User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Remember the kids when parents are ill
When evaluating and treating adults, keep in mind that mental disorders may cause functional impairment that hinders parents’ ability to care for dependent children. Certain disorders—such as schizophrenia, bipolar disorder, and major depressive disorder—are often associated with substantial functional impairment.1 Failure to consider the impact of the functional impairment on a parent’s caretaking responsibilities can place dependent children at risk.
Don’t forget the kids. Ask adult patients with psychiatric conditions if they have dependent children in their care. If they do, determine if any functional impairment resulting from the mental disorder is impacting the patient’s ability to care for the children or places children at imminent risk of harm.
Keep in mind that parents who are experiencing delusions or suicidal ideation may harbor thoughts of filicide.2 Maintain a high degree of suspicion when evaluating and managing such patients, and inquire directly about filicidal thoughts.
Use techniques similar to those for assessing suicidal thoughts. Begin with a normalizing statement such as, “Sometimes, when people have symptoms like those you are describing, they have thoughts of harming their children.” Then follow up with a direct question using unambiguous language: “Have you had any thoughts of hurting or killing your children?” When patients endorse such thoughts, explore whether they have formulated a plan and assess whether they intend to follow through with it.3
When a patient with dependent children requires hospital admission or admits to caretaking inability, ensure that proper arrangements are made for his or her children’s care. Contact the spouse, grandparent, guardian, or social services agency.
Remember that not all dependents are children. An increasing number of elderly persons are being cared for by their adult children. Caretaking can be a major psychosocial stressor that triggers the onset or exacerbation of a psychiatric disorder—such as a major depressive episode—that leads to functional impairment.4
1. Hales RE, Yudofsky SC, eds. Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing; 2003.
2. Jennings KD, Ross S, Popper S, Elmore M. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord 1999;54:21-8.
3. Shea SC. Psychiatric interviewing: the art of understanding (2nd ed). Philadelphia: WB Saunders; 1998.
4. Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed). New York: Lippincott Williams & Wilkins; 2004.
Dr. Campbell is assistant professor, department of psychiatry, Case Western Reserve University School of Medicine, Cleveland, OH, and is residency program director and director of clinical services, department of psychiatry, University Hospitals of Cleveland.
When evaluating and treating adults, keep in mind that mental disorders may cause functional impairment that hinders parents’ ability to care for dependent children. Certain disorders—such as schizophrenia, bipolar disorder, and major depressive disorder—are often associated with substantial functional impairment.1 Failure to consider the impact of the functional impairment on a parent’s caretaking responsibilities can place dependent children at risk.
Don’t forget the kids. Ask adult patients with psychiatric conditions if they have dependent children in their care. If they do, determine if any functional impairment resulting from the mental disorder is impacting the patient’s ability to care for the children or places children at imminent risk of harm.
Keep in mind that parents who are experiencing delusions or suicidal ideation may harbor thoughts of filicide.2 Maintain a high degree of suspicion when evaluating and managing such patients, and inquire directly about filicidal thoughts.
Use techniques similar to those for assessing suicidal thoughts. Begin with a normalizing statement such as, “Sometimes, when people have symptoms like those you are describing, they have thoughts of harming their children.” Then follow up with a direct question using unambiguous language: “Have you had any thoughts of hurting or killing your children?” When patients endorse such thoughts, explore whether they have formulated a plan and assess whether they intend to follow through with it.3
When a patient with dependent children requires hospital admission or admits to caretaking inability, ensure that proper arrangements are made for his or her children’s care. Contact the spouse, grandparent, guardian, or social services agency.
Remember that not all dependents are children. An increasing number of elderly persons are being cared for by their adult children. Caretaking can be a major psychosocial stressor that triggers the onset or exacerbation of a psychiatric disorder—such as a major depressive episode—that leads to functional impairment.4
When evaluating and treating adults, keep in mind that mental disorders may cause functional impairment that hinders parents’ ability to care for dependent children. Certain disorders—such as schizophrenia, bipolar disorder, and major depressive disorder—are often associated with substantial functional impairment.1 Failure to consider the impact of the functional impairment on a parent’s caretaking responsibilities can place dependent children at risk.
Don’t forget the kids. Ask adult patients with psychiatric conditions if they have dependent children in their care. If they do, determine if any functional impairment resulting from the mental disorder is impacting the patient’s ability to care for the children or places children at imminent risk of harm.
Keep in mind that parents who are experiencing delusions or suicidal ideation may harbor thoughts of filicide.2 Maintain a high degree of suspicion when evaluating and managing such patients, and inquire directly about filicidal thoughts.
Use techniques similar to those for assessing suicidal thoughts. Begin with a normalizing statement such as, “Sometimes, when people have symptoms like those you are describing, they have thoughts of harming their children.” Then follow up with a direct question using unambiguous language: “Have you had any thoughts of hurting or killing your children?” When patients endorse such thoughts, explore whether they have formulated a plan and assess whether they intend to follow through with it.3
When a patient with dependent children requires hospital admission or admits to caretaking inability, ensure that proper arrangements are made for his or her children’s care. Contact the spouse, grandparent, guardian, or social services agency.
Remember that not all dependents are children. An increasing number of elderly persons are being cared for by their adult children. Caretaking can be a major psychosocial stressor that triggers the onset or exacerbation of a psychiatric disorder—such as a major depressive episode—that leads to functional impairment.4
1. Hales RE, Yudofsky SC, eds. Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing; 2003.
2. Jennings KD, Ross S, Popper S, Elmore M. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord 1999;54:21-8.
3. Shea SC. Psychiatric interviewing: the art of understanding (2nd ed). Philadelphia: WB Saunders; 1998.
4. Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed). New York: Lippincott Williams & Wilkins; 2004.
Dr. Campbell is assistant professor, department of psychiatry, Case Western Reserve University School of Medicine, Cleveland, OH, and is residency program director and director of clinical services, department of psychiatry, University Hospitals of Cleveland.
1. Hales RE, Yudofsky SC, eds. Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing; 2003.
2. Jennings KD, Ross S, Popper S, Elmore M. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord 1999;54:21-8.
3. Shea SC. Psychiatric interviewing: the art of understanding (2nd ed). Philadelphia: WB Saunders; 1998.
4. Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed). New York: Lippincott Williams & Wilkins; 2004.
Dr. Campbell is assistant professor, department of psychiatry, Case Western Reserve University School of Medicine, Cleveland, OH, and is residency program director and director of clinical services, department of psychiatry, University Hospitals of Cleveland.
Be prepared, not panicked, when served with a subpoena
On Friday morning, Dr. G receives a subpoena requesting one of her patient’s records be available for court the following Monday. This is Dr. G’s first subpoena. The timetable is short, and she is uncertain about what to do and whom to ask for advice.
If you experience circumstances similar to Dr. G’s, these suggestions can acquaint you with the legal system, its processes, and jargon.
Subpoena or summons? A summons may mean a lawsuit was filed against you. A subpoena means you’re called to appear as a witness and/or provide the court with records or information.1 Inform your personal attorney or your facility’s legal team about the subpoena and provide a copy.
Identify the sender. Find out if a judge, attorney, or prosecutor issued the legal notice. Ask the issuer for information such as the case details and type of information expected from you. Confirm that you treated this particular patient or saw him or her in consultation.
Don’t ignore it. Subpoenas and summonses are court orders. You can be served twice, and if you disregard these notices you can be held in contempt of court. You could be fined or imprisoned, but judges usually are more interested in having witnesses comply than in inflicting punishment.
If the date, time, or location of the court appearance is not convenient, work with the court to reschedule and obtain these arrangements in writing. If you experience difficulty, contact the judge’s clerk directly.
Privacy concerns. Your attorney and the American Psychiatric Association’s legal consultation plan (available at www.psych.org/ecp/membership.cfm) can help you act in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations and applicable state laws. Your malpractice insurer can also offer guidance.
Request your patient’s consent to release your records to the court. If the patient refuses to consent, clearly explain to the court your inability to release confidential records. If your records or testimony are crucial for the case, the judge may still order you to comply.
Some subpoenas have exclusion criteria that exempt psychiatric and/or psychotherapeutic notes. If you believe your records qualify as exempt, you must submit a letter to the court explaining the reasons.
Requirements to comply. A subpoena may require testimony only, records only, or both. If you are required to testify, clarify whether you are to appear at trial, deposition, or both. In court, discuss only facts pertaining to the case, not your opinion.
1. Reiley DG, Guldner GT, Leinen AL. “You are commanded to appear:” The subpoena and the emergency medicine resident. Ann Emerg Med 2003;42(6):843-6.
Mariam Bekhit, MD, is child psychiatry fellow, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark.
Anwar Ghali, MD, is chairman, psychiatric department, Trinitas Hospital, Elizabeth, NJ.
On Friday morning, Dr. G receives a subpoena requesting one of her patient’s records be available for court the following Monday. This is Dr. G’s first subpoena. The timetable is short, and she is uncertain about what to do and whom to ask for advice.
If you experience circumstances similar to Dr. G’s, these suggestions can acquaint you with the legal system, its processes, and jargon.
Subpoena or summons? A summons may mean a lawsuit was filed against you. A subpoena means you’re called to appear as a witness and/or provide the court with records or information.1 Inform your personal attorney or your facility’s legal team about the subpoena and provide a copy.
Identify the sender. Find out if a judge, attorney, or prosecutor issued the legal notice. Ask the issuer for information such as the case details and type of information expected from you. Confirm that you treated this particular patient or saw him or her in consultation.
Don’t ignore it. Subpoenas and summonses are court orders. You can be served twice, and if you disregard these notices you can be held in contempt of court. You could be fined or imprisoned, but judges usually are more interested in having witnesses comply than in inflicting punishment.
If the date, time, or location of the court appearance is not convenient, work with the court to reschedule and obtain these arrangements in writing. If you experience difficulty, contact the judge’s clerk directly.
Privacy concerns. Your attorney and the American Psychiatric Association’s legal consultation plan (available at www.psych.org/ecp/membership.cfm) can help you act in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations and applicable state laws. Your malpractice insurer can also offer guidance.
Request your patient’s consent to release your records to the court. If the patient refuses to consent, clearly explain to the court your inability to release confidential records. If your records or testimony are crucial for the case, the judge may still order you to comply.
Some subpoenas have exclusion criteria that exempt psychiatric and/or psychotherapeutic notes. If you believe your records qualify as exempt, you must submit a letter to the court explaining the reasons.
Requirements to comply. A subpoena may require testimony only, records only, or both. If you are required to testify, clarify whether you are to appear at trial, deposition, or both. In court, discuss only facts pertaining to the case, not your opinion.
On Friday morning, Dr. G receives a subpoena requesting one of her patient’s records be available for court the following Monday. This is Dr. G’s first subpoena. The timetable is short, and she is uncertain about what to do and whom to ask for advice.
If you experience circumstances similar to Dr. G’s, these suggestions can acquaint you with the legal system, its processes, and jargon.
Subpoena or summons? A summons may mean a lawsuit was filed against you. A subpoena means you’re called to appear as a witness and/or provide the court with records or information.1 Inform your personal attorney or your facility’s legal team about the subpoena and provide a copy.
Identify the sender. Find out if a judge, attorney, or prosecutor issued the legal notice. Ask the issuer for information such as the case details and type of information expected from you. Confirm that you treated this particular patient or saw him or her in consultation.
Don’t ignore it. Subpoenas and summonses are court orders. You can be served twice, and if you disregard these notices you can be held in contempt of court. You could be fined or imprisoned, but judges usually are more interested in having witnesses comply than in inflicting punishment.
If the date, time, or location of the court appearance is not convenient, work with the court to reschedule and obtain these arrangements in writing. If you experience difficulty, contact the judge’s clerk directly.
Privacy concerns. Your attorney and the American Psychiatric Association’s legal consultation plan (available at www.psych.org/ecp/membership.cfm) can help you act in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations and applicable state laws. Your malpractice insurer can also offer guidance.
Request your patient’s consent to release your records to the court. If the patient refuses to consent, clearly explain to the court your inability to release confidential records. If your records or testimony are crucial for the case, the judge may still order you to comply.
Some subpoenas have exclusion criteria that exempt psychiatric and/or psychotherapeutic notes. If you believe your records qualify as exempt, you must submit a letter to the court explaining the reasons.
Requirements to comply. A subpoena may require testimony only, records only, or both. If you are required to testify, clarify whether you are to appear at trial, deposition, or both. In court, discuss only facts pertaining to the case, not your opinion.
1. Reiley DG, Guldner GT, Leinen AL. “You are commanded to appear:” The subpoena and the emergency medicine resident. Ann Emerg Med 2003;42(6):843-6.
Mariam Bekhit, MD, is child psychiatry fellow, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark.
Anwar Ghali, MD, is chairman, psychiatric department, Trinitas Hospital, Elizabeth, NJ.
1. Reiley DG, Guldner GT, Leinen AL. “You are commanded to appear:” The subpoena and the emergency medicine resident. Ann Emerg Med 2003;42(6):843-6.
Mariam Bekhit, MD, is child psychiatry fellow, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark.
Anwar Ghali, MD, is chairman, psychiatric department, Trinitas Hospital, Elizabeth, NJ.
Does chronic pain shrink the brain?
Chronic pain—particularly lower back pain—is frustrating to both patient and clinician. Because most cases lack an obvious physical explanation, the doctor may wonder if the patient is faking or exaggerating—that the pain is “in the patient’s head.” Studies suggest this cerebral component may exist—but not in ways you might expect.
How the brain processes pain
According to traditional belief, the brain passively receives noxious signals from injured tissue (nociceptive) or damaged nerve (neuropathic). Extensive—some would say excessive—tests are often conducted in search of a bone or muscle injury that might explain the pain.
Functional imaging studies across 15 years have shown activity in various brain regions when subjects feel pain. In addition to the somatosensory cortex, pain also activates brain areas involved with mood, attention, and anxiety. More important, the brain does not passively receive signals from the periphery but can inhibit ascending signals with endogenous opioids, such as endorphins and enkephalins.
Apkarian et alPosttraumatic stress disorder: Nature and nurture?, May 2004.)
Drugs. Medications and other substances taken to alleviate pain might also reduce gray matter. Excessive alcohol and opioid use have long-term adverse effects on the CNS.3 Is treatment or self-medication mildly toxic to the brain?
Overuse atrophy. Apkarian et al1 propose that cortical loss may be secondary to overuse. They suggest that persistent pain perception— and the resultant negative affect and stress—causes an excitotoxic and inflammatory state that wears out portions of the brain circuitry. If this is true, then chronic pain itself causes cerebral atrophy.
Whatever the explanation, this study indicates that chronic lower back pain pathology extends beyond the lower back.
Related resources
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70.
- International Association for the Study of Pain. www.iasp-pain.org.
1. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5.
2. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079-91.
3. Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med 2002;347:843-5.
Dr. Higgins is clinical associate professor of family medicine and psychiatry, Medical University of South Carolina, Charleston ([email protected]).
Chronic pain—particularly lower back pain—is frustrating to both patient and clinician. Because most cases lack an obvious physical explanation, the doctor may wonder if the patient is faking or exaggerating—that the pain is “in the patient’s head.” Studies suggest this cerebral component may exist—but not in ways you might expect.
How the brain processes pain
According to traditional belief, the brain passively receives noxious signals from injured tissue (nociceptive) or damaged nerve (neuropathic). Extensive—some would say excessive—tests are often conducted in search of a bone or muscle injury that might explain the pain.
Functional imaging studies across 15 years have shown activity in various brain regions when subjects feel pain. In addition to the somatosensory cortex, pain also activates brain areas involved with mood, attention, and anxiety. More important, the brain does not passively receive signals from the periphery but can inhibit ascending signals with endogenous opioids, such as endorphins and enkephalins.
Apkarian et alPosttraumatic stress disorder: Nature and nurture?, May 2004.)
Drugs. Medications and other substances taken to alleviate pain might also reduce gray matter. Excessive alcohol and opioid use have long-term adverse effects on the CNS.3 Is treatment or self-medication mildly toxic to the brain?
Overuse atrophy. Apkarian et al1 propose that cortical loss may be secondary to overuse. They suggest that persistent pain perception— and the resultant negative affect and stress—causes an excitotoxic and inflammatory state that wears out portions of the brain circuitry. If this is true, then chronic pain itself causes cerebral atrophy.
Whatever the explanation, this study indicates that chronic lower back pain pathology extends beyond the lower back.
Related resources
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70.
- International Association for the Study of Pain. www.iasp-pain.org.
Chronic pain—particularly lower back pain—is frustrating to both patient and clinician. Because most cases lack an obvious physical explanation, the doctor may wonder if the patient is faking or exaggerating—that the pain is “in the patient’s head.” Studies suggest this cerebral component may exist—but not in ways you might expect.
How the brain processes pain
According to traditional belief, the brain passively receives noxious signals from injured tissue (nociceptive) or damaged nerve (neuropathic). Extensive—some would say excessive—tests are often conducted in search of a bone or muscle injury that might explain the pain.
Functional imaging studies across 15 years have shown activity in various brain regions when subjects feel pain. In addition to the somatosensory cortex, pain also activates brain areas involved with mood, attention, and anxiety. More important, the brain does not passively receive signals from the periphery but can inhibit ascending signals with endogenous opioids, such as endorphins and enkephalins.
Apkarian et alPosttraumatic stress disorder: Nature and nurture?, May 2004.)
Drugs. Medications and other substances taken to alleviate pain might also reduce gray matter. Excessive alcohol and opioid use have long-term adverse effects on the CNS.3 Is treatment or self-medication mildly toxic to the brain?
Overuse atrophy. Apkarian et al1 propose that cortical loss may be secondary to overuse. They suggest that persistent pain perception— and the resultant negative affect and stress—causes an excitotoxic and inflammatory state that wears out portions of the brain circuitry. If this is true, then chronic pain itself causes cerebral atrophy.
Whatever the explanation, this study indicates that chronic lower back pain pathology extends beyond the lower back.
Related resources
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70.
- International Association for the Study of Pain. www.iasp-pain.org.
1. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5.
2. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079-91.
3. Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med 2002;347:843-5.
Dr. Higgins is clinical associate professor of family medicine and psychiatry, Medical University of South Carolina, Charleston ([email protected]).
1. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5.
2. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079-91.
3. Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med 2002;347:843-5.
Dr. Higgins is clinical associate professor of family medicine and psychiatry, Medical University of South Carolina, Charleston ([email protected]).
Vacationing vs. abandoning
Hospitalized patient hangs himself; estate blames vacationing psychiatrist
Los Angeles County (CA) Superior Court
Police took a 34-year-old man to an inpatient psychiatric facility after they found him walking naked on a city street. The hospital admitted him on a 72-hour involuntary hold because of his suicidal thoughts, although the psychiatrist did not believe he intended to kill himself. The patient had never attempted suicide before. The psychiatrist ordered treatment with risperidone and monitoring every 30 minutes.
Two days later, at the beginning of the psychiatrist’s vacation, the hospital started a 14-day hold process. After 3 days, the on-call psychiatrist documented the patient’s refusal to communicate and take medication, but the patient denied suicidal thinking.
After 3 more days, staff discovered the patient sitting unconscious on the floor next to the toilet, with his pants wrapped around his neck and tied to a grab bar. Staff attempted cardiopulmonary resuscitation and called paramedics, but the patient was dead.
The patient’s estate claimed that the hospital and first treating psychiatrist did not take appropriate measures to prevent the suicide. It charged the hospital with negligence in failing to have a breakaway grab bar and claimed the original psychiatrist did not adequately communicate the patient’s status with the covering psychiatrist before leaving on vacation.
The defense claimed the patient was not at high risk for suicide and that the standard of care is to communicate information regarding high-risk patients to the covering psychiatrist. The original psychiatrist also claimed the patient was doing well when he left for vacation.
- The jury decided for the defense
Dr. Grant’s observations
Patients and their families may feel abandoned in their psychiatrists’ absence. But this absence does not legally constitute abandonment unless:
- a doctor-patient relationship exists
- the doctor terminates the relationship
- there is a need for continuing care
- termination lacks reasonable notice so arrangements for continuing care cannot be made.
- Ensure that a system for getting urgent information to covering psychiatrists is in place.
- Verify that the covering psychiatrist knows he or she is responsible for your patients in emergency distress—including interviewing, reviewing records, and documenting treatment. His or her role is not just to fill space until you return.
- Tell emergency-prone patients the dates you’ll be unavailable and give them the contact information for the covering psychiatrist.
- Inform the covering psychiatrist about patients at high risk for suicide, decompensation, or hospitalization.
While travel is at times necessary, psychiatrists must ensure that emergency-prone patients have access to care in their absence (Box). You can delegate this responsibility to a covering psychiatrist, but choose him or her wisely. Selecting a physician you know is incapable of providing sound treatment is considered negligent. The primary psychiatrist cannot be held responsible for a substitute psychiatrist’s negligence if the choice of substitute is viewed as a competent delegation.
Cases are selected by Current Psychiatry's editors from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Hospitalized patient hangs himself; estate blames vacationing psychiatrist
Los Angeles County (CA) Superior Court
Police took a 34-year-old man to an inpatient psychiatric facility after they found him walking naked on a city street. The hospital admitted him on a 72-hour involuntary hold because of his suicidal thoughts, although the psychiatrist did not believe he intended to kill himself. The patient had never attempted suicide before. The psychiatrist ordered treatment with risperidone and monitoring every 30 minutes.
Two days later, at the beginning of the psychiatrist’s vacation, the hospital started a 14-day hold process. After 3 days, the on-call psychiatrist documented the patient’s refusal to communicate and take medication, but the patient denied suicidal thinking.
After 3 more days, staff discovered the patient sitting unconscious on the floor next to the toilet, with his pants wrapped around his neck and tied to a grab bar. Staff attempted cardiopulmonary resuscitation and called paramedics, but the patient was dead.
The patient’s estate claimed that the hospital and first treating psychiatrist did not take appropriate measures to prevent the suicide. It charged the hospital with negligence in failing to have a breakaway grab bar and claimed the original psychiatrist did not adequately communicate the patient’s status with the covering psychiatrist before leaving on vacation.
The defense claimed the patient was not at high risk for suicide and that the standard of care is to communicate information regarding high-risk patients to the covering psychiatrist. The original psychiatrist also claimed the patient was doing well when he left for vacation.
- The jury decided for the defense
Dr. Grant’s observations
Patients and their families may feel abandoned in their psychiatrists’ absence. But this absence does not legally constitute abandonment unless:
- a doctor-patient relationship exists
- the doctor terminates the relationship
- there is a need for continuing care
- termination lacks reasonable notice so arrangements for continuing care cannot be made.
- Ensure that a system for getting urgent information to covering psychiatrists is in place.
- Verify that the covering psychiatrist knows he or she is responsible for your patients in emergency distress—including interviewing, reviewing records, and documenting treatment. His or her role is not just to fill space until you return.
- Tell emergency-prone patients the dates you’ll be unavailable and give them the contact information for the covering psychiatrist.
- Inform the covering psychiatrist about patients at high risk for suicide, decompensation, or hospitalization.
While travel is at times necessary, psychiatrists must ensure that emergency-prone patients have access to care in their absence (Box). You can delegate this responsibility to a covering psychiatrist, but choose him or her wisely. Selecting a physician you know is incapable of providing sound treatment is considered negligent. The primary psychiatrist cannot be held responsible for a substitute psychiatrist’s negligence if the choice of substitute is viewed as a competent delegation.
Hospitalized patient hangs himself; estate blames vacationing psychiatrist
Los Angeles County (CA) Superior Court
Police took a 34-year-old man to an inpatient psychiatric facility after they found him walking naked on a city street. The hospital admitted him on a 72-hour involuntary hold because of his suicidal thoughts, although the psychiatrist did not believe he intended to kill himself. The patient had never attempted suicide before. The psychiatrist ordered treatment with risperidone and monitoring every 30 minutes.
Two days later, at the beginning of the psychiatrist’s vacation, the hospital started a 14-day hold process. After 3 days, the on-call psychiatrist documented the patient’s refusal to communicate and take medication, but the patient denied suicidal thinking.
After 3 more days, staff discovered the patient sitting unconscious on the floor next to the toilet, with his pants wrapped around his neck and tied to a grab bar. Staff attempted cardiopulmonary resuscitation and called paramedics, but the patient was dead.
The patient’s estate claimed that the hospital and first treating psychiatrist did not take appropriate measures to prevent the suicide. It charged the hospital with negligence in failing to have a breakaway grab bar and claimed the original psychiatrist did not adequately communicate the patient’s status with the covering psychiatrist before leaving on vacation.
The defense claimed the patient was not at high risk for suicide and that the standard of care is to communicate information regarding high-risk patients to the covering psychiatrist. The original psychiatrist also claimed the patient was doing well when he left for vacation.
- The jury decided for the defense
Dr. Grant’s observations
Patients and their families may feel abandoned in their psychiatrists’ absence. But this absence does not legally constitute abandonment unless:
- a doctor-patient relationship exists
- the doctor terminates the relationship
- there is a need for continuing care
- termination lacks reasonable notice so arrangements for continuing care cannot be made.
- Ensure that a system for getting urgent information to covering psychiatrists is in place.
- Verify that the covering psychiatrist knows he or she is responsible for your patients in emergency distress—including interviewing, reviewing records, and documenting treatment. His or her role is not just to fill space until you return.
- Tell emergency-prone patients the dates you’ll be unavailable and give them the contact information for the covering psychiatrist.
- Inform the covering psychiatrist about patients at high risk for suicide, decompensation, or hospitalization.
While travel is at times necessary, psychiatrists must ensure that emergency-prone patients have access to care in their absence (Box). You can delegate this responsibility to a covering psychiatrist, but choose him or her wisely. Selecting a physician you know is incapable of providing sound treatment is considered negligent. The primary psychiatrist cannot be held responsible for a substitute psychiatrist’s negligence if the choice of substitute is viewed as a competent delegation.
Cases are selected by Current Psychiatry's editors from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry's editors from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Turning psychiatric emergencies into opportunities
Like it or not, we deal with psychiatric emergencies. Emergencies—by definition—come at inconvenient times. Everybody is upset. None of the so-called “community resources” are available when we need them. And we usually have trouble getting paid for responding.
On the other hand, being asked to evaluate and treat patients with emergency psychiatric problems enables us to use all of our training and experience. In the adrenaline-charged emergency department (ED), our informed decisions can make a tremendous difference in the lives of patients and their families.
In this issue, Drs. Gabrielle Melin and Kristin Vickers-Douglas describe a practical workup of patients who arrive at the ED with acute psychiatric illness (page 14). They emphasize how to:
- conduct a sufficient workup for medical and psychiatric illness
- develop a therapeutic alliance with patients under trying circumstances
- protect staff and ourselves, as well as patients, from harm.
As Drs. Melin and Vickers-Douglas explain, “In the high-pressure ED, a sufficient workup for complicated medical conditions lies somewhere between extensive/unnecessary and inadequate. Thus, determining an exact diagnosis is not as important as establishing a diagnostic category to guide emergency treatment.” Similarly, adopting a pragmatic attitude can help us balance emergencies’ frustrations with their opportunities.
P. S. Last month I announced a contest asking for creative ideas for the Craig and Frances Lindner Center of HOPE, a psychiatric treatment center to be built in Cincinnati. Suggestions can be clinical, architectural, financial, or anything else. We will award $1,000 for the best idea.
The entry deadline is midnight Dec. 31, 2005. Send your suggestions to [email protected].
Like it or not, we deal with psychiatric emergencies. Emergencies—by definition—come at inconvenient times. Everybody is upset. None of the so-called “community resources” are available when we need them. And we usually have trouble getting paid for responding.
On the other hand, being asked to evaluate and treat patients with emergency psychiatric problems enables us to use all of our training and experience. In the adrenaline-charged emergency department (ED), our informed decisions can make a tremendous difference in the lives of patients and their families.
In this issue, Drs. Gabrielle Melin and Kristin Vickers-Douglas describe a practical workup of patients who arrive at the ED with acute psychiatric illness (page 14). They emphasize how to:
- conduct a sufficient workup for medical and psychiatric illness
- develop a therapeutic alliance with patients under trying circumstances
- protect staff and ourselves, as well as patients, from harm.
As Drs. Melin and Vickers-Douglas explain, “In the high-pressure ED, a sufficient workup for complicated medical conditions lies somewhere between extensive/unnecessary and inadequate. Thus, determining an exact diagnosis is not as important as establishing a diagnostic category to guide emergency treatment.” Similarly, adopting a pragmatic attitude can help us balance emergencies’ frustrations with their opportunities.
P. S. Last month I announced a contest asking for creative ideas for the Craig and Frances Lindner Center of HOPE, a psychiatric treatment center to be built in Cincinnati. Suggestions can be clinical, architectural, financial, or anything else. We will award $1,000 for the best idea.
The entry deadline is midnight Dec. 31, 2005. Send your suggestions to [email protected].
Like it or not, we deal with psychiatric emergencies. Emergencies—by definition—come at inconvenient times. Everybody is upset. None of the so-called “community resources” are available when we need them. And we usually have trouble getting paid for responding.
On the other hand, being asked to evaluate and treat patients with emergency psychiatric problems enables us to use all of our training and experience. In the adrenaline-charged emergency department (ED), our informed decisions can make a tremendous difference in the lives of patients and their families.
In this issue, Drs. Gabrielle Melin and Kristin Vickers-Douglas describe a practical workup of patients who arrive at the ED with acute psychiatric illness (page 14). They emphasize how to:
- conduct a sufficient workup for medical and psychiatric illness
- develop a therapeutic alliance with patients under trying circumstances
- protect staff and ourselves, as well as patients, from harm.
As Drs. Melin and Vickers-Douglas explain, “In the high-pressure ED, a sufficient workup for complicated medical conditions lies somewhere between extensive/unnecessary and inadequate. Thus, determining an exact diagnosis is not as important as establishing a diagnostic category to guide emergency treatment.” Similarly, adopting a pragmatic attitude can help us balance emergencies’ frustrations with their opportunities.
P. S. Last month I announced a contest asking for creative ideas for the Craig and Frances Lindner Center of HOPE, a psychiatric treatment center to be built in Cincinnati. Suggestions can be clinical, architectural, financial, or anything else. We will award $1,000 for the best idea.
The entry deadline is midnight Dec. 31, 2005. Send your suggestions to [email protected].
A ‘bad’ boy’s behavior problems
History: impulsive and distractible
For 2 years Mark, age 11, has been treated for attention-deficit/hyperactivity disorder (ADHD). His initial symptoms included inattention, hyperactivity, distractibility, short attention span, failure to follow instructions, poor organization, and intruding on others. He often picks fights with his 7-year-old brother, mildly injuring him on one occasion. His teacher recently punished him for roaming the classroom and distracting his classmates.
