User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Ramelteon
In clinical trials, ramelteon has helped patients fall asleep more quickly. Whereas other sleep-promoting medications sedate through effects on gamma-butyric acid (GABA) receptors, ramelteon interacts with melatonin receptors to regulate sleep patterns. It is FDA-approved for treating insomnia characterized by sleep-onset difficulty (Table 1)
Table 1
Ramelteon: Fast facts
| Brand name: |
| Rozerem |
| Class: |
| Nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia characterized by sleep-onset difficulty |
| Approval date: |
| August 18, 2005 |
| Manufacturer: |
| Takeda Pharmaceuticals North America |
| Dosing form: |
| 8-mg tablets |
| Recommended dosage: |
| 8 mg within 30 minutes of going to bed |
| Additional prescribing information: |
| www.rozerem.com |
How it works
Ramelteon, a melatonin receptor agonist, has high affinity for the MT1 and MT2 (melatonin) receptors. Although the precise mechanism by which ramelteon affects sleep remains unknown, its effect on sleep is hypothesized to be similar to that of the neurohormone melatonin.
Melatonin is important to maintaining the circadian rhythm that underlies the sleep-wake cycle. Sunlight influences neurohormones that mediate daytime-specific physiologic events. An increase in melatonin—a change that accompanies darkness—is believed to mediate changes in physiology that are characteristic of nighttime. Melatonin thus may be more of a circadian “clock” regulator than a sedative.
Ramelteon shows some features of melatonin that differentiate it from the GABA-related sedating agents. Both ramelteon and melatonin lack abuse potential and a dose-response relationship.
Pharmacokinetics
Ramelteon is absorbed rapidly from the GI tract and reaches median peak concentrations within 30 to 90 minutes of dosing. Taking ramelteon with a high-fat meal reduces its maximum concentration by 22% and slows hypnotic onset by approximately 45 minutes.
The drug is metabolized mostly through the 1A2 isoenzyme of the cytochrome P (CYP)-450 system, although CYP 2C and 3A4 isoenzymes are also involved. About 90% of the dose is excreted.
Ramelteon’s elimination half-life averages 1 to 2.6 hours, so blood levels upon awakening will likely be too low to cause residual effects. Interestingly, in one placebo-controlled study,1 subjects who received a single 64-mg dose reported significantly reduced alertness and diminished ability to concentrate upon awakening. Subjects who took a 16-mg dose did not report this effect. Whether this finding is clinically relevant or relates to a residual effect, sedation, or cognitive impairment is unclear.
Efficacy
In a randomized, double-blind, placebo-controlled trial, ramelteon shortened sleep latency (time between going to bed and falling asleep) among patients with transient insomnia.
Roth et al1 studied 375 healthy adults ages 35 to 60 who reported sleeping 6.5 to 8.5 hours nightly and usually taking ≥30 minutes to fall asleep. In sleep research centers, subjects received one dose of ramelteon, 16 or 64 mg, or placebo 30 minutes before bedtime.
Mean latency to persistent sleep, measured with polysomnography, was 10 minutes shorter among both ramelteon dosage groups than among the placebo group. Mean total sleep time was 11 to 14 minutes longer among both ramelteon groups based on polysomnography, although subjective sleep estimates the next morning were similar among all three groups.
Roth et al2 also assessed efficacy of ramelteon across 5 weeks among 829 older patients (mean age 72) with insomnia (as defined by DSM-IV-TR) for ≥3 months, total nightly sleep time ≤6.5 hours for 3 nights, and self-reported sleep latency ≥45 minutes nightly for ≥3 nights.
Mean sleep latency decreased 25 to 30 minutes among subjects taking ramelteon, 4 or 8 mg nightly, compared with a mean 15-minute decrease among the placebo group. Average total sleep time was 5 to 8 minutes longer among both ramelteon groups compared with placebo.
Subjects in both ramelteon groups then received placebo for 1 week, during which time their mean latency to persistent sleep improved further or stayed the same. This suggests that ramelteon did not cause rebound insomnia.
Safety and tolerability
Ramelteon was generally well tolerated in clinical and preclinical trials. Headaches (7% of subjects), somnolence (5%), dizziness (5%), fatigue (4%), nausea (3%), exacerbated insomnia (3%), and upper respiratory tract infection (3%) were most commonly reported.3 Less-common effects included diarrhea, myalgia, depression, dysgeusia, arthralgia, influenza, and blood cortisol decrease.
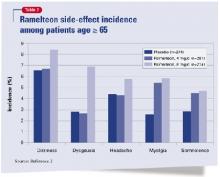
The most common side effects among subjects age ≥65 were dizziness, dysgeusia, headaches, myalgia, and somnolence (Table 2). These occurred less frequently over 5 weeks among patients taking 4 mg/d than among those who took 8 mg/d, the FDA-approved dosage.
