantri et al10 found that dullness to percussion had a positive predictive value of only 55% but a negative predictive value of 97%, suggesting that the absence of the sign is very helpful in ruling out an effusion.
According to classic textbook descriptions,26 percussive sounds penetrate a maximum of 6 cm (2 cm of body wall thickness and 4 cm of lung), and at least 500 mL of fluid must be present in order to be able to detect an effusion by physical examination.2,8 Most of these descriptions are based on original studies done in cadavers more than 100 years ago.
The auscultatory percussion technique was first described by Laennec and used to delineate the size of several organs by placing the stethoscope directly above the structure to be outlined, followed by percussion from the periphery towards the organ of interest. The original technique was subsequently modified for the examination of the chest by Guarino.27
Although auscultatory percussion was used initially to try to detect lung lesions, masses and consolidations, Guarino and Guarino12 found this technique to be highly effective in detecting pleural effusion. In a prospective blinded study in 118 patients, this method was highly (95%) sensitive and 100% specific in detecting pleural effusion, even in patients with obesity, pneumonia, or other pleural abnormalities. Of note, their findings suggested that auscultatory percussion can detect as little as 50 mL of pleural fluid.
Bohadana et al13 compared auscultatory and conventional percussion with chest radiographic findings in 281 patients. They found that auscultatory percussion was 100% sensitive for detecting large pleural effusions.
However, when Bourke et al14 compared conventional and auscultatory percussion in 21 patients with abnormal radiographs, both methods had low sensitivity (15.4% vs 19.2%) but high specificity (97.3% vs 85.1%, respectively). It is important to mention that in this series only a few patients had a pleural effusion.
McDermott et al16 compared conventional and auscultatory percussion in detecting pleural effusion in 14 hospitalized patients, using ultrasonography instead of chest radiography as the gold standard for comparison. The findings on auscultatory percussion correlated better with the findings on ultrasonography than did those on conventional percussion. The authors gave no information about sensitivity or specificity.
Kalantri et al10 found that auscultatory percussion had a sensitivity of 58% and a specificity of 85%.
Auscultation
Originally described by Laennec (who invented the stethoscope),19 auscultation is perhaps the physical examination technique most used to detect pleural effusion.
Lichtenstein et al15 performed a study of auscultation in critically ill patients and found it to have a very low sensitivity (42%) but a higher specificity (90%), with an overall diagnostic accuracy of 60%. Of note, compared with chest radiography, auscultatory findings had similar sensitivity but higher accuracy.
Absent or diminished breath sounds strongly suggest an effusion.19
Egophonism. Laennec also described egophonism as a pathognomonic sign associated with a moderate degree of effusion. The word egophony comes from the Greek “ego,” which means goat; it is used to describe the change in the pronounced sound of E to A. The mechanism responsible for finding this sign in massive pleural effusions is probably upward displacement and compression or consolidation of the lung at the top of the effusion.
However, if the effusion is small, the consolidation will not be large enough to produce this sign. Similarly, other lung conditions associated with large consolidations may produce egophonism without a pleural effusion. Little is known about the predictive value of this sign, and significant interob-server variability needs to be taken into account.28
Pleural rub. Pleural effusions that result from any disease that causes direct inflammation of the pleurae can be associated with a pleural rub. This sound, classically described as rubbing of unoiled leather, is pathognomonic of pleural disease but not of pleural effusion. In fact, a pleural rub will disappear once an effusion develops. Little is known about the accuracy of this finding; in the study by Kalantri et al it had a very low sensitivity (5%) but a very high specificity (99%).10 The differential diagnosis includes pleuritis, pneumonia, mesothelioma, and tumors that metastasize to the pleura.
In detecting and evaluating pleural effusion, technology has not replaced clinical skills. Yet, despite centuries of lore, data are limited on the role of the physical examination and on its accuracy compared with other noninvasive tests such as conventional chest radiography or ultrasonography.
The following is an overview of the value of the clinical history and physical examination in detecting pleural effusion and a brief review of the available information regarding its accuracy compared with other diagnostic methods.
