User login
Zika virus update: A rapidly moving target
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
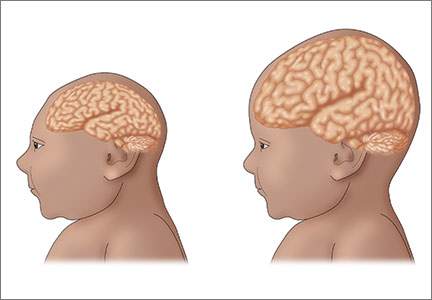
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
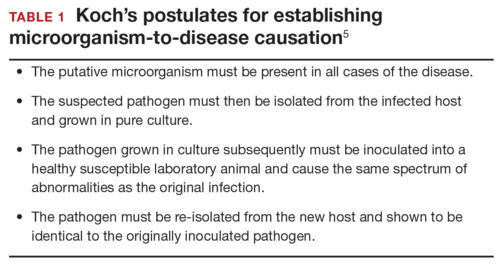
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.
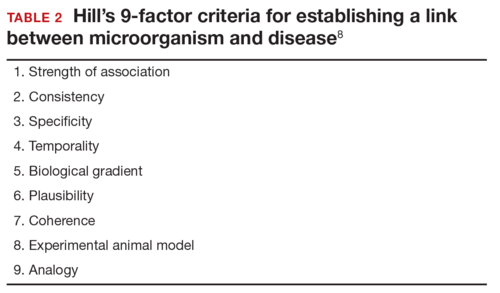
Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.
How should ZV infection be confirmed in adults and newborns?
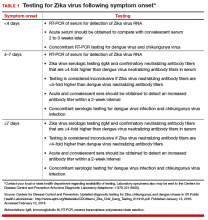
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.

How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.

How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
In this Article
- Confirming Zika virus infection
- Zika virus and Guillain-Barré syndrome
- Preventing sexual transmission
What insect repellents are safe during pregnancy?
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Zika virus: Counseling considerations for this emerging perinatal threat
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
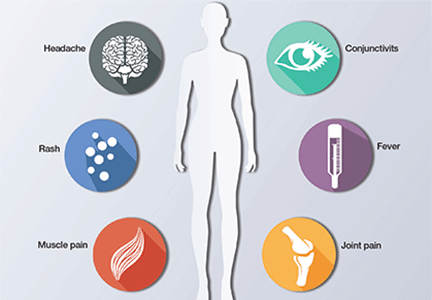
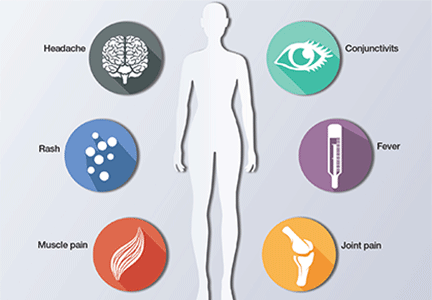
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus infection in the news
- CDC: Zika virus disease cases by US state or territory, updated periodically
- CDC: Q&As for ObGyns on pregnant women and Zika virus, 2/9/16
- CDC: Zika virus infection among US pregnant travelers, 2/26/16
- CDC: Interim guidelines for health care providers caring for infants and children with possible Zika virus infection, 2/19/16
- SMFM statement: Ultrasound screening for fetal microcephaly following Zika virus exposure, 2/16/16
- FDA approves first Zika diagnostic test for commercial use. Newsweek, 2/26/16
- NIH accelerates timeline for human trials of Zika vaccine. The Washington Post, 2/17/16
- Patient resource: Zika virus and pregnancy fact sheet from MotherToBaby.org
- Zika virus article collection from New England Journal of Medicine
- Zika infection diagnosed in 18 pregnant US women who traveled to Zika-affected areas
- FDA grants emergency approval to new 3-in-1 lab test for Zika
- ACOG Practice Advisory: Updated interim guidance for care of women of reproductive age during a Zika virus outbreak, 3/31/16
- MMWR: Patterns in Zika virus testing and infection, 4/22/16
- What insect repellents are safe during pregnancy? 5/19/16
- Zika virus and complications: Q&A from WHO, 5/31/16
- WHO strengthens guidelines to prevent sexual transmission of Zika virus, 5/31/16
- Ultrasound screening for fetal microcephaly following Zika virus exposure (from AJOG), 6/1/16
- CDC: Interim guidance for interpretation of Zika virus antibody test results, 6/3/16
- First Zika vaccine to begin testing in human trials, The Washington Post, 6/20/16
- NIH launches the Zika in Infants and Pregnancy (ZIP) international study, 6/21/16
CASE 1: Pregnant traveler asks: Should I be tested for Zika virus?
