User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Hookahs aren’t a safe alternative to cigarettes
Repositioning psychotherapy as a neurobiological intervention
Despite its well-documented efficacy in a myriad of neuropsychiatric conditions, psychotherapy has never been able to shrug off an unwarranted aura of fuzziness as a legitimate medical intervention. To many uninformed people, psychotherapy isn’t a “real” treatment, such as medication or a surgical procedure. It often is referred to as “talk therapy,” which provokes skepticism, even snickering, because talking is a ubiquitous social activity.
Psychotherapy is sometimes perceived as a scam—that is, a placebo packaged and propagated as treatment; after all, how can spoken words “heal” the wounds of the soul? Paradoxically, skepticism about the vague and mysterious mechanism of action of psychotherapy might make things worse by adding to the stigma that mental illness is a spurious “all-in-your-head” complaint and not a genuine medical disorder.
Giving psychotherapy the respect it deserves
To the chagrin of many in our field, this image problem persists, despite psychotherapy having helped millions of people. It is widely used in conjunction with pharmacotherapy by psychiatrists and nurse practitioners, and as a sole therapeutic modality by psychologists and other therapists. This image problem has emboldened health insurance companies to arbitrarily limit reimbursement for psychotherapy, compared with psychopharmacology, to the detriment of patients who often can benefit significantly from psychotherapy alone, without medication.
So, how can psychotherapy capture the respect it deserves as a vital and valid therapeutic modality?
For one, psychotherapy has to be evidence-based and rigorously proven to be superior to placebo—the same standard that drugs are held to before they are approved by the FDA. But there are hundreds of psychotherapies, of which only a minority are evidence-based (eg, cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal therapy), based on findings of controlled trials in which they were documented to be efficacious.
There is a dearth of data about the safety and tolerability of, and indications for, specific psychotherapies—criteria that are major factors in determining whether the FDA approves a medication. Also, dosing of psychotherapy remains ambiguous, subjective, and lacking solid clinical guidelines, and the frequency of visits, duration of each visit, and need for maintenance of psychotherapy lack solid scientific evidence.
Patients therefore seem to receive psychotherapy for as long as health insurance pays for it, even if they need more of it. Frequency of treatment is determined by the therapist, or at the convenience of the patient or the therapist. Long-term psychotherapy—1 or 2 years—once was common, but short-term psychotherapy of fewer than 10 sessions has become the rage since managed care curtailed reimbursement. It is curious that, although most practitioners agree that serious psychiatric disorders can require ongoing, even lifelong maintenance of a drug beyond the acute phase, no one ever argues for indefinite continuation of psychotherapy (although Sigmund Freud did discuss terminable and interminable psychoanalysis).
Rx: A ’makeover’
Psychotherapy needs to be reconceptualized, rebranded, and repositioned as a neurobiological treatment—because, in fact, that’s what it is. This notion goes hand-in-hand with unimpeachable evidence that the mind is an integral component of the brain and mental illness is generated from genetic or environmentally-induced dysregulation of neurobiological homeostasis.
An important line of evidence for the neurologic effects of psychotherapy are studies of positron-emission tomography showing that psychotherapy induces changes in specific brain regions that are identical to changes induced by drug therapy.1,2 The component activities of psychotherapy—verbal and nonverbal communication, evocation of memories, empathizing, challenging, connecting the dots, triggering insights, and reducing anguish—are transduced into instantaneous neuroplastic changes, which can be lasting and lead to corrective modification of the neural circuitry of feelings, thinking, and behavior.
Most non-neuroscientists might not be aware that the brain changes continuously, moment to moment, forming dendritic spines that immediately encode verbal and nonverbal memories in response to experiences throughout life. A skilled psychotherapist exploits this biological property of the brain to modify its molecular and cellular structure to relieve the anguish and psychopathology of its avatar, the mind.
What might silence the skeptics?
Psychotherapy legitimately could be relabeled “neuropsychotherapy” to indicate that it has an impact on the neural structure and function that underpin the “psyche”—that collection of thoughts, feelings, memories, and impulses that are a product of activity in specific brain pathways, just as other brain pathways produce movement and sensation. Future studies, using innovative biotechnological imaging methods, will demonstrate the tangible neurobiological impact of neuropsychotherapy and erase the skepticism that shrouds its nature. At that point, neuropsychotherapy will get the respect it deserves as a tool to heal the mind by repairing the brain.
1. Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58(7):631-640.
2. Baxter LR Jr, Schwartz JM, Bergman KS, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(9):681-689.
Despite its well-documented efficacy in a myriad of neuropsychiatric conditions, psychotherapy has never been able to shrug off an unwarranted aura of fuzziness as a legitimate medical intervention. To many uninformed people, psychotherapy isn’t a “real” treatment, such as medication or a surgical procedure. It often is referred to as “talk therapy,” which provokes skepticism, even snickering, because talking is a ubiquitous social activity.
Psychotherapy is sometimes perceived as a scam—that is, a placebo packaged and propagated as treatment; after all, how can spoken words “heal” the wounds of the soul? Paradoxically, skepticism about the vague and mysterious mechanism of action of psychotherapy might make things worse by adding to the stigma that mental illness is a spurious “all-in-your-head” complaint and not a genuine medical disorder.
Giving psychotherapy the respect it deserves
To the chagrin of many in our field, this image problem persists, despite psychotherapy having helped millions of people. It is widely used in conjunction with pharmacotherapy by psychiatrists and nurse practitioners, and as a sole therapeutic modality by psychologists and other therapists. This image problem has emboldened health insurance companies to arbitrarily limit reimbursement for psychotherapy, compared with psychopharmacology, to the detriment of patients who often can benefit significantly from psychotherapy alone, without medication.
So, how can psychotherapy capture the respect it deserves as a vital and valid therapeutic modality?
For one, psychotherapy has to be evidence-based and rigorously proven to be superior to placebo—the same standard that drugs are held to before they are approved by the FDA. But there are hundreds of psychotherapies, of which only a minority are evidence-based (eg, cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal therapy), based on findings of controlled trials in which they were documented to be efficacious.
There is a dearth of data about the safety and tolerability of, and indications for, specific psychotherapies—criteria that are major factors in determining whether the FDA approves a medication. Also, dosing of psychotherapy remains ambiguous, subjective, and lacking solid clinical guidelines, and the frequency of visits, duration of each visit, and need for maintenance of psychotherapy lack solid scientific evidence.
Patients therefore seem to receive psychotherapy for as long as health insurance pays for it, even if they need more of it. Frequency of treatment is determined by the therapist, or at the convenience of the patient or the therapist. Long-term psychotherapy—1 or 2 years—once was common, but short-term psychotherapy of fewer than 10 sessions has become the rage since managed care curtailed reimbursement. It is curious that, although most practitioners agree that serious psychiatric disorders can require ongoing, even lifelong maintenance of a drug beyond the acute phase, no one ever argues for indefinite continuation of psychotherapy (although Sigmund Freud did discuss terminable and interminable psychoanalysis).
Rx: A ’makeover’
Psychotherapy needs to be reconceptualized, rebranded, and repositioned as a neurobiological treatment—because, in fact, that’s what it is. This notion goes hand-in-hand with unimpeachable evidence that the mind is an integral component of the brain and mental illness is generated from genetic or environmentally-induced dysregulation of neurobiological homeostasis.
An important line of evidence for the neurologic effects of psychotherapy are studies of positron-emission tomography showing that psychotherapy induces changes in specific brain regions that are identical to changes induced by drug therapy.1,2 The component activities of psychotherapy—verbal and nonverbal communication, evocation of memories, empathizing, challenging, connecting the dots, triggering insights, and reducing anguish—are transduced into instantaneous neuroplastic changes, which can be lasting and lead to corrective modification of the neural circuitry of feelings, thinking, and behavior.
Most non-neuroscientists might not be aware that the brain changes continuously, moment to moment, forming dendritic spines that immediately encode verbal and nonverbal memories in response to experiences throughout life. A skilled psychotherapist exploits this biological property of the brain to modify its molecular and cellular structure to relieve the anguish and psychopathology of its avatar, the mind.
What might silence the skeptics?
Psychotherapy legitimately could be relabeled “neuropsychotherapy” to indicate that it has an impact on the neural structure and function that underpin the “psyche”—that collection of thoughts, feelings, memories, and impulses that are a product of activity in specific brain pathways, just as other brain pathways produce movement and sensation. Future studies, using innovative biotechnological imaging methods, will demonstrate the tangible neurobiological impact of neuropsychotherapy and erase the skepticism that shrouds its nature. At that point, neuropsychotherapy will get the respect it deserves as a tool to heal the mind by repairing the brain.
Despite its well-documented efficacy in a myriad of neuropsychiatric conditions, psychotherapy has never been able to shrug off an unwarranted aura of fuzziness as a legitimate medical intervention. To many uninformed people, psychotherapy isn’t a “real” treatment, such as medication or a surgical procedure. It often is referred to as “talk therapy,” which provokes skepticism, even snickering, because talking is a ubiquitous social activity.
Psychotherapy is sometimes perceived as a scam—that is, a placebo packaged and propagated as treatment; after all, how can spoken words “heal” the wounds of the soul? Paradoxically, skepticism about the vague and mysterious mechanism of action of psychotherapy might make things worse by adding to the stigma that mental illness is a spurious “all-in-your-head” complaint and not a genuine medical disorder.
Giving psychotherapy the respect it deserves
To the chagrin of many in our field, this image problem persists, despite psychotherapy having helped millions of people. It is widely used in conjunction with pharmacotherapy by psychiatrists and nurse practitioners, and as a sole therapeutic modality by psychologists and other therapists. This image problem has emboldened health insurance companies to arbitrarily limit reimbursement for psychotherapy, compared with psychopharmacology, to the detriment of patients who often can benefit significantly from psychotherapy alone, without medication.
So, how can psychotherapy capture the respect it deserves as a vital and valid therapeutic modality?
For one, psychotherapy has to be evidence-based and rigorously proven to be superior to placebo—the same standard that drugs are held to before they are approved by the FDA. But there are hundreds of psychotherapies, of which only a minority are evidence-based (eg, cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal therapy), based on findings of controlled trials in which they were documented to be efficacious.
There is a dearth of data about the safety and tolerability of, and indications for, specific psychotherapies—criteria that are major factors in determining whether the FDA approves a medication. Also, dosing of psychotherapy remains ambiguous, subjective, and lacking solid clinical guidelines, and the frequency of visits, duration of each visit, and need for maintenance of psychotherapy lack solid scientific evidence.
Patients therefore seem to receive psychotherapy for as long as health insurance pays for it, even if they need more of it. Frequency of treatment is determined by the therapist, or at the convenience of the patient or the therapist. Long-term psychotherapy—1 or 2 years—once was common, but short-term psychotherapy of fewer than 10 sessions has become the rage since managed care curtailed reimbursement. It is curious that, although most practitioners agree that serious psychiatric disorders can require ongoing, even lifelong maintenance of a drug beyond the acute phase, no one ever argues for indefinite continuation of psychotherapy (although Sigmund Freud did discuss terminable and interminable psychoanalysis).
Rx: A ’makeover’
Psychotherapy needs to be reconceptualized, rebranded, and repositioned as a neurobiological treatment—because, in fact, that’s what it is. This notion goes hand-in-hand with unimpeachable evidence that the mind is an integral component of the brain and mental illness is generated from genetic or environmentally-induced dysregulation of neurobiological homeostasis.
An important line of evidence for the neurologic effects of psychotherapy are studies of positron-emission tomography showing that psychotherapy induces changes in specific brain regions that are identical to changes induced by drug therapy.1,2 The component activities of psychotherapy—verbal and nonverbal communication, evocation of memories, empathizing, challenging, connecting the dots, triggering insights, and reducing anguish—are transduced into instantaneous neuroplastic changes, which can be lasting and lead to corrective modification of the neural circuitry of feelings, thinking, and behavior.
Most non-neuroscientists might not be aware that the brain changes continuously, moment to moment, forming dendritic spines that immediately encode verbal and nonverbal memories in response to experiences throughout life. A skilled psychotherapist exploits this biological property of the brain to modify its molecular and cellular structure to relieve the anguish and psychopathology of its avatar, the mind.
What might silence the skeptics?
Psychotherapy legitimately could be relabeled “neuropsychotherapy” to indicate that it has an impact on the neural structure and function that underpin the “psyche”—that collection of thoughts, feelings, memories, and impulses that are a product of activity in specific brain pathways, just as other brain pathways produce movement and sensation. Future studies, using innovative biotechnological imaging methods, will demonstrate the tangible neurobiological impact of neuropsychotherapy and erase the skepticism that shrouds its nature. At that point, neuropsychotherapy will get the respect it deserves as a tool to heal the mind by repairing the brain.
1. Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58(7):631-640.
2. Baxter LR Jr, Schwartz JM, Bergman KS, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(9):681-689.
1. Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58(7):631-640.
