User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
When does benign shyness become social anxiety, a treatable disorder?
Since the appearance of social anxiety disorder (SAD) in the DSM-III in 1980, research on its prevalence, characteristics, and treatment have grown (Box 11,2). In addition to the name, the definition of SAD has changed over the years; as a result, its prevalence has increased in recent cohort studies. This has led to debate over whether the experience of shyness is being over-pathologized, or whether SAD has been underdiagnosed in earlier decades. Those who argue that shyness is being over-pathologized note that it is a normal human experience that has evolutionary functions (eg, preventing engagement in harmful social relationships3). Others argue that a high degree of shyness is not beneficial in terms of evolution because it causes the individual to be shunned, so to speak, by society.4
Why worry about ‘over-pathologizing’?
The medicalization of shyness might be a reflection of Western societal values of assertiveness and gregariousness; other societies that value modesty and reticence do not over-pathologize shyness.5 It is important not to assume that someone who is shy necessarily has a “pathologic” level of social anxiety, especially because some people who are shy view that condition as a positive quality, much like sensitivity and conscientiousness.5
The broader issue of what constitutes a mental disorder arises in this debate. A “disorder” is a socially constructed label that describes a set of symptoms occurring together and its associated behaviors, not a real entity with etiological homogeneity.6 Labeling emotional problems “disordered” assumes that happiness is the natural homeostatic state, and distressing emotional states are abnormal and need to be changed.7 A diagnostic label can help improve communication and understand maladaptive behaviors; if that label is reified, however, it can lead to assumptions that the etiology, course, and treatment response are known. Proponents of the diagnostic psychiatric nomenclature have acknowledged the dangers of over-pathologizing normal experiences of living (such as fear) by way of diagnostic labeling.8
Determining when shyness becomes a clinically significant problem—what we call SAD here—demands a delicate distinction that has important implications for treatment. On one hand, if shyness is over-pathologized, persons who neither desire nor need treatment might be subjected to unnecessary and costly intervention. On the other hand, if SAD is underdiagnosed, some persons will not receive treatment that might be beneficial to them.
In this article, we review the similarities and differences between shyness and SAD, and provide recommendations for determining when shyness becomes a more clinically significant problem. We also highlight the importance of this distinction as it pertains to management, and provide suggestions for treatment approaches.
SAD: Definition, prevalence
SAD is defined as a significant fear of embarrassment or humiliation in social or performance-based situations, to a point at which the affected person often avoids these situations or endures them only with a high level of distress9 (Table 1, and Box 2). SAD can be distinguished from other anxiety disorders based on the source and content of the fear (ie, the source being social interaction or performance situations, and the content being a fear that one will show a behavior that will cause embarrassment). SAD also should be distinguished from autism spectrum disorders, in which persons have limited social communication capabilities and inadequate age-appropriate social relationships.
SAD is most highly comorbid with mood and anxiety disorders, with rates of at least 30% in clinical samples.10 The disorder also is highly comorbid with avoidant personality disorder—to a point at which it is argued that they are one and the same disorder.11
As with other psychiatric disorders, anxiety must cause significant impairment or distress. What constitutes significant impairment or distress is subjective, and the arbitrary nature of this criterion can influence estimates of the prevalence of SAD. For example, prevalence ranges as widely as 1.9% to 20.4% when different cut-offs are used for distress ratings and the number of impaired domains.12
The prevalence of SAD varies from 1 epidemiological study to another (ie, the Epidemiological Catchment Area [ECA] Study and the National Comorbidity Survey [NCS])—in part, a consequence of the differing definitions of significant impairment or distress. The ECA study assessed the clinical significance of each symptom in anxiety disorders; the NCS assessed overall clinical significance of the disorder. When the clinical significance criterion was applied at the symptom level to the NCS dataset (as was done in the ECA study), 1-year prevalence decreased by 50% (from 7.4% to 3.7%).13 The manner in which significant impairment or distress is defined (ie, conservatively or liberally) impacts whether social anxiety symptoms are classified as disordered or non-disordered.
Shyness: Definition, prevalence
Shyness often refers to 1) anxiety, inhibition, reticence, or a combination of these findings, in social and interpersonal situations, and 2) a fear of negative evaluation by others.14 It is a normal facet of personality that combines the experience of social anxiety and inhibited behavior,15 and also has been described as a stable temperament.16 Shyness is common; in the NCS study,17 26% of women and 19% of men characterized themselves as “very shy”; in the NCS Adolescent study,18 nearly 50% of adolescents self-identified as shy.
Persons who are shy tend to self-report greater social anxiety and embarrassment in social situations than non-shy persons do; they also might experience greater autonomic reactivity—especially blushing—in social or performance situations.15 Furthermore, shy persons are more likely to have axis I comorbidity and traits of introversion and neuroticism, compared with non-shy persons.14
Research suggests that temperament and behavioral inhibition are risk factors for mood and anxiety disorders, and appear to have a particularly strong relationship with SAD.19 A recent prospective study showed that shyness tends to increase steeply in toddlerhood, then stabilizes in childhood. Shyness in childhood—but not toddlerhood—is predictive of anxiety, depression, and poorer social skills in adolescence.20
A qualitative, or just quantitative, difference?
It is clear that SAD and shyness share several features—including anxiety and embarrassment—in social interactions. This raises a question: Are SAD and shyness distinct qualitatively, or do they represent points along a continuum, with SAD being an extreme form of shyness?
Continuum hypothesis. Support for the continuum hypothesis includes evidence that SAD and shyness share several features, including autonomic arousal, deficits in social skills (eg, aversion of gaze, difficulty initiating and maintaining conversation), avoidance of social situations, and fear of negative evaluation.21,22 In addition, both shyness and SAD are highly heritable,23 and mothers of shy children have a significantly higher rate of SAD than non-shy children do.24 No familial or genetic studies have compared heritability and familial aggregation in shyness and SAD.
According to the continuum hypothesis, if SAD is an extreme form of shyness, all (or nearly all) persons who have a diagnosis of SAD also would be characterized as shy. However, only approximately one-half of such persons report having been shy in childhood.17 Less than one-quarter of shy persons meet criteria for SAD.14,18 Because many persons who are shy do not meet criteria for SAD, and many who have SAD were not considered shy earlier in life, it has been suggested that this supports a qualitative distinction.
Qualitative distinctiveness. Despite having similarities, several features distinguish the experience of SAD from that of shyness. Compared with shyness, a SAD diagnosis is associated with:
- greater comorbidity
- greater severity of avoidance and impairment
- poorer quality of life.18,21,25
Studies that compared SAD, shyness without SAD, and non-shyness have shown that the shyness without SAD group more closely resembles the non-shy group than the SAD group—particularly with regard to impairment, presence of substance use, and other behavioral problems.18,25
Given the evidence, experts have concluded that shyness and a SAD diagnosis are overlapping yet different constructs that encapsulate qualitative and quantitative differences.25 There is a spectrum of shyness that ranges from a normative level to a higher level that overlaps the experience of SAD, but the 2 states represent different constructs.25
Guidance for making an assessment. Because of similarities in anxiety, embarrassment, and other symptoms in social situations, the best way to determine whether shyness crosses the line into a clinically significant problem is to assess the severity of the anxiety and associated degree of impairment and distress. More severe anxiety paired with distress about having anxiety and significant impairment in multiple areas of functioning might indicate more problematic social anxiety—a diagnosis of SAD—not just “normal” shyness.
It is important to take into account the environmental and cultural context of a patient’s distress and impairment because these features might fall within a normal range, given immediate circumstances (such as speaking in front of a large audience when one is not normally called on to do so, to a degree that does not interfere with general social functioning6).
What is considered a normative range depends on the developmental stage:
- Among children, a greater level of shyness might be considered more normative when it manifests during developmental stages in which separation anxiety appears.
- Among adolescents, a greater level of shyness might be considered normative especially during early adolescence (when social relationships become more important), and during times of transition (ie, entering high school).
- In adulthood, a greater level of normative shyness or social anxiety might be present during a major life change (eg, beginning to date again after the loss of a lengthy marriage or romantic relationship).
Assessment tools
Assessment tools can help you differentiate normal shyness from SAD. Several empirically-validated rating scales exist, including clinician-rated and self-report scales.
Liebowitz Social Anxiety Scale26 rates the severity of fear and avoidance in a variety of social interaction and performance-based situations. However, it was developed primarily as a clinician-rated scale and might be more burdensome to complete in practice. In addition, it does not provide cut-offs to indicate when more clinically significant anxiety might be likely.
Clinically Useful Social Anxiety Disorder Outcome Scale (CUSADOS)27 and Mini-Social Phobia Inventory (Mini-SPIN)28 are brief self-report scales that provide cut-offs to suggest further assessment is warranted. A cut-off score of 16 on the CUSADOS suggests the presence of SAD with 73% diagnostic efficiency.
One disadvantage to relying on a rating scale alone is the narrow focus on symptoms. Given that shyness and SAD share similar symptoms, it is necessary to assess the degree of impairment related to these symptoms to determine whether the problem is clinically significant. The overly narrow focus on symptoms utilized by the biomedical approach has been criticized for contributing to the medicalization of normal shyness.5
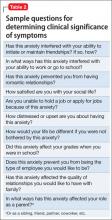
Diagnostic interviews, such as the Structured Clinical Interview for DSM-IV Axis I Disorders29 include sections on SAD that assess avoidance and impairment/distress associated with anxiety. Because these interviews may increase the time burden during an office visit, there are several general questions outside of a structured interview that you can ask, such as: “Has this anxiety interfered with your ability to initiate or maintain friendships? If so, how?” (Table 2). Persons with clinically significant social anxiety, rather than shyness, tend to report greater effects on their relationships and on work or school performance, as well as greater distress about having that anxiety.
Treatment approaches based on distinctions
Exercise care in making the distinction between normal shyness and dysfunctional and impairing levels of anxiety characteristic of SAD, because persons who display normal shyness but who are overdiagnosed might feel stigmatized by a diagnostic label.5 Also, overpathologizing shyness takes what is a social problem out of context, and could promote treatment strategies that might not be helpful or effective.30
Unnecessary diagnosis might lead to unnecessary treatment, such as prescribing an antidepressant or benzodiazepine. Avoiding such a situation is important, because of the side effects associated with medication and the potential for dependence and withdrawal effects with benzodiazepines.
Persons who exhibit normal shyness do not require medical treatment and, often, do not want it. However, some people may be interested in improving their ability to function in social interactions. Self-help approaches or brief psychotherapy (eg, cognitive-behavioral therapy [CBT]) should be the first step—and might be all that is necessary.
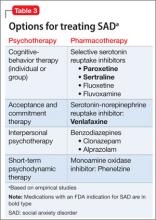
The opposite side of the problem. Under-recognition of clinically significant social anxiety can lead to under-treatment, which is common even in patients with a SAD diagnosis.31 Treatment options include CBT, medication, and CBT combined with medication (Table 3):
- several studies have demonstrated the short- and long-term efficacy of CBT alone for SAD
- medication alone has been efficacious in the short-term, but less efficacious than CBT in the long-term
- combined treatment also has been shown to be more efficacious than CBT or medication alone in the short-term
- there is evidence to suggest that CBT alone is more efficacious in the long-term compared with combined treatment.a
CBT is recommended as an appropriate first-line option, especially for mild and moderate SAD; it is the preferred initial treatment option of the United Kingdom’s National Institute for Health and Care Excellence (NICE). For more severe presentations (such as the presence of comorbidity) or when a patient did not respond to an adequate course of CBT, combined treatment might be an option—the goal being to taper the medication and continue CBT as a longer-term treatment. Research has shown that continuing CBT while discontinuing medication helps prevent relapse.32,33
Appropriate pharmacotherapy options include selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.34 Increasingly, benzodiazepines are considered less desirable; they are not recommended for routine use in SAD in the NICE guidelines. Those guidelines call for continuing pharmacotherapy for 6 months when a patient responds to treatment within 3 months, then discontinuing medication with the aid of CBT.
Bottom Line
The severity of anxiety and associated impairment and distress are the main variables that differentiate normal shyness and clinically significant social anxiety. Taking care not to over-pathologize normal shyness and common social anxiety concerns or underdiagnose severe, impairing social anxiety disorder has important implications for treatment—and for whether a patient needs treatment at all.
Related Resources
National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment, and treatment of social anxiety disorder. http://guidance.nice.org.uk/cg159.
• Hofmann SG, DiBartolo PM, eds. Social anxiety: clinical, developmental, and social perspectives, 2nd ed. London, United Kingdom: Academic Press; 2010.
• The Shyness Institute. www.shyness.com.
Drug Brand Names
Alprazolam • Xanax Clonazepam • Klonopin Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Phenelzine • Nardil
Sertraline • Zoloft Venlafaxine • Effexor
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Kristy L. Dalrymple, PhD, discusses, treating social anxiety disorder. Dr. Dalrymple is Staff Psychologist, Department of Psychiatry, Rhode Island Hospital, and Assistant Professor of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, Rhode Island.
1. Bruce LC, Coles ME, Heimberg RG. Social phobia and social anxiety disorder: effect of disorder name on recommendation for treatment. Am J Psychiatry. 2012;169(5):538.
2. Bögels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168-189.
3. Wakefield JC, Horwitz AV, Schmitz MF. Are we overpathologizing the socially anxious? Social phobia from a harmful dysfunction perspective. Can J Psychiatry. 2005;50(6):317-319.
4. Campbell-Sills L, Stein MB. Justifying the diagnostic status of social phobia: a reply to Wakefield, Horwitz, and Schmitz. Can J Psychiatry. 2005;50(6):320-323.
5. Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133-153.
6. Wakefield JC. The DSM-5 debate over the bereavement exclusion: psychiatric diagnosis and the future of empirically supported treatment. Clin Psychol Rev. 2013; 33(7):825-845.
7. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York, NY: Guilford Press; 2012.
8. Kupfer DJ, First MB, Regier DA, eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Dalrymple KL, Zimmerman M. Does comorbid social anxiety disorder impact the clinical presentation of principal major depressive disorder? J Affect Disord. 2007;100:241-247.
11. Dalrymple KL. Issues and controversies surrounding the diagnosis and treatment of social anxiety disorder. Expert Rev Neurother. 2012;12(8):993-1008.
12. Furmark T, Tillfors M, Everz PO, et al. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416-424.
13. Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115-123.
14. Heiser NA, Turner SM, Beidel DC. Shyness: relationship to social phobia and other psychiatric disorders. Behav Res Ther. 2003;41:209-221.
15. Hofmann SG, Moscovitch DA, Hyo-Jin K. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiology. 2006;61:134-142.
16. Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Hofmann SG, DiBartolo PM, eds. From social anxiety to social phobia: multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001:216-234.
17. Cox BJ, MacPherson PS, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019-1027.
18. Burstein M, Ameli-Grillon L, Merikangas KR. Shyness versus social phobia in US youth. Pediatrics. 2011;128:917-925.
19. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety. 2008;25:357-367.
20. Karevold E, Ystrom E, Coplan RJ, et al. A prospective longitudinal study of shyness from infancy to adolescence: stability, age-related changes, and prediction of socio-emotional functioning. J Abnorm Child Psychol. 2012; 40:1167-1177.
21. Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. J Anxiety Disord. 2002;16:585-598.
22. Schneier FR, Blanco C, Antia SX, et al. The social anxiety spectrum. Psychiatr Clin N Am. 2002;25:757-774.
23. Stein MB, Chavira DA, Jang KL. Bringing up bashful baby: developmental pathways to social phobia. Psychiatr Clin N Am. 2001;24:797-818.
24. Cooper PJ, Eke M. Childhood shyness and maternal social phobia: a community study. Br J Psychiatry. 1999;174:439-443.
25. Heiser NA, Turner SM, Beidel DC, et al. Differentiating social phobia from shyness. J Anxiety Disord. 2009;23:469-476.
26. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141-173.
27. Dalrymple, KL, Martinez J, Tepe E, et al. A clinically useful social anxiety disorder outcome scale. Compr Psychiatry. 2013;54(7):758-765.
28. Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14(2):137-140.
29. First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997.
30. Conrad P. Medicalization and social control. Ann Rev Sociology. 1992;18:209-232.
31. Zimmerman M, Chelminski I. Clinician recognition of anxiety disorders in depressed outpatients. J Psychiatr Res. 2003;37:325-333.
32. Gelernter CS, Uhde TW, Cimbolic P, et al. Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry. 1991;48:938-945.
33. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(suppl 5):34-41.
34. Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol. 2013;16:235-249.
Since the appearance of social anxiety disorder (SAD) in the DSM-III in 1980, research on its prevalence, characteristics, and treatment have grown (Box 11,2). In addition to the name, the definition of SAD has changed over the years; as a result, its prevalence has increased in recent cohort studies. This has led to debate over whether the experience of shyness is being over-pathologized, or whether SAD has been underdiagnosed in earlier decades. Those who argue that shyness is being over-pathologized note that it is a normal human experience that has evolutionary functions (eg, preventing engagement in harmful social relationships3). Others argue that a high degree of shyness is not beneficial in terms of evolution because it causes the individual to be shunned, so to speak, by society.4
Why worry about ‘over-pathologizing’?
The medicalization of shyness might be a reflection of Western societal values of assertiveness and gregariousness; other societies that value modesty and reticence do not over-pathologize shyness.5 It is important not to assume that someone who is shy necessarily has a “pathologic” level of social anxiety, especially because some people who are shy view that condition as a positive quality, much like sensitivity and conscientiousness.5
The broader issue of what constitutes a mental disorder arises in this debate. A “disorder” is a socially constructed label that describes a set of symptoms occurring together and its associated behaviors, not a real entity with etiological homogeneity.6 Labeling emotional problems “disordered” assumes that happiness is the natural homeostatic state, and distressing emotional states are abnormal and need to be changed.7 A diagnostic label can help improve communication and understand maladaptive behaviors; if that label is reified, however, it can lead to assumptions that the etiology, course, and treatment response are known. Proponents of the diagnostic psychiatric nomenclature have acknowledged the dangers of over-pathologizing normal experiences of living (such as fear) by way of diagnostic labeling.8
Determining when shyness becomes a clinically significant problem—what we call SAD here—demands a delicate distinction that has important implications for treatment. On one hand, if shyness is over-pathologized, persons who neither desire nor need treatment might be subjected to unnecessary and costly intervention. On the other hand, if SAD is underdiagnosed, some persons will not receive treatment that might be beneficial to them.
In this article, we review the similarities and differences between shyness and SAD, and provide recommendations for determining when shyness becomes a more clinically significant problem. We also highlight the importance of this distinction as it pertains to management, and provide suggestions for treatment approaches.