None of Mark’s symptoms suggest mania. His family has no history of mood disorders, but his father has been diagnosed with substance dependence.
Mark’s psychiatrist had prescribed an extended-release amphetamine salts preparation, 10 mg/d. Soon after, Mark began experiencing stomachaches, insomnia, facial flushing, and headaches. The dosage was reduced to 5 mg/d, but Mark stopped taking the medication after less than 3 weeks. Cognitive-behavioral therapy and classroom modifications were then tried for 11 months, but Mark’s behavior worsened. His symptoms now include inattention, distractibility, excessive talking, restlessness, and impulsivity.
The authors’ observations
Children and adolescents often present with excessive talking, distractibility, increased activity or restlessness, and loss of normal functioning. Distinguishing these ADHD symptoms from those of bipolar, disruptive, learning, movement, anxiety, substance-related or other mental disorders can be challenging.How to reduce mania risk when prescribing stimulants,” October 2005, at www.currentpsychiatry.com.)
Pediatric bipolar disorder often goes undetected because DSM-IV-TR criteria—established for adults—may not apply to youths. Children are more likely to present with a mixed mood state, less-distinct periods between episodes, grandiosity, irritability, and a chronic, continuous course.2 By contrast, bipolar adults often present with a sudden classic manic episode, elation, and euphoria.2 Adults also usually have relatively stable periods between episodes and tend to have comorbid substance dependence, panic, or eating disorders.
Mark’s symptoms still suggest ADHD. His inattention started before age 7, and his teacher is mostly concerned about his hyperactive, impulsive, and disruptive behavior. Mark’s mother, teacher, and psychiatrist feel confident that the ADHD diagnosis is correct, so we decide against comprehensive reassessment or prescribing a mood stabilizer. Mark’s mother opts to try the nonstimulant ADHD medication atomoxetine rather than a different stimulant.
Treatment: a moving experience
Mark’s psychiatrist prescribes atomoxetine, 18 mg/d for 4 days, then increases the dosage to 25 mg/d after finding that the boy could tolerate the medication.
Two weeks after starting atomoxetine, Mark becomes agitated and activated, and voices suicidal thoughts on one occasion. Without warning, while his mother is driving him to school, he opens the door of the moving vehicle and tries to jump out. His mother stops him by calling his name, yelling “No,” and slamming on the brakes. Mark tells her that he is “bad” and wants to die. She has no idea what prompted this behavior.
Mark also has become more oppositional and defiant, and his temper tantrums and destruction of household items are more frequent. He continues to behave aggressively toward his younger brother, often breaking some of his favorite toys. Mark also shows elevated and expansive mood, irritability, pressured speech, inflated self-esteem, and psychomotor agitation—symptoms consistent with a manic episode. At one visit, Mark tells his psychia trist, “I feel great! I can do anything.”
The authors’ observations
Mark, who had been diagnosed as having ADHD, began showing manic activation and suicidal thinking 2 weeks after starting atomoxetine. Whether he showed de novo suicidal behavior or reckless behavior associated with mania is unclear.
Atomoxetine-induced mania is not a new finding.2,5 During clinical trials, 2% of patients reported mood swings and 8% reported irritability. Subsequent experience indicates the risk of mood destabilization may be as high as 33%.5
Atomoxetine, a nonstimulant medication indicated for treating pediatric and adult ADHD, is a potent norepinephrine reuptake inhibitor. Reanalysis of the atomoxetine clinical trial database showed a slightly but statistically significant higher risk of suicidal behavior and thoughts in children and adolescents compared with placebo.6 No deaths from suicide were reported. The FDA subsequently ordered a black box warning on atomoxetine’s label instructing physicians, patients, and families to watch closely for suicidality symptoms with atomoxetine use.
Atomoxetine is safe and effective for pediatric ADHD, provided youths are properly monitored. Be careful, however, when prescribing atomoxetine to youths with a personal or family history of mood disorder.
FDA also is reviewing data on all drugs indicated for treating ADHD to determine whether they cause suicidality, new-onset mental disorders, or other psychiatric adverse events.7
Assessing medication risk
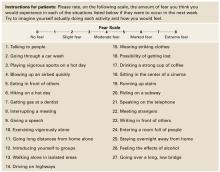
All youths being treated for a mood disorder and/or ADHD must be assessed for suicide risk, but how to most effectively perform this assessment is unclear. Organizations representing pediatrics and child and adolescent psychiatry have not yet incorporated FDA’s medication guidelines regarding pediatric suicidality—released earlier this year—into their guidelines (Table 1). As a result, most physicians follow pediatric patients less frequently than FDA now advises.
Atomoxetine’s receptor profile resembles that of antidepressants, which also are labeled with a black box warning describing increased suicidality risk when used in children and adolescents. Risk of suicidal behavior is highest within 10 days of starting antidepressants, and a significant risk remains throughout the first month. The suicidality rate appears to drop after that time.9,10
Follow FDA patient monitoring guidelines for antidepressants when prescribing atomoxetine to youths—particularly given the prospective labeling change. Atomoxetine’s manufacturer is expected to release a patient monitoring guideline unique to this drug.
Table 1
FDA guidelines for monitoring pediatric antidepressant use
| After starting an antidepressant, patients should see their doctor: |
|
| Source: Reference 8 |
Suicidality: finding other causes
Suicidality is more prevalent in bipolar disorder than in other mental disorders,2,4 and ADHD and mania often co-exist (Box 1).11,12 Mania induced by medication might explain suicidality or other behavior changes in some youths, but activation, mania, behavior change, or suicidality can result from the primary or comorbid disorder rather than the medication.
No deaths by suicide were reported among the FDA-reviewed studies of antidepressant use in children and adolescents. Fatal suicidal behavior has been reported in adolescents not treated with medications.14
FDA cites 12 features that point to suicide risk in youths (Box 2).8-10 Seven features suggest both ADHD and mania, which overlap to the point of diagnostic distraction.
As many as 20% of children diagnosed with ADHD also meet DSM-IV-TR criteria for bipolar disorder.
When bipolar disorder is the initial diagnosis, 30% to 40% of adolescents and 70% to 90% of prepubertal children may meet ADHD criteria.
Prepubescent major depression carries a 50% lifetime risk of developing mania.
Source: References 3, 11-13
- New or more thoughts of suicide
- Suicide attempts
- New or worsened depression
- New or worsened anxiety
- Feeling agitated or restless*
- Panic attacks
- Difficulty sleeping (insomnia)*
- New or worsened irritability*
- Aggressive, angry, or violent behavior*
- Acting on dangerous impulses*
- Extreme hyperactivity in actions and talking (hypomania or mania)*
- Other unusual behavior changes*
* Suggest both ADHD and mania
Source: References 8-10
The authors’ observations
Consider a broad differential diagnosis when evaluating inattention, hyperactivity, and impulsivity in children. Family medical history, corroborative clinical interviews, past and current behavioral rating scores, and psychological testing can help confirm an ADHD diagnosis (Table 2).
A careful patient interview, watching for diagnostic clues, taking a confirmatory history, and attention to key symptoms can help you discern ADHD from mania. Rule out unexplored diagnoses such as substance abuse, disturbed relationships, medical illness, and other mental disorders. Having the family and teachers track the youth’s longitudinal mood, energy, sleep, and actions may confirm a mood disorder.
Elated mood or grandiosity indicate mania. Irritable hyperactivity is seen more frequently in mania, whereas general hyperactivity tends to be present in ADHD. Childhood depression often heralds bipolar disorder.
Suspected medication-induced suicidality may call for stopping the offending agent, but determining whether a mental disorder or medication is causing suicidal thoughts can be difficult.
Try stopping the suspected offending drug first. If the youth remains suicidal after 1 week, a thorough biopsychosocial reassessment may guide future options including inpatient care, intensive outpatient psychotherapy, monitoring, and cautious use of antidepressant and/or antimanic medications.
Suicide risk requires clinician vigilance. As we learn from the FDA’s warnings, each treatment episode confers new risk and underscores the importance of watching for risk factors that may predict suicide (Table 3).
Table 2
What to include in an ADHD evaluation
| Histories: psychosocial, developmental, medical, educational, substance use and/or family |
| Clinical interviews with the child or adolescent. Corroborative interviews with parents, guardians, teachers, others |
| Rating scales assessing past behavior: Instruments completed by multiple sources such as the youth, family members or guardian, former teachers, others |
| Rating scales of current behavior: Instruments completed by youth, parents or guardian, former teachers, siblings, significant others |
| Psychological testing: Psychoeducational evaluation, personality inventory, intelligence assessment, and/or a continuous performance test. ADHD diagnosis remains clinical, and no evaluation should rely too heavily on “objective tests” for a definitive diagnosis |
Table 3
Risk factors that may predict suicide in youths
| Older (pubertal) age |
| Male gender |
| Mania |
| Mixed mood state |
| Psychosis |
| Victim of sexual or physical abuse |
| Co-occurring disruptive disorders |
| Comorbid substance abuse |
| Impulsivity |
| Easy access to means, such as firearms, lethal toxins, or medications |
| Lack of family support |
| Acute stressors |
| Family history of suicide |
| Source: Adapted from reference 2. |
Continued treatment: no more medication
Mark’s psychiatrist immediately stops atomoxetine. The boy’s mother, a psychiatric nurse, declines a trial of divalproex because she fears drug toxicity. Mark’s suicidality and agitation resolve over 1 week, and he returns to baseline function, leading us to believe his mania was medication-induced.
One year later, Mark takes no medications. He is behaving well at school and made the honor roll this fall. His teacher reports that Mark is “smart, well liked, but talks excessively,” though she says his talking is “not as out of control” as it was a year ago.
Mark recently began playing soccer as an outlet for his hyperactivity. He has not been penalized on the soccer field but is occasionally “over the edge,” pushing and shoving other players. When frustrated at home he has short outbursts, slams doors, and yells at his brother without being physically aggressive.
Mark’s office visits are infrequent, but he recently asked his mother to take him to his psychiatrist and counselor. His mother realizes he may soon need medication, but she wants to wait.
Related resources
- U.S. Food and Drug Administration. List of drugs receiving a boxed warning, other product labeling changes, and a medication guide pertaining to pediatric suicidality. www.fda.gov/cder/drug/antidepressants/MDD_alldruglist.pdf.
- Eli Lilly and Co. Questions and answers about the Strattera (atomoxetine) label change: a guide for patients and parents. www.strattera.com/1_5_news/Q&A_Strattera_label_update.pdf.
- U.S. Food and Drug Administration. Public health advisory: Suicidal thinking in children and adolescents being treated with Strattera (atomoxetine).www.fda.gov/cder/drug/advisory/atomoxetine.htm.
- Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294(16):2064-74.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall XR
- Atomoxetine • Strattera
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Rappley MD. Clinical practice: attention deficit-hyperactivity disorder. N Engl J Med 2005;352:165-73.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Scheffer RE, Apps JN. ADHD or bipolar disease? Age-specific manic symptoms are key. Current Psychiatry 2005;4(5):42-52.
4. Schapiro NA. Bipolar disorders in children and adolescents. J Pediatr Health Care 2005;19:131-41.
5. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114:895-6.
6. Eli Lilly and Co Questions and answers about the Strattera (atomoxetine) label change: a guide for patients and parents. Available at: http://www.strattera.com/1_5_news/Q&A_Strattera_label_update.pdf. Accessed Oct. 16, 2005.
7. Mathews AW, Abboud L. FDA offers more details on relabeling: concern that ADHD drugs may cause adverse events stemmed from few reports. Wall Street Journal June 30, 2005 D4.
8. U.S. Food and Drug Administration. The FDA required medication guide about using antidepressants in children and teenagers. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIMedicationGuide.htm. Accessed July 1, 2005.
9. U.S. Food and Drug Administration public health advisory October 15, 2004: Suicidality in children and adolescents being treated with antidepressant medications. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm. Accessed July 1, 2005.
10. U.S. Food and Drug Administration public health advisory: Labeling change request letter for antidepressant medications. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIlabelChange.htm. Accessed July 1, 2005.
11. Wozniak J, Biederman J, Kiely K, et al. Manic-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34:867-76.
12. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35:997-1008.
13. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82 (suppl):S59-S69.
14. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338-43.
History: impulsive and distractible
For 2 years Mark, age 11, has been treated for attention-deficit/hyperactivity disorder (ADHD). His initial symptoms included inattention, hyperactivity, distractibility, short attention span, failure to follow instructions, poor organization, and intruding on others. He often picks fights with his 7-year-old brother, mildly injuring him on one occasion. His teacher recently punished him for roaming the classroom and distracting his classmates.
None of Mark’s symptoms suggest mania. His family has no history of mood disorders, but his father has been diagnosed with substance dependence.
Mark’s psychiatrist had prescribed an extended-release amphetamine salts preparation, 10 mg/d. Soon after, Mark began experiencing stomachaches, insomnia, facial flushing, and headaches. The dosage was reduced to 5 mg/d, but Mark stopped taking the medication after less than 3 weeks. Cognitive-behavioral therapy and classroom modifications were then tried for 11 months, but Mark’s behavior worsened. His symptoms now include inattention, distractibility, excessive talking, restlessness, and impulsivity.
The authors’ observations
Children and adolescents often present with excessive talking, distractibility, increased activity or restlessness, and loss of normal functioning. Distinguishing these ADHD symptoms from those of bipolar, disruptive, learning, movement, anxiety, substance-related or other mental disorders can be challenging.How to reduce mania risk when prescribing stimulants,” October 2005, at www.currentpsychiatry.com.)
Pediatric bipolar disorder often goes undetected because DSM-IV-TR criteria—established for adults—may not apply to youths. Children are more likely to present with a mixed mood state, less-distinct periods between episodes, grandiosity, irritability, and a chronic, continuous course.2 By contrast, bipolar adults often present with a sudden classic manic episode, elation, and euphoria.2 Adults also usually have relatively stable periods between episodes and tend to have comorbid substance dependence, panic, or eating disorders.
Mark’s symptoms still suggest ADHD. His inattention started before age 7, and his teacher is mostly concerned about his hyperactive, impulsive, and disruptive behavior. Mark’s mother, teacher, and psychiatrist feel confident that the ADHD diagnosis is correct, so we decide against comprehensive reassessment or prescribing a mood stabilizer. Mark’s mother opts to try the nonstimulant ADHD medication atomoxetine rather than a different stimulant.
Treatment: a moving experience
Mark’s psychiatrist prescribes atomoxetine, 18 mg/d for 4 days, then increases the dosage to 25 mg/d after finding that the boy could tolerate the medication.
Two weeks after starting atomoxetine, Mark becomes agitated and activated, and voices suicidal thoughts on one occasion. Without warning, while his mother is driving him to school, he opens the door of the moving vehicle and tries to jump out. His mother stops him by calling his name, yelling “No,” and slamming on the brakes. Mark tells her that he is “bad” and wants to die. She has no idea what prompted this behavior.
Mark also has become more oppositional and defiant, and his temper tantrums and destruction of household items are more frequent. He continues to behave aggressively toward his younger brother, often breaking some of his favorite toys. Mark also shows elevated and expansive mood, irritability, pressured speech, inflated self-esteem, and psychomotor agitation—symptoms consistent with a manic episode. At one visit, Mark tells his psychia trist, “I feel great! I can do anything.”
The authors’ observations
Mark, who had been diagnosed as having ADHD, began showing manic activation and suicidal thinking 2 weeks after starting atomoxetine. Whether he showed de novo suicidal behavior or reckless behavior associated with mania is unclear.
Atomoxetine-induced mania is not a new finding.2,5 During clinical trials, 2% of patients reported mood swings and 8% reported irritability. Subsequent experience indicates the risk of mood destabilization may be as high as 33%.5
Atomoxetine, a nonstimulant medication indicated for treating pediatric and adult ADHD, is a potent norepinephrine reuptake inhibitor. Reanalysis of the atomoxetine clinical trial database showed a slightly but statistically significant higher risk of suicidal behavior and thoughts in children and adolescents compared with placebo.6 No deaths from suicide were reported. The FDA subsequently ordered a black box warning on atomoxetine’s label instructing physicians, patients, and families to watch closely for suicidality symptoms with atomoxetine use.
Atomoxetine is safe and effective for pediatric ADHD, provided youths are properly monitored. Be careful, however, when prescribing atomoxetine to youths with a personal or family history of mood disorder.
FDA also is reviewing data on all drugs indicated for treating ADHD to determine whether they cause suicidality, new-onset mental disorders, or other psychiatric adverse events.7
Assessing medication risk
All youths being treated for a mood disorder and/or ADHD must be assessed for suicide risk, but how to most effectively perform this assessment is unclear. Organizations representing pediatrics and child and adolescent psychiatry have not yet incorporated FDA’s medication guidelines regarding pediatric suicidality—released earlier this year—into their guidelines (Table 1). As a result, most physicians follow pediatric patients less frequently than FDA now advises.
Atomoxetine’s receptor profile resembles that of antidepressants, which also are labeled with a black box warning describing increased suicidality risk when used in children and adolescents. Risk of suicidal behavior is highest within 10 days of starting antidepressants, and a significant risk remains throughout the first month. The suicidality rate appears to drop after that time.9,10
Follow FDA patient monitoring guidelines for antidepressants when prescribing atomoxetine to youths—particularly given the prospective labeling change. Atomoxetine’s manufacturer is expected to release a patient monitoring guideline unique to this drug.
Table 1
FDA guidelines for monitoring pediatric antidepressant use
| After starting an antidepressant, patients should see their doctor: |
|
| Source: Reference 8 |
Suicidality: finding other causes
Suicidality is more prevalent in bipolar disorder than in other mental disorders,2,4 and ADHD and mania often co-exist (Box 1).11,12 Mania induced by medication might explain suicidality or other behavior changes in some youths, but activation, mania, behavior change, or suicidality can result from the primary or comorbid disorder rather than the medication.
No deaths by suicide were reported among the FDA-reviewed studies of antidepressant use in children and adolescents. Fatal suicidal behavior has been reported in adolescents not treated with medications.14
FDA cites 12 features that point to suicide risk in youths (Box 2).8-10 Seven features suggest both ADHD and mania, which overlap to the point of diagnostic distraction.
As many as 20% of children diagnosed with ADHD also meet DSM-IV-TR criteria for bipolar disorder.
When bipolar disorder is the initial diagnosis, 30% to 40% of adolescents and 70% to 90% of prepubertal children may meet ADHD criteria.
Prepubescent major depression carries a 50% lifetime risk of developing mania.
Source: References 3, 11-13
- New or more thoughts of suicide
- Suicide attempts
- New or worsened depression
- New or worsened anxiety
- Feeling agitated or restless*
- Panic attacks
- Difficulty sleeping (insomnia)*
- New or worsened irritability*
- Aggressive, angry, or violent behavior*
- Acting on dangerous impulses*
- Extreme hyperactivity in actions and talking (hypomania or mania)*
- Other unusual behavior changes*
* Suggest both ADHD and mania
Source: References 8-10
The authors’ observations
Consider a broad differential diagnosis when evaluating inattention, hyperactivity, and impulsivity in children. Family medical history, corroborative clinical interviews, past and current behavioral rating scores, and psychological testing can help confirm an ADHD diagnosis (Table 2).
A careful patient interview, watching for diagnostic clues, taking a confirmatory history, and attention to key symptoms can help you discern ADHD from mania. Rule out unexplored diagnoses such as substance abuse, disturbed relationships, medical illness, and other mental disorders. Having the family and teachers track the youth’s longitudinal mood, energy, sleep, and actions may confirm a mood disorder.
Elated mood or grandiosity indicate mania. Irritable hyperactivity is seen more frequently in mania, whereas general hyperactivity tends to be present in ADHD. Childhood depression often heralds bipolar disorder.
Suspected medication-induced suicidality may call for stopping the offending agent, but determining whether a mental disorder or medication is causing suicidal thoughts can be difficult.
Try stopping the suspected offending drug first. If the youth remains suicidal after 1 week, a thorough biopsychosocial reassessment may guide future options including inpatient care, intensive outpatient psychotherapy, monitoring, and cautious use of antidepressant and/or antimanic medications.
Suicide risk requires clinician vigilance. As we learn from the FDA’s warnings, each treatment episode confers new risk and underscores the importance of watching for risk factors that may predict suicide (Table 3).
Table 2
What to include in an ADHD evaluation
| Histories: psychosocial, developmental, medical, educational, substance use and/or family |
| Clinical interviews with the child or adolescent. Corroborative interviews with parents, guardians, teachers, others |
| Rating scales assessing past behavior: Instruments completed by multiple sources such as the youth, family members or guardian, former teachers, others |
| Rating scales of current behavior: Instruments completed by youth, parents or guardian, former teachers, siblings, significant others |
| Psychological testing: Psychoeducational evaluation, personality inventory, intelligence assessment, and/or a continuous performance test. ADHD diagnosis remains clinical, and no evaluation should rely too heavily on “objective tests” for a definitive diagnosis |
Table 3
Risk factors that may predict suicide in youths
| Older (pubertal) age |
| Male gender |
| Mania |
| Mixed mood state |
| Psychosis |
| Victim of sexual or physical abuse |
| Co-occurring disruptive disorders |
| Comorbid substance abuse |
| Impulsivity |
| Easy access to means, such as firearms, lethal toxins, or medications |
| Lack of family support |
| Acute stressors |
| Family history of suicide |
| Source: Adapted from reference 2. |
Continued treatment: no more medication
Mark’s psychiatrist immediately stops atomoxetine. The boy’s mother, a psychiatric nurse, declines a trial of divalproex because she fears drug toxicity. Mark’s suicidality and agitation resolve over 1 week, and he returns to baseline function, leading us to believe his mania was medication-induced.
One year later, Mark takes no medications. He is behaving well at school and made the honor roll this fall. His teacher reports that Mark is “smart, well liked, but talks excessively,” though she says his talking is “not as out of control” as it was a year ago.
Mark recently began playing soccer as an outlet for his hyperactivity. He has not been penalized on the soccer field but is occasionally “over the edge,” pushing and shoving other players. When frustrated at home he has short outbursts, slams doors, and yells at his brother without being physically aggressive.
Mark’s office visits are infrequent, but he recently asked his mother to take him to his psychiatrist and counselor. His mother realizes he may soon need medication, but she wants to wait.
Related resources
- U.S. Food and Drug Administration. List of drugs receiving a boxed warning, other product labeling changes, and a medication guide pertaining to pediatric suicidality. www.fda.gov/cder/drug/antidepressants/MDD_alldruglist.pdf.
- Eli Lilly and Co. Questions and answers about the Strattera (atomoxetine) label change: a guide for patients and parents. www.strattera.com/1_5_news/Q&A_Strattera_label_update.pdf.
- U.S. Food and Drug Administration. Public health advisory: Suicidal thinking in children and adolescents being treated with Strattera (atomoxetine).www.fda.gov/cder/drug/advisory/atomoxetine.htm.
- Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294(16):2064-74.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall XR
- Atomoxetine • Strattera
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: impulsive and distractible
For 2 years Mark, age 11, has been treated for attention-deficit/hyperactivity disorder (ADHD). His initial symptoms included inattention, hyperactivity, distractibility, short attention span, failure to follow instructions, poor organization, and intruding on others. He often picks fights with his 7-year-old brother, mildly injuring him on one occasion. His teacher recently punished him for roaming the classroom and distracting his classmates.
None of Mark’s symptoms suggest mania. His family has no history of mood disorders, but his father has been diagnosed with substance dependence.
Mark’s psychiatrist had prescribed an extended-release amphetamine salts preparation, 10 mg/d. Soon after, Mark began experiencing stomachaches, insomnia, facial flushing, and headaches. The dosage was reduced to 5 mg/d, but Mark stopped taking the medication after less than 3 weeks. Cognitive-behavioral therapy and classroom modifications were then tried for 11 months, but Mark’s behavior worsened. His symptoms now include inattention, distractibility, excessive talking, restlessness, and impulsivity.
The authors’ observations
Children and adolescents often present with excessive talking, distractibility, increased activity or restlessness, and loss of normal functioning. Distinguishing these ADHD symptoms from those of bipolar, disruptive, learning, movement, anxiety, substance-related or other mental disorders can be challenging.How to reduce mania risk when prescribing stimulants,” October 2005, at www.currentpsychiatry.com.)
Pediatric bipolar disorder often goes undetected because DSM-IV-TR criteria—established for adults—may not apply to youths. Children are more likely to present with a mixed mood state, less-distinct periods between episodes, grandiosity, irritability, and a chronic, continuous course.2 By contrast, bipolar adults often present with a sudden classic manic episode, elation, and euphoria.2 Adults also usually have relatively stable periods between episodes and tend to have comorbid substance dependence, panic, or eating disorders.
Mark’s symptoms still suggest ADHD. His inattention started before age 7, and his teacher is mostly concerned about his hyperactive, impulsive, and disruptive behavior. Mark’s mother, teacher, and psychiatrist feel confident that the ADHD diagnosis is correct, so we decide against comprehensive reassessment or prescribing a mood stabilizer. Mark’s mother opts to try the nonstimulant ADHD medication atomoxetine rather than a different stimulant.
Treatment: a moving experience
Mark’s psychiatrist prescribes atomoxetine, 18 mg/d for 4 days, then increases the dosage to 25 mg/d after finding that the boy could tolerate the medication.
Two weeks after starting atomoxetine, Mark becomes agitated and activated, and voices suicidal thoughts on one occasion. Without warning, while his mother is driving him to school, he opens the door of the moving vehicle and tries to jump out. His mother stops him by calling his name, yelling “No,” and slamming on the brakes. Mark tells her that he is “bad” and wants to die. She has no idea what prompted this behavior.
Mark also has become more oppositional and defiant, and his temper tantrums and destruction of household items are more frequent. He continues to behave aggressively toward his younger brother, often breaking some of his favorite toys. Mark also shows elevated and expansive mood, irritability, pressured speech, inflated self-esteem, and psychomotor agitation—symptoms consistent with a manic episode. At one visit, Mark tells his psychia trist, “I feel great! I can do anything.”
The authors’ observations
Mark, who had been diagnosed as having ADHD, began showing manic activation and suicidal thinking 2 weeks after starting atomoxetine. Whether he showed de novo suicidal behavior or reckless behavior associated with mania is unclear.
Atomoxetine-induced mania is not a new finding.2,5 During clinical trials, 2% of patients reported mood swings and 8% reported irritability. Subsequent experience indicates the risk of mood destabilization may be as high as 33%.5
Atomoxetine, a nonstimulant medication indicated for treating pediatric and adult ADHD, is a potent norepinephrine reuptake inhibitor. Reanalysis of the atomoxetine clinical trial database showed a slightly but statistically significant higher risk of suicidal behavior and thoughts in children and adolescents compared with placebo.6 No deaths from suicide were reported. The FDA subsequently ordered a black box warning on atomoxetine’s label instructing physicians, patients, and families to watch closely for suicidality symptoms with atomoxetine use.
Atomoxetine is safe and effective for pediatric ADHD, provided youths are properly monitored. Be careful, however, when prescribing atomoxetine to youths with a personal or family history of mood disorder.
FDA also is reviewing data on all drugs indicated for treating ADHD to determine whether they cause suicidality, new-onset mental disorders, or other psychiatric adverse events.7
Assessing medication risk
All youths being treated for a mood disorder and/or ADHD must be assessed for suicide risk, but how to most effectively perform this assessment is unclear. Organizations representing pediatrics and child and adolescent psychiatry have not yet incorporated FDA’s medication guidelines regarding pediatric suicidality—released earlier this year—into their guidelines (Table 1). As a result, most physicians follow pediatric patients less frequently than FDA now advises.
Atomoxetine’s receptor profile resembles that of antidepressants, which also are labeled with a black box warning describing increased suicidality risk when used in children and adolescents. Risk of suicidal behavior is highest within 10 days of starting antidepressants, and a significant risk remains throughout the first month. The suicidality rate appears to drop after that time.9,10
Follow FDA patient monitoring guidelines for antidepressants when prescribing atomoxetine to youths—particularly given the prospective labeling change. Atomoxetine’s manufacturer is expected to release a patient monitoring guideline unique to this drug.
Table 1
FDA guidelines for monitoring pediatric antidepressant use
| After starting an antidepressant, patients should see their doctor: |
|
| Source: Reference 8 |
Suicidality: finding other causes
Suicidality is more prevalent in bipolar disorder than in other mental disorders,2,4 and ADHD and mania often co-exist (Box 1).11,12 Mania induced by medication might explain suicidality or other behavior changes in some youths, but activation, mania, behavior change, or suicidality can result from the primary or comorbid disorder rather than the medication.
No deaths by suicide were reported among the FDA-reviewed studies of antidepressant use in children and adolescents. Fatal suicidal behavior has been reported in adolescents not treated with medications.14
FDA cites 12 features that point to suicide risk in youths (Box 2).8-10 Seven features suggest both ADHD and mania, which overlap to the point of diagnostic distraction.
As many as 20% of children diagnosed with ADHD also meet DSM-IV-TR criteria for bipolar disorder.
When bipolar disorder is the initial diagnosis, 30% to 40% of adolescents and 70% to 90% of prepubertal children may meet ADHD criteria.
Prepubescent major depression carries a 50% lifetime risk of developing mania.
Source: References 3, 11-13
- New or more thoughts of suicide
- Suicide attempts
- New or worsened depression
- New or worsened anxiety
- Feeling agitated or restless*
- Panic attacks
- Difficulty sleeping (insomnia)*
- New or worsened irritability*
- Aggressive, angry, or violent behavior*
- Acting on dangerous impulses*
- Extreme hyperactivity in actions and talking (hypomania or mania)*
- Other unusual behavior changes*
* Suggest both ADHD and mania
Source: References 8-10
The authors’ observations
Consider a broad differential diagnosis when evaluating inattention, hyperactivity, and impulsivity in children. Family medical history, corroborative clinical interviews, past and current behavioral rating scores, and psychological testing can help confirm an ADHD diagnosis (Table 2).
A careful patient interview, watching for diagnostic clues, taking a confirmatory history, and attention to key symptoms can help you discern ADHD from mania. Rule out unexplored diagnoses such as substance abuse, disturbed relationships, medical illness, and other mental disorders. Having the family and teachers track the youth’s longitudinal mood, energy, sleep, and actions may confirm a mood disorder.
Elated mood or grandiosity indicate mania. Irritable hyperactivity is seen more frequently in mania, whereas general hyperactivity tends to be present in ADHD. Childhood depression often heralds bipolar disorder.
Suspected medication-induced suicidality may call for stopping the offending agent, but determining whether a mental disorder or medication is causing suicidal thoughts can be difficult.