2. Baxter LR Jr, Schwartz JM, Bergman KS, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(9):681-689.
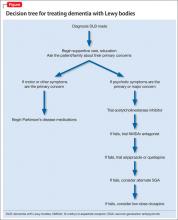
Treating Alzheimer's disease
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Expanding medication options for pediatric ADHD
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
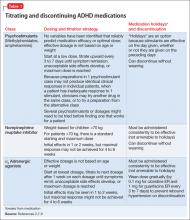
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
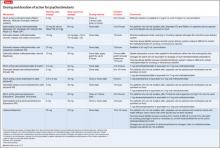
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
Levomilnacipran for the treatment of major depressive disorder
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3
Levomilnacipran generally was reported to be safe and well tolerated. The most common adverse events leading to discontinuation in the levomilnacipran group were nausea, vomiting, change in systolic and diastolic blood pressure, and increase in heart rate. The favorable tolerability profile of levomilnacipran may relate to the 2-fold greater potency for NE reuptake inhibition compared with 5-HT reuptake inhibition.3
The second study was an 11-week, fixed-dose trial of levomilnacipran using 40, 80, or 120 mg. A total of 724 outpatients age 18 to 65 who met DSM-IV-TR criteria for MDD and who had an ongoing episode of depression lasting >8 weeks were randomly assigned to receive placebo (n = 179) or levomilnacipran at 40 mg (n = 181), 80 mg (n = 181), or 120 mg (n = 183) once daily for 8 weeks of double-blind treatment followed by a 2-week, double-blind taper of the drug.5 The primary efficacy parameter was change from baseline on the MADRS and the secondary efficacy parameter was change from baseline in SDS total score. HDRS-17, CGI-I, and CGI-S were included as secondary outcome measures.5
Significant difference in MADRS total score were seen in the levomilnacipran group compared with the placebo group (least mean squared difference: 40 mg/d, −3.23; 80 mg/d, −3.99; and 120 mg/d, −4.86). Higher dosages produced a numerically greater change and significant separation from placebo occurred sooner in the 80-mg and 120-mg groups compared with the 40-mg group.5
Significant differences vs placebo were consistently observed across secondary outcome measures for the higher dosages of levomilnacipran, and improvement in SDS total score was noted in all levomilnacipran groups compared with the placebo group. When dosed at 120 mg/d, levomilnacipran produced significant improvement vs placebo on all SDS subscales and was as well tolerated as the 80 mg dosage.5
No new safety concerns were observed in the study. A dose-response relationship in tolerability was not demonstrated and the number of patients reporting adverse events and who discontinued participation because of adverse events was higher in the 80-mg group than in the 40-mg and 120-mg groups.5
Tolerability
Overall, levomilnacipran was well tolerated in clinical trials, during which 2,673 subjects were exposed to the drug—translating to 942 patient-years of exposure. These patients ranged in age from 18 to 78; 825 of these subjects were enrolled in long-term studies for 1 year. Dosing of levomilnacipran during these studies ranged from 40 to 120 mg once daily, without regard to food.1
Nine percent of patients who received levomilnacipran in short-term studies discontinued because of adverse events, compared with 3% of patients who received placebo. The most common adverse event reported was nausea; other common adverse events reported included constipation, hyperhidrosis, elevated heart rate, erectile dysfunction, tachycardia, palpitations, and vomiting. Of these events, only erectile dysfunction and urinary hesitation were dose-related.1 Among levomilnacipran-treated female patients, <2% reported adverse events related to sexual dysfunction.
All SNRIs have well established associations with elevation in blood pressure and heart rate. Levomilnacipran resulted in a mean increase of 3 mm HG in systolic and 3.2 mm Hg in diastolic blood pressure in short-term, placebo-controlled trials.1
Orthostatic hypotension was observed in 11.6% of patients in the levomilnacipran groups, compared with 9.7% in placebo groups in all short-term studies. Orthostatic reductions of blood pressure occurred in 5.8%, 6.1%, and 9.8% of levomilnacipran-treated patients with dosages of 40, 80, and 120 mg/d respectively, indicating a dose-dependent adverse event. A mean increase in heart rate of 7.2 beats per minute (bpm) also was seen in short-term studies in the levomilnacipran-treated group compared with 0.3 bpm in the placebo-treated group.1 Clinicians should monitor blood pressure and heart rate routinely because of potential increases seen in some subjects in these studies, which excluded those who had significant cardiovascular disease.
Unique clinical issues
Both in-vitro and in-vivo studies found that levomilnacipran exhibited more potency for NE reuptake inhibition than for 5-HT reuptake inhibition at the lowest effective dosage (10 mg/kg). However as the dosage was increased (20 mg/kg and 40 mg/kg), it was equally potent at NE and 5-HT reuptake inhibition. This is in contrast to venlafaxine, which demonstrates a similar, but opposite, effect in terms of potentiation at the 5-HT and NE reuptake pumps.2
The greater noradrenergic effect of levomilnacipran could lend itself to treating certain subgroups of patients whose symptoms are believed to be related to deficiencies in NE (eg, lassitude).4 This concept is theoretical, and was not explicitly studied in clinical trials and the drug is not labeled in this way.
Contraindications
Contraindications to levomilnacipran are similar to those seen with SSRIs and SNRIs, including concomitant use of a monoamine oxidase inhibitor (MAOI) and the use of the levomilnacipran within 14 days of stopping an MAOI. Contraindications unique to levomilnacipran include hypersensitivity to levomilnacipran, milnacipran, or any component specific to the formulation; and uncontrolled narrow-angle glaucoma.1
Dosing
The recommended dosage range of levomilnacipran is 40 to 120 mg once
daily with or without food. The capsules should be swallowed whole and should not be opened or crushed. As with most psychotropics, levomilnacipran should be taken at approximately the same time each day.1
The manufacturer recommends an initial dose of levomilnacipran of 20 mg once daily for 2 days, increased to 40 mg once daily. Based on efficacy and tolerably, levomilnacipran can be increased in increments of 40 mg every 2 days.
Dosage adjustment is recommended for patients with moderate or severe renal impairment; and the maintenance dosage should not exceed 80 mg and 40 mg respectively in these populations. As with many antidepressants, gradual dosage reduction is recommended to avoid discontinuation symptoms.
Bottom Line
Levomilnacipran is FDA-approved for treating major depressive disorder in adults. In 2 randomized controlled trials, the drug showed a significant separation from placebo. Levomilnacipran generally was reported to be safe and well tolerated; common adverse events were nausea, vomiting, changes in blood pressure, and an increase in heart rate.
Related Resources
- Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? [published online September 8, 2013]. Int J Clin Pract. doi: 10.1111/ijcp.12298.
- Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
Drug Brand Names
Duloxetine • Cymbalta Milnacipran • Savella
Ketoconazole • Nizoral Venlafaxine • Effexor Levomilnacipran • Fetzima
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Drs. Kazanchi and Malhotra report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fetzima [package insert]. St. Louis, MO: Forest Laboratories; 2013.
2. Auclair AL, Martel JC, Assié MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338-347.
3. Montgomery SA, Mansuy L, Ruth A, et al. Efficacy and Safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2013;74(4):363-369.
4. Kasper S, Meshkat D, Kutzelnigg A. Improvement of the noradrenergic symptom cluster following treatment with milnacipran. Neuropsychiatric Dis Treat. 2011; 7(suppl 1):21-27.
5. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
6. Hutt AJ, Vanetová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facultatis Pharmaceuticae Universitatis Comenianae. 2003; 50(7):23.
7. U.S. Food and Drug Administration. Development of new stereoisomeric drugs. Published May 1, 1992. http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm#.UKHEWm4ZyYE.email. Accessed October 8, 2013.
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3
Levomilnacipran generally was reported to be safe and well tolerated. The most common adverse events leading to discontinuation in the levomilnacipran group were nausea, vomiting, change in systolic and diastolic blood pressure, and increase in heart rate. The favorable tolerability profile of levomilnacipran may relate to the 2-fold greater potency for NE reuptake inhibition compared with 5-HT reuptake inhibition.3
The second study was an 11-week, fixed-dose trial of levomilnacipran using 40, 80, or 120 mg. A total of 724 outpatients age 18 to 65 who met DSM-IV-TR criteria for MDD and who had an ongoing episode of depression lasting >8 weeks were randomly assigned to receive placebo (n = 179) or levomilnacipran at 40 mg (n = 181), 80 mg (n = 181), or 120 mg (n = 183) once daily for 8 weeks of double-blind treatment followed by a 2-week, double-blind taper of the drug.5 The primary efficacy parameter was change from baseline on the MADRS and the secondary efficacy parameter was change from baseline in SDS total score. HDRS-17, CGI-I, and CGI-S were included as secondary outcome measures.5
Significant difference in MADRS total score were seen in the levomilnacipran group compared with the placebo group (least mean squared difference: 40 mg/d, −3.23; 80 mg/d, −3.99; and 120 mg/d, −4.86). Higher dosages produced a numerically greater change and significant separation from placebo occurred sooner in the 80-mg and 120-mg groups compared with the 40-mg group.5
Significant differences vs placebo were consistently observed across secondary outcome measures for the higher dosages of levomilnacipran, and improvement in SDS total score was noted in all levomilnacipran groups compared with the placebo group. When dosed at 120 mg/d, levomilnacipran produced significant improvement vs placebo on all SDS subscales and was as well tolerated as the 80 mg dosage.5
No new safety concerns were observed in the study. A dose-response relationship in tolerability was not demonstrated and the number of patients reporting adverse events and who discontinued participation because of adverse events was higher in the 80-mg group than in the 40-mg and 120-mg groups.5
Tolerability
Overall, levomilnacipran was well tolerated in clinical trials, during which 2,673 subjects were exposed to the drug—translating to 942 patient-years of exposure. These patients ranged in age from 18 to 78; 825 of these subjects were enrolled in long-term studies for 1 year. Dosing of levomilnacipran during these studies ranged from 40 to 120 mg once daily, without regard to food.1
Nine percent of patients who received levomilnacipran in short-term studies discontinued because of adverse events, compared with 3% of patients who received placebo. The most common adverse event reported was nausea; other common adverse events reported included constipation, hyperhidrosis, elevated heart rate, erectile dysfunction, tachycardia, palpitations, and vomiting. Of these events, only erectile dysfunction and urinary hesitation were dose-related.1 Among levomilnacipran-treated female patients, <2% reported adverse events related to sexual dysfunction.
All SNRIs have well established associations with elevation in blood pressure and heart rate. Levomilnacipran resulted in a mean increase of 3 mm HG in systolic and 3.2 mm Hg in diastolic blood pressure in short-term, placebo-controlled trials.1
Orthostatic hypotension was observed in 11.6% of patients in the levomilnacipran groups, compared with 9.7% in placebo groups in all short-term studies. Orthostatic reductions of blood pressure occurred in 5.8%, 6.1%, and 9.8% of levomilnacipran-treated patients with dosages of 40, 80, and 120 mg/d respectively, indicating a dose-dependent adverse event. A mean increase in heart rate of 7.2 beats per minute (bpm) also was seen in short-term studies in the levomilnacipran-treated group compared with 0.3 bpm in the placebo-treated group.1 Clinicians should monitor blood pressure and heart rate routinely because of potential increases seen in some subjects in these studies, which excluded those who had significant cardiovascular disease.
Unique clinical issues
Both in-vitro and in-vivo studies found that levomilnacipran exhibited more potency for NE reuptake inhibition than for 5-HT reuptake inhibition at the lowest effective dosage (10 mg/kg). However as the dosage was increased (20 mg/kg and 40 mg/kg), it was equally potent at NE and 5-HT reuptake inhibition. This is in contrast to venlafaxine, which demonstrates a similar, but opposite, effect in terms of potentiation at the 5-HT and NE reuptake pumps.2
The greater noradrenergic effect of levomilnacipran could lend itself to treating certain subgroups of patients whose symptoms are believed to be related to deficiencies in NE (eg, lassitude).4 This concept is theoretical, and was not explicitly studied in clinical trials and the drug is not labeled in this way.
Contraindications
Contraindications to levomilnacipran are similar to those seen with SSRIs and SNRIs, including concomitant use of a monoamine oxidase inhibitor (MAOI) and the use of the levomilnacipran within 14 days of stopping an MAOI. Contraindications unique to levomilnacipran include hypersensitivity to levomilnacipran, milnacipran, or any component specific to the formulation; and uncontrolled narrow-angle glaucoma.1
Dosing
The recommended dosage range of levomilnacipran is 40 to 120 mg once
daily with or without food. The capsules should be swallowed whole and should not be opened or crushed. As with most psychotropics, levomilnacipran should be taken at approximately the same time each day.1
The manufacturer recommends an initial dose of levomilnacipran of 20 mg once daily for 2 days, increased to 40 mg once daily. Based on efficacy and tolerably, levomilnacipran can be increased in increments of 40 mg every 2 days.
Dosage adjustment is recommended for patients with moderate or severe renal impairment; and the maintenance dosage should not exceed 80 mg and 40 mg respectively in these populations. As with many antidepressants, gradual dosage reduction is recommended to avoid discontinuation symptoms.
Bottom Line
Levomilnacipran is FDA-approved for treating major depressive disorder in adults. In 2 randomized controlled trials, the drug showed a significant separation from placebo. Levomilnacipran generally was reported to be safe and well tolerated; common adverse events were nausea, vomiting, changes in blood pressure, and an increase in heart rate.
Related Resources
- Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? [published online September 8, 2013]. Int J Clin Pract. doi: 10.1111/ijcp.12298.
- Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
Drug Brand Names
Duloxetine • Cymbalta Milnacipran • Savella
Ketoconazole • Nizoral Venlafaxine • Effexor Levomilnacipran • Fetzima
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Drs. Kazanchi and Malhotra report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3
Levomilnacipran generally was reported to be safe and well tolerated. The most common adverse events leading to discontinuation in the levomilnacipran group were nausea, vomiting, change in systolic and diastolic blood pressure, and increase in heart rate. The favorable tolerability profile of levomilnacipran may relate to the 2-fold greater potency for NE reuptake inhibition compared with 5-HT reuptake inhibition.3
The second study was an 11-week, fixed-dose trial of levomilnacipran using 40, 80, or 120 mg. A total of 724 outpatients age 18 to 65 who met DSM-IV-TR criteria for MDD and who had an ongoing episode of depression lasting >8 weeks were randomly assigned to receive placebo (n = 179) or levomilnacipran at 40 mg (n = 181), 80 mg (n = 181), or 120 mg (n = 183) once daily for 8 weeks of double-blind treatment followed by a 2-week, double-blind taper of the drug.5 The primary efficacy parameter was change from baseline on the MADRS and the secondary efficacy parameter was change from baseline in SDS total score. HDRS-17, CGI-I, and CGI-S were included as secondary outcome measures.5
Significant difference in MADRS total score were seen in the levomilnacipran group compared with the placebo group (least mean squared difference: 40 mg/d, −3.23; 80 mg/d, −3.99; and 120 mg/d, −4.86). Higher dosages produced a numerically greater change and significant separation from placebo occurred sooner in the 80-mg and 120-mg groups compared with the 40-mg group.5
Significant differences vs placebo were consistently observed across secondary outcome measures for the higher dosages of levomilnacipran, and improvement in SDS total score was noted in all levomilnacipran groups compared with the placebo group. When dosed at 120 mg/d, levomilnacipran produced significant improvement vs placebo on all SDS subscales and was as well tolerated as the 80 mg dosage.5
No new safety concerns were observed in the study. A dose-response relationship in tolerability was not demonstrated and the number of patients reporting adverse events and who discontinued participation because of adverse events was higher in the 80-mg group than in the 40-mg and 120-mg groups.5
Tolerability
Overall, levomilnacipran was well tolerated in clinical trials, during which 2,673 subjects were exposed to the drug—translating to 942 patient-years of exposure. These patients ranged in age from 18 to 78; 825 of these subjects were enrolled in long-term studies for 1 year. Dosing of levomilnacipran during these studies ranged from 40 to 120 mg once daily, without regard to food.1
Nine percent of patients who received levomilnacipran in short-term studies discontinued because of adverse events, compared with 3% of patients who received placebo. The most common adverse event reported was nausea; other common adverse events reported included constipation, hyperhidrosis, elevated heart rate, erectile dysfunction, tachycardia, palpitations, and vomiting. Of these events, only erectile dysfunction and urinary hesitation were dose-related.1 Among levomilnacipran-treated female patients, <2% reported adverse events related to sexual dysfunction.
