User login
A 40-year-old premenopausal woman presents with a palpable lump in her left breast. She first noted it 2 months ago on self-examination, and it has steadily grown in size regardless of the phase of her menstrual cycle.
The patient has never undergone mammography. Her menarche was at age 12. At age 35, she had one child (whom she breastfed) after a normal first full-term pregnancy. She took oral contraceptives for 10 years before her pregnancy. She has no other medical problems. She has no family history of breast or ovarian cancer.
On examination, her breasts are slightly asymmetric, without skin discoloration, tenderness, swelling, nipple retraction, or discharge. A 1.5- to 2-cm, rubbery, mobile lump can be felt in the left breast at about the 2 o’clock position. No axillary lymph nodes can be palpated. The rest of her examination is normal.
BREAST CANCER MUST BE RULED OUT
Benign breast disease is found in approximately 90% of women 20 to 50 years of age who come to a physician with a breast problem.1
Nevertheless, breast cancer is of major concern. It is the most common type of cancer in women in the United States, responsible for an estimated 194,440 new cases and 40,610 deaths in 2009. It is also the leading cause of cancer-related death in women age 45 to 55 years in this country.2,3
Breast cancer is most common in postmenopausal women, its incidence rising sharply after the age of 45 and leveling off at age 75. The median age at diagnosis is 61 years. Still, 1.9% of breast cancers in women are diagnosed at age 20 to 34, 10.6% at age 35 to 44, and 22.4% at age 45 to 54.4
Thus, it is paramount to perform a thorough assessment and workup of women who have breast lumps, regardless of their age. Doing so allows breast cancer to be detected at an early stage. The 5-year survival rate is 98.0% for women with localized disease, 83.6% with regional disease, and 23.4% with distant disease.4
WHAT IS THE APPROPRIATE WORKUP?
1. Which of the following are appropriate in the workup of this patient?
- Mammography
- Ultrasonography
- Percutaneous needle biopsy of the lesion
- Magnetic resonance imaging (MRI) of the brain
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Positron emission tomography (PET)
She should undergo mammography, ultrasonography, and percutaneous needle biopsy.
Physical findings that suggest breast cancer include a hard, isolated, sometimes nonmobile lump, serosanguinous nipple discharge, and unilateral nipple retraction. Peau d’orange skin discoloration can occur. A scaly, vesicular, or ulcerated rash with or without pruritus, burning, irritation, or pain of the nipple or skin (Paget disease of the breast) is found in 1% to 3% of breast cancers and may be initially dismissed as mastitis.5,6 Palpable enlarged axillary lymph nodes can suggest invasive breast cancer.
Mammography is recommended in all cases of suspicious breast lumps. In a patient with a palpable lump, diagnostic mammography has a positive predictive value of 21.8%, a specificity of 85.8%, and a sensitivity of 87.7%, which are higher values than in a patient without signs or symptoms.7
The BIRADS score. Mammographic findings are summarized using a scoring system devised by the American College of Radiology called BIRADS (Breast Imaging Reporting and Data System). This system is based on mass irregularity, density, spiculation, and presence or absence of microcalcifications. It standardizes the results of mammography, gives an estimate of the risk of breast cancer, and recommends the frequency of follow-up examinations.8 Scores range from 0 to 6:
- 0—Incomplete assessment warranting additional evaluation
- 1—Completely negative mammogram
- 2—Benign lesion
- 3—Requires follow-up mammogram at 6 months
- 4—Risk of cancer is 2% to 95%; core biopsy needed
- 5—Risk of cancer is more than 95%; core biopsy needed
- 6—Cases that have already been proven to be malignant.
Ultrasonography is also done if a suspicious lesion is found on mammography or physical examination. It helps differentiate between solid and cystic masses. If a mass is identified as a cyst, ultrasonography can further characterize it as simple, complicated-simple, or complex. Simple cysts and complicated-simple cysts are unlikely to be malignant.9,10 Complex cysts or cysts associated with solid tissue are evaluated by biopsy.
Percutaneous needle biopsy should be done for a definitive diagnosis of most suspicious breast masses.
MRI can sometimes provide more accurate information about the possibility of multifocal breast cancer by revealing additional lesions missed on mammography or ultrasonography. It is also useful in determining more accurately the size of the breast tumor and looking for any possible contralateral lesions. In addition, it can sometimes detect enlarged axillary lymph nodes. However, it has poor specificity for breast cancer and may lead to additional and sometimes unnecessary diagnostic tests, which can delay treatment.
MRI’s role is therefore not clearly established, but it is commonly used in clinical practice. It is argued that workup of MRI findings may help in planning more accurate surgical procedures and may prevent reoperations. Based on retrospective analyses, results of breast MRI may lead to altered surgical treatment in approximately 13% of patients.11
Interestingly, a recent randomized trial showed no difference in reoperation rates between patients who underwent MRI before surgery vs those who did not. However, diagnostic workup of new MRI findings was not mandated by the study protocol, making the results of this trial difficult to interpret.12
DIFFERENTIAL DIAGNOSIS
2. Which of the following is in the differential diagnosis of a woman presenting with a breast abnormality?
- Fibrocystic changes
- Breast cyst
- Ductal ectasia
- Simple fibroadenoma
- Intraductal papilloma
- Ductal carcinoma in situ
- Mastitis
- Infiltrating ductal carcinoma
- Phyllodes tumor
All of these choices are part of the differential diagnosis.
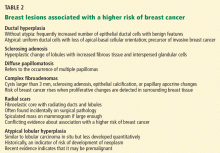
Benign breast lesions
Simple fibroadenoma, one of the most common proliferative lesions, is not associated with a higher risk of developing breast cancer.
Fibrocystic changes are the most common nonproliferative lesions. Occasionally breast pain, nipple discharge, or significant lumpiness that varies during the course of the menstrual cycle can occur. The nipple discharge in women with fibrocystic changes is physiologic and pale green to brown in color. It can also be yellow, whitish, clear, or bloody. Bloody nipple discharge is considered pathologic and suggests a process other than fibrocystic changes, necessitating further workup. However, bloody discharge is not always a sign of malignancy, as it can have a benign cause as well.
Ductal ectasia, another nonproliferative lesion, is a result of dilation of subareolar ducts that contain fluid with a crystalline material. It can penetrate the duct, forming a nodule, which causes pain and occasionally fever.
Precancerous and cancerous lesions
Ductal carcinoma in situ is a true neoplasm that has not yet developed the ability to invade through the basement membrane of the ducts. The likelihood of progression to invasive breast cancer depends on the histologic grade, the tumor size, and the patient’s age.
Lobular carcinoma in situ arises from lobules and terminal ducts of breast tissue. Much controversy surrounds this type of tumor, which was thought to be a marker of increased risk of developing ipsilateral and contralateral breast cancer and not to be a malignant lesion itself.15 However, there is emerging evidence to suggest that a pleomorphic variant of lobular carcinoma in situ is associated with development of breast cancer in the same site as the lesion, whereas a nonpleomorphic form is a marker of increased risk of ipsilateral and contralateral breast cancer.16
Invasive ductal and lobular carcinomas are the true invasive breast cancers, with a potential to metastasize.
Phyllodes tumors are uncommon fibroepithelial lesions that account for less than 1% of all breast neoplasms. The median age at presentation is 45 years.17 Despite the historical name “cystosarcoma phyllodes,” these lesions are not true sarcomas and have stromal and epithelial components.
These tumors display very heterogeneous behavior and, based on predefined histologic criteria, are often classified as benign, borderline, or malignant. Benign phyllodes tumors are similar to fibroadenomas in both histology and prognosis, making their diagnosis challenging. The most aggressive phyllodes tumors lose their epithelial component and have high metastatic potential. These tumors often have a biphasic growth pattern, and women may present with a smooth, round, well-defined breast lump that was stable for many years but then started to grow rapidly.17
Surgical resection with wide margins is the primary management of these tumors.18
Mastitis, ie, inflammation of the breast tissue, often presents with symptoms of breast erythema, swelling, tenderness, and nipple discharge. It may be secondary to infection (most often in lactating women) or other causes such as radiation or underlying malignancy. A complication of infectious mastitis is formation of a breast abscess. Underlying malignancy, especially inflammatory breast cancer, is a common cause of noninfectious mastitis and is very important to recognize.19
RISK FACTORS FOR BREAST CANCER
3. Which of the following are risk factors for breast cancer?
- Menarche before age 12
- Female sex
- Personal history of breast cancer
- Obesity
- Never having had children, or having given birth for the first time at an older age
- Older age
- History of hormone replacement therapy with estrogen and progesterone
- Family history of breast cancer
All of these choices are risk factors for breast cancer.
