User login
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
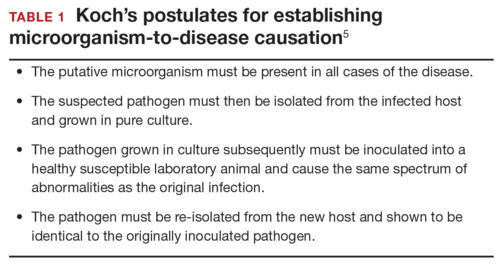
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.
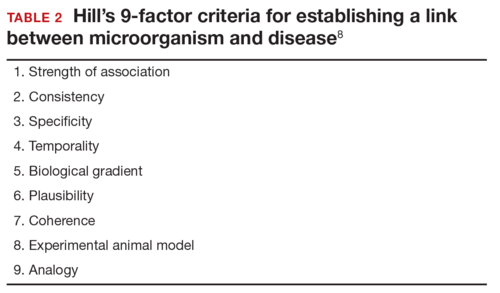
Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.
How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.

How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.

How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
In this Article
- Confirming Zika virus infection
- Zika virus and Guillain-Barré syndrome
- Preventing sexual transmission