All SNRIs have well established associations with elevation in blood pressure and heart rate. Levomilnacipran resulted in a mean increase of 3 mm HG in systolic and 3.2 mm Hg in diastolic blood pressure in short-term, placebo-controlled trials.1
Orthostatic hypotension was observed in 11.6% of patients in the levomilnacipran groups, compared with 9.7% in placebo groups in all short-term studies. Orthostatic reductions of blood pressure occurred in 5.8%, 6.1%, and 9.8% of levomilnacipran-treated patients with dosages of 40, 80, and 120 mg/d respectively, indicating a dose-dependent adverse event. A mean increase in heart rate of 7.2 beats per minute (bpm) also was seen in short-term studies in the levomilnacipran-treated group compared with 0.3 bpm in the placebo-treated group.1 Clinicians should monitor blood pressure and heart rate routinely because of potential increases seen in some subjects in these studies, which excluded those who had significant cardiovascular disease.
Unique clinical issues
Both in-vitro and in-vivo studies found that levomilnacipran exhibited more potency for NE reuptake inhibition than for 5-HT reuptake inhibition at the lowest effective dosage (10 mg/kg). However as the dosage was increased (20 mg/kg and 40 mg/kg), it was equally potent at NE and 5-HT reuptake inhibition. This is in contrast to venlafaxine, which demonstrates a similar, but opposite, effect in terms of potentiation at the 5-HT and NE reuptake pumps.2
The greater noradrenergic effect of levomilnacipran could lend itself to treating certain subgroups of patients whose symptoms are believed to be related to deficiencies in NE (eg, lassitude).4 This concept is theoretical, and was not explicitly studied in clinical trials and the drug is not labeled in this way.
Contraindications
Contraindications to levomilnacipran are similar to those seen with SSRIs and SNRIs, including concomitant use of a monoamine oxidase inhibitor (MAOI) and the use of the levomilnacipran within 14 days of stopping an MAOI. Contraindications unique to levomilnacipran include hypersensitivity to levomilnacipran, milnacipran, or any component specific to the formulation; and uncontrolled narrow-angle glaucoma.1
Dosing
The recommended dosage range of levomilnacipran is 40 to 120 mg once
daily with or without food. The capsules should be swallowed whole and should not be opened or crushed. As with most psychotropics, levomilnacipran should be taken at approximately the same time each day.1
The manufacturer recommends an initial dose of levomilnacipran of 20 mg once daily for 2 days, increased to 40 mg once daily. Based on efficacy and tolerably, levomilnacipran can be increased in increments of 40 mg every 2 days.
Dosage adjustment is recommended for patients with moderate or severe renal impairment; and the maintenance dosage should not exceed 80 mg and 40 mg respectively in these populations. As with many antidepressants, gradual dosage reduction is recommended to avoid discontinuation symptoms.
Bottom Line
Levomilnacipran is FDA-approved for treating major depressive disorder in adults. In 2 randomized controlled trials, the drug showed a significant separation from placebo. Levomilnacipran generally was reported to be safe and well tolerated; common adverse events were nausea, vomiting, changes in blood pressure, and an increase in heart rate.
Related Resources
- Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? [published online September 8, 2013]. Int J Clin Pract. doi: 10.1111/ijcp.12298.
- Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
Drug Brand Names
Duloxetine • Cymbalta Milnacipran • Savella
Ketoconazole • Nizoral Venlafaxine • Effexor Levomilnacipran • Fetzima
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Drs. Kazanchi and Malhotra report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fetzima [package insert]. St. Louis, MO: Forest Laboratories; 2013.
2. Auclair AL, Martel JC, Assié MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338-347.
3. Montgomery SA, Mansuy L, Ruth A, et al. Efficacy and Safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2013;74(4):363-369.
4. Kasper S, Meshkat D, Kutzelnigg A. Improvement of the noradrenergic symptom cluster following treatment with milnacipran. Neuropsychiatric Dis Treat. 2011; 7(suppl 1):21-27.
5. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
6. Hutt AJ, Vanetová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facultatis Pharmaceuticae Universitatis Comenianae. 2003; 50(7):23.
7. U.S. Food and Drug Administration. Development of new stereoisomeric drugs. Published May 1, 1992. http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm#.UKHEWm4ZyYE.email. Accessed October 8, 2013.
1. Fetzima [package insert]. St. Louis, MO: Forest Laboratories; 2013.
2. Auclair AL, Martel JC, Assié MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338-347.
3. Montgomery SA, Mansuy L, Ruth A, et al. Efficacy and Safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2013;74(4):363-369.
4. Kasper S, Meshkat D, Kutzelnigg A. Improvement of the noradrenergic symptom cluster following treatment with milnacipran. Neuropsychiatric Dis Treat. 2011; 7(suppl 1):21-27.
5. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
6. Hutt AJ, Vanetová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facultatis Pharmaceuticae Universitatis Comenianae. 2003; 50(7):23.
7. U.S. Food and Drug Administration. Development of new stereoisomeric drugs. Published May 1, 1992. http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm#.UKHEWm4ZyYE.email. Accessed October 8, 2013.
Dementia, bizarre creatures, and a white knight to the rescue
CASE Strange creatures
Ms. L, age 78, is admitted to the inpatient unit for treatment of psychosis and behavioral changes. In the months before this admission, she had visited the emergency room several times for recurrent falls. CT scans of the head show no acute changes; brain and spinal MRI reveal evidence of chronic white matter disease and degenerative changes of the spine. Medical workup is unremarkable and includes evaluation for syncope and ambulation impairments related to degenerative disease of the hip joints.
Ms. L and her family are instructed to follow-up with her primary care physician and a neurologist for neuromuscular workup.
She next presents to her primary care physician, describing hallucinations of strangers walking around her house. Over a few weeks, hallucinations expand to include a fixed hallucination of creatures that she describes as having qualities of insects and plants, “piling up” around her. She describes tactile hallucinations of these creatures crawling on her skin, and she tracks their movements around her. She complains of vivid visual hallucinations of these creatures spinning webs across the room and she says she keeps the lights on at night. Ms. L becomes anxious and depressed, and her insomnia becomes worse.
She is referred for outpatient psychiatric evaluation and treatment.
Ms. L’s family notes lapses of short-term memory, disorganization, and difficulty with tasks such as cooking because she has trouble following steps. These deficits come and go, with periods when she is functional and others during which she experiences considerable confusion. The family is uncertain when these signs and symptoms first appeared, but are clear that these deficits are having an impact on her day-to-day life. She can conduct activities of daily living, but with increasing difficulty—and only with help from her husband for tasks that require complex order and movement.
Over several months, Ms. L’s gait stability decreases and she begins to rely on a walker to keep from falling. On the Montreal Cognitive Assessment screening for cognitive dysfunction, she scores 19 out of 30 (normal range >25). This suggests cognitive impairment greater than expected for her age, compared with normal controls, and, when coupled with her functional impairment, raises the possibility of a diagnosis of dementia with Lewy bodies (DLB).
a) donepezil
b) memantine
c) quetiapine
d) low-dose clozapine
The authors’ observations
Limited literature exists of placebo-controlled, large-scale studies on DLB treatment. Cholinesterase inhibitors have shown some symptomatic benefit, including for hallucinations.1-3 Memantine, an N-methyl-d-aspartate receptor blocker, shows mixed results.4 Many studies explore the use of neuroleptics for treating hallucinations in psychosis in Parkinson’s disease and Parkinson’s disease dementia (PDD) but, in DLB, the literature primarily consists of case reports.2 Much of DLB treatment is inferred and intermixed with studies on PDD.5,6
Low-dose clozapine has become a standard treatment for psychosis in Parkinson’s disease based on the findings of several trials.6 Despite its side-effect profile, clozapine has been shown to ameliorate hallucinations in PDD without exacerbating parkinsonian symptoms,7,8 and is the only medication with proven efficacy in PDD.2 The French Clozapine Parkinson Study Group demonstrated relief of psychotic symptoms of Parkinson’s disease with clozapine, 6.25 mg/d.9 The Clozapine Study Group found complete resolution of hallucinations in some patients within 1 day of initiating clozapine. Among patients in this study who did not see immediate benefit, most showed significant improvement of psychotic symptoms in 1 or 2 weeks.10
TREATMENT Few options
Ms. L’s psychiatrist and primary care physician start her on a series of medications. Donepezil is initiated for suspected dementia. We begin a trial of quetiapine to address the hallucinations, but the drug makes her movement symptoms worse. Risperidone also is tried but, again, the drugs make movement symptoms, particularly gait instability, tremor, and rigidity worse without alleviating the hallucinations. Neuroleptics seem to exacerbate confusion. Because of worsening depressive symptoms and our concern over possible pseudodementia, we try several selective serotonin reuptake inhibitors (SSRIs) and mirtazapine. Antidepressants have little effect on her depressive symptoms and do not improve hallucinations or insomnia.
Ms. L’s signs and symptoms become worse over the next few months, with more severe hallucinations, agitation, insomnia, and gait instability. Her agitation over the hallucinations increases and she begins pouring bleach around herself in bed and spraying her house with toxic bug spray. Ms. L’s family brings her to the hospital after they observe her scratching the hallucinatory creatures off of her skin with a razor blade and trying to pry them out of her mouth with a piece of metal.
In the hospital, medical and neurologic workups rule out organic causes for her symptoms and signs. MRI is consistent with imaging from 6 months earlier. Focal neurologic signs are absent. Blood work is within normal limits, failing to reveal any pathology that would suggest a cause for her symptoms and signs, such as syphilis, vitamin deficiency, and Lyme disease.
Ms. L’s symptoms were consistent with consensus guideline criteria for a clinical diagnosis of DLB (Table 1).11-18
She is started on low-dose quetiapine, which she tolerates poorly with worsening confusion, rigidity, tremor, and gait instability. Because other agents failed, Ms. L’s providers and family decide on a trial of clozapine.
Within 24 hours after the first dose of clozapine, 25 mg, sleep improves, the tactile component of hallucinations diminish, and she begins to spend increasing periods of time “observing the creatures” rather than fighting with them.
Over the next few days, Ms. L’s attitude towards the creatures changes. Now, as she sits observing them intently, the hallucinations evolve: rather than tormenting her and causing distress, the plant-creatures burst apart and a miniature knight on horseback charges out. The rest of the creatures then gather into a rank and file and the knight leads them to the nearest exit.
Clozapine is titrated to 50 mg/d, which she tolerates well without exacerbation of cognitive symptoms or movement disorder. The only notable adverse effect at the time of her discharge is sialorrhea.
with an antipsychotic?
a) start low and go slow
b) monitor her heart rate and blood pressure
c) readminister the Montreal Cognitive Assessment
d) all of the above
The authors’ observations
Ideally, in psychosis, antipsychotics eliminate positive symptoms such as hallucinations and delusions. In DLB, the aim is to alleviate the agitation and suffering brought on by the psychotic symptoms without exacerbating other motor and cognitive symptoms. The hallucinations are obstinate, and it is a well-known quality of this disorder that patients are exceptionally susceptible to a range of antipsychotic side effects including cognitive impairment, fatigue, neuroleptic malignant syndrome, and parkinsonism.19
Treatment in DLB requires trial and error, and medications with fewer associated risks should be administered first. Patients with DLB treated with neuroleptics have an increased risk of death compared with those who are not treated.19 Moreover, prescribing information for clozapine includes a black-box warning that the drug:
- is not approved for dementia-related psychosis and
- is associated with an increased risk of death in elderly patients with these conditions, similar to what is seen with other neuroleptics.20
Despite these well-known concerns, it remains difficult for clinicians not to try to treat the distress caused by these symptoms.
We chose clozapine for Ms. L because:
- other neuroleptics failed
- acetylcholinesterase inhibitors did not alleviate Ms. L’s psychosis and associated behavioral disturbance
- there is substantial evidence that the drug can be effective in Parkinson’s disease with psychosis.
There is controversy regarding use of clozapine in DLB. In one case series, clozapine trigger extreme neuroleptic reactions in some patients, similar to what occurs with other second-generation antipsychotics.21 Another case series provides examples of the drug’s efficacy in treating hallucinations and delusions with minimal adverse effects.22
It is important to emphasize that Ms. L’s hallucinations did not go away; rather, they changed to a more benign presentation that she could manage and, occasionally, found pleasant. Ultimately, her agitation—the primary target of treatment—improved markedly with the arrival of the knight in shining armor.