POTENTIAL CAUSES ARE MANY
The pleurae consist of two membranes that protect the lungs, allow them to move, contribute to their shape, and prevent the alveoli at the pleural surface from becoming overdistended. Between the visceral pleura (covering the lung) and the parietal pleura (covering the diaphragm and the chest wall) is the pleural space.
In healthy adults, the pleural space contains an estimated 5 to 10 mL of pleural fluid (0.1 mg/kg body weight).1 Pleural effusion is an accumulation of an abnormal amount of fluid in the pleural space.
Although the potential causes are many, the most common are congestive heart failure, pneumonia (40% of patients hospitalized with pneumonia have pleural effusion),2,3 cancer, and pulmonary embolism.4
Because many diseases affecting different organs can cause a pleural effusion, we cannot overemphasize the importance of a thorough history and physical examination to uncover clues that will help identify its cause and narrow the diagnostic workup. For example, significant weight loss and cachexia could be due to cancer, and joint, skin, or eye symptoms could be due to a connective tissue disorder.
A thorough review of the patient’s medications is mandatory, since several medications (eg, amiodarone [Cordarone], methotrexate [Rheumatrex, Trexall], and nitrofurantoin [Macrobid]) can be associated with exudative effusions. In addition, the patient’s occupational history must be ascertained, since exposure to asbestos can raise the suspicion of a malignant disease of the pleura such as mesothelioma.
SYMPTOMS ARE NEITHER SENSITIVE NOR SPECIFIC
The symptoms of pleural effusion are neither sensitive nor specific, and many patients have manifestations of the underlying process but not of the effusion itself. The most common symptoms directly related to effusion are cough, dyspnea, and pleuritic chest pain.5
Cough. Many patients with a pleural effusion have a dry, nonproductive cough, a consequence of inflammation of the pleurae or compression of the bronchial walls. Although this symptom is rarely helpful in diagnosing a pleural effusion, if accompanied by purulent sputum it suggests pneumonia, and if complicated by hemoptysis it suggests cancer or pulmonary embolism.
Dyspnea is a consequence of a combination of a restrictive lung defect, a ventilation-perfusion mismatch, and a decrease in cardiac output. Although large pleural effusions reduce lung volume and are generally associated with dyspnea, the symptoms may be out of proportion to the size of the effusion, and patients with small to moderate effusions may also have shortness of breath if their baseline lung function is poor.2
Chest pain accompanying a pleural effusion suggests inflammation of the parietal pleura,6 but could be due to cancer in the chest wall and ribs—or to a benign disease of the thoracic wall such as rib fracture or costo-chondritis.
Pain of pleural origin can remain localized to the adjacent area of the chest, but sometimes it is referred to other areas. If the diaphragmatic pleura is involved, the pain is in many cases referred to the ipsilateral shoulder.5 Pain may also be referred to the abdomen.
Pleuritic chest pain is described as being worse with deep inspiration or when lying down. It is common in patients with pulmonary embolism, parapneumonic effusion, or viral pleurisy, but it can also occur in patients with pneumothorax or pericarditis. A dull, aching chest pain may be due to an underlying pleural malignancy.7
PHYSICAL EXAMINATION: LONG TRADITION, FEW DATA
Our knowledge of the role of physical examination in detecting pleural effusion is still based mostly on expert opinion and on small case series.8,9
Patterson et al11 prospectively compared physical examination (including auscultation, percussion, and tactile fremitus) with bedside ultrasonography and found that physical examination had a lower sensitivity (53% vs 80%, respectively) but a similar specificity (71%).
Bigger effusions are easier to detect
The physical findings are related to the volume of fluid in the pleural effusion and its effects on the chest wall, diaphragm, and lungs. Physical findings are generally normal if less than 300 mL of fluid is present, whereas large effusions (> 1,500 mL) can be associated with significant asymmetry of chest expansion and bulging of intercostal spaces.