Family history
The overall relative risk of developing breast cancer in a woman with a first-degree relative with the disease is 1.7. However, the relative risk is about 3 if the first-degree relative developed breast cancer before menopause, and 9 if the first-degree relative developed bilateral breast cancer before menopause.5
Estrogen exposure
The duration and amount of estrogen exposure are also risk factors. For example, menarche before age 12 and menopause after age 55 are associated with a higher risk. Women who go through menopause after age 55 have a twofold higher risk of breast cancer compared with women who go through menopause at an early age. Pregnancy before age 30 lowers the risk of breast cancer; late first full-term pregnancy or nulliparity increases it. Lactation, on the other hand, has a protective effect.5
Oral contraceptives have traditionally been thought to increase the risk of breast cancer. In the 1990s, a meta-analysis involving 153,506 women found that those who had used oral contraceptives had a 24% higher risk of developing breast cancer.26 However, this association has come into question since newer oral contraceptive pills containing different progestins and lower amounts of estrogen have become available. In fact, recent studies showed no link between oral contraceptive use and breast cancer.27,28 Nevertheless, women at higher risk of developing breast cancer are advised not to use oral contraceptives.
Hormone replacement therapy with estrogen and progesterone was found to increase the risk of breast cancer by 26% in the Women’s Health Initiative (WHI) study, which involved 16,608 healthy women followed for a median of 5.6 years.29
In a study reported separately, the WHI investigators randomized 10,739 women who had undergone hysterectomy to receive either hormone replacement therapy with unopposed estrogen (which is feasible only in women without a uterus) or placebo. They found no increase in the risk of invasive breast cancer in women on hormone replacement therapy with estrogen alone. In fact, the study showed a trend towards a modest reduction of this risk (odds ratio 0.77; 95% confidence interval 0.59–1.01).30
After the results of the WHI were published, the use of hormone replacement therapy in postmenopausal women declined significantly. And in 2003—1 year later—the incidence of breast cancer had dropped by 6.7%.31
Most experts now recommend that estrogen-progestin combinations be used only selectively to treat the symptoms of menopause, and only for the short term.
Other risk factors
Other factors found to modestly increase the risk of breast cancer include:
- Alcohol use
- Obesity
- Radiation exposure. Patients are at higher risk of breast cancer 15 to 20 years after receiving upper-mantle radiotherapy for Hodgkin lymphoma.5
Case continues: Bad news on mammography, ultrasonography, biopsy
The patient undergoes mammography, which shows a 2.5-cm spiculated lesion with areas of calcifications (BIRADS score of 5). Subsequently, ultrasonography confirms that the suspicious mass is not a cyst. Ultrasound-guided core needle biopsy reveals that the lesion is a high-grade invasive ductal carcinoma. The tumor is positive for both estrogen and progesterone receptors and negative for HER2/neu overexpression.
STAGING EVALUATION
4. Given these findings, what is the next step to take?
- CT of the chest, abdomen, and pelvis
- MRI of the brain
- PET
- Referral to a surgeon for a possible mastectomy with sentinel lymph node dissection
- Referral to a surgeon for a possible lumpectomy with sentinel lymph node dissection
At this point, the patient should be referred to a surgeon for possible mastectomy or lumpectomy.
Women who appear clinically to have early breast cancer, such as in this case, should have a complete blood count, comprehensive metabolic panel, and chest x-ray as their initial staging evaluation. No further studies are recommended unless the findings on history, physical examination, or the above testing suggest possible metastases.
Mastectomy vs lumpectomy
Early-stage breast cancer is managed with definitive surgery. The two options are mastectomy and breast conservation therapy, the latter involving lumpectomy followed by breast radiation therapy.
Multiple randomized studies comparing mastectomy and lumpectomy showed no difference in survival rates, but patients in the lumpectomy groups had higher rates of local recurrence.32 Breast radiation therapy after lumpectomy lowered the rates of local recurrence and breast cancer death.33 Therefore, most patients can opt to undergo either lumpectomy with radiation or mastectomy, depending on personal preference.
However, mastectomy rather than breast conservation therapy is still recommended in cases of prior radiation therapy, inability to achieve negative surgical margins (as in cases of large tumors), multicentric disease (cancer in separate breast quadrants), or multiple areas of calcifications. Mastectomy is also preferred in most pregnant women unless the diagnosis of breast cancer is made in the third trimester and radiation therapy can be given after delivery. Patients who have large lesions in a small breast may also choose mastectomy with breast reconstruction rather than breast conservation therapy. Patients with a history of scleroderma are encouraged to undergo mastectomy because of increased toxicity from radiation treatment.
Sentinel vs axillary lymph node dissection
Knowledge of axillary lymph node involvement is important because it determines the stage in the tumor-node-metastasis (TNM) system, and it influences the choice of further therapy. Therefore, all patients with nonmetastatic invasive breast cancer must have their axillary lymph nodes sampled.
Conventionally, this involves axillary lymph node dissection. Unfortunately, upper extremity lymphedema develops in 6% to 30% of patients within the first 3 years, and in 49% of patients after 20 years following axillary lymph node dissection.34
Sentinel lymph node dissection was developed to minimize this complication. This procedure involves the injection of a blue dye, isosulfan blue (Lymphazurin), around the edge of the tumor or in the dermis overlying the tumor. The most proximal axillary lymph nodes that stain blue are dissected. Alternatively, a radioactive colloid (most commonly technetium sulfur colloid agents) may be injected, allowing sentinel lymph nodes to be identified by lymphoscintigraphy. If no metastases are found in the sentinel lymph nodes, axillary lymph node dissection is not performed.
A prospective study in 536 women found that at 5 years of follow-up, lymphedema developed in only 5% of patients after sentinel lymph node dissection compared with 16% of those who underwent axillary lymph node dissection (P < .001), with comparable outcomes in terms of disease recurrence.35
Case continues: Patient undergoes surgery
The patient elects to undergo lumpectomy with sentinel lymph node dissection. Pathologic review of the resection specimen reveals a 2.5-cm poorly differentiated invasive ductal carcinoma. Sentinel lymph node dissection shows metastases, and therefore axillary lymph node dissection is performed. One of eight lymph nodes removed is positive for metastases. All surgical margins are negative.
POSTOPERATIVE CARE
5. What would be the next step for our patient?
- Radiation followed by observation
- Tamoxifen (Nolvadex) for 5 years
- Observation only
- Chemotherapy followed by radiation therapy and 5 years of tamoxifen
She should receive chemotherapy, followed by radiation therapy and then tamoxifen for 5 years.
Chemotherapy. Almost all patients who have lymph-node-positive disease are advised to undergo chemotherapy.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a metaanalysis of 194 randomized trials that compared adjuvant chemotherapy and no treatment in early-stage breast cancer. Chemotherapy led to a 10% absolute improvement in survival at 15 years for women younger than 50 years and 3% in women age 51 to 69.36
Indications for chemotherapy include axillary lymph node involvement, locally advanced disease, and other risk factors for recurrence such as young age at diagnosis, strong positive family history of breast cancer, prior history of breast cancer, or lymph-node-negative, estrogen-receptor-negative tumors that are larger than 1 cm in diameter.
The Oncotype DX assay is a new tool to help oncologists decide whether to use chemotherapy in cases of estrogen-receptor-positive breast cancer, in which the benefit of chemotherapy is uncertain. It is a polymerase chain reaction assay that measures the expression of 16 cancer-specific genes and five reference genes within the breast tumor. Based on the pattern of expression of these genes, breast cancer can be characterized as low-risk, intermediate-risk, or high-risk. Patients in the high-risk group have a high chance of cancer recurrence and benefit from chemotherapy. Patients in the low-risk group are unlikely to have a recurrence or to benefit from chemotherapy.37 It is far less clear if patients in the intermediate-risk group benefit from chemotherapy, but this assay might eventually prove useful in deciding for or against chemotherapy in this group of patients as well.38 The Oncotype DX assay is presently being studied in a clinical trial.