SAD: Definition, prevalence
SAD is defined as a significant fear of embarrassment or humiliation in social or performance-based situations, to a point at which the affected person often avoids these situations or endures them only with a high level of distress9 (Table 1, and Box 2). SAD can be distinguished from other anxiety disorders based on the source and content of the fear (ie, the source being social interaction or performance situations, and the content being a fear that one will show a behavior that will cause embarrassment). SAD also should be distinguished from autism spectrum disorders, in which persons have limited social communication capabilities and inadequate age-appropriate social relationships.
SAD is most highly comorbid with mood and anxiety disorders, with rates of at least 30% in clinical samples.10 The disorder also is highly comorbid with avoidant personality disorder—to a point at which it is argued that they are one and the same disorder.11
As with other psychiatric disorders, anxiety must cause significant impairment or distress. What constitutes significant impairment or distress is subjective, and the arbitrary nature of this criterion can influence estimates of the prevalence of SAD. For example, prevalence ranges as widely as 1.9% to 20.4% when different cut-offs are used for distress ratings and the number of impaired domains.12
The prevalence of SAD varies from 1 epidemiological study to another (ie, the Epidemiological Catchment Area [ECA] Study and the National Comorbidity Survey [NCS])—in part, a consequence of the differing definitions of significant impairment or distress. The ECA study assessed the clinical significance of each symptom in anxiety disorders; the NCS assessed overall clinical significance of the disorder. When the clinical significance criterion was applied at the symptom level to the NCS dataset (as was done in the ECA study), 1-year prevalence decreased by 50% (from 7.4% to 3.7%).13 The manner in which significant impairment or distress is defined (ie, conservatively or liberally) impacts whether social anxiety symptoms are classified as disordered or non-disordered.
Shyness: Definition, prevalence
Shyness often refers to 1) anxiety, inhibition, reticence, or a combination of these findings, in social and interpersonal situations, and 2) a fear of negative evaluation by others.14 It is a normal facet of personality that combines the experience of social anxiety and inhibited behavior,15 and also has been described as a stable temperament.16 Shyness is common; in the NCS study,17 26% of women and 19% of men characterized themselves as “very shy”; in the NCS Adolescent study,18 nearly 50% of adolescents self-identified as shy.
Persons who are shy tend to self-report greater social anxiety and embarrassment in social situations than non-shy persons do; they also might experience greater autonomic reactivity—especially blushing—in social or performance situations.15 Furthermore, shy persons are more likely to have axis I comorbidity and traits of introversion and neuroticism, compared with non-shy persons.14
Research suggests that temperament and behavioral inhibition are risk factors for mood and anxiety disorders, and appear to have a particularly strong relationship with SAD.19 A recent prospective study showed that shyness tends to increase steeply in toddlerhood, then stabilizes in childhood. Shyness in childhood—but not toddlerhood—is predictive of anxiety, depression, and poorer social skills in adolescence.20
A qualitative, or just quantitative, difference?
It is clear that SAD and shyness share several features—including anxiety and embarrassment—in social interactions. This raises a question: Are SAD and shyness distinct qualitatively, or do they represent points along a continuum, with SAD being an extreme form of shyness?
Continuum hypothesis. Support for the continuum hypothesis includes evidence that SAD and shyness share several features, including autonomic arousal, deficits in social skills (eg, aversion of gaze, difficulty initiating and maintaining conversation), avoidance of social situations, and fear of negative evaluation.21,22 In addition, both shyness and SAD are highly heritable,23 and mothers of shy children have a significantly higher rate of SAD than non-shy children do.24 No familial or genetic studies have compared heritability and familial aggregation in shyness and SAD.
According to the continuum hypothesis, if SAD is an extreme form of shyness, all (or nearly all) persons who have a diagnosis of SAD also would be characterized as shy. However, only approximately one-half of such persons report having been shy in childhood.17 Less than one-quarter of shy persons meet criteria for SAD.14,18 Because many persons who are shy do not meet criteria for SAD, and many who have SAD were not considered shy earlier in life, it has been suggested that this supports a qualitative distinction.
Qualitative distinctiveness. Despite having similarities, several features distinguish the experience of SAD from that of shyness. Compared with shyness, a SAD diagnosis is associated with:
- greater comorbidity
- greater severity of avoidance and impairment
- poorer quality of life.18,21,25
Studies that compared SAD, shyness without SAD, and non-shyness have shown that the shyness without SAD group more closely resembles the non-shy group than the SAD group—particularly with regard to impairment, presence of substance use, and other behavioral problems.18,25
Given the evidence, experts have concluded that shyness and a SAD diagnosis are overlapping yet different constructs that encapsulate qualitative and quantitative differences.25 There is a spectrum of shyness that ranges from a normative level to a higher level that overlaps the experience of SAD, but the 2 states represent different constructs.25
Guidance for making an assessment. Because of similarities in anxiety, embarrassment, and other symptoms in social situations, the best way to determine whether shyness crosses the line into a clinically significant problem is to assess the severity of the anxiety and associated degree of impairment and distress. More severe anxiety paired with distress about having anxiety and significant impairment in multiple areas of functioning might indicate more problematic social anxiety—a diagnosis of SAD—not just “normal” shyness.
It is important to take into account the environmental and cultural context of a patient’s distress and impairment because these features might fall within a normal range, given immediate circumstances (such as speaking in front of a large audience when one is not normally called on to do so, to a degree that does not interfere with general social functioning6).
What is considered a normative range depends on the developmental stage:
- Among children, a greater level of shyness might be considered more normative when it manifests during developmental stages in which separation anxiety appears.
- Among adolescents, a greater level of shyness might be considered normative especially during early adolescence (when social relationships become more important), and during times of transition (ie, entering high school).
- In adulthood, a greater level of normative shyness or social anxiety might be present during a major life change (eg, beginning to date again after the loss of a lengthy marriage or romantic relationship).
Assessment tools
Assessment tools can help you differentiate normal shyness from SAD. Several empirically-validated rating scales exist, including clinician-rated and self-report scales.
Liebowitz Social Anxiety Scale26 rates the severity of fear and avoidance in a variety of social interaction and performance-based situations. However, it was developed primarily as a clinician-rated scale and might be more burdensome to complete in practice. In addition, it does not provide cut-offs to indicate when more clinically significant anxiety might be likely.
Clinically Useful Social Anxiety Disorder Outcome Scale (CUSADOS)27 and Mini-Social Phobia Inventory (Mini-SPIN)28 are brief self-report scales that provide cut-offs to suggest further assessment is warranted. A cut-off score of 16 on the CUSADOS suggests the presence of SAD with 73% diagnostic efficiency.
One disadvantage to relying on a rating scale alone is the narrow focus on symptoms. Given that shyness and SAD share similar symptoms, it is necessary to assess the degree of impairment related to these symptoms to determine whether the problem is clinically significant. The overly narrow focus on symptoms utilized by the biomedical approach has been criticized for contributing to the medicalization of normal shyness.5
Diagnostic interviews, such as the Structured Clinical Interview for DSM-IV Axis I Disorders29 include sections on SAD that assess avoidance and impairment/distress associated with anxiety. Because these interviews may increase the time burden during an office visit, there are several general questions outside of a structured interview that you can ask, such as: “Has this anxiety interfered with your ability to initiate or maintain friendships? If so, how?” (Table 2). Persons with clinically significant social anxiety, rather than shyness, tend to report greater effects on their relationships and on work or school performance, as well as greater distress about having that anxiety.
Treatment approaches based on distinctions
Exercise care in making the distinction between normal shyness and dysfunctional and impairing levels of anxiety characteristic of SAD, because persons who display normal shyness but who are overdiagnosed might feel stigmatized by a diagnostic label.5 Also, overpathologizing shyness takes what is a social problem out of context, and could promote treatment strategies that might not be helpful or effective.30
Unnecessary diagnosis might lead to unnecessary treatment, such as prescribing an antidepressant or benzodiazepine. Avoiding such a situation is important, because of the side effects associated with medication and the potential for dependence and withdrawal effects with benzodiazepines.
Persons who exhibit normal shyness do not require medical treatment and, often, do not want it. However, some people may be interested in improving their ability to function in social interactions. Self-help approaches or brief psychotherapy (eg, cognitive-behavioral therapy [CBT]) should be the first step—and might be all that is necessary.
The opposite side of the problem. Under-recognition of clinically significant social anxiety can lead to under-treatment, which is common even in patients with a SAD diagnosis.31 Treatment options include CBT, medication, and CBT combined with medication (Table 3):
- several studies have demonstrated the short- and long-term efficacy of CBT alone for SAD
- medication alone has been efficacious in the short-term, but less efficacious than CBT in the long-term
- combined treatment also has been shown to be more efficacious than CBT or medication alone in the short-term
- there is evidence to suggest that CBT alone is more efficacious in the long-term compared with combined treatment.a
CBT is recommended as an appropriate first-line option, especially for mild and moderate SAD; it is the preferred initial treatment option of the United Kingdom’s National Institute for Health and Care Excellence (NICE). For more severe presentations (such as the presence of comorbidity) or when a patient did not respond to an adequate course of CBT, combined treatment might be an option—the goal being to taper the medication and continue CBT as a longer-term treatment. Research has shown that continuing CBT while discontinuing medication helps prevent relapse.32,33
Appropriate pharmacotherapy options include selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.34 Increasingly, benzodiazepines are considered less desirable; they are not recommended for routine use in SAD in the NICE guidelines. Those guidelines call for continuing pharmacotherapy for 6 months when a patient responds to treatment within 3 months, then discontinuing medication with the aid of CBT.
Bottom Line
The severity of anxiety and associated impairment and distress are the main variables that differentiate normal shyness and clinically significant social anxiety. Taking care not to over-pathologize normal shyness and common social anxiety concerns or underdiagnose severe, impairing social anxiety disorder has important implications for treatment—and for whether a patient needs treatment at all.
Related Resources
National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment, and treatment of social anxiety disorder. http://guidance.nice.org.uk/cg159.
• Hofmann SG, DiBartolo PM, eds. Social anxiety: clinical, developmental, and social perspectives, 2nd ed. London, United Kingdom: Academic Press; 2010.
• The Shyness Institute. www.shyness.com.
Drug Brand Names
Alprazolam • Xanax Clonazepam • Klonopin Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Phenelzine • Nardil
Sertraline • Zoloft Venlafaxine • Effexor
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Kristy L. Dalrymple, PhD, discusses, treating social anxiety disorder. Dr. Dalrymple is Staff Psychologist, Department of Psychiatry, Rhode Island Hospital, and Assistant Professor of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, Rhode Island.
Since the appearance of social anxiety disorder (SAD) in the DSM-III in 1980, research on its prevalence, characteristics, and treatment have grown (Box 11,2). In addition to the name, the definition of SAD has changed over the years; as a result, its prevalence has increased in recent cohort studies. This has led to debate over whether the experience of shyness is being over-pathologized, or whether SAD has been underdiagnosed in earlier decades. Those who argue that shyness is being over-pathologized note that it is a normal human experience that has evolutionary functions (eg, preventing engagement in harmful social relationships3). Others argue that a high degree of shyness is not beneficial in terms of evolution because it causes the individual to be shunned, so to speak, by society.4
Why worry about ‘over-pathologizing’?
The medicalization of shyness might be a reflection of Western societal values of assertiveness and gregariousness; other societies that value modesty and reticence do not over-pathologize shyness.5 It is important not to assume that someone who is shy necessarily has a “pathologic” level of social anxiety, especially because some people who are shy view that condition as a positive quality, much like sensitivity and conscientiousness.5
The broader issue of what constitutes a mental disorder arises in this debate. A “disorder” is a socially constructed label that describes a set of symptoms occurring together and its associated behaviors, not a real entity with etiological homogeneity.6 Labeling emotional problems “disordered” assumes that happiness is the natural homeostatic state, and distressing emotional states are abnormal and need to be changed.7 A diagnostic label can help improve communication and understand maladaptive behaviors; if that label is reified, however, it can lead to assumptions that the etiology, course, and treatment response are known. Proponents of the diagnostic psychiatric nomenclature have acknowledged the dangers of over-pathologizing normal experiences of living (such as fear) by way of diagnostic labeling.8
Determining when shyness becomes a clinically significant problem—what we call SAD here—demands a delicate distinction that has important implications for treatment. On one hand, if shyness is over-pathologized, persons who neither desire nor need treatment might be subjected to unnecessary and costly intervention. On the other hand, if SAD is underdiagnosed, some persons will not receive treatment that might be beneficial to them.
In this article, we review the similarities and differences between shyness and SAD, and provide recommendations for determining when shyness becomes a more clinically significant problem. We also highlight the importance of this distinction as it pertains to management, and provide suggestions for treatment approaches.
SAD: Definition, prevalence
SAD is defined as a significant fear of embarrassment or humiliation in social or performance-based situations, to a point at which the affected person often avoids these situations or endures them only with a high level of distress9 (Table 1, and Box 2). SAD can be distinguished from other anxiety disorders based on the source and content of the fear (ie, the source being social interaction or performance situations, and the content being a fear that one will show a behavior that will cause embarrassment). SAD also should be distinguished from autism spectrum disorders, in which persons have limited social communication capabilities and inadequate age-appropriate social relationships.
SAD is most highly comorbid with mood and anxiety disorders, with rates of at least 30% in clinical samples.10 The disorder also is highly comorbid with avoidant personality disorder—to a point at which it is argued that they are one and the same disorder.11
As with other psychiatric disorders, anxiety must cause significant impairment or distress. What constitutes significant impairment or distress is subjective, and the arbitrary nature of this criterion can influence estimates of the prevalence of SAD. For example, prevalence ranges as widely as 1.9% to 20.4% when different cut-offs are used for distress ratings and the number of impaired domains.12
The prevalence of SAD varies from 1 epidemiological study to another (ie, the Epidemiological Catchment Area [ECA] Study and the National Comorbidity Survey [NCS])—in part, a consequence of the differing definitions of significant impairment or distress. The ECA study assessed the clinical significance of each symptom in anxiety disorders; the NCS assessed overall clinical significance of the disorder. When the clinical significance criterion was applied at the symptom level to the NCS dataset (as was done in the ECA study), 1-year prevalence decreased by 50% (from 7.4% to 3.7%).13 The manner in which significant impairment or distress is defined (ie, conservatively or liberally) impacts whether social anxiety symptoms are classified as disordered or non-disordered.
Shyness: Definition, prevalence
Shyness often refers to 1) anxiety, inhibition, reticence, or a combination of these findings, in social and interpersonal situations, and 2) a fear of negative evaluation by others.14 It is a normal facet of personality that combines the experience of social anxiety and inhibited behavior,15 and also has been described as a stable temperament.16 Shyness is common; in the NCS study,17 26% of women and 19% of men characterized themselves as “very shy”; in the NCS Adolescent study,18 nearly 50% of adolescents self-identified as shy.
Persons who are shy tend to self-report greater social anxiety and embarrassment in social situations than non-shy persons do; they also might experience greater autonomic reactivity—especially blushing—in social or performance situations.15 Furthermore, shy persons are more likely to have axis I comorbidity and traits of introversion and neuroticism, compared with non-shy persons.14
Research suggests that temperament and behavioral inhibition are risk factors for mood and anxiety disorders, and appear to have a particularly strong relationship with SAD.19 A recent prospective study showed that shyness tends to increase steeply in toddlerhood, then stabilizes in childhood. Shyness in childhood—but not toddlerhood—is predictive of anxiety, depression, and poorer social skills in adolescence.20
A qualitative, or just quantitative, difference?
It is clear that SAD and shyness share several features—including anxiety and embarrassment—in social interactions. This raises a question: Are SAD and shyness distinct qualitatively, or do they represent points along a continuum, with SAD being an extreme form of shyness?
Continuum hypothesis. Support for the continuum hypothesis includes evidence that SAD and shyness share several features, including autonomic arousal, deficits in social skills (eg, aversion of gaze, difficulty initiating and maintaining conversation), avoidance of social situations, and fear of negative evaluation.21,22 In addition, both shyness and SAD are highly heritable,23 and mothers of shy children have a significantly higher rate of SAD than non-shy children do.24 No familial or genetic studies have compared heritability and familial aggregation in shyness and SAD.
According to the continuum hypothesis, if SAD is an extreme form of shyness, all (or nearly all) persons who have a diagnosis of SAD also would be characterized as shy. However, only approximately one-half of such persons report having been shy in childhood.17 Less than one-quarter of shy persons meet criteria for SAD.14,18 Because many persons who are shy do not meet criteria for SAD, and many who have SAD were not considered shy earlier in life, it has been suggested that this supports a qualitative distinction.
Qualitative distinctiveness. Despite having similarities, several features distinguish the experience of SAD from that of shyness. Compared with shyness, a SAD diagnosis is associated with:
- greater comorbidity
- greater severity of avoidance and impairment
- poorer quality of life.18,21,25
Studies that compared SAD, shyness without SAD, and non-shyness have shown that the shyness without SAD group more closely resembles the non-shy group than the SAD group—particularly with regard to impairment, presence of substance use, and other behavioral problems.18,25
Given the evidence, experts have concluded that shyness and a SAD diagnosis are overlapping yet different constructs that encapsulate qualitative and quantitative differences.25 There is a spectrum of shyness that ranges from a normative level to a higher level that overlaps the experience of SAD, but the 2 states represent different constructs.25
Guidance for making an assessment. Because of similarities in anxiety, embarrassment, and other symptoms in social situations, the best way to determine whether shyness crosses the line into a clinically significant problem is to assess the severity of the anxiety and associated degree of impairment and distress. More severe anxiety paired with distress about having anxiety and significant impairment in multiple areas of functioning might indicate more problematic social anxiety—a diagnosis of SAD—not just “normal” shyness.
It is important to take into account the environmental and cultural context of a patient’s distress and impairment because these features might fall within a normal range, given immediate circumstances (such as speaking in front of a large audience when one is not normally called on to do so, to a degree that does not interfere with general social functioning6).
What is considered a normative range depends on the developmental stage:
- Among children, a greater level of shyness might be considered more normative when it manifests during developmental stages in which separation anxiety appears.
- Among adolescents, a greater level of shyness might be considered normative especially during early adolescence (when social relationships become more important), and during times of transition (ie, entering high school).
- In adulthood, a greater level of normative shyness or social anxiety might be present during a major life change (eg, beginning to date again after the loss of a lengthy marriage or romantic relationship).
Assessment tools
Assessment tools can help you differentiate normal shyness from SAD. Several empirically-validated rating scales exist, including clinician-rated and self-report scales.
Liebowitz Social Anxiety Scale26 rates the severity of fear and avoidance in a variety of social interaction and performance-based situations. However, it was developed primarily as a clinician-rated scale and might be more burdensome to complete in practice. In addition, it does not provide cut-offs to indicate when more clinically significant anxiety might be likely.