A 28-year-old Hispanic woman (G3P2) at 15 weeks’ gestation visits your office for a routine prenatal care appointment. She reports having returned from a 3-week holiday in Brazil 2 days ago, and she is concerned about having experienced fever, malaise, arthralgias, and a disseminated erythematous rash. She has since heard about the Zika virus and asks you if she and her baby are in danger and whether she should be tested for the disease.
What should you tell this patient?
The Zika virus is an RNA Flavivirus, transmitted primarily by the Aedes aegypti mosquito.1 This virus is closely related to the organisms that cause dengue fever, yellow fever, chikungunya infection, and West Nile infection. By feeding on infected prey, mosquitoes can transmit the virus to humans through bites. They breed near pools of stagnant water, can survive both indoors and outdoors, and prefer to be near people. These mosquitoes bite mostly during daylight hours, so it is essential that people use insect repellent throughout the day while in endemic areas.2 These mosquitoes live only in tropical regions; however, the Aedes albopictus mosquito, also known as the Asian tiger mosquito, lives in temperate regions and can transmit the Zika virus as well3 (FIGURE 1).
| FIGURE 1 Aedes aegypti and Aedes albopictus mosquitoes | ||

| 
| |
Aedes aegypti (left) and Aedes albopictus (right) mosquitoes. Aedes mosquitoes are the main transmission vector for the Zika virus. | ||
|
The Zika virus was first discovered in 1947 when it was isolated from a rhesus monkey in Uganda. It subsequently spread to Southeast Asia and eventually caused major outbreaks in the Yap Islands of Micronesia (2007)4 and French Polynesia (2013).5 In 2015, local transmission of the Zika virus infection was noted in Brazil, and, most recently, a pandemic of Zika virus infection has occurred throughout South America, Central America, and the Caribbean islands. To date, local mosquito-borne virus transmission has not occurred in the continental United States, although at least 82 cases acquired during travel to infected areas have been reported.6
Additionally, there have been rare cases involving spread of this virus from infected blood transfusions and through sexual contact.7 In February 2016, the first case of locally acquired Zika virus infection was reported in Texas following sexual transmission of the disease.8
Clinical manifestations of Zika virus infection
Eighty percent of patients infected with Zika virus remain asymptomatic. The illness is short-lived, occurring 2 to 12 days following the mosquito bite, and infected individuals usually do not require hospitalization or experience serious morbidity. When symptoms are present, they typically include low-grade fever (37.8° to 38.5°C), maculopapular rash, arthralgias of the hands and feet, and nonpurulent conjunctivitis. Patients also may experience headache, retro-orbital pain, myalgia, and, rarely, abdominal pain, nausea, vomiting, diarrhea, ulcerations of mucous membranes, and pruritus.9 Guillain-Barré syndrome has been reported in association with Zika virus infection10; however, a definitive cause-effect relationship has not been proven.
If a pregnant woman is infected with the Zika virus, perinatal transmission can occur, either through uteroplacental transmission or vertically from mother to child at the time of delivery. Zika virus RNA has been detected in blood, amniotic fluid, semen, saliva, cerebrospinal fluid, urine, and breast milk. Although the virus has been shown to be present in breast milk, there has been no evidence of viral replication in milk or reported transmission in breastfed infants.11 Pregnant women are not known to have increased susceptibility to Zika virus infection when compared with the general population, and there is no evidence to suggest pregnant women will have a more serious illness if infected.
The Zika virus has been strongly associated with congenital microcephaly and fetal loss among women infected during pregnancy.12 Following the recent large outbreak in Brazil, an alarmingly high number of Brazilian newborns with microcephaly have been observed. The total now exceeds 4,000. Because of these ominous findings, fetuses and neonates born to women with a history of infection should be evaluated for adverse effects of congenital infection.
Management strategies for Zika virus exposure during pregnancy
The incidence of Zika virus infection during pregnancy remains unknown. However, a pregnant woman may be infected in any trimester, and maternal-fetal transmission of the virus can occur throughout pregnancy. If a patient is pregnant and has travelled to areas of Zika virus transmission, or has had unprotected sexual contact with a partner who has had exposure, she should be carefully screened with a detailed review of systems and ultrasonography to evaluate for fetal microcephaly or intracranial calcifications. The US Centers for Disease Control and Prevention (CDC) initially recommended that, if a patient exhibited 2 or more symptoms consistent with Zika virus infection within 2 weeks of exposure or if sonographic evidence revealed fetal microcephaly or intracranial calcifications, she should be tested for Zika virus infection.11
More recently, the CDC issued new guidelines recommending that even asymptomatic women with exposure have serologic testing for infection and that all exposed women undergo serial ultrasound assessments.13 The CDC also recommends offering retesting in the mid second trimester for women who were exposed very early in gestation.