Radiation therapy after mastectomy is recommended in patients who have breast tumors larger than 5 cm or metastases to more than three axillary lymph nodes.39
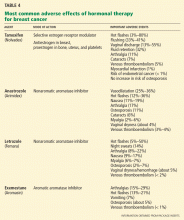
Antiestrogen therapy. After chemotherapy, patients with estrogen-receptor-positive cancers also receive 5 years of antiestrogen therapy. Available antiestrogen agents for such patients include tamoxifen, which is a selective estrogen receptor modulator, and drugs called aromatase inhibitors that block conversion of androgens to estrogens in peripheral tissues. Anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) are examples of available aromatase inhibitors. Premenopausal women are treated with tamoxifen, and postmenopausal women are offered aromatase inhibitors.
Table 4 lists the most common adverse effects of these agents. Aromatase inhibitors are associated with a higher risk of osteoporosis and arthralgia, while tamoxifen increases the risks of thromboembolism, endometrial cancer, and vaginal discharge. Both agents may produce menopausal symptoms such as hot flashes and mood swings.
Case continues: Seven years later, metastases in the spine
The patient achieves a complete remission. She is seen for a routine visit 7 years after diagnosis. She now reports mid-back pain that has worsened over the last 2 months. A bone scan reveals diffuse metastatic disease in the spine and in both humeral bones. CT of the chest, abdomen, and pelvis is negative for visceral metastases. Bone marrow aspiration and biopsy study show marrow infiltration by adenocarcinoma that stains positive for estrogen receptors and negative for HER2. The patient otherwise feels well and has no other symptoms.
WHAT TREATMENT FOR METASTATIC BREAST CANCER?
6. What should you now do for our patient?
- Discuss end-of-life care and refer her to a hospice program
- Educate the patient that no options for treatment exist and recommend enrolling in a phase I clinical trial
- Refer her to an oncologist for consideration of chemotherapy
- Refer her to an oncologist for consideration of endocrine treatment
She should be referred to an oncologist for consideration of endocrine treatment.
The most common sites of breast cancer metastases are the bones, followed by the liver and lungs. Metastatic breast cancer almost always is incurable. However, treatment can palliate symptoms.
Although a randomized trial of treatment vs best supportive care has never been done, many believe that treatment may improve survival. 40 The median survival of patients treated with standard therapy is about 3 years if the breast cancer is estrogen-receptor-positive and 2 years if it is estrogen-receptor-negative, but survival rates vary widely from patient to patient.41,42
Standard therapy or enrollment in a clinical phase II or III trial is indicated for this patient before considering enrollment in a phase I clinical trial or supportive care alone.
Endocrine therapy is the first-line therapy in women with estrogen-receptor-positive metastatic breast cancer. Postmenopausal women usually receive an aromatase inhibitor first.43,44 Response to endocrine therapy usually takes weeks to months but may last for several years.
Premenopausal women with estrogen-receptor-positive breast cancer also receive ovarian ablation therapy (oophorectomy or chemical ovarian ablation) with gonadotropin-releasing hormone agonists.
In addition, most patients with bone involvement are treated with high doses of intravenous bisphosphonates, which can reduce skeletal complications.45
Chemotherapy is reserved for patients with estrogen-receptor-negative breast cancer and those with cancer that progresses despite treatment with multiple antiestrogen agents. The time to response when chemotherapy is used is quicker, but the duration of response is usually shorter, lasting on average less than 1 year.37
Trastuzumab (Herceptin), a monoclonal humanized murine antibody to the extracellular domain of the HER2 protein, is indicated in patients with HER2-overexpressing tumors.46,47
STABLE 2 YEARS LATER
The patient was started on letrozole and a bisphosphonate, zolendronic acid (Zometa). Ovarian ablation was initiated with goserelin (Zoladex) given monthly. A bone scan performed 2 months after starting treatment showed improvement in bony metastases. She also noted significant improvement in pain. Her disease remains stable 2 years after starting endocrine therapy.
- Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 1999; 130:651–657.
- Petrelli NJ, Winer EP, Brahmer J, et al. Clinical cancer advances 2009: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:6052–6069.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008; 58:71–96.
- National Cancer Institute. SEER Stat Fact Sheets. www.seer.cancer.gov/statfacts/html/breast.html#ref09. Accessed June 7, 2010.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ; the publishers of the journal Oncology. Cancer Management: A multidisciplinary Approach. Medical, Surgical & Radiation Oncology. 11th ed. CMP Medica; 2008.
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187:171–177.
- Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst 2002; 94:1151–1159.
- American College of Radiology. Breast Imaging Reporting and Data System: BIRADS Atlas. 4th ed. Reston, VA: American College of Radiology; 2003.
- Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184:1260–1265.
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003; 227:183–191.
- Schell AM, Rosenkranz K, Lewis PJ. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 2009; 192:1438–1444.
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375:563–571.
- Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007; 13:115–121.
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985; 312:146–151.
- Page DL, Kidd TE, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991; 22:1232–1239.
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductallobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002; 15:1044–1050.
- Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw 2007; 5:324–330.
- Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996; 77:910–916.
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009; 15:367–380.
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 1999; 64:963–970.
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90:606–611.
- Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999; 286:2528–2531.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–1727.
- Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States). Cancer Causes Control 1997; 8:65–72.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002; 346:2025–2032.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333:1456–1461.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26:5213–5219.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826.
- Albain KS, Barlow WE, Shak S, et al; Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65.
- Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44:989–990.
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005; 104:1742–1750.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101–2109.
- Gamucci T, D’Ottavio AM, Magnolfi E, et al. Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer. Br J Cancer 2007; 97:1040–1045.
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18:3758–3767.
- Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335:1785–1791.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792.
A 40-year-old premenopausal woman presents with a palpable lump in her left breast. She first noted it 2 months ago on self-examination, and it has steadily grown in size regardless of the phase of her menstrual cycle.
The patient has never undergone mammography. Her menarche was at age 12. At age 35, she had one child (whom she breastfed) after a normal first full-term pregnancy. She took oral contraceptives for 10 years before her pregnancy. She has no other medical problems. She has no family history of breast or ovarian cancer.
On examination, her breasts are slightly asymmetric, without skin discoloration, tenderness, swelling, nipple retraction, or discharge. A 1.5- to 2-cm, rubbery, mobile lump can be felt in the left breast at about the 2 o’clock position. No axillary lymph nodes can be palpated. The rest of her examination is normal.
BREAST CANCER MUST BE RULED OUT
Benign breast disease is found in approximately 90% of women 20 to 50 years of age who come to a physician with a breast problem.1
Nevertheless, breast cancer is of major concern. It is the most common type of cancer in women in the United States, responsible for an estimated 194,440 new cases and 40,610 deaths in 2009. It is also the leading cause of cancer-related death in women age 45 to 55 years in this country.2,3
Breast cancer is most common in postmenopausal women, its incidence rising sharply after the age of 45 and leveling off at age 75. The median age at diagnosis is 61 years. Still, 1.9% of breast cancers in women are diagnosed at age 20 to 34, 10.6% at age 35 to 44, and 22.4% at age 45 to 54.4
Thus, it is paramount to perform a thorough assessment and workup of women who have breast lumps, regardless of their age. Doing so allows breast cancer to be detected at an early stage. The 5-year survival rate is 98.0% for women with localized disease, 83.6% with regional disease, and 23.4% with distant disease.4
WHAT IS THE APPROPRIATE WORKUP?
1. Which of the following are appropriate in the workup of this patient?
- Mammography
- Ultrasonography
- Percutaneous needle biopsy of the lesion
- Magnetic resonance imaging (MRI) of the brain
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Positron emission tomography (PET)
She should undergo mammography, ultrasonography, and percutaneous needle biopsy.
Physical findings that suggest breast cancer include a hard, isolated, sometimes nonmobile lump, serosanguinous nipple discharge, and unilateral nipple retraction. Peau d’orange skin discoloration can occur. A scaly, vesicular, or ulcerated rash with or without pruritus, burning, irritation, or pain of the nipple or skin (Paget disease of the breast) is found in 1% to 3% of breast cancers and may be initially dismissed as mastitis.5,6 Palpable enlarged axillary lymph nodes can suggest invasive breast cancer.