Clinically Useful Social Anxiety Disorder Outcome Scale (CUSADOS)27 and Mini-Social Phobia Inventory (Mini-SPIN)28 are brief self-report scales that provide cut-offs to suggest further assessment is warranted. A cut-off score of 16 on the CUSADOS suggests the presence of SAD with 73% diagnostic efficiency.
One disadvantage to relying on a rating scale alone is the narrow focus on symptoms. Given that shyness and SAD share similar symptoms, it is necessary to assess the degree of impairment related to these symptoms to determine whether the problem is clinically significant. The overly narrow focus on symptoms utilized by the biomedical approach has been criticized for contributing to the medicalization of normal shyness.5
Diagnostic interviews, such as the Structured Clinical Interview for DSM-IV Axis I Disorders29 include sections on SAD that assess avoidance and impairment/distress associated with anxiety. Because these interviews may increase the time burden during an office visit, there are several general questions outside of a structured interview that you can ask, such as: “Has this anxiety interfered with your ability to initiate or maintain friendships? If so, how?” (Table 2). Persons with clinically significant social anxiety, rather than shyness, tend to report greater effects on their relationships and on work or school performance, as well as greater distress about having that anxiety.
Treatment approaches based on distinctions
Exercise care in making the distinction between normal shyness and dysfunctional and impairing levels of anxiety characteristic of SAD, because persons who display normal shyness but who are overdiagnosed might feel stigmatized by a diagnostic label.5 Also, overpathologizing shyness takes what is a social problem out of context, and could promote treatment strategies that might not be helpful or effective.30
Unnecessary diagnosis might lead to unnecessary treatment, such as prescribing an antidepressant or benzodiazepine. Avoiding such a situation is important, because of the side effects associated with medication and the potential for dependence and withdrawal effects with benzodiazepines.
Persons who exhibit normal shyness do not require medical treatment and, often, do not want it. However, some people may be interested in improving their ability to function in social interactions. Self-help approaches or brief psychotherapy (eg, cognitive-behavioral therapy [CBT]) should be the first step—and might be all that is necessary.
The opposite side of the problem. Under-recognition of clinically significant social anxiety can lead to under-treatment, which is common even in patients with a SAD diagnosis.31 Treatment options include CBT, medication, and CBT combined with medication (Table 3):
- several studies have demonstrated the short- and long-term efficacy of CBT alone for SAD
- medication alone has been efficacious in the short-term, but less efficacious than CBT in the long-term
- combined treatment also has been shown to be more efficacious than CBT or medication alone in the short-term
- there is evidence to suggest that CBT alone is more efficacious in the long-term compared with combined treatment.a
CBT is recommended as an appropriate first-line option, especially for mild and moderate SAD; it is the preferred initial treatment option of the United Kingdom’s National Institute for Health and Care Excellence (NICE). For more severe presentations (such as the presence of comorbidity) or when a patient did not respond to an adequate course of CBT, combined treatment might be an option—the goal being to taper the medication and continue CBT as a longer-term treatment. Research has shown that continuing CBT while discontinuing medication helps prevent relapse.32,33
Appropriate pharmacotherapy options include selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.34 Increasingly, benzodiazepines are considered less desirable; they are not recommended for routine use in SAD in the NICE guidelines. Those guidelines call for continuing pharmacotherapy for 6 months when a patient responds to treatment within 3 months, then discontinuing medication with the aid of CBT.
Bottom Line
The severity of anxiety and associated impairment and distress are the main variables that differentiate normal shyness and clinically significant social anxiety. Taking care not to over-pathologize normal shyness and common social anxiety concerns or underdiagnose severe, impairing social anxiety disorder has important implications for treatment—and for whether a patient needs treatment at all.
Related Resources
National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment, and treatment of social anxiety disorder. http://guidance.nice.org.uk/cg159.
• Hofmann SG, DiBartolo PM, eds. Social anxiety: clinical, developmental, and social perspectives, 2nd ed. London, United Kingdom: Academic Press; 2010.
• The Shyness Institute. www.shyness.com.
Drug Brand Names
Alprazolam • Xanax Clonazepam • Klonopin Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Phenelzine • Nardil
Sertraline • Zoloft Venlafaxine • Effexor
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Kristy L. Dalrymple, PhD, discusses, treating social anxiety disorder. Dr. Dalrymple is Staff Psychologist, Department of Psychiatry, Rhode Island Hospital, and Assistant Professor of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, Rhode Island.
1. Bruce LC, Coles ME, Heimberg RG. Social phobia and social anxiety disorder: effect of disorder name on recommendation for treatment. Am J Psychiatry. 2012;169(5):538.
2. Bögels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168-189.
3. Wakefield JC, Horwitz AV, Schmitz MF. Are we overpathologizing the socially anxious? Social phobia from a harmful dysfunction perspective. Can J Psychiatry. 2005;50(6):317-319.
4. Campbell-Sills L, Stein MB. Justifying the diagnostic status of social phobia: a reply to Wakefield, Horwitz, and Schmitz. Can J Psychiatry. 2005;50(6):320-323.
5. Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133-153.
6. Wakefield JC. The DSM-5 debate over the bereavement exclusion: psychiatric diagnosis and the future of empirically supported treatment. Clin Psychol Rev. 2013; 33(7):825-845.
7. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York, NY: Guilford Press; 2012.
8. Kupfer DJ, First MB, Regier DA, eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Dalrymple KL, Zimmerman M. Does comorbid social anxiety disorder impact the clinical presentation of principal major depressive disorder? J Affect Disord. 2007;100:241-247.
11. Dalrymple KL. Issues and controversies surrounding the diagnosis and treatment of social anxiety disorder. Expert Rev Neurother. 2012;12(8):993-1008.
12. Furmark T, Tillfors M, Everz PO, et al. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416-424.
13. Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115-123.
14. Heiser NA, Turner SM, Beidel DC. Shyness: relationship to social phobia and other psychiatric disorders. Behav Res Ther. 2003;41:209-221.
15. Hofmann SG, Moscovitch DA, Hyo-Jin K. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiology. 2006;61:134-142.
16. Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Hofmann SG, DiBartolo PM, eds. From social anxiety to social phobia: multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001:216-234.
17. Cox BJ, MacPherson PS, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019-1027.
18. Burstein M, Ameli-Grillon L, Merikangas KR. Shyness versus social phobia in US youth. Pediatrics. 2011;128:917-925.
19. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety. 2008;25:357-367.
20. Karevold E, Ystrom E, Coplan RJ, et al. A prospective longitudinal study of shyness from infancy to adolescence: stability, age-related changes, and prediction of socio-emotional functioning. J Abnorm Child Psychol. 2012; 40:1167-1177.
21. Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. J Anxiety Disord. 2002;16:585-598.
22. Schneier FR, Blanco C, Antia SX, et al. The social anxiety spectrum. Psychiatr Clin N Am. 2002;25:757-774.
23. Stein MB, Chavira DA, Jang KL. Bringing up bashful baby: developmental pathways to social phobia. Psychiatr Clin N Am. 2001;24:797-818.
24. Cooper PJ, Eke M. Childhood shyness and maternal social phobia: a community study. Br J Psychiatry. 1999;174:439-443.
25. Heiser NA, Turner SM, Beidel DC, et al. Differentiating social phobia from shyness. J Anxiety Disord. 2009;23:469-476.
26. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141-173.
27. Dalrymple, KL, Martinez J, Tepe E, et al. A clinically useful social anxiety disorder outcome scale. Compr Psychiatry. 2013;54(7):758-765.
28. Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14(2):137-140.
29. First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997.
30. Conrad P. Medicalization and social control. Ann Rev Sociology. 1992;18:209-232.
31. Zimmerman M, Chelminski I. Clinician recognition of anxiety disorders in depressed outpatients. J Psychiatr Res. 2003;37:325-333.
32. Gelernter CS, Uhde TW, Cimbolic P, et al. Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry. 1991;48:938-945.
33. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(suppl 5):34-41.
34. Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol. 2013;16:235-249.
1. Bruce LC, Coles ME, Heimberg RG. Social phobia and social anxiety disorder: effect of disorder name on recommendation for treatment. Am J Psychiatry. 2012;169(5):538.
2. Bögels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168-189.
3. Wakefield JC, Horwitz AV, Schmitz MF. Are we overpathologizing the socially anxious? Social phobia from a harmful dysfunction perspective. Can J Psychiatry. 2005;50(6):317-319.
4. Campbell-Sills L, Stein MB. Justifying the diagnostic status of social phobia: a reply to Wakefield, Horwitz, and Schmitz. Can J Psychiatry. 2005;50(6):320-323.
5. Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133-153.
6. Wakefield JC. The DSM-5 debate over the bereavement exclusion: psychiatric diagnosis and the future of empirically supported treatment. Clin Psychol Rev. 2013; 33(7):825-845.
7. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York, NY: Guilford Press; 2012.
8. Kupfer DJ, First MB, Regier DA, eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Dalrymple KL, Zimmerman M. Does comorbid social anxiety disorder impact the clinical presentation of principal major depressive disorder? J Affect Disord. 2007;100:241-247.
11. Dalrymple KL. Issues and controversies surrounding the diagnosis and treatment of social anxiety disorder. Expert Rev Neurother. 2012;12(8):993-1008.
12. Furmark T, Tillfors M, Everz PO, et al. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416-424.
13. Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115-123.
14. Heiser NA, Turner SM, Beidel DC. Shyness: relationship to social phobia and other psychiatric disorders. Behav Res Ther. 2003;41:209-221.
15. Hofmann SG, Moscovitch DA, Hyo-Jin K. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiology. 2006;61:134-142.
16. Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Hofmann SG, DiBartolo PM, eds. From social anxiety to social phobia: multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001:216-234.
17. Cox BJ, MacPherson PS, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019-1027.
18. Burstein M, Ameli-Grillon L, Merikangas KR. Shyness versus social phobia in US youth. Pediatrics. 2011;128:917-925.
19. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety. 2008;25:357-367.
20. Karevold E, Ystrom E, Coplan RJ, et al. A prospective longitudinal study of shyness from infancy to adolescence: stability, age-related changes, and prediction of socio-emotional functioning. J Abnorm Child Psychol. 2012; 40:1167-1177.
21. Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. J Anxiety Disord. 2002;16:585-598.
22. Schneier FR, Blanco C, Antia SX, et al. The social anxiety spectrum. Psychiatr Clin N Am. 2002;25:757-774.
23. Stein MB, Chavira DA, Jang KL. Bringing up bashful baby: developmental pathways to social phobia. Psychiatr Clin N Am. 2001;24:797-818.
24. Cooper PJ, Eke M. Childhood shyness and maternal social phobia: a community study. Br J Psychiatry. 1999;174:439-443.
25. Heiser NA, Turner SM, Beidel DC, et al. Differentiating social phobia from shyness. J Anxiety Disord. 2009;23:469-476.
26. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141-173.
27. Dalrymple, KL, Martinez J, Tepe E, et al. A clinically useful social anxiety disorder outcome scale. Compr Psychiatry. 2013;54(7):758-765.
28. Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14(2):137-140.
29. First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997.
30. Conrad P. Medicalization and social control. Ann Rev Sociology. 1992;18:209-232.
31. Zimmerman M, Chelminski I. Clinician recognition of anxiety disorders in depressed outpatients. J Psychiatr Res. 2003;37:325-333.
32. Gelernter CS, Uhde TW, Cimbolic P, et al. Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry. 1991;48:938-945.
33. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(suppl 5):34-41.
34. Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol. 2013;16:235-249.
Overcoming medication nonadherence in schizophrenia: Strategies that can reduce harm
Medication nonadherence is a common problem when treating patients with schizophrenia that can worsen prognosis and lead to sub-optimal treatment outcomes. In this article, we discuss common reasons for nonadherence and describe evidence-based treatments intended to increase adherence and improve outcomes (Box).1-6
Common reasons for nonadherence
The primary predictor of future nonadherence is a history of nonadherence. It is important to understand patients’ reasons for nonadherence so that practical and evidence-based solutions can be implemented into the treatment plans of individual patients.
The 2009 Expert Consensus Guidelines on Adherence Problems in Patients with Serious and Persistent Mental Illness divided variables related to nonadherence into 3 categories:
- those that lie within the patient (intrinsic)
- those that are related to the patient’s relationship with healthcare providers, family, or caregivers (extrinsic)
- those that are related to the healthcare delivery system (extrinsic).7
Among intrinsic variables, studies have shown a correlation between nonadherence and education level, lower socioeconomic status, homelessness, and male sex.7 (The Expert Consensus Guidelines considered homelessness to be an intrinsic factor because it was used as a demographic variable in the studies.)
Cognitive and negative symptoms associated with schizophrenia are an intrinsic risk factor for nonadherence because patients might not remember when or how to take medication.7 In a study by Freudenreich and co-workers8 of 81 outpatients who had a diagnosis of schizophrenia, the presence of negative symptoms predicted a negative attitude toward psychotropic medications. Poor insight might be the result of cognitive dysfunction associated with schizophrenia, and often is due to a lack of awareness of the importance of taking medications.
Limited insight into the need for treatment can be problematic early in the course of the illness when it may be directly related to positive symptoms. Perkins and colleagues9 demonstrated that patients recovering from a first psychotic episode who had limited insight into their illness and lacked desire to seek treatment were less adherent with medication. In another study, 5% of psychiatrists surveyed thought that many of their patients with schizophrenia were nonadherent because those patients did not believe that medications were effective or useful.10
Comorbid substance abuse disorders can contribute to medication nonadherence. In an analysis of 6,731 patients with schizophrenia, Novick and co-workers reported that alcohol dependence and substance abuse in the previous month predicted medication nonadherence.11 Hunt and colleagues demonstrated that, among 99 nonadherent patients with schizophrenia, time to first readmission was shorter for patients with comorbid substance abuse disorders compared with patients who had a diagnosis of schizophrenia only. Over the 4-year study period, the 28 patients who had a dual diagnosis (schizophrenia and substance abuse) accounted for 57% of all hospital readmissions.12
Several variables that affect medication adherence are related to the patient’s relationship with healthcare providers, family, caregivers, and the service delivery system.7 These include:
- the perceived stigma of being given a diagnosis of a serious mental illness
- adverse effects related to medications
- poor social and family support
- difficulty gaining access to mental health services.7,10
Societal stigma associated with seeking treatment from a mental health professional may contribute to nonadherence in some patients. In 1 study,13 36% of people surveyed would not want to work closely with a person who has a serious mental illness.
Adverse effects contribute significantly to nonadherence
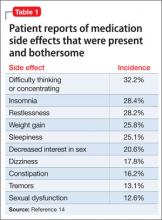
Limited treatment options (which may be expensive) can make it difficult to manage the adverse effects of antipsychotics. In a cross-sectional survey of 876 patients, investigators reported that: 1) <50% of patients were adherent with medication, and 2) 80% experienced ≥1 side effect that was reported to be “somewhat bothersome” in self-ratings (Table 1).14 Extrapyramidal symptoms (EPS) and agitation were most strongly associated with nonadherence; weight gain, akathisia, and sexual dysfunction also were associated with nonadherence.14 This study did not distinguish adverse effects associated with first-generation antipsychotics (FGAs) from those associated with second-generation antipsychotics (SGAs), even though 71.7% of patients studied were taking an SGA.
A meta-analysis by Leucht and co-workers15 compared 15 antipsychotics (the FGAs haloperidol and chlorpromazine and 13 SGAs) for efficacy and tolerability in schizophrenia. Haloperidol had the highest rate of discontinuation for any
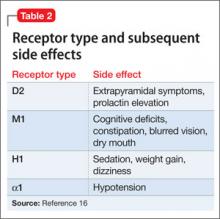
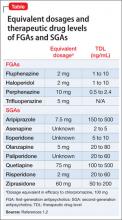
Antipsychotic binding affinities to dopamine 2 (D2), serotonin 2A (5-HT2A), histamine (H1), and other receptors have an impact on a medication’s side-effect profile. Because of individual patient characteristics, you might be faced with choosing a medication that has a lower risk of EPS but a higher risk of weight gain and metabolic complications—or the inverse. Understanding binding affinities, side-effect profiles, and how to minimize or utilize adverse effects (ie, giving a drug that is approved to treat schizophrenia and is associated with weight gain to a patient with schizophrenia who has lost weight) may lead to greater adherence (Table 216 and Table 317).
Adequate support is essential
The therapeutic alliance plays a key role in patients’ attitudes toward taking medication. Magura and colleagues18 found that one-third of psychiatric patients (13% of whom had a diagnosis of schizophrenia) reported that their psychiatrist did not spend enough time with them explaining side effects, and felt “rushed.”
Patients with schizophrenia often require access to social support systems provided by family members, friends, and community agencies that provide case management and attendant care services. Patients who are adherent to medication tend to have greater perceived family involvement in medication treatment, and tend to have been raised in a family that had more of a positive attitude toward medication.19
In our practice, we have observed that recent state and federal budget cuts have resulted in patients having greater difficulty gaining access to case management and attendant care services, which then leads to increased rates of medication nonadherence. Be aware that variables such as limited office hours, financial hardship, and cultural and language barriers can compromise a patient’s ability to seek and continue care.
In the following section, we lay out techniques for improving adherence in patients with schizophrenia.
Employ general and specific strategies to boost adherence
How can you raise medication adherence concerns with patients, keeping in mind that they often overestimate their adherence?
Ask. Some clinicians ask questions such as “Are you taking your medication?”, although a more effective approach might be to ask how the patient is taking his (her) medication. Asking questions such as “When do you take your medication?” and “In the past week, how many doses do you think you missed?” might be more effective ways to inquire about adherence.7
The Expert Consensus Guidelines recommend asking patients about medication adherence monthly for those who are stable, doing well, and believed to be adherent. For those who are new to a practice or who are not doing well, inquire about medication adherence at least weekly.7
In our practice, patients who are unstable but do not require inpatient hospitalization typically are seen more often in the clinic, or are referred to intensive outpatient or partial hospitalization programs. If an unstable patient is unable to come in for more frequent appointments, we arrange phone conferences between her and her provider. If a patient is not doing well and has a case manager, we often ask that case manager to visit the patient, in person, more often than he (she) would otherwise.
Take a nonjudgmental approach when raising these issues with patients. Questions such as “We all forget to take our medication sometimes; do you?” help to normalize nonadherence, and improve the therapeutic alliance, and might result in the patient being more honest with the clinician.7 Because patients may be apprehensive about discussing adverse events, clinicians must be proactive about improving the therapeutic alliance and making patients feel comfortable when discussing sensitive topics. Clinicians should try to convey the idea that, although adherence is a concern, so is quality of life. A clinicians’ willingness to take a flexible approach that is nonpunitive nor authoritarian can aid the therapeutic alliance and improve overall adherence.