Treatment recommendations
If neuropsychiatric symptoms in DLB are the primary concern of the patient and family, we recommend the following:
- Begin treatment with a cholinesterase inhibitor. The best evidence exists for rivastigmine and donepezil. These drugs have a low risk of side effects, which are primarily gastrointestinal effects with some reports of worsening extrapyramidal symptoms.23-25
- If the patient obtains minimal benefit or develops a significant adverse effect from cholinesterase inhibitors, consider memantine. Its efficacy is under examination and results are mixed; it can be used in combination with cholinesterase inhibitors.26-28
- If psychotic symptoms are upsetting and refractory to other therapies, consider antipsychotics. Avoid first-generation antipsychotics. The American Psychiatric Association recommends aripiprazole or quetiapine initially, although there is little evidence comparing neuroleptics in DLB.29 Because of its risks, reserve clozapine for refractory cases. An exception might be made for patients sensitive to extrapyramidal effects, in whom clozapine could be considered earlier.
There are no formal neuroleptic dosing guidelines beyond a general urging towards minimalism. Mosimann and McKeith30 recommend clozapine, 12.5 mg/d; olanzapine, 2.5 mg/d; risperidone, 0.25 mg/d; or quetiapine, 12.5 mg/d. Such dosages might be effective while producing only minimal side effects.9,31
SSRIs and other antidepressants have not been shown to improve neuropsychiatric symptoms, and often are poorly tolerated.32
One study found efficacy with electroconvulsive therapy and transcranial magnetic stimulation in treatment-resistant patients.33
In addition to these treatments, nonpharmaceutical interventions should be employed from the earliest stages of diagnosis and treatment (Table 2). See the Figure for an algorithm for treating DLB. These include educational and behavioral interventions, social support, psychological interventions, and environmental therapies and modifications.
OUTCOME New friends
The creatures return from time to time, Ms. L reports, but are no longer upsetting because the white knight (a sort of mental deus ex machina) leads the once-terrifying things away. She describes the hallucination as a kind of zoological observation, refers to the creatures that once horrified her as “her friends,” and chuckles as she observes their natural history. This new, far more benign hallucination becomes a mainstay of her symptoms, and she is discharged to the care of her husband and family.
Soon after her discharge, her hallucinations resolved completely, but returned briefly when Ms. L resumed smoking cigarettes because smoking is known to lower clozapine serum levels.34
Bottom Line
Consider a low dosage of a neuroleptic when a patient suffers significant distress and behavioral disturbance related to psychotic symptoms in dementia with Lewy bodies and those problems are not relieved by other agents. Low-dose clozapine is an option for refractory psychotic symptoms or in patients with severe extrapyramidal sensitivity. Start low, and go slow.
Related Resources
- Bishnoi RJ, Grossberg GT, Manepalli J. Differentiating Alzheimer’s disease from dementia with Lewy bodies. Current Psychiatry. 2012;11(11):22-27.
- McKeith I, Emre M. Management of Parkinson’s disease dementia and dementia with Lewy bodies. In: Emre M, ed. Cognitive impairment and dementia in Parkinson’s disease. Oxford, United Kingdom: Oxford University Press; 2010:245-256.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Clozapine • Clozaril Olanzapine • Zyprexa
Donepezil • Aricept Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Memantine • Namenda Rivastigmine • Exelon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerized assessment system. Dement Geriatr Cogn Disord. 2002; 13(3):183-192.
2. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164(10):1491-1498.
3. McKeith IG, Wesnes KA, Perry E, et al. Hallucinations predict attentional improvements with rivastigmine in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;18(1):94-100.
4. Emre M, Tsolaki , Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
5. Aarsland D, Ballard C, Walker Z, et al. Clinical trials of dementia with Lewy bodies and Parkinson’s disease dementia. Curr Neurol Neurosci Rep. 2012;12(5):492-501.
6. Drach LM. Drug treatment of dementia with Lewy bodies and Parkinson’s disease dementia--common features and differences [in German]. Med Monatsschr Pharm. 2011; 34(2):47-52.
7. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol. 2007;17(3):165-171.
8. Marti MJ, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254(suppl 5):41-48.
9. French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson’s disease. Lancet. 1999;353(9169):2041-2042.
10. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15(2):201-211.
11. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
12. McKeith IG, Ballard CG, Perry RH et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54(5):1050-1058.
13. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(suppl 3):417-423.
15. Geser F, Wenning GK, Poewe W, et al. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. 2005;20(suppl 12):S11-20.
16. Latoo J, Jan F. Dementia with Lewy bodies: clinical review. British Journal of Medical Practioners. 2008;1(1):10-14.
17. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333-341.
18. Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. SIC Task Force Appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467-486.
19. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
20. Clozapine Monitoring Guidelines. 2008. http://www.clozapineregistry.com/resuming_treatment_after_interruption.pdf.ashx. Accessed October 31, 2013.
21. Burke WJ, Pfeiffer RF, McComb RD. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 1998;10(2):227-229.
22. Chacko RC, Hurley RA, Jankovic J. Clozapine use in diffuse Lewy body disease. J Neuropsychiatry Clin Neurosci. 1993;5(2):206-208.
23. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036
24. Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investiagors. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled trial. Ann Neurol. 2012; 72(1):41-52.
25. Ukai K, Aleksic B, Ishihara R, et al. Efficacy of donepezil for the treatment of visual and multiple sensory hallucinations in dementia with Lewy bodies. Clinical Neuropsychopharmacology and Therapeutics. 2011;2:56-58.
26. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
27. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-284.
28. Mathys ML, McCarrell J, Sleeper RB, et al. Visual hallucinations treated with the reinitiation of memantine in a patient with Lewy body dementia. Ann Pharmacother. 2013;47(2):e10.
29. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed November 1, 2013.
30. Mosimann U, McKeith IG. Dementia with lewy bodies—diagnosis and treatment. Swiss Med Wkly. 2003;133:131-142.
31. Baskys A, Davis P Atypical antipsychotic quetiapine in the treatment of the psychosis associated with Lewy body dementia. Neurobiol Aging. 2002;23:S63.
32. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24(4):306-364.
33. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
34. van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169-172.
CASE Strange creatures
Ms. L, age 78, is admitted to the inpatient unit for treatment of psychosis and behavioral changes. In the months before this admission, she had visited the emergency room several times for recurrent falls. CT scans of the head show no acute changes; brain and spinal MRI reveal evidence of chronic white matter disease and degenerative changes of the spine. Medical workup is unremarkable and includes evaluation for syncope and ambulation impairments related to degenerative disease of the hip joints.
Ms. L and her family are instructed to follow-up with her primary care physician and a neurologist for neuromuscular workup.
She next presents to her primary care physician, describing hallucinations of strangers walking around her house. Over a few weeks, hallucinations expand to include a fixed hallucination of creatures that she describes as having qualities of insects and plants, “piling up” around her. She describes tactile hallucinations of these creatures crawling on her skin, and she tracks their movements around her. She complains of vivid visual hallucinations of these creatures spinning webs across the room and she says she keeps the lights on at night. Ms. L becomes anxious and depressed, and her insomnia becomes worse.
She is referred for outpatient psychiatric evaluation and treatment.
Ms. L’s family notes lapses of short-term memory, disorganization, and difficulty with tasks such as cooking because she has trouble following steps. These deficits come and go, with periods when she is functional and others during which she experiences considerable confusion. The family is uncertain when these signs and symptoms first appeared, but are clear that these deficits are having an impact on her day-to-day life. She can conduct activities of daily living, but with increasing difficulty—and only with help from her husband for tasks that require complex order and movement.
Over several months, Ms. L’s gait stability decreases and she begins to rely on a walker to keep from falling. On the Montreal Cognitive Assessment screening for cognitive dysfunction, she scores 19 out of 30 (normal range >25). This suggests cognitive impairment greater than expected for her age, compared with normal controls, and, when coupled with her functional impairment, raises the possibility of a diagnosis of dementia with Lewy bodies (DLB).
a) donepezil
b) memantine
c) quetiapine
d) low-dose clozapine
The authors’ observations
Limited literature exists of placebo-controlled, large-scale studies on DLB treatment. Cholinesterase inhibitors have shown some symptomatic benefit, including for hallucinations.1-3 Memantine, an N-methyl-d-aspartate receptor blocker, shows mixed results.4 Many studies explore the use of neuroleptics for treating hallucinations in psychosis in Parkinson’s disease and Parkinson’s disease dementia (PDD) but, in DLB, the literature primarily consists of case reports.2 Much of DLB treatment is inferred and intermixed with studies on PDD.5,6
Low-dose clozapine has become a standard treatment for psychosis in Parkinson’s disease based on the findings of several trials.6 Despite its side-effect profile, clozapine has been shown to ameliorate hallucinations in PDD without exacerbating parkinsonian symptoms,7,8 and is the only medication with proven efficacy in PDD.2 The French Clozapine Parkinson Study Group demonstrated relief of psychotic symptoms of Parkinson’s disease with clozapine, 6.25 mg/d.9 The Clozapine Study Group found complete resolution of hallucinations in some patients within 1 day of initiating clozapine. Among patients in this study who did not see immediate benefit, most showed significant improvement of psychotic symptoms in 1 or 2 weeks.10
TREATMENT Few options
Ms. L’s psychiatrist and primary care physician start her on a series of medications. Donepezil is initiated for suspected dementia. We begin a trial of quetiapine to address the hallucinations, but the drug makes her movement symptoms worse. Risperidone also is tried but, again, the drugs make movement symptoms, particularly gait instability, tremor, and rigidity worse without alleviating the hallucinations. Neuroleptics seem to exacerbate confusion. Because of worsening depressive symptoms and our concern over possible pseudodementia, we try several selective serotonin reuptake inhibitors (SSRIs) and mirtazapine. Antidepressants have little effect on her depressive symptoms and do not improve hallucinations or insomnia.
Ms. L’s signs and symptoms become worse over the next few months, with more severe hallucinations, agitation, insomnia, and gait instability. Her agitation over the hallucinations increases and she begins pouring bleach around herself in bed and spraying her house with toxic bug spray. Ms. L’s family brings her to the hospital after they observe her scratching the hallucinatory creatures off of her skin with a razor blade and trying to pry them out of her mouth with a piece of metal.
In the hospital, medical and neurologic workups rule out organic causes for her symptoms and signs. MRI is consistent with imaging from 6 months earlier. Focal neurologic signs are absent. Blood work is within normal limits, failing to reveal any pathology that would suggest a cause for her symptoms and signs, such as syphilis, vitamin deficiency, and Lyme disease.
Ms. L’s symptoms were consistent with consensus guideline criteria for a clinical diagnosis of DLB (Table 1).11-18
She is started on low-dose quetiapine, which she tolerates poorly with worsening confusion, rigidity, tremor, and gait instability. Because other agents failed, Ms. L’s providers and family decide on a trial of clozapine.
Within 24 hours after the first dose of clozapine, 25 mg, sleep improves, the tactile component of hallucinations diminish, and she begins to spend increasing periods of time “observing the creatures” rather than fighting with them.
Over the next few days, Ms. L’s attitude towards the creatures changes. Now, as she sits observing them intently, the hallucinations evolve: rather than tormenting her and causing distress, the plant-creatures burst apart and a miniature knight on horseback charges out. The rest of the creatures then gather into a rank and file and the knight leads them to the nearest exit.
Clozapine is titrated to 50 mg/d, which she tolerates well without exacerbation of cognitive symptoms or movement disorder. The only notable adverse effect at the time of her discharge is sialorrhea.
with an antipsychotic?
a) start low and go slow
b) monitor her heart rate and blood pressure
c) readminister the Montreal Cognitive Assessment
d) all of the above
The authors’ observations
Ideally, in psychosis, antipsychotics eliminate positive symptoms such as hallucinations and delusions. In DLB, the aim is to alleviate the agitation and suffering brought on by the psychotic symptoms without exacerbating other motor and cognitive symptoms. The hallucinations are obstinate, and it is a well-known quality of this disorder that patients are exceptionally susceptible to a range of antipsychotic side effects including cognitive impairment, fatigue, neuroleptic malignant syndrome, and parkinsonism.19
Treatment in DLB requires trial and error, and medications with fewer associated risks should be administered first. Patients with DLB treated with neuroleptics have an increased risk of death compared with those who are not treated.19 Moreover, prescribing information for clozapine includes a black-box warning that the drug:
- is not approved for dementia-related psychosis and
- is associated with an increased risk of death in elderly patients with these conditions, similar to what is seen with other neuroleptics.20
Despite these well-known concerns, it remains difficult for clinicians not to try to treat the distress caused by these symptoms.
We chose clozapine for Ms. L because:
- other neuroleptics failed
- acetylcholinesterase inhibitors did not alleviate Ms. L’s psychosis and associated behavioral disturbance
- there is substantial evidence that the drug can be effective in Parkinson’s disease with psychosis.
There is controversy regarding use of clozapine in DLB. In one case series, clozapine trigger extreme neuroleptic reactions in some patients, similar to what occurs with other second-generation antipsychotics.21 Another case series provides examples of the drug’s efficacy in treating hallucinations and delusions with minimal adverse effects.22
It is important to emphasize that Ms. L’s hallucinations did not go away; rather, they changed to a more benign presentation that she could manage and, occasionally, found pleasant. Ultimately, her agitation—the primary target of treatment—improved markedly with the arrival of the knight in shining armor.
Treatment recommendations
If neuropsychiatric symptoms in DLB are the primary concern of the patient and family, we recommend the following:
- Begin treatment with a cholinesterase inhibitor. The best evidence exists for rivastigmine and donepezil. These drugs have a low risk of side effects, which are primarily gastrointestinal effects with some reports of worsening extrapyramidal symptoms.23-25
- If the patient obtains minimal benefit or develops a significant adverse effect from cholinesterase inhibitors, consider memantine. Its efficacy is under examination and results are mixed; it can be used in combination with cholinesterase inhibitors.26-28
- If psychotic symptoms are upsetting and refractory to other therapies, consider antipsychotics. Avoid first-generation antipsychotics. The American Psychiatric Association recommends aripiprazole or quetiapine initially, although there is little evidence comparing neuroleptics in DLB.29 Because of its risks, reserve clozapine for refractory cases. An exception might be made for patients sensitive to extrapyramidal effects, in whom clozapine could be considered earlier.