The best diagnostic test for infection is reverse transcriptase-polymerase chain reaction (RT-PCR), and, ideally, it should be completed within 4 days of symptom onset. Beyond 4 days after symptom onset, testing for Zika virus immunoglobulin M (IgM)-specific antibody and neutralizing antibody should be performed in addition to the RT-PCR test. At times, interpretation of antibody testing can be problematic because cross-reaction with related arboviruses is common. Moreover, Zika viremia decreases rapidly over time; therefore, if serum is collected even 5 to 7 days after symptom onset, a negative test does not definitively exclude infection (TABLE 1).
In the United States, local health departments should be contacted to facilitate testing, as the tests described above are not currently commercially available. If the local health department is unable to perform this testing, clinicians should contact the CDC’s Division of Vector-Borne Diseases (telephone: 1-970-221-6400) or visit their website (http://www.cdc.gov/ncezid/dvbd/specimensub/arboviral-shipping.html) for detailed instructions on specimen submission.
Testing is not indicated for women without a history of travel to areas where Zika virus infection is endemic or without a history of unprotected sexual contact with someone who has been exposed to the infection.
Following the delivery of a live infant to an infected or exposed mother, detailed histopathologic evaluation of the placenta and umbilical cord should be performed. Frozen sections of placental and cord tissue should be tested for Zika virus RNA, and cord serum should be tested for Zika and dengue virus IgM and neutralizing antibodies. In cases of fetal loss in the setting of relevant travel history or exposure (particularly maternal symptoms or sonographic evidence of microcephaly), RT-PCR testing and immunohistochemistry should be completed on fetal tissues, umbilical cord, and placenta.2
Treatment is supportive
At present, there is no vaccine for the Zika virus, and no hyperimmune globulin or anti‑ viral chemotherapy is available. Treatment is therefore supportive. Patients should be encouraged to rest and maintain hydration. The preferred antipyretic and analgesic is acetaminophen (650 mg orally every 6 hours or 1,000 mg orally every 8 hours). Aspirin should be avoided until dengue infection has been ruled out because of the related risk of bleeding with hemorrhagic fever. Nonsteroidal anti-inflammatory drugs should be avoided in the second half of pregnancy because of their effect on fetal renal blood flow (oligohydramnios) and stricture of the ductus arteriosus.
CASE 1 Continued
Given this patient’s recent travel, exposure to mosquito-borne illness, and clinical manifestations of malaise, rash, and joint pain, you proceed with serologic testing. The RT-PCR test is positive for Zika virus.
What should be the next step in the management of this patient?
Prenatal diagnosis and fetal surveillance
The recent epidemic of microcephaly and poor pregnancy outcomes reported in Brazil has been alarming and demonstrates an almost 20-fold increase in incidence of this condition between 2014–2015.14 Careful surveillance is needed for this birth defect and other poor pregnancy outcomes in association with the Zika virus. To date, a direct causal relationship between Zika virus infection and microcephaly has not been unequivocally established15; however; these microcephaly cases have yet to be attributed to any other cause (FIGURE 2)
| FIGURE 2 Microcephaly: associated with Zika virus infection in pregnancy |

|
Illustration depicts a child with congenital microcephaly (left) and one with head circumference within the mean SD (right). |
Following the outbreak in Brazil, a task force and registry were established to investigate microcephaly and other birth defects associated with Zika virus infection. In one small investigation, 35 cases of microcephaly were reported, and 71% of the infants were seriously affected (head circumference >3 SD below the mean). Fifty percent of babies had at least one neurologic abnormality, and, of the 27 patients who had neuroimaging studies, all had distinct abnormalities, including widespread brain calcifications and cell migration abnormalities, such as lissencephaly, pachgyria, and ventriculomegaly due to cortical atrophy.16
In addition to microcephaly, fetal ultrasound monitoring has revealed focal brain abnormalities, such as asymmetric cerebral hemispheres, ventriculomegaly, displacement of the midline, failure to visualize the corpus callosum, failure of thalamic development, and the presence of intraocular and brain calcifications.17
In collaboration with the CDC, the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal-Medicine have developed guidelines to monitor fetal growth in women with laboratory evidence of Zika virus infection.18 Recommendations include having a detailed anatomy ultrasound and serial growth sonograms every 3 to 4 weeks, along with referral to a maternal-fetal medicine or infectious disease specialist.