Be sensitive to financial, cultural, and language variables that can affect access to care. The Expert Consensus Guidelines recommend asking patients if they can afford their medication. In our practice, we have seen patients with schizophrenia discharged from the hospital only to be readmitted 1 month later because they could not afford to fill their prescriptions.
It is important to have translation services available, in person or by phone, for patients who do not speak English. Furthermore, it is important to understand the limitations that your practice might place on access to care. Ask patients if they have ever had trouble making an appointment when they needed to be seen, or if they called the office with a question and did not receive an answer in a timely fashion; doing so allows you to assess the practice’s ability to meet patients’ needs and helps you build a therapeutic alliance.
Make objective assessments. It is important for practitioners to not base their assessment of medication adherence solely on subjective findings. Asking patients to bring in their medication bottles for pill counts and checking with the patients’ pharmacies for information about refill frequency can provide some objective data. Electronic monitoring systems use microprocessors inserted into bottle caps to record the occurrence and timing of each bottle opening. Studies show that these electronic monitoring systems are the gold standard for determining medication adherence and could be used in cases where it is unclear if the patient is taking his (her) medication.7,20 Such systems have successfully monitored medication adherence in clinical trials, but their use in clinical practice is complicated by ethical and legal considerations and cost issues.
Simplify the regimen. Using medications with once-daily dosing, for example, can help improve adherence. Pfeiffer and co-workers21 found that patients whose medication regimens were changed from once daily to more than once daily experienced a decrease in medication adherence. Conversely, a decrease in dosing frequency was significantly associated with improved adherence. More than once-daily dosing was only weakly associated with poorer adherence among patients already on a stable regimen.
Discussing positive and negative aspects of past medication trials with a patient and inquiring if she prefers a specific medication can be an effective way to build the therapeutic relationship and help with adherence.
Direct patients to psychosocial interventions. These can be broadly classified as:
- educational approaches
- group therapy approaches
- family interventions
- cognitive treatments
- combination approaches.
Psychoeducational approaches have limited effect on improving adherence when delivered to individual patients. However, 1 study showed that psychoeducation was effective at improving adherence when extended to include the patient’s family.22
Motivational interviewing techniques, behavioral approaches, and family interventions are effective at increasing medication adherence. One study looked at the value of training a patient-identified informant to supervise and administer medication. This person, usually a family member or close support, was responsible for obtaining medication from the pharmacy, administering the medication, and recording adherence. After 1 year, 67% of patients who used an informant were adherent, compared with only 45% in the group that did not have informant support.22 Case managers, attendant care workers, home health nurses, and assertive community treatment (ACT) teams also can participate in this manner; it is important, therefore, for you to be aware of the resources available in your community and to understand your role as patient advocate.
Substance abuse is a strong risk factor for nonadherence among patients with schizophrenia,18 which makes it important to assess patients for substance use and encourage those who do abuse to seek treatment. Although 1 study showed no correlation between Alcoholics Anonymous (AA) attendance and medication adherence,12 many AA and Narcotics Anonymous groups do not discuss psychiatric medications during group meetings. Magura and colleagues encouraged the use of “dual focus” groups that involve mental health professionals and addiction treatment specialists discussing mental health and substance abuse issues at the same setting.18
Prescribe long-acting injectable antipsychotics. Typically, long-acting injectable antipsychotics (LAIs) are reserved for patients who have a history of nonadherence. In a small study (N = 97) comparing LAI risperidone and oral risperidone or oral haloperidol, patients treated with an LAI had significantly fewer all-cause discontinuations (26.0%, compared with 70.2%) at 24 months.23 The Adherence to Treatment and Therapeutic Strategies in Schizophrenic Patients study examined 1,848 patients with schizophrenia and reached similar findings regarding LAI antipsychotics.24 (Note: Aripiprazole, fluphenazine, haloperidol, olanzapine, and paliperidone also are available in an LAI formulation.)
Bottom Line
Antipsychotic nonadherence in schizophrenia is a major problem for patients, families, and society. Being able to identify patients at risk for nonadherence, understanding the reasons for their nonadherence, and seeking practical solutions to the problem are all the responsibility of the treating physician. Psychoeducation, addressing substance abuse, modifying dosing, and using long-acting injectable antipsychotics may help improve adherence.
Related Resources
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- National Alliance on Mental Illness. www.nami.org.
- Assertive Community Treatment (ACT) Organization. www.actassociation.org.
Drug Brand Names
Aripiprazole • Abilify Chlorpromazine • Thorazine Clozapine • Clozaril Fluphenazine • Permitil Haloperidol • Haldol Iloperidone • Fanapt Olanzapine • Zyprexa Paliperidone • Invega Perphenazine • Trilafon Quetiapine • Seroquel Risperidone • Risperdal Ziprasidone • Geodon
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Dr. McKnight reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305-2312.
2. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
3. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
4. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
5. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemial Drug Saf. 2003;12(5):423-424.
6. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891.
7. Velligan D, Weiden P, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness. J Psychiatr Pract. 2010;16(1):34-45.
8. Freudenreich O, Cather C, Evins A, et al. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. J Clin Psychiatry. 2004;65(10):1372-1376.
9. Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63.
10. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121-132.
11. Novick D, Haro J, Suarez D, et al. Predictors and clinical consequences of nonadherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113.
12. Hunt GE, Bergen J, Bashir M, et al. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival four years after a relapse. Schizophr Res. 2002;54(3):253-264.
13. McGinty EE, Webster DW, Barry CL. Effects of news media messages about mass shootings on attitudes toward persons with serious mental illness and public support for gun control policies. Am J Psychiatry. 2013;170(5):494-501.
14. DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;1382(9896): 951-962.
16. Robinson DS. Antipsychotics: pharmacology and clinical decision making. Primary Psychiatry. 2007;14(10):23-25.
17. Robinson D, Correll CU, Kane JM, et al. Practical dosing strategies in the treatment of schizophrenia. CNS Spectr. 2010;15:4(suppl 6):1-16.
18. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
19. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181.
20. Byerly M, Nakonezny P, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437-452.
21. Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207-1210.
22. Farooq S, Nazar Z, Irfan M, et al. Schizophrenia medication adherence in a resource-poor setting: randomized controlled trial of supervised treatment in out-patients for schizophrenia (STOPS). Br J Psychiatry. 2011;199(6):467-472.
23. Emsley R, Oosthuizen P, Koen L, et al. Oral vs injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
24. Gutierrez-Casares JR, Canãs F, Rodriguez-Morales A, et al. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327-337.
Medication nonadherence is a common problem when treating patients with schizophrenia that can worsen prognosis and lead to sub-optimal treatment outcomes. In this article, we discuss common reasons for nonadherence and describe evidence-based treatments intended to increase adherence and improve outcomes (Box).1-6
Common reasons for nonadherence
The primary predictor of future nonadherence is a history of nonadherence. It is important to understand patients’ reasons for nonadherence so that practical and evidence-based solutions can be implemented into the treatment plans of individual patients.
The 2009 Expert Consensus Guidelines on Adherence Problems in Patients with Serious and Persistent Mental Illness divided variables related to nonadherence into 3 categories:
- those that lie within the patient (intrinsic)
- those that are related to the patient’s relationship with healthcare providers, family, or caregivers (extrinsic)
- those that are related to the healthcare delivery system (extrinsic).7
Among intrinsic variables, studies have shown a correlation between nonadherence and education level, lower socioeconomic status, homelessness, and male sex.7 (The Expert Consensus Guidelines considered homelessness to be an intrinsic factor because it was used as a demographic variable in the studies.)
Cognitive and negative symptoms associated with schizophrenia are an intrinsic risk factor for nonadherence because patients might not remember when or how to take medication.7 In a study by Freudenreich and co-workers8 of 81 outpatients who had a diagnosis of schizophrenia, the presence of negative symptoms predicted a negative attitude toward psychotropic medications. Poor insight might be the result of cognitive dysfunction associated with schizophrenia, and often is due to a lack of awareness of the importance of taking medications.
Limited insight into the need for treatment can be problematic early in the course of the illness when it may be directly related to positive symptoms. Perkins and colleagues9 demonstrated that patients recovering from a first psychotic episode who had limited insight into their illness and lacked desire to seek treatment were less adherent with medication. In another study, 5% of psychiatrists surveyed thought that many of their patients with schizophrenia were nonadherent because those patients did not believe that medications were effective or useful.10
Comorbid substance abuse disorders can contribute to medication nonadherence. In an analysis of 6,731 patients with schizophrenia, Novick and co-workers reported that alcohol dependence and substance abuse in the previous month predicted medication nonadherence.11 Hunt and colleagues demonstrated that, among 99 nonadherent patients with schizophrenia, time to first readmission was shorter for patients with comorbid substance abuse disorders compared with patients who had a diagnosis of schizophrenia only. Over the 4-year study period, the 28 patients who had a dual diagnosis (schizophrenia and substance abuse) accounted for 57% of all hospital readmissions.12
Several variables that affect medication adherence are related to the patient’s relationship with healthcare providers, family, caregivers, and the service delivery system.7 These include:
- the perceived stigma of being given a diagnosis of a serious mental illness
- adverse effects related to medications
- poor social and family support
- difficulty gaining access to mental health services.7,10
Societal stigma associated with seeking treatment from a mental health professional may contribute to nonadherence in some patients. In 1 study,13 36% of people surveyed would not want to work closely with a person who has a serious mental illness.
Adverse effects contribute significantly to nonadherence
Limited treatment options (which may be expensive) can make it difficult to manage the adverse effects of antipsychotics. In a cross-sectional survey of 876 patients, investigators reported that: 1) <50% of patients were adherent with medication, and 2) 80% experienced ≥1 side effect that was reported to be “somewhat bothersome” in self-ratings (Table 1).14 Extrapyramidal symptoms (EPS) and agitation were most strongly associated with nonadherence; weight gain, akathisia, and sexual dysfunction also were associated with nonadherence.14 This study did not distinguish adverse effects associated with first-generation antipsychotics (FGAs) from those associated with second-generation antipsychotics (SGAs), even though 71.7% of patients studied were taking an SGA.
A meta-analysis by Leucht and co-workers15 compared 15 antipsychotics (the FGAs haloperidol and chlorpromazine and 13 SGAs) for efficacy and tolerability in schizophrenia. Haloperidol had the highest rate of discontinuation for any
Antipsychotic binding affinities to dopamine 2 (D2), serotonin 2A (5-HT2A), histamine (H1), and other receptors have an impact on a medication’s side-effect profile. Because of individual patient characteristics, you might be faced with choosing a medication that has a lower risk of EPS but a higher risk of weight gain and metabolic complications—or the inverse. Understanding binding affinities, side-effect profiles, and how to minimize or utilize adverse effects (ie, giving a drug that is approved to treat schizophrenia and is associated with weight gain to a patient with schizophrenia who has lost weight) may lead to greater adherence (Table 216 and Table 317).
Adequate support is essential
The therapeutic alliance plays a key role in patients’ attitudes toward taking medication. Magura and colleagues18 found that one-third of psychiatric patients (13% of whom had a diagnosis of schizophrenia) reported that their psychiatrist did not spend enough time with them explaining side effects, and felt “rushed.”
Patients with schizophrenia often require access to social support systems provided by family members, friends, and community agencies that provide case management and attendant care services. Patients who are adherent to medication tend to have greater perceived family involvement in medication treatment, and tend to have been raised in a family that had more of a positive attitude toward medication.19
In our practice, we have observed that recent state and federal budget cuts have resulted in patients having greater difficulty gaining access to case management and attendant care services, which then leads to increased rates of medication nonadherence. Be aware that variables such as limited office hours, financial hardship, and cultural and language barriers can compromise a patient’s ability to seek and continue care.
In the following section, we lay out techniques for improving adherence in patients with schizophrenia.
Employ general and specific strategies to boost adherence
How can you raise medication adherence concerns with patients, keeping in mind that they often overestimate their adherence?
Ask. Some clinicians ask questions such as “Are you taking your medication?”, although a more effective approach might be to ask how the patient is taking his (her) medication. Asking questions such as “When do you take your medication?” and “In the past week, how many doses do you think you missed?” might be more effective ways to inquire about adherence.7
The Expert Consensus Guidelines recommend asking patients about medication adherence monthly for those who are stable, doing well, and believed to be adherent. For those who are new to a practice or who are not doing well, inquire about medication adherence at least weekly.7
In our practice, patients who are unstable but do not require inpatient hospitalization typically are seen more often in the clinic, or are referred to intensive outpatient or partial hospitalization programs. If an unstable patient is unable to come in for more frequent appointments, we arrange phone conferences between her and her provider. If a patient is not doing well and has a case manager, we often ask that case manager to visit the patient, in person, more often than he (she) would otherwise.
Take a nonjudgmental approach when raising these issues with patients. Questions such as “We all forget to take our medication sometimes; do you?” help to normalize nonadherence, and improve the therapeutic alliance, and might result in the patient being more honest with the clinician.7 Because patients may be apprehensive about discussing adverse events, clinicians must be proactive about improving the therapeutic alliance and making patients feel comfortable when discussing sensitive topics. Clinicians should try to convey the idea that, although adherence is a concern, so is quality of life. A clinicians’ willingness to take a flexible approach that is nonpunitive nor authoritarian can aid the therapeutic alliance and improve overall adherence.
Be sensitive to financial, cultural, and language variables that can affect access to care. The Expert Consensus Guidelines recommend asking patients if they can afford their medication. In our practice, we have seen patients with schizophrenia discharged from the hospital only to be readmitted 1 month later because they could not afford to fill their prescriptions.
It is important to have translation services available, in person or by phone, for patients who do not speak English. Furthermore, it is important to understand the limitations that your practice might place on access to care. Ask patients if they have ever had trouble making an appointment when they needed to be seen, or if they called the office with a question and did not receive an answer in a timely fashion; doing so allows you to assess the practice’s ability to meet patients’ needs and helps you build a therapeutic alliance.
Make objective assessments. It is important for practitioners to not base their assessment of medication adherence solely on subjective findings. Asking patients to bring in their medication bottles for pill counts and checking with the patients’ pharmacies for information about refill frequency can provide some objective data. Electronic monitoring systems use microprocessors inserted into bottle caps to record the occurrence and timing of each bottle opening. Studies show that these electronic monitoring systems are the gold standard for determining medication adherence and could be used in cases where it is unclear if the patient is taking his (her) medication.7,20 Such systems have successfully monitored medication adherence in clinical trials, but their use in clinical practice is complicated by ethical and legal considerations and cost issues.
Simplify the regimen. Using medications with once-daily dosing, for example, can help improve adherence. Pfeiffer and co-workers21 found that patients whose medication regimens were changed from once daily to more than once daily experienced a decrease in medication adherence. Conversely, a decrease in dosing frequency was significantly associated with improved adherence. More than once-daily dosing was only weakly associated with poorer adherence among patients already on a stable regimen.
Discussing positive and negative aspects of past medication trials with a patient and inquiring if she prefers a specific medication can be an effective way to build the therapeutic relationship and help with adherence.
Direct patients to psychosocial interventions. These can be broadly classified as:
- educational approaches
- group therapy approaches
- family interventions
- cognitive treatments
- combination approaches.
Psychoeducational approaches have limited effect on improving adherence when delivered to individual patients. However, 1 study showed that psychoeducation was effective at improving adherence when extended to include the patient’s family.22
Motivational interviewing techniques, behavioral approaches, and family interventions are effective at increasing medication adherence. One study looked at the value of training a patient-identified informant to supervise and administer medication. This person, usually a family member or close support, was responsible for obtaining medication from the pharmacy, administering the medication, and recording adherence. After 1 year, 67% of patients who used an informant were adherent, compared with only 45% in the group that did not have informant support.22 Case managers, attendant care workers, home health nurses, and assertive community treatment (ACT) teams also can participate in this manner; it is important, therefore, for you to be aware of the resources available in your community and to understand your role as patient advocate.
Substance abuse is a strong risk factor for nonadherence among patients with schizophrenia,18 which makes it important to assess patients for substance use and encourage those who do abuse to seek treatment. Although 1 study showed no correlation between Alcoholics Anonymous (AA) attendance and medication adherence,12 many AA and Narcotics Anonymous groups do not discuss psychiatric medications during group meetings. Magura and colleagues encouraged the use of “dual focus” groups that involve mental health professionals and addiction treatment specialists discussing mental health and substance abuse issues at the same setting.18
Prescribe long-acting injectable antipsychotics. Typically, long-acting injectable antipsychotics (LAIs) are reserved for patients who have a history of nonadherence. In a small study (N = 97) comparing LAI risperidone and oral risperidone or oral haloperidol, patients treated with an LAI had significantly fewer all-cause discontinuations (26.0%, compared with 70.2%) at 24 months.23 The Adherence to Treatment and Therapeutic Strategies in Schizophrenic Patients study examined 1,848 patients with schizophrenia and reached similar findings regarding LAI antipsychotics.24 (Note: Aripiprazole, fluphenazine, haloperidol, olanzapine, and paliperidone also are available in an LAI formulation.)
Bottom Line
Antipsychotic nonadherence in schizophrenia is a major problem for patients, families, and society. Being able to identify patients at risk for nonadherence, understanding the reasons for their nonadherence, and seeking practical solutions to the problem are all the responsibility of the treating physician. Psychoeducation, addressing substance abuse, modifying dosing, and using long-acting injectable antipsychotics may help improve adherence.
Related Resources
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- National Alliance on Mental Illness. www.nami.org.
- Assertive Community Treatment (ACT) Organization. www.actassociation.org.
Drug Brand Names
Aripiprazole • Abilify Chlorpromazine • Thorazine Clozapine • Clozaril Fluphenazine • Permitil Haloperidol • Haldol Iloperidone • Fanapt Olanzapine • Zyprexa Paliperidone • Invega Perphenazine • Trilafon Quetiapine • Seroquel Risperidone • Risperdal Ziprasidone • Geodon
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Dr. McKnight reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Medication nonadherence is a common problem when treating patients with schizophrenia that can worsen prognosis and lead to sub-optimal treatment outcomes. In this article, we discuss common reasons for nonadherence and describe evidence-based treatments intended to increase adherence and improve outcomes (Box).1-6
Common reasons for nonadherence
The primary predictor of future nonadherence is a history of nonadherence. It is important to understand patients’ reasons for nonadherence so that practical and evidence-based solutions can be implemented into the treatment plans of individual patients.
The 2009 Expert Consensus Guidelines on Adherence Problems in Patients with Serious and Persistent Mental Illness divided variables related to nonadherence into 3 categories:
- those that lie within the patient (intrinsic)
- those that are related to the patient’s relationship with healthcare providers, family, or caregivers (extrinsic)
- those that are related to the healthcare delivery system (extrinsic).7
Among intrinsic variables, studies have shown a correlation between nonadherence and education level, lower socioeconomic status, homelessness, and male sex.7 (The Expert Consensus Guidelines considered homelessness to be an intrinsic factor because it was used as a demographic variable in the studies.)