There are no formal neuroleptic dosing guidelines beyond a general urging towards minimalism. Mosimann and McKeith30 recommend clozapine, 12.5 mg/d; olanzapine, 2.5 mg/d; risperidone, 0.25 mg/d; or quetiapine, 12.5 mg/d. Such dosages might be effective while producing only minimal side effects.9,31
SSRIs and other antidepressants have not been shown to improve neuropsychiatric symptoms, and often are poorly tolerated.32
One study found efficacy with electroconvulsive therapy and transcranial magnetic stimulation in treatment-resistant patients.33
In addition to these treatments, nonpharmaceutical interventions should be employed from the earliest stages of diagnosis and treatment (Table 2). See the Figure for an algorithm for treating DLB. These include educational and behavioral interventions, social support, psychological interventions, and environmental therapies and modifications.
OUTCOME New friends
The creatures return from time to time, Ms. L reports, but are no longer upsetting because the white knight (a sort of mental deus ex machina) leads the once-terrifying things away. She describes the hallucination as a kind of zoological observation, refers to the creatures that once horrified her as “her friends,” and chuckles as she observes their natural history. This new, far more benign hallucination becomes a mainstay of her symptoms, and she is discharged to the care of her husband and family.
Soon after her discharge, her hallucinations resolved completely, but returned briefly when Ms. L resumed smoking cigarettes because smoking is known to lower clozapine serum levels.34
Bottom Line
Consider a low dosage of a neuroleptic when a patient suffers significant distress and behavioral disturbance related to psychotic symptoms in dementia with Lewy bodies and those problems are not relieved by other agents. Low-dose clozapine is an option for refractory psychotic symptoms or in patients with severe extrapyramidal sensitivity. Start low, and go slow.
Related Resources
- Bishnoi RJ, Grossberg GT, Manepalli J. Differentiating Alzheimer’s disease from dementia with Lewy bodies. Current Psychiatry. 2012;11(11):22-27.
- McKeith I, Emre M. Management of Parkinson’s disease dementia and dementia with Lewy bodies. In: Emre M, ed. Cognitive impairment and dementia in Parkinson’s disease. Oxford, United Kingdom: Oxford University Press; 2010:245-256.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Clozapine • Clozaril Olanzapine • Zyprexa
Donepezil • Aricept Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Memantine • Namenda Rivastigmine • Exelon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Strange creatures
Ms. L, age 78, is admitted to the inpatient unit for treatment of psychosis and behavioral changes. In the months before this admission, she had visited the emergency room several times for recurrent falls. CT scans of the head show no acute changes; brain and spinal MRI reveal evidence of chronic white matter disease and degenerative changes of the spine. Medical workup is unremarkable and includes evaluation for syncope and ambulation impairments related to degenerative disease of the hip joints.
Ms. L and her family are instructed to follow-up with her primary care physician and a neurologist for neuromuscular workup.
She next presents to her primary care physician, describing hallucinations of strangers walking around her house. Over a few weeks, hallucinations expand to include a fixed hallucination of creatures that she describes as having qualities of insects and plants, “piling up” around her. She describes tactile hallucinations of these creatures crawling on her skin, and she tracks their movements around her. She complains of vivid visual hallucinations of these creatures spinning webs across the room and she says she keeps the lights on at night. Ms. L becomes anxious and depressed, and her insomnia becomes worse.
She is referred for outpatient psychiatric evaluation and treatment.
Ms. L’s family notes lapses of short-term memory, disorganization, and difficulty with tasks such as cooking because she has trouble following steps. These deficits come and go, with periods when she is functional and others during which she experiences considerable confusion. The family is uncertain when these signs and symptoms first appeared, but are clear that these deficits are having an impact on her day-to-day life. She can conduct activities of daily living, but with increasing difficulty—and only with help from her husband for tasks that require complex order and movement.
Over several months, Ms. L’s gait stability decreases and she begins to rely on a walker to keep from falling. On the Montreal Cognitive Assessment screening for cognitive dysfunction, she scores 19 out of 30 (normal range >25). This suggests cognitive impairment greater than expected for her age, compared with normal controls, and, when coupled with her functional impairment, raises the possibility of a diagnosis of dementia with Lewy bodies (DLB).
a) donepezil
b) memantine
c) quetiapine
d) low-dose clozapine
The authors’ observations
Limited literature exists of placebo-controlled, large-scale studies on DLB treatment. Cholinesterase inhibitors have shown some symptomatic benefit, including for hallucinations.1-3 Memantine, an N-methyl-d-aspartate receptor blocker, shows mixed results.4 Many studies explore the use of neuroleptics for treating hallucinations in psychosis in Parkinson’s disease and Parkinson’s disease dementia (PDD) but, in DLB, the literature primarily consists of case reports.2 Much of DLB treatment is inferred and intermixed with studies on PDD.5,6
Low-dose clozapine has become a standard treatment for psychosis in Parkinson’s disease based on the findings of several trials.6 Despite its side-effect profile, clozapine has been shown to ameliorate hallucinations in PDD without exacerbating parkinsonian symptoms,7,8 and is the only medication with proven efficacy in PDD.2 The French Clozapine Parkinson Study Group demonstrated relief of psychotic symptoms of Parkinson’s disease with clozapine, 6.25 mg/d.9 The Clozapine Study Group found complete resolution of hallucinations in some patients within 1 day of initiating clozapine. Among patients in this study who did not see immediate benefit, most showed significant improvement of psychotic symptoms in 1 or 2 weeks.10
TREATMENT Few options
Ms. L’s psychiatrist and primary care physician start her on a series of medications. Donepezil is initiated for suspected dementia. We begin a trial of quetiapine to address the hallucinations, but the drug makes her movement symptoms worse. Risperidone also is tried but, again, the drugs make movement symptoms, particularly gait instability, tremor, and rigidity worse without alleviating the hallucinations. Neuroleptics seem to exacerbate confusion. Because of worsening depressive symptoms and our concern over possible pseudodementia, we try several selective serotonin reuptake inhibitors (SSRIs) and mirtazapine. Antidepressants have little effect on her depressive symptoms and do not improve hallucinations or insomnia.
Ms. L’s signs and symptoms become worse over the next few months, with more severe hallucinations, agitation, insomnia, and gait instability. Her agitation over the hallucinations increases and she begins pouring bleach around herself in bed and spraying her house with toxic bug spray. Ms. L’s family brings her to the hospital after they observe her scratching the hallucinatory creatures off of her skin with a razor blade and trying to pry them out of her mouth with a piece of metal.
In the hospital, medical and neurologic workups rule out organic causes for her symptoms and signs. MRI is consistent with imaging from 6 months earlier. Focal neurologic signs are absent. Blood work is within normal limits, failing to reveal any pathology that would suggest a cause for her symptoms and signs, such as syphilis, vitamin deficiency, and Lyme disease.
Ms. L’s symptoms were consistent with consensus guideline criteria for a clinical diagnosis of DLB (Table 1).11-18
She is started on low-dose quetiapine, which she tolerates poorly with worsening confusion, rigidity, tremor, and gait instability. Because other agents failed, Ms. L’s providers and family decide on a trial of clozapine.
Within 24 hours after the first dose of clozapine, 25 mg, sleep improves, the tactile component of hallucinations diminish, and she begins to spend increasing periods of time “observing the creatures” rather than fighting with them.
Over the next few days, Ms. L’s attitude towards the creatures changes. Now, as she sits observing them intently, the hallucinations evolve: rather than tormenting her and causing distress, the plant-creatures burst apart and a miniature knight on horseback charges out. The rest of the creatures then gather into a rank and file and the knight leads them to the nearest exit.
Clozapine is titrated to 50 mg/d, which she tolerates well without exacerbation of cognitive symptoms or movement disorder. The only notable adverse effect at the time of her discharge is sialorrhea.
with an antipsychotic?
a) start low and go slow
b) monitor her heart rate and blood pressure
c) readminister the Montreal Cognitive Assessment
d) all of the above
The authors’ observations
Ideally, in psychosis, antipsychotics eliminate positive symptoms such as hallucinations and delusions. In DLB, the aim is to alleviate the agitation and suffering brought on by the psychotic symptoms without exacerbating other motor and cognitive symptoms. The hallucinations are obstinate, and it is a well-known quality of this disorder that patients are exceptionally susceptible to a range of antipsychotic side effects including cognitive impairment, fatigue, neuroleptic malignant syndrome, and parkinsonism.19
Treatment in DLB requires trial and error, and medications with fewer associated risks should be administered first. Patients with DLB treated with neuroleptics have an increased risk of death compared with those who are not treated.19 Moreover, prescribing information for clozapine includes a black-box warning that the drug:
- is not approved for dementia-related psychosis and
- is associated with an increased risk of death in elderly patients with these conditions, similar to what is seen with other neuroleptics.20
Despite these well-known concerns, it remains difficult for clinicians not to try to treat the distress caused by these symptoms.
We chose clozapine for Ms. L because:
- other neuroleptics failed
- acetylcholinesterase inhibitors did not alleviate Ms. L’s psychosis and associated behavioral disturbance
- there is substantial evidence that the drug can be effective in Parkinson’s disease with psychosis.
There is controversy regarding use of clozapine in DLB. In one case series, clozapine trigger extreme neuroleptic reactions in some patients, similar to what occurs with other second-generation antipsychotics.21 Another case series provides examples of the drug’s efficacy in treating hallucinations and delusions with minimal adverse effects.22
It is important to emphasize that Ms. L’s hallucinations did not go away; rather, they changed to a more benign presentation that she could manage and, occasionally, found pleasant. Ultimately, her agitation—the primary target of treatment—improved markedly with the arrival of the knight in shining armor.
Treatment recommendations
If neuropsychiatric symptoms in DLB are the primary concern of the patient and family, we recommend the following:
- Begin treatment with a cholinesterase inhibitor. The best evidence exists for rivastigmine and donepezil. These drugs have a low risk of side effects, which are primarily gastrointestinal effects with some reports of worsening extrapyramidal symptoms.23-25
- If the patient obtains minimal benefit or develops a significant adverse effect from cholinesterase inhibitors, consider memantine. Its efficacy is under examination and results are mixed; it can be used in combination with cholinesterase inhibitors.26-28
- If psychotic symptoms are upsetting and refractory to other therapies, consider antipsychotics. Avoid first-generation antipsychotics. The American Psychiatric Association recommends aripiprazole or quetiapine initially, although there is little evidence comparing neuroleptics in DLB.29 Because of its risks, reserve clozapine for refractory cases. An exception might be made for patients sensitive to extrapyramidal effects, in whom clozapine could be considered earlier.
There are no formal neuroleptic dosing guidelines beyond a general urging towards minimalism. Mosimann and McKeith30 recommend clozapine, 12.5 mg/d; olanzapine, 2.5 mg/d; risperidone, 0.25 mg/d; or quetiapine, 12.5 mg/d. Such dosages might be effective while producing only minimal side effects.9,31
SSRIs and other antidepressants have not been shown to improve neuropsychiatric symptoms, and often are poorly tolerated.32
One study found efficacy with electroconvulsive therapy and transcranial magnetic stimulation in treatment-resistant patients.33
In addition to these treatments, nonpharmaceutical interventions should be employed from the earliest stages of diagnosis and treatment (Table 2). See the Figure for an algorithm for treating DLB. These include educational and behavioral interventions, social support, psychological interventions, and environmental therapies and modifications.
OUTCOME New friends
The creatures return from time to time, Ms. L reports, but are no longer upsetting because the white knight (a sort of mental deus ex machina) leads the once-terrifying things away. She describes the hallucination as a kind of zoological observation, refers to the creatures that once horrified her as “her friends,” and chuckles as she observes their natural history. This new, far more benign hallucination becomes a mainstay of her symptoms, and she is discharged to the care of her husband and family.
Soon after her discharge, her hallucinations resolved completely, but returned briefly when Ms. L resumed smoking cigarettes because smoking is known to lower clozapine serum levels.34
Bottom Line
Consider a low dosage of a neuroleptic when a patient suffers significant distress and behavioral disturbance related to psychotic symptoms in dementia with Lewy bodies and those problems are not relieved by other agents. Low-dose clozapine is an option for refractory psychotic symptoms or in patients with severe extrapyramidal sensitivity. Start low, and go slow.
Related Resources
- Bishnoi RJ, Grossberg GT, Manepalli J. Differentiating Alzheimer’s disease from dementia with Lewy bodies. Current Psychiatry. 2012;11(11):22-27.
- McKeith I, Emre M. Management of Parkinson’s disease dementia and dementia with Lewy bodies. In: Emre M, ed. Cognitive impairment and dementia in Parkinson’s disease. Oxford, United Kingdom: Oxford University Press; 2010:245-256.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Clozapine • Clozaril Olanzapine • Zyprexa
Donepezil • Aricept Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Memantine • Namenda Rivastigmine • Exelon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerized assessment system. Dement Geriatr Cogn Disord. 2002; 13(3):183-192.
2. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164(10):1491-1498.
3. McKeith IG, Wesnes KA, Perry E, et al. Hallucinations predict attentional improvements with rivastigmine in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;18(1):94-100.
4. Emre M, Tsolaki , Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
5. Aarsland D, Ballard C, Walker Z, et al. Clinical trials of dementia with Lewy bodies and Parkinson’s disease dementia. Curr Neurol Neurosci Rep. 2012;12(5):492-501.
6. Drach LM. Drug treatment of dementia with Lewy bodies and Parkinson’s disease dementia--common features and differences [in German]. Med Monatsschr Pharm. 2011; 34(2):47-52.
7. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol. 2007;17(3):165-171.
8. Marti MJ, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254(suppl 5):41-48.
9. French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson’s disease. Lancet. 1999;353(9169):2041-2042.
10. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15(2):201-211.
11. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
12. McKeith IG, Ballard CG, Perry RH et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54(5):1050-1058.
13. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(suppl 3):417-423.