Inspection
Although inspection of the chest is not very helpful in detecting a pleural effusion, it can provide other relevant information such as the respiratory rate and the breathing position adopted by the patient (patients with a large pleural effusion may have orthopnea); it can also reveal abnormalities in the shape of the thorax such as the increased anteroposterior diameter (“barrel shape”) seen in patients with chronic obstructive pulmonary disease.18
In addition, by inspection we can assess the expansion of the thorax. The utility of inspecting chest expansion to detect lung restriction was first noted by Laennec19 in 1821. A simple method of evaluating chest expansion is to place a measuring tape around the chest at the level of the nipples to compare the circumference at end-inspiration and at end-expiration.20 In the absence of emphysema, the difference should be at least 2 inches. An expansion of 1.5 inches or less is considered abnormal.21 More relevant to pleural effusion than the amount of overall chest expansion is whether the expansion is symmetrical, which we can assess by palpation.
Palpation
Signs of pleural effusion that can be detected by palpation include asymmetric chest expansion and asymmetric tactile fremitus.
Other signs. Palpation of the chest can also help in detecting underlying disease of the thorax sometimes associated with pleurisy or pleural effusions. Chest wall tumors or skin abscesses may be related to underlying empyema, localized tenderness may be associated with rib fractures or costochondritis, and crepitus may be due to subcutaneous emphysema.
Percussion
The chest can be percussed directly with the tips of the fingers of one hand or indirectly by placing a third finger against the surface to be percussed. There are two main techniques used to detect pleural effusions: comparative percussion and auscultatory percussion.
Since other conditions such as consolidation of the lung and atelectasis can also be associated with dullness to percussion, some authors advocate percussion in the lateral supine position to detect a shift in the dullness that would indicate movement of fluid in the chest.25
The sensitivity of comparative chest percussion and its accuracy related to the size of the effusion are unknown. Kalantri et al10 found that dullness to percussion had a positive predictive value of only 55% but a negative predictive value of 97%, suggesting that the absence of the sign is very helpful in ruling out an effusion.
According to classic textbook descriptions,26 percussive sounds penetrate a maximum of 6 cm (2 cm of body wall thickness and 4 cm of lung), and at least 500 mL of fluid must be present in order to be able to detect an effusion by physical examination.2,8 Most of these descriptions are based on original studies done in cadavers more than 100 years ago.
The auscultatory percussion technique was first described by Laennec and used to delineate the size of several organs by placing the stethoscope directly above the structure to be outlined, followed by percussion from the periphery towards the organ of interest. The original technique was subsequently modified for the examination of the chest by Guarino.27
Although auscultatory percussion was used initially to try to detect lung lesions, masses and consolidations, Guarino and Guarino12 found this technique to be highly effective in detecting pleural effusion. In a prospective blinded study in 118 patients, this method was highly (95%) sensitive and 100% specific in detecting pleural effusion, even in patients with obesity, pneumonia, or other pleural abnormalities. Of note, their findings suggested that auscultatory percussion can detect as little as 50 mL of pleural fluid.
Bohadana et al13 compared auscultatory and conventional percussion with chest radiographic findings in 281 patients. They found that auscultatory percussion was 100% sensitive for detecting large pleural effusions.
However, when Bourke et al14 compared conventional and auscultatory percussion in 21 patients with abnormal radiographs, both methods had low sensitivity (15.4% vs 19.2%) but high specificity (97.3% vs 85.1%, respectively). It is important to mention that in this series only a few patients had a pleural effusion.
McDermott et al16 compared conventional and auscultatory percussion in detecting pleural effusion in 14 hospitalized patients, using ultrasonography instead of chest radiography as the gold standard for comparison. The findings on auscultatory percussion correlated better with the findings on ultrasonography than did those on conventional percussion. The authors gave no information about sensitivity or specificity.
Kalantri et al10 found that auscultatory percussion had a sensitivity of 58% and a specificity of 85%.
Auscultation
Originally described by Laennec (who invented the stethoscope),19 auscultation is perhaps the physical examination technique most used to detect pleural effusion.
Lichtenstein et al15 performed a study of auscultation in critically ill patients and found it to have a very low sensitivity (42%) but a higher specificity (90%), with an overall diagnostic accuracy of 60%. Of note, compared with chest radiography, auscultatory findings had similar sensitivity but higher accuracy.