Cognitive and negative symptoms associated with schizophrenia are an intrinsic risk factor for nonadherence because patients might not remember when or how to take medication.7 In a study by Freudenreich and co-workers8 of 81 outpatients who had a diagnosis of schizophrenia, the presence of negative symptoms predicted a negative attitude toward psychotropic medications. Poor insight might be the result of cognitive dysfunction associated with schizophrenia, and often is due to a lack of awareness of the importance of taking medications.
Limited insight into the need for treatment can be problematic early in the course of the illness when it may be directly related to positive symptoms. Perkins and colleagues9 demonstrated that patients recovering from a first psychotic episode who had limited insight into their illness and lacked desire to seek treatment were less adherent with medication. In another study, 5% of psychiatrists surveyed thought that many of their patients with schizophrenia were nonadherent because those patients did not believe that medications were effective or useful.10
Comorbid substance abuse disorders can contribute to medication nonadherence. In an analysis of 6,731 patients with schizophrenia, Novick and co-workers reported that alcohol dependence and substance abuse in the previous month predicted medication nonadherence.11 Hunt and colleagues demonstrated that, among 99 nonadherent patients with schizophrenia, time to first readmission was shorter for patients with comorbid substance abuse disorders compared with patients who had a diagnosis of schizophrenia only. Over the 4-year study period, the 28 patients who had a dual diagnosis (schizophrenia and substance abuse) accounted for 57% of all hospital readmissions.12
Several variables that affect medication adherence are related to the patient’s relationship with healthcare providers, family, caregivers, and the service delivery system.7 These include:
- the perceived stigma of being given a diagnosis of a serious mental illness
- adverse effects related to medications
- poor social and family support
- difficulty gaining access to mental health services.7,10
Societal stigma associated with seeking treatment from a mental health professional may contribute to nonadherence in some patients. In 1 study,13 36% of people surveyed would not want to work closely with a person who has a serious mental illness.
Adverse effects contribute significantly to nonadherence
Limited treatment options (which may be expensive) can make it difficult to manage the adverse effects of antipsychotics. In a cross-sectional survey of 876 patients, investigators reported that: 1) <50% of patients were adherent with medication, and 2) 80% experienced ≥1 side effect that was reported to be “somewhat bothersome” in self-ratings (Table 1).14 Extrapyramidal symptoms (EPS) and agitation were most strongly associated with nonadherence; weight gain, akathisia, and sexual dysfunction also were associated with nonadherence.14 This study did not distinguish adverse effects associated with first-generation antipsychotics (FGAs) from those associated with second-generation antipsychotics (SGAs), even though 71.7% of patients studied were taking an SGA.
A meta-analysis by Leucht and co-workers15 compared 15 antipsychotics (the FGAs haloperidol and chlorpromazine and 13 SGAs) for efficacy and tolerability in schizophrenia. Haloperidol had the highest rate of discontinuation for any
Antipsychotic binding affinities to dopamine 2 (D2), serotonin 2A (5-HT2A), histamine (H1), and other receptors have an impact on a medication’s side-effect profile. Because of individual patient characteristics, you might be faced with choosing a medication that has a lower risk of EPS but a higher risk of weight gain and metabolic complications—or the inverse. Understanding binding affinities, side-effect profiles, and how to minimize or utilize adverse effects (ie, giving a drug that is approved to treat schizophrenia and is associated with weight gain to a patient with schizophrenia who has lost weight) may lead to greater adherence (Table 216 and Table 317).
Adequate support is essential
The therapeutic alliance plays a key role in patients’ attitudes toward taking medication. Magura and colleagues18 found that one-third of psychiatric patients (13% of whom had a diagnosis of schizophrenia) reported that their psychiatrist did not spend enough time with them explaining side effects, and felt “rushed.”
Patients with schizophrenia often require access to social support systems provided by family members, friends, and community agencies that provide case management and attendant care services. Patients who are adherent to medication tend to have greater perceived family involvement in medication treatment, and tend to have been raised in a family that had more of a positive attitude toward medication.19
In our practice, we have observed that recent state and federal budget cuts have resulted in patients having greater difficulty gaining access to case management and attendant care services, which then leads to increased rates of medication nonadherence. Be aware that variables such as limited office hours, financial hardship, and cultural and language barriers can compromise a patient’s ability to seek and continue care.
In the following section, we lay out techniques for improving adherence in patients with schizophrenia.
Employ general and specific strategies to boost adherence
How can you raise medication adherence concerns with patients, keeping in mind that they often overestimate their adherence?
Ask. Some clinicians ask questions such as “Are you taking your medication?”, although a more effective approach might be to ask how the patient is taking his (her) medication. Asking questions such as “When do you take your medication?” and “In the past week, how many doses do you think you missed?” might be more effective ways to inquire about adherence.7
The Expert Consensus Guidelines recommend asking patients about medication adherence monthly for those who are stable, doing well, and believed to be adherent. For those who are new to a practice or who are not doing well, inquire about medication adherence at least weekly.7
In our practice, patients who are unstable but do not require inpatient hospitalization typically are seen more often in the clinic, or are referred to intensive outpatient or partial hospitalization programs. If an unstable patient is unable to come in for more frequent appointments, we arrange phone conferences between her and her provider. If a patient is not doing well and has a case manager, we often ask that case manager to visit the patient, in person, more often than he (she) would otherwise.
Take a nonjudgmental approach when raising these issues with patients. Questions such as “We all forget to take our medication sometimes; do you?” help to normalize nonadherence, and improve the therapeutic alliance, and might result in the patient being more honest with the clinician.7 Because patients may be apprehensive about discussing adverse events, clinicians must be proactive about improving the therapeutic alliance and making patients feel comfortable when discussing sensitive topics. Clinicians should try to convey the idea that, although adherence is a concern, so is quality of life. A clinicians’ willingness to take a flexible approach that is nonpunitive nor authoritarian can aid the therapeutic alliance and improve overall adherence.
Be sensitive to financial, cultural, and language variables that can affect access to care. The Expert Consensus Guidelines recommend asking patients if they can afford their medication. In our practice, we have seen patients with schizophrenia discharged from the hospital only to be readmitted 1 month later because they could not afford to fill their prescriptions.
It is important to have translation services available, in person or by phone, for patients who do not speak English. Furthermore, it is important to understand the limitations that your practice might place on access to care. Ask patients if they have ever had trouble making an appointment when they needed to be seen, or if they called the office with a question and did not receive an answer in a timely fashion; doing so allows you to assess the practice’s ability to meet patients’ needs and helps you build a therapeutic alliance.
Make objective assessments. It is important for practitioners to not base their assessment of medication adherence solely on subjective findings. Asking patients to bring in their medication bottles for pill counts and checking with the patients’ pharmacies for information about refill frequency can provide some objective data. Electronic monitoring systems use microprocessors inserted into bottle caps to record the occurrence and timing of each bottle opening. Studies show that these electronic monitoring systems are the gold standard for determining medication adherence and could be used in cases where it is unclear if the patient is taking his (her) medication.7,20 Such systems have successfully monitored medication adherence in clinical trials, but their use in clinical practice is complicated by ethical and legal considerations and cost issues.
Simplify the regimen. Using medications with once-daily dosing, for example, can help improve adherence. Pfeiffer and co-workers21 found that patients whose medication regimens were changed from once daily to more than once daily experienced a decrease in medication adherence. Conversely, a decrease in dosing frequency was significantly associated with improved adherence. More than once-daily dosing was only weakly associated with poorer adherence among patients already on a stable regimen.
Discussing positive and negative aspects of past medication trials with a patient and inquiring if she prefers a specific medication can be an effective way to build the therapeutic relationship and help with adherence.
Direct patients to psychosocial interventions. These can be broadly classified as:
- educational approaches
- group therapy approaches
- family interventions
- cognitive treatments
- combination approaches.
Psychoeducational approaches have limited effect on improving adherence when delivered to individual patients. However, 1 study showed that psychoeducation was effective at improving adherence when extended to include the patient’s family.22
Motivational interviewing techniques, behavioral approaches, and family interventions are effective at increasing medication adherence. One study looked at the value of training a patient-identified informant to supervise and administer medication. This person, usually a family member or close support, was responsible for obtaining medication from the pharmacy, administering the medication, and recording adherence. After 1 year, 67% of patients who used an informant were adherent, compared with only 45% in the group that did not have informant support.22 Case managers, attendant care workers, home health nurses, and assertive community treatment (ACT) teams also can participate in this manner; it is important, therefore, for you to be aware of the resources available in your community and to understand your role as patient advocate.
Substance abuse is a strong risk factor for nonadherence among patients with schizophrenia,18 which makes it important to assess patients for substance use and encourage those who do abuse to seek treatment. Although 1 study showed no correlation between Alcoholics Anonymous (AA) attendance and medication adherence,12 many AA and Narcotics Anonymous groups do not discuss psychiatric medications during group meetings. Magura and colleagues encouraged the use of “dual focus” groups that involve mental health professionals and addiction treatment specialists discussing mental health and substance abuse issues at the same setting.18
Prescribe long-acting injectable antipsychotics. Typically, long-acting injectable antipsychotics (LAIs) are reserved for patients who have a history of nonadherence. In a small study (N = 97) comparing LAI risperidone and oral risperidone or oral haloperidol, patients treated with an LAI had significantly fewer all-cause discontinuations (26.0%, compared with 70.2%) at 24 months.23 The Adherence to Treatment and Therapeutic Strategies in Schizophrenic Patients study examined 1,848 patients with schizophrenia and reached similar findings regarding LAI antipsychotics.24 (Note: Aripiprazole, fluphenazine, haloperidol, olanzapine, and paliperidone also are available in an LAI formulation.)
Bottom Line
Antipsychotic nonadherence in schizophrenia is a major problem for patients, families, and society. Being able to identify patients at risk for nonadherence, understanding the reasons for their nonadherence, and seeking practical solutions to the problem are all the responsibility of the treating physician. Psychoeducation, addressing substance abuse, modifying dosing, and using long-acting injectable antipsychotics may help improve adherence.
Related Resources
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- National Alliance on Mental Illness. www.nami.org.
- Assertive Community Treatment (ACT) Organization. www.actassociation.org.
Drug Brand Names
Aripiprazole • Abilify Chlorpromazine • Thorazine Clozapine • Clozaril Fluphenazine • Permitil Haloperidol • Haldol Iloperidone • Fanapt Olanzapine • Zyprexa Paliperidone • Invega Perphenazine • Trilafon Quetiapine • Seroquel Risperidone • Risperdal Ziprasidone • Geodon
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Dr. McKnight reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305-2312.
2. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
3. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
4. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
5. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemial Drug Saf. 2003;12(5):423-424.
6. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891.
7. Velligan D, Weiden P, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness. J Psychiatr Pract. 2010;16(1):34-45.
8. Freudenreich O, Cather C, Evins A, et al. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. J Clin Psychiatry. 2004;65(10):1372-1376.
9. Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63.
10. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121-132.
11. Novick D, Haro J, Suarez D, et al. Predictors and clinical consequences of nonadherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113.
12. Hunt GE, Bergen J, Bashir M, et al. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival four years after a relapse. Schizophr Res. 2002;54(3):253-264.
13. McGinty EE, Webster DW, Barry CL. Effects of news media messages about mass shootings on attitudes toward persons with serious mental illness and public support for gun control policies. Am J Psychiatry. 2013;170(5):494-501.
14. DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;1382(9896): 951-962.
16. Robinson DS. Antipsychotics: pharmacology and clinical decision making. Primary Psychiatry. 2007;14(10):23-25.
17. Robinson D, Correll CU, Kane JM, et al. Practical dosing strategies in the treatment of schizophrenia. CNS Spectr. 2010;15:4(suppl 6):1-16.
18. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
19. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181.
20. Byerly M, Nakonezny P, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437-452.
21. Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207-1210.
22. Farooq S, Nazar Z, Irfan M, et al. Schizophrenia medication adherence in a resource-poor setting: randomized controlled trial of supervised treatment in out-patients for schizophrenia (STOPS). Br J Psychiatry. 2011;199(6):467-472.
23. Emsley R, Oosthuizen P, Koen L, et al. Oral vs injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
24. Gutierrez-Casares JR, Canãs F, Rodriguez-Morales A, et al. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327-337.
1. Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305-2312.
2. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
3. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
4. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
5. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemial Drug Saf. 2003;12(5):423-424.
6. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891.
7. Velligan D, Weiden P, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness. J Psychiatr Pract. 2010;16(1):34-45.
8. Freudenreich O, Cather C, Evins A, et al. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. J Clin Psychiatry. 2004;65(10):1372-1376.
9. Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63.
10. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121-132.
11. Novick D, Haro J, Suarez D, et al. Predictors and clinical consequences of nonadherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113.
12. Hunt GE, Bergen J, Bashir M, et al. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival four years after a relapse. Schizophr Res. 2002;54(3):253-264.
13. McGinty EE, Webster DW, Barry CL. Effects of news media messages about mass shootings on attitudes toward persons with serious mental illness and public support for gun control policies. Am J Psychiatry. 2013;170(5):494-501.
14. DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;1382(9896): 951-962.
16. Robinson DS. Antipsychotics: pharmacology and clinical decision making. Primary Psychiatry. 2007;14(10):23-25.
17. Robinson D, Correll CU, Kane JM, et al. Practical dosing strategies in the treatment of schizophrenia. CNS Spectr. 2010;15:4(suppl 6):1-16.
18. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
19. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181.
20. Byerly M, Nakonezny P, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437-452.
21. Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207-1210.
22. Farooq S, Nazar Z, Irfan M, et al. Schizophrenia medication adherence in a resource-poor setting: randomized controlled trial of supervised treatment in out-patients for schizophrenia (STOPS). Br J Psychiatry. 2011;199(6):467-472.
23. Emsley R, Oosthuizen P, Koen L, et al. Oral vs injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
24. Gutierrez-Casares JR, Canãs F, Rodriguez-Morales A, et al. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327-337.
More on the sensitivity of the SOAPP
We thank Dr. Ted Jones for his letter and comments about our article, “Chronic non-cancer pain and substance use disorders: Challenges and strategies” (Current Psychiatry, July 2013, p. 35-41; http://bit.ly/162NTCO). Dr. Jones correctly points out that we referred to the SOAPP-R and not to SOAPP (Current Psychiatry, Comments and Controversies, “Did the authors slip on SOAPP?” October 2013, p. 40; http://bit.ly/18YeV2C). This was an oversight and typing mistake.
However, we cannot agree with Dr. Jones’ comments about the sensitivity of SOAPP as we stated it. Dr. Jones says that we asserted the sensitivity of the tool to be 90%—we wrote, “A survey of 48 patients by Moore et al found the combination of a clinical interview and the Screener and Opioid Assessment for Patient with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD.”
The text in the article by Moore and colleagues1 states, “Combining the clinical interview with the SOAPP increased sensitivity to 0.90.” We believe that these 2 statements basically say the same thing. Whether the patients might or might not represent a substance use disorder is not clear from the article by Moore and colleagues, and probably is clearer to the authors than to the readers.
We stand corrected regarding the incorrectly presented version of the screening tool, but we believe we were correct when writing about the sensitivity of this tool in combination with a clinical interview.
Mark Juska, MD
Fellow
Richard Balon, MD
Professor
Department of Psychiatry and Behavioral Neurosciences
Department of Anesthesiology
Wayne State University
Detroit, Michigan
Reference
1. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
We thank Dr. Ted Jones for his letter and comments about our article, “Chronic non-cancer pain and substance use disorders: Challenges and strategies” (Current Psychiatry, July 2013, p. 35-41; http://bit.ly/162NTCO). Dr. Jones correctly points out that we referred to the SOAPP-R and not to SOAPP (Current Psychiatry, Comments and Controversies, “Did the authors slip on SOAPP?” October 2013, p. 40; http://bit.ly/18YeV2C). This was an oversight and typing mistake.
However, we cannot agree with Dr. Jones’ comments about the sensitivity of SOAPP as we stated it. Dr. Jones says that we asserted the sensitivity of the tool to be 90%—we wrote, “A survey of 48 patients by Moore et al found the combination of a clinical interview and the Screener and Opioid Assessment for Patient with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD.”
The text in the article by Moore and colleagues1 states, “Combining the clinical interview with the SOAPP increased sensitivity to 0.90.” We believe that these 2 statements basically say the same thing. Whether the patients might or might not represent a substance use disorder is not clear from the article by Moore and colleagues, and probably is clearer to the authors than to the readers.
We stand corrected regarding the incorrectly presented version of the screening tool, but we believe we were correct when writing about the sensitivity of this tool in combination with a clinical interview.
Mark Juska, MD
Fellow
Richard Balon, MD
Professor
Department of Psychiatry and Behavioral Neurosciences
Department of Anesthesiology
Wayne State University
Detroit, Michigan
Reference
1. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
We thank Dr. Ted Jones for his letter and comments about our article, “Chronic non-cancer pain and substance use disorders: Challenges and strategies” (Current Psychiatry, July 2013, p. 35-41; http://bit.ly/162NTCO). Dr. Jones correctly points out that we referred to the SOAPP-R and not to SOAPP (Current Psychiatry, Comments and Controversies, “Did the authors slip on SOAPP?” October 2013, p. 40; http://bit.ly/18YeV2C). This was an oversight and typing mistake.
However, we cannot agree with Dr. Jones’ comments about the sensitivity of SOAPP as we stated it. Dr. Jones says that we asserted the sensitivity of the tool to be 90%—we wrote, “A survey of 48 patients by Moore et al found the combination of a clinical interview and the Screener and Opioid Assessment for Patient with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD.”
The text in the article by Moore and colleagues1 states, “Combining the clinical interview with the SOAPP increased sensitivity to 0.90.” We believe that these 2 statements basically say the same thing. Whether the patients might or might not represent a substance use disorder is not clear from the article by Moore and colleagues, and probably is clearer to the authors than to the readers.
We stand corrected regarding the incorrectly presented version of the screening tool, but we believe we were correct when writing about the sensitivity of this tool in combination with a clinical interview.
Mark Juska, MD
Fellow
Richard Balon, MD
Professor
Department of Psychiatry and Behavioral Neurosciences
Department of Anesthesiology
Wayne State University
Detroit, Michigan
Reference
1. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
The saga of psychiatric serendipities continues…
We read Dr. Nasrallah’s editorial in the September 2013 issue (Current Psychiatry, From the Editor, “A saga of psychiatric serendipities…” September 2013, p. 7-8, 54; http://bit.ly/1dLiqhc) with great interest and excitement.