15. Geser F, Wenning GK, Poewe W, et al. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. 2005;20(suppl 12):S11-20.
16. Latoo J, Jan F. Dementia with Lewy bodies: clinical review. British Journal of Medical Practioners. 2008;1(1):10-14.
17. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333-341.
18. Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. SIC Task Force Appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467-486.
19. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
20. Clozapine Monitoring Guidelines. 2008. http://www.clozapineregistry.com/resuming_treatment_after_interruption.pdf.ashx. Accessed October 31, 2013.
21. Burke WJ, Pfeiffer RF, McComb RD. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 1998;10(2):227-229.
22. Chacko RC, Hurley RA, Jankovic J. Clozapine use in diffuse Lewy body disease. J Neuropsychiatry Clin Neurosci. 1993;5(2):206-208.
23. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036
24. Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investiagors. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled trial. Ann Neurol. 2012; 72(1):41-52.
25. Ukai K, Aleksic B, Ishihara R, et al. Efficacy of donepezil for the treatment of visual and multiple sensory hallucinations in dementia with Lewy bodies. Clinical Neuropsychopharmacology and Therapeutics. 2011;2:56-58.
26. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
27. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-284.
28. Mathys ML, McCarrell J, Sleeper RB, et al. Visual hallucinations treated with the reinitiation of memantine in a patient with Lewy body dementia. Ann Pharmacother. 2013;47(2):e10.
29. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed November 1, 2013.
30. Mosimann U, McKeith IG. Dementia with lewy bodies—diagnosis and treatment. Swiss Med Wkly. 2003;133:131-142.
31. Baskys A, Davis P Atypical antipsychotic quetiapine in the treatment of the psychosis associated with Lewy body dementia. Neurobiol Aging. 2002;23:S63.
32. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24(4):306-364.
33. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
34. van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169-172.
1. Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the Cognitive Drug Research computerized assessment system. Dement Geriatr Cogn Disord. 2002; 13(3):183-192.
2. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164(10):1491-1498.
3. McKeith IG, Wesnes KA, Perry E, et al. Hallucinations predict attentional improvements with rivastigmine in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;18(1):94-100.
4. Emre M, Tsolaki , Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
5. Aarsland D, Ballard C, Walker Z, et al. Clinical trials of dementia with Lewy bodies and Parkinson’s disease dementia. Curr Neurol Neurosci Rep. 2012;12(5):492-501.
6. Drach LM. Drug treatment of dementia with Lewy bodies and Parkinson’s disease dementia--common features and differences [in German]. Med Monatsschr Pharm. 2011; 34(2):47-52.
7. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol. 2007;17(3):165-171.
8. Marti MJ, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254(suppl 5):41-48.
9. French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson’s disease. Lancet. 1999;353(9169):2041-2042.
10. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord. 2000;15(2):201-211.
11. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
12. McKeith IG, Ballard CG, Perry RH et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54(5):1050-1058.
13. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(suppl 3):417-423.
15. Geser F, Wenning GK, Poewe W, et al. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. 2005;20(suppl 12):S11-20.
16. Latoo J, Jan F. Dementia with Lewy bodies: clinical review. British Journal of Medical Practioners. 2008;1(1):10-14.
17. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333-341.
18. Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. SIC Task Force Appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18(5):467-486.
19. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
20. Clozapine Monitoring Guidelines. 2008. http://www.clozapineregistry.com/resuming_treatment_after_interruption.pdf.ashx. Accessed October 31, 2013.
21. Burke WJ, Pfeiffer RF, McComb RD. Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 1998;10(2):227-229.
22. Chacko RC, Hurley RA, Jankovic J. Clozapine use in diffuse Lewy body disease. J Neuropsychiatry Clin Neurosci. 1993;5(2):206-208.
23. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036
24. Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investiagors. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled trial. Ann Neurol. 2012; 72(1):41-52.
25. Ukai K, Aleksic B, Ishihara R, et al. Efficacy of donepezil for the treatment of visual and multiple sensory hallucinations in dementia with Lewy bodies. Clinical Neuropsychopharmacology and Therapeutics. 2011;2:56-58.
26. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
27. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281-284.
28. Mathys ML, McCarrell J, Sleeper RB, et al. Visual hallucinations treated with the reinitiation of memantine in a patient with Lewy body dementia. Ann Pharmacother. 2013;47(2):e10.
29. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd ed. http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243205&PDFSource=6. Accessed November 1, 2013.
30. Mosimann U, McKeith IG. Dementia with lewy bodies—diagnosis and treatment. Swiss Med Wkly. 2003;133:131-142.
31. Baskys A, Davis P Atypical antipsychotic quetiapine in the treatment of the psychosis associated with Lewy body dementia. Neurobiol Aging. 2002;23:S63.
32. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24(4):306-364.
33. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
34. van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169-172.
Are you admitting malpractice if you apologize to a patient?
Dear Dr. Mossman:
Recently, my prescribing error caused a patient to get very sick. I feel terrible. I want to tell my patient I’m sorry, but I’ve heard that a lawyer could use my “confession” to prove I’ve committed malpractice. If I apologize, could my words come back to haunt me if a lawsuit is filed?
Submitted by “Dr. E”
As several faiths have long recognized, apologies are important social acts that express our awareness of and obligations to each other. In recent years, psychologists have established how apologies confer emotional benefits on those who give and receive them.1 Offering a sincere apology can be the right thing to do and a beneficial act for both the apologizing and the injured parties.
Traditionally, physicians have avoided apologizing for errors that harmed patients. Part of the reluctance stemmed from pride or wanting to avoid shame. But as Dr. E’s question suggests, doctors also have feared—and lawyers have advised—that apologizing might compromise a malpractice defense.2
Attitudes have changed in recent years, however. Increasingly, practitioners, medical organizations, and risk management entities are telling physicians they should apologize for errors, and many states have laws that mitigate adverse legal consequences of saying “I’m sorry.”
In response to Dr. E’s questions, I’ll examine:
• ethical and professional obligations following unexpected outcomes
• physicians’ reasons for being reluctant to apologize
• the benefits of apologizing
• legal protections for apologies.
Owning up: Ethical and professional expectations
Current codes of medical ethics say that physicians should tell patients when mistakes and misjudgments have caused harm. “It is a fundamental ethical requirement that a physician should at all times deal honestly and openly with patients,” states the American Medical Association’s Code of Ethics. When a patient suffers because of a medical error, “the physician is ethically required to inform the patient of all the facts necessary to ensure” the patient can “make informed decisions regarding future medical care.”3
The National Patient Safety Foundation,4 the American College of Physicians,5 and the Joint Commission (the agency that provides official accreditation of thousands of healthcare organizations) have voiced similar positions for years. Since 2001, the Joint Commission has required that practitioners and medical facilities tell patients and families “about the outcomes of care, treatment, and services ... including unanticipated outcomes.”6
Reluctance is understandable
Although these recommendations and policies suggest that telling patients about medical errors is an established professional expectation, physicians remain reluctant to apologize to patients for emotional and legal reasons that are easy to understand.
Apologizing is hard. On one hand, research shows that refusing to apologize sometimes increases feelings of empowerment and control, and can boost self-esteem more than apologizing does.7 On the other, apologizing often requires one to acknowledge a failure or betrayal of trust and to experience guilt, shame, embarrassment, or fear that one’s apology will be met with anger or rejection.8
Physicians historically have treated errors as personal failures. Apologizing in a medical context can feel like saying, “I am incompetent.”9,10 The law has reinforced this attitude. As the Mississippi Supreme Court put it, “Medical malpractice is legal fault by a physician or surgeon. It arises from the failure of a physician to provide the quality of care required by law” (emphasis added).11
Some lawyers continue to advise physicians not to make admissions that could be used in a malpractice case. Their reasoning: If a doctor does something that adversely affects a malpractice insurer’s ability to defend the case, the insurer might not provide liability coverage for the adverse event.12
Emotional and legal benefits
Against this no-apology stance is a growing body of theoretical, empirical, and practical arguments favoring apologies for medical errors. Case studies suggest that anger is behind much medical malpractice litigation and that physicians’ apologies—which reduce anger and increase communication—might reduce patients’ motivations to sue.13 Apologies sometimes lead to forgiveness, an emotional state that “can provide victims and offenders with many important benefits, including enhanced psychological well-being ... and greater physiological health.”14 Apologies do this by mitigating the injured party’s negative emotional states and diminishing rumination about the transgression and perceived harm severity.
The practical argument favoring apologizing is that it may defuse feelings that lead to lawsuits and reduce the size of payouts. Experimental studies suggest that apologizing leads to earlier satisfaction and closure, faster settlements, and lower damage payments. When apologies include admissions of fault, injured parties feel greater respect for and less need to punish those who have harmed them, are more willing to forgive, and are more likely to accept settlement offers.15
Hospitals in Pennsylvania, Kentucky, and Michigan have found that sincere apologies and effective error disclosure programs reduce malpractice payouts and lead to faster settlements.16 As some plaintiffs’ lawyers point out, being honest and forthright and fixing the injured parties’ problems can quickly defuse a lawsuit. One attorney explained things this way: “We never sue the nice, contrite doctors. Their patients never call our offices. But the doctors who are poor communicators and abandon their patients get sued all the time. Their patients come to our offices looking for answers.”17
Apology laws: Protection from your own words
The belief that apologies by physicians can help patients emotionally and reduce malpractice litigation has led state legislatures to enact so-called apology laws in many jurisdictions in the United States.18 The general point of these rules and statutes is to prevent later use of doctors’ words in litigation. States differ substantially in the scope and type of protection that their laws offer. Some states prohibit doctors’ apologies for adverse outcomes from being used in litigation to prove negligence, while others only exclude expressions of sympathy or offers to pay for corrective treatment. Selected language from several states’ apology laws appears in the Table.19-23
Do apology laws work? Recent research by economists Ho and Liu indicates that they do. Comparing payouts in states with and without apology laws, they conclude that “apology laws have the greatest reduction in average payment size and settlement time in cases involving more severe patient outcomes,”13 such as obstetrics and anesthesia cases, cases that involve infants, and cases in which physicians improperly manage or fail to properly diagnose an illness.24
The practical impact of apologizing for psychiatric malpractice cases is unclear, but forensic psychiatrists Marilyn Price and Patricia Recupero believe that, following some unexpected outcomes, thoughtful expressions of sympathy, regret, and—if the outcome resulted from an error—apologies may be appropriate. Price and Recupero caution that such conversations should occur as part of broader programs that investigate unanticipated adverse events and provide education and coaching about appropriate ways to make disclosures. Clinicians also should consult with legal counsel, risk management officers, and liability insurance carriers before initiating such disclosures.25
Bottom Line
Apologizing for medical errors may mitigate malpractice liability and can help injured parties and physicians feel better. Whether plaintiffs can use apologies as evidence of malpractice depends on state laws and rules of evidence. Before you apologize for an unanticipated outcome, discuss the situation with your legal counsel, risk management officers, and insurers.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Douglas Mossman, MD, talks about who you should consult before apologizing to a patient for a bad outcome. Dr. Mossman is Professor of Clinical Psychiatry and Director, Division of Forensic Psychiatry, University of Cincinnati College of Medicine, Cincinnati, Ohio.
1. McCullough ME, Sandage SJ, Brown SW, et al. Interpersonal forgiving in close relationships: II. Theoretical elaboration and measurement. J Pers Soc Psychol. 1998;75:1586-1603.
2. O’Reilly KB. “I’m sorry”: why is that so hard for doctors to say? http://www.amednews.com/article/20100201/profession/302019937/4. Published February 1, 2010. Accessed September 30, 2013.
3. American Medical Association. AMA Code of Medical Ethics, Opinion 8.12 – Patient information. http://www.ama-assn.org//ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion812.page. Published June 1994. Accessed September 30, 2013.
4. Hickson GB, Pichert JW. Disclosure and apology. http://www.npsf.org/wp-content/uploads/2011/10/RG_SUPS_After_Mod1_Hickson.pdf. Accessed October 4, 2013.
5. Snyder L, American College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1, pt 2):73-104.
6. ECRI Institute. Disclosure of unanticipated outcomes. In: Healthcare risk control Supplement A, Risk analysis. Plymouth Meeting, PA: ECRI; 2002.
7. Okimoto TG, Wenzel M, Hedrick K. Refusing to apologize can have psychological benefits (and we issue no mea culpa for this research finding). Eur J Soc Psychol. 2013;43:22-31.
8. Lazare A. On apology. New York, NY: Oxford University Press; 2004.
9. Hilfiker D. Facing our mistakes. N Engl J Med. 1984;310:
118-122.
10. Leape LL. Error in medicine. JAMA. 1994;272:1851-1857.
11. Hall v. Hilbun, 466 So.2d 856 (Miss. 1985).
12. Kern SI. You continue to face exposure if you apologize. http://medicaleconomics.modernmedicine.com/medical-economics/news/modernmedicine/modern-medicine-now/you-continue-face-exposure-if-you-apologiz. Published September 24, 2010. Accessed October 1, 2013.
13. Ho B, Liu E. Does sorry work? The impact of apology laws on medical malpractice. J Risk Uncertain. 2011;43(2):141-167.
14. Fehr R, Gelfand MJ, Nag M. The road to forgiveness: a meta-analytic synthesis of its situational and dispositional correlates. Psychol Bull. 2010;136:894-914.
15. Robbennolt JK. Apologies and settlement. Court Review. 2009;45:90-97.
16. Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assoc. 2012;112(5):302-306.
17. Wojcieszak D, Banja J, Houk C. The sorry works! coalition: making the case for full disclosure. Jt Comm J Qual Patient Saf. 2006;32(6):344-350.
18. National Conference of State Legislatures. Medical liability/Medical malpractice laws. http://www.ncsl.org/issues-research/banking/medical-liability-medical-malpractice-laws.aspx. Published August 15, 2011. Accessed October 4, 2013.