Mammography is recommended in all cases of suspicious breast lumps. In a patient with a palpable lump, diagnostic mammography has a positive predictive value of 21.8%, a specificity of 85.8%, and a sensitivity of 87.7%, which are higher values than in a patient without signs or symptoms.7
The BIRADS score. Mammographic findings are summarized using a scoring system devised by the American College of Radiology called BIRADS (Breast Imaging Reporting and Data System). This system is based on mass irregularity, density, spiculation, and presence or absence of microcalcifications. It standardizes the results of mammography, gives an estimate of the risk of breast cancer, and recommends the frequency of follow-up examinations.8 Scores range from 0 to 6:
- 0—Incomplete assessment warranting additional evaluation
- 1—Completely negative mammogram
- 2—Benign lesion
- 3—Requires follow-up mammogram at 6 months
- 4—Risk of cancer is 2% to 95%; core biopsy needed
- 5—Risk of cancer is more than 95%; core biopsy needed
- 6—Cases that have already been proven to be malignant.
Ultrasonography is also done if a suspicious lesion is found on mammography or physical examination. It helps differentiate between solid and cystic masses. If a mass is identified as a cyst, ultrasonography can further characterize it as simple, complicated-simple, or complex. Simple cysts and complicated-simple cysts are unlikely to be malignant.9,10 Complex cysts or cysts associated with solid tissue are evaluated by biopsy.
Percutaneous needle biopsy should be done for a definitive diagnosis of most suspicious breast masses.
MRI can sometimes provide more accurate information about the possibility of multifocal breast cancer by revealing additional lesions missed on mammography or ultrasonography. It is also useful in determining more accurately the size of the breast tumor and looking for any possible contralateral lesions. In addition, it can sometimes detect enlarged axillary lymph nodes. However, it has poor specificity for breast cancer and may lead to additional and sometimes unnecessary diagnostic tests, which can delay treatment.
MRI’s role is therefore not clearly established, but it is commonly used in clinical practice. It is argued that workup of MRI findings may help in planning more accurate surgical procedures and may prevent reoperations. Based on retrospective analyses, results of breast MRI may lead to altered surgical treatment in approximately 13% of patients.11
Interestingly, a recent randomized trial showed no difference in reoperation rates between patients who underwent MRI before surgery vs those who did not. However, diagnostic workup of new MRI findings was not mandated by the study protocol, making the results of this trial difficult to interpret.12
DIFFERENTIAL DIAGNOSIS
2. Which of the following is in the differential diagnosis of a woman presenting with a breast abnormality?
- Fibrocystic changes
- Breast cyst
- Ductal ectasia
- Simple fibroadenoma
- Intraductal papilloma
- Ductal carcinoma in situ
- Mastitis
- Infiltrating ductal carcinoma
- Phyllodes tumor
All of these choices are part of the differential diagnosis.
Benign breast lesions
Simple fibroadenoma, one of the most common proliferative lesions, is not associated with a higher risk of developing breast cancer.
Fibrocystic changes are the most common nonproliferative lesions. Occasionally breast pain, nipple discharge, or significant lumpiness that varies during the course of the menstrual cycle can occur. The nipple discharge in women with fibrocystic changes is physiologic and pale green to brown in color. It can also be yellow, whitish, clear, or bloody. Bloody nipple discharge is considered pathologic and suggests a process other than fibrocystic changes, necessitating further workup. However, bloody discharge is not always a sign of malignancy, as it can have a benign cause as well.
Ductal ectasia, another nonproliferative lesion, is a result of dilation of subareolar ducts that contain fluid with a crystalline material. It can penetrate the duct, forming a nodule, which causes pain and occasionally fever.
Precancerous and cancerous lesions
Ductal carcinoma in situ is a true neoplasm that has not yet developed the ability to invade through the basement membrane of the ducts. The likelihood of progression to invasive breast cancer depends on the histologic grade, the tumor size, and the patient’s age.
Lobular carcinoma in situ arises from lobules and terminal ducts of breast tissue. Much controversy surrounds this type of tumor, which was thought to be a marker of increased risk of developing ipsilateral and contralateral breast cancer and not to be a malignant lesion itself.15 However, there is emerging evidence to suggest that a pleomorphic variant of lobular carcinoma in situ is associated with development of breast cancer in the same site as the lesion, whereas a nonpleomorphic form is a marker of increased risk of ipsilateral and contralateral breast cancer.16
Invasive ductal and lobular carcinomas are the true invasive breast cancers, with a potential to metastasize.
Phyllodes tumors are uncommon fibroepithelial lesions that account for less than 1% of all breast neoplasms. The median age at presentation is 45 years.17 Despite the historical name “cystosarcoma phyllodes,” these lesions are not true sarcomas and have stromal and epithelial components.
These tumors display very heterogeneous behavior and, based on predefined histologic criteria, are often classified as benign, borderline, or malignant. Benign phyllodes tumors are similar to fibroadenomas in both histology and prognosis, making their diagnosis challenging. The most aggressive phyllodes tumors lose their epithelial component and have high metastatic potential. These tumors often have a biphasic growth pattern, and women may present with a smooth, round, well-defined breast lump that was stable for many years but then started to grow rapidly.17
Surgical resection with wide margins is the primary management of these tumors.18
Mastitis, ie, inflammation of the breast tissue, often presents with symptoms of breast erythema, swelling, tenderness, and nipple discharge. It may be secondary to infection (most often in lactating women) or other causes such as radiation or underlying malignancy. A complication of infectious mastitis is formation of a breast abscess. Underlying malignancy, especially inflammatory breast cancer, is a common cause of noninfectious mastitis and is very important to recognize.19
RISK FACTORS FOR BREAST CANCER
3. Which of the following are risk factors for breast cancer?
- Menarche before age 12
- Female sex
- Personal history of breast cancer
- Obesity
- Never having had children, or having given birth for the first time at an older age
- Older age
- History of hormone replacement therapy with estrogen and progesterone
- Family history of breast cancer
All of these choices are risk factors for breast cancer.
Family history
The overall relative risk of developing breast cancer in a woman with a first-degree relative with the disease is 1.7. However, the relative risk is about 3 if the first-degree relative developed breast cancer before menopause, and 9 if the first-degree relative developed bilateral breast cancer before menopause.5
Estrogen exposure
The duration and amount of estrogen exposure are also risk factors. For example, menarche before age 12 and menopause after age 55 are associated with a higher risk. Women who go through menopause after age 55 have a twofold higher risk of breast cancer compared with women who go through menopause at an early age. Pregnancy before age 30 lowers the risk of breast cancer; late first full-term pregnancy or nulliparity increases it. Lactation, on the other hand, has a protective effect.5
Oral contraceptives have traditionally been thought to increase the risk of breast cancer. In the 1990s, a meta-analysis involving 153,506 women found that those who had used oral contraceptives had a 24% higher risk of developing breast cancer.26 However, this association has come into question since newer oral contraceptive pills containing different progestins and lower amounts of estrogen have become available. In fact, recent studies showed no link between oral contraceptive use and breast cancer.27,28 Nevertheless, women at higher risk of developing breast cancer are advised not to use oral contraceptives.
Hormone replacement therapy with estrogen and progesterone was found to increase the risk of breast cancer by 26% in the Women’s Health Initiative (WHI) study, which involved 16,608 healthy women followed for a median of 5.6 years.29
In a study reported separately, the WHI investigators randomized 10,739 women who had undergone hysterectomy to receive either hormone replacement therapy with unopposed estrogen (which is feasible only in women without a uterus) or placebo. They found no increase in the risk of invasive breast cancer in women on hormone replacement therapy with estrogen alone. In fact, the study showed a trend towards a modest reduction of this risk (odds ratio 0.77; 95% confidence interval 0.59–1.01).30
After the results of the WHI were published, the use of hormone replacement therapy in postmenopausal women declined significantly. And in 2003—1 year later—the incidence of breast cancer had dropped by 6.7%.31
Most experts now recommend that estrogen-progestin combinations be used only selectively to treat the symptoms of menopause, and only for the short term.