This is an important topic. When we consider mood disorders, John Cade’s discovery of lithium is another example of serendipity and a good measure of clinical judgment involving a handful of patients.1 Given that most of these discoveries involve small numbers of patients in studies that do not have a double-blind control methodology, it is disappointing that, in today’s academic world, almost no journals will accept pilot studies or case reports that are not seen as crucial for a breakthroughs or discovery. We want to add our voice to that of Dr. Nasrallah and ask people to rethink this policy, which, ultimately, stifles creativity and the progress of new psychiatric treatments.
Ronald Brenner, MD
Chair
Subramoniam Madhusoodanan, MD
Associate Chair
Department of Psychiatry
St. John’s Episcopal Hospital
Far Rockaway, New York
Reference
1. Cade JF. Lithium salts in the treatment of excitement. Med J Aust. 1949;2(10):349-352.
We read Dr. Nasrallah’s editorial in the September 2013 issue (Current Psychiatry, From the Editor, “A saga of psychiatric serendipities…” September 2013, p. 7-8, 54; http://bit.ly/1dLiqhc) with great interest and excitement.
This is an important topic. When we consider mood disorders, John Cade’s discovery of lithium is another example of serendipity and a good measure of clinical judgment involving a handful of patients.1 Given that most of these discoveries involve small numbers of patients in studies that do not have a double-blind control methodology, it is disappointing that, in today’s academic world, almost no journals will accept pilot studies or case reports that are not seen as crucial for a breakthroughs or discovery. We want to add our voice to that of Dr. Nasrallah and ask people to rethink this policy, which, ultimately, stifles creativity and the progress of new psychiatric treatments.
Ronald Brenner, MD
Chair
Subramoniam Madhusoodanan, MD
Associate Chair
Department of Psychiatry
St. John’s Episcopal Hospital
Far Rockaway, New York
Reference
1. Cade JF. Lithium salts in the treatment of excitement. Med J Aust. 1949;2(10):349-352.
We read Dr. Nasrallah’s editorial in the September 2013 issue (Current Psychiatry, From the Editor, “A saga of psychiatric serendipities…” September 2013, p. 7-8, 54; http://bit.ly/1dLiqhc) with great interest and excitement.
This is an important topic. When we consider mood disorders, John Cade’s discovery of lithium is another example of serendipity and a good measure of clinical judgment involving a handful of patients.1 Given that most of these discoveries involve small numbers of patients in studies that do not have a double-blind control methodology, it is disappointing that, in today’s academic world, almost no journals will accept pilot studies or case reports that are not seen as crucial for a breakthroughs or discovery. We want to add our voice to that of Dr. Nasrallah and ask people to rethink this policy, which, ultimately, stifles creativity and the progress of new psychiatric treatments.
Ronald Brenner, MD
Chair
Subramoniam Madhusoodanan, MD
Associate Chair
Department of Psychiatry
St. John’s Episcopal Hospital
Far Rockaway, New York
Reference
1. Cade JF. Lithium salts in the treatment of excitement. Med J Aust. 1949;2(10):349-352.
Lessons on the path from clinician to forensic expert
As physicians, we strive to heal suffering; as psychiatry trainees, we are taught to relieve that suffering through careful assessment, development of rapport, and empathic care. What then of the forensic expert, whose role is to provide the courts with objective assessment of the “defendant,” free of a therapeutic alliance1,2? Learning to navigate between these different roles is a necessary part of forensic training.2
In my journey to become a forensic psychiatrist equipped to treat adults and youth, I’ve had the good fortune to learn from those who appear to have mastered this balancing act. In this article, I present some of those lessons, with the hope that they will resonate with others—both those who are forensically inclined and those who wish to ease the jolt of being subpoenaed to appear before the court.
A day spent in the system
One of my earliest forensic experiences occurred during my training at Johns Hopkins, when I worked in the municipal court. I learned several lessons when I was assigned to pre-screen a defendant for competency to stand trial3 and criminal responsibility,4 both determined by the court but often informed by forensic evaluation.
Lesson #1: Answer only the question that you have been asked. En route to call for the defendant, I scanned my “how-to” guides and was relieved to learn that I was not to serve as decision-maker or treating clinician.5 I realized that I was not being asked to determine guilt or even give treatment recommendations; having a circumscribed task made that first evaluation less overwhelming. Learning to answer only the question you are being asked is a valuable lesson—one that ought to be remembered by those preparing for forensic evaluations and court testimony.
Lesson #2: There is a place for role induction. Entering a nearly empty office at municipal court, I sat behind a large metal desk and waited for the defendant. When he arrived, dressed in orange and escorted by the armed court officer, I rose to my feet awkwardly. I thought that I should shake hands with him, but stopped my hand in mid-air when I saw his handcuffed wrists.
As the guard knelt to chain the defendant’s ankle shackle to the floor, I waited patiently. Once the guard was outside, I introduced myself and read from my script. I explained the purpose of the evaluation and informed him that, unlike a
physician-patient relationship, this evaluation would not be confidential and would be shared with the court in a written report. Although the content of this introductory segment was in stark contrast to my usual patient encounters, this role induction6 was not. The purpose of role induction in a forensic setting is not to affect prognosis, yet such explanation is necessary to maintain ethical boundaries.1
Lesson #3: Know your phenomenology. Proceeding with the evaluation, I inquired about aspects of the defendant’s life. I attempted to assess his knowledge of the charges against him and how the court works,3 and obtained his account of the reported criminal events.4 Having an interest in psychotic illness and an appreciation for Jaspers’ descriptions of psychiatric phenomenology,7 I confidently delved into questions about the source, number, quality, and content of the voices he reported hearing.
Although not fail-proof, knowledge of phenomenology is necessary to discern whether reported symptoms should be trusted.8,9 In his writings10 and during my brief mentorship by him, Phillip Resnick, MD, stressed the importance of being able to detect malingering through knowledge of classic phenomenology and by maintaining a healthy level of suspicion.
Lesson #4: Impartiality is difficult but necessary. I concluded the interview, thanked the defendant, and asked if he had any questions. He declined. I motioned for the court officer to enter the room, unshackle the defendant from the floor, and escort him out. Exiting the room, I turned off the lights and shut the heavy door. The coldness of the physical environment seemed a metaphor for how I felt during the evaluation: In seeking the “truth,”11 had I lost a vital humanistic element?
Performing that early assessment, I felt as if such work challenged the reason I had decided to enter the medical profession. I struggled to see how such objective work contributed to relieving human suffering.
Now, only slightly more seasoned in this trade, I have a better appreciation for this necessarily impartial work. Although the role of the treating provider and the role of the forensic evaluator are distinct,12 both can be rewarding and both provide a valuable service.
Service in the name of Justice
I believe that, by presenting assessments free of bias, one can further the goal of justice: Forensic psychiatry provides the courts with the means to better understand and gain access to the mental health system. The task seemed daunting at first; now, I welcome opportunities to make such contributions to the fair and just treatment of all people.
Disclosure
Dr. Graham reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Academy of Psychiatry and the Law. AAPL ethical guidelines for the practice of forensic psychiatry (adopted 2005). http://www.aapl.org/ethics.htm. Accessed August 21, 2013.
2. Strasburger LH, Gutheil TG, Brodsky A. On wearing two hats: role conflict in serving as both psychotherapist and expert witness. Am J Psychiatry. 1997;154(4):448-456.
3. Mossman D, Noffsinger SG, Ash P, et al. AAPL Practice Guideline for the forensic psychiatric evaluation of competence to stand trial. J Am Acad Psychiatry Law. 2007;35(4 suppl): S3-S72.
4. Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL Practice Guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law. 2002; 30(2 suppl):S3-S40.
5. Rappeport JR. Differences between forensic and general psychiatry. Am J Psychiatry. 1982;139(3):331-334.
6. Chisolm MS, Lyketsos CG. Systematic psychiatric evaluation: a step-by-step guide to applying The Perspectives of Psychiatry. Baltimore, MD: The Johns Hopkins University Press; 2002.
7. Jaspers K. Allgemeine psychopathologie. Berlin, Germany: J Springer; 1913.
8. Soliman S, Resnick PJ. Feigning in adjudicative competence valuations. Behav Sci Law. 2010;28:614-629.
9. Taylor FK. The role of phenomenology in psychiatry. Br J Psychiatry. 1967;113:765-770.
10. Resnick PJ. My favorite tips for detecting malingering and violence risk. Psychiatr Clin North Am. 2007;30(2):227-232
11. Palermo GB. Forensic mental health experts in the court—an ethical dilemma. Int J Offender Ther Comp Criminol. 2003;47(2):122-125.
12. Appelbaum PS. A theory of ethics for forensic psychiatry. J Am Acad Psychiatry Law. 1997;25(3):233-247.
As physicians, we strive to heal suffering; as psychiatry trainees, we are taught to relieve that suffering through careful assessment, development of rapport, and empathic care. What then of the forensic expert, whose role is to provide the courts with objective assessment of the “defendant,” free of a therapeutic alliance1,2? Learning to navigate between these different roles is a necessary part of forensic training.2
In my journey to become a forensic psychiatrist equipped to treat adults and youth, I’ve had the good fortune to learn from those who appear to have mastered this balancing act. In this article, I present some of those lessons, with the hope that they will resonate with others—both those who are forensically inclined and those who wish to ease the jolt of being subpoenaed to appear before the court.
A day spent in the system
One of my earliest forensic experiences occurred during my training at Johns Hopkins, when I worked in the municipal court. I learned several lessons when I was assigned to pre-screen a defendant for competency to stand trial3 and criminal responsibility,4 both determined by the court but often informed by forensic evaluation.
Lesson #1: Answer only the question that you have been asked. En route to call for the defendant, I scanned my “how-to” guides and was relieved to learn that I was not to serve as decision-maker or treating clinician.5 I realized that I was not being asked to determine guilt or even give treatment recommendations; having a circumscribed task made that first evaluation less overwhelming. Learning to answer only the question you are being asked is a valuable lesson—one that ought to be remembered by those preparing for forensic evaluations and court testimony.
Lesson #2: There is a place for role induction. Entering a nearly empty office at municipal court, I sat behind a large metal desk and waited for the defendant. When he arrived, dressed in orange and escorted by the armed court officer, I rose to my feet awkwardly. I thought that I should shake hands with him, but stopped my hand in mid-air when I saw his handcuffed wrists.
As the guard knelt to chain the defendant’s ankle shackle to the floor, I waited patiently. Once the guard was outside, I introduced myself and read from my script. I explained the purpose of the evaluation and informed him that, unlike a
physician-patient relationship, this evaluation would not be confidential and would be shared with the court in a written report. Although the content of this introductory segment was in stark contrast to my usual patient encounters, this role induction6 was not. The purpose of role induction in a forensic setting is not to affect prognosis, yet such explanation is necessary to maintain ethical boundaries.1
Lesson #3: Know your phenomenology. Proceeding with the evaluation, I inquired about aspects of the defendant’s life. I attempted to assess his knowledge of the charges against him and how the court works,3 and obtained his account of the reported criminal events.4 Having an interest in psychotic illness and an appreciation for Jaspers’ descriptions of psychiatric phenomenology,7 I confidently delved into questions about the source, number, quality, and content of the voices he reported hearing.
Although not fail-proof, knowledge of phenomenology is necessary to discern whether reported symptoms should be trusted.8,9 In his writings10 and during my brief mentorship by him, Phillip Resnick, MD, stressed the importance of being able to detect malingering through knowledge of classic phenomenology and by maintaining a healthy level of suspicion.
Lesson #4: Impartiality is difficult but necessary. I concluded the interview, thanked the defendant, and asked if he had any questions. He declined. I motioned for the court officer to enter the room, unshackle the defendant from the floor, and escort him out. Exiting the room, I turned off the lights and shut the heavy door. The coldness of the physical environment seemed a metaphor for how I felt during the evaluation: In seeking the “truth,”11 had I lost a vital humanistic element?
Performing that early assessment, I felt as if such work challenged the reason I had decided to enter the medical profession. I struggled to see how such objective work contributed to relieving human suffering.
Now, only slightly more seasoned in this trade, I have a better appreciation for this necessarily impartial work. Although the role of the treating provider and the role of the forensic evaluator are distinct,12 both can be rewarding and both provide a valuable service.
Service in the name of Justice
I believe that, by presenting assessments free of bias, one can further the goal of justice: Forensic psychiatry provides the courts with the means to better understand and gain access to the mental health system. The task seemed daunting at first; now, I welcome opportunities to make such contributions to the fair and just treatment of all people.
Disclosure
Dr. Graham reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
As physicians, we strive to heal suffering; as psychiatry trainees, we are taught to relieve that suffering through careful assessment, development of rapport, and empathic care. What then of the forensic expert, whose role is to provide the courts with objective assessment of the “defendant,” free of a therapeutic alliance1,2? Learning to navigate between these different roles is a necessary part of forensic training.2
In my journey to become a forensic psychiatrist equipped to treat adults and youth, I’ve had the good fortune to learn from those who appear to have mastered this balancing act. In this article, I present some of those lessons, with the hope that they will resonate with others—both those who are forensically inclined and those who wish to ease the jolt of being subpoenaed to appear before the court.
A day spent in the system
One of my earliest forensic experiences occurred during my training at Johns Hopkins, when I worked in the municipal court. I learned several lessons when I was assigned to pre-screen a defendant for competency to stand trial3 and criminal responsibility,4 both determined by the court but often informed by forensic evaluation.
Lesson #1: Answer only the question that you have been asked. En route to call for the defendant, I scanned my “how-to” guides and was relieved to learn that I was not to serve as decision-maker or treating clinician.5 I realized that I was not being asked to determine guilt or even give treatment recommendations; having a circumscribed task made that first evaluation less overwhelming. Learning to answer only the question you are being asked is a valuable lesson—one that ought to be remembered by those preparing for forensic evaluations and court testimony.
Lesson #2: There is a place for role induction. Entering a nearly empty office at municipal court, I sat behind a large metal desk and waited for the defendant. When he arrived, dressed in orange and escorted by the armed court officer, I rose to my feet awkwardly. I thought that I should shake hands with him, but stopped my hand in mid-air when I saw his handcuffed wrists.
As the guard knelt to chain the defendant’s ankle shackle to the floor, I waited patiently. Once the guard was outside, I introduced myself and read from my script. I explained the purpose of the evaluation and informed him that, unlike a
physician-patient relationship, this evaluation would not be confidential and would be shared with the court in a written report. Although the content of this introductory segment was in stark contrast to my usual patient encounters, this role induction6 was not. The purpose of role induction in a forensic setting is not to affect prognosis, yet such explanation is necessary to maintain ethical boundaries.1
Lesson #3: Know your phenomenology. Proceeding with the evaluation, I inquired about aspects of the defendant’s life. I attempted to assess his knowledge of the charges against him and how the court works,3 and obtained his account of the reported criminal events.4 Having an interest in psychotic illness and an appreciation for Jaspers’ descriptions of psychiatric phenomenology,7 I confidently delved into questions about the source, number, quality, and content of the voices he reported hearing.
Although not fail-proof, knowledge of phenomenology is necessary to discern whether reported symptoms should be trusted.8,9 In his writings10 and during my brief mentorship by him, Phillip Resnick, MD, stressed the importance of being able to detect malingering through knowledge of classic phenomenology and by maintaining a healthy level of suspicion.
Lesson #4: Impartiality is difficult but necessary. I concluded the interview, thanked the defendant, and asked if he had any questions. He declined. I motioned for the court officer to enter the room, unshackle the defendant from the floor, and escort him out. Exiting the room, I turned off the lights and shut the heavy door. The coldness of the physical environment seemed a metaphor for how I felt during the evaluation: In seeking the “truth,”11 had I lost a vital humanistic element?
Performing that early assessment, I felt as if such work challenged the reason I had decided to enter the medical profession. I struggled to see how such objective work contributed to relieving human suffering.
Now, only slightly more seasoned in this trade, I have a better appreciation for this necessarily impartial work. Although the role of the treating provider and the role of the forensic evaluator are distinct,12 both can be rewarding and both provide a valuable service.
Service in the name of Justice
I believe that, by presenting assessments free of bias, one can further the goal of justice: Forensic psychiatry provides the courts with the means to better understand and gain access to the mental health system. The task seemed daunting at first; now, I welcome opportunities to make such contributions to the fair and just treatment of all people.
Disclosure
Dr. Graham reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Academy of Psychiatry and the Law. AAPL ethical guidelines for the practice of forensic psychiatry (adopted 2005). http://www.aapl.org/ethics.htm. Accessed August 21, 2013.
2. Strasburger LH, Gutheil TG, Brodsky A. On wearing two hats: role conflict in serving as both psychotherapist and expert witness. Am J Psychiatry. 1997;154(4):448-456.
3. Mossman D, Noffsinger SG, Ash P, et al. AAPL Practice Guideline for the forensic psychiatric evaluation of competence to stand trial. J Am Acad Psychiatry Law. 2007;35(4 suppl): S3-S72.
4. Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL Practice Guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law. 2002; 30(2 suppl):S3-S40.
5. Rappeport JR. Differences between forensic and general psychiatry. Am J Psychiatry. 1982;139(3):331-334.
6. Chisolm MS, Lyketsos CG. Systematic psychiatric evaluation: a step-by-step guide to applying The Perspectives of Psychiatry. Baltimore, MD: The Johns Hopkins University Press; 2002.
7. Jaspers K. Allgemeine psychopathologie. Berlin, Germany: J Springer; 1913.
8. Soliman S, Resnick PJ. Feigning in adjudicative competence valuations. Behav Sci Law. 2010;28:614-629.
9. Taylor FK. The role of phenomenology in psychiatry. Br J Psychiatry. 1967;113:765-770.
10. Resnick PJ. My favorite tips for detecting malingering and violence risk. Psychiatr Clin North Am. 2007;30(2):227-232
11. Palermo GB. Forensic mental health experts in the court—an ethical dilemma. Int J Offender Ther Comp Criminol. 2003;47(2):122-125.
12. Appelbaum PS. A theory of ethics for forensic psychiatry. J Am Acad Psychiatry Law. 1997;25(3):233-247.
1. American Academy of Psychiatry and the Law. AAPL ethical guidelines for the practice of forensic psychiatry (adopted 2005). http://www.aapl.org/ethics.htm. Accessed August 21, 2013.
2. Strasburger LH, Gutheil TG, Brodsky A. On wearing two hats: role conflict in serving as both psychotherapist and expert witness. Am J Psychiatry. 1997;154(4):448-456.
3. Mossman D, Noffsinger SG, Ash P, et al. AAPL Practice Guideline for the forensic psychiatric evaluation of competence to stand trial. J Am Acad Psychiatry Law. 2007;35(4 suppl): S3-S72.