Absent or diminished breath sounds strongly suggest an effusion.19
Egophonism. Laennec also described egophonism as a pathognomonic sign associated with a moderate degree of effusion. The word egophony comes from the Greek “ego,” which means goat; it is used to describe the change in the pronounced sound of E to A. The mechanism responsible for finding this sign in massive pleural effusions is probably upward displacement and compression or consolidation of the lung at the top of the effusion.
However, if the effusion is small, the consolidation will not be large enough to produce this sign. Similarly, other lung conditions associated with large consolidations may produce egophonism without a pleural effusion. Little is known about the predictive value of this sign, and significant interob-server variability needs to be taken into account.28
Pleural rub. Pleural effusions that result from any disease that causes direct inflammation of the pleurae can be associated with a pleural rub. This sound, classically described as rubbing of unoiled leather, is pathognomonic of pleural disease but not of pleural effusion. In fact, a pleural rub will disappear once an effusion develops. Little is known about the accuracy of this finding; in the study by Kalantri et al it had a very low sensitivity (5%) but a very high specificity (99%).10 The differential diagnosis includes pleuritis, pneumonia, mesothelioma, and tumors that metastasize to the pleura.
- Noppen M, De Waele M, Li R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162:1023–1026.
- Bouros D. Pleural Disease. New York: Marcel Dekker, 2004.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3:75–80.
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346:1971–1977.
- Light RW. Pleural Diseases, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest 1995; 107:1598–1603.
- Leopold SS, Hopkins HU. Leopold’s Principles and Methods of Physical Diagnosis, 3d ed. Philadelphia: W.B. Saunders, 1965.
- Norris GW, Landis HRM, Montgomery CM, Krumbhaar EB. Diseases of the Chest and the Principles of Physical Diagnosis, 4th ed, rev. Philadelphia, W.B. Saunders, 1929.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Patterson LA, Costantino TG, Satz WA. Diagnosing pleural effusion: a prospective comparison of physical examination with bedside ultra-sonography [abstract]. Ann Emerg Med 2004; 44:S112.
- Guarino JR, Guarino JC. Auscultatory percussion: a simple method to detect pleural effusion. J Gen Intern Med 1994; 9:71–74.
- Bohadana AB, Coimbra FT, Santiago JR. Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion. Respiration 1986; 50:218–225.
- Bourke S, Nunes D, Stafford F, Hurley G, Graham I. Percussion of the chest re-visited: a comparison of the diagnostic value of auscultatory and conventional chest percussion. Ir J Med Sci 1989; 158:82–84.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100:9–15.
- McDermott TD, McCarthy M, Chestnut T, Schumann L. A comparison of conventional percussion and auscultation percussion in the detection of pleural effusions of hospitalized patients. J Am Acad Nurse Pract 1997; 9:483–486.
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441.
- Pierce JA, Ebert RV. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am J Med 1958; 25:13–22.
- Laennec RTH. A Treatise On the Disease[s] of the Chest. New York: Library of the New York Academy of Medicine and Hafner Publishing Company, 1962.
- Bockenhauer SE, Chen H, Julliard KN, Weedon J. Measuring thoracic excursion: reliability of the cloth tape measure technique. J Am Osteopath Assoc 2007; 107:191–196.
- Fries JF. The reactive enthesopathies. Dis Mon 1985; 31 1:1–46.
- McGee SR. Evidence-based physical diagnosis. Philadelphia: W.B. Saunders, 2001.
- Auenbrugger L, Forbes J. On percussion of the chest: being a translation of Auenbrugger’s original treatise entitled “Inventum novum ex percussione thoracis humani, ut signo abstrusos interni pectoris morbos detegendi” (Vienna, 1761). Baltimore: The Johns Hopkins Press, 1936.
- Auenbrugger L, Neuburger M. Inventum Novum. London: Reprinted for Dawsons of Pall Mall, 1966.
- Gilbert VE. Shifting percussion dullness of the chest: a sign of pleural effusion. South Med J 1997; 90:1255–1256.
- Weil A. Handbuch und Atlas der topographischen Percussion nebst einer Darstellung der Lehre vom Percussionsschall. Leipzig: Vogel, 1880.