Try stopping the suspected offending drug first. If the youth remains suicidal after 1 week, a thorough biopsychosocial reassessment may guide future options including inpatient care, intensive outpatient psychotherapy, monitoring, and cautious use of antidepressant and/or antimanic medications.
Suicide risk requires clinician vigilance. As we learn from the FDA’s warnings, each treatment episode confers new risk and underscores the importance of watching for risk factors that may predict suicide (Table 3).
Table 2
What to include in an ADHD evaluation
| Histories: psychosocial, developmental, medical, educational, substance use and/or family |
| Clinical interviews with the child or adolescent. Corroborative interviews with parents, guardians, teachers, others |
| Rating scales assessing past behavior: Instruments completed by multiple sources such as the youth, family members or guardian, former teachers, others |
| Rating scales of current behavior: Instruments completed by youth, parents or guardian, former teachers, siblings, significant others |
| Psychological testing: Psychoeducational evaluation, personality inventory, intelligence assessment, and/or a continuous performance test. ADHD diagnosis remains clinical, and no evaluation should rely too heavily on “objective tests” for a definitive diagnosis |
Table 3
Risk factors that may predict suicide in youths
| Older (pubertal) age |
| Male gender |
| Mania |
| Mixed mood state |
| Psychosis |
| Victim of sexual or physical abuse |
| Co-occurring disruptive disorders |
| Comorbid substance abuse |
| Impulsivity |
| Easy access to means, such as firearms, lethal toxins, or medications |
| Lack of family support |
| Acute stressors |
| Family history of suicide |
| Source: Adapted from reference 2. |
Continued treatment: no more medication
Mark’s psychiatrist immediately stops atomoxetine. The boy’s mother, a psychiatric nurse, declines a trial of divalproex because she fears drug toxicity. Mark’s suicidality and agitation resolve over 1 week, and he returns to baseline function, leading us to believe his mania was medication-induced.
One year later, Mark takes no medications. He is behaving well at school and made the honor roll this fall. His teacher reports that Mark is “smart, well liked, but talks excessively,” though she says his talking is “not as out of control” as it was a year ago.
Mark recently began playing soccer as an outlet for his hyperactivity. He has not been penalized on the soccer field but is occasionally “over the edge,” pushing and shoving other players. When frustrated at home he has short outbursts, slams doors, and yells at his brother without being physically aggressive.
Mark’s office visits are infrequent, but he recently asked his mother to take him to his psychiatrist and counselor. His mother realizes he may soon need medication, but she wants to wait.
Related resources
- U.S. Food and Drug Administration. List of drugs receiving a boxed warning, other product labeling changes, and a medication guide pertaining to pediatric suicidality. www.fda.gov/cder/drug/antidepressants/MDD_alldruglist.pdf.
- Eli Lilly and Co. Questions and answers about the Strattera (atomoxetine) label change: a guide for patients and parents. www.strattera.com/1_5_news/Q&A_Strattera_label_update.pdf.
- U.S. Food and Drug Administration. Public health advisory: Suicidal thinking in children and adolescents being treated with Strattera (atomoxetine).www.fda.gov/cder/drug/advisory/atomoxetine.htm.
- Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294(16):2064-74.
Drug brand names
- Divalproex sodium • Depakote
- Mixed amphetamine salts • Adderall XR
- Atomoxetine • Strattera
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Rappley MD. Clinical practice: attention deficit-hyperactivity disorder. N Engl J Med 2005;352:165-73.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Scheffer RE, Apps JN. ADHD or bipolar disease? Age-specific manic symptoms are key. Current Psychiatry 2005;4(5):42-52.
4. Schapiro NA. Bipolar disorders in children and adolescents. J Pediatr Health Care 2005;19:131-41.
5. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114:895-6.
6. Eli Lilly and Co Questions and answers about the Strattera (atomoxetine) label change: a guide for patients and parents. Available at: http://www.strattera.com/1_5_news/Q&A_Strattera_label_update.pdf. Accessed Oct. 16, 2005.
7. Mathews AW, Abboud L. FDA offers more details on relabeling: concern that ADHD drugs may cause adverse events stemmed from few reports. Wall Street Journal June 30, 2005 D4.
8. U.S. Food and Drug Administration. The FDA required medication guide about using antidepressants in children and teenagers. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIMedicationGuide.htm. Accessed July 1, 2005.
9. U.S. Food and Drug Administration public health advisory October 15, 2004: Suicidality in children and adolescents being treated with antidepressant medications. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm. Accessed July 1, 2005.
10. U.S. Food and Drug Administration public health advisory: Labeling change request letter for antidepressant medications. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIlabelChange.htm. Accessed July 1, 2005.
11. Wozniak J, Biederman J, Kiely K, et al. Manic-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34:867-76.
12. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35:997-1008.
13. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82 (suppl):S59-S69.
14. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338-43.
1. Rappley MD. Clinical practice: attention deficit-hyperactivity disorder. N Engl J Med 2005;352:165-73.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Scheffer RE, Apps JN. ADHD or bipolar disease? Age-specific manic symptoms are key. Current Psychiatry 2005;4(5):42-52.
4. Schapiro NA. Bipolar disorders in children and adolescents. J Pediatr Health Care 2005;19:131-41.
5. Henderson TA, Hartman K. Aggression, mania, and hypomania induction associated with atomoxetine. Pediatrics 2004;114:895-6.
6. Eli Lilly and Co Questions and answers about the Strattera (atomoxetine) label change: a guide for patients and parents. Available at: http://www.strattera.com/1_5_news/Q&A_Strattera_label_update.pdf. Accessed Oct. 16, 2005.
7. Mathews AW, Abboud L. FDA offers more details on relabeling: concern that ADHD drugs may cause adverse events stemmed from few reports. Wall Street Journal June 30, 2005 D4.
8. U.S. Food and Drug Administration. The FDA required medication guide about using antidepressants in children and teenagers. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIMedicationGuide.htm. Accessed July 1, 2005.
9. U.S. Food and Drug Administration public health advisory October 15, 2004: Suicidality in children and adolescents being treated with antidepressant medications. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm. Accessed July 1, 2005.
10. U.S. Food and Drug Administration public health advisory: Labeling change request letter for antidepressant medications. Available at: http://www.fda.gov/cder/drug/antidepressants/SSRIlabelChange.htm. Accessed July 1, 2005.
11. Wozniak J, Biederman J, Kiely K, et al. Manic-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry 1995;34:867-76.
12. Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry 1996;35:997-1008.
13. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82 (suppl):S59-S69.
14. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338-43.
Are undiagnosed eating disorders keeping your patients sick?
The internist next door asks you about his patient with bulimia, who routinely has potassium levels of 2.0 mEq/L. “She admits it’s a problem but thinks she’ll get fat if she stops purging. What can I tell her to get her into treatment?”
That afternoon, your longtime patient Mr. J—age 56 with depression, obesity, and hypertension—arrives for his appointment. With the day’s earlier conversation in mind, you ask him if he has an eating problem. Staring at the floor, he describes a lifelong battle with nighttime eating binges, which he has never mentioned to you before.
Mr. J may have concealed his binge eating because of shame or ambivalence about stopping a psychologically protective behavior. And his eating disorder may be complicating his depression treatment.
But outpatient psychiatrists can often manage patients like Mr. J in consultation with a nutritionist and primary care physician. Eating disorders are treatable,1,2 and many patients can recover. This article describes how to identify eating disorders so that treatment can begin.
Psychiatric comorbidity
Eating disorders are common in outpatient practice (Box)3,4 and coexist with a variety of psychiatric diagnoses (Table 1). For example, in 248 women with anorexia, bulimia, or unspecified eating disorders, 74% had another Axis I disorder, including:
- anxiety disorders (54%)
- affective disorders (52%)
- substance-related disorders (25%).
Eating disorders also are much more common in persons who present with psychiatric problems than in the general population. For example:
- Among 62 patients with a primary diagnosis of obsessive-compulsive disorder, 13% had anorexia or bulimia nervosa and another 18% met subthreshold criteria.6
- In 257 female patients with anxiety disorders, nearly 12% also met criteria for a possible eating disorder.7
Some 40% of persons with eating disorders meet DSM-IV-TR criteria for anorexia or bulimia nervosa. The other 60%—with eating disorder, not otherwise specified (ED-NOS)—are divided nearly evenly between binge eating disorder and subsyndromal anorexia or bulimia. Outpatient psychiatrists see these eating disorders most often.
Anorexia and bulimia nervosa prevalence rates are estimated to be 0.3% and 1%, respectively.3 But including ED-NOS patients increases the overall eating disorder prevalence to 2% to 3%—equal to or greater than the combined rates of schizophrenia and bipolar I disorder.
Although considered “subsyndromal,” ED-NOS patients suffer psychopathology, impairment, and medical comorbidity similar to those of persons who meet DSM-IV-TR criteria for anorexia or bulimia nervosa.4
Common psychiatric comorbidities in patients with eating disorders
| Disorder | Comorbidities |
|---|---|
| Anorexia and bulimia nervosa | Anxiety disorders (social phobia, PTSD, OCD) |
| Mood disorders (major depressive disorder, dysthymia, bipolar disorder) | |
| Substance use disorders (more common in patients who binge and/or purge) | |
| Personality disorders (cluster C more common in restricting anorexia, cluster B more common in patients who binge and purge) | |
| Binge eating disorder | Anxiety disorders (PTSD) |
| Mood disorders (major depressive disorder, bipolar disorder) | |
| PTSD: posttraumatic stress disorder | |
| OCD: obsessive-compulsive disorder | |
Who has eating disorders?
Most eating disorder patients are adolescent girls or young women with pronounced body image dissatisfaction. Other patients include:
Atypical young women. Some young women—usually Asian—meet most criteria for anorexia nervosa but lack the characteristic drive for thinness. They tend to have less psychopathology and better prognosis than typical female patients.9
Boys and men. Female-to-male ratios are approximately 11:1 for anorexia, 5:1 for bulimia, and 3:1 for binge eating disorder. Men and boys with eating disorders are similar to their female counterparts but are more likely to report:
- comorbid substance abuse
- having begun weight loss and purging in response to teasing or concerns about health, sports performance, or gay relationships, rather than appearance.10
Middle-aged to late-life. Midlife onset of eating disorders may be precipitated by losses or concerns about aging. In the elderly, eating disorders may be manifestations of complicated bereavement, and ruling out medical causes of weight loss is crucial in this age group.
Night-eating syndrome. Some patients eat at least 25% of daily calories after the evening meal. They experience insomnia, morning anorexia, and sometimes amnesia for the nocturnal eating episodes. Anxiety, depression, or sleep disorders may be contributing factors.12
Identifying eating disorders
Screening. No formal guidelines recommend which psychiatric patients to screen for eating disorders. We suggest screening any patients who are over- or underweight or have eating disorder risk factors, such as:
- young women (teens and early 20s)
- athletes in certain sports (gymnastics, ballet, figure skating, running, body building, wrestling)
- history of childhood sexual abuse.13
Interviewing. Evaluate all 4 illness domains—nutritional, medical, psychological, and social. Because patients often do not volunteer information, ask about:
- symptoms and complications
- onset and development of eating and weight problems
- history of being teased or criticized about weight
- weight history (premorbid, lowest, highest, and preferred weights).
DSM-IV-TR defines binging as consuming a large quantity of food in a discrete time and feeling out of control of eating. Ask specifically how the patient defines “binge,” and seek details of a typical binge. Also ask about compensatory behaviors (purging by vomiting or using laxatives or diuretics). Is the patient abusing ipecac, diet pills, or thyroid hormone? Does he or she fast or exercise compulsively (such as even while ill)?
Eating and exercise patterns. Ask the patient to recall everything eaten in the past 24 hours. This history can help estimate caloric intake and may reveal problematic eating patterns. For example, does the patient:
- avoid certain foods, consider others to be “safe,” or use diet products, gum, or mints?
- engage in food rituals, such as slow eating, hoarding food, or eating odd combinations?
- steal food, weigh him/herself frequently, or visit pro-anorexia/pro-bulimia Web sites?
Table 2
Potential medical complications of anorexia and bulimia nervosa
| Organ system | Symptoms | Signs, syndromes, laboratory abnormalities |
|---|---|---|
| Cardiovascular | Palpitations, dyspnea, chest pain, dizziness | Bradycardia, orthostasis, acrocyanosis |
| Prolonged PR and QTc intervals on ECG, mitral valve prolapse, cardiomyopathy in ipecac abusers | ||
| CNS | Anxious, depressed, or irritable mood; obsessiveness; cognitive deficits; seizures (rare) | Enlarged ventricles on CT or MRI, deficits on neuropsychological testing, abnormal EEG, signs of peripheral neuropathy |
| Dermatologic | Hair loss, dry skin | Xerosis, carotenoderma, cheilitis, lanugo, brittle hair and nails, Russell’s sign (callus on dorsum of hand used to induce vomiting) |
| Endocrine | Fatigue, cold intolerance | Hypothermia, hypoglycemia, hypercortisolemia, ↓ T3 and T4 |
| Gastrointestinal | Bloating, constipation, spontaneous vomiting, reflux, abdominal pain, heartburn, hematemesis | Abnormal bowel sounds, delayed gastric emptying, superior mesenteric artery syndrome, pancreatitis |
| In patients who vomit: Mallory-Weiss tears, Barrett’s esophagus, occult blood in stool, ↑ amylase, gingivitis, dental caries, sialadenosis, perimolysis | ||
| Genitourinary | Polyuria, oliguria | ↑ BUN, nephrolithiasis, hypokalemic nephropathy, renal failure (rare). |
| Hematologic | Fatigue, bruising | Anemia; ↓ numbers of WBCs, RBCs and platelets; ↓ ferritin, B12, folate |
| Metabolic | Weakness, cardiac or CNS manifestations | ↓ K, Na, Mg, phosphate; ↑ cholesterol; metabolic alkalosis (from vomiting), or acidosis (from laxatives); thiamin and niacin deficiencies (rare). |
| Musculoskeletal | Weakness, cramps, bone pain | Wasting, ↑ CK (rare), decreased bone mineral density, pathologic fractures |
| Reproductive | Amenorrhea, ↓ libido, infertility, ↑ pregnancy, neonatal complications | Arrested sexual development; ↓ estrogen or testosterone; prepubertal levels of LH and FSH |
Interview adjuncts
Assessment tools. In addition to patient interviews, some clinicians use self-report scales to screen for eating disorders or to monitor treatment. Reliable and valid self-report questionnaires include the Eating Disorder Examination-Q (36 items),14 Eating Disorder Inventory (91 items),15 and Eating Attitudes Test (26 items).16
The Eating Attitudes Test takes 10 minutes to complete and is widely used for screening. A cut-off score of 20 indicates a potential eating disorder and the need for a follow-up interview.
Self-report diaries can help identify binge eating triggers—usually dietary restriction combined with interpersonal stressors. Ask the patient to record all meals, snacks, binges, purges, and exercise activities, plus time of day and associated feelings, thoughts, and situations. Diaries can also reveal maladaptive thoughts, such as body image distortion, and problematic coping strategies, such as purging or excessive exercising.
Medical workup
Measure height and weight, calculate body mass index, and check vital signs (including supine and standing blood pressure and pulse) and hydration status. Perform a neurologic exam, particularly for peripheral neuropathy, and check for cardiac, dermatologic, and GI complications (Tables 2 and 3). Include a dental examination if the patient admits or you suspect self-induced vomiting.
If treating eating disorders’ medical consequences is beyond the scope of your practice, refer the patient for evaluation by a physician with this experience.
Table 3
Common medical complications of binge eating disorder
| Obesity (body mass index>30) and related comorbidities: |
| Hypertension |
| Diabetes mellitus |
| Hyperlipidemia |
| Increased cardiovascular mortality |
| Obstructive sleep apnea |
| Degenerative arthritis |
| Gastroesophageal reflux symptoms and complications |
- Weight for height in women: 100 lbs for the first 5 feet, +5 lbs/inch over 5 feet
- Weight for height in men: 106 lbs for the first 5 feet, +6 lbs/inch over 5 feet.
For adolescents with suspected anorexia nervosa, estimate expected body weight from individual growth curves or standard growth charts posted on the Centers for Disease Control and Prevention Web site (see Related resources).
Note that the DSM-IV-TR weight criterion for anorexia of “less than 85% of expected” is an example, not an absolute cutoff. Anorexia nervosa would be an appropriate diagnosis for a patient who weighs more than 85% of expected weight but has lost substantial weight and meets the other diagnostic criteria.
BMI
Laboratory tests vary, depending on patients’ suspected eating disorders (Table 4). In 214 outpatient women with anorexia, the most common abnormalities were anemia (38.6%), leukocytopenia (34.4%), hyponatremia (19.7%) and hypokalemia (19.7%).17 With few exceptions, abnormal values are not predicted by the apparent degree of undernutrition.
Table 4
Laboratory studies for patients with suspected eating disorders
| For whom | Recommended tests |
|---|---|
| All eating disorder patients | Comprehensive metabolic panel (electrolytes, glucose, albumin, measures of hepatic and renal function), complete blood count, urinalysis, ECG, TSH |
| Add for patients with anorexia | Serum magnesium, phosphate, calcium; creatinine clearance; chest radiography; estrogen in women, testosterone in men; DEXA bone density scan; consider echocardiography, brain MRI; screen urine for unreported substances of abuse |
| Add for patients with bulimia and purging type anorexia | Serum magnesium, phosphate, calcium; DEXA scan if patient is amenorrheic or has history of anorexia; amylase (fractionated, if possible); consider fecal occult blood, urine for electrolytes and laxatives, urine drug screen |
| Add for patients with binge eating disorder | Fasting blood glucose, fasting lipid profile |
From diagnosis to treatment
Talking with patients. Discussing abnormal lab results with patients can be therapeutic. In our experience, recovered patients often report that worry about medical complications was their primary reason to seek treatment for eating disorders.
Relate the patient’s cognitive, mood, and physical symptoms to abnormal eating behavior, then present the eating disorder diagnosis as the beginning of treatment. For example, you could praise Mr. J for his courage in revealing his binge eating and tell him that identifying this problem is the first step toward solving it. Not only can he overcome binge eating, but treatment will also likely improve his mood, weight, and blood pressure.
Eating disorder patients who are medically stable, motivated for treatment, have good support, and are able and willing to come for frequent appointments are good candidates for outpatient eating disorder treatment.
For clinicians
- Standard growth charts. National Center for Health Statistics. Centers for Disease Control and Prevention. www.cdc.gov/growthcharts.
- Brewerton TD. Clinical handbook of eating disorders: an integrated approach. New York: Marcel Dekker; 2004.
- Work group on eating disorders. Practice guideline for the treatment of patients with eating disorders (2nd ed.). Washington, DC: American Psychiatric Publishing; 2000. Available at: http://www.psych.org/psych_pract/treatg/pg/eating_revisebook_index.cfm.
- Zerbe KJ. The body betrayed: a deeper understanding of women, eating disorders, and treatment. Carlsbad, CA: Gürze Books; 1995.
- National Eating Disorders Association. www.nationaleatingdisorders.org.
- National Association of Anorexia Nervosa and Associated Disorders. www.anad.org.
1. Reas Dl, Williamson DA, Martin CK, Zucker NL. Duration of illness predicts outcome for bulimia nervosa: a long-term outcome study. Int J Eat Disord 2000;27:428-34.
2. Nielsen S, Moller-Madsen S, Isager T, et al. Standardized mortality in eating disorders—a quantitative summary of previously published and new evidence. J Psychosom Res 1998;44:413-34.
3. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34(4):383-96.
4. Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175-82.
5. Milos G, Spindler A, Schnyder U. Psychiatric comorbidity and eating disorder inventory (EDI) profiles in eating disorder patients. Can J Psychiatry 2004;49:179-84.
6. Rubenstein CS, Pigott TA, L’ Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
7. Becker CB, DeViva JC, Zayfert C. Eating disorder symptoms among female anxiety disorder patients in clinical practice: the importance of anxiety comorbidity assessment. J Anxiety Disord 2004;18(3):255-74.
8. Keys A, Brozek J, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
9. Ramacciotti CE, Dell’Osso L, Paoli RA, et al. Characteristics of eating disorder patients without a drive for thinness. Int J Eat Disord 2002;32:206-12.
10. Andersen AE. Males with eating disorders: medical considerations. In: Mehler PS, Andersen AE (eds). Eating disorders: a guide to medical care and complications. Baltimore: The Johns Hopkins University Press; 1999;214-26.
11. Lantzouni E, Frank GR, Golden NH, Shenker RI. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health 2002;31(2):162-5.
12. Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eating Disord 2001;30:193-203.
13. Jacobi C, Morris L, de Zwaan M. Overview of risk factors for anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton, TD (ed). Clinical handbook of eating disorders: An integrated approach. New York: Marcel Dekker; 2004;183-208.
14. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord 1994;16:363-70.
15. Garner DM. Eating Disorder Inventory-2 professional manual. Odessa, FL: Psychological Assessment Resources; 1991.
16. Garner DM. Psychoeducational principles in treatment. In: Garner DM, Garfinkel PE (eds). Handbook of treatment for eating disorders (2nd ed). New York: Guilford Press; 1997;145-77.
17. Miller KK, Grinspoon SK, Ciampa J, et al. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 2005;165(5):561-6.
The internist next door asks you about his patient with bulimia, who routinely has potassium levels of 2.0 mEq/L. “She admits it’s a problem but thinks she’ll get fat if she stops purging. What can I tell her to get her into treatment?”
That afternoon, your longtime patient Mr. J—age 56 with depression, obesity, and hypertension—arrives for his appointment. With the day’s earlier conversation in mind, you ask him if he has an eating problem. Staring at the floor, he describes a lifelong battle with nighttime eating binges, which he has never mentioned to you before.
Mr. J may have concealed his binge eating because of shame or ambivalence about stopping a psychologically protective behavior. And his eating disorder may be complicating his depression treatment.
But outpatient psychiatrists can often manage patients like Mr. J in consultation with a nutritionist and primary care physician. Eating disorders are treatable,1,2 and many patients can recover. This article describes how to identify eating disorders so that treatment can begin.
Psychiatric comorbidity
Eating disorders are common in outpatient practice (Box)3,4 and coexist with a variety of psychiatric diagnoses (Table 1). For example, in 248 women with anorexia, bulimia, or unspecified eating disorders, 74% had another Axis I disorder, including:
- anxiety disorders (54%)
- affective disorders (52%)
- substance-related disorders (25%).
Eating disorders also are much more common in persons who present with psychiatric problems than in the general population. For example:
- Among 62 patients with a primary diagnosis of obsessive-compulsive disorder, 13% had anorexia or bulimia nervosa and another 18% met subthreshold criteria.6
- In 257 female patients with anxiety disorders, nearly 12% also met criteria for a possible eating disorder.7
Some 40% of persons with eating disorders meet DSM-IV-TR criteria for anorexia or bulimia nervosa. The other 60%—with eating disorder, not otherwise specified (ED-NOS)—are divided nearly evenly between binge eating disorder and subsyndromal anorexia or bulimia. Outpatient psychiatrists see these eating disorders most often.
Anorexia and bulimia nervosa prevalence rates are estimated to be 0.3% and 1%, respectively.3 But including ED-NOS patients increases the overall eating disorder prevalence to 2% to 3%—equal to or greater than the combined rates of schizophrenia and bipolar I disorder.
Although considered “subsyndromal,” ED-NOS patients suffer psychopathology, impairment, and medical comorbidity similar to those of persons who meet DSM-IV-TR criteria for anorexia or bulimia nervosa.4
Common psychiatric comorbidities in patients with eating disorders
| Disorder | Comorbidities |
|---|---|
| Anorexia and bulimia nervosa | Anxiety disorders (social phobia, PTSD, OCD) |
| Mood disorders (major depressive disorder, dysthymia, bipolar disorder) | |
| Substance use disorders (more common in patients who binge and/or purge) | |
| Personality disorders (cluster C more common in restricting anorexia, cluster B more common in patients who binge and purge) | |
| Binge eating disorder | Anxiety disorders (PTSD) |
| Mood disorders (major depressive disorder, bipolar disorder) | |
| PTSD: posttraumatic stress disorder | |
| OCD: obsessive-compulsive disorder | |
Who has eating disorders?
Most eating disorder patients are adolescent girls or young women with pronounced body image dissatisfaction. Other patients include:
Atypical young women. Some young women—usually Asian—meet most criteria for anorexia nervosa but lack the characteristic drive for thinness. They tend to have less psychopathology and better prognosis than typical female patients.9
Boys and men. Female-to-male ratios are approximately 11:1 for anorexia, 5:1 for bulimia, and 3:1 for binge eating disorder. Men and boys with eating disorders are similar to their female counterparts but are more likely to report:
- comorbid substance abuse
- having begun weight loss and purging in response to teasing or concerns about health, sports performance, or gay relationships, rather than appearance.10
Middle-aged to late-life. Midlife onset of eating disorders may be precipitated by losses or concerns about aging. In the elderly, eating disorders may be manifestations of complicated bereavement, and ruling out medical causes of weight loss is crucial in this age group.
Night-eating syndrome. Some patients eat at least 25% of daily calories after the evening meal. They experience insomnia, morning anorexia, and sometimes amnesia for the nocturnal eating episodes. Anxiety, depression, or sleep disorders may be contributing factors.12
Identifying eating disorders
Screening. No formal guidelines recommend which psychiatric patients to screen for eating disorders. We suggest screening any patients who are over- or underweight or have eating disorder risk factors, such as:
- young women (teens and early 20s)
- athletes in certain sports (gymnastics, ballet, figure skating, running, body building, wrestling)
- history of childhood sexual abuse.13
Interviewing. Evaluate all 4 illness domains—nutritional, medical, psychological, and social. Because patients often do not volunteer information, ask about:
- symptoms and complications
- onset and development of eating and weight problems
- history of being teased or criticized about weight
- weight history (premorbid, lowest, highest, and preferred weights).
DSM-IV-TR defines binging as consuming a large quantity of food in a discrete time and feeling out of control of eating. Ask specifically how the patient defines “binge,” and seek details of a typical binge. Also ask about compensatory behaviors (purging by vomiting or using laxatives or diuretics). Is the patient abusing ipecac, diet pills, or thyroid hormone? Does he or she fast or exercise compulsively (such as even while ill)?
Eating and exercise patterns. Ask the patient to recall everything eaten in the past 24 hours. This history can help estimate caloric intake and may reveal problematic eating patterns. For example, does the patient:
- avoid certain foods, consider others to be “safe,” or use diet products, gum, or mints?
- engage in food rituals, such as slow eating, hoarding food, or eating odd combinations?
- steal food, weigh him/herself frequently, or visit pro-anorexia/pro-bulimia Web sites?
Table 2
Potential medical complications of anorexia and bulimia nervosa
| Organ system | Symptoms | Signs, syndromes, laboratory abnormalities |
|---|---|---|
| Cardiovascular | Palpitations, dyspnea, chest pain, dizziness | Bradycardia, orthostasis, acrocyanosis |
| Prolonged PR and QTc intervals on ECG, mitral valve prolapse, cardiomyopathy in ipecac abusers | ||
| CNS | Anxious, depressed, or irritable mood; obsessiveness; cognitive deficits; seizures (rare) | Enlarged ventricles on CT or MRI, deficits on neuropsychological testing, abnormal EEG, signs of peripheral neuropathy |
| Dermatologic | Hair loss, dry skin | Xerosis, carotenoderma, cheilitis, lanugo, brittle hair and nails, Russell’s sign (callus on dorsum of hand used to induce vomiting) |
| Endocrine | Fatigue, cold intolerance | Hypothermia, hypoglycemia, hypercortisolemia, ↓ T3 and T4 |
| Gastrointestinal | Bloating, constipation, spontaneous vomiting, reflux, abdominal pain, heartburn, hematemesis | Abnormal bowel sounds, delayed gastric emptying, superior mesenteric artery syndrome, pancreatitis |
| In patients who vomit: Mallory-Weiss tears, Barrett’s esophagus, occult blood in stool, ↑ amylase, gingivitis, dental caries, sialadenosis, perimolysis | ||
| Genitourinary | Polyuria, oliguria | ↑ BUN, nephrolithiasis, hypokalemic nephropathy, renal failure (rare). |
| Hematologic | Fatigue, bruising | Anemia; ↓ numbers of WBCs, RBCs and platelets; ↓ ferritin, B12, folate |
| Metabolic | Weakness, cardiac or CNS manifestations | ↓ K, Na, Mg, phosphate; ↑ cholesterol; metabolic alkalosis (from vomiting), or acidosis (from laxatives); thiamin and niacin deficiencies (rare). |
| Musculoskeletal | Weakness, cramps, bone pain | Wasting, ↑ CK (rare), decreased bone mineral density, pathologic fractures |
| Reproductive | Amenorrhea, ↓ libido, infertility, ↑ pregnancy, neonatal complications | Arrested sexual development; ↓ estrogen or testosterone; prepubertal levels of LH and FSH |
Interview adjuncts
Assessment tools. In addition to patient interviews, some clinicians use self-report scales to screen for eating disorders or to monitor treatment. Reliable and valid self-report questionnaires include the Eating Disorder Examination-Q (36 items),14 Eating Disorder Inventory (91 items),15 and Eating Attitudes Test (26 items).16
The Eating Attitudes Test takes 10 minutes to complete and is widely used for screening. A cut-off score of 20 indicates a potential eating disorder and the need for a follow-up interview.
Self-report diaries can help identify binge eating triggers—usually dietary restriction combined with interpersonal stressors. Ask the patient to record all meals, snacks, binges, purges, and exercise activities, plus time of day and associated feelings, thoughts, and situations. Diaries can also reveal maladaptive thoughts, such as body image distortion, and problematic coping strategies, such as purging or excessive exercising.
Medical workup
Measure height and weight, calculate body mass index, and check vital signs (including supine and standing blood pressure and pulse) and hydration status. Perform a neurologic exam, particularly for peripheral neuropathy, and check for cardiac, dermatologic, and GI complications (Tables 2 and 3). Include a dental examination if the patient admits or you suspect self-induced vomiting.
If treating eating disorders’ medical consequences is beyond the scope of your practice, refer the patient for evaluation by a physician with this experience.
Table 3
Common medical complications of binge eating disorder
| Obesity (body mass index>30) and related comorbidities: |
| Hypertension |
| Diabetes mellitus |
| Hyperlipidemia |
| Increased cardiovascular mortality |
| Obstructive sleep apnea |
| Degenerative arthritis |
| Gastroesophageal reflux symptoms and complications |
- Weight for height in women: 100 lbs for the first 5 feet, +5 lbs/inch over 5 feet
- Weight for height in men: 106 lbs for the first 5 feet, +6 lbs/inch over 5 feet.