Other risk factors
Other factors found to modestly increase the risk of breast cancer include:
- Alcohol use
- Obesity
- Radiation exposure. Patients are at higher risk of breast cancer 15 to 20 years after receiving upper-mantle radiotherapy for Hodgkin lymphoma.5
Case continues: Bad news on mammography, ultrasonography, biopsy
The patient undergoes mammography, which shows a 2.5-cm spiculated lesion with areas of calcifications (BIRADS score of 5). Subsequently, ultrasonography confirms that the suspicious mass is not a cyst. Ultrasound-guided core needle biopsy reveals that the lesion is a high-grade invasive ductal carcinoma. The tumor is positive for both estrogen and progesterone receptors and negative for HER2/neu overexpression.
STAGING EVALUATION
4. Given these findings, what is the next step to take?
- CT of the chest, abdomen, and pelvis
- MRI of the brain
- PET
- Referral to a surgeon for a possible mastectomy with sentinel lymph node dissection
- Referral to a surgeon for a possible lumpectomy with sentinel lymph node dissection
At this point, the patient should be referred to a surgeon for possible mastectomy or lumpectomy.
Women who appear clinically to have early breast cancer, such as in this case, should have a complete blood count, comprehensive metabolic panel, and chest x-ray as their initial staging evaluation. No further studies are recommended unless the findings on history, physical examination, or the above testing suggest possible metastases.
Mastectomy vs lumpectomy
Early-stage breast cancer is managed with definitive surgery. The two options are mastectomy and breast conservation therapy, the latter involving lumpectomy followed by breast radiation therapy.
Multiple randomized studies comparing mastectomy and lumpectomy showed no difference in survival rates, but patients in the lumpectomy groups had higher rates of local recurrence.32 Breast radiation therapy after lumpectomy lowered the rates of local recurrence and breast cancer death.33 Therefore, most patients can opt to undergo either lumpectomy with radiation or mastectomy, depending on personal preference.
However, mastectomy rather than breast conservation therapy is still recommended in cases of prior radiation therapy, inability to achieve negative surgical margins (as in cases of large tumors), multicentric disease (cancer in separate breast quadrants), or multiple areas of calcifications. Mastectomy is also preferred in most pregnant women unless the diagnosis of breast cancer is made in the third trimester and radiation therapy can be given after delivery. Patients who have large lesions in a small breast may also choose mastectomy with breast reconstruction rather than breast conservation therapy. Patients with a history of scleroderma are encouraged to undergo mastectomy because of increased toxicity from radiation treatment.
Sentinel vs axillary lymph node dissection
Knowledge of axillary lymph node involvement is important because it determines the stage in the tumor-node-metastasis (TNM) system, and it influences the choice of further therapy. Therefore, all patients with nonmetastatic invasive breast cancer must have their axillary lymph nodes sampled.
Conventionally, this involves axillary lymph node dissection. Unfortunately, upper extremity lymphedema develops in 6% to 30% of patients within the first 3 years, and in 49% of patients after 20 years following axillary lymph node dissection.34
Sentinel lymph node dissection was developed to minimize this complication. This procedure involves the injection of a blue dye, isosulfan blue (Lymphazurin), around the edge of the tumor or in the dermis overlying the tumor. The most proximal axillary lymph nodes that stain blue are dissected. Alternatively, a radioactive colloid (most commonly technetium sulfur colloid agents) may be injected, allowing sentinel lymph nodes to be identified by lymphoscintigraphy. If no metastases are found in the sentinel lymph nodes, axillary lymph node dissection is not performed.
A prospective study in 536 women found that at 5 years of follow-up, lymphedema developed in only 5% of patients after sentinel lymph node dissection compared with 16% of those who underwent axillary lymph node dissection (P < .001), with comparable outcomes in terms of disease recurrence.35
Case continues: Patient undergoes surgery
The patient elects to undergo lumpectomy with sentinel lymph node dissection. Pathologic review of the resection specimen reveals a 2.5-cm poorly differentiated invasive ductal carcinoma. Sentinel lymph node dissection shows metastases, and therefore axillary lymph node dissection is performed. One of eight lymph nodes removed is positive for metastases. All surgical margins are negative.
POSTOPERATIVE CARE
5. What would be the next step for our patient?
- Radiation followed by observation
- Tamoxifen (Nolvadex) for 5 years
- Observation only
- Chemotherapy followed by radiation therapy and 5 years of tamoxifen
She should receive chemotherapy, followed by radiation therapy and then tamoxifen for 5 years.
Chemotherapy. Almost all patients who have lymph-node-positive disease are advised to undergo chemotherapy.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a metaanalysis of 194 randomized trials that compared adjuvant chemotherapy and no treatment in early-stage breast cancer. Chemotherapy led to a 10% absolute improvement in survival at 15 years for women younger than 50 years and 3% in women age 51 to 69.36
Indications for chemotherapy include axillary lymph node involvement, locally advanced disease, and other risk factors for recurrence such as young age at diagnosis, strong positive family history of breast cancer, prior history of breast cancer, or lymph-node-negative, estrogen-receptor-negative tumors that are larger than 1 cm in diameter.
The Oncotype DX assay is a new tool to help oncologists decide whether to use chemotherapy in cases of estrogen-receptor-positive breast cancer, in which the benefit of chemotherapy is uncertain. It is a polymerase chain reaction assay that measures the expression of 16 cancer-specific genes and five reference genes within the breast tumor. Based on the pattern of expression of these genes, breast cancer can be characterized as low-risk, intermediate-risk, or high-risk. Patients in the high-risk group have a high chance of cancer recurrence and benefit from chemotherapy. Patients in the low-risk group are unlikely to have a recurrence or to benefit from chemotherapy.37 It is far less clear if patients in the intermediate-risk group benefit from chemotherapy, but this assay might eventually prove useful in deciding for or against chemotherapy in this group of patients as well.38 The Oncotype DX assay is presently being studied in a clinical trial.
Radiation therapy after mastectomy is recommended in patients who have breast tumors larger than 5 cm or metastases to more than three axillary lymph nodes.39
Antiestrogen therapy. After chemotherapy, patients with estrogen-receptor-positive cancers also receive 5 years of antiestrogen therapy. Available antiestrogen agents for such patients include tamoxifen, which is a selective estrogen receptor modulator, and drugs called aromatase inhibitors that block conversion of androgens to estrogens in peripheral tissues. Anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) are examples of available aromatase inhibitors. Premenopausal women are treated with tamoxifen, and postmenopausal women are offered aromatase inhibitors.
Table 4 lists the most common adverse effects of these agents. Aromatase inhibitors are associated with a higher risk of osteoporosis and arthralgia, while tamoxifen increases the risks of thromboembolism, endometrial cancer, and vaginal discharge. Both agents may produce menopausal symptoms such as hot flashes and mood swings.
Case continues: Seven years later, metastases in the spine
The patient achieves a complete remission. She is seen for a routine visit 7 years after diagnosis. She now reports mid-back pain that has worsened over the last 2 months. A bone scan reveals diffuse metastatic disease in the spine and in both humeral bones. CT of the chest, abdomen, and pelvis is negative for visceral metastases. Bone marrow aspiration and biopsy study show marrow infiltration by adenocarcinoma that stains positive for estrogen receptors and negative for HER2. The patient otherwise feels well and has no other symptoms.
WHAT TREATMENT FOR METASTATIC BREAST CANCER?
6. What should you now do for our patient?
- Discuss end-of-life care and refer her to a hospice program
- Educate the patient that no options for treatment exist and recommend enrolling in a phase I clinical trial
- Refer her to an oncologist for consideration of chemotherapy
- Refer her to an oncologist for consideration of endocrine treatment
She should be referred to an oncologist for consideration of endocrine treatment.
The most common sites of breast cancer metastases are the bones, followed by the liver and lungs. Metastatic breast cancer almost always is incurable. However, treatment can palliate symptoms.
Although a randomized trial of treatment vs best supportive care has never been done, many believe that treatment may improve survival. 40 The median survival of patients treated with standard therapy is about 3 years if the breast cancer is estrogen-receptor-positive and 2 years if it is estrogen-receptor-negative, but survival rates vary widely from patient to patient.41,42
Standard therapy or enrollment in a clinical phase II or III trial is indicated for this patient before considering enrollment in a phase I clinical trial or supportive care alone.
Endocrine therapy is the first-line therapy in women with estrogen-receptor-positive metastatic breast cancer. Postmenopausal women usually receive an aromatase inhibitor first.43,44 Response to endocrine therapy usually takes weeks to months but may last for several years.
