User login
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
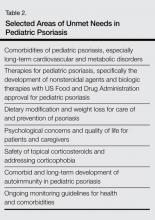
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
Practice Points
- The majority of children with psoriasis have severe disease, scalp involvement, and a family history.
- Pediatric psoriasis is associated with metabolic syndrome, especially obesity.
- Anxiety and depression may be noted in children with psoriasis as well as their caregivers.