User login
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
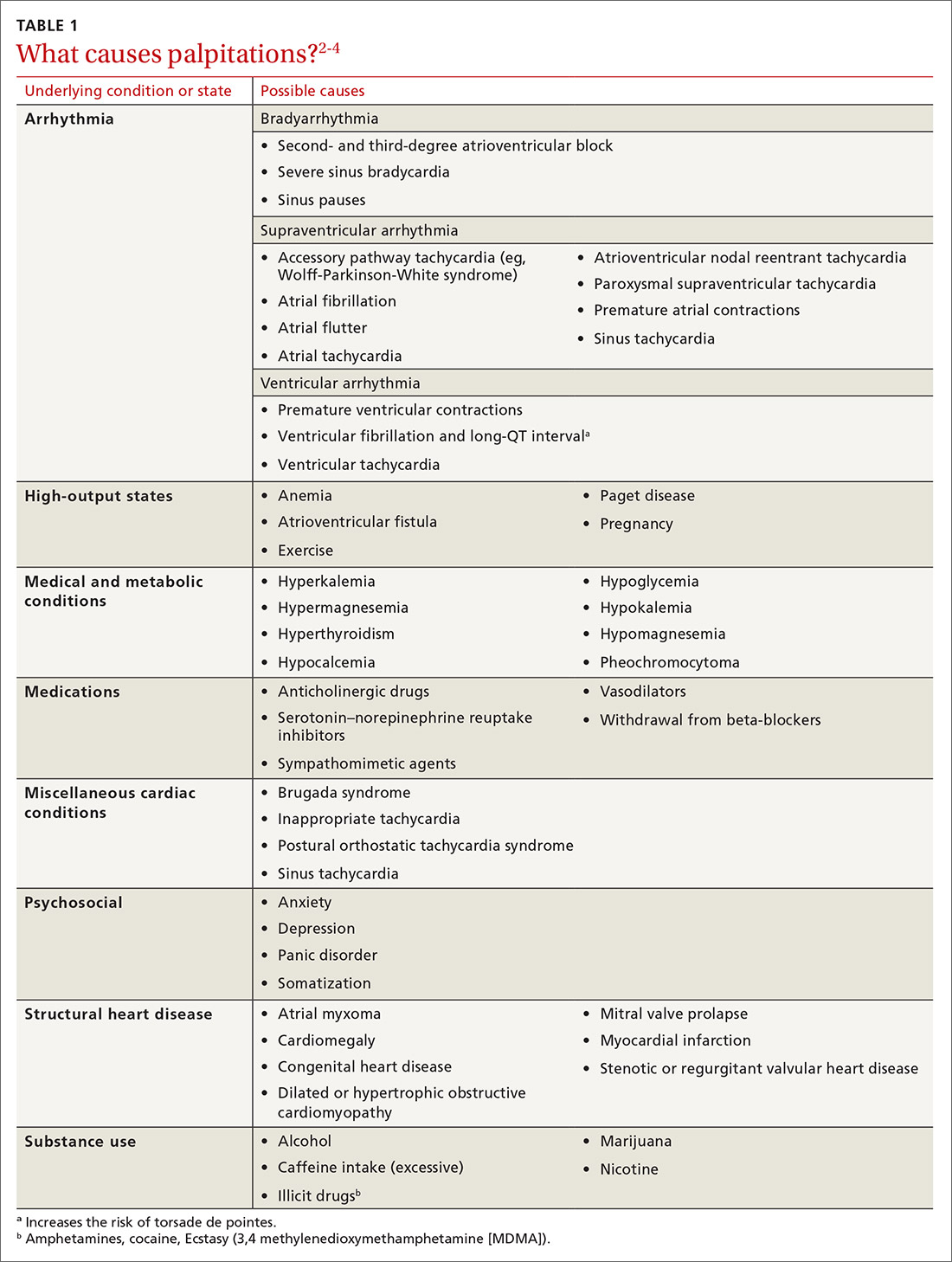
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
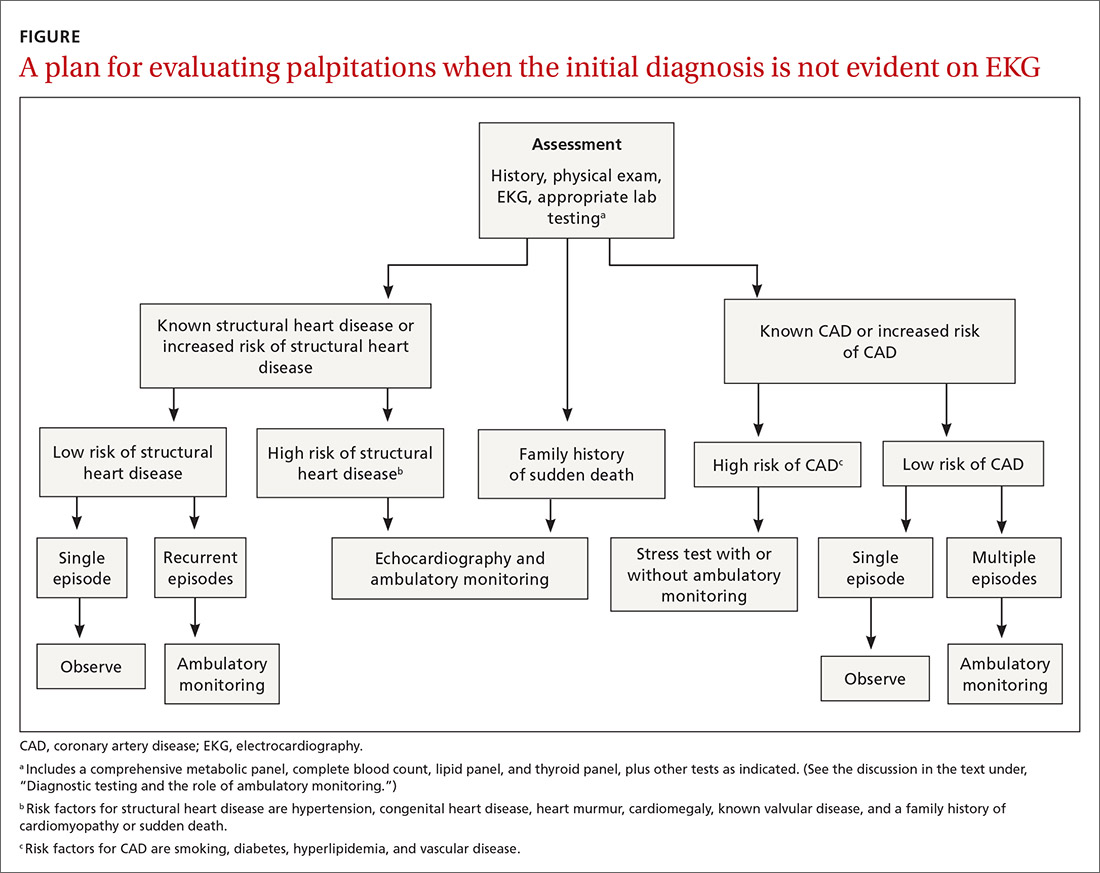
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
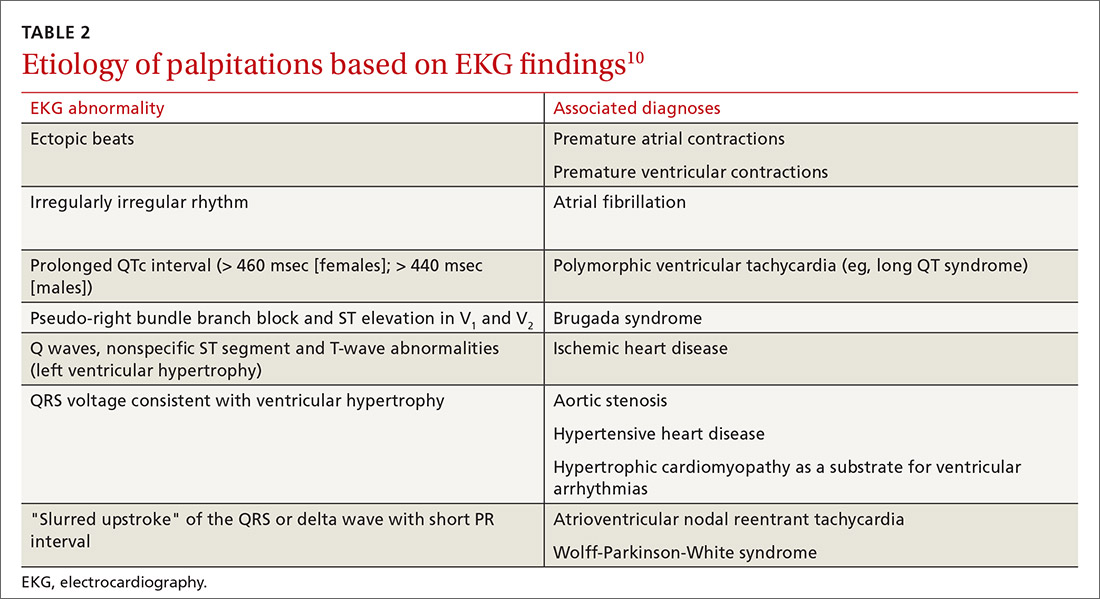
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)

Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).

Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).

Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)

Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).

Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).

Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; [email protected].
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
PRACTICE RECOMMENDATIONS
› Order echocardiography for patients who have palpitations and risk factors for structural heart disease. C
› Order stress testing for patients who have exertional symptoms or multiple risk factors for coronary artery disease. C
› Evaluate all patients who have syncope associated with their palpitations for a cardiac cause. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series