Ramelteon also showed no abuse potential compared with triazolam and placebo in a trial of 14 patients with a history of anxiolytic or sedative/hypnotic abuse.4
Contraindications
Do not give ramelteon to patients taking fluvoxamine. The antidepressant has been shown to raise serum ramelteon approximately 70-fold, thus substantially increasing the risk of ramelteon-associated adverse events.3
Ramelteon has shown teratogenicity in animals, though at doses far exceeding human levels. Still, as with other sleep-promoting medications, avoid prescribing ramelteon to expectant mothers.
Concomitant use of a strong CYP enzyme inducer such as rifampin may increase ramelteon metabolism and reduce serum ramelteon, which might decrease its efficacy in some cases. Whether increasing the ramelteon dosage counters this interaction is unknown.
Strong CYP 2C9 inhibitors such as fluconazole or strong CYP 3A4 inhibitors such as ketoconazole can raise serum ramelteon and might increase the risk of adverse events in some persons.
Dosing
Start ramelteon at 8 mg nightly, and tell patients to take it within 30 minutes of going to bed.
Because high-fat food slows its absorption, advise patients not to take ramelteon within 1 hour of eating a high-fat meal.
Ramelteon’s efficacy and side effects do not appear to be dose-dependent when given at 8 to 64 mg/d. Whether dosages >64 mg/d increase side-effect risk or therapeutic effect is unknown.
As with other hypnotics, supplement ramelteon therapy with sleep hygiene education and relaxation techniques.
Clinical implications
Ramelteon appears to help patients who have trouble falling asleep.
Because no other prescription medication targets melatonin neurotransmitters, no precedent and little data exist to guide patient choice, dosing, and treatment duration. Effects of ramelteon use >5 weeks are unknown. Clinical use and future research should uncover more information about ramelteon’s properties.
Related resources
- Ramelteon Web site. www.rozerem.com.
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9:25-39.
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev 2005;9:5-9.
Drug brand names
- Fluconazole • Diflucan
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Ramelteon • Rozerem
- Rifampin • Rifadin
- Triazolam • Halcion
Disclosures
Dr. Krystal receives research/grant support, is a consultant to, or is a speaker for Cephalon, Cyberonics, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Mecta Corp., Merck and Co., Neurocrine Biosciences, Neurogen Corp., Neuronetics, Organon, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon Pharmaceuticals, Takeda Pharmaceuticals North America, and TransOral Pharmaceuticals.
1. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
2. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomnia in elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
3. Rozerem prescribing information. Takeda Pharmaceuticals North America, 2005.
4. Griffiths R, Seuss P. Ramelteon and triazolam in humans: behavioral effects and abuse potential (poster). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
In clinical trials, ramelteon has helped patients fall asleep more quickly. Whereas other sleep-promoting medications sedate through effects on gamma-butyric acid (GABA) receptors, ramelteon interacts with melatonin receptors to regulate sleep patterns. It is FDA-approved for treating insomnia characterized by sleep-onset difficulty (Table 1)
Table 1
Ramelteon: Fast facts
| Brand name: |
| Rozerem |
| Class: |
| Nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia characterized by sleep-onset difficulty |
| Approval date: |
| August 18, 2005 |
| Manufacturer: |
| Takeda Pharmaceuticals North America |
| Dosing form: |
| 8-mg tablets |
| Recommended dosage: |
| 8 mg within 30 minutes of going to bed |
| Additional prescribing information: |
| www.rozerem.com |
How it works
Ramelteon, a melatonin receptor agonist, has high affinity for the MT1 and MT2 (melatonin) receptors. Although the precise mechanism by which ramelteon affects sleep remains unknown, its effect on sleep is hypothesized to be similar to that of the neurohormone melatonin.
Melatonin is important to maintaining the circadian rhythm that underlies the sleep-wake cycle. Sunlight influences neurohormones that mediate daytime-specific physiologic events. An increase in melatonin—a change that accompanies darkness—is believed to mediate changes in physiology that are characteristic of nighttime. Melatonin thus may be more of a circadian “clock” regulator than a sedative.
Ramelteon shows some features of melatonin that differentiate it from the GABA-related sedating agents. Both ramelteon and melatonin lack abuse potential and a dose-response relationship.
Pharmacokinetics
Ramelteon is absorbed rapidly from the GI tract and reaches median peak concentrations within 30 to 90 minutes of dosing. Taking ramelteon with a high-fat meal reduces its maximum concentration by 22% and slows hypnotic onset by approximately 45 minutes.
The drug is metabolized mostly through the 1A2 isoenzyme of the cytochrome P (CYP)-450 system, although CYP 2C and 3A4 isoenzymes are also involved. About 90% of the dose is excreted.