4. Giorgi-Guarnieri D, Janofsky J, Keram E, et al. AAPL Practice Guideline for forensic psychiatric evaluation of defendants raising the insanity defense. J Am Acad Psychiatry Law. 2002; 30(2 suppl):S3-S40.
5. Rappeport JR. Differences between forensic and general psychiatry. Am J Psychiatry. 1982;139(3):331-334.
6. Chisolm MS, Lyketsos CG. Systematic psychiatric evaluation: a step-by-step guide to applying The Perspectives of Psychiatry. Baltimore, MD: The Johns Hopkins University Press; 2002.
7. Jaspers K. Allgemeine psychopathologie. Berlin, Germany: J Springer; 1913.
8. Soliman S, Resnick PJ. Feigning in adjudicative competence valuations. Behav Sci Law. 2010;28:614-629.
9. Taylor FK. The role of phenomenology in psychiatry. Br J Psychiatry. 1967;113:765-770.
10. Resnick PJ. My favorite tips for detecting malingering and violence risk. Psychiatr Clin North Am. 2007;30(2):227-232
11. Palermo GB. Forensic mental health experts in the court—an ethical dilemma. Int J Offender Ther Comp Criminol. 2003;47(2):122-125.
12. Appelbaum PS. A theory of ethics for forensic psychiatry. J Am Acad Psychiatry Law. 1997;25(3):233-247.
Treatment-resistant depression
Shyness or social anxiety disorder?
6 ‘D’s: Next steps after an insufficient antipsychotic response
There are a lack of research on, and strategies for dealing with, an insufficient response to antipsychotics. Treatment often is guided by what is described in published case reports or anecdotal evidence, rather than the findings of systematic studies.
We propose that a patient be considered “difficult-to-treat” or “treatment-resistant” after experiencing limited or negative responses to 3 different antipsychotics—with ≥1 being a second-generation antipsychotic (SGA)—that the patient has taken for at least 6 to 8 weeks at the maximum recommended dosage. Furthermore, switching to clozapine is an important strategy; do not consider it solely a last resort.
These 6 ‘D’s can remind you of other problems to consider when evaluating a treatment-resistant patient.
Diagnosis. Is the diagnosis, including the presence of comorbid conditions, accurate? Are significant psychosocial stressors undermining treatment response? Treat any comorbid conditions and consider instituting adjunctive psychosocial interventions, including cognitive-behavioral therapy.
Dosage. Have the patient try the maximum recommended dosage if he (she) can tolerate it. Equivalent dosages of antipsychotics are shown in the Table,1,2 and can guide off-label use of higher dosages. Research does not support use of chlorpromazine equivalents >1,000 mg/d, and usually should not be employed. If using a higher than normal dosage, perform an ECG before the increase.3
Beneficial and adverse effects should be monitored carefully. Reduce the dosage after 3 months if the risk-benefit ratio does not justify the higher dosage.3
Duration. Try a treatment for at least 6 weeks at the maximum tolerated
dosage—even extending it to 12 weeks—before considering abandoning it because of insufficient response.
Drug interactions. Use a drug interaction tool to ensure drug-drug interactions are not reducing antipsychotic levels. A recent increase in smoking or decrease in caffeine intake can reduce the blood level of olanzapine and clozapine.4 Ultra-rapid metabolizers of cytochrome P450 isoenzymes may have a lower blood level of antipsychotic.
Consider pharmacogenetic testing in patients in whom you observe an unexpected lack of efficacy or adverse effects at customary dosages.
Double up. You might need to add another medication to the antipsychotic. Symptoms might help determine which medication to add:
- a combination of an SGA and a first-generation antipsychotic may be more effective than antipsychotic monotherapy5
- for prominent negative symptoms, consider using 2 SGAs of different potency together. Use caution when prescribing ziprasidone with another antipsychotic because this could prolong the QTc interval
- if a mood stabilizer is appropriate, consider lamotrigine because of its possible potentiating effect on SGAs6
- benzodiazepines can be used to reduce agitation or anxiety but are ineffective for psychosis.7
Drug levels. Measurement of the blood level of the drug is most useful when administering clozapine; focus on the clozapine, not on the norclozapine level that also is reported. Ensure a clozapine level of 350 to 600 ng/mL.
Therapeutic levels have been established for most antipsychotics (Table).1,2 Occasionally, knowing these levels can be helpful in evaluating patients for potential problems with absorption and metabolism of the drug, and with nonadherence.
Disclosures
Drs. Faden and Pinninti report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Mago receives grant/research support from Bristol-Myers Squibb, Forest Institute, Genomind, and Shire.
1. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693.
2. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
3. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. http://www.rcpsych.ac.uk/files/pdfversion/CR138.pdf. Published May 2006. Accessed March 26, 2013.
4. Pinninti NR, Mago R, de Leon J. Coffee, cigarettes and meds: what are the metabolic effects? Psychiatric Times. 2005;22(6):20-23.
5. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
6. Citrome L. Treatment-resistant schizophrenia: what can we do about it? Current Psychiatry. 2011;10(6):52-58.
7. Volz A, Khorsand V, Gillies D, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1): CD006391.
There are a lack of research on, and strategies for dealing with, an insufficient response to antipsychotics. Treatment often is guided by what is described in published case reports or anecdotal evidence, rather than the findings of systematic studies.
We propose that a patient be considered “difficult-to-treat” or “treatment-resistant” after experiencing limited or negative responses to 3 different antipsychotics—with ≥1 being a second-generation antipsychotic (SGA)—that the patient has taken for at least 6 to 8 weeks at the maximum recommended dosage. Furthermore, switching to clozapine is an important strategy; do not consider it solely a last resort.
These 6 ‘D’s can remind you of other problems to consider when evaluating a treatment-resistant patient.
Diagnosis. Is the diagnosis, including the presence of comorbid conditions, accurate? Are significant psychosocial stressors undermining treatment response? Treat any comorbid conditions and consider instituting adjunctive psychosocial interventions, including cognitive-behavioral therapy.
Dosage. Have the patient try the maximum recommended dosage if he (she) can tolerate it. Equivalent dosages of antipsychotics are shown in the Table,1,2 and can guide off-label use of higher dosages. Research does not support use of chlorpromazine equivalents >1,000 mg/d, and usually should not be employed. If using a higher than normal dosage, perform an ECG before the increase.3
Beneficial and adverse effects should be monitored carefully. Reduce the dosage after 3 months if the risk-benefit ratio does not justify the higher dosage.3
Duration. Try a treatment for at least 6 weeks at the maximum tolerated
dosage—even extending it to 12 weeks—before considering abandoning it because of insufficient response.
Drug interactions. Use a drug interaction tool to ensure drug-drug interactions are not reducing antipsychotic levels. A recent increase in smoking or decrease in caffeine intake can reduce the blood level of olanzapine and clozapine.4 Ultra-rapid metabolizers of cytochrome P450 isoenzymes may have a lower blood level of antipsychotic.
Consider pharmacogenetic testing in patients in whom you observe an unexpected lack of efficacy or adverse effects at customary dosages.
Double up. You might need to add another medication to the antipsychotic. Symptoms might help determine which medication to add:
- a combination of an SGA and a first-generation antipsychotic may be more effective than antipsychotic monotherapy5
- for prominent negative symptoms, consider using 2 SGAs of different potency together. Use caution when prescribing ziprasidone with another antipsychotic because this could prolong the QTc interval
- if a mood stabilizer is appropriate, consider lamotrigine because of its possible potentiating effect on SGAs6
- benzodiazepines can be used to reduce agitation or anxiety but are ineffective for psychosis.7
Drug levels. Measurement of the blood level of the drug is most useful when administering clozapine; focus on the clozapine, not on the norclozapine level that also is reported. Ensure a clozapine level of 350 to 600 ng/mL.
Therapeutic levels have been established for most antipsychotics (Table).1,2 Occasionally, knowing these levels can be helpful in evaluating patients for potential problems with absorption and metabolism of the drug, and with nonadherence.
Disclosures
Drs. Faden and Pinninti report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Mago receives grant/research support from Bristol-Myers Squibb, Forest Institute, Genomind, and Shire.
There are a lack of research on, and strategies for dealing with, an insufficient response to antipsychotics. Treatment often is guided by what is described in published case reports or anecdotal evidence, rather than the findings of systematic studies.
We propose that a patient be considered “difficult-to-treat” or “treatment-resistant” after experiencing limited or negative responses to 3 different antipsychotics—with ≥1 being a second-generation antipsychotic (SGA)—that the patient has taken for at least 6 to 8 weeks at the maximum recommended dosage. Furthermore, switching to clozapine is an important strategy; do not consider it solely a last resort.
These 6 ‘D’s can remind you of other problems to consider when evaluating a treatment-resistant patient.
Diagnosis. Is the diagnosis, including the presence of comorbid conditions, accurate? Are significant psychosocial stressors undermining treatment response? Treat any comorbid conditions and consider instituting adjunctive psychosocial interventions, including cognitive-behavioral therapy.
Dosage. Have the patient try the maximum recommended dosage if he (she) can tolerate it. Equivalent dosages of antipsychotics are shown in the Table,1,2 and can guide off-label use of higher dosages. Research does not support use of chlorpromazine equivalents >1,000 mg/d, and usually should not be employed. If using a higher than normal dosage, perform an ECG before the increase.3
Beneficial and adverse effects should be monitored carefully. Reduce the dosage after 3 months if the risk-benefit ratio does not justify the higher dosage.3
Duration. Try a treatment for at least 6 weeks at the maximum tolerated
dosage—even extending it to 12 weeks—before considering abandoning it because of insufficient response.
Drug interactions. Use a drug interaction tool to ensure drug-drug interactions are not reducing antipsychotic levels. A recent increase in smoking or decrease in caffeine intake can reduce the blood level of olanzapine and clozapine.4 Ultra-rapid metabolizers of cytochrome P450 isoenzymes may have a lower blood level of antipsychotic.
Consider pharmacogenetic testing in patients in whom you observe an unexpected lack of efficacy or adverse effects at customary dosages.
Double up. You might need to add another medication to the antipsychotic. Symptoms might help determine which medication to add:
- a combination of an SGA and a first-generation antipsychotic may be more effective than antipsychotic monotherapy5
- for prominent negative symptoms, consider using 2 SGAs of different potency together. Use caution when prescribing ziprasidone with another antipsychotic because this could prolong the QTc interval
- if a mood stabilizer is appropriate, consider lamotrigine because of its possible potentiating effect on SGAs6
- benzodiazepines can be used to reduce agitation or anxiety but are ineffective for psychosis.7
Drug levels. Measurement of the blood level of the drug is most useful when administering clozapine; focus on the clozapine, not on the norclozapine level that also is reported. Ensure a clozapine level of 350 to 600 ng/mL.
Therapeutic levels have been established for most antipsychotics (Table).1,2 Occasionally, knowing these levels can be helpful in evaluating patients for potential problems with absorption and metabolism of the drug, and with nonadherence.
Disclosures
Drs. Faden and Pinninti report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Mago receives grant/research support from Bristol-Myers Squibb, Forest Institute, Genomind, and Shire.
1. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693.
2. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
3. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. http://www.rcpsych.ac.uk/files/pdfversion/CR138.pdf. Published May 2006. Accessed March 26, 2013.
4. Pinninti NR, Mago R, de Leon J. Coffee, cigarettes and meds: what are the metabolic effects? Psychiatric Times. 2005;22(6):20-23.
5. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
6. Citrome L. Treatment-resistant schizophrenia: what can we do about it? Current Psychiatry. 2011;10(6):52-58.
7. Volz A, Khorsand V, Gillies D, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1): CD006391.
1. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693.
2. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
3. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. http://www.rcpsych.ac.uk/files/pdfversion/CR138.pdf. Published May 2006. Accessed March 26, 2013.
4. Pinninti NR, Mago R, de Leon J. Coffee, cigarettes and meds: what are the metabolic effects? Psychiatric Times. 2005;22(6):20-23.
5. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
6. Citrome L. Treatment-resistant schizophrenia: what can we do about it? Current Psychiatry. 2011;10(6):52-58.
7. Volz A, Khorsand V, Gillies D, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1): CD006391.
Auditory musical hallucinations: When a patient complains, ‘I hear a symphony!’
Nonpsychotic auditory musical hallucinations—hearing singing voices, musical tones, song lyrics, or instrumental music—occur in >20% of outpatients who have a diagnosis of an anxiety, affective, or schizophrenic disorder, with the highest prevalence (41%) in patients with obsessive-compulsive disorder (OCD).1 OCD comorbidity with other psychiatric disorders increases the frequency of auditory musical hallucinations. Auditory musical hallucinations mainly affect older (mean age, 61.5 years) females who have tinnitus and severe, high-frequency, sensorineural hearing loss.1 Auditory musical hallucinations occur in psychiatric diseases, ictal states of complex partial seizures, abnormalities of the auditory cortex, thalamic infarcts, subarachnoid hemorrhage, tumors of the brain stem, intoxication, and progressive deafness.1,2
What patients report hearing
Some patients identify 1 musical instrument that dominates others. The musical tones are reported to have a vibrating quality, similar to the sound produced by blowing air through a paper-covered comb. Some patients hear singing voices, predominantly deep in tone, although the words usually are not clear.
Patients with auditory musical hallucinations associated with deafness may not have dementia or psychosis. Both sensorineural and conductive involvement indicates a mixed type of deafness. Pure tone audiograms show a bilateral loss of >30 decibels, affecting the higher and lower ranges.2,3 Cerebral atrophy and microangiopathic changes are common co-occurring findings on MRI.
Treatment options
Reassure your patient that the experience is not necessarily associated with a psychotic disorder. Perform a complete history, physical, and neurologic examination. Rule out unilateral symptoms, tinnitus, and hearing loss. If she (he) is experiencing unilateral symptoms, pulsatile tinnitus, unilateral hearing loss, and a constant feeling of unsteadiness, further evaluation is necessary to exclude underlying pathology. Treating concurrent insomnia, depression, or anxiety might resolve the hallucinations.4
Nonpharmacotherapeutic treatments include hearing amplification, and masking tinnitus with a hearing aid emitting low-volume music or sounds of nature (ie, rainfall).4 Two cases have reported successful carbamazepine therapy; 2 other cases demonstrated success with clomipramine.5 Frequently, symptoms spontaneously remit.
Consider electroconvulsive therapy (ECT) for patients with musical hallucinations that are refractory to medical treatment and cause distress; 3 patients with concurrent major depressive disorder showed improvement after ECT.6 Antipsychotics are not recommended as first-line treatment.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hermesh H, Konas S, Shiloh R, et al. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65(2):191-197.
2. Schakenraad SM, Teunisse RJ, Olde Rikkert MG. Musical hallucinations in psychiatric patients. Int J Geriatr Psychiatry. 2006;21(4):394-397.
3. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65.
4. Zegarra NM, Cuetter AC, Briones DF, et al. Nonpsychotic auditory musical hallucinations in elderly persons with progressive deafness. Clin Geriatr. 2007;15(11):33-37.
5. Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68-e70.
6. Wengel SP, Burke WJ, Holemon D. Musical hallucinations. The sounds of silence? J Am Geriatr Soc. 1989;37(2):163-166.
Nonpsychotic auditory musical hallucinations—hearing singing voices, musical tones, song lyrics, or instrumental music—occur in >20% of outpatients who have a diagnosis of an anxiety, affective, or schizophrenic disorder, with the highest prevalence (41%) in patients with obsessive-compulsive disorder (OCD).1 OCD comorbidity with other psychiatric disorders increases the frequency of auditory musical hallucinations. Auditory musical hallucinations mainly affect older (mean age, 61.5 years) females who have tinnitus and severe, high-frequency, sensorineural hearing loss.1 Auditory musical hallucinations occur in psychiatric diseases, ictal states of complex partial seizures, abnormalities of the auditory cortex, thalamic infarcts, subarachnoid hemorrhage, tumors of the brain stem, intoxication, and progressive deafness.1,2
What patients report hearing
Some patients identify 1 musical instrument that dominates others. The musical tones are reported to have a vibrating quality, similar to the sound produced by blowing air through a paper-covered comb. Some patients hear singing voices, predominantly deep in tone, although the words usually are not clear.
Patients with auditory musical hallucinations associated with deafness may not have dementia or psychosis. Both sensorineural and conductive involvement indicates a mixed type of deafness. Pure tone audiograms show a bilateral loss of >30 decibels, affecting the higher and lower ranges.2,3 Cerebral atrophy and microangiopathic changes are common co-occurring findings on MRI.
Treatment options
Reassure your patient that the experience is not necessarily associated with a psychotic disorder. Perform a complete history, physical, and neurologic examination. Rule out unilateral symptoms, tinnitus, and hearing loss. If she (he) is experiencing unilateral symptoms, pulsatile tinnitus, unilateral hearing loss, and a constant feeling of unsteadiness, further evaluation is necessary to exclude underlying pathology. Treating concurrent insomnia, depression, or anxiety might resolve the hallucinations.4
Nonpharmacotherapeutic treatments include hearing amplification, and masking tinnitus with a hearing aid emitting low-volume music or sounds of nature (ie, rainfall).4 Two cases have reported successful carbamazepine therapy; 2 other cases demonstrated success with clomipramine.5 Frequently, symptoms spontaneously remit.
Consider electroconvulsive therapy (ECT) for patients with musical hallucinations that are refractory to medical treatment and cause distress; 3 patients with concurrent major depressive disorder showed improvement after ECT.6 Antipsychotics are not recommended as first-line treatment.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nonpsychotic auditory musical hallucinations—hearing singing voices, musical tones, song lyrics, or instrumental music—occur in >20% of outpatients who have a diagnosis of an anxiety, affective, or schizophrenic disorder, with the highest prevalence (41%) in patients with obsessive-compulsive disorder (OCD).1 OCD comorbidity with other psychiatric disorders increases the frequency of auditory musical hallucinations. Auditory musical hallucinations mainly affect older (mean age, 61.5 years) females who have tinnitus and severe, high-frequency, sensorineural hearing loss.1 Auditory musical hallucinations occur in psychiatric diseases, ictal states of complex partial seizures, abnormalities of the auditory cortex, thalamic infarcts, subarachnoid hemorrhage, tumors of the brain stem, intoxication, and progressive deafness.1,2
What patients report hearing
Some patients identify 1 musical instrument that dominates others. The musical tones are reported to have a vibrating quality, similar to the sound produced by blowing air through a paper-covered comb. Some patients hear singing voices, predominantly deep in tone, although the words usually are not clear.
Patients with auditory musical hallucinations associated with deafness may not have dementia or psychosis. Both sensorineural and conductive involvement indicates a mixed type of deafness. Pure tone audiograms show a bilateral loss of >30 decibels, affecting the higher and lower ranges.2,3 Cerebral atrophy and microangiopathic changes are common co-occurring findings on MRI.