For adolescents with suspected anorexia nervosa, estimate expected body weight from individual growth curves or standard growth charts posted on the Centers for Disease Control and Prevention Web site (see Related resources).
Note that the DSM-IV-TR weight criterion for anorexia of “less than 85% of expected” is an example, not an absolute cutoff. Anorexia nervosa would be an appropriate diagnosis for a patient who weighs more than 85% of expected weight but has lost substantial weight and meets the other diagnostic criteria.
BMI
Laboratory tests vary, depending on patients’ suspected eating disorders (Table 4). In 214 outpatient women with anorexia, the most common abnormalities were anemia (38.6%), leukocytopenia (34.4%), hyponatremia (19.7%) and hypokalemia (19.7%).17 With few exceptions, abnormal values are not predicted by the apparent degree of undernutrition.
Table 4
Laboratory studies for patients with suspected eating disorders
| For whom | Recommended tests |
|---|---|
| All eating disorder patients | Comprehensive metabolic panel (electrolytes, glucose, albumin, measures of hepatic and renal function), complete blood count, urinalysis, ECG, TSH |
| Add for patients with anorexia | Serum magnesium, phosphate, calcium; creatinine clearance; chest radiography; estrogen in women, testosterone in men; DEXA bone density scan; consider echocardiography, brain MRI; screen urine for unreported substances of abuse |
| Add for patients with bulimia and purging type anorexia | Serum magnesium, phosphate, calcium; DEXA scan if patient is amenorrheic or has history of anorexia; amylase (fractionated, if possible); consider fecal occult blood, urine for electrolytes and laxatives, urine drug screen |
| Add for patients with binge eating disorder | Fasting blood glucose, fasting lipid profile |
From diagnosis to treatment
Talking with patients. Discussing abnormal lab results with patients can be therapeutic. In our experience, recovered patients often report that worry about medical complications was their primary reason to seek treatment for eating disorders.
Relate the patient’s cognitive, mood, and physical symptoms to abnormal eating behavior, then present the eating disorder diagnosis as the beginning of treatment. For example, you could praise Mr. J for his courage in revealing his binge eating and tell him that identifying this problem is the first step toward solving it. Not only can he overcome binge eating, but treatment will also likely improve his mood, weight, and blood pressure.
Eating disorder patients who are medically stable, motivated for treatment, have good support, and are able and willing to come for frequent appointments are good candidates for outpatient eating disorder treatment.
For clinicians
- Standard growth charts. National Center for Health Statistics. Centers for Disease Control and Prevention. www.cdc.gov/growthcharts.
- Brewerton TD. Clinical handbook of eating disorders: an integrated approach. New York: Marcel Dekker; 2004.
- Work group on eating disorders. Practice guideline for the treatment of patients with eating disorders (2nd ed.). Washington, DC: American Psychiatric Publishing; 2000. Available at: http://www.psych.org/psych_pract/treatg/pg/eating_revisebook_index.cfm.
- Zerbe KJ. The body betrayed: a deeper understanding of women, eating disorders, and treatment. Carlsbad, CA: Gürze Books; 1995.
- National Eating Disorders Association. www.nationaleatingdisorders.org.
- National Association of Anorexia Nervosa and Associated Disorders. www.anad.org.
The internist next door asks you about his patient with bulimia, who routinely has potassium levels of 2.0 mEq/L. “She admits it’s a problem but thinks she’ll get fat if she stops purging. What can I tell her to get her into treatment?”
That afternoon, your longtime patient Mr. J—age 56 with depression, obesity, and hypertension—arrives for his appointment. With the day’s earlier conversation in mind, you ask him if he has an eating problem. Staring at the floor, he describes a lifelong battle with nighttime eating binges, which he has never mentioned to you before.
Mr. J may have concealed his binge eating because of shame or ambivalence about stopping a psychologically protective behavior. And his eating disorder may be complicating his depression treatment.
But outpatient psychiatrists can often manage patients like Mr. J in consultation with a nutritionist and primary care physician. Eating disorders are treatable,1,2 and many patients can recover. This article describes how to identify eating disorders so that treatment can begin.
Psychiatric comorbidity
Eating disorders are common in outpatient practice (Box)3,4 and coexist with a variety of psychiatric diagnoses (Table 1). For example, in 248 women with anorexia, bulimia, or unspecified eating disorders, 74% had another Axis I disorder, including:
- anxiety disorders (54%)
- affective disorders (52%)
- substance-related disorders (25%).
Eating disorders also are much more common in persons who present with psychiatric problems than in the general population. For example:
- Among 62 patients with a primary diagnosis of obsessive-compulsive disorder, 13% had anorexia or bulimia nervosa and another 18% met subthreshold criteria.6
- In 257 female patients with anxiety disorders, nearly 12% also met criteria for a possible eating disorder.7
Some 40% of persons with eating disorders meet DSM-IV-TR criteria for anorexia or bulimia nervosa. The other 60%—with eating disorder, not otherwise specified (ED-NOS)—are divided nearly evenly between binge eating disorder and subsyndromal anorexia or bulimia. Outpatient psychiatrists see these eating disorders most often.
Anorexia and bulimia nervosa prevalence rates are estimated to be 0.3% and 1%, respectively.3 But including ED-NOS patients increases the overall eating disorder prevalence to 2% to 3%—equal to or greater than the combined rates of schizophrenia and bipolar I disorder.
Although considered “subsyndromal,” ED-NOS patients suffer psychopathology, impairment, and medical comorbidity similar to those of persons who meet DSM-IV-TR criteria for anorexia or bulimia nervosa.4
Common psychiatric comorbidities in patients with eating disorders
| Disorder | Comorbidities |
|---|---|
| Anorexia and bulimia nervosa | Anxiety disorders (social phobia, PTSD, OCD) |
| Mood disorders (major depressive disorder, dysthymia, bipolar disorder) | |
| Substance use disorders (more common in patients who binge and/or purge) | |
| Personality disorders (cluster C more common in restricting anorexia, cluster B more common in patients who binge and purge) | |
| Binge eating disorder | Anxiety disorders (PTSD) |
| Mood disorders (major depressive disorder, bipolar disorder) | |
| PTSD: posttraumatic stress disorder | |
| OCD: obsessive-compulsive disorder | |
Who has eating disorders?
Most eating disorder patients are adolescent girls or young women with pronounced body image dissatisfaction. Other patients include:
Atypical young women. Some young women—usually Asian—meet most criteria for anorexia nervosa but lack the characteristic drive for thinness. They tend to have less psychopathology and better prognosis than typical female patients.9
Boys and men. Female-to-male ratios are approximately 11:1 for anorexia, 5:1 for bulimia, and 3:1 for binge eating disorder. Men and boys with eating disorders are similar to their female counterparts but are more likely to report:
- comorbid substance abuse
- having begun weight loss and purging in response to teasing or concerns about health, sports performance, or gay relationships, rather than appearance.10
Middle-aged to late-life. Midlife onset of eating disorders may be precipitated by losses or concerns about aging. In the elderly, eating disorders may be manifestations of complicated bereavement, and ruling out medical causes of weight loss is crucial in this age group.
Night-eating syndrome. Some patients eat at least 25% of daily calories after the evening meal. They experience insomnia, morning anorexia, and sometimes amnesia for the nocturnal eating episodes. Anxiety, depression, or sleep disorders may be contributing factors.12
Identifying eating disorders
Screening. No formal guidelines recommend which psychiatric patients to screen for eating disorders. We suggest screening any patients who are over- or underweight or have eating disorder risk factors, such as:
- young women (teens and early 20s)
- athletes in certain sports (gymnastics, ballet, figure skating, running, body building, wrestling)
- history of childhood sexual abuse.13
Interviewing. Evaluate all 4 illness domains—nutritional, medical, psychological, and social. Because patients often do not volunteer information, ask about:
- symptoms and complications
- onset and development of eating and weight problems
- history of being teased or criticized about weight
- weight history (premorbid, lowest, highest, and preferred weights).
DSM-IV-TR defines binging as consuming a large quantity of food in a discrete time and feeling out of control of eating. Ask specifically how the patient defines “binge,” and seek details of a typical binge. Also ask about compensatory behaviors (purging by vomiting or using laxatives or diuretics). Is the patient abusing ipecac, diet pills, or thyroid hormone? Does he or she fast or exercise compulsively (such as even while ill)?
Eating and exercise patterns. Ask the patient to recall everything eaten in the past 24 hours. This history can help estimate caloric intake and may reveal problematic eating patterns. For example, does the patient:
- avoid certain foods, consider others to be “safe,” or use diet products, gum, or mints?
- engage in food rituals, such as slow eating, hoarding food, or eating odd combinations?
- steal food, weigh him/herself frequently, or visit pro-anorexia/pro-bulimia Web sites?
Table 2
Potential medical complications of anorexia and bulimia nervosa
| Organ system | Symptoms | Signs, syndromes, laboratory abnormalities |
|---|---|---|
| Cardiovascular | Palpitations, dyspnea, chest pain, dizziness | Bradycardia, orthostasis, acrocyanosis |
| Prolonged PR and QTc intervals on ECG, mitral valve prolapse, cardiomyopathy in ipecac abusers | ||
| CNS | Anxious, depressed, or irritable mood; obsessiveness; cognitive deficits; seizures (rare) | Enlarged ventricles on CT or MRI, deficits on neuropsychological testing, abnormal EEG, signs of peripheral neuropathy |
| Dermatologic | Hair loss, dry skin | Xerosis, carotenoderma, cheilitis, lanugo, brittle hair and nails, Russell’s sign (callus on dorsum of hand used to induce vomiting) |
| Endocrine | Fatigue, cold intolerance | Hypothermia, hypoglycemia, hypercortisolemia, ↓ T3 and T4 |
| Gastrointestinal | Bloating, constipation, spontaneous vomiting, reflux, abdominal pain, heartburn, hematemesis | Abnormal bowel sounds, delayed gastric emptying, superior mesenteric artery syndrome, pancreatitis |
| In patients who vomit: Mallory-Weiss tears, Barrett’s esophagus, occult blood in stool, ↑ amylase, gingivitis, dental caries, sialadenosis, perimolysis | ||
| Genitourinary | Polyuria, oliguria | ↑ BUN, nephrolithiasis, hypokalemic nephropathy, renal failure (rare). |
| Hematologic | Fatigue, bruising | Anemia; ↓ numbers of WBCs, RBCs and platelets; ↓ ferritin, B12, folate |
| Metabolic | Weakness, cardiac or CNS manifestations | ↓ K, Na, Mg, phosphate; ↑ cholesterol; metabolic alkalosis (from vomiting), or acidosis (from laxatives); thiamin and niacin deficiencies (rare). |
| Musculoskeletal | Weakness, cramps, bone pain | Wasting, ↑ CK (rare), decreased bone mineral density, pathologic fractures |
| Reproductive | Amenorrhea, ↓ libido, infertility, ↑ pregnancy, neonatal complications | Arrested sexual development; ↓ estrogen or testosterone; prepubertal levels of LH and FSH |
Interview adjuncts
Assessment tools. In addition to patient interviews, some clinicians use self-report scales to screen for eating disorders or to monitor treatment. Reliable and valid self-report questionnaires include the Eating Disorder Examination-Q (36 items),14 Eating Disorder Inventory (91 items),15 and Eating Attitudes Test (26 items).16
The Eating Attitudes Test takes 10 minutes to complete and is widely used for screening. A cut-off score of 20 indicates a potential eating disorder and the need for a follow-up interview.
Self-report diaries can help identify binge eating triggers—usually dietary restriction combined with interpersonal stressors. Ask the patient to record all meals, snacks, binges, purges, and exercise activities, plus time of day and associated feelings, thoughts, and situations. Diaries can also reveal maladaptive thoughts, such as body image distortion, and problematic coping strategies, such as purging or excessive exercising.
Medical workup
Measure height and weight, calculate body mass index, and check vital signs (including supine and standing blood pressure and pulse) and hydration status. Perform a neurologic exam, particularly for peripheral neuropathy, and check for cardiac, dermatologic, and GI complications (Tables 2 and 3). Include a dental examination if the patient admits or you suspect self-induced vomiting.
If treating eating disorders’ medical consequences is beyond the scope of your practice, refer the patient for evaluation by a physician with this experience.
Table 3
Common medical complications of binge eating disorder
| Obesity (body mass index>30) and related comorbidities: |
| Hypertension |
| Diabetes mellitus |
| Hyperlipidemia |
| Increased cardiovascular mortality |
| Obstructive sleep apnea |
| Degenerative arthritis |
| Gastroesophageal reflux symptoms and complications |
- Weight for height in women: 100 lbs for the first 5 feet, +5 lbs/inch over 5 feet
- Weight for height in men: 106 lbs for the first 5 feet, +6 lbs/inch over 5 feet.
For adolescents with suspected anorexia nervosa, estimate expected body weight from individual growth curves or standard growth charts posted on the Centers for Disease Control and Prevention Web site (see Related resources).
Note that the DSM-IV-TR weight criterion for anorexia of “less than 85% of expected” is an example, not an absolute cutoff. Anorexia nervosa would be an appropriate diagnosis for a patient who weighs more than 85% of expected weight but has lost substantial weight and meets the other diagnostic criteria.
BMI
Laboratory tests vary, depending on patients’ suspected eating disorders (Table 4). In 214 outpatient women with anorexia, the most common abnormalities were anemia (38.6%), leukocytopenia (34.4%), hyponatremia (19.7%) and hypokalemia (19.7%).17 With few exceptions, abnormal values are not predicted by the apparent degree of undernutrition.
Table 4
Laboratory studies for patients with suspected eating disorders
| For whom | Recommended tests |
|---|---|
| All eating disorder patients | Comprehensive metabolic panel (electrolytes, glucose, albumin, measures of hepatic and renal function), complete blood count, urinalysis, ECG, TSH |
| Add for patients with anorexia | Serum magnesium, phosphate, calcium; creatinine clearance; chest radiography; estrogen in women, testosterone in men; DEXA bone density scan; consider echocardiography, brain MRI; screen urine for unreported substances of abuse |
| Add for patients with bulimia and purging type anorexia | Serum magnesium, phosphate, calcium; DEXA scan if patient is amenorrheic or has history of anorexia; amylase (fractionated, if possible); consider fecal occult blood, urine for electrolytes and laxatives, urine drug screen |
| Add for patients with binge eating disorder | Fasting blood glucose, fasting lipid profile |
From diagnosis to treatment
Talking with patients. Discussing abnormal lab results with patients can be therapeutic. In our experience, recovered patients often report that worry about medical complications was their primary reason to seek treatment for eating disorders.
Relate the patient’s cognitive, mood, and physical symptoms to abnormal eating behavior, then present the eating disorder diagnosis as the beginning of treatment. For example, you could praise Mr. J for his courage in revealing his binge eating and tell him that identifying this problem is the first step toward solving it. Not only can he overcome binge eating, but treatment will also likely improve his mood, weight, and blood pressure.
Eating disorder patients who are medically stable, motivated for treatment, have good support, and are able and willing to come for frequent appointments are good candidates for outpatient eating disorder treatment.
For clinicians
- Standard growth charts. National Center for Health Statistics. Centers for Disease Control and Prevention. www.cdc.gov/growthcharts.
- Brewerton TD. Clinical handbook of eating disorders: an integrated approach. New York: Marcel Dekker; 2004.
- Work group on eating disorders. Practice guideline for the treatment of patients with eating disorders (2nd ed.). Washington, DC: American Psychiatric Publishing; 2000. Available at: http://www.psych.org/psych_pract/treatg/pg/eating_revisebook_index.cfm.
- Zerbe KJ. The body betrayed: a deeper understanding of women, eating disorders, and treatment. Carlsbad, CA: Gürze Books; 1995.
- National Eating Disorders Association. www.nationaleatingdisorders.org.
- National Association of Anorexia Nervosa and Associated Disorders. www.anad.org.
1. Reas Dl, Williamson DA, Martin CK, Zucker NL. Duration of illness predicts outcome for bulimia nervosa: a long-term outcome study. Int J Eat Disord 2000;27:428-34.
2. Nielsen S, Moller-Madsen S, Isager T, et al. Standardized mortality in eating disorders—a quantitative summary of previously published and new evidence. J Psychosom Res 1998;44:413-34.
3. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34(4):383-96.
4. Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175-82.
5. Milos G, Spindler A, Schnyder U. Psychiatric comorbidity and eating disorder inventory (EDI) profiles in eating disorder patients. Can J Psychiatry 2004;49:179-84.
6. Rubenstein CS, Pigott TA, L’ Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
7. Becker CB, DeViva JC, Zayfert C. Eating disorder symptoms among female anxiety disorder patients in clinical practice: the importance of anxiety comorbidity assessment. J Anxiety Disord 2004;18(3):255-74.
8. Keys A, Brozek J, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
9. Ramacciotti CE, Dell’Osso L, Paoli RA, et al. Characteristics of eating disorder patients without a drive for thinness. Int J Eat Disord 2002;32:206-12.
10. Andersen AE. Males with eating disorders: medical considerations. In: Mehler PS, Andersen AE (eds). Eating disorders: a guide to medical care and complications. Baltimore: The Johns Hopkins University Press; 1999;214-26.
11. Lantzouni E, Frank GR, Golden NH, Shenker RI. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health 2002;31(2):162-5.
12. Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eating Disord 2001;30:193-203.
13. Jacobi C, Morris L, de Zwaan M. Overview of risk factors for anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton, TD (ed). Clinical handbook of eating disorders: An integrated approach. New York: Marcel Dekker; 2004;183-208.
14. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord 1994;16:363-70.
15. Garner DM. Eating Disorder Inventory-2 professional manual. Odessa, FL: Psychological Assessment Resources; 1991.
16. Garner DM. Psychoeducational principles in treatment. In: Garner DM, Garfinkel PE (eds). Handbook of treatment for eating disorders (2nd ed). New York: Guilford Press; 1997;145-77.
17. Miller KK, Grinspoon SK, Ciampa J, et al. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 2005;165(5):561-6.
1. Reas Dl, Williamson DA, Martin CK, Zucker NL. Duration of illness predicts outcome for bulimia nervosa: a long-term outcome study. Int J Eat Disord 2000;27:428-34.
2. Nielsen S, Moller-Madsen S, Isager T, et al. Standardized mortality in eating disorders—a quantitative summary of previously published and new evidence. J Psychosom Res 1998;44:413-34.
3. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34(4):383-96.
4. Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175-82.
5. Milos G, Spindler A, Schnyder U. Psychiatric comorbidity and eating disorder inventory (EDI) profiles in eating disorder patients. Can J Psychiatry 2004;49:179-84.
6. Rubenstein CS, Pigott TA, L’ Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
7. Becker CB, DeViva JC, Zayfert C. Eating disorder symptoms among female anxiety disorder patients in clinical practice: the importance of anxiety comorbidity assessment. J Anxiety Disord 2004;18(3):255-74.
8. Keys A, Brozek J, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
9. Ramacciotti CE, Dell’Osso L, Paoli RA, et al. Characteristics of eating disorder patients without a drive for thinness. Int J Eat Disord 2002;32:206-12.
10. Andersen AE. Males with eating disorders: medical considerations. In: Mehler PS, Andersen AE (eds). Eating disorders: a guide to medical care and complications. Baltimore: The Johns Hopkins University Press; 1999;214-26.
11. Lantzouni E, Frank GR, Golden NH, Shenker RI. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health 2002;31(2):162-5.
12. Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eating Disord 2001;30:193-203.
13. Jacobi C, Morris L, de Zwaan M. Overview of risk factors for anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton, TD (ed). Clinical handbook of eating disorders: An integrated approach. New York: Marcel Dekker; 2004;183-208.
14. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord 1994;16:363-70.
15. Garner DM. Eating Disorder Inventory-2 professional manual. Odessa, FL: Psychological Assessment Resources; 1991.
16. Garner DM. Psychoeducational principles in treatment. In: Garner DM, Garfinkel PE (eds). Handbook of treatment for eating disorders (2nd ed). New York: Guilford Press; 1997;145-77.
17. Miller KK, Grinspoon SK, Ciampa J, et al. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 2005;165(5):561-6.
Panic attacks: Help sufferers recover with cognitive-behavioral therapy
With panic attacks, alarming physiologic symptoms mount swiftly—tachycardia, chest pain, sweating, trembling, smothering or choking, dizziness, fear of losing control or going crazy—even fear of dying.1 Patients constantly fear the next attack, worry about its consequences, and change their behaviors to avoid or withdraw from anxiety-provoking situations.
To relieve their suffering, cognitive-behavioral therapy (CBT) may offer benefits you would not realize with medication alone. CBT can:
- improve long-term patient outcomes
- enhance medication management
- boost treatment response when medication alone is inadequate
- ease drug discontinuation.2
Whether you or a CBT-trained psychotherapist guides the sessions, you can achieve optimal results for your patients with panic disorder.
How Effective is CBT?
Panic disorder is chronic, often disabling, and characterized by spontaneous, unpredictable panic attacks (Boxes 1 and 23-11). When treated with CBT, about three-quarters of patients become panic-free and maintain treatment gains at follow-up, and one-half become both panic-free and free of excess anxiety.9
Typical therapy is 12 individual, once-weekly visits for psycho-education, relaxation, and breathing training; cognitive restructuring; and exposure therapies.
Briefer protocols, “reduced therapist contact,”12 and group therapy13 also can help patients and in some studies have been as beneficial as 12 weeks of individual therapy. Although trained psychotherapists have higher success rates than nonbehaviorists when treating panic patients, nonbehaviorists also can provide effective therapy after relatively brief training.14
American Psychiatric Association15 treatment guidelines recommend medications—such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and benzodiazepines—as well as CBT as first-line therapies for panic disorder. Other treatment guidelines concur16 and note that CBT is more cost-effective than medications.
In comparison studies, CBT has been at least as effective for panic symptoms as SSRIs,17,18 TCAs,19 and alprazolam.20 Antidepressants are the preferred drug for panic disorder16 because they lack benzodiazepines’ dependence and abuse potential.
Providing medication during CBT may maintain patients’ therapeutic gains better than CBT alone if the medication is continued after CBT is completed. Interestingly, patients who use benzodiazepines during CBT may have higher relapse rates than those who do not use benzodiazepines, particularly when the benzodiazepines are withdrawn.9
CBT produces improvement rates similar to those of pharmacologic treatment at one-quarter to one-half the cost in the first year. Patients also appear to have better clinical outcomes if they receive CBT while SSRIs or benzodiazepines are being discontinued, compared with simply stopping the medications.8
Panic attacks typically begin between ages 10 and 40. The cause is unknown, but evidence points to multiple factors, including heredity, neurobiology, provocations, and psychological conditioning (Box 2).3-9 prevalence is approximately 5%,10 and about three-fourths of panic disorder patients are female.11
Comorbidity. Up to 50% of persons with panic disorder also experience agoraphobia.1 Depression, other anxiety disorders, and substance abuse may complicate the clinical picture.
BIOLOGICAL THEORIES
Genetics. About 10% of persons who experience panic attacks have first-degree relatives with panic disorder. Twin studies suggest heritability of up to 43%
Neurobiology. Anxiety responses appear to be organized at different neuroanatomic levels:
- automatic responses by periaqueductal grey matter or locus coeruleus
- practiced responses by the amygdala and septohippocampal regions
- cognitively complex responses by higher cortical regions.
The hypothalamus mediates neurohormonal responses. Panic disorder patients’ response to SSRIs, tricyclic antidepressants, and benzodiazepines suggest a link with neurotransmitters serotonin, norepinephrine, and GABA. Adenosine, cannabinoids, neuropeptides, hormones, neurotrophins, cytokines, and cellular mediators may also be involved.
Provocation. Panic disorder may have a physiologic mechanism. When exposed in the laboratory to panicogenic substances (such as carbon dioxide, sodium lactate, yohimbine, and caffeine), persons with panic disorders experience greater numbers of panic attacks than do those without panic disorders. These laboratory-induced panic attacks resemble real attacks, and anti-panic medications block the induced panic attacks.
PSYCHOLOGICAL THEORIES
The cognitive-behavioral model postulates that panic disorder patients:
- have a predisposed vulnerability to respond with physiologic arousal to negative stressors
- tend to see anxiety symptoms as harmful
- have negative and catastrophizing cognitions about those symptoms.
With conditioning, patients associate early physiologic arousal with other panic symptoms as the arousal progresses. Ultimately, they become hypervigilant for symptoms and develop a learned escalation of anxiety and apprehension (with accompanying negative cognitions) when the early symptoms re-occur.
Source: References 3-9
CBT Candidates
To diagnose panic disorder, conduct a thorough psychiatric evaluation that includes assessing for comorbid mental and substance use disorders. The history and physical exam are essential to rule out medical causes of the patient’s symptoms, such as heart disease causing dizziness or palpitations. Asking patients to keep panic attack records can help you identify panic symptoms’ frequency and triggers.9
An assessment tool such as the Albany Panic and Phobia Questionnaire (Figure) can be a useful starting point. It has 27 items and three subscales to quantify a patient’s fear of agoraphobic situations, social phobia situations, and situations that produce bodily sensations (interoceptive symptoms). Items on the interoceptive subscale include activities such as exercising vigorously, ingesting caffeine, and experiencing intense emotion.21 Using the Anxiety Sensitivity Index is another assessment option.22
Not all patients with panic attacks respond well to CBT; predictors of poor response in clinical trials have included:
- severe baseline panic symptoms, personality disorders, and possibly depressed mood
- marital dissatisfaction
- low motivation for treatment.2,9
Figure Patient assessment: Albany Panic and Phobia Questionnaire
Source: Reprinted with permission from Rapee RM, Craske MG, Barlow DH. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The Albany Panic and Phobia Questionnaire. Anxiety 1995;1:114-22. Copyright 1995, Wiley-Liss, Inc.
Cognitive Therapies for Panic Disorder
Psychoeducation. Begin by defining and explaining anxiety, panic attacks, panic disorder, and any comorbid psychopathology the patient may have (agoraphobia, depression). Explain panic symptoms as physiologic and psychological responses to stressors.
Address the patient’s fears that anxiety’s physiologic symptoms represent a serious or undiagnosed medical disorder or that a panic attack could cause serious harm. Assign self-help and reading materials to reinforce this discussion (see Related resources). Finally, explain the rationale for using CBT to treat panic symptoms.
Use cognitive restructuring to address faulty or irrational information-processing patterns that underlie pathologic anxiety. Identify automatic thought patterns (such as catastrophizing, overgeneralization, all-or-nothing thinking, and personalization), then provide a careful “reality check,” in which you systematically substitute a more-rational thought process.
Have the patient keep a self-monitoring diary to help you assess thought patterns and re-direct irrational thoughts. A diary may identify anxiety-provoking scenarios on which to focus therapy. Encourage patients to document anxiety events using the “triple-column” technique:23
- column 1: circumstances of the anxiety or panic
- column 2: their emotional state at the time
- column 3: any thoughts they can identify.
Instruct them to log this data while experiencing symptoms or immediately afterward. Later, during the intervention phase, patients can record how they tried to restructure their thoughts and any consequent mood changes. Review diary entries with them during subsequent sessions.
Exposure Therapy
Graduated exposure and response prevention (ERP) is the core component of CBT for panic disorder. ERP exercises require the patient to confront anxiety-producing stimuli while agreeing not to engage in maladaptive behavior that avoids, prematurely reduces, or prevents the anxiety. The stimuli may be external cues—such as bridges, stores, or heights—or interoceptive cues such as dizziness, tachycardia, or tachypnea.
Creating fear hierarchies. To begin, we recommend that you work with the patient to create lists of all external situations and interoceptive stimuli that cause him or her anxiety. Separating the stimuli into two lists helps patients recognize that their bodily stimuli are at least as important as environmental stimuli in promoting a panic attack.
The patient then rates each stimulus using a Subjective Units of Distress scale (SUDS)—assigning 0 to 100 points from no anxiety to overwhelming anxiety—and ranks items on the lists from mildest to worst anxiety. Instruct patients to rate the distress they would feel if they could not escape from the stimuli.
First experience. After the hierarchies are created, the therapist introduces the patient to exposure therapy by choosing an item that causes mild to moderate anxiety. Starting at this anxiety level, patients are likely to succeed with their first exercise without feeling overwhelmed. The therapist teaches the patient about the process, then begins the exposure by helping the patient create and confront the very scenario (or a representation of that scenario) that causes anxiety.
When working on interoceptive cues, various exercises can be used to reproduce bother-some bodily symptoms, such as:
- running up a flight of stairs or running in place to generate tachycardia
- purposefully hyperventilating to produce lightheadedness.
- spinning in place to create dizziness.
The patient agrees not to actively attempt to escape the scenario but to tolerate and perhaps even focus on the anxiety (Box 3). Using “safety cues”—such as leaning against a wall or keeping eyes closed—is also forbidden. Patients soon see that the anxiety does not last indefinitely but begins to diminish fairly rapidly.
Reaching the goal. After repeated exposure sessions, anxiety associated with a stimulus begins to extinguish. Having experienced the success of tolerating a previously difficult stimuli and feeling much less anxious, the patient is ready to take on increasingly difficult tasks. The therapist also assigns the patient “homework” to practice exposure exercises already mastered during sessions. As exposure therapy progresses, the patient takes a larger role in designing and executing sessions. The goal is for the patient to learn to become his or her own behaviorist and to intervene early when panic symptoms begin.
Resist temptation to rescue patients from their anxiety during exposure sessions, such as by chatting about the weather or current events or providing other distractions. To extinguish the link between the stimuli and anxiety, the patient must experience anxiety all the way through the exercise—preferably giving ongoing Subjective Units of Distress (SUDS) ratings—until symptoms inevitably wane and cease.
Similarly, avoid assigning exposure homework to be done “until you can’t stand it anymore, then take a rest.” Although well-intentioned, allowing the patient to escape the exposure when anxiety peaks increases conditioned anxiety and strongly reinforces avoidance behaviors.
Other Behavioral Techniques
Imaginal exposure sessions can be created using visualizations of feared stimuli, gradually presented as with in vivo exposure. For example, as you recount a target scenario, ask the patient to imagine a progression of events or bodily cues that have led to panic attacks. The patient supplies SUDS ratings and refrains from imagining an avoidance or maladaptive response. You can tape-record the session for homework and assign the patient to listen to it and participate daily.
Imaginal exposure may help treat phobic avoidance (such as agoraphobic symptoms), but study results have been disappointing in panic symptoms.24 However, this approach may help reluctant patients initiate in vivo exposure therapy.