Premenopausal women with estrogen-receptor-positive breast cancer also receive ovarian ablation therapy (oophorectomy or chemical ovarian ablation) with gonadotropin-releasing hormone agonists.
In addition, most patients with bone involvement are treated with high doses of intravenous bisphosphonates, which can reduce skeletal complications.45
Chemotherapy is reserved for patients with estrogen-receptor-negative breast cancer and those with cancer that progresses despite treatment with multiple antiestrogen agents. The time to response when chemotherapy is used is quicker, but the duration of response is usually shorter, lasting on average less than 1 year.37
Trastuzumab (Herceptin), a monoclonal humanized murine antibody to the extracellular domain of the HER2 protein, is indicated in patients with HER2-overexpressing tumors.46,47
STABLE 2 YEARS LATER
The patient was started on letrozole and a bisphosphonate, zolendronic acid (Zometa). Ovarian ablation was initiated with goserelin (Zoladex) given monthly. A bone scan performed 2 months after starting treatment showed improvement in bony metastases. She also noted significant improvement in pain. Her disease remains stable 2 years after starting endocrine therapy.
A 40-year-old premenopausal woman presents with a palpable lump in her left breast. She first noted it 2 months ago on self-examination, and it has steadily grown in size regardless of the phase of her menstrual cycle.
The patient has never undergone mammography. Her menarche was at age 12. At age 35, she had one child (whom she breastfed) after a normal first full-term pregnancy. She took oral contraceptives for 10 years before her pregnancy. She has no other medical problems. She has no family history of breast or ovarian cancer.
On examination, her breasts are slightly asymmetric, without skin discoloration, tenderness, swelling, nipple retraction, or discharge. A 1.5- to 2-cm, rubbery, mobile lump can be felt in the left breast at about the 2 o’clock position. No axillary lymph nodes can be palpated. The rest of her examination is normal.
BREAST CANCER MUST BE RULED OUT
Benign breast disease is found in approximately 90% of women 20 to 50 years of age who come to a physician with a breast problem.1
Nevertheless, breast cancer is of major concern. It is the most common type of cancer in women in the United States, responsible for an estimated 194,440 new cases and 40,610 deaths in 2009. It is also the leading cause of cancer-related death in women age 45 to 55 years in this country.2,3
Breast cancer is most common in postmenopausal women, its incidence rising sharply after the age of 45 and leveling off at age 75. The median age at diagnosis is 61 years. Still, 1.9% of breast cancers in women are diagnosed at age 20 to 34, 10.6% at age 35 to 44, and 22.4% at age 45 to 54.4
Thus, it is paramount to perform a thorough assessment and workup of women who have breast lumps, regardless of their age. Doing so allows breast cancer to be detected at an early stage. The 5-year survival rate is 98.0% for women with localized disease, 83.6% with regional disease, and 23.4% with distant disease.4
WHAT IS THE APPROPRIATE WORKUP?
1. Which of the following are appropriate in the workup of this patient?
- Mammography
- Ultrasonography
- Percutaneous needle biopsy of the lesion
- Magnetic resonance imaging (MRI) of the brain
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Positron emission tomography (PET)
She should undergo mammography, ultrasonography, and percutaneous needle biopsy.
Physical findings that suggest breast cancer include a hard, isolated, sometimes nonmobile lump, serosanguinous nipple discharge, and unilateral nipple retraction. Peau d’orange skin discoloration can occur. A scaly, vesicular, or ulcerated rash with or without pruritus, burning, irritation, or pain of the nipple or skin (Paget disease of the breast) is found in 1% to 3% of breast cancers and may be initially dismissed as mastitis.5,6 Palpable enlarged axillary lymph nodes can suggest invasive breast cancer.
Mammography is recommended in all cases of suspicious breast lumps. In a patient with a palpable lump, diagnostic mammography has a positive predictive value of 21.8%, a specificity of 85.8%, and a sensitivity of 87.7%, which are higher values than in a patient without signs or symptoms.7
The BIRADS score. Mammographic findings are summarized using a scoring system devised by the American College of Radiology called BIRADS (Breast Imaging Reporting and Data System). This system is based on mass irregularity, density, spiculation, and presence or absence of microcalcifications. It standardizes the results of mammography, gives an estimate of the risk of breast cancer, and recommends the frequency of follow-up examinations.8 Scores range from 0 to 6:
- 0—Incomplete assessment warranting additional evaluation
- 1—Completely negative mammogram
- 2—Benign lesion
- 3—Requires follow-up mammogram at 6 months
- 4—Risk of cancer is 2% to 95%; core biopsy needed
- 5—Risk of cancer is more than 95%; core biopsy needed
- 6—Cases that have already been proven to be malignant.
Ultrasonography is also done if a suspicious lesion is found on mammography or physical examination. It helps differentiate between solid and cystic masses. If a mass is identified as a cyst, ultrasonography can further characterize it as simple, complicated-simple, or complex. Simple cysts and complicated-simple cysts are unlikely to be malignant.9,10 Complex cysts or cysts associated with solid tissue are evaluated by biopsy.
Percutaneous needle biopsy should be done for a definitive diagnosis of most suspicious breast masses.
MRI can sometimes provide more accurate information about the possibility of multifocal breast cancer by revealing additional lesions missed on mammography or ultrasonography. It is also useful in determining more accurately the size of the breast tumor and looking for any possible contralateral lesions. In addition, it can sometimes detect enlarged axillary lymph nodes. However, it has poor specificity for breast cancer and may lead to additional and sometimes unnecessary diagnostic tests, which can delay treatment.
MRI’s role is therefore not clearly established, but it is commonly used in clinical practice. It is argued that workup of MRI findings may help in planning more accurate surgical procedures and may prevent reoperations. Based on retrospective analyses, results of breast MRI may lead to altered surgical treatment in approximately 13% of patients.11
Interestingly, a recent randomized trial showed no difference in reoperation rates between patients who underwent MRI before surgery vs those who did not. However, diagnostic workup of new MRI findings was not mandated by the study protocol, making the results of this trial difficult to interpret.12
DIFFERENTIAL DIAGNOSIS
2. Which of the following is in the differential diagnosis of a woman presenting with a breast abnormality?
- Fibrocystic changes
- Breast cyst
- Ductal ectasia
- Simple fibroadenoma
- Intraductal papilloma
- Ductal carcinoma in situ
- Mastitis
- Infiltrating ductal carcinoma
- Phyllodes tumor
All of these choices are part of the differential diagnosis.
Benign breast lesions
Simple fibroadenoma, one of the most common proliferative lesions, is not associated with a higher risk of developing breast cancer.
Fibrocystic changes are the most common nonproliferative lesions. Occasionally breast pain, nipple discharge, or significant lumpiness that varies during the course of the menstrual cycle can occur. The nipple discharge in women with fibrocystic changes is physiologic and pale green to brown in color. It can also be yellow, whitish, clear, or bloody. Bloody nipple discharge is considered pathologic and suggests a process other than fibrocystic changes, necessitating further workup. However, bloody discharge is not always a sign of malignancy, as it can have a benign cause as well.
Ductal ectasia, another nonproliferative lesion, is a result of dilation of subareolar ducts that contain fluid with a crystalline material. It can penetrate the duct, forming a nodule, which causes pain and occasionally fever.
Precancerous and cancerous lesions
Ductal carcinoma in situ is a true neoplasm that has not yet developed the ability to invade through the basement membrane of the ducts. The likelihood of progression to invasive breast cancer depends on the histologic grade, the tumor size, and the patient’s age.
Lobular carcinoma in situ arises from lobules and terminal ducts of breast tissue. Much controversy surrounds this type of tumor, which was thought to be a marker of increased risk of developing ipsilateral and contralateral breast cancer and not to be a malignant lesion itself.15 However, there is emerging evidence to suggest that a pleomorphic variant of lobular carcinoma in situ is associated with development of breast cancer in the same site as the lesion, whereas a nonpleomorphic form is a marker of increased risk of ipsilateral and contralateral breast cancer.16
Invasive ductal and lobular carcinomas are the true invasive breast cancers, with a potential to metastasize.
Phyllodes tumors are uncommon fibroepithelial lesions that account for less than 1% of all breast neoplasms. The median age at presentation is 45 years.17 Despite the historical name “cystosarcoma phyllodes,” these lesions are not true sarcomas and have stromal and epithelial components.