Treatment options
Reassure your patient that the experience is not necessarily associated with a psychotic disorder. Perform a complete history, physical, and neurologic examination. Rule out unilateral symptoms, tinnitus, and hearing loss. If she (he) is experiencing unilateral symptoms, pulsatile tinnitus, unilateral hearing loss, and a constant feeling of unsteadiness, further evaluation is necessary to exclude underlying pathology. Treating concurrent insomnia, depression, or anxiety might resolve the hallucinations.4
Nonpharmacotherapeutic treatments include hearing amplification, and masking tinnitus with a hearing aid emitting low-volume music or sounds of nature (ie, rainfall).4 Two cases have reported successful carbamazepine therapy; 2 other cases demonstrated success with clomipramine.5 Frequently, symptoms spontaneously remit.
Consider electroconvulsive therapy (ECT) for patients with musical hallucinations that are refractory to medical treatment and cause distress; 3 patients with concurrent major depressive disorder showed improvement after ECT.6 Antipsychotics are not recommended as first-line treatment.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hermesh H, Konas S, Shiloh R, et al. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65(2):191-197.
2. Schakenraad SM, Teunisse RJ, Olde Rikkert MG. Musical hallucinations in psychiatric patients. Int J Geriatr Psychiatry. 2006;21(4):394-397.
3. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65.
4. Zegarra NM, Cuetter AC, Briones DF, et al. Nonpsychotic auditory musical hallucinations in elderly persons with progressive deafness. Clin Geriatr. 2007;15(11):33-37.
5. Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68-e70.
6. Wengel SP, Burke WJ, Holemon D. Musical hallucinations. The sounds of silence? J Am Geriatr Soc. 1989;37(2):163-166.
1. Hermesh H, Konas S, Shiloh R, et al. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65(2):191-197.
2. Schakenraad SM, Teunisse RJ, Olde Rikkert MG. Musical hallucinations in psychiatric patients. Int J Geriatr Psychiatry. 2006;21(4):394-397.
3. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65.
4. Zegarra NM, Cuetter AC, Briones DF, et al. Nonpsychotic auditory musical hallucinations in elderly persons with progressive deafness. Clin Geriatr. 2007;15(11):33-37.
5. Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68-e70.
6. Wengel SP, Burke WJ, Holemon D. Musical hallucinations. The sounds of silence? J Am Geriatr Soc. 1989;37(2):163-166.
SSRIs in pregnancy: What should you tell your depressed patient?
Mrs. D is a 28-year-old married woman who became depressed after her first pregnancy. The depression was treated successfully with paroxetine, 20 mg/d. Before beginning treatment, she reported low mood, spent most of the day in bed, was unable to care for herself, and confessed to thoughts of harming her child.
Mrs. D presents to your clinic asking whether she should continue her selective serotonin reuptake inhibitor (SSRI) because she and her husband are thinking about having a second child. Recently, she tells you, she saw a news article suggesting that antidepressants show little benefit, and she is concerned that her baby might have a heart defect if she continues paroxetine.
Mrs. D wants to discontinue her medication, but her husband thought she should discuss doing so with you first. During this visit she takes a pregnancy test, which is positive. She wants to know what to do.
women experience depression; 3.8% of pregnant women receive an SSRI.1 SSRIs are the most commonly prescribed antidepressants during pregnancy, but their use remains controversial. There is disagreement about the maternal and neonatal risks of untreated depression and SSRI exposure.2-10 Media reports of studies demonstrating adverse effects associated with SSRIs may generate fear among women, possibly prompting them to self-discontinue medication.
Evidence of risks and benefits
Clinicians should be aware of possible adverse effects of SSRI use and untreated depression (Table).2-10 The available data precludes definitive associations between untreated depression and poor outcomes (Box). Studies of SSRI use during pregnancy have shown conflicting results for all potential outcomes. Absolute risk, with the exception of neonatal adaptation syndrome, is estimated to be small. Neonatal adaptation syndrome—which is characterized by jitteriness, poor muscle tone, weak cries, respiratory distress, hypoglycemia, low Apgar scores, and seizures—occurs in 15% to 30% of infants born to mothers taking SSRIs, but it is transient and resolves during the first weeks of life.
Treatment recommendations
Given the conflicting nature of the evidence, treatment plans should be individualized, weighing the risks and benefits of treatment and the patient’s beliefs and psychiatric history. Consider severity of symptoms and history, including effective therapy and history of relapse. For women with mild or moderate depression, cognitive-behavioral therapy might be an appropriate first-line therapy. However, non-pharmacotherapeutic interventions might not relieve severe depression or be available to all women. When discontinuing an SSRI before pregnancy, counsel the patient to not discontinue the medication abruptly and provide an appropriate taper schedule. See Related Resources for detailed recommendations from the American Psychiatric Association and the American College of Obstetricians and Gynecologists.
Reviewing the SSRI literature regarding pregnancy
Sertraline, paroxetine, citalopram, and fluoxetine are the most studied SSRIs during pregnancy; little information is available on escitalopram and fluvoxamine.11 Prescribing preference generally is given to the medications with the most evidence; paroxetine may be an exception. In 2005, the FDA requested a change in paroxetine’s pregnancy category from C to D, indicating that adequate studies demonstrated a risk of congenital cardiac malformations.11 Additional studies have been conducted, and the teratogenicity of paroxetine is debatable. A recent review reports 8 studies that suggest a malformation risk, compared with 15 studies that show no risk.12
The American Academy of Pediatrics considers SSRIs to be compatible with breast-feeding.13 The best-studied drugs include sertraline and paroxetine. Fluoxetine should be avoided when possible because a long elimination half-life can cause the drug to accumulate in the newborn, increasing the risk of irritability, hypertonia, sedation, and poor suckle.7
There is no best SSRI for all pregnant women. Risks and benefits, including previous treatment success and failure, should be taken into account before starting or switching therapy. Whenever possible, consider monotherapy to avoid compounding the risk of harm.
Related Resources
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31:403-413.
- MGH Center for Women’s Mental Health. www.womensmentalhealth.org.
Drug Brand Names
Citalopram • Celexa Escitalopram • Lexapro Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Sertraline • Zoloft
Disclosures
Dr. Leino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Ellingrod receives grant support from the National Institute of Mental Health.
1. Alwan S, Reefhuis J, Rasmussen SA, et al. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51(2):264-270.
2. Domar AD, Moragianni VA, Ryley DA, et al. The risks of selective serotonin reuptake inhibitor use in infertile women: a review of the impact on fertility, pregnancy, neonatal health and beyond. Hum Reprod. 20113;28(1):160-171.
3. Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15(1):1-14.
4. Spinelli M. Antidepressant treatment during pregnancy. Am J Psychiatry. 2012;169(2):121-124.
5. Oyebode F, Rastogi A, Berrisford G, et al. Psychotropics in pregnancy: safety and other considerations. Pharmacol Ther. 2012;135(1):71-77.
6. Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 2013;127(2):94-114.
7. Sie SD, Wennink JM, van Driel JJ, et al. Maternal use of SSRIs, SNRIs and NaSSAs: practical recommendations during pregnancy and lactation. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F472-476.
8. Jimenez-Solem E, Andersen JT, Petersen M, et al. SSRI use during pregnancy and risk of stillbirth and neonatal mortality. Am J Psychiatry. 2013;170(3):299-304.
9. Nikfar S, Rahimi R, Hendoiee N, et al. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: a systematic review and updated meta-analysis. Daru. 2012;20(1):75.
10. Stephansson O, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of stillbirth and infant mortality. JAMA. 2013;309(1):48-54.
11. U.S. Food and Drug Administration. Public health advisory: paroxetine. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/Public HealthAdvisories/ucm051731.htm. Published December 8, 2005. Accessed September 27, 2013.
12. Koren G, Nordeng H. Antidepressant use during pregnancy: the benefit-risk ratio. Am J Obstet Gynecol. 2012;207(3):157-163.
13. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776-789.
Mrs. D is a 28-year-old married woman who became depressed after her first pregnancy. The depression was treated successfully with paroxetine, 20 mg/d. Before beginning treatment, she reported low mood, spent most of the day in bed, was unable to care for herself, and confessed to thoughts of harming her child.
Mrs. D presents to your clinic asking whether she should continue her selective serotonin reuptake inhibitor (SSRI) because she and her husband are thinking about having a second child. Recently, she tells you, she saw a news article suggesting that antidepressants show little benefit, and she is concerned that her baby might have a heart defect if she continues paroxetine.
Mrs. D wants to discontinue her medication, but her husband thought she should discuss doing so with you first. During this visit she takes a pregnancy test, which is positive. She wants to know what to do.
women experience depression; 3.8% of pregnant women receive an SSRI.1 SSRIs are the most commonly prescribed antidepressants during pregnancy, but their use remains controversial. There is disagreement about the maternal and neonatal risks of untreated depression and SSRI exposure.2-10 Media reports of studies demonstrating adverse effects associated with SSRIs may generate fear among women, possibly prompting them to self-discontinue medication.
Evidence of risks and benefits
Clinicians should be aware of possible adverse effects of SSRI use and untreated depression (Table).2-10 The available data precludes definitive associations between untreated depression and poor outcomes (Box). Studies of SSRI use during pregnancy have shown conflicting results for all potential outcomes. Absolute risk, with the exception of neonatal adaptation syndrome, is estimated to be small. Neonatal adaptation syndrome—which is characterized by jitteriness, poor muscle tone, weak cries, respiratory distress, hypoglycemia, low Apgar scores, and seizures—occurs in 15% to 30% of infants born to mothers taking SSRIs, but it is transient and resolves during the first weeks of life.
Treatment recommendations
Given the conflicting nature of the evidence, treatment plans should be individualized, weighing the risks and benefits of treatment and the patient’s beliefs and psychiatric history. Consider severity of symptoms and history, including effective therapy and history of relapse. For women with mild or moderate depression, cognitive-behavioral therapy might be an appropriate first-line therapy. However, non-pharmacotherapeutic interventions might not relieve severe depression or be available to all women. When discontinuing an SSRI before pregnancy, counsel the patient to not discontinue the medication abruptly and provide an appropriate taper schedule. See Related Resources for detailed recommendations from the American Psychiatric Association and the American College of Obstetricians and Gynecologists.
Reviewing the SSRI literature regarding pregnancy
Sertraline, paroxetine, citalopram, and fluoxetine are the most studied SSRIs during pregnancy; little information is available on escitalopram and fluvoxamine.11 Prescribing preference generally is given to the medications with the most evidence; paroxetine may be an exception. In 2005, the FDA requested a change in paroxetine’s pregnancy category from C to D, indicating that adequate studies demonstrated a risk of congenital cardiac malformations.11 Additional studies have been conducted, and the teratogenicity of paroxetine is debatable. A recent review reports 8 studies that suggest a malformation risk, compared with 15 studies that show no risk.12
The American Academy of Pediatrics considers SSRIs to be compatible with breast-feeding.13 The best-studied drugs include sertraline and paroxetine. Fluoxetine should be avoided when possible because a long elimination half-life can cause the drug to accumulate in the newborn, increasing the risk of irritability, hypertonia, sedation, and poor suckle.7
There is no best SSRI for all pregnant women. Risks and benefits, including previous treatment success and failure, should be taken into account before starting or switching therapy. Whenever possible, consider monotherapy to avoid compounding the risk of harm.
Related Resources
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31:403-413.
- MGH Center for Women’s Mental Health. www.womensmentalhealth.org.
Drug Brand Names
Citalopram • Celexa Escitalopram • Lexapro Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Sertraline • Zoloft
Disclosures
Dr. Leino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Ellingrod receives grant support from the National Institute of Mental Health.
Mrs. D is a 28-year-old married woman who became depressed after her first pregnancy. The depression was treated successfully with paroxetine, 20 mg/d. Before beginning treatment, she reported low mood, spent most of the day in bed, was unable to care for herself, and confessed to thoughts of harming her child.
Mrs. D presents to your clinic asking whether she should continue her selective serotonin reuptake inhibitor (SSRI) because she and her husband are thinking about having a second child. Recently, she tells you, she saw a news article suggesting that antidepressants show little benefit, and she is concerned that her baby might have a heart defect if she continues paroxetine.
Mrs. D wants to discontinue her medication, but her husband thought she should discuss doing so with you first. During this visit she takes a pregnancy test, which is positive. She wants to know what to do.
women experience depression; 3.8% of pregnant women receive an SSRI.1 SSRIs are the most commonly prescribed antidepressants during pregnancy, but their use remains controversial. There is disagreement about the maternal and neonatal risks of untreated depression and SSRI exposure.2-10 Media reports of studies demonstrating adverse effects associated with SSRIs may generate fear among women, possibly prompting them to self-discontinue medication.
Evidence of risks and benefits
Clinicians should be aware of possible adverse effects of SSRI use and untreated depression (Table).2-10 The available data precludes definitive associations between untreated depression and poor outcomes (Box). Studies of SSRI use during pregnancy have shown conflicting results for all potential outcomes. Absolute risk, with the exception of neonatal adaptation syndrome, is estimated to be small. Neonatal adaptation syndrome—which is characterized by jitteriness, poor muscle tone, weak cries, respiratory distress, hypoglycemia, low Apgar scores, and seizures—occurs in 15% to 30% of infants born to mothers taking SSRIs, but it is transient and resolves during the first weeks of life.
Treatment recommendations
Given the conflicting nature of the evidence, treatment plans should be individualized, weighing the risks and benefits of treatment and the patient’s beliefs and psychiatric history. Consider severity of symptoms and history, including effective therapy and history of relapse. For women with mild or moderate depression, cognitive-behavioral therapy might be an appropriate first-line therapy. However, non-pharmacotherapeutic interventions might not relieve severe depression or be available to all women. When discontinuing an SSRI before pregnancy, counsel the patient to not discontinue the medication abruptly and provide an appropriate taper schedule. See Related Resources for detailed recommendations from the American Psychiatric Association and the American College of Obstetricians and Gynecologists.
Reviewing the SSRI literature regarding pregnancy
Sertraline, paroxetine, citalopram, and fluoxetine are the most studied SSRIs during pregnancy; little information is available on escitalopram and fluvoxamine.11 Prescribing preference generally is given to the medications with the most evidence; paroxetine may be an exception. In 2005, the FDA requested a change in paroxetine’s pregnancy category from C to D, indicating that adequate studies demonstrated a risk of congenital cardiac malformations.11 Additional studies have been conducted, and the teratogenicity of paroxetine is debatable. A recent review reports 8 studies that suggest a malformation risk, compared with 15 studies that show no risk.12
The American Academy of Pediatrics considers SSRIs to be compatible with breast-feeding.13 The best-studied drugs include sertraline and paroxetine. Fluoxetine should be avoided when possible because a long elimination half-life can cause the drug to accumulate in the newborn, increasing the risk of irritability, hypertonia, sedation, and poor suckle.7
There is no best SSRI for all pregnant women. Risks and benefits, including previous treatment success and failure, should be taken into account before starting or switching therapy. Whenever possible, consider monotherapy to avoid compounding the risk of harm.
Related Resources
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31:403-413.
- MGH Center for Women’s Mental Health. www.womensmentalhealth.org.
Drug Brand Names
Citalopram • Celexa Escitalopram • Lexapro Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Sertraline • Zoloft
Disclosures
Dr. Leino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Ellingrod receives grant support from the National Institute of Mental Health.
1. Alwan S, Reefhuis J, Rasmussen SA, et al. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51(2):264-270.
2. Domar AD, Moragianni VA, Ryley DA, et al. The risks of selective serotonin reuptake inhibitor use in infertile women: a review of the impact on fertility, pregnancy, neonatal health and beyond. Hum Reprod. 20113;28(1):160-171.
3. Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15(1):1-14.
4. Spinelli M. Antidepressant treatment during pregnancy. Am J Psychiatry. 2012;169(2):121-124.
5. Oyebode F, Rastogi A, Berrisford G, et al. Psychotropics in pregnancy: safety and other considerations. Pharmacol Ther. 2012;135(1):71-77.
6. Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 2013;127(2):94-114.
7. Sie SD, Wennink JM, van Driel JJ, et al. Maternal use of SSRIs, SNRIs and NaSSAs: practical recommendations during pregnancy and lactation. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F472-476.
8. Jimenez-Solem E, Andersen JT, Petersen M, et al. SSRI use during pregnancy and risk of stillbirth and neonatal mortality. Am J Psychiatry. 2013;170(3):299-304.
9. Nikfar S, Rahimi R, Hendoiee N, et al. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: a systematic review and updated meta-analysis. Daru. 2012;20(1):75.
10. Stephansson O, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of stillbirth and infant mortality. JAMA. 2013;309(1):48-54.
11. U.S. Food and Drug Administration. Public health advisory: paroxetine. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/Public HealthAdvisories/ucm051731.htm. Published December 8, 2005. Accessed September 27, 2013.
12. Koren G, Nordeng H. Antidepressant use during pregnancy: the benefit-risk ratio. Am J Obstet Gynecol. 2012;207(3):157-163.
13. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776-789.
1. Alwan S, Reefhuis J, Rasmussen SA, et al. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51(2):264-270.
2. Domar AD, Moragianni VA, Ryley DA, et al. The risks of selective serotonin reuptake inhibitor use in infertile women: a review of the impact on fertility, pregnancy, neonatal health and beyond. Hum Reprod. 20113;28(1):160-171.
3. Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15(1):1-14.
4. Spinelli M. Antidepressant treatment during pregnancy. Am J Psychiatry. 2012;169(2):121-124.
5. Oyebode F, Rastogi A, Berrisford G, et al. Psychotropics in pregnancy: safety and other considerations. Pharmacol Ther. 2012;135(1):71-77.
6. Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 2013;127(2):94-114.
7. Sie SD, Wennink JM, van Driel JJ, et al. Maternal use of SSRIs, SNRIs and NaSSAs: practical recommendations during pregnancy and lactation. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F472-476.
8. Jimenez-Solem E, Andersen JT, Petersen M, et al. SSRI use during pregnancy and risk of stillbirth and neonatal mortality. Am J Psychiatry. 2013;170(3):299-304.
9. Nikfar S, Rahimi R, Hendoiee N, et al. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: a systematic review and updated meta-analysis. Daru. 2012;20(1):75.
10. Stephansson O, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of stillbirth and infant mortality. JAMA. 2013;309(1):48-54.
11. U.S. Food and Drug Administration. Public health advisory: paroxetine. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/Public HealthAdvisories/ucm051731.htm. Published December 8, 2005. Accessed September 27, 2013.
12. Koren G, Nordeng H. Antidepressant use during pregnancy: the benefit-risk ratio. Am J Obstet Gynecol. 2012;207(3):157-163.
13. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776-789.