User login
VANCOUVER, B.C. – Manipulating the skin microbiome might one day help control atopic dermatitis, Dr. Thomas Bieber said at the World Congress of Dermatology.
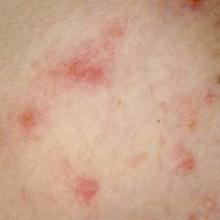
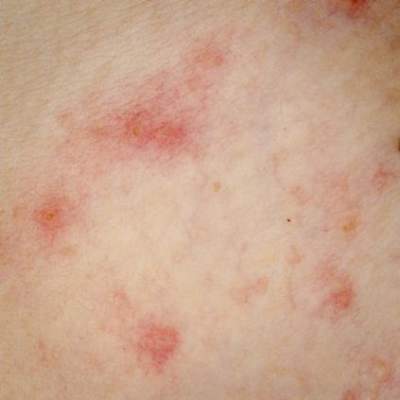
Scientists used to focus mainly on pathogens when considering how skin microbes affect atopic dermatitis (AD), said Dr. Bieber, professor of dermatology at the University of Bonn (Germany). But while Staphylococcus aureus is “the constant companion” of patients with AD, data from recent studies have shown that commensal microorganisms found on and within healthy skin undergo a “dialogue” with epithelial cells to help regulate antimicrobial peptides, which in turn inhibit pathogen growth, he said.
Flares in atopic dermatitis are marked by dramatic rises in S. aureus and corresponding decreases in commensal microbes, he noted. Whether an AD flare causes this microbial shift or results from it remains unclear, “but based on this knowledge, we are thinking about strategies to modulate and restore the microbiome. We have discovered a new kind of link to how the microbiome might affect atopic dermatitis,” Dr. Bieber explained.
In the future, such strategies might include formulating emollients to rebalance microbial diversity, he said, pointing to the landmark trial of fecal transplantation in patients with recurrent Clostridium difficile infections (N. Engl. J. Med. 2013;368:407-15). “That study was very impressive – the protocol was stopped due to the overwhelming benefits,” he said. “In terms of skin, could we imagine something similar?”
Restoring skin microbial diversity also might help prevent flares and worsening of AD, said Dr. Bieber. “We can control atopic dermatitis by controlling inflammation, dryness, improving the microbiota with appropriate emollients, and trying to hit early and efficiently,” he said. “We should not only think in terms of treatment, but in terms of prevention. Proactive management of atopic dermatitis does not restore or improve the diversity of the microbiome. It is probably not sufficient, and it’s just one part of the approach.”
Other future treatments for AD might target the dendritic cells that normally “bridge” the innate and adaptive immune systems, or the toll-like receptors of innate immune cells, which help regulate production of antimicrobial peptides by commensal microorganisms, Dr. Bieber said.
Recent research has shown that Th2 T cells inhibit antimicrobial peptide production in patients with atopic dermatitis, and that their dendritic cells have a “defective sensing mechanism toward staphylococcal-derived products,” he noted. “They are kind of neutralized or paralyzed, are not able to induce th17 immune response, and are not able to turn on antimicrobial production in the skin.” Restoring this sensing mechanism might one day help reestablish the normal, healthy diversity of the epidermal microbiome and prevent or alleviate AD, he said.
Dr. Bieber reported serving as a consultant, advisory board member, or speaker bureau member for L’Oreal, Galderma, Sanofi, Regeneron, Pfizer, and Novartis.
VANCOUVER, B.C. – Manipulating the skin microbiome might one day help control atopic dermatitis, Dr. Thomas Bieber said at the World Congress of Dermatology.
Scientists used to focus mainly on pathogens when considering how skin microbes affect atopic dermatitis (AD), said Dr. Bieber, professor of dermatology at the University of Bonn (Germany). But while Staphylococcus aureus is “the constant companion” of patients with AD, data from recent studies have shown that commensal microorganisms found on and within healthy skin undergo a “dialogue” with epithelial cells to help regulate antimicrobial peptides, which in turn inhibit pathogen growth, he said.
Flares in atopic dermatitis are marked by dramatic rises in S. aureus and corresponding decreases in commensal microbes, he noted. Whether an AD flare causes this microbial shift or results from it remains unclear, “but based on this knowledge, we are thinking about strategies to modulate and restore the microbiome. We have discovered a new kind of link to how the microbiome might affect atopic dermatitis,” Dr. Bieber explained.