- Guarino JR. Auscultatory percussion, a new aid in the examination of the chest. J Kansas Med Soc 1974; 75:193–194.
- Sapira JD. About egophony. Chest 1995; 108:865–867.
- Noppen M, De Waele M, Li R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med 2000; 162:1023–1026.
- Bouros D. Pleural Disease. New York: Marcel Dekker, 2004.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3:75–80.
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346:1971–1977.
- Light RW. Pleural Diseases, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest 1995; 107:1598–1603.
- Leopold SS, Hopkins HU. Leopold’s Principles and Methods of Physical Diagnosis, 3d ed. Philadelphia: W.B. Saunders, 1965.
- Norris GW, Landis HRM, Montgomery CM, Krumbhaar EB. Diseases of the Chest and the Principles of Physical Diagnosis, 4th ed, rev. Philadelphia, W.B. Saunders, 1929.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Patterson LA, Costantino TG, Satz WA. Diagnosing pleural effusion: a prospective comparison of physical examination with bedside ultra-sonography [abstract]. Ann Emerg Med 2004; 44:S112.
- Guarino JR, Guarino JC. Auscultatory percussion: a simple method to detect pleural effusion. J Gen Intern Med 1994; 9:71–74.
- Bohadana AB, Coimbra FT, Santiago JR. Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion. Respiration 1986; 50:218–225.
- Bourke S, Nunes D, Stafford F, Hurley G, Graham I. Percussion of the chest re-visited: a comparison of the diagnostic value of auscultatory and conventional chest percussion. Ir J Med Sci 1989; 158:82–84.
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100:9–15.
- McDermott TD, McCarthy M, Chestnut T, Schumann L. A comparison of conventional percussion and auscultation percussion in the detection of pleural effusions of hospitalized patients. J Am Acad Nurse Pract 1997; 9:483–486.
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:436–441.
- Pierce JA, Ebert RV. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am J Med 1958; 25:13–22.
- Laennec RTH. A Treatise On the Disease[s] of the Chest. New York: Library of the New York Academy of Medicine and Hafner Publishing Company, 1962.
- Bockenhauer SE, Chen H, Julliard KN, Weedon J. Measuring thoracic excursion: reliability of the cloth tape measure technique. J Am Osteopath Assoc 2007; 107:191–196.
- Fries JF. The reactive enthesopathies. Dis Mon 1985; 31 1:1–46.
- McGee SR. Evidence-based physical diagnosis. Philadelphia: W.B. Saunders, 2001.
- Auenbrugger L, Forbes J. On percussion of the chest: being a translation of Auenbrugger’s original treatise entitled “Inventum novum ex percussione thoracis humani, ut signo abstrusos interni pectoris morbos detegendi” (Vienna, 1761). Baltimore: The Johns Hopkins Press, 1936.
- Auenbrugger L, Neuburger M. Inventum Novum. London: Reprinted for Dawsons of Pall Mall, 1966.
- Gilbert VE. Shifting percussion dullness of the chest: a sign of pleural effusion. South Med J 1997; 90:1255–1256.
- Weil A. Handbuch und Atlas der topographischen Percussion nebst einer Darstellung der Lehre vom Percussionsschall. Leipzig: Vogel, 1880.
- Guarino JR. Auscultatory percussion, a new aid in the examination of the chest. J Kansas Med Soc 1974; 75:193–194.
- Sapira JD. About egophony. Chest 1995; 108:865–867.
KEY POINTS
- The potential causes of pleural effusion are many and include congestive heart failure, pneumonia, cancer, and pulmonary embolism.
- Cardinal symptoms of pleural effusion are cough, chest pain, and dyspnea, but these are not very sensitive or specific.
- Common signs of pleural effusion are asymmetric chest expansion, asymmetric tactile fremitus, dullness to percussion, absent or diminished breath sounds, and rubs. The larger the effusion, the more sensitive these signs are.
- Some have advocated auscultatory percussion (tapping on the manubrium while listening on the patient’s back) as being more sensitive than conventional percussion for detecting the dullness to percussion of pleural effusion.