Relaxation training—such as progressive muscle relaxation, visual imagery, or autogenic protocols—has shown mixed results in treating panic.25,26 Relaxation may help patients cope with panic’s physiologic arousal, but it is not suitable as a singular intervention.
Breathing retraining. Because hyperventilation and panic symptoms are related, instruction and practice in slow, diaphragmatic breathing has long been a component of CBT for panic symptoms. Little evidence supports breathing retraining,9 although Meuret et al27,28 have described a respiratory feedback paradigm that may reduce panic symptoms in appropriately selected patients.
Many studies that have assessed breathing retraining as monotherapy for panic have had methodologic flaws.27
Building a Therapeutic Alliance
Successful therapists have been found to use empathic listening more than directives and explanations in the first therapy session.9 They understand the suffering from panic disorder and the value of listening as patients explain their symptoms, thoughts, and feelings. The rapport built during this initial interaction can help sustain motivation as the therapist then takes charge of subsequent sessions.
Among important skills for CBT therapists, Seligman29 includes empathy, caring, warmth, and active listening, as well as the ability to:
- be a teacher, scientist, and co-investigator
- demystify treatment
- engage clients as “active, knowledgeable, and responsible partners” in their therapy.
Finally, although CBT clinicians suggest tasks and interventions for this “shared endeavor,” patients are primarily responsible for change.
- Anxiety Disorders Association of America. www.adaa.org.
- Craske M, Barlow D, Cary NC. Mastery of your anxiety and panic, (3rd ed). Therapist guide and client workbook. Oxford, UK: Oxford University Press; 2000.
- Otto MW. Stopping anxiety medication: panic control therapy for benzodiazepine discontinuation. Therapist guide and patient workbook. Oxford, UK: Oxford University Press; 2004.
- The Panic Center. Patient diaries and other self-help resources. www.paniccenter.net
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 1994.
2. Otto MW, Deckersbach T. Cognitive-behavioral therapy for panic disorder: Theory, strategies, and outcome. In: Rosenbaum JF, Pollack M (eds). Panic disorder and its treatment. New York: Marcel Dekker; 1998.
3. Hettema, JM, Neale, MC, Kendler, KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001;158(10):1568-78.
4. Kendler KS, Gardner CO, Prescott CA. Panic syndromes in a population-based sample of male and female twins. Psychol Med 2001;31:989-1000.
5. Sandford JJ, Argyropoulos SV, Nutt DJ. The psychobiology of anxiolytic drugs. Part I: basic neurobiology. Pharmacol Ther 2000;88:197-212.
6. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol 2003;70:83-244.
7. Sanderson WC, Rego SA. Empirically supported psychological treatment of panic disorder and agoraphobia. Medscape. Available at www.medscape.com/viewprogram/350_pnt. Accessed Nov. 8, 2005.
8. Rayburn NR, Otto MW. Cognitive-behavioral therapy for panic disorder: a review of treatment elements, strategies, and outcomes. CNS Spectr 2003;8(5):356-62.
9. Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH (ed). Clinical handbook of psychological disorders: A step-by-step treatment manual. New York: Guilford Press; 2001;1-59.
10. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry 2005;62:593-602.
11. Rapee RM, Barlow DH. Generalized anxiety disorders, panic disorders, and phobias. In: Sutker PB, Adams HE (eds). Comprehensive handbook of psychopathology (3rd ed). New York: Kluwer Academic/Plenum; 2001.
12. Cote G, Gauthier JG, Laberge B, et al. Reduced therapist contact in the cognitive behavioral treatment of panic disorder. Behav Ther 1994;25:123-45.
13. Telch MJ, Lucas JA, Schmidt NB, et al. Group cognitive-behavioral treatment of panic disorder. Behav Res Ther 1993;31:279-28.
14. Welkowitz LA, Papp LA, Cloitre M, et al. Cognitive-behavioral therapy for panic disorder delivered by psychopharmacologically oriented clinicians. J Nerv Ment Dis 1991;179:473-77.
15. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Washington, DC: American Psychiatric Association; 1998.
16. Royal Australian and New Zealand College of Psychiatrists. Australian and New Zealand clinical practice guidelines for the treatment of panic disorder and agoraphobia. Aust NZ J Psychiatry 2003;37:641-56.Available at: www.ranzcp.org/publicarea/cpg.asp. Accessed Aug. 24, 2005.
17. Black DW, Wesner R, Bowers W, Gabel J. A comparison of fluvoxamine, cognitive therapy, and placebo in the treatment of panic disorder. Arch Gen Psychiatry 1993;31:383-94.
18. Dannon PN, Gon-Usishkin M, Gelbert A, et al. Cognitive behavioral group therapy in panic disorder patients: The efficacy of CBGT versus drug treatment. Ann Clin Psychiatry 2004;16:41-6.
19. Clark DM, Salkovskis PM, Hackmann A, et al. A comparison of cognitive therapy, applied relaxation, and imipramine in the treatment of panic disorder. Br J Psychiatry 1994;164:759-69.
20. Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavior therapy in treatment of panic disorder. J Consult Clin Psychol 1990;58:77-84.
21. Rapee RM, Craske MG, Barlow DH. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The Albany Panic and Phobia Questionnaire. Anxiety 1995;1:114-22.
22. Reiss S, Peterson R, Gursky D, McNally R. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behav Res Ther 1986;24:1-8.
23. Burns DD. Feeling good: The new mood therapy. New York: William Morrow and Co.; 1980.
24. Clum GA, Watkins PL, Borden JW, et al. A comparison of guided imaginal coping and imaginal exposure in the treatment of panic disorder. J Rational-Emotive & Cognitive Behavior Therapy 1993;11(4):179-93.
25. Craske MG, Brown TA, Barlow DH. Behavioral treatment of panic disorder: A two year follow-up. Behav Ther 1991;22:289-304.
26. Ost LG, Westling BE. Applied relaxation vs cognitive behavior therapy in the treatment of panic disorder. Behav Res Ther 1995;33:145-58.
27. Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training for treating panic disorder: useful intervention or impediment. Behav Mod 2003;27(5):731-54.
28. Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. J Clin Psychol 2004;60:197-207.
29. Seligman L. Systems, strategies, and skills of counseling and psychotherapy. Saddle River, NJ: Prentice-Hall; 2001.
With panic attacks, alarming physiologic symptoms mount swiftly—tachycardia, chest pain, sweating, trembling, smothering or choking, dizziness, fear of losing control or going crazy—even fear of dying.1 Patients constantly fear the next attack, worry about its consequences, and change their behaviors to avoid or withdraw from anxiety-provoking situations.
To relieve their suffering, cognitive-behavioral therapy (CBT) may offer benefits you would not realize with medication alone. CBT can:
- improve long-term patient outcomes
- enhance medication management
- boost treatment response when medication alone is inadequate
- ease drug discontinuation.2
Whether you or a CBT-trained psychotherapist guides the sessions, you can achieve optimal results for your patients with panic disorder.
How Effective is CBT?
Panic disorder is chronic, often disabling, and characterized by spontaneous, unpredictable panic attacks (Boxes 1 and 23-11). When treated with CBT, about three-quarters of patients become panic-free and maintain treatment gains at follow-up, and one-half become both panic-free and free of excess anxiety.9
Typical therapy is 12 individual, once-weekly visits for psycho-education, relaxation, and breathing training; cognitive restructuring; and exposure therapies.
Briefer protocols, “reduced therapist contact,”12 and group therapy13 also can help patients and in some studies have been as beneficial as 12 weeks of individual therapy. Although trained psychotherapists have higher success rates than nonbehaviorists when treating panic patients, nonbehaviorists also can provide effective therapy after relatively brief training.14
American Psychiatric Association15 treatment guidelines recommend medications—such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and benzodiazepines—as well as CBT as first-line therapies for panic disorder. Other treatment guidelines concur16 and note that CBT is more cost-effective than medications.
In comparison studies, CBT has been at least as effective for panic symptoms as SSRIs,17,18 TCAs,19 and alprazolam.20 Antidepressants are the preferred drug for panic disorder16 because they lack benzodiazepines’ dependence and abuse potential.
Providing medication during CBT may maintain patients’ therapeutic gains better than CBT alone if the medication is continued after CBT is completed. Interestingly, patients who use benzodiazepines during CBT may have higher relapse rates than those who do not use benzodiazepines, particularly when the benzodiazepines are withdrawn.9
CBT produces improvement rates similar to those of pharmacologic treatment at one-quarter to one-half the cost in the first year. Patients also appear to have better clinical outcomes if they receive CBT while SSRIs or benzodiazepines are being discontinued, compared with simply stopping the medications.8
Panic attacks typically begin between ages 10 and 40. The cause is unknown, but evidence points to multiple factors, including heredity, neurobiology, provocations, and psychological conditioning (Box 2).3-9 prevalence is approximately 5%,10 and about three-fourths of panic disorder patients are female.11
Comorbidity. Up to 50% of persons with panic disorder also experience agoraphobia.1 Depression, other anxiety disorders, and substance abuse may complicate the clinical picture.
BIOLOGICAL THEORIES
Genetics. About 10% of persons who experience panic attacks have first-degree relatives with panic disorder. Twin studies suggest heritability of up to 43%
Neurobiology. Anxiety responses appear to be organized at different neuroanatomic levels:
- automatic responses by periaqueductal grey matter or locus coeruleus
- practiced responses by the amygdala and septohippocampal regions
- cognitively complex responses by higher cortical regions.
The hypothalamus mediates neurohormonal responses. Panic disorder patients’ response to SSRIs, tricyclic antidepressants, and benzodiazepines suggest a link with neurotransmitters serotonin, norepinephrine, and GABA. Adenosine, cannabinoids, neuropeptides, hormones, neurotrophins, cytokines, and cellular mediators may also be involved.
Provocation. Panic disorder may have a physiologic mechanism. When exposed in the laboratory to panicogenic substances (such as carbon dioxide, sodium lactate, yohimbine, and caffeine), persons with panic disorders experience greater numbers of panic attacks than do those without panic disorders. These laboratory-induced panic attacks resemble real attacks, and anti-panic medications block the induced panic attacks.
PSYCHOLOGICAL THEORIES
The cognitive-behavioral model postulates that panic disorder patients:
- have a predisposed vulnerability to respond with physiologic arousal to negative stressors
- tend to see anxiety symptoms as harmful
- have negative and catastrophizing cognitions about those symptoms.
With conditioning, patients associate early physiologic arousal with other panic symptoms as the arousal progresses. Ultimately, they become hypervigilant for symptoms and develop a learned escalation of anxiety and apprehension (with accompanying negative cognitions) when the early symptoms re-occur.
Source: References 3-9
CBT Candidates
To diagnose panic disorder, conduct a thorough psychiatric evaluation that includes assessing for comorbid mental and substance use disorders. The history and physical exam are essential to rule out medical causes of the patient’s symptoms, such as heart disease causing dizziness or palpitations. Asking patients to keep panic attack records can help you identify panic symptoms’ frequency and triggers.9
An assessment tool such as the Albany Panic and Phobia Questionnaire (Figure) can be a useful starting point. It has 27 items and three subscales to quantify a patient’s fear of agoraphobic situations, social phobia situations, and situations that produce bodily sensations (interoceptive symptoms). Items on the interoceptive subscale include activities such as exercising vigorously, ingesting caffeine, and experiencing intense emotion.21 Using the Anxiety Sensitivity Index is another assessment option.22
Not all patients with panic attacks respond well to CBT; predictors of poor response in clinical trials have included:
- severe baseline panic symptoms, personality disorders, and possibly depressed mood
- marital dissatisfaction
- low motivation for treatment.2,9
Figure Patient assessment: Albany Panic and Phobia Questionnaire
Source: Reprinted with permission from Rapee RM, Craske MG, Barlow DH. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The Albany Panic and Phobia Questionnaire. Anxiety 1995;1:114-22. Copyright 1995, Wiley-Liss, Inc.
Cognitive Therapies for Panic Disorder
Psychoeducation. Begin by defining and explaining anxiety, panic attacks, panic disorder, and any comorbid psychopathology the patient may have (agoraphobia, depression). Explain panic symptoms as physiologic and psychological responses to stressors.
Address the patient’s fears that anxiety’s physiologic symptoms represent a serious or undiagnosed medical disorder or that a panic attack could cause serious harm. Assign self-help and reading materials to reinforce this discussion (see Related resources). Finally, explain the rationale for using CBT to treat panic symptoms.
Use cognitive restructuring to address faulty or irrational information-processing patterns that underlie pathologic anxiety. Identify automatic thought patterns (such as catastrophizing, overgeneralization, all-or-nothing thinking, and personalization), then provide a careful “reality check,” in which you systematically substitute a more-rational thought process.
Have the patient keep a self-monitoring diary to help you assess thought patterns and re-direct irrational thoughts. A diary may identify anxiety-provoking scenarios on which to focus therapy. Encourage patients to document anxiety events using the “triple-column” technique:23
- column 1: circumstances of the anxiety or panic
- column 2: their emotional state at the time
- column 3: any thoughts they can identify.
Instruct them to log this data while experiencing symptoms or immediately afterward. Later, during the intervention phase, patients can record how they tried to restructure their thoughts and any consequent mood changes. Review diary entries with them during subsequent sessions.
Exposure Therapy
Graduated exposure and response prevention (ERP) is the core component of CBT for panic disorder. ERP exercises require the patient to confront anxiety-producing stimuli while agreeing not to engage in maladaptive behavior that avoids, prematurely reduces, or prevents the anxiety. The stimuli may be external cues—such as bridges, stores, or heights—or interoceptive cues such as dizziness, tachycardia, or tachypnea.
Creating fear hierarchies. To begin, we recommend that you work with the patient to create lists of all external situations and interoceptive stimuli that cause him or her anxiety. Separating the stimuli into two lists helps patients recognize that their bodily stimuli are at least as important as environmental stimuli in promoting a panic attack.
The patient then rates each stimulus using a Subjective Units of Distress scale (SUDS)—assigning 0 to 100 points from no anxiety to overwhelming anxiety—and ranks items on the lists from mildest to worst anxiety. Instruct patients to rate the distress they would feel if they could not escape from the stimuli.
First experience. After the hierarchies are created, the therapist introduces the patient to exposure therapy by choosing an item that causes mild to moderate anxiety. Starting at this anxiety level, patients are likely to succeed with their first exercise without feeling overwhelmed. The therapist teaches the patient about the process, then begins the exposure by helping the patient create and confront the very scenario (or a representation of that scenario) that causes anxiety.
When working on interoceptive cues, various exercises can be used to reproduce bother-some bodily symptoms, such as:
- running up a flight of stairs or running in place to generate tachycardia
- purposefully hyperventilating to produce lightheadedness.
- spinning in place to create dizziness.
The patient agrees not to actively attempt to escape the scenario but to tolerate and perhaps even focus on the anxiety (Box 3). Using “safety cues”—such as leaning against a wall or keeping eyes closed—is also forbidden. Patients soon see that the anxiety does not last indefinitely but begins to diminish fairly rapidly.
Reaching the goal. After repeated exposure sessions, anxiety associated with a stimulus begins to extinguish. Having experienced the success of tolerating a previously difficult stimuli and feeling much less anxious, the patient is ready to take on increasingly difficult tasks. The therapist also assigns the patient “homework” to practice exposure exercises already mastered during sessions. As exposure therapy progresses, the patient takes a larger role in designing and executing sessions. The goal is for the patient to learn to become his or her own behaviorist and to intervene early when panic symptoms begin.
Resist temptation to rescue patients from their anxiety during exposure sessions, such as by chatting about the weather or current events or providing other distractions. To extinguish the link between the stimuli and anxiety, the patient must experience anxiety all the way through the exercise—preferably giving ongoing Subjective Units of Distress (SUDS) ratings—until symptoms inevitably wane and cease.
Similarly, avoid assigning exposure homework to be done “until you can’t stand it anymore, then take a rest.” Although well-intentioned, allowing the patient to escape the exposure when anxiety peaks increases conditioned anxiety and strongly reinforces avoidance behaviors.
Other Behavioral Techniques
Imaginal exposure sessions can be created using visualizations of feared stimuli, gradually presented as with in vivo exposure. For example, as you recount a target scenario, ask the patient to imagine a progression of events or bodily cues that have led to panic attacks. The patient supplies SUDS ratings and refrains from imagining an avoidance or maladaptive response. You can tape-record the session for homework and assign the patient to listen to it and participate daily.
Imaginal exposure may help treat phobic avoidance (such as agoraphobic symptoms), but study results have been disappointing in panic symptoms.24 However, this approach may help reluctant patients initiate in vivo exposure therapy.
Relaxation training—such as progressive muscle relaxation, visual imagery, or autogenic protocols—has shown mixed results in treating panic.25,26 Relaxation may help patients cope with panic’s physiologic arousal, but it is not suitable as a singular intervention.
Breathing retraining. Because hyperventilation and panic symptoms are related, instruction and practice in slow, diaphragmatic breathing has long been a component of CBT for panic symptoms. Little evidence supports breathing retraining,9 although Meuret et al27,28 have described a respiratory feedback paradigm that may reduce panic symptoms in appropriately selected patients.
Many studies that have assessed breathing retraining as monotherapy for panic have had methodologic flaws.27
Building a Therapeutic Alliance
Successful therapists have been found to use empathic listening more than directives and explanations in the first therapy session.9 They understand the suffering from panic disorder and the value of listening as patients explain their symptoms, thoughts, and feelings. The rapport built during this initial interaction can help sustain motivation as the therapist then takes charge of subsequent sessions.
Among important skills for CBT therapists, Seligman29 includes empathy, caring, warmth, and active listening, as well as the ability to:
- be a teacher, scientist, and co-investigator
- demystify treatment
- engage clients as “active, knowledgeable, and responsible partners” in their therapy.
Finally, although CBT clinicians suggest tasks and interventions for this “shared endeavor,” patients are primarily responsible for change.
- Anxiety Disorders Association of America. www.adaa.org.
- Craske M, Barlow D, Cary NC. Mastery of your anxiety and panic, (3rd ed). Therapist guide and client workbook. Oxford, UK: Oxford University Press; 2000.
- Otto MW. Stopping anxiety medication: panic control therapy for benzodiazepine discontinuation. Therapist guide and patient workbook. Oxford, UK: Oxford University Press; 2004.
- The Panic Center. Patient diaries and other self-help resources. www.paniccenter.net
With panic attacks, alarming physiologic symptoms mount swiftly—tachycardia, chest pain, sweating, trembling, smothering or choking, dizziness, fear of losing control or going crazy—even fear of dying.1 Patients constantly fear the next attack, worry about its consequences, and change their behaviors to avoid or withdraw from anxiety-provoking situations.
To relieve their suffering, cognitive-behavioral therapy (CBT) may offer benefits you would not realize with medication alone. CBT can:
- improve long-term patient outcomes
- enhance medication management
- boost treatment response when medication alone is inadequate
- ease drug discontinuation.2
Whether you or a CBT-trained psychotherapist guides the sessions, you can achieve optimal results for your patients with panic disorder.
How Effective is CBT?
Panic disorder is chronic, often disabling, and characterized by spontaneous, unpredictable panic attacks (Boxes 1 and 23-11). When treated with CBT, about three-quarters of patients become panic-free and maintain treatment gains at follow-up, and one-half become both panic-free and free of excess anxiety.9
Typical therapy is 12 individual, once-weekly visits for psycho-education, relaxation, and breathing training; cognitive restructuring; and exposure therapies.
Briefer protocols, “reduced therapist contact,”12 and group therapy13 also can help patients and in some studies have been as beneficial as 12 weeks of individual therapy. Although trained psychotherapists have higher success rates than nonbehaviorists when treating panic patients, nonbehaviorists also can provide effective therapy after relatively brief training.14
American Psychiatric Association15 treatment guidelines recommend medications—such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and benzodiazepines—as well as CBT as first-line therapies for panic disorder. Other treatment guidelines concur16 and note that CBT is more cost-effective than medications.
In comparison studies, CBT has been at least as effective for panic symptoms as SSRIs,17,18 TCAs,19 and alprazolam.20 Antidepressants are the preferred drug for panic disorder16 because they lack benzodiazepines’ dependence and abuse potential.
Providing medication during CBT may maintain patients’ therapeutic gains better than CBT alone if the medication is continued after CBT is completed. Interestingly, patients who use benzodiazepines during CBT may have higher relapse rates than those who do not use benzodiazepines, particularly when the benzodiazepines are withdrawn.9
CBT produces improvement rates similar to those of pharmacologic treatment at one-quarter to one-half the cost in the first year. Patients also appear to have better clinical outcomes if they receive CBT while SSRIs or benzodiazepines are being discontinued, compared with simply stopping the medications.8
Panic attacks typically begin between ages 10 and 40. The cause is unknown, but evidence points to multiple factors, including heredity, neurobiology, provocations, and psychological conditioning (Box 2).3-9 prevalence is approximately 5%,10 and about three-fourths of panic disorder patients are female.11
Comorbidity. Up to 50% of persons with panic disorder also experience agoraphobia.1 Depression, other anxiety disorders, and substance abuse may complicate the clinical picture.
BIOLOGICAL THEORIES
Genetics. About 10% of persons who experience panic attacks have first-degree relatives with panic disorder. Twin studies suggest heritability of up to 43%
Neurobiology. Anxiety responses appear to be organized at different neuroanatomic levels:
- automatic responses by periaqueductal grey matter or locus coeruleus
- practiced responses by the amygdala and septohippocampal regions
- cognitively complex responses by higher cortical regions.
The hypothalamus mediates neurohormonal responses. Panic disorder patients’ response to SSRIs, tricyclic antidepressants, and benzodiazepines suggest a link with neurotransmitters serotonin, norepinephrine, and GABA. Adenosine, cannabinoids, neuropeptides, hormones, neurotrophins, cytokines, and cellular mediators may also be involved.
Provocation. Panic disorder may have a physiologic mechanism. When exposed in the laboratory to panicogenic substances (such as carbon dioxide, sodium lactate, yohimbine, and caffeine), persons with panic disorders experience greater numbers of panic attacks than do those without panic disorders. These laboratory-induced panic attacks resemble real attacks, and anti-panic medications block the induced panic attacks.
PSYCHOLOGICAL THEORIES
The cognitive-behavioral model postulates that panic disorder patients:
- have a predisposed vulnerability to respond with physiologic arousal to negative stressors
- tend to see anxiety symptoms as harmful
- have negative and catastrophizing cognitions about those symptoms.
With conditioning, patients associate early physiologic arousal with other panic symptoms as the arousal progresses. Ultimately, they become hypervigilant for symptoms and develop a learned escalation of anxiety and apprehension (with accompanying negative cognitions) when the early symptoms re-occur.
Source: References 3-9
CBT Candidates
To diagnose panic disorder, conduct a thorough psychiatric evaluation that includes assessing for comorbid mental and substance use disorders. The history and physical exam are essential to rule out medical causes of the patient’s symptoms, such as heart disease causing dizziness or palpitations. Asking patients to keep panic attack records can help you identify panic symptoms’ frequency and triggers.9
An assessment tool such as the Albany Panic and Phobia Questionnaire (Figure) can be a useful starting point. It has 27 items and three subscales to quantify a patient’s fear of agoraphobic situations, social phobia situations, and situations that produce bodily sensations (interoceptive symptoms). Items on the interoceptive subscale include activities such as exercising vigorously, ingesting caffeine, and experiencing intense emotion.21 Using the Anxiety Sensitivity Index is another assessment option.22
Not all patients with panic attacks respond well to CBT; predictors of poor response in clinical trials have included:
- severe baseline panic symptoms, personality disorders, and possibly depressed mood
- marital dissatisfaction
- low motivation for treatment.2,9
Figure Patient assessment: Albany Panic and Phobia Questionnaire
Source: Reprinted with permission from Rapee RM, Craske MG, Barlow DH. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The Albany Panic and Phobia Questionnaire. Anxiety 1995;1:114-22. Copyright 1995, Wiley-Liss, Inc.
Cognitive Therapies for Panic Disorder
Psychoeducation. Begin by defining and explaining anxiety, panic attacks, panic disorder, and any comorbid psychopathology the patient may have (agoraphobia, depression). Explain panic symptoms as physiologic and psychological responses to stressors.
Address the patient’s fears that anxiety’s physiologic symptoms represent a serious or undiagnosed medical disorder or that a panic attack could cause serious harm. Assign self-help and reading materials to reinforce this discussion (see Related resources). Finally, explain the rationale for using CBT to treat panic symptoms.
Use cognitive restructuring to address faulty or irrational information-processing patterns that underlie pathologic anxiety. Identify automatic thought patterns (such as catastrophizing, overgeneralization, all-or-nothing thinking, and personalization), then provide a careful “reality check,” in which you systematically substitute a more-rational thought process.
Have the patient keep a self-monitoring diary to help you assess thought patterns and re-direct irrational thoughts. A diary may identify anxiety-provoking scenarios on which to focus therapy. Encourage patients to document anxiety events using the “triple-column” technique:23
- column 1: circumstances of the anxiety or panic
- column 2: their emotional state at the time
- column 3: any thoughts they can identify.
Instruct them to log this data while experiencing symptoms or immediately afterward. Later, during the intervention phase, patients can record how they tried to restructure their thoughts and any consequent mood changes. Review diary entries with them during subsequent sessions.
Exposure Therapy
Graduated exposure and response prevention (ERP) is the core component of CBT for panic disorder. ERP exercises require the patient to confront anxiety-producing stimuli while agreeing not to engage in maladaptive behavior that avoids, prematurely reduces, or prevents the anxiety. The stimuli may be external cues—such as bridges, stores, or heights—or interoceptive cues such as dizziness, tachycardia, or tachypnea.
Creating fear hierarchies. To begin, we recommend that you work with the patient to create lists of all external situations and interoceptive stimuli that cause him or her anxiety. Separating the stimuli into two lists helps patients recognize that their bodily stimuli are at least as important as environmental stimuli in promoting a panic attack.
The patient then rates each stimulus using a Subjective Units of Distress scale (SUDS)—assigning 0 to 100 points from no anxiety to overwhelming anxiety—and ranks items on the lists from mildest to worst anxiety. Instruct patients to rate the distress they would feel if they could not escape from the stimuli.
First experience. After the hierarchies are created, the therapist introduces the patient to exposure therapy by choosing an item that causes mild to moderate anxiety. Starting at this anxiety level, patients are likely to succeed with their first exercise without feeling overwhelmed. The therapist teaches the patient about the process, then begins the exposure by helping the patient create and confront the very scenario (or a representation of that scenario) that causes anxiety.
When working on interoceptive cues, various exercises can be used to reproduce bother-some bodily symptoms, such as:
- running up a flight of stairs or running in place to generate tachycardia
- purposefully hyperventilating to produce lightheadedness.
- spinning in place to create dizziness.
The patient agrees not to actively attempt to escape the scenario but to tolerate and perhaps even focus on the anxiety (Box 3). Using “safety cues”—such as leaning against a wall or keeping eyes closed—is also forbidden. Patients soon see that the anxiety does not last indefinitely but begins to diminish fairly rapidly.
Reaching the goal. After repeated exposure sessions, anxiety associated with a stimulus begins to extinguish. Having experienced the success of tolerating a previously difficult stimuli and feeling much less anxious, the patient is ready to take on increasingly difficult tasks. The therapist also assigns the patient “homework” to practice exposure exercises already mastered during sessions. As exposure therapy progresses, the patient takes a larger role in designing and executing sessions. The goal is for the patient to learn to become his or her own behaviorist and to intervene early when panic symptoms begin.
Resist temptation to rescue patients from their anxiety during exposure sessions, such as by chatting about the weather or current events or providing other distractions. To extinguish the link between the stimuli and anxiety, the patient must experience anxiety all the way through the exercise—preferably giving ongoing Subjective Units of Distress (SUDS) ratings—until symptoms inevitably wane and cease.
Similarly, avoid assigning exposure homework to be done “until you can’t stand it anymore, then take a rest.” Although well-intentioned, allowing the patient to escape the exposure when anxiety peaks increases conditioned anxiety and strongly reinforces avoidance behaviors.
Other Behavioral Techniques
Imaginal exposure sessions can be created using visualizations of feared stimuli, gradually presented as with in vivo exposure. For example, as you recount a target scenario, ask the patient to imagine a progression of events or bodily cues that have led to panic attacks. The patient supplies SUDS ratings and refrains from imagining an avoidance or maladaptive response. You can tape-record the session for homework and assign the patient to listen to it and participate daily.
Imaginal exposure may help treat phobic avoidance (such as agoraphobic symptoms), but study results have been disappointing in panic symptoms.24 However, this approach may help reluctant patients initiate in vivo exposure therapy.
Relaxation training—such as progressive muscle relaxation, visual imagery, or autogenic protocols—has shown mixed results in treating panic.25,26 Relaxation may help patients cope with panic’s physiologic arousal, but it is not suitable as a singular intervention.
Breathing retraining. Because hyperventilation and panic symptoms are related, instruction and practice in slow, diaphragmatic breathing has long been a component of CBT for panic symptoms. Little evidence supports breathing retraining,9 although Meuret et al27,28 have described a respiratory feedback paradigm that may reduce panic symptoms in appropriately selected patients.
Many studies that have assessed breathing retraining as monotherapy for panic have had methodologic flaws.27
Building a Therapeutic Alliance
Successful therapists have been found to use empathic listening more than directives and explanations in the first therapy session.9 They understand the suffering from panic disorder and the value of listening as patients explain their symptoms, thoughts, and feelings. The rapport built during this initial interaction can help sustain motivation as the therapist then takes charge of subsequent sessions.
Among important skills for CBT therapists, Seligman29 includes empathy, caring, warmth, and active listening, as well as the ability to:
- be a teacher, scientist, and co-investigator
- demystify treatment
- engage clients as “active, knowledgeable, and responsible partners” in their therapy.
Finally, although CBT clinicians suggest tasks and interventions for this “shared endeavor,” patients are primarily responsible for change.
- Anxiety Disorders Association of America. www.adaa.org.
- Craske M, Barlow D, Cary NC. Mastery of your anxiety and panic, (3rd ed). Therapist guide and client workbook. Oxford, UK: Oxford University Press; 2000.
- Otto MW. Stopping anxiety medication: panic control therapy for benzodiazepine discontinuation. Therapist guide and patient workbook. Oxford, UK: Oxford University Press; 2004.
- The Panic Center. Patient diaries and other self-help resources. www.paniccenter.net
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 1994.
2. Otto MW, Deckersbach T. Cognitive-behavioral therapy for panic disorder: Theory, strategies, and outcome. In: Rosenbaum JF, Pollack M (eds). Panic disorder and its treatment. New York: Marcel Dekker; 1998.