These tumors display very heterogeneous behavior and, based on predefined histologic criteria, are often classified as benign, borderline, or malignant. Benign phyllodes tumors are similar to fibroadenomas in both histology and prognosis, making their diagnosis challenging. The most aggressive phyllodes tumors lose their epithelial component and have high metastatic potential. These tumors often have a biphasic growth pattern, and women may present with a smooth, round, well-defined breast lump that was stable for many years but then started to grow rapidly.17
Surgical resection with wide margins is the primary management of these tumors.18
Mastitis, ie, inflammation of the breast tissue, often presents with symptoms of breast erythema, swelling, tenderness, and nipple discharge. It may be secondary to infection (most often in lactating women) or other causes such as radiation or underlying malignancy. A complication of infectious mastitis is formation of a breast abscess. Underlying malignancy, especially inflammatory breast cancer, is a common cause of noninfectious mastitis and is very important to recognize.19
RISK FACTORS FOR BREAST CANCER
3. Which of the following are risk factors for breast cancer?
- Menarche before age 12
- Female sex
- Personal history of breast cancer
- Obesity
- Never having had children, or having given birth for the first time at an older age
- Older age
- History of hormone replacement therapy with estrogen and progesterone
- Family history of breast cancer
All of these choices are risk factors for breast cancer.
Family history
The overall relative risk of developing breast cancer in a woman with a first-degree relative with the disease is 1.7. However, the relative risk is about 3 if the first-degree relative developed breast cancer before menopause, and 9 if the first-degree relative developed bilateral breast cancer before menopause.5
Estrogen exposure
The duration and amount of estrogen exposure are also risk factors. For example, menarche before age 12 and menopause after age 55 are associated with a higher risk. Women who go through menopause after age 55 have a twofold higher risk of breast cancer compared with women who go through menopause at an early age. Pregnancy before age 30 lowers the risk of breast cancer; late first full-term pregnancy or nulliparity increases it. Lactation, on the other hand, has a protective effect.5
Oral contraceptives have traditionally been thought to increase the risk of breast cancer. In the 1990s, a meta-analysis involving 153,506 women found that those who had used oral contraceptives had a 24% higher risk of developing breast cancer.26 However, this association has come into question since newer oral contraceptive pills containing different progestins and lower amounts of estrogen have become available. In fact, recent studies showed no link between oral contraceptive use and breast cancer.27,28 Nevertheless, women at higher risk of developing breast cancer are advised not to use oral contraceptives.
Hormone replacement therapy with estrogen and progesterone was found to increase the risk of breast cancer by 26% in the Women’s Health Initiative (WHI) study, which involved 16,608 healthy women followed for a median of 5.6 years.29
In a study reported separately, the WHI investigators randomized 10,739 women who had undergone hysterectomy to receive either hormone replacement therapy with unopposed estrogen (which is feasible only in women without a uterus) or placebo. They found no increase in the risk of invasive breast cancer in women on hormone replacement therapy with estrogen alone. In fact, the study showed a trend towards a modest reduction of this risk (odds ratio 0.77; 95% confidence interval 0.59–1.01).30
After the results of the WHI were published, the use of hormone replacement therapy in postmenopausal women declined significantly. And in 2003—1 year later—the incidence of breast cancer had dropped by 6.7%.31
Most experts now recommend that estrogen-progestin combinations be used only selectively to treat the symptoms of menopause, and only for the short term.
Other risk factors
Other factors found to modestly increase the risk of breast cancer include:
- Alcohol use
- Obesity
- Radiation exposure. Patients are at higher risk of breast cancer 15 to 20 years after receiving upper-mantle radiotherapy for Hodgkin lymphoma.5
Case continues: Bad news on mammography, ultrasonography, biopsy
The patient undergoes mammography, which shows a 2.5-cm spiculated lesion with areas of calcifications (BIRADS score of 5). Subsequently, ultrasonography confirms that the suspicious mass is not a cyst. Ultrasound-guided core needle biopsy reveals that the lesion is a high-grade invasive ductal carcinoma. The tumor is positive for both estrogen and progesterone receptors and negative for HER2/neu overexpression.
STAGING EVALUATION
4. Given these findings, what is the next step to take?
- CT of the chest, abdomen, and pelvis
- MRI of the brain
- PET
- Referral to a surgeon for a possible mastectomy with sentinel lymph node dissection
- Referral to a surgeon for a possible lumpectomy with sentinel lymph node dissection
At this point, the patient should be referred to a surgeon for possible mastectomy or lumpectomy.
Women who appear clinically to have early breast cancer, such as in this case, should have a complete blood count, comprehensive metabolic panel, and chest x-ray as their initial staging evaluation. No further studies are recommended unless the findings on history, physical examination, or the above testing suggest possible metastases.
Mastectomy vs lumpectomy
Early-stage breast cancer is managed with definitive surgery. The two options are mastectomy and breast conservation therapy, the latter involving lumpectomy followed by breast radiation therapy.
Multiple randomized studies comparing mastectomy and lumpectomy showed no difference in survival rates, but patients in the lumpectomy groups had higher rates of local recurrence.32 Breast radiation therapy after lumpectomy lowered the rates of local recurrence and breast cancer death.33 Therefore, most patients can opt to undergo either lumpectomy with radiation or mastectomy, depending on personal preference.
However, mastectomy rather than breast conservation therapy is still recommended in cases of prior radiation therapy, inability to achieve negative surgical margins (as in cases of large tumors), multicentric disease (cancer in separate breast quadrants), or multiple areas of calcifications. Mastectomy is also preferred in most pregnant women unless the diagnosis of breast cancer is made in the third trimester and radiation therapy can be given after delivery. Patients who have large lesions in a small breast may also choose mastectomy with breast reconstruction rather than breast conservation therapy. Patients with a history of scleroderma are encouraged to undergo mastectomy because of increased toxicity from radiation treatment.
Sentinel vs axillary lymph node dissection
Knowledge of axillary lymph node involvement is important because it determines the stage in the tumor-node-metastasis (TNM) system, and it influences the choice of further therapy. Therefore, all patients with nonmetastatic invasive breast cancer must have their axillary lymph nodes sampled.
Conventionally, this involves axillary lymph node dissection. Unfortunately, upper extremity lymphedema develops in 6% to 30% of patients within the first 3 years, and in 49% of patients after 20 years following axillary lymph node dissection.34
Sentinel lymph node dissection was developed to minimize this complication. This procedure involves the injection of a blue dye, isosulfan blue (Lymphazurin), around the edge of the tumor or in the dermis overlying the tumor. The most proximal axillary lymph nodes that stain blue are dissected. Alternatively, a radioactive colloid (most commonly technetium sulfur colloid agents) may be injected, allowing sentinel lymph nodes to be identified by lymphoscintigraphy. If no metastases are found in the sentinel lymph nodes, axillary lymph node dissection is not performed.
A prospective study in 536 women found that at 5 years of follow-up, lymphedema developed in only 5% of patients after sentinel lymph node dissection compared with 16% of those who underwent axillary lymph node dissection (P < .001), with comparable outcomes in terms of disease recurrence.35
Case continues: Patient undergoes surgery
The patient elects to undergo lumpectomy with sentinel lymph node dissection. Pathologic review of the resection specimen reveals a 2.5-cm poorly differentiated invasive ductal carcinoma. Sentinel lymph node dissection shows metastases, and therefore axillary lymph node dissection is performed. One of eight lymph nodes removed is positive for metastases. All surgical margins are negative.
POSTOPERATIVE CARE
5. What would be the next step for our patient?
- Radiation followed by observation
- Tamoxifen (Nolvadex) for 5 years
- Observation only
- Chemotherapy followed by radiation therapy and 5 years of tamoxifen
She should receive chemotherapy, followed by radiation therapy and then tamoxifen for 5 years.
Chemotherapy. Almost all patients who have lymph-node-positive disease are advised to undergo chemotherapy.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a metaanalysis of 194 randomized trials that compared adjuvant chemotherapy and no treatment in early-stage breast cancer. Chemotherapy led to a 10% absolute improvement in survival at 15 years for women younger than 50 years and 3% in women age 51 to 69.36
Indications for chemotherapy include axillary lymph node involvement, locally advanced disease, and other risk factors for recurrence such as young age at diagnosis, strong positive family history of breast cancer, prior history of breast cancer, or lymph-node-negative, estrogen-receptor-negative tumors that are larger than 1 cm in diameter.