In the future, such strategies might include formulating emollients to rebalance microbial diversity, he said, pointing to the landmark trial of fecal transplantation in patients with recurrent Clostridium difficile infections (N. Engl. J. Med. 2013;368:407-15). “That study was very impressive – the protocol was stopped due to the overwhelming benefits,” he said. “In terms of skin, could we imagine something similar?”
Restoring skin microbial diversity also might help prevent flares and worsening of AD, said Dr. Bieber. “We can control atopic dermatitis by controlling inflammation, dryness, improving the microbiota with appropriate emollients, and trying to hit early and efficiently,” he said. “We should not only think in terms of treatment, but in terms of prevention. Proactive management of atopic dermatitis does not restore or improve the diversity of the microbiome. It is probably not sufficient, and it’s just one part of the approach.”
Other future treatments for AD might target the dendritic cells that normally “bridge” the innate and adaptive immune systems, or the toll-like receptors of innate immune cells, which help regulate production of antimicrobial peptides by commensal microorganisms, Dr. Bieber said.
Recent research has shown that Th2 T cells inhibit antimicrobial peptide production in patients with atopic dermatitis, and that their dendritic cells have a “defective sensing mechanism toward staphylococcal-derived products,” he noted. “They are kind of neutralized or paralyzed, are not able to induce th17 immune response, and are not able to turn on antimicrobial production in the skin.” Restoring this sensing mechanism might one day help reestablish the normal, healthy diversity of the epidermal microbiome and prevent or alleviate AD, he said.
Dr. Bieber reported serving as a consultant, advisory board member, or speaker bureau member for L’Oreal, Galderma, Sanofi, Regeneron, Pfizer, and Novartis.
VANCOUVER, B.C. – Manipulating the skin microbiome might one day help control atopic dermatitis, Dr. Thomas Bieber said at the World Congress of Dermatology.
Scientists used to focus mainly on pathogens when considering how skin microbes affect atopic dermatitis (AD), said Dr. Bieber, professor of dermatology at the University of Bonn (Germany). But while Staphylococcus aureus is “the constant companion” of patients with AD, data from recent studies have shown that commensal microorganisms found on and within healthy skin undergo a “dialogue” with epithelial cells to help regulate antimicrobial peptides, which in turn inhibit pathogen growth, he said.
Flares in atopic dermatitis are marked by dramatic rises in S. aureus and corresponding decreases in commensal microbes, he noted. Whether an AD flare causes this microbial shift or results from it remains unclear, “but based on this knowledge, we are thinking about strategies to modulate and restore the microbiome. We have discovered a new kind of link to how the microbiome might affect atopic dermatitis,” Dr. Bieber explained.
In the future, such strategies might include formulating emollients to rebalance microbial diversity, he said, pointing to the landmark trial of fecal transplantation in patients with recurrent Clostridium difficile infections (N. Engl. J. Med. 2013;368:407-15). “That study was very impressive – the protocol was stopped due to the overwhelming benefits,” he said. “In terms of skin, could we imagine something similar?”
Restoring skin microbial diversity also might help prevent flares and worsening of AD, said Dr. Bieber. “We can control atopic dermatitis by controlling inflammation, dryness, improving the microbiota with appropriate emollients, and trying to hit early and efficiently,” he said. “We should not only think in terms of treatment, but in terms of prevention. Proactive management of atopic dermatitis does not restore or improve the diversity of the microbiome. It is probably not sufficient, and it’s just one part of the approach.”
Other future treatments for AD might target the dendritic cells that normally “bridge” the innate and adaptive immune systems, or the toll-like receptors of innate immune cells, which help regulate production of antimicrobial peptides by commensal microorganisms, Dr. Bieber said.
Recent research has shown that Th2 T cells inhibit antimicrobial peptide production in patients with atopic dermatitis, and that their dendritic cells have a “defective sensing mechanism toward staphylococcal-derived products,” he noted. “They are kind of neutralized or paralyzed, are not able to induce th17 immune response, and are not able to turn on antimicrobial production in the skin.” Restoring this sensing mechanism might one day help reestablish the normal, healthy diversity of the epidermal microbiome and prevent or alleviate AD, he said.
Dr. Bieber reported serving as a consultant, advisory board member, or speaker bureau member for L’Oreal, Galderma, Sanofi, Regeneron, Pfizer, and Novartis.
EXPERT ANALYSIS FROM WCD 2015