3. Hettema, JM, Neale, MC, Kendler, KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001;158(10):1568-78.
4. Kendler KS, Gardner CO, Prescott CA. Panic syndromes in a population-based sample of male and female twins. Psychol Med 2001;31:989-1000.
5. Sandford JJ, Argyropoulos SV, Nutt DJ. The psychobiology of anxiolytic drugs. Part I: basic neurobiology. Pharmacol Ther 2000;88:197-212.
6. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol 2003;70:83-244.
7. Sanderson WC, Rego SA. Empirically supported psychological treatment of panic disorder and agoraphobia. Medscape. Available at www.medscape.com/viewprogram/350_pnt. Accessed Nov. 8, 2005.
8. Rayburn NR, Otto MW. Cognitive-behavioral therapy for panic disorder: a review of treatment elements, strategies, and outcomes. CNS Spectr 2003;8(5):356-62.
9. Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH (ed). Clinical handbook of psychological disorders: A step-by-step treatment manual. New York: Guilford Press; 2001;1-59.
10. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry 2005;62:593-602.
11. Rapee RM, Barlow DH. Generalized anxiety disorders, panic disorders, and phobias. In: Sutker PB, Adams HE (eds). Comprehensive handbook of psychopathology (3rd ed). New York: Kluwer Academic/Plenum; 2001.
12. Cote G, Gauthier JG, Laberge B, et al. Reduced therapist contact in the cognitive behavioral treatment of panic disorder. Behav Ther 1994;25:123-45.
13. Telch MJ, Lucas JA, Schmidt NB, et al. Group cognitive-behavioral treatment of panic disorder. Behav Res Ther 1993;31:279-28.
14. Welkowitz LA, Papp LA, Cloitre M, et al. Cognitive-behavioral therapy for panic disorder delivered by psychopharmacologically oriented clinicians. J Nerv Ment Dis 1991;179:473-77.
15. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Washington, DC: American Psychiatric Association; 1998.
16. Royal Australian and New Zealand College of Psychiatrists. Australian and New Zealand clinical practice guidelines for the treatment of panic disorder and agoraphobia. Aust NZ J Psychiatry 2003;37:641-56.Available at: www.ranzcp.org/publicarea/cpg.asp. Accessed Aug. 24, 2005.
17. Black DW, Wesner R, Bowers W, Gabel J. A comparison of fluvoxamine, cognitive therapy, and placebo in the treatment of panic disorder. Arch Gen Psychiatry 1993;31:383-94.
18. Dannon PN, Gon-Usishkin M, Gelbert A, et al. Cognitive behavioral group therapy in panic disorder patients: The efficacy of CBGT versus drug treatment. Ann Clin Psychiatry 2004;16:41-6.
19. Clark DM, Salkovskis PM, Hackmann A, et al. A comparison of cognitive therapy, applied relaxation, and imipramine in the treatment of panic disorder. Br J Psychiatry 1994;164:759-69.
20. Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavior therapy in treatment of panic disorder. J Consult Clin Psychol 1990;58:77-84.
21. Rapee RM, Craske MG, Barlow DH. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The Albany Panic and Phobia Questionnaire. Anxiety 1995;1:114-22.
22. Reiss S, Peterson R, Gursky D, McNally R. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behav Res Ther 1986;24:1-8.
23. Burns DD. Feeling good: The new mood therapy. New York: William Morrow and Co.; 1980.
24. Clum GA, Watkins PL, Borden JW, et al. A comparison of guided imaginal coping and imaginal exposure in the treatment of panic disorder. J Rational-Emotive & Cognitive Behavior Therapy 1993;11(4):179-93.
25. Craske MG, Brown TA, Barlow DH. Behavioral treatment of panic disorder: A two year follow-up. Behav Ther 1991;22:289-304.
26. Ost LG, Westling BE. Applied relaxation vs cognitive behavior therapy in the treatment of panic disorder. Behav Res Ther 1995;33:145-58.
27. Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training for treating panic disorder: useful intervention or impediment. Behav Mod 2003;27(5):731-54.
28. Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. J Clin Psychol 2004;60:197-207.
29. Seligman L. Systems, strategies, and skills of counseling and psychotherapy. Saddle River, NJ: Prentice-Hall; 2001.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 1994.
2. Otto MW, Deckersbach T. Cognitive-behavioral therapy for panic disorder: Theory, strategies, and outcome. In: Rosenbaum JF, Pollack M (eds). Panic disorder and its treatment. New York: Marcel Dekker; 1998.
3. Hettema, JM, Neale, MC, Kendler, KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001;158(10):1568-78.
4. Kendler KS, Gardner CO, Prescott CA. Panic syndromes in a population-based sample of male and female twins. Psychol Med 2001;31:989-1000.
5. Sandford JJ, Argyropoulos SV, Nutt DJ. The psychobiology of anxiolytic drugs. Part I: basic neurobiology. Pharmacol Ther 2000;88:197-212.
6. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol 2003;70:83-244.
7. Sanderson WC, Rego SA. Empirically supported psychological treatment of panic disorder and agoraphobia. Medscape. Available at www.medscape.com/viewprogram/350_pnt. Accessed Nov. 8, 2005.
8. Rayburn NR, Otto MW. Cognitive-behavioral therapy for panic disorder: a review of treatment elements, strategies, and outcomes. CNS Spectr 2003;8(5):356-62.
9. Craske MG, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH (ed). Clinical handbook of psychological disorders: A step-by-step treatment manual. New York: Guilford Press; 2001;1-59.
10. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry 2005;62:593-602.
11. Rapee RM, Barlow DH. Generalized anxiety disorders, panic disorders, and phobias. In: Sutker PB, Adams HE (eds). Comprehensive handbook of psychopathology (3rd ed). New York: Kluwer Academic/Plenum; 2001.
12. Cote G, Gauthier JG, Laberge B, et al. Reduced therapist contact in the cognitive behavioral treatment of panic disorder. Behav Ther 1994;25:123-45.
13. Telch MJ, Lucas JA, Schmidt NB, et al. Group cognitive-behavioral treatment of panic disorder. Behav Res Ther 1993;31:279-28.
14. Welkowitz LA, Papp LA, Cloitre M, et al. Cognitive-behavioral therapy for panic disorder delivered by psychopharmacologically oriented clinicians. J Nerv Ment Dis 1991;179:473-77.
15. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Washington, DC: American Psychiatric Association; 1998.
16. Royal Australian and New Zealand College of Psychiatrists. Australian and New Zealand clinical practice guidelines for the treatment of panic disorder and agoraphobia. Aust NZ J Psychiatry 2003;37:641-56.Available at: www.ranzcp.org/publicarea/cpg.asp. Accessed Aug. 24, 2005.
17. Black DW, Wesner R, Bowers W, Gabel J. A comparison of fluvoxamine, cognitive therapy, and placebo in the treatment of panic disorder. Arch Gen Psychiatry 1993;31:383-94.
18. Dannon PN, Gon-Usishkin M, Gelbert A, et al. Cognitive behavioral group therapy in panic disorder patients: The efficacy of CBGT versus drug treatment. Ann Clin Psychiatry 2004;16:41-6.
19. Clark DM, Salkovskis PM, Hackmann A, et al. A comparison of cognitive therapy, applied relaxation, and imipramine in the treatment of panic disorder. Br J Psychiatry 1994;164:759-69.
20. Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavior therapy in treatment of panic disorder. J Consult Clin Psychol 1990;58:77-84.
21. Rapee RM, Craske MG, Barlow DH. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The Albany Panic and Phobia Questionnaire. Anxiety 1995;1:114-22.
22. Reiss S, Peterson R, Gursky D, McNally R. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behav Res Ther 1986;24:1-8.
23. Burns DD. Feeling good: The new mood therapy. New York: William Morrow and Co.; 1980.
24. Clum GA, Watkins PL, Borden JW, et al. A comparison of guided imaginal coping and imaginal exposure in the treatment of panic disorder. J Rational-Emotive & Cognitive Behavior Therapy 1993;11(4):179-93.
25. Craske MG, Brown TA, Barlow DH. Behavioral treatment of panic disorder: A two year follow-up. Behav Ther 1991;22:289-304.
26. Ost LG, Westling BE. Applied relaxation vs cognitive behavior therapy in the treatment of panic disorder. Behav Res Ther 1995;33:145-58.
27. Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training for treating panic disorder: useful intervention or impediment. Behav Mod 2003;27(5):731-54.
28. Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. J Clin Psychol 2004;60:197-207.
29. Seligman L. Systems, strategies, and skills of counseling and psychotherapy. Saddle River, NJ: Prentice-Hall; 2001.
Bipolar disorder: New strategy for checking serum valproate
Valproate’s well-accepted therapeutic range for treating epilepsy—50 to 100 mcg/mL—was adopted for bipolar disorder treatment without rigorous evaluation of serum levels and response relationships. Because most literature on monitoring serum valproate refers to its use as an anticonvulsant, you may wonder:
- When should I measure serum valproate in bipolar patients?
- What do serum valproate levels mean in their clinical care?
To answer these questions, we discuss when to monitor serum valproate, whether routinely or in specific situations. We then review studies that show how serum levels affect valproate’s efficacy and safety in three phases of bipolar disorder management: acute mania, maintenance therapy, and acute depression.
Is monitoring overused?
Some neurologists consider serum levels nonessential—and, in some cases, overused—when valproate is used as an anticonvulsant for healthy patients.1,2 A multicenter, randomized controlled trial evaluating the impact of antiepileptic drug monitoring on patient outcomes3 supports this notion, at least in part. Serum monitoring did not improve therapeutic outcome, suggesting that patients with epilepsy could be satisfactorily treated by adjusting dosages based on clinical response.
On the other hand, American Psychiatric Association (APA) guidelines for bipolar disorder suggest routine serum monitoring every 6 months along with other hematologic and hepatic assessments, or more frequently if necessary. The APA recommends maintaining serum valproate levels of 50 to 125 mcg/mL when treating:
- acutely manic patients
- outpatients
- the elderly
- patients who are hypomanic or euthymic.4
Table 1
4 situations where serum valproate monitoring may be clinically useful
| To establish a baseline effective level in individual patients |
| To assess lack or loss of efficacy, including patient adherence |
| When drug-drug interactions increase or decrease valproate clearance (such as with aspirin, carbamazepine, felbamate, or phenytoin)5 |
| When dose-dependent side effects occur (such as alopecia, elevated liver function, thrombocytopenia, or pancreatitis) |
Effective levels in acute mania
In one of the first randomized, double-blind, placebo-controlled trials to examine valproate use in adults with acute mania, Pope et al6 used the epilepsy reference range to adjust dosages. Patients (n=17) initially received valproate, 750 mg/d, and dosages were then adjusted to serum levels of 50 to 100 mcg/mL. Nineteen patients received placebo. Mean (SD) baseline Young Mania Rating Scale (YMRS) scores for the valproate and placebo groups were 28.2 (5.8) and 28.6 (6.9), respectively.
Patients receiving valproate showed the greatest symptomatic improvement—as indicated by YMRS scores—within 1 to 4 days of achieving a serum level ≥50 mcg/mL. Serum valproate for all patients was maintained at >50 mcg/mL, which limits our ability to draw conclusions about a minimum level associated with efficacy.
Minimum threshold for efficacy. In another randomized, double-blind, placebo-controlled study of acute mania, Bowden et al7 compared the efficacy of divalproex (n=69) versus lithium (n=36) or placebo (n=74) given for 3 weeks. Patients met criteria for manic disorder using the Schedule for Affective Disorders and Schizophrenia (SADS) and had Mania Rating Scale scores (derived from the SADS) of at least 14.
Those in the divalproex group received 750 mg/d for 2 days, then 1,000 mg/d for 3 days. Dosages were then adjusted to target a serum level of 150 mcg/mL, unless limited by side effects. Mean serum valproate levels on days 8 and 21 were 77 and 93.2 mcg/mL, respectively. Marked improvement, defined as ≥50% reduction in Mania Rating Scale scores, was seen in 48% of the divalproex group, compared with 25% of the placebo group.
The authors then analyzed the relationship between serum valproate levels and clinical response and tolerability.8 At day 5, patients with serum valproate ≥45 mcg/mL were 2 to 7 times more likely to show 20% or greater improvement in SADS mania subscales (manic syndrome, and behavior and ideation).
This study provided a minimum threshold for valproate efficacy in bipolar mania—45 to 50 mcg/mL—but not a level above which further clinical benefit would not be gained.
Optimum serum ranges. Allen et al9 recently conducted a post hoc analysis of pooled intent-to-treat data from three randomized, fixed dose, placebo-controlled studies of divalproex for acute mania. Subjects were stratified into a placebo group (n=171) and six serum valproate ranges:
- ≤55 mcg/mL (n=35)
- >55 to 71.3 mcg/mL (n=32)
- >71.3 to 85 mcg/mL (n=36)
- >85 to 94 mcg/mL (n=34)
- >94 to 107 mcg/mL (n=33)
- >107 mcg/mL (n=33).
Loading for rapid response. Patients with acute mania may respond sooner when loading doses are used to attain therapeutic serum valproate levels.
Keck et al10 examined time to onset of improvement in adults with acute mania (N=19) receiving oral loading doses of valproate (20 mg/kg/d in divided doses for 5 days) to rapidly attain valproate levels ≥50 mcg/mL. Ten (53%) patients who received at least 1 loading dose showed a ≥50% reduction in MRS scores and the greatest improvement across the first 3 days.
Hirschfeld et al11 also reported that patients’ symptoms began to improve sooner when divalproex was given at 30 mg/kg/d on days 1 and 2, and 20 mg/kg/d on days 3 to 10 (n=20), compared with standard titration (750 mg/d on days 1 and 2, and gradual dose titration on days 3 to 10 [n=20]).
Discussion. In acute mania, evidence suggests that patients with serum valproate ≥45 to 50 mcg/mL may show greater clinical improvement than patients with lower serum levels. Loading doses may achieve a minimum therapeutic serum level more quickly, yielding faster clinical improvement. A serum level >90 mcg/mL may confer additional benefit.
Although a minimum serum level has been recommended, no data have established a maximum level beyond which further clinical improvement would not be observed.
In maintenance therapy
What serum valproate levels are most effective for bipolar maintenance therapy? Some evidence is emerging.
Bowden et al12 compared divalproex (n=187), lithium (n=90), and placebo (n=92) in a 52-week, double-blind, parallel-group study of bipolar adult outpatients who met recovery criteria 3 months after an index manic episode. Divalproex dosages were adjusted to achieve trough serum concentrations between 71 and 125 mcg/mL. Mean (SD) and median serum valproate levels were 84.8 (29.9) mcg/mL and 83.9 mcg/mL, respectively. Serum valproate levels significantly correlated with Mania Rating Scale scores. No minimum threshold for efficacy was reported.
Thirteen subjects in the divalproex group were then stratified into 4 categories:
- nontherapeutic (
- low therapeutic (50 to 74.9 mcg/mL)
- medium therapeutic (75 to 99.9 mcg/mL)
- high therapeutic (>100 mcg/mL).
Discussion. Serum valproate levels of 75 to 100 mcg/mL may be most effective in preventing subsequent mood episodes with acceptable tolerability. Prospective, longitudinal studies are needed to better establish a therapeutic range for valproate in bipolar maintenance therapy.
In bipolar depression
Little evidence supports a therapeutic serum valproate range for treating acute bipolar depression.
In an 8-week, double-blind study, Davis et al14 randomly assigned adults with bipolar depression to divalproex (n=13) or placebo (n=12). Bipolar depression diagnoses were confirmed using the Structured Clinical Interview for DSM-IV, and patients were required to have a Hamilton Rating Scale for Depression (HRSD) score ≥16.
Valproate was started at 500 mg/d and titrated to serum levels of 50 to 150 mcg/mL. Mean (SD) serum valproate levels at weeks 4 and 8 were 80 (9.3) mcg/mL and 81 (19.2) mcg/mL, respectively. Remission rate (defined a priori as a >50% improvement and total HRSD score 15 In Sachs’ 8-week study, the mean (SD) valproate level was 61.5 (42.8) mcg/mL.
Discussion. The relationship between serum valproate and therapeutic efficacy in acute bipolar depression—and the range of levels considered therapeutic—are undefined. For now we recommend that individual patients’ clinical response and tolerability guide optimum serum valproate in acute bipolar depression (Box).16
When evaluating serum valproate levels–especially for assessing adherence–be careful to:
- obtain blood samples 12 hours after the most recent dose to accurately assess serum trough concentrations
- account for valproate’s saturation of protein binding sites and increased free fraction with increased serum concentration.16
Valproate clearance is increased when more free drug is available for metabolism, and this may result in disproportionately lower steady-state serum concentrations. Smaller increases in total valproate after dosage increases may be misinterpreted as medication nonadherence.
High levels and safety
High serum valproate levels may increase the risk and frequency of side effects. For example, serum levels >125 mcg/mL have been associated with:
- increased nausea, vomiting, dizziness, and sedation in acutely manic patients8
- weight gain and reduced platelets and white blood cells in patients receiving valproate as maintenance treatment.12
In the loading dose study by Hirschfeld et al,11 patients receiving divalproex, 20 to 30 mg/kg/d, did not experience a higher frequency or severity of side effects compared with patients receiving standard titration. Keck et al10 also reported minimal valproate-related side effects in their open-label study. Neither study suggested an upper-limit valproate level associated with increased side effects.
Discussion. Serum valproate >125 mcg/mL has been associated with increased side effects (Table 2), but more studies are needed.
Table 2
For bipolar disorder, suggested serum valproate therapeutic ranges*
| Serum valproate (mcg/mL) | |||
|---|---|---|---|
| Lower level | Upper level | Comments | |
| Acute mania | 45 to 50 | 125 | Upper level based on tolerability, not efficacy |
| Maintenance | 75 | 100 | Levels based primarily on retrospective analysis |
| Acute bipolar | Not established | Not established | |
| * Based on available data | |||
Clinical recommendations
Carefully consider when to monitor serum valproate levels in your patients with bipolar disorder:
- Obtaining routine serum levels can be expensive, and no data support the cost-effectiveness of this approach in bipolar disorder.
- Individualize valproate dosing; a specific patient’s therapeutic range may differ from another’s or from those published in the literature or used by a clinical laboratory.
- Monitoring serum valproate levels does not replace the need to adjust dosages based on patients’ therapeutic response and tolerance.
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry2002; 159(suppl 4):1–50.
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- Carbamazepine • Tegretol, Equetro
- Divalproex sodium • Depakote
- Felbamate • Felbatol
- Phenytoin • Dilantin
Dr. Kaneria reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Patel is a consultant to and speaker for Eli Lilly and Co. and a speaker for Pfizer.
Dr. Keck receives research support from or is a consultant to or advisor for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Co., Organon, Ortho-McNeil Pharmaceutical, Merck & Co., Pfizer, Shire, and UCB Pharma.
1. Glauser TA, Pippenger CE. Controversies in blood-level monitoring: reexamining its role in the treatment of epilepsy. Epilepsia 2000;41(suppl 8):S6-S15.
2. Pellock JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991;41:961-4.
3. Jannuzzi G, Cian P, Fattore C, et al. A multicenter randomized controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia 2000;41:222-30.
4. AmericanPsychiatric Association Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159(suppl 4):1-50.
5. Depakote (divalproex sodium) package insert Abbott Park, IL: Abbott Laboratories; October 2005.
6. Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry 1991;48:62-8.
7. Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA 1994;271:918-24.
8. Bowden CL, Janicak PG, Orsulak P, et al. Relation of serum valproate concentration to response in mania. Am J Psychiatry 1996;153:765-70.
9. Allen MH, Baker J, Wozniak PJ. Relationship of serum valproate level to response in mania (abstract presentation). New York: American Psychiatric Association annual meeting, 2004.
10. Keck PE, Jr, McElroy SL, Tugrul KC, Bennett JA. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry 1993;54:305-8.
11. Hirschfeld RM, Allen MH, McEvoy JP, et al. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry 1999;60:815-18.
12. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
13. Keck PE, Jr, Bowden CL, Meinhold JM, et al. Relationship between serum valproate and lithium levels and efficacy and tolerability in bipolar maintenance therapy. Int J Psychiatry Clin Pract (in press).
14. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord 2005;85:259-66.
15. Sachs GS, Collins MA, Altshuler LL, et al. Divalproex sodium versus placebo for the treatment of bipolar depression (abstract presentation). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2001.
16. Wilder BJ. Pharmacokinetics of valproate and carbamazepine. J Clin Psychopharmacol 1992;12(suppl 1):64S-68S.
Valproate’s well-accepted therapeutic range for treating epilepsy—50 to 100 mcg/mL—was adopted for bipolar disorder treatment without rigorous evaluation of serum levels and response relationships. Because most literature on monitoring serum valproate refers to its use as an anticonvulsant, you may wonder:
- When should I measure serum valproate in bipolar patients?
- What do serum valproate levels mean in their clinical care?
To answer these questions, we discuss when to monitor serum valproate, whether routinely or in specific situations. We then review studies that show how serum levels affect valproate’s efficacy and safety in three phases of bipolar disorder management: acute mania, maintenance therapy, and acute depression.
Is monitoring overused?
Some neurologists consider serum levels nonessential—and, in some cases, overused—when valproate is used as an anticonvulsant for healthy patients.1,2 A multicenter, randomized controlled trial evaluating the impact of antiepileptic drug monitoring on patient outcomes3 supports this notion, at least in part. Serum monitoring did not improve therapeutic outcome, suggesting that patients with epilepsy could be satisfactorily treated by adjusting dosages based on clinical response.
On the other hand, American Psychiatric Association (APA) guidelines for bipolar disorder suggest routine serum monitoring every 6 months along with other hematologic and hepatic assessments, or more frequently if necessary. The APA recommends maintaining serum valproate levels of 50 to 125 mcg/mL when treating:
- acutely manic patients
- outpatients
- the elderly
- patients who are hypomanic or euthymic.4
Table 1
4 situations where serum valproate monitoring may be clinically useful
| To establish a baseline effective level in individual patients |
| To assess lack or loss of efficacy, including patient adherence |
| When drug-drug interactions increase or decrease valproate clearance (such as with aspirin, carbamazepine, felbamate, or phenytoin)5 |
| When dose-dependent side effects occur (such as alopecia, elevated liver function, thrombocytopenia, or pancreatitis) |
Effective levels in acute mania
In one of the first randomized, double-blind, placebo-controlled trials to examine valproate use in adults with acute mania, Pope et al6 used the epilepsy reference range to adjust dosages. Patients (n=17) initially received valproate, 750 mg/d, and dosages were then adjusted to serum levels of 50 to 100 mcg/mL. Nineteen patients received placebo. Mean (SD) baseline Young Mania Rating Scale (YMRS) scores for the valproate and placebo groups were 28.2 (5.8) and 28.6 (6.9), respectively.
Patients receiving valproate showed the greatest symptomatic improvement—as indicated by YMRS scores—within 1 to 4 days of achieving a serum level ≥50 mcg/mL. Serum valproate for all patients was maintained at >50 mcg/mL, which limits our ability to draw conclusions about a minimum level associated with efficacy.
Minimum threshold for efficacy. In another randomized, double-blind, placebo-controlled study of acute mania, Bowden et al7 compared the efficacy of divalproex (n=69) versus lithium (n=36) or placebo (n=74) given for 3 weeks. Patients met criteria for manic disorder using the Schedule for Affective Disorders and Schizophrenia (SADS) and had Mania Rating Scale scores (derived from the SADS) of at least 14.
Those in the divalproex group received 750 mg/d for 2 days, then 1,000 mg/d for 3 days. Dosages were then adjusted to target a serum level of 150 mcg/mL, unless limited by side effects. Mean serum valproate levels on days 8 and 21 were 77 and 93.2 mcg/mL, respectively. Marked improvement, defined as ≥50% reduction in Mania Rating Scale scores, was seen in 48% of the divalproex group, compared with 25% of the placebo group.
The authors then analyzed the relationship between serum valproate levels and clinical response and tolerability.8 At day 5, patients with serum valproate ≥45 mcg/mL were 2 to 7 times more likely to show 20% or greater improvement in SADS mania subscales (manic syndrome, and behavior and ideation).
This study provided a minimum threshold for valproate efficacy in bipolar mania—45 to 50 mcg/mL—but not a level above which further clinical benefit would not be gained.
Optimum serum ranges. Allen et al9 recently conducted a post hoc analysis of pooled intent-to-treat data from three randomized, fixed dose, placebo-controlled studies of divalproex for acute mania. Subjects were stratified into a placebo group (n=171) and six serum valproate ranges:
- ≤55 mcg/mL (n=35)
- >55 to 71.3 mcg/mL (n=32)
- >71.3 to 85 mcg/mL (n=36)
- >85 to 94 mcg/mL (n=34)
- >94 to 107 mcg/mL (n=33)
- >107 mcg/mL (n=33).
Loading for rapid response. Patients with acute mania may respond sooner when loading doses are used to attain therapeutic serum valproate levels.
Keck et al10 examined time to onset of improvement in adults with acute mania (N=19) receiving oral loading doses of valproate (20 mg/kg/d in divided doses for 5 days) to rapidly attain valproate levels ≥50 mcg/mL. Ten (53%) patients who received at least 1 loading dose showed a ≥50% reduction in MRS scores and the greatest improvement across the first 3 days.
Hirschfeld et al11 also reported that patients’ symptoms began to improve sooner when divalproex was given at 30 mg/kg/d on days 1 and 2, and 20 mg/kg/d on days 3 to 10 (n=20), compared with standard titration (750 mg/d on days 1 and 2, and gradual dose titration on days 3 to 10 [n=20]).
Discussion. In acute mania, evidence suggests that patients with serum valproate ≥45 to 50 mcg/mL may show greater clinical improvement than patients with lower serum levels. Loading doses may achieve a minimum therapeutic serum level more quickly, yielding faster clinical improvement. A serum level >90 mcg/mL may confer additional benefit.
Although a minimum serum level has been recommended, no data have established a maximum level beyond which further clinical improvement would not be observed.
In maintenance therapy
What serum valproate levels are most effective for bipolar maintenance therapy? Some evidence is emerging.
Bowden et al12 compared divalproex (n=187), lithium (n=90), and placebo (n=92) in a 52-week, double-blind, parallel-group study of bipolar adult outpatients who met recovery criteria 3 months after an index manic episode. Divalproex dosages were adjusted to achieve trough serum concentrations between 71 and 125 mcg/mL. Mean (SD) and median serum valproate levels were 84.8 (29.9) mcg/mL and 83.9 mcg/mL, respectively. Serum valproate levels significantly correlated with Mania Rating Scale scores. No minimum threshold for efficacy was reported.
Thirteen subjects in the divalproex group were then stratified into 4 categories:
- nontherapeutic (
- low therapeutic (50 to 74.9 mcg/mL)
- medium therapeutic (75 to 99.9 mcg/mL)
- high therapeutic (>100 mcg/mL).
Discussion. Serum valproate levels of 75 to 100 mcg/mL may be most effective in preventing subsequent mood episodes with acceptable tolerability. Prospective, longitudinal studies are needed to better establish a therapeutic range for valproate in bipolar maintenance therapy.
In bipolar depression
Little evidence supports a therapeutic serum valproate range for treating acute bipolar depression.
In an 8-week, double-blind study, Davis et al14 randomly assigned adults with bipolar depression to divalproex (n=13) or placebo (n=12). Bipolar depression diagnoses were confirmed using the Structured Clinical Interview for DSM-IV, and patients were required to have a Hamilton Rating Scale for Depression (HRSD) score ≥16.
Valproate was started at 500 mg/d and titrated to serum levels of 50 to 150 mcg/mL. Mean (SD) serum valproate levels at weeks 4 and 8 were 80 (9.3) mcg/mL and 81 (19.2) mcg/mL, respectively. Remission rate (defined a priori as a >50% improvement and total HRSD score 15 In Sachs’ 8-week study, the mean (SD) valproate level was 61.5 (42.8) mcg/mL.
Discussion. The relationship between serum valproate and therapeutic efficacy in acute bipolar depression—and the range of levels considered therapeutic—are undefined. For now we recommend that individual patients’ clinical response and tolerability guide optimum serum valproate in acute bipolar depression (Box).16
When evaluating serum valproate levels–especially for assessing adherence–be careful to:
- obtain blood samples 12 hours after the most recent dose to accurately assess serum trough concentrations
- account for valproate’s saturation of protein binding sites and increased free fraction with increased serum concentration.16
Valproate clearance is increased when more free drug is available for metabolism, and this may result in disproportionately lower steady-state serum concentrations. Smaller increases in total valproate after dosage increases may be misinterpreted as medication nonadherence.
High levels and safety
High serum valproate levels may increase the risk and frequency of side effects. For example, serum levels >125 mcg/mL have been associated with:
- increased nausea, vomiting, dizziness, and sedation in acutely manic patients8
- weight gain and reduced platelets and white blood cells in patients receiving valproate as maintenance treatment.12
In the loading dose study by Hirschfeld et al,11 patients receiving divalproex, 20 to 30 mg/kg/d, did not experience a higher frequency or severity of side effects compared with patients receiving standard titration. Keck et al10 also reported minimal valproate-related side effects in their open-label study. Neither study suggested an upper-limit valproate level associated with increased side effects.
Discussion. Serum valproate >125 mcg/mL has been associated with increased side effects (Table 2), but more studies are needed.
Table 2
For bipolar disorder, suggested serum valproate therapeutic ranges*
| Serum valproate (mcg/mL) | |||
|---|---|---|---|
| Lower level | Upper level | Comments | |
| Acute mania | 45 to 50 | 125 | Upper level based on tolerability, not efficacy |
| Maintenance | 75 | 100 | Levels based primarily on retrospective analysis |
| Acute bipolar | Not established | Not established | |
| * Based on available data | |||
Clinical recommendations
Carefully consider when to monitor serum valproate levels in your patients with bipolar disorder:
- Obtaining routine serum levels can be expensive, and no data support the cost-effectiveness of this approach in bipolar disorder.
- Individualize valproate dosing; a specific patient’s therapeutic range may differ from another’s or from those published in the literature or used by a clinical laboratory.
- Monitoring serum valproate levels does not replace the need to adjust dosages based on patients’ therapeutic response and tolerance.
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry2002; 159(suppl 4):1–50.
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- Carbamazepine • Tegretol, Equetro
- Divalproex sodium • Depakote
- Felbamate • Felbatol
- Phenytoin • Dilantin
Dr. Kaneria reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Patel is a consultant to and speaker for Eli Lilly and Co. and a speaker for Pfizer.