If the pregnancy is beyond 15 weeks’ gestational age, an amniocentesis should be performed in symptomatic patients and in those with abnormal ultrasound findings. Amniotic fluid should be tested for Zika virus with RT-PCR (FIGURE 3).12 The sensitivity and specificity of amniotic fluid RT-PCR in detecting congenital infection, as well as the predictive value of a fetal anomaly, remain unknown at this time. For this reason, a patient must be counseled carefully regarding the benefits of confirming intrauterine infection versus the slight risks of premature rupture of membranes, infection, and pregnancy loss related to amniocentesis.
Once diagnosed, microcephaly cannot be “fixed.” However, pregnancy termination is an option that some parents may choose once they are aware of the diagnosis and prognosis of microcephaly. Moreover, even for parents who would not choose abortion, there may be considerable value in being prepared for the care of a severely disabled child. Microcephaly has many possible causes, Zika virus infection being just one. Others include genetic syndromes and other congenital infections, such as cytomegalovirus (CMV) infection and toxoplasmosis. Amniocentesis therefore may help the clinician sort through these causes. For both CMV infection and toxoplasmosis, certain antenatal treatments may be helpful in lessening the severity of fetal injury.
CASE 2 Pregnant patient has travel plans
A 34-year-old woman (G1P0) presents to you for her first prenatal visit. She mentions she plans to take a cruise through the Eastern Caribbean in 2 weeks. Following the history and physical examination, what should you tell this patient?
Perinatal counseling: Limiting exposure is best
As mentioned, there is currently no treatment, prophylactic medication, or vaccination for Zika virus infection. Because of the virus’s significant associations with adverse pregnancy outcomes, birth defects, and fetal loss, the CDC has issued a travel advisory urging pregnant women to avoid travel to areas when Zika virus infection is prevalent. Currently, Zika virus outbreaks are occurring throughout South and Central America, the Pacific Islands, and Africa, and the infection is expected to spread, mainly due to international air travel. If travel to these areas is inevitable, women should take rigorous precautions to avoid exposure to mosquito bites and infection (TABLE 2).
If a woman was infected with laboratory-confirmed Zika virus infection in a prior pregnancy, she should not be at risk for congenital infection during her next pregnancy. This is mainly because the period of viremia is short-lived and lasts approximately 5 to 7 days.2
Further, based on documented sexual transmission of the virus, pregnant women should abstain from sexual activity or should consistently and correctly use condoms with partners who have Zika virus infection or exposure to the virus until further evidence is available.
Stay informed
Zika virus infection is now pandemic; it has evolved from an isolated disease of the tropics to one that is sweeping the Western hemisphere. It is being reported daily in new locations around the world. Given the unsettling association of Zika virus infection with birth defects, careful obstetric surveillance of exposed or symptomatic patients is imperative. Clinicians must carefully screen patients with potential risk of exposure and be prepared to offer appropriate perinatal counseling and diagnostic testing during pregnancy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983.
- Centers for Disease Control and Prevention. Zika virus. Atlanta, GA: US Dept of Health and Human Services; 2015. http://www.cdc.gov/zika/index.html. Accessed February 12, 2016.
- Bogoch II, Brady OJ, Kraemer MU, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):pii:20751.
- Centers for Disease Control and Prevention. Zika virus disease in the United States, 2015–2016. http://www.cdc.gov/zika/geo/united-states.html. Accessed February 12, 2016.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. http://www.dallascounty.org/department/hhs /press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf. Accessed February 3, 2016.
- Ministry of Health, Manuatu Hauora. Zika virus. http://www.health.govt.nz/our-work/diseases-and-conditions/zika -virus. Accessed January 13, 2016.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:4–6.
- Centers for Disease Control and Prevention. Zika virus: transmission. http://www.cdc.gov/zika/transmission/index.html. Accessed January 20, 2016.
- Petersen EE, Staples JE, Meaney-Delamn, D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127.
- Pan American Health Organization, World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1,2015. http://www.paho.org/hq/index.php?option=com_doc man&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 13, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association -with-microcephaly-rapid-risk-assessment.pdf. Accessed January 13, 2016.
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al; Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential associations with microcephaly and Guillain-Barré syndrome. December 10, 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association.
In this Article
- Management strategies for pregnant patients with Zika virus exposure
- Fetal surveillance
- Perinatal counseling on exposure prevention
- Algorithm for evaluation and management