19. Conn Gen Stat Ann §52-184d(b).
20. Fla Stat §90.4026(2).
21. Ill Comp Stat §5/8-1901.
22. NC Gen Stat §8C-1, Rule 413.
23. Tex. Civ. Prac. & Rem. Code §18.061.
24. Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empir Leg Stud. 2011;8:179-199.
25. Price M, Recupero PR. Risk management. In: Sharfstein SS, Dickerson FB, Oldham JM, eds. Textbook of hospital psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:411-412.
Dear Dr. Mossman:
Recently, my prescribing error caused a patient to get very sick. I feel terrible. I want to tell my patient I’m sorry, but I’ve heard that a lawyer could use my “confession” to prove I’ve committed malpractice. If I apologize, could my words come back to haunt me if a lawsuit is filed?
Submitted by “Dr. E”
As several faiths have long recognized, apologies are important social acts that express our awareness of and obligations to each other. In recent years, psychologists have established how apologies confer emotional benefits on those who give and receive them.1 Offering a sincere apology can be the right thing to do and a beneficial act for both the apologizing and the injured parties.
Traditionally, physicians have avoided apologizing for errors that harmed patients. Part of the reluctance stemmed from pride or wanting to avoid shame. But as Dr. E’s question suggests, doctors also have feared—and lawyers have advised—that apologizing might compromise a malpractice defense.2
Attitudes have changed in recent years, however. Increasingly, practitioners, medical organizations, and risk management entities are telling physicians they should apologize for errors, and many states have laws that mitigate adverse legal consequences of saying “I’m sorry.”
In response to Dr. E’s questions, I’ll examine:
• ethical and professional obligations following unexpected outcomes
• physicians’ reasons for being reluctant to apologize
• the benefits of apologizing
• legal protections for apologies.
Owning up: Ethical and professional expectations
Current codes of medical ethics say that physicians should tell patients when mistakes and misjudgments have caused harm. “It is a fundamental ethical requirement that a physician should at all times deal honestly and openly with patients,” states the American Medical Association’s Code of Ethics. When a patient suffers because of a medical error, “the physician is ethically required to inform the patient of all the facts necessary to ensure” the patient can “make informed decisions regarding future medical care.”3
The National Patient Safety Foundation,4 the American College of Physicians,5 and the Joint Commission (the agency that provides official accreditation of thousands of healthcare organizations) have voiced similar positions for years. Since 2001, the Joint Commission has required that practitioners and medical facilities tell patients and families “about the outcomes of care, treatment, and services ... including unanticipated outcomes.”6
Reluctance is understandable
Although these recommendations and policies suggest that telling patients about medical errors is an established professional expectation, physicians remain reluctant to apologize to patients for emotional and legal reasons that are easy to understand.
Apologizing is hard. On one hand, research shows that refusing to apologize sometimes increases feelings of empowerment and control, and can boost self-esteem more than apologizing does.7 On the other, apologizing often requires one to acknowledge a failure or betrayal of trust and to experience guilt, shame, embarrassment, or fear that one’s apology will be met with anger or rejection.8
Physicians historically have treated errors as personal failures. Apologizing in a medical context can feel like saying, “I am incompetent.”9,10 The law has reinforced this attitude. As the Mississippi Supreme Court put it, “Medical malpractice is legal fault by a physician or surgeon. It arises from the failure of a physician to provide the quality of care required by law” (emphasis added).11
Some lawyers continue to advise physicians not to make admissions that could be used in a malpractice case. Their reasoning: If a doctor does something that adversely affects a malpractice insurer’s ability to defend the case, the insurer might not provide liability coverage for the adverse event.12
Emotional and legal benefits
Against this no-apology stance is a growing body of theoretical, empirical, and practical arguments favoring apologies for medical errors. Case studies suggest that anger is behind much medical malpractice litigation and that physicians’ apologies—which reduce anger and increase communication—might reduce patients’ motivations to sue.13 Apologies sometimes lead to forgiveness, an emotional state that “can provide victims and offenders with many important benefits, including enhanced psychological well-being ... and greater physiological health.”14 Apologies do this by mitigating the injured party’s negative emotional states and diminishing rumination about the transgression and perceived harm severity.
The practical argument favoring apologizing is that it may defuse feelings that lead to lawsuits and reduce the size of payouts. Experimental studies suggest that apologizing leads to earlier satisfaction and closure, faster settlements, and lower damage payments. When apologies include admissions of fault, injured parties feel greater respect for and less need to punish those who have harmed them, are more willing to forgive, and are more likely to accept settlement offers.15
Hospitals in Pennsylvania, Kentucky, and Michigan have found that sincere apologies and effective error disclosure programs reduce malpractice payouts and lead to faster settlements.16 As some plaintiffs’ lawyers point out, being honest and forthright and fixing the injured parties’ problems can quickly defuse a lawsuit. One attorney explained things this way: “We never sue the nice, contrite doctors. Their patients never call our offices. But the doctors who are poor communicators and abandon their patients get sued all the time. Their patients come to our offices looking for answers.”17
Apology laws: Protection from your own words
The belief that apologies by physicians can help patients emotionally and reduce malpractice litigation has led state legislatures to enact so-called apology laws in many jurisdictions in the United States.18 The general point of these rules and statutes is to prevent later use of doctors’ words in litigation. States differ substantially in the scope and type of protection that their laws offer. Some states prohibit doctors’ apologies for adverse outcomes from being used in litigation to prove negligence, while others only exclude expressions of sympathy or offers to pay for corrective treatment. Selected language from several states’ apology laws appears in the Table.19-23
Do apology laws work? Recent research by economists Ho and Liu indicates that they do. Comparing payouts in states with and without apology laws, they conclude that “apology laws have the greatest reduction in average payment size and settlement time in cases involving more severe patient outcomes,”13 such as obstetrics and anesthesia cases, cases that involve infants, and cases in which physicians improperly manage or fail to properly diagnose an illness.24
The practical impact of apologizing for psychiatric malpractice cases is unclear, but forensic psychiatrists Marilyn Price and Patricia Recupero believe that, following some unexpected outcomes, thoughtful expressions of sympathy, regret, and—if the outcome resulted from an error—apologies may be appropriate. Price and Recupero caution that such conversations should occur as part of broader programs that investigate unanticipated adverse events and provide education and coaching about appropriate ways to make disclosures. Clinicians also should consult with legal counsel, risk management officers, and liability insurance carriers before initiating such disclosures.25
Bottom Line
Apologizing for medical errors may mitigate malpractice liability and can help injured parties and physicians feel better. Whether plaintiffs can use apologies as evidence of malpractice depends on state laws and rules of evidence. Before you apologize for an unanticipated outcome, discuss the situation with your legal counsel, risk management officers, and insurers.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Douglas Mossman, MD, talks about who you should consult before apologizing to a patient for a bad outcome. Dr. Mossman is Professor of Clinical Psychiatry and Director, Division of Forensic Psychiatry, University of Cincinnati College of Medicine, Cincinnati, Ohio.
Dear Dr. Mossman:
Recently, my prescribing error caused a patient to get very sick. I feel terrible. I want to tell my patient I’m sorry, but I’ve heard that a lawyer could use my “confession” to prove I’ve committed malpractice. If I apologize, could my words come back to haunt me if a lawsuit is filed?
Submitted by “Dr. E”
As several faiths have long recognized, apologies are important social acts that express our awareness of and obligations to each other. In recent years, psychologists have established how apologies confer emotional benefits on those who give and receive them.1 Offering a sincere apology can be the right thing to do and a beneficial act for both the apologizing and the injured parties.
Traditionally, physicians have avoided apologizing for errors that harmed patients. Part of the reluctance stemmed from pride or wanting to avoid shame. But as Dr. E’s question suggests, doctors also have feared—and lawyers have advised—that apologizing might compromise a malpractice defense.2
Attitudes have changed in recent years, however. Increasingly, practitioners, medical organizations, and risk management entities are telling physicians they should apologize for errors, and many states have laws that mitigate adverse legal consequences of saying “I’m sorry.”
In response to Dr. E’s questions, I’ll examine:
• ethical and professional obligations following unexpected outcomes
• physicians’ reasons for being reluctant to apologize
• the benefits of apologizing
• legal protections for apologies.
Owning up: Ethical and professional expectations
Current codes of medical ethics say that physicians should tell patients when mistakes and misjudgments have caused harm. “It is a fundamental ethical requirement that a physician should at all times deal honestly and openly with patients,” states the American Medical Association’s Code of Ethics. When a patient suffers because of a medical error, “the physician is ethically required to inform the patient of all the facts necessary to ensure” the patient can “make informed decisions regarding future medical care.”3
The National Patient Safety Foundation,4 the American College of Physicians,5 and the Joint Commission (the agency that provides official accreditation of thousands of healthcare organizations) have voiced similar positions for years. Since 2001, the Joint Commission has required that practitioners and medical facilities tell patients and families “about the outcomes of care, treatment, and services ... including unanticipated outcomes.”6
Reluctance is understandable
Although these recommendations and policies suggest that telling patients about medical errors is an established professional expectation, physicians remain reluctant to apologize to patients for emotional and legal reasons that are easy to understand.
Apologizing is hard. On one hand, research shows that refusing to apologize sometimes increases feelings of empowerment and control, and can boost self-esteem more than apologizing does.7 On the other, apologizing often requires one to acknowledge a failure or betrayal of trust and to experience guilt, shame, embarrassment, or fear that one’s apology will be met with anger or rejection.8
Physicians historically have treated errors as personal failures. Apologizing in a medical context can feel like saying, “I am incompetent.”9,10 The law has reinforced this attitude. As the Mississippi Supreme Court put it, “Medical malpractice is legal fault by a physician or surgeon. It arises from the failure of a physician to provide the quality of care required by law” (emphasis added).11
Some lawyers continue to advise physicians not to make admissions that could be used in a malpractice case. Their reasoning: If a doctor does something that adversely affects a malpractice insurer’s ability to defend the case, the insurer might not provide liability coverage for the adverse event.12
Emotional and legal benefits
Against this no-apology stance is a growing body of theoretical, empirical, and practical arguments favoring apologies for medical errors. Case studies suggest that anger is behind much medical malpractice litigation and that physicians’ apologies—which reduce anger and increase communication—might reduce patients’ motivations to sue.13 Apologies sometimes lead to forgiveness, an emotional state that “can provide victims and offenders with many important benefits, including enhanced psychological well-being ... and greater physiological health.”14 Apologies do this by mitigating the injured party’s negative emotional states and diminishing rumination about the transgression and perceived harm severity.
The practical argument favoring apologizing is that it may defuse feelings that lead to lawsuits and reduce the size of payouts. Experimental studies suggest that apologizing leads to earlier satisfaction and closure, faster settlements, and lower damage payments. When apologies include admissions of fault, injured parties feel greater respect for and less need to punish those who have harmed them, are more willing to forgive, and are more likely to accept settlement offers.15
Hospitals in Pennsylvania, Kentucky, and Michigan have found that sincere apologies and effective error disclosure programs reduce malpractice payouts and lead to faster settlements.16 As some plaintiffs’ lawyers point out, being honest and forthright and fixing the injured parties’ problems can quickly defuse a lawsuit. One attorney explained things this way: “We never sue the nice, contrite doctors. Their patients never call our offices. But the doctors who are poor communicators and abandon their patients get sued all the time. Their patients come to our offices looking for answers.”17
Apology laws: Protection from your own words
The belief that apologies by physicians can help patients emotionally and reduce malpractice litigation has led state legislatures to enact so-called apology laws in many jurisdictions in the United States.18 The general point of these rules and statutes is to prevent later use of doctors’ words in litigation. States differ substantially in the scope and type of protection that their laws offer. Some states prohibit doctors’ apologies for adverse outcomes from being used in litigation to prove negligence, while others only exclude expressions of sympathy or offers to pay for corrective treatment. Selected language from several states’ apology laws appears in the Table.19-23
Do apology laws work? Recent research by economists Ho and Liu indicates that they do. Comparing payouts in states with and without apology laws, they conclude that “apology laws have the greatest reduction in average payment size and settlement time in cases involving more severe patient outcomes,”13 such as obstetrics and anesthesia cases, cases that involve infants, and cases in which physicians improperly manage or fail to properly diagnose an illness.24
The practical impact of apologizing for psychiatric malpractice cases is unclear, but forensic psychiatrists Marilyn Price and Patricia Recupero believe that, following some unexpected outcomes, thoughtful expressions of sympathy, regret, and—if the outcome resulted from an error—apologies may be appropriate. Price and Recupero caution that such conversations should occur as part of broader programs that investigate unanticipated adverse events and provide education and coaching about appropriate ways to make disclosures. Clinicians also should consult with legal counsel, risk management officers, and liability insurance carriers before initiating such disclosures.25
Bottom Line
Apologizing for medical errors may mitigate malpractice liability and can help injured parties and physicians feel better. Whether plaintiffs can use apologies as evidence of malpractice depends on state laws and rules of evidence. Before you apologize for an unanticipated outcome, discuss the situation with your legal counsel, risk management officers, and insurers.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Douglas Mossman, MD, talks about who you should consult before apologizing to a patient for a bad outcome. Dr. Mossman is Professor of Clinical Psychiatry and Director, Division of Forensic Psychiatry, University of Cincinnati College of Medicine, Cincinnati, Ohio.
1. McCullough ME, Sandage SJ, Brown SW, et al. Interpersonal forgiving in close relationships: II. Theoretical elaboration and measurement. J Pers Soc Psychol. 1998;75:1586-1603.
2. O’Reilly KB. “I’m sorry”: why is that so hard for doctors to say? http://www.amednews.com/article/20100201/profession/302019937/4. Published February 1, 2010. Accessed September 30, 2013.
3. American Medical Association. AMA Code of Medical Ethics, Opinion 8.12 – Patient information. http://www.ama-assn.org//ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion812.page. Published June 1994. Accessed September 30, 2013.
4. Hickson GB, Pichert JW. Disclosure and apology. http://www.npsf.org/wp-content/uploads/2011/10/RG_SUPS_After_Mod1_Hickson.pdf. Accessed October 4, 2013.