Dr. Keck receives research support from or is a consultant to or advisor for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Co., Organon, Ortho-McNeil Pharmaceutical, Merck & Co., Pfizer, Shire, and UCB Pharma.
Valproate’s well-accepted therapeutic range for treating epilepsy—50 to 100 mcg/mL—was adopted for bipolar disorder treatment without rigorous evaluation of serum levels and response relationships. Because most literature on monitoring serum valproate refers to its use as an anticonvulsant, you may wonder:
- When should I measure serum valproate in bipolar patients?
- What do serum valproate levels mean in their clinical care?
To answer these questions, we discuss when to monitor serum valproate, whether routinely or in specific situations. We then review studies that show how serum levels affect valproate’s efficacy and safety in three phases of bipolar disorder management: acute mania, maintenance therapy, and acute depression.
Is monitoring overused?
Some neurologists consider serum levels nonessential—and, in some cases, overused—when valproate is used as an anticonvulsant for healthy patients.1,2 A multicenter, randomized controlled trial evaluating the impact of antiepileptic drug monitoring on patient outcomes3 supports this notion, at least in part. Serum monitoring did not improve therapeutic outcome, suggesting that patients with epilepsy could be satisfactorily treated by adjusting dosages based on clinical response.
On the other hand, American Psychiatric Association (APA) guidelines for bipolar disorder suggest routine serum monitoring every 6 months along with other hematologic and hepatic assessments, or more frequently if necessary. The APA recommends maintaining serum valproate levels of 50 to 125 mcg/mL when treating:
- acutely manic patients
- outpatients
- the elderly
- patients who are hypomanic or euthymic.4
Table 1
4 situations where serum valproate monitoring may be clinically useful
| To establish a baseline effective level in individual patients |
| To assess lack or loss of efficacy, including patient adherence |
| When drug-drug interactions increase or decrease valproate clearance (such as with aspirin, carbamazepine, felbamate, or phenytoin)5 |
| When dose-dependent side effects occur (such as alopecia, elevated liver function, thrombocytopenia, or pancreatitis) |
Effective levels in acute mania
In one of the first randomized, double-blind, placebo-controlled trials to examine valproate use in adults with acute mania, Pope et al6 used the epilepsy reference range to adjust dosages. Patients (n=17) initially received valproate, 750 mg/d, and dosages were then adjusted to serum levels of 50 to 100 mcg/mL. Nineteen patients received placebo. Mean (SD) baseline Young Mania Rating Scale (YMRS) scores for the valproate and placebo groups were 28.2 (5.8) and 28.6 (6.9), respectively.
Patients receiving valproate showed the greatest symptomatic improvement—as indicated by YMRS scores—within 1 to 4 days of achieving a serum level ≥50 mcg/mL. Serum valproate for all patients was maintained at >50 mcg/mL, which limits our ability to draw conclusions about a minimum level associated with efficacy.
Minimum threshold for efficacy. In another randomized, double-blind, placebo-controlled study of acute mania, Bowden et al7 compared the efficacy of divalproex (n=69) versus lithium (n=36) or placebo (n=74) given for 3 weeks. Patients met criteria for manic disorder using the Schedule for Affective Disorders and Schizophrenia (SADS) and had Mania Rating Scale scores (derived from the SADS) of at least 14.
Those in the divalproex group received 750 mg/d for 2 days, then 1,000 mg/d for 3 days. Dosages were then adjusted to target a serum level of 150 mcg/mL, unless limited by side effects. Mean serum valproate levels on days 8 and 21 were 77 and 93.2 mcg/mL, respectively. Marked improvement, defined as ≥50% reduction in Mania Rating Scale scores, was seen in 48% of the divalproex group, compared with 25% of the placebo group.
The authors then analyzed the relationship between serum valproate levels and clinical response and tolerability.8 At day 5, patients with serum valproate ≥45 mcg/mL were 2 to 7 times more likely to show 20% or greater improvement in SADS mania subscales (manic syndrome, and behavior and ideation).
This study provided a minimum threshold for valproate efficacy in bipolar mania—45 to 50 mcg/mL—but not a level above which further clinical benefit would not be gained.
Optimum serum ranges. Allen et al9 recently conducted a post hoc analysis of pooled intent-to-treat data from three randomized, fixed dose, placebo-controlled studies of divalproex for acute mania. Subjects were stratified into a placebo group (n=171) and six serum valproate ranges:
- ≤55 mcg/mL (n=35)
- >55 to 71.3 mcg/mL (n=32)
- >71.3 to 85 mcg/mL (n=36)
- >85 to 94 mcg/mL (n=34)
- >94 to 107 mcg/mL (n=33)
- >107 mcg/mL (n=33).
Loading for rapid response. Patients with acute mania may respond sooner when loading doses are used to attain therapeutic serum valproate levels.
Keck et al10 examined time to onset of improvement in adults with acute mania (N=19) receiving oral loading doses of valproate (20 mg/kg/d in divided doses for 5 days) to rapidly attain valproate levels ≥50 mcg/mL. Ten (53%) patients who received at least 1 loading dose showed a ≥50% reduction in MRS scores and the greatest improvement across the first 3 days.
Hirschfeld et al11 also reported that patients’ symptoms began to improve sooner when divalproex was given at 30 mg/kg/d on days 1 and 2, and 20 mg/kg/d on days 3 to 10 (n=20), compared with standard titration (750 mg/d on days 1 and 2, and gradual dose titration on days 3 to 10 [n=20]).
Discussion. In acute mania, evidence suggests that patients with serum valproate ≥45 to 50 mcg/mL may show greater clinical improvement than patients with lower serum levels. Loading doses may achieve a minimum therapeutic serum level more quickly, yielding faster clinical improvement. A serum level >90 mcg/mL may confer additional benefit.
Although a minimum serum level has been recommended, no data have established a maximum level beyond which further clinical improvement would not be observed.
In maintenance therapy
What serum valproate levels are most effective for bipolar maintenance therapy? Some evidence is emerging.
Bowden et al12 compared divalproex (n=187), lithium (n=90), and placebo (n=92) in a 52-week, double-blind, parallel-group study of bipolar adult outpatients who met recovery criteria 3 months after an index manic episode. Divalproex dosages were adjusted to achieve trough serum concentrations between 71 and 125 mcg/mL. Mean (SD) and median serum valproate levels were 84.8 (29.9) mcg/mL and 83.9 mcg/mL, respectively. Serum valproate levels significantly correlated with Mania Rating Scale scores. No minimum threshold for efficacy was reported.
Thirteen subjects in the divalproex group were then stratified into 4 categories:
- nontherapeutic (
- low therapeutic (50 to 74.9 mcg/mL)
- medium therapeutic (75 to 99.9 mcg/mL)
- high therapeutic (>100 mcg/mL).
Discussion. Serum valproate levels of 75 to 100 mcg/mL may be most effective in preventing subsequent mood episodes with acceptable tolerability. Prospective, longitudinal studies are needed to better establish a therapeutic range for valproate in bipolar maintenance therapy.
In bipolar depression
Little evidence supports a therapeutic serum valproate range for treating acute bipolar depression.
In an 8-week, double-blind study, Davis et al14 randomly assigned adults with bipolar depression to divalproex (n=13) or placebo (n=12). Bipolar depression diagnoses were confirmed using the Structured Clinical Interview for DSM-IV, and patients were required to have a Hamilton Rating Scale for Depression (HRSD) score ≥16.
Valproate was started at 500 mg/d and titrated to serum levels of 50 to 150 mcg/mL. Mean (SD) serum valproate levels at weeks 4 and 8 were 80 (9.3) mcg/mL and 81 (19.2) mcg/mL, respectively. Remission rate (defined a priori as a >50% improvement and total HRSD score 15 In Sachs’ 8-week study, the mean (SD) valproate level was 61.5 (42.8) mcg/mL.
Discussion. The relationship between serum valproate and therapeutic efficacy in acute bipolar depression—and the range of levels considered therapeutic—are undefined. For now we recommend that individual patients’ clinical response and tolerability guide optimum serum valproate in acute bipolar depression (Box).16
When evaluating serum valproate levels–especially for assessing adherence–be careful to:
- obtain blood samples 12 hours after the most recent dose to accurately assess serum trough concentrations
- account for valproate’s saturation of protein binding sites and increased free fraction with increased serum concentration.16
Valproate clearance is increased when more free drug is available for metabolism, and this may result in disproportionately lower steady-state serum concentrations. Smaller increases in total valproate after dosage increases may be misinterpreted as medication nonadherence.
High levels and safety
High serum valproate levels may increase the risk and frequency of side effects. For example, serum levels >125 mcg/mL have been associated with:
- increased nausea, vomiting, dizziness, and sedation in acutely manic patients8
- weight gain and reduced platelets and white blood cells in patients receiving valproate as maintenance treatment.12
In the loading dose study by Hirschfeld et al,11 patients receiving divalproex, 20 to 30 mg/kg/d, did not experience a higher frequency or severity of side effects compared with patients receiving standard titration. Keck et al10 also reported minimal valproate-related side effects in their open-label study. Neither study suggested an upper-limit valproate level associated with increased side effects.
Discussion. Serum valproate >125 mcg/mL has been associated with increased side effects (Table 2), but more studies are needed.
Table 2
For bipolar disorder, suggested serum valproate therapeutic ranges*
| Serum valproate (mcg/mL) | |||
|---|---|---|---|
| Lower level | Upper level | Comments | |
| Acute mania | 45 to 50 | 125 | Upper level based on tolerability, not efficacy |
| Maintenance | 75 | 100 | Levels based primarily on retrospective analysis |
| Acute bipolar | Not established | Not established | |
| * Based on available data | |||
Clinical recommendations
Carefully consider when to monitor serum valproate levels in your patients with bipolar disorder:
- Obtaining routine serum levels can be expensive, and no data support the cost-effectiveness of this approach in bipolar disorder.
- Individualize valproate dosing; a specific patient’s therapeutic range may differ from another’s or from those published in the literature or used by a clinical laboratory.
- Monitoring serum valproate levels does not replace the need to adjust dosages based on patients’ therapeutic response and tolerance.
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry2002; 159(suppl 4):1–50.
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- Carbamazepine • Tegretol, Equetro
- Divalproex sodium • Depakote
- Felbamate • Felbatol
- Phenytoin • Dilantin
Dr. Kaneria reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Patel is a consultant to and speaker for Eli Lilly and Co. and a speaker for Pfizer.
Dr. Keck receives research support from or is a consultant to or advisor for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Co., Organon, Ortho-McNeil Pharmaceutical, Merck & Co., Pfizer, Shire, and UCB Pharma.
1. Glauser TA, Pippenger CE. Controversies in blood-level monitoring: reexamining its role in the treatment of epilepsy. Epilepsia 2000;41(suppl 8):S6-S15.
2. Pellock JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991;41:961-4.
3. Jannuzzi G, Cian P, Fattore C, et al. A multicenter randomized controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia 2000;41:222-30.
4. AmericanPsychiatric Association Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159(suppl 4):1-50.
5. Depakote (divalproex sodium) package insert Abbott Park, IL: Abbott Laboratories; October 2005.
6. Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry 1991;48:62-8.
7. Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA 1994;271:918-24.
8. Bowden CL, Janicak PG, Orsulak P, et al. Relation of serum valproate concentration to response in mania. Am J Psychiatry 1996;153:765-70.
9. Allen MH, Baker J, Wozniak PJ. Relationship of serum valproate level to response in mania (abstract presentation). New York: American Psychiatric Association annual meeting, 2004.
10. Keck PE, Jr, McElroy SL, Tugrul KC, Bennett JA. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry 1993;54:305-8.
11. Hirschfeld RM, Allen MH, McEvoy JP, et al. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry 1999;60:815-18.
12. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
13. Keck PE, Jr, Bowden CL, Meinhold JM, et al. Relationship between serum valproate and lithium levels and efficacy and tolerability in bipolar maintenance therapy. Int J Psychiatry Clin Pract (in press).
14. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord 2005;85:259-66.
15. Sachs GS, Collins MA, Altshuler LL, et al. Divalproex sodium versus placebo for the treatment of bipolar depression (abstract presentation). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2001.
16. Wilder BJ. Pharmacokinetics of valproate and carbamazepine. J Clin Psychopharmacol 1992;12(suppl 1):64S-68S.
1. Glauser TA, Pippenger CE. Controversies in blood-level monitoring: reexamining its role in the treatment of epilepsy. Epilepsia 2000;41(suppl 8):S6-S15.
2. Pellock JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991;41:961-4.
3. Jannuzzi G, Cian P, Fattore C, et al. A multicenter randomized controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia 2000;41:222-30.
4. AmericanPsychiatric Association Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159(suppl 4):1-50.
5. Depakote (divalproex sodium) package insert Abbott Park, IL: Abbott Laboratories; October 2005.
6. Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry 1991;48:62-8.
7. Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA 1994;271:918-24.
8. Bowden CL, Janicak PG, Orsulak P, et al. Relation of serum valproate concentration to response in mania. Am J Psychiatry 1996;153:765-70.
9. Allen MH, Baker J, Wozniak PJ. Relationship of serum valproate level to response in mania (abstract presentation). New York: American Psychiatric Association annual meeting, 2004.
10. Keck PE, Jr, McElroy SL, Tugrul KC, Bennett JA. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry 1993;54:305-8.
11. Hirschfeld RM, Allen MH, McEvoy JP, et al. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry 1999;60:815-18.
12. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
13. Keck PE, Jr, Bowden CL, Meinhold JM, et al. Relationship between serum valproate and lithium levels and efficacy and tolerability in bipolar maintenance therapy. Int J Psychiatry Clin Pract (in press).
14. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord 2005;85:259-66.
15. Sachs GS, Collins MA, Altshuler LL, et al. Divalproex sodium versus placebo for the treatment of bipolar depression (abstract presentation). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2001.
16. Wilder BJ. Pharmacokinetics of valproate and carbamazepine. J Clin Psychopharmacol 1992;12(suppl 1):64S-68S.
When every minute counts: What workup is sufficient for diagnosis under pressure?
Police officers bring Mr. A, age 25, to the emergency department (ED) in handcuffs after an alleged assault at work. He is calm but will provide no information about himself. ED staff don’t know if he has been using illicit substances, is on medications, or has any medical conditions.
Mr. A says the FBI is after him, but he makes no threats to ED staff. He talks about milking cows on a farm and of hearing animal sounds, though he lives in the city. After about 30 minutes, he consents to a lab draw and provides a urine sample.
Because no charges are pending and Mr. A is semi-cooperative, police remove his handcuffs and leave him in the care of two ED security officers. He is given something to eat and drink and seems fairly content. He asks how long he will need to stay in the room but does not demand to leave.
In a fast-paced ED, physicians might not notice signs of psychiatric illness, such as Mr. A’s paranoid and delusional thinking. By being familiar with techniques to manage patients’ psychiatric emergencies, you can help your ED colleagues:
- establish working psychiatric diagnoses and medical causes of psychiatric symptoms in the fast-paced ED
- maintain a safe ED environment for patients and clinicians.
What ed patients want
To understand how ED patients feel, put yourself in Mr. A’s shoes. You were at work and began to hallucinate. You believed your boss was out to harm you, and in fear you made comments perceived as threatening.
The next thing you know, you’re in a police car with handcuffs on. All of your coworkers witnessed your embarrassment. Now you are in a small ED room, wondering what’s going to happen next. Are you going to be put in a straight jacket and a padded room?
Patients may experience anxiety-provoking thoughts whether they come to the ED voluntarily or involuntarily. Fear and confusion can affect their behavior in the ED, and how providers respond to patients in crisis can escalate or de-escalate an already-difficult situation.
Psychiatric illness in the ed
Mr. A may have a psychiatric disorder, as do at least 3% of patients seen in EDs.1 This figure may be low, however:
- Kunen et al2 asserted that EDs are underdiagnosing psychiatric disorders, given a U.S. Department of Health and Human Services 1999 estimate that 20% to 28% of Americans have psychiatric illnesses. Using ED discharge records across 6 months in three emergency departments, the authors found the psychiatric diagnosis rate to be 5.27% in 33,000 ED visits.
- Another study, done in a university teaching hospital ED, showed that ED physicians trained to focus on patients’ presenting problems often missed comorbid medical or psychiatric illnesses.
In the randomized, controlled trial by Schriger et al,3 218 patients with nonspecific complaints suggesting occult psychiatric illness (such as chronic headache, abdominal pain, or back pain) completed the Primary Care Evaluation of Mental Disorders (PRIME-MD) questionnaire. This 27-item self-report asks questions about mood, alcohol use, obsessive-compulsive symptoms, phobias, and somatoform symptoms.
Participants were then randomly assigned to “report” or “nonreport” groups, depending on whether or not ED physicians received their PRIME-MD scores. Even when informed of patients’ psychiatric symptoms, ED physicians rarely diagnosed or treated psychiatric disorders. Lack of mental status documentation and psychiatric interviews was apparent, the authors noted.
Case continued: a toxic cocktail
Mr. A’s urine drug screen and lab results are positive for benzodiazepines, methamphetamines, and cannabis. The staff decide Mr. A will require further observation and detoxification, and he is told this. A bed is not available at the hospital, however, and calls to nearby facilities find no empty beds.
As time passes, Mr. A shows signs of agitation and arousal. He paces the examination room—his jaw clenched and his face flushed—and begins raising his voice, asking to be discharged.
Recommendations. Unpleasantness is sometimes unavoidable, but no one in the ED has tried to create an alliance with Mr. A (Box). Try to make patients’ ED experiences as positive as possible. Make it clear that you share a common goal: to help the patient feel better. In fact, psychiatric patients and emergency psychiatrists have similar ideas about what constitutes quality ED care. When surveyed,4 ED patients said they preferred:
- verbal interventions compared with medications
- a collaborative approach with ED physicians
- having medications selected for their specific problems, medication experiences, and choices
- benzodiazepines rather than conventional antipsychotics such as haloperidol.
Treat patients with respect, and preserve their sense of dignity
Offer patients choices when reasonable to help them feel they have some control
Strongly (and early) encourage smokers to accept nicotine replacement to avoid withdrawal and heightened arousal
Offer food, beverages, a blanket, or other comfort measures that would not compromise safety (do not give hot coffee, in case the patient throws the cup at someone)
Allow patients to call a loved one, friend, or pastor (offer a cordless phone to avoid strangulation attempts)
Allow relatives or friends to sit and talk with the patient if this would not compromise safety
Keep patients informed on what is going on and why
Answer questions asked by the patient and family or friends
Offer oral medications first
Get to know your security staff well
A pragmatic workup
Medical illnesses such as delirium, stroke, drug toxicity, or urinary tract infections can trigger or worsen psychiatric illness (Table 1).5 Comorbidities such as diabetes, hypertension, obesity, and heart disease are common in patients with psychiatric disorders, and psychotropics can cause or exacerbate these conditions.
In the high-pressure ED, a sufficient workup for complicated medical conditions lies somewhere between extensive/unnecessary and inadequate. Thus, determining an exact diagnosis is not as important as establishing a diagnostic category to guide emergency treatment.
Think of psychiatric disorders as they are organized in DSM-IV-TR—mood, anxiety, psychotic, substance use/withdrawal/intoxication, cognitive, adjustment, somatoform, and personality disorders—and whether they are primary or secondary to a general medical condition or substance use. For example:
- Anxiety disorder secondary to a general medical condition means the history, physical exam, or lab reports suggest a medical condition is the direct physiologic cause of the mood disturbance.
- Methamphetamine-induced psychotic disorder would be the diagnosis if methamphetamines are presumed to be causing a patient’s psychotic symptoms.
Hospitalization. ED staff often develop a treatment plan based on a patient’s clinical picture and a working diagnosis. The plan hinges on deciding if the patient needs to be admitted to the hospital. Admission may be warranted for life-threatening medical conditions or safety issues, such as threats to self or others or inability to care for oneself at home. Other issues come into play—such as starting or changing medications and follow-up to ensure continuity of care—if you decide to discharge the patient.
Even after medical clearance, patients in the psychiatric emergency service may have underlying medical illnesses (Table 2).6
Table 1
Medical disorders that can cause psychiatric symptoms
| Medical/toxic disorders | Examples |
|---|---|
| Alcohol and drugs of abuse | Amphetamines (including methamphetamine), cocaine, heroin, Jimson weed, ketamine, marijuana, MDMA (‘Ecstasy’), LSD, PCP |
| Prescription drugs | Antibiotics, anticholinergics, anticonvulsants, antihypertensives, benzodiazepines, chemotherapeutic agents, cimetidine, corticosteroids, digitalis, narcotics, propranolol, sleep medications, tricyclic antidepressants |
| CNS disease | Hypertensive encephalopathy, intracranial aneurysm, metastases, normal pressure hydrocephalus, postictal nonconvulsive status, primary CNS infection, seizure disorders, stroke, subdural hematoma, tumor |
| Infections | Acute rheumatic fever, diphtheria, malaria, Legionnaires’ disease, pneumonia, Rocky Mountain spotted fever, sepsis, syphilis, typhoid fever, urinary tract infection |
| Metabolic/endocrine disorders | Adrenal disease, diabetic ketoacidosis, hepatic encephalopathy, hypoglycemia, pituitary dysfunction, renal disease, serum electrolyte imbalances (sodium, potassium, calcium), thyroid disease, vitamin deficiencies, Wilson’s disease |
| Cardiopulmonary disease | Arrhythmias, congestive heart failure, COPD/asthma, myocardial infarction, pulmonary embolism |
| Miscellaneous | Anemia, lupus, multiple sclerosis, temporal arteritis, vasculitis |
| Source: Reprinted with permission from Williams ER, Shepherd SM. Medical clearance of psychiatric patients. Emerg Med Clin North Am 2000;18(2):185-90. Copyright 2000, Elsevier. | |
Table 2
Reasonable medical assessment in psychiatric emergencies
| DO |
| Obtain a medical history, the best determinant of medical need |
| Listen to patients. If they say they have a medical condition, believe them; if they say they don’t, try to believe them |
| Thoroughly check vital signs |
| Conduct a focused physical examination |
| Maintain a high index of suspicion |
Be selective with laboratory testing. Check:
|
| DON’T |
| Order blanket laboratory screening |
| Order an ECG in healthy young patients in the absence of clinical findings |
| Order chest radiography in the absence of known disease/exposure/symptoms |
| Source: Reprinted from Currier GW. Medical assessment on the psychiatric emergency service. Psychiatric Issues in Emergency Care Settings 2004;3(July):17, with permission from Cliggott Publishing Group of CMP Healthcare Media. Copyright 2004. |
Overwhelming demand
In the study of ED patient preferences,4 one-fifth of patients said they went to the ED because they lacked access to routine mental health care. Therefore, besides psychiatric conditions caused by medical illnesses, ED physicians can see patients with any primary psychiatric diagnosis, including mood and anxiety disorders and psychosis.
Under pressures of time and limited collateral information, ED staff must:
- individualize psychiatric treatment
- consider use of medications and/or restraints
- rule out life-threatening causes for psychiatric symptoms
- stabilize patients and prevent injury to self and others.
These tasks are becoming increasingly difficult as more and more patients present to emergency rooms. Nationally, ED visits increased from 19 million in 1992 to 108 million in 2000, according to the U.S. Department of Health and Human Services.1
Psychiatric patients are seeking ED care in greater numbers, and the number of those staying longer than anticipated (“boarding”) also has increased, according to a 2004 survey of 340 physicians by the American College of Emergency Physicians, American Psychiatric Association, National Mental Health Association, and National Alliance for the Mentally Ill. Surveyed physicians blamed inadequate Medicaid funding and bed shortages for the increasing ED visits.7
In crowded emergency rooms, where patients wait longer and longer to be seen, the influx of acutely ill psychiatric patients increases the risks of agitation, violence, and injury, as well as litigation.8
Case continued: going up in smoke
Recognizing Mr. A’s arousal, ED staff tries to reassure him and offers him food, something to drink, a phone Call, and a magazine. When these attempts fail to de-escalate his agitation, staff offers to make him more comfortable by giving oral lorazepam, which he adamantly refuses. He is told again that he must stay until a transfer facility is found for him.
Mr. A then demands to go outside “for a smoke.” When he is told ED patients cannot leave to smoke and is offered nicotine replacement, he begins to scream and lunges at one of the security officers. He is extremely strong, and additional officers are summoned. He retreats inside the room, slams the door, shatters the door window with a chair, and begins punching the broken glass. He slides to the floor in a vasovagal reaction at the sight of his bleeding hands but soon becomes combative again.
Staff give Mr. A IM haloperidol, 10 mg, and lorazepam, 2 mg, to manage his extreme agitation and place him in physical restraints to protect him and others. Within 25 to 30 minutes he is calm, and a safe environment has been re-established. The lacerations on his hands are sutured, and he is admitted to an inpatient psychiatric hospital for further stabilization and treatment.
No place for complacency
Mr. A’s experience illustrates how situations can become dangerous when precautions are not taken. Five steps can help you prepare and protect yourself when evaluating patients in the ED:
- seek the patient history
- evaluate the context in which the patient is being assessed
- identify arousal states (fear, anger, confusion, and humiliation)
- structure the interview for safety
- keep your guard up during the clinical encounter.9
Risk is high when law enforcement officers bring a patient to the ED. Be on guard, even if the patient is 80 years old and in a wheelchair. Complacency has no place in the ED; prepare as much as you can before interviewing the patient.
When restraints are needed. Involuntary medication and/or restraints may be necessary when reasonable interventions have failed, the patient will not cooperate, and he or she is exhibiting behavior/symptoms that could result in injury. Approximately 10% to 20% of psychiatric patients require physical or chemical restraint in the ED.10
Expert consensus guidelines suggest starting with verbal intervention, voluntary medication, and show of force, although emergency medication may be a reasonable first treatment (Algorithm).11 Offer oral medication first; IM medications carry risks including acute dystonia and akathisia, although these can be treated.
Lorazepam, 1 to 2 mg oral/IM, combined with haloperidol, 2 to 5 mg oral/IM, is a reasonable start in most cases. If the patient remains extremely agitated, the same medications and dosages can be repeated 30 to 60 minutes after the initial administration.12
Conventional oral/IM agents are usually more readily available in the ED than atypical antipsychotics, which must be ordered from the pharmacy. Recent FDA black-box warnings also emphasize that atypical antipsychotics are approved only for treating schizophrenia, acute manic and mixed episodes of bipolar I disorder, and for maintenance treatment in bipolar disorder. When compared with placebo, atypical antipsychotics have been associated with:
- increased risk for cerebrovascular events in elderly patients with dementia
- death in elderly patients with dementia-related psychosis.
Atypicals may be more appropriate than conventional antipsychotics for emergency treatment of agitation and aggression in some patients with complicating medical conditions or histories. For example, avoid high-potency conventional antipsychotics in patients with a history of extrapyramidal side effects and in those with mental retardation/developmental delay.11 Similarly, avoid benzodiazepines in patients with chronic obstructive pulmonary disease (COPD) or a history of drug-seeking behavior or drug abuse.
Of course not all psychiatric interventions in the ED are involuntary. For example, the ED physician may start an antidepressant for a patient diagnosed with mild to moderate depression for whom hospitalization is not indicated. Characteristics of patients who may be good candidates for starting antidepressants in the ED include a clear diagnosis, no substance abuse, low suicide risk, no psychosis or agitation, available social supports, clear follow-up plan, desire to begin treatment, and ability to pay for and obtain medications.13
Algorithm Consensus guideline for treating a behavioral emergency
Related resources
- Allen MH, Currier GW, Hughes DH, et al. The Expert Consensus Guideline Series: Treatment of behavioral emergencies. Postgrad Med 2001;May(Spec No):1-88.
- American College of Emergency Physicians. www.acep.org
- National Alliance on Mental Illness. www.nami.org
Drug brand names
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McCaig LF, Ly N. National Hospital Ambulatory Medical Care Survey: 2000 emergency department summary. Adv Data 2002;327:1-27.
2. Kunen S, Niederhauser R, Smith PO, et al. Race disparities in psychiatric rates in emergency departments. J Consult Clin Psychol 2005;73(1):116-126.
3. Schriger DL, Gibbons PS, Langone CA, et al. Enabling the diagnosis of occult psychiatric illness in the emergency department: a randomized, controlled trial of the computerized, self-administered PRIME-MD diagnostic system. Ann Emerg Med 2001;37(2):132-40.
4. Allen M, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract 2003;9(1):39-58.
5. Williams ER, Shepherd SM. Medical clearance of psychiatric patients. Emerg Med Clin North Am 2000;18(2):185-98.
6. Allen MH, Currier GW. Medical assessment on the psychiatric emergency service. New Dir Ment Health Serv 1999;82:21-8.
7. Mulligan K. ER docs report large increase in psychiatric patients. Psychiatr News 2004;39(12):10.-
8. Karcz A, Holbrook J, Auerbach BS, et al. Preventability of malpractice claims in emergency medicine: a closed claims study. Ann Emerg Med 1990;19(8):865-73.
9. Battaglia J. Is this patient dangerous? 5 steps to assess risk for violence. Current Psychiatry 2004;3(2):14-21.
10. De Fruyt J, Demyttenaere K. Rapid tranquilization: new approaches in the emergency treatment of behavioral disturbances. Eur Psychiatry 2004;19:243-9.
11. Allen MH, Currier GW, Hughes DH, et al. The Expert Consensus Guideline Series: Treatment of behavioral emergencies. Postgrad Med 2001;May(Spec No):1-88.
12. Hughes DH. Acute psychopharmacological management of the aggressive psychotic patient. Psychiatr Serv 1999;50(9):1135-7.
13. Glick RL. Starting antidepressant treatment in the emergency setting. Psychiatric Issues in Emergency Care Settings 2004;3(2):6-10.
Police officers bring Mr. A, age 25, to the emergency department (ED) in handcuffs after an alleged assault at work. He is calm but will provide no information about himself. ED staff don’t know if he has been using illicit substances, is on medications, or has any medical conditions.
Mr. A says the FBI is after him, but he makes no threats to ED staff. He talks about milking cows on a farm and of hearing animal sounds, though he lives in the city. After about 30 minutes, he consents to a lab draw and provides a urine sample.
Because no charges are pending and Mr. A is semi-cooperative, police remove his handcuffs and leave him in the care of two ED security officers. He is given something to eat and drink and seems fairly content. He asks how long he will need to stay in the room but does not demand to leave.
In a fast-paced ED, physicians might not notice signs of psychiatric illness, such as Mr. A’s paranoid and delusional thinking. By being familiar with techniques to manage patients’ psychiatric emergencies, you can help your ED colleagues:
- establish working psychiatric diagnoses and medical causes of psychiatric symptoms in the fast-paced ED
- maintain a safe ED environment for patients and clinicians.