The Oncotype DX assay is a new tool to help oncologists decide whether to use chemotherapy in cases of estrogen-receptor-positive breast cancer, in which the benefit of chemotherapy is uncertain. It is a polymerase chain reaction assay that measures the expression of 16 cancer-specific genes and five reference genes within the breast tumor. Based on the pattern of expression of these genes, breast cancer can be characterized as low-risk, intermediate-risk, or high-risk. Patients in the high-risk group have a high chance of cancer recurrence and benefit from chemotherapy. Patients in the low-risk group are unlikely to have a recurrence or to benefit from chemotherapy.37 It is far less clear if patients in the intermediate-risk group benefit from chemotherapy, but this assay might eventually prove useful in deciding for or against chemotherapy in this group of patients as well.38 The Oncotype DX assay is presently being studied in a clinical trial.
Radiation therapy after mastectomy is recommended in patients who have breast tumors larger than 5 cm or metastases to more than three axillary lymph nodes.39
Antiestrogen therapy. After chemotherapy, patients with estrogen-receptor-positive cancers also receive 5 years of antiestrogen therapy. Available antiestrogen agents for such patients include tamoxifen, which is a selective estrogen receptor modulator, and drugs called aromatase inhibitors that block conversion of androgens to estrogens in peripheral tissues. Anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) are examples of available aromatase inhibitors. Premenopausal women are treated with tamoxifen, and postmenopausal women are offered aromatase inhibitors.
Table 4 lists the most common adverse effects of these agents. Aromatase inhibitors are associated with a higher risk of osteoporosis and arthralgia, while tamoxifen increases the risks of thromboembolism, endometrial cancer, and vaginal discharge. Both agents may produce menopausal symptoms such as hot flashes and mood swings.
Case continues: Seven years later, metastases in the spine
The patient achieves a complete remission. She is seen for a routine visit 7 years after diagnosis. She now reports mid-back pain that has worsened over the last 2 months. A bone scan reveals diffuse metastatic disease in the spine and in both humeral bones. CT of the chest, abdomen, and pelvis is negative for visceral metastases. Bone marrow aspiration and biopsy study show marrow infiltration by adenocarcinoma that stains positive for estrogen receptors and negative for HER2. The patient otherwise feels well and has no other symptoms.
WHAT TREATMENT FOR METASTATIC BREAST CANCER?
6. What should you now do for our patient?
- Discuss end-of-life care and refer her to a hospice program
- Educate the patient that no options for treatment exist and recommend enrolling in a phase I clinical trial
- Refer her to an oncologist for consideration of chemotherapy
- Refer her to an oncologist for consideration of endocrine treatment
She should be referred to an oncologist for consideration of endocrine treatment.
The most common sites of breast cancer metastases are the bones, followed by the liver and lungs. Metastatic breast cancer almost always is incurable. However, treatment can palliate symptoms.
Although a randomized trial of treatment vs best supportive care has never been done, many believe that treatment may improve survival. 40 The median survival of patients treated with standard therapy is about 3 years if the breast cancer is estrogen-receptor-positive and 2 years if it is estrogen-receptor-negative, but survival rates vary widely from patient to patient.41,42
Standard therapy or enrollment in a clinical phase II or III trial is indicated for this patient before considering enrollment in a phase I clinical trial or supportive care alone.
Endocrine therapy is the first-line therapy in women with estrogen-receptor-positive metastatic breast cancer. Postmenopausal women usually receive an aromatase inhibitor first.43,44 Response to endocrine therapy usually takes weeks to months but may last for several years.
Premenopausal women with estrogen-receptor-positive breast cancer also receive ovarian ablation therapy (oophorectomy or chemical ovarian ablation) with gonadotropin-releasing hormone agonists.
In addition, most patients with bone involvement are treated with high doses of intravenous bisphosphonates, which can reduce skeletal complications.45
Chemotherapy is reserved for patients with estrogen-receptor-negative breast cancer and those with cancer that progresses despite treatment with multiple antiestrogen agents. The time to response when chemotherapy is used is quicker, but the duration of response is usually shorter, lasting on average less than 1 year.37
Trastuzumab (Herceptin), a monoclonal humanized murine antibody to the extracellular domain of the HER2 protein, is indicated in patients with HER2-overexpressing tumors.46,47
STABLE 2 YEARS LATER
The patient was started on letrozole and a bisphosphonate, zolendronic acid (Zometa). Ovarian ablation was initiated with goserelin (Zoladex) given monthly. A bone scan performed 2 months after starting treatment showed improvement in bony metastases. She also noted significant improvement in pain. Her disease remains stable 2 years after starting endocrine therapy.
- Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 1999; 130:651–657.
- Petrelli NJ, Winer EP, Brahmer J, et al. Clinical cancer advances 2009: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:6052–6069.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008; 58:71–96.
- National Cancer Institute. SEER Stat Fact Sheets. www.seer.cancer.gov/statfacts/html/breast.html#ref09. Accessed June 7, 2010.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ; the publishers of the journal Oncology. Cancer Management: A multidisciplinary Approach. Medical, Surgical & Radiation Oncology. 11th ed. CMP Medica; 2008.
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187:171–177.
- Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst 2002; 94:1151–1159.
- American College of Radiology. Breast Imaging Reporting and Data System: BIRADS Atlas. 4th ed. Reston, VA: American College of Radiology; 2003.
- Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184:1260–1265.
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003; 227:183–191.
- Schell AM, Rosenkranz K, Lewis PJ. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 2009; 192:1438–1444.
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375:563–571.
- Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007; 13:115–121.
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985; 312:146–151.
- Page DL, Kidd TE, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991; 22:1232–1239.
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductallobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002; 15:1044–1050.
- Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw 2007; 5:324–330.
- Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996; 77:910–916.
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009; 15:367–380.
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 1999; 64:963–970.
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90:606–611.
- Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999; 286:2528–2531.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–1727.
- Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States). Cancer Causes Control 1997; 8:65–72.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002; 346:2025–2032.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333:1456–1461.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26:5213–5219.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826.
- Albain KS, Barlow WE, Shak S, et al; Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65.
- Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44:989–990.
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005; 104:1742–1750.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101–2109.
- Gamucci T, D’Ottavio AM, Magnolfi E, et al. Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer. Br J Cancer 2007; 97:1040–1045.
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18:3758–3767.
- Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335:1785–1791.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792.
- Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 1999; 130:651–657.
- Petrelli NJ, Winer EP, Brahmer J, et al. Clinical cancer advances 2009: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:6052–6069.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008; 58:71–96.
- National Cancer Institute. SEER Stat Fact Sheets. www.seer.cancer.gov/statfacts/html/breast.html#ref09. Accessed June 7, 2010.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ; the publishers of the journal Oncology. Cancer Management: A multidisciplinary Approach. Medical, Surgical & Radiation Oncology. 11th ed. CMP Medica; 2008.
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187:171–177.
- Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst 2002; 94:1151–1159.
- American College of Radiology. Breast Imaging Reporting and Data System: BIRADS Atlas. 4th ed. Reston, VA: American College of Radiology; 2003.
- Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184:1260–1265.
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003; 227:183–191.
- Schell AM, Rosenkranz K, Lewis PJ. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 2009; 192:1438–1444.
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375:563–571.
- Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007; 13:115–121.
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985; 312:146–151.
- Page DL, Kidd TE, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991; 22:1232–1239.
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductallobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002; 15:1044–1050.
- Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw 2007; 5:324–330.
- Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996; 77:910–916.
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009; 15:367–380.
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 1999; 64:963–970.
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90:606–611.
- Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999; 286:2528–2531.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–1727.
- Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States). Cancer Causes Control 1997; 8:65–72.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002; 346:2025–2032.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333:1456–1461.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26:5213–5219.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826.
- Albain KS, Barlow WE, Shak S, et al; Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65.
- Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44:989–990.
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005; 104:1742–1750.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101–2109.
- Gamucci T, D’Ottavio AM, Magnolfi E, et al. Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer. Br J Cancer 2007; 97:1040–1045.
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18:3758–3767.
- Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335:1785–1791.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792.