5. Snyder L, American College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1, pt 2):73-104.
6. ECRI Institute. Disclosure of unanticipated outcomes. In: Healthcare risk control Supplement A, Risk analysis. Plymouth Meeting, PA: ECRI; 2002.
7. Okimoto TG, Wenzel M, Hedrick K. Refusing to apologize can have psychological benefits (and we issue no mea culpa for this research finding). Eur J Soc Psychol. 2013;43:22-31.
8. Lazare A. On apology. New York, NY: Oxford University Press; 2004.
9. Hilfiker D. Facing our mistakes. N Engl J Med. 1984;310:
118-122.
10. Leape LL. Error in medicine. JAMA. 1994;272:1851-1857.
11. Hall v. Hilbun, 466 So.2d 856 (Miss. 1985).
12. Kern SI. You continue to face exposure if you apologize. http://medicaleconomics.modernmedicine.com/medical-economics/news/modernmedicine/modern-medicine-now/you-continue-face-exposure-if-you-apologiz. Published September 24, 2010. Accessed October 1, 2013.
13. Ho B, Liu E. Does sorry work? The impact of apology laws on medical malpractice. J Risk Uncertain. 2011;43(2):141-167.
14. Fehr R, Gelfand MJ, Nag M. The road to forgiveness: a meta-analytic synthesis of its situational and dispositional correlates. Psychol Bull. 2010;136:894-914.
15. Robbennolt JK. Apologies and settlement. Court Review. 2009;45:90-97.
16. Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assoc. 2012;112(5):302-306.
17. Wojcieszak D, Banja J, Houk C. The sorry works! coalition: making the case for full disclosure. Jt Comm J Qual Patient Saf. 2006;32(6):344-350.
18. National Conference of State Legislatures. Medical liability/Medical malpractice laws. http://www.ncsl.org/issues-research/banking/medical-liability-medical-malpractice-laws.aspx. Published August 15, 2011. Accessed October 4, 2013.
19. Conn Gen Stat Ann §52-184d(b).
20. Fla Stat §90.4026(2).
21. Ill Comp Stat §5/8-1901.
22. NC Gen Stat §8C-1, Rule 413.
23. Tex. Civ. Prac. & Rem. Code §18.061.
24. Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empir Leg Stud. 2011;8:179-199.
25. Price M, Recupero PR. Risk management. In: Sharfstein SS, Dickerson FB, Oldham JM, eds. Textbook of hospital psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:411-412.
1. McCullough ME, Sandage SJ, Brown SW, et al. Interpersonal forgiving in close relationships: II. Theoretical elaboration and measurement. J Pers Soc Psychol. 1998;75:1586-1603.
2. O’Reilly KB. “I’m sorry”: why is that so hard for doctors to say? http://www.amednews.com/article/20100201/profession/302019937/4. Published February 1, 2010. Accessed September 30, 2013.
3. American Medical Association. AMA Code of Medical Ethics, Opinion 8.12 – Patient information. http://www.ama-assn.org//ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion812.page. Published June 1994. Accessed September 30, 2013.
4. Hickson GB, Pichert JW. Disclosure and apology. http://www.npsf.org/wp-content/uploads/2011/10/RG_SUPS_After_Mod1_Hickson.pdf. Accessed October 4, 2013.
5. Snyder L, American College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1, pt 2):73-104.
6. ECRI Institute. Disclosure of unanticipated outcomes. In: Healthcare risk control Supplement A, Risk analysis. Plymouth Meeting, PA: ECRI; 2002.
7. Okimoto TG, Wenzel M, Hedrick K. Refusing to apologize can have psychological benefits (and we issue no mea culpa for this research finding). Eur J Soc Psychol. 2013;43:22-31.
8. Lazare A. On apology. New York, NY: Oxford University Press; 2004.
9. Hilfiker D. Facing our mistakes. N Engl J Med. 1984;310:
118-122.
10. Leape LL. Error in medicine. JAMA. 1994;272:1851-1857.
11. Hall v. Hilbun, 466 So.2d 856 (Miss. 1985).
12. Kern SI. You continue to face exposure if you apologize. http://medicaleconomics.modernmedicine.com/medical-economics/news/modernmedicine/modern-medicine-now/you-continue-face-exposure-if-you-apologiz. Published September 24, 2010. Accessed October 1, 2013.
13. Ho B, Liu E. Does sorry work? The impact of apology laws on medical malpractice. J Risk Uncertain. 2011;43(2):141-167.
14. Fehr R, Gelfand MJ, Nag M. The road to forgiveness: a meta-analytic synthesis of its situational and dispositional correlates. Psychol Bull. 2010;136:894-914.
15. Robbennolt JK. Apologies and settlement. Court Review. 2009;45:90-97.
16. Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assoc. 2012;112(5):302-306.
17. Wojcieszak D, Banja J, Houk C. The sorry works! coalition: making the case for full disclosure. Jt Comm J Qual Patient Saf. 2006;32(6):344-350.
18. National Conference of State Legislatures. Medical liability/Medical malpractice laws. http://www.ncsl.org/issues-research/banking/medical-liability-medical-malpractice-laws.aspx. Published August 15, 2011. Accessed October 4, 2013.
19. Conn Gen Stat Ann §52-184d(b).
20. Fla Stat §90.4026(2).
21. Ill Comp Stat §5/8-1901.
22. NC Gen Stat §8C-1, Rule 413.
23. Tex. Civ. Prac. & Rem. Code §18.061.
24. Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empir Leg Stud. 2011;8:179-199.
25. Price M, Recupero PR. Risk management. In: Sharfstein SS, Dickerson FB, Oldham JM, eds. Textbook of hospital psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:411-412.
Out of the cupboard and into the clinic: Nutmeg-induced mood disorder
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
Head banging: Cause for worry, or normal childhood development?
Most children who head bang—rhythmic movement of the head against a solid object, marked by compulsive repetitiveness1—usually are normal, healthy, well-cared-for children, in whom no cause for this activity can be determined. More common in boys than in girls, childhood head banging usually starts when the child is age 18 months, but he (she) should grow out of it by age 4.1,2 Nevertheless, you should be prepared to provide careful, targeted evaluation when presented with a child who head bangs, and discuss with parents or caregivers the possibility of a nonphysiologic cause, such as disruptions or discord in the home.
First concern: Is this normal?
Although head banging is seen in 5% to 15% of healthy children,1 children who are mentally retarded, blind, deaf, or autistic are more likely to participate in head banging.1 There also may be a familial predisposition; head banging is more frequent among cousins of children who bang their heads.1 Some studies have found that socioeconomic status, birth order, response to music, and motor development are correlated with head banging.1
Leung and colleagues1 propose that head banging is an integral part of normal development; a tension-releasing maneuver; an attention-seeking device; and a form of pain relief in response to acute illnesses. Fatigue, hunger, teething, or discomfort from a wet diaper can increase the tendency to head bang.
How does it happen?
Head banging generally occurs before sleep. The child will repeatedly bang his head—usually the frontal-parietal region—against a pillow, headboard, or railing of a crib 60 to 80 times per minute.1 This repetitive motion may continue for a few minutes or as long as an hour. While head banging, the child does not seem to experience pain or discomfort, but may appear relaxed or happy. Although this habit appears alarming (calluses, bruises, abrasions, and contusions may occur—especially in children with mental retardation)1,2, there rarely is significant head damage.
Talking to concerned parents
Head banging can be confused with typical temper tantrums, spasmus nutans (triad of pendular nystagmus, head nodding, and torticollis), and infantile myoclonic seizures (sudden dropping of the head and flexion of the arms).1 Take a detailed history and careful evaluation of the parent-child relationship to uncover any underlying causes, such as an unhappy home environment (eg, divorce or neglect). A complete physical examination may reveal an ear infection, visual problems, deafness, cerebral palsy, mental retardation, or evidence of abuse.
Psychotropic medication is not recommended. Treatment options include:1
• treating underlying abnormalities, such as otitis media
• padding the sides of the crib
• providing auditory stimulation, including allowing the child to participate in rhythmic actions during the day3
• fitting the child for a protective helmet.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Leung AK, Robson WL. Head banging. J Singapore Paediatr Soc. 1990;32(1-2):14-17.
2. Kravitz H, Rosenthal V, Teplitz Z, et al. A study of head-banging in infants and children. Dis Nerv Syst. 1960;21:203-208.
3. Ryan NM. Body rocking, head banging, and head rolling: an analysis of rhythmic motor activities in normal infants. Pediatr Nurs. 1983;9(4):281-285, 296.
Most children who head bang—rhythmic movement of the head against a solid object, marked by compulsive repetitiveness1—usually are normal, healthy, well-cared-for children, in whom no cause for this activity can be determined. More common in boys than in girls, childhood head banging usually starts when the child is age 18 months, but he (she) should grow out of it by age 4.1,2 Nevertheless, you should be prepared to provide careful, targeted evaluation when presented with a child who head bangs, and discuss with parents or caregivers the possibility of a nonphysiologic cause, such as disruptions or discord in the home.
First concern: Is this normal?
Although head banging is seen in 5% to 15% of healthy children,1 children who are mentally retarded, blind, deaf, or autistic are more likely to participate in head banging.1 There also may be a familial predisposition; head banging is more frequent among cousins of children who bang their heads.1 Some studies have found that socioeconomic status, birth order, response to music, and motor development are correlated with head banging.1
Leung and colleagues1 propose that head banging is an integral part of normal development; a tension-releasing maneuver; an attention-seeking device; and a form of pain relief in response to acute illnesses. Fatigue, hunger, teething, or discomfort from a wet diaper can increase the tendency to head bang.
How does it happen?
Head banging generally occurs before sleep. The child will repeatedly bang his head—usually the frontal-parietal region—against a pillow, headboard, or railing of a crib 60 to 80 times per minute.1 This repetitive motion may continue for a few minutes or as long as an hour. While head banging, the child does not seem to experience pain or discomfort, but may appear relaxed or happy. Although this habit appears alarming (calluses, bruises, abrasions, and contusions may occur—especially in children with mental retardation)1,2, there rarely is significant head damage.
Talking to concerned parents
Head banging can be confused with typical temper tantrums, spasmus nutans (triad of pendular nystagmus, head nodding, and torticollis), and infantile myoclonic seizures (sudden dropping of the head and flexion of the arms).1 Take a detailed history and careful evaluation of the parent-child relationship to uncover any underlying causes, such as an unhappy home environment (eg, divorce or neglect). A complete physical examination may reveal an ear infection, visual problems, deafness, cerebral palsy, mental retardation, or evidence of abuse.
Psychotropic medication is not recommended. Treatment options include:1
• treating underlying abnormalities, such as otitis media
• padding the sides of the crib
• providing auditory stimulation, including allowing the child to participate in rhythmic actions during the day3
• fitting the child for a protective helmet.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Most children who head bang—rhythmic movement of the head against a solid object, marked by compulsive repetitiveness1—usually are normal, healthy, well-cared-for children, in whom no cause for this activity can be determined. More common in boys than in girls, childhood head banging usually starts when the child is age 18 months, but he (she) should grow out of it by age 4.1,2 Nevertheless, you should be prepared to provide careful, targeted evaluation when presented with a child who head bangs, and discuss with parents or caregivers the possibility of a nonphysiologic cause, such as disruptions or discord in the home.
First concern: Is this normal?
Although head banging is seen in 5% to 15% of healthy children,1 children who are mentally retarded, blind, deaf, or autistic are more likely to participate in head banging.1 There also may be a familial predisposition; head banging is more frequent among cousins of children who bang their heads.1 Some studies have found that socioeconomic status, birth order, response to music, and motor development are correlated with head banging.1
Leung and colleagues1 propose that head banging is an integral part of normal development; a tension-releasing maneuver; an attention-seeking device; and a form of pain relief in response to acute illnesses. Fatigue, hunger, teething, or discomfort from a wet diaper can increase the tendency to head bang.
How does it happen?
Head banging generally occurs before sleep. The child will repeatedly bang his head—usually the frontal-parietal region—against a pillow, headboard, or railing of a crib 60 to 80 times per minute.1 This repetitive motion may continue for a few minutes or as long as an hour. While head banging, the child does not seem to experience pain or discomfort, but may appear relaxed or happy. Although this habit appears alarming (calluses, bruises, abrasions, and contusions may occur—especially in children with mental retardation)1,2, there rarely is significant head damage.
Talking to concerned parents
Head banging can be confused with typical temper tantrums, spasmus nutans (triad of pendular nystagmus, head nodding, and torticollis), and infantile myoclonic seizures (sudden dropping of the head and flexion of the arms).1 Take a detailed history and careful evaluation of the parent-child relationship to uncover any underlying causes, such as an unhappy home environment (eg, divorce or neglect). A complete physical examination may reveal an ear infection, visual problems, deafness, cerebral palsy, mental retardation, or evidence of abuse.
Psychotropic medication is not recommended. Treatment options include:1
• treating underlying abnormalities, such as otitis media
• padding the sides of the crib
• providing auditory stimulation, including allowing the child to participate in rhythmic actions during the day3
• fitting the child for a protective helmet.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Leung AK, Robson WL. Head banging. J Singapore Paediatr Soc. 1990;32(1-2):14-17.
2. Kravitz H, Rosenthal V, Teplitz Z, et al. A study of head-banging in infants and children. Dis Nerv Syst. 1960;21:203-208.
3. Ryan NM. Body rocking, head banging, and head rolling: an analysis of rhythmic motor activities in normal infants. Pediatr Nurs. 1983;9(4):281-285, 296.
1. Leung AK, Robson WL. Head banging. J Singapore Paediatr Soc. 1990;32(1-2):14-17.
2. Kravitz H, Rosenthal V, Teplitz Z, et al. A study of head-banging in infants and children. Dis Nerv Syst. 1960;21:203-208.
3. Ryan NM. Body rocking, head banging, and head rolling: an analysis of rhythmic motor activities in normal infants. Pediatr Nurs. 1983;9(4):281-285, 296.