What ed patients want
To understand how ED patients feel, put yourself in Mr. A’s shoes. You were at work and began to hallucinate. You believed your boss was out to harm you, and in fear you made comments perceived as threatening.
The next thing you know, you’re in a police car with handcuffs on. All of your coworkers witnessed your embarrassment. Now you are in a small ED room, wondering what’s going to happen next. Are you going to be put in a straight jacket and a padded room?
Patients may experience anxiety-provoking thoughts whether they come to the ED voluntarily or involuntarily. Fear and confusion can affect their behavior in the ED, and how providers respond to patients in crisis can escalate or de-escalate an already-difficult situation.
Psychiatric illness in the ed
Mr. A may have a psychiatric disorder, as do at least 3% of patients seen in EDs.1 This figure may be low, however:
- Kunen et al2 asserted that EDs are underdiagnosing psychiatric disorders, given a U.S. Department of Health and Human Services 1999 estimate that 20% to 28% of Americans have psychiatric illnesses. Using ED discharge records across 6 months in three emergency departments, the authors found the psychiatric diagnosis rate to be 5.27% in 33,000 ED visits.
- Another study, done in a university teaching hospital ED, showed that ED physicians trained to focus on patients’ presenting problems often missed comorbid medical or psychiatric illnesses.
In the randomized, controlled trial by Schriger et al,3 218 patients with nonspecific complaints suggesting occult psychiatric illness (such as chronic headache, abdominal pain, or back pain) completed the Primary Care Evaluation of Mental Disorders (PRIME-MD) questionnaire. This 27-item self-report asks questions about mood, alcohol use, obsessive-compulsive symptoms, phobias, and somatoform symptoms.
Participants were then randomly assigned to “report” or “nonreport” groups, depending on whether or not ED physicians received their PRIME-MD scores. Even when informed of patients’ psychiatric symptoms, ED physicians rarely diagnosed or treated psychiatric disorders. Lack of mental status documentation and psychiatric interviews was apparent, the authors noted.
Case continued: a toxic cocktail
Mr. A’s urine drug screen and lab results are positive for benzodiazepines, methamphetamines, and cannabis. The staff decide Mr. A will require further observation and detoxification, and he is told this. A bed is not available at the hospital, however, and calls to nearby facilities find no empty beds.
As time passes, Mr. A shows signs of agitation and arousal. He paces the examination room—his jaw clenched and his face flushed—and begins raising his voice, asking to be discharged.
Recommendations. Unpleasantness is sometimes unavoidable, but no one in the ED has tried to create an alliance with Mr. A (Box). Try to make patients’ ED experiences as positive as possible. Make it clear that you share a common goal: to help the patient feel better. In fact, psychiatric patients and emergency psychiatrists have similar ideas about what constitutes quality ED care. When surveyed,4 ED patients said they preferred:
- verbal interventions compared with medications
- a collaborative approach with ED physicians
- having medications selected for their specific problems, medication experiences, and choices
- benzodiazepines rather than conventional antipsychotics such as haloperidol.
Treat patients with respect, and preserve their sense of dignity
Offer patients choices when reasonable to help them feel they have some control
Strongly (and early) encourage smokers to accept nicotine replacement to avoid withdrawal and heightened arousal
Offer food, beverages, a blanket, or other comfort measures that would not compromise safety (do not give hot coffee, in case the patient throws the cup at someone)
Allow patients to call a loved one, friend, or pastor (offer a cordless phone to avoid strangulation attempts)
Allow relatives or friends to sit and talk with the patient if this would not compromise safety
Keep patients informed on what is going on and why
Answer questions asked by the patient and family or friends
Offer oral medications first
Get to know your security staff well
A pragmatic workup
Medical illnesses such as delirium, stroke, drug toxicity, or urinary tract infections can trigger or worsen psychiatric illness (Table 1).5 Comorbidities such as diabetes, hypertension, obesity, and heart disease are common in patients with psychiatric disorders, and psychotropics can cause or exacerbate these conditions.
In the high-pressure ED, a sufficient workup for complicated medical conditions lies somewhere between extensive/unnecessary and inadequate. Thus, determining an exact diagnosis is not as important as establishing a diagnostic category to guide emergency treatment.
Think of psychiatric disorders as they are organized in DSM-IV-TR—mood, anxiety, psychotic, substance use/withdrawal/intoxication, cognitive, adjustment, somatoform, and personality disorders—and whether they are primary or secondary to a general medical condition or substance use. For example:
- Anxiety disorder secondary to a general medical condition means the history, physical exam, or lab reports suggest a medical condition is the direct physiologic cause of the mood disturbance.
- Methamphetamine-induced psychotic disorder would be the diagnosis if methamphetamines are presumed to be causing a patient’s psychotic symptoms.
Hospitalization. ED staff often develop a treatment plan based on a patient’s clinical picture and a working diagnosis. The plan hinges on deciding if the patient needs to be admitted to the hospital. Admission may be warranted for life-threatening medical conditions or safety issues, such as threats to self or others or inability to care for oneself at home. Other issues come into play—such as starting or changing medications and follow-up to ensure continuity of care—if you decide to discharge the patient.
Even after medical clearance, patients in the psychiatric emergency service may have underlying medical illnesses (Table 2).6
Table 1
Medical disorders that can cause psychiatric symptoms
| Medical/toxic disorders | Examples |
|---|---|
| Alcohol and drugs of abuse | Amphetamines (including methamphetamine), cocaine, heroin, Jimson weed, ketamine, marijuana, MDMA (‘Ecstasy’), LSD, PCP |
| Prescription drugs | Antibiotics, anticholinergics, anticonvulsants, antihypertensives, benzodiazepines, chemotherapeutic agents, cimetidine, corticosteroids, digitalis, narcotics, propranolol, sleep medications, tricyclic antidepressants |
| CNS disease | Hypertensive encephalopathy, intracranial aneurysm, metastases, normal pressure hydrocephalus, postictal nonconvulsive status, primary CNS infection, seizure disorders, stroke, subdural hematoma, tumor |
| Infections | Acute rheumatic fever, diphtheria, malaria, Legionnaires’ disease, pneumonia, Rocky Mountain spotted fever, sepsis, syphilis, typhoid fever, urinary tract infection |
| Metabolic/endocrine disorders | Adrenal disease, diabetic ketoacidosis, hepatic encephalopathy, hypoglycemia, pituitary dysfunction, renal disease, serum electrolyte imbalances (sodium, potassium, calcium), thyroid disease, vitamin deficiencies, Wilson’s disease |
| Cardiopulmonary disease | Arrhythmias, congestive heart failure, COPD/asthma, myocardial infarction, pulmonary embolism |
| Miscellaneous | Anemia, lupus, multiple sclerosis, temporal arteritis, vasculitis |
| Source: Reprinted with permission from Williams ER, Shepherd SM. Medical clearance of psychiatric patients. Emerg Med Clin North Am 2000;18(2):185-90. Copyright 2000, Elsevier. | |
Table 2
Reasonable medical assessment in psychiatric emergencies
| DO |
| Obtain a medical history, the best determinant of medical need |
| Listen to patients. If they say they have a medical condition, believe them; if they say they don’t, try to believe them |
| Thoroughly check vital signs |
| Conduct a focused physical examination |
| Maintain a high index of suspicion |
Be selective with laboratory testing. Check:
|
| DON’T |
| Order blanket laboratory screening |
| Order an ECG in healthy young patients in the absence of clinical findings |
| Order chest radiography in the absence of known disease/exposure/symptoms |
| Source: Reprinted from Currier GW. Medical assessment on the psychiatric emergency service. Psychiatric Issues in Emergency Care Settings 2004;3(July):17, with permission from Cliggott Publishing Group of CMP Healthcare Media. Copyright 2004. |
Overwhelming demand
In the study of ED patient preferences,4 one-fifth of patients said they went to the ED because they lacked access to routine mental health care. Therefore, besides psychiatric conditions caused by medical illnesses, ED physicians can see patients with any primary psychiatric diagnosis, including mood and anxiety disorders and psychosis.
Under pressures of time and limited collateral information, ED staff must:
- individualize psychiatric treatment
- consider use of medications and/or restraints
- rule out life-threatening causes for psychiatric symptoms
- stabilize patients and prevent injury to self and others.
These tasks are becoming increasingly difficult as more and more patients present to emergency rooms. Nationally, ED visits increased from 19 million in 1992 to 108 million in 2000, according to the U.S. Department of Health and Human Services.1
Psychiatric patients are seeking ED care in greater numbers, and the number of those staying longer than anticipated (“boarding”) also has increased, according to a 2004 survey of 340 physicians by the American College of Emergency Physicians, American Psychiatric Association, National Mental Health Association, and National Alliance for the Mentally Ill. Surveyed physicians blamed inadequate Medicaid funding and bed shortages for the increasing ED visits.7
In crowded emergency rooms, where patients wait longer and longer to be seen, the influx of acutely ill psychiatric patients increases the risks of agitation, violence, and injury, as well as litigation.8
Case continued: going up in smoke
Recognizing Mr. A’s arousal, ED staff tries to reassure him and offers him food, something to drink, a phone Call, and a magazine. When these attempts fail to de-escalate his agitation, staff offers to make him more comfortable by giving oral lorazepam, which he adamantly refuses. He is told again that he must stay until a transfer facility is found for him.
Mr. A then demands to go outside “for a smoke.” When he is told ED patients cannot leave to smoke and is offered nicotine replacement, he begins to scream and lunges at one of the security officers. He is extremely strong, and additional officers are summoned. He retreats inside the room, slams the door, shatters the door window with a chair, and begins punching the broken glass. He slides to the floor in a vasovagal reaction at the sight of his bleeding hands but soon becomes combative again.
Staff give Mr. A IM haloperidol, 10 mg, and lorazepam, 2 mg, to manage his extreme agitation and place him in physical restraints to protect him and others. Within 25 to 30 minutes he is calm, and a safe environment has been re-established. The lacerations on his hands are sutured, and he is admitted to an inpatient psychiatric hospital for further stabilization and treatment.
No place for complacency
Mr. A’s experience illustrates how situations can become dangerous when precautions are not taken. Five steps can help you prepare and protect yourself when evaluating patients in the ED:
- seek the patient history
- evaluate the context in which the patient is being assessed
- identify arousal states (fear, anger, confusion, and humiliation)
- structure the interview for safety
- keep your guard up during the clinical encounter.9
Risk is high when law enforcement officers bring a patient to the ED. Be on guard, even if the patient is 80 years old and in a wheelchair. Complacency has no place in the ED; prepare as much as you can before interviewing the patient.
When restraints are needed. Involuntary medication and/or restraints may be necessary when reasonable interventions have failed, the patient will not cooperate, and he or she is exhibiting behavior/symptoms that could result in injury. Approximately 10% to 20% of psychiatric patients require physical or chemical restraint in the ED.10
Expert consensus guidelines suggest starting with verbal intervention, voluntary medication, and show of force, although emergency medication may be a reasonable first treatment (Algorithm).11 Offer oral medication first; IM medications carry risks including acute dystonia and akathisia, although these can be treated.
Lorazepam, 1 to 2 mg oral/IM, combined with haloperidol, 2 to 5 mg oral/IM, is a reasonable start in most cases. If the patient remains extremely agitated, the same medications and dosages can be repeated 30 to 60 minutes after the initial administration.12
Conventional oral/IM agents are usually more readily available in the ED than atypical antipsychotics, which must be ordered from the pharmacy. Recent FDA black-box warnings also emphasize that atypical antipsychotics are approved only for treating schizophrenia, acute manic and mixed episodes of bipolar I disorder, and for maintenance treatment in bipolar disorder. When compared with placebo, atypical antipsychotics have been associated with:
- increased risk for cerebrovascular events in elderly patients with dementia
- death in elderly patients with dementia-related psychosis.
Atypicals may be more appropriate than conventional antipsychotics for emergency treatment of agitation and aggression in some patients with complicating medical conditions or histories. For example, avoid high-potency conventional antipsychotics in patients with a history of extrapyramidal side effects and in those with mental retardation/developmental delay.11 Similarly, avoid benzodiazepines in patients with chronic obstructive pulmonary disease (COPD) or a history of drug-seeking behavior or drug abuse.
Of course not all psychiatric interventions in the ED are involuntary. For example, the ED physician may start an antidepressant for a patient diagnosed with mild to moderate depression for whom hospitalization is not indicated. Characteristics of patients who may be good candidates for starting antidepressants in the ED include a clear diagnosis, no substance abuse, low suicide risk, no psychosis or agitation, available social supports, clear follow-up plan, desire to begin treatment, and ability to pay for and obtain medications.13
Algorithm Consensus guideline for treating a behavioral emergency
Related resources
- Allen MH, Currier GW, Hughes DH, et al. The Expert Consensus Guideline Series: Treatment of behavioral emergencies. Postgrad Med 2001;May(Spec No):1-88.
- American College of Emergency Physicians. www.acep.org
- National Alliance on Mental Illness. www.nami.org
Drug brand names
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Police officers bring Mr. A, age 25, to the emergency department (ED) in handcuffs after an alleged assault at work. He is calm but will provide no information about himself. ED staff don’t know if he has been using illicit substances, is on medications, or has any medical conditions.
Mr. A says the FBI is after him, but he makes no threats to ED staff. He talks about milking cows on a farm and of hearing animal sounds, though he lives in the city. After about 30 minutes, he consents to a lab draw and provides a urine sample.
Because no charges are pending and Mr. A is semi-cooperative, police remove his handcuffs and leave him in the care of two ED security officers. He is given something to eat and drink and seems fairly content. He asks how long he will need to stay in the room but does not demand to leave.
In a fast-paced ED, physicians might not notice signs of psychiatric illness, such as Mr. A’s paranoid and delusional thinking. By being familiar with techniques to manage patients’ psychiatric emergencies, you can help your ED colleagues:
- establish working psychiatric diagnoses and medical causes of psychiatric symptoms in the fast-paced ED
- maintain a safe ED environment for patients and clinicians.
What ed patients want
To understand how ED patients feel, put yourself in Mr. A’s shoes. You were at work and began to hallucinate. You believed your boss was out to harm you, and in fear you made comments perceived as threatening.
The next thing you know, you’re in a police car with handcuffs on. All of your coworkers witnessed your embarrassment. Now you are in a small ED room, wondering what’s going to happen next. Are you going to be put in a straight jacket and a padded room?
Patients may experience anxiety-provoking thoughts whether they come to the ED voluntarily or involuntarily. Fear and confusion can affect their behavior in the ED, and how providers respond to patients in crisis can escalate or de-escalate an already-difficult situation.
Psychiatric illness in the ed
Mr. A may have a psychiatric disorder, as do at least 3% of patients seen in EDs.1 This figure may be low, however:
- Kunen et al2 asserted that EDs are underdiagnosing psychiatric disorders, given a U.S. Department of Health and Human Services 1999 estimate that 20% to 28% of Americans have psychiatric illnesses. Using ED discharge records across 6 months in three emergency departments, the authors found the psychiatric diagnosis rate to be 5.27% in 33,000 ED visits.
- Another study, done in a university teaching hospital ED, showed that ED physicians trained to focus on patients’ presenting problems often missed comorbid medical or psychiatric illnesses.
In the randomized, controlled trial by Schriger et al,3 218 patients with nonspecific complaints suggesting occult psychiatric illness (such as chronic headache, abdominal pain, or back pain) completed the Primary Care Evaluation of Mental Disorders (PRIME-MD) questionnaire. This 27-item self-report asks questions about mood, alcohol use, obsessive-compulsive symptoms, phobias, and somatoform symptoms.
Participants were then randomly assigned to “report” or “nonreport” groups, depending on whether or not ED physicians received their PRIME-MD scores. Even when informed of patients’ psychiatric symptoms, ED physicians rarely diagnosed or treated psychiatric disorders. Lack of mental status documentation and psychiatric interviews was apparent, the authors noted.
Case continued: a toxic cocktail
Mr. A’s urine drug screen and lab results are positive for benzodiazepines, methamphetamines, and cannabis. The staff decide Mr. A will require further observation and detoxification, and he is told this. A bed is not available at the hospital, however, and calls to nearby facilities find no empty beds.
As time passes, Mr. A shows signs of agitation and arousal. He paces the examination room—his jaw clenched and his face flushed—and begins raising his voice, asking to be discharged.
Recommendations. Unpleasantness is sometimes unavoidable, but no one in the ED has tried to create an alliance with Mr. A (Box). Try to make patients’ ED experiences as positive as possible. Make it clear that you share a common goal: to help the patient feel better. In fact, psychiatric patients and emergency psychiatrists have similar ideas about what constitutes quality ED care. When surveyed,4 ED patients said they preferred:
- verbal interventions compared with medications
- a collaborative approach with ED physicians
- having medications selected for their specific problems, medication experiences, and choices
- benzodiazepines rather than conventional antipsychotics such as haloperidol.
Treat patients with respect, and preserve their sense of dignity
Offer patients choices when reasonable to help them feel they have some control
Strongly (and early) encourage smokers to accept nicotine replacement to avoid withdrawal and heightened arousal
Offer food, beverages, a blanket, or other comfort measures that would not compromise safety (do not give hot coffee, in case the patient throws the cup at someone)
Allow patients to call a loved one, friend, or pastor (offer a cordless phone to avoid strangulation attempts)
Allow relatives or friends to sit and talk with the patient if this would not compromise safety
Keep patients informed on what is going on and why
Answer questions asked by the patient and family or friends
Offer oral medications first
Get to know your security staff well
A pragmatic workup
Medical illnesses such as delirium, stroke, drug toxicity, or urinary tract infections can trigger or worsen psychiatric illness (Table 1).5 Comorbidities such as diabetes, hypertension, obesity, and heart disease are common in patients with psychiatric disorders, and psychotropics can cause or exacerbate these conditions.
In the high-pressure ED, a sufficient workup for complicated medical conditions lies somewhere between extensive/unnecessary and inadequate. Thus, determining an exact diagnosis is not as important as establishing a diagnostic category to guide emergency treatment.
Think of psychiatric disorders as they are organized in DSM-IV-TR—mood, anxiety, psychotic, substance use/withdrawal/intoxication, cognitive, adjustment, somatoform, and personality disorders—and whether they are primary or secondary to a general medical condition or substance use. For example:
- Anxiety disorder secondary to a general medical condition means the history, physical exam, or lab reports suggest a medical condition is the direct physiologic cause of the mood disturbance.
- Methamphetamine-induced psychotic disorder would be the diagnosis if methamphetamines are presumed to be causing a patient’s psychotic symptoms.
Hospitalization. ED staff often develop a treatment plan based on a patient’s clinical picture and a working diagnosis. The plan hinges on deciding if the patient needs to be admitted to the hospital. Admission may be warranted for life-threatening medical conditions or safety issues, such as threats to self or others or inability to care for oneself at home. Other issues come into play—such as starting or changing medications and follow-up to ensure continuity of care—if you decide to discharge the patient.
Even after medical clearance, patients in the psychiatric emergency service may have underlying medical illnesses (Table 2).6
Table 1
Medical disorders that can cause psychiatric symptoms
| Medical/toxic disorders | Examples |
|---|---|
| Alcohol and drugs of abuse | Amphetamines (including methamphetamine), cocaine, heroin, Jimson weed, ketamine, marijuana, MDMA (‘Ecstasy’), LSD, PCP |
| Prescription drugs | Antibiotics, anticholinergics, anticonvulsants, antihypertensives, benzodiazepines, chemotherapeutic agents, cimetidine, corticosteroids, digitalis, narcotics, propranolol, sleep medications, tricyclic antidepressants |
| CNS disease | Hypertensive encephalopathy, intracranial aneurysm, metastases, normal pressure hydrocephalus, postictal nonconvulsive status, primary CNS infection, seizure disorders, stroke, subdural hematoma, tumor |
| Infections | Acute rheumatic fever, diphtheria, malaria, Legionnaires’ disease, pneumonia, Rocky Mountain spotted fever, sepsis, syphilis, typhoid fever, urinary tract infection |
| Metabolic/endocrine disorders | Adrenal disease, diabetic ketoacidosis, hepatic encephalopathy, hypoglycemia, pituitary dysfunction, renal disease, serum electrolyte imbalances (sodium, potassium, calcium), thyroid disease, vitamin deficiencies, Wilson’s disease |
| Cardiopulmonary disease | Arrhythmias, congestive heart failure, COPD/asthma, myocardial infarction, pulmonary embolism |
| Miscellaneous | Anemia, lupus, multiple sclerosis, temporal arteritis, vasculitis |
| Source: Reprinted with permission from Williams ER, Shepherd SM. Medical clearance of psychiatric patients. Emerg Med Clin North Am 2000;18(2):185-90. Copyright 2000, Elsevier. | |
Table 2
Reasonable medical assessment in psychiatric emergencies
| DO |
| Obtain a medical history, the best determinant of medical need |
| Listen to patients. If they say they have a medical condition, believe them; if they say they don’t, try to believe them |
| Thoroughly check vital signs |
| Conduct a focused physical examination |
| Maintain a high index of suspicion |
Be selective with laboratory testing. Check:
|
| DON’T |
| Order blanket laboratory screening |
| Order an ECG in healthy young patients in the absence of clinical findings |
| Order chest radiography in the absence of known disease/exposure/symptoms |
| Source: Reprinted from Currier GW. Medical assessment on the psychiatric emergency service. Psychiatric Issues in Emergency Care Settings 2004;3(July):17, with permission from Cliggott Publishing Group of CMP Healthcare Media. Copyright 2004. |
Overwhelming demand
In the study of ED patient preferences,4 one-fifth of patients said they went to the ED because they lacked access to routine mental health care. Therefore, besides psychiatric conditions caused by medical illnesses, ED physicians can see patients with any primary psychiatric diagnosis, including mood and anxiety disorders and psychosis.
Under pressures of time and limited collateral information, ED staff must:
- individualize psychiatric treatment
- consider use of medications and/or restraints
- rule out life-threatening causes for psychiatric symptoms
- stabilize patients and prevent injury to self and others.
These tasks are becoming increasingly difficult as more and more patients present to emergency rooms. Nationally, ED visits increased from 19 million in 1992 to 108 million in 2000, according to the U.S. Department of Health and Human Services.1
Psychiatric patients are seeking ED care in greater numbers, and the number of those staying longer than anticipated (“boarding”) also has increased, according to a 2004 survey of 340 physicians by the American College of Emergency Physicians, American Psychiatric Association, National Mental Health Association, and National Alliance for the Mentally Ill. Surveyed physicians blamed inadequate Medicaid funding and bed shortages for the increasing ED visits.7
In crowded emergency rooms, where patients wait longer and longer to be seen, the influx of acutely ill psychiatric patients increases the risks of agitation, violence, and injury, as well as litigation.8
Case continued: going up in smoke
Recognizing Mr. A’s arousal, ED staff tries to reassure him and offers him food, something to drink, a phone Call, and a magazine. When these attempts fail to de-escalate his agitation, staff offers to make him more comfortable by giving oral lorazepam, which he adamantly refuses. He is told again that he must stay until a transfer facility is found for him.
Mr. A then demands to go outside “for a smoke.” When he is told ED patients cannot leave to smoke and is offered nicotine replacement, he begins to scream and lunges at one of the security officers. He is extremely strong, and additional officers are summoned. He retreats inside the room, slams the door, shatters the door window with a chair, and begins punching the broken glass. He slides to the floor in a vasovagal reaction at the sight of his bleeding hands but soon becomes combative again.
Staff give Mr. A IM haloperidol, 10 mg, and lorazepam, 2 mg, to manage his extreme agitation and place him in physical restraints to protect him and others. Within 25 to 30 minutes he is calm, and a safe environment has been re-established. The lacerations on his hands are sutured, and he is admitted to an inpatient psychiatric hospital for further stabilization and treatment.
No place for complacency
Mr. A’s experience illustrates how situations can become dangerous when precautions are not taken. Five steps can help you prepare and protect yourself when evaluating patients in the ED:
- seek the patient history
- evaluate the context in which the patient is being assessed
- identify arousal states (fear, anger, confusion, and humiliation)
- structure the interview for safety
- keep your guard up during the clinical encounter.9
Risk is high when law enforcement officers bring a patient to the ED. Be on guard, even if the patient is 80 years old and in a wheelchair. Complacency has no place in the ED; prepare as much as you can before interviewing the patient.
When restraints are needed. Involuntary medication and/or restraints may be necessary when reasonable interventions have failed, the patient will not cooperate, and he or she is exhibiting behavior/symptoms that could result in injury. Approximately 10% to 20% of psychiatric patients require physical or chemical restraint in the ED.10
Expert consensus guidelines suggest starting with verbal intervention, voluntary medication, and show of force, although emergency medication may be a reasonable first treatment (Algorithm).11 Offer oral medication first; IM medications carry risks including acute dystonia and akathisia, although these can be treated.
Lorazepam, 1 to 2 mg oral/IM, combined with haloperidol, 2 to 5 mg oral/IM, is a reasonable start in most cases. If the patient remains extremely agitated, the same medications and dosages can be repeated 30 to 60 minutes after the initial administration.12
Conventional oral/IM agents are usually more readily available in the ED than atypical antipsychotics, which must be ordered from the pharmacy. Recent FDA black-box warnings also emphasize that atypical antipsychotics are approved only for treating schizophrenia, acute manic and mixed episodes of bipolar I disorder, and for maintenance treatment in bipolar disorder. When compared with placebo, atypical antipsychotics have been associated with:
- increased risk for cerebrovascular events in elderly patients with dementia
- death in elderly patients with dementia-related psychosis.
Atypicals may be more appropriate than conventional antipsychotics for emergency treatment of agitation and aggression in some patients with complicating medical conditions or histories. For example, avoid high-potency conventional antipsychotics in patients with a history of extrapyramidal side effects and in those with mental retardation/developmental delay.11 Similarly, avoid benzodiazepines in patients with chronic obstructive pulmonary disease (COPD) or a history of drug-seeking behavior or drug abuse.
Of course not all psychiatric interventions in the ED are involuntary. For example, the ED physician may start an antidepressant for a patient diagnosed with mild to moderate depression for whom hospitalization is not indicated. Characteristics of patients who may be good candidates for starting antidepressants in the ED include a clear diagnosis, no substance abuse, low suicide risk, no psychosis or agitation, available social supports, clear follow-up plan, desire to begin treatment, and ability to pay for and obtain medications.13
Algorithm Consensus guideline for treating a behavioral emergency
Related resources
- Allen MH, Currier GW, Hughes DH, et al. The Expert Consensus Guideline Series: Treatment of behavioral emergencies. Postgrad Med 2001;May(Spec No):1-88.
- American College of Emergency Physicians. www.acep.org
- National Alliance on Mental Illness. www.nami.org
Drug brand names
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McCaig LF, Ly N. National Hospital Ambulatory Medical Care Survey: 2000 emergency department summary. Adv Data 2002;327:1-27.
2. Kunen S, Niederhauser R, Smith PO, et al. Race disparities in psychiatric rates in emergency departments. J Consult Clin Psychol 2005;73(1):116-126.
3. Schriger DL, Gibbons PS, Langone CA, et al. Enabling the diagnosis of occult psychiatric illness in the emergency department: a randomized, controlled trial of the computerized, self-administered PRIME-MD diagnostic system. Ann Emerg Med 2001;37(2):132-40.
4. Allen M, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract 2003;9(1):39-58.
5. Williams ER, Shepherd SM. Medical clearance of psychiatric patients. Emerg Med Clin North Am 2000;18(2):185-98.
6. Allen MH, Currier GW. Medical assessment on the psychiatric emergency service. New Dir Ment Health Serv 1999;82:21-8.
7. Mulligan K. ER docs report large increase in psychiatric patients. Psychiatr News 2004;39(12):10.-
8. Karcz A, Holbrook J, Auerbach BS, et al. Preventability of malpractice claims in emergency medicine: a closed claims study. Ann Emerg Med 1990;19(8):865-73.
9. Battaglia J. Is this patient dangerous? 5 steps to assess risk for violence. Current Psychiatry 2004;3(2):14-21.
10. De Fruyt J, Demyttenaere K. Rapid tranquilization: new approaches in the emergency treatment of behavioral disturbances. Eur Psychiatry 2004;19:243-9.
11. Allen MH, Currier GW, Hughes DH, et al. The Expert Consensus Guideline Series: Treatment of behavioral emergencies. Postgrad Med 2001;May(Spec No):1-88.
12. Hughes DH. Acute psychopharmacological management of the aggressive psychotic patient. Psychiatr Serv 1999;50(9):1135-7.
13. Glick RL. Starting antidepressant treatment in the emergency setting. Psychiatric Issues in Emergency Care Settings 2004;3(2):6-10.
1. McCaig LF, Ly N. National Hospital Ambulatory Medical Care Survey: 2000 emergency department summary. Adv Data 2002;327:1-27.
2. Kunen S, Niederhauser R, Smith PO, et al. Race disparities in psychiatric rates in emergency departments. J Consult Clin Psychol 2005;73(1):116-126.
3. Schriger DL, Gibbons PS, Langone CA, et al. Enabling the diagnosis of occult psychiatric illness in the emergency department: a randomized, controlled trial of the computerized, self-administered PRIME-MD diagnostic system. Ann Emerg Med 2001;37(2):132-40.
4. Allen M, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract 2003;9(1):39-58.
5. Williams ER, Shepherd SM. Medical clearance of psychiatric patients. Emerg Med Clin North Am 2000;18(2):185-98.
6. Allen MH, Currier GW. Medical assessment on the psychiatric emergency service. New Dir Ment Health Serv 1999;82:21-8.
7. Mulligan K. ER docs report large increase in psychiatric patients. Psychiatr News 2004;39(12):10.-
8. Karcz A, Holbrook J, Auerbach BS, et al. Preventability of malpractice claims in emergency medicine: a closed claims study. Ann Emerg Med 1990;19(8):865-73.
9. Battaglia J. Is this patient dangerous? 5 steps to assess risk for violence. Current Psychiatry 2004;3(2):14-21.
10. De Fruyt J, Demyttenaere K. Rapid tranquilization: new approaches in the emergency treatment of behavioral disturbances. Eur Psychiatry 2004;19:243-9.
11. Allen MH, Currier GW, Hughes DH, et al. The Expert Consensus Guideline Series: Treatment of behavioral emergencies. Postgrad Med 2001;May(Spec No):1-88.
12. Hughes DH. Acute psychopharmacological management of the aggressive psychotic patient. Psychiatr Serv 1999;50(9):1135-7.
13. Glick RL. Starting antidepressant treatment in the emergency setting. Psychiatric Issues in Emergency Care Settings 2004;3(2):6-10.