Ramelteon’s elimination half-life averages 1 to 2.6 hours, so blood levels upon awakening will likely be too low to cause residual effects. Interestingly, in one placebo-controlled study,1 subjects who received a single 64-mg dose reported significantly reduced alertness and diminished ability to concentrate upon awakening. Subjects who took a 16-mg dose did not report this effect. Whether this finding is clinically relevant or relates to a residual effect, sedation, or cognitive impairment is unclear.
Efficacy
In a randomized, double-blind, placebo-controlled trial, ramelteon shortened sleep latency (time between going to bed and falling asleep) among patients with transient insomnia.
Roth et al1 studied 375 healthy adults ages 35 to 60 who reported sleeping 6.5 to 8.5 hours nightly and usually taking ≥30 minutes to fall asleep. In sleep research centers, subjects received one dose of ramelteon, 16 or 64 mg, or placebo 30 minutes before bedtime.
Mean latency to persistent sleep, measured with polysomnography, was 10 minutes shorter among both ramelteon dosage groups than among the placebo group. Mean total sleep time was 11 to 14 minutes longer among both ramelteon groups based on polysomnography, although subjective sleep estimates the next morning were similar among all three groups.
Roth et al2 also assessed efficacy of ramelteon across 5 weeks among 829 older patients (mean age 72) with insomnia (as defined by DSM-IV-TR) for ≥3 months, total nightly sleep time ≤6.5 hours for 3 nights, and self-reported sleep latency ≥45 minutes nightly for ≥3 nights.
Mean sleep latency decreased 25 to 30 minutes among subjects taking ramelteon, 4 or 8 mg nightly, compared with a mean 15-minute decrease among the placebo group. Average total sleep time was 5 to 8 minutes longer among both ramelteon groups compared with placebo.
Subjects in both ramelteon groups then received placebo for 1 week, during which time their mean latency to persistent sleep improved further or stayed the same. This suggests that ramelteon did not cause rebound insomnia.
Safety and tolerability
Ramelteon was generally well tolerated in clinical and preclinical trials. Headaches (7% of subjects), somnolence (5%), dizziness (5%), fatigue (4%), nausea (3%), exacerbated insomnia (3%), and upper respiratory tract infection (3%) were most commonly reported.3 Less-common effects included diarrhea, myalgia, depression, dysgeusia, arthralgia, influenza, and blood cortisol decrease.
The most common side effects among subjects age ≥65 were dizziness, dysgeusia, headaches, myalgia, and somnolence (Table 2). These occurred less frequently over 5 weeks among patients taking 4 mg/d than among those who took 8 mg/d, the FDA-approved dosage.
Ramelteon also showed no abuse potential compared with triazolam and placebo in a trial of 14 patients with a history of anxiolytic or sedative/hypnotic abuse.4
Contraindications
Do not give ramelteon to patients taking fluvoxamine. The antidepressant has been shown to raise serum ramelteon approximately 70-fold, thus substantially increasing the risk of ramelteon-associated adverse events.3
Ramelteon has shown teratogenicity in animals, though at doses far exceeding human levels. Still, as with other sleep-promoting medications, avoid prescribing ramelteon to expectant mothers.
Concomitant use of a strong CYP enzyme inducer such as rifampin may increase ramelteon metabolism and reduce serum ramelteon, which might decrease its efficacy in some cases. Whether increasing the ramelteon dosage counters this interaction is unknown.
Strong CYP 2C9 inhibitors such as fluconazole or strong CYP 3A4 inhibitors such as ketoconazole can raise serum ramelteon and might increase the risk of adverse events in some persons.
Dosing
Start ramelteon at 8 mg nightly, and tell patients to take it within 30 minutes of going to bed.
Because high-fat food slows its absorption, advise patients not to take ramelteon within 1 hour of eating a high-fat meal.
Ramelteon’s efficacy and side effects do not appear to be dose-dependent when given at 8 to 64 mg/d. Whether dosages >64 mg/d increase side-effect risk or therapeutic effect is unknown.
As with other hypnotics, supplement ramelteon therapy with sleep hygiene education and relaxation techniques.
Clinical implications
Ramelteon appears to help patients who have trouble falling asleep.
Because no other prescription medication targets melatonin neurotransmitters, no precedent and little data exist to guide patient choice, dosing, and treatment duration. Effects of ramelteon use >5 weeks are unknown. Clinical use and future research should uncover more information about ramelteon’s properties.
Related resources
- Ramelteon Web site. www.rozerem.com.
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9:25-39.
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev 2005;9:5-9.
Drug brand names
- Fluconazole • Diflucan
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Ramelteon • Rozerem
- Rifampin • Rifadin
- Triazolam • Halcion
Disclosures
Dr. Krystal receives research/grant support, is a consultant to, or is a speaker for Cephalon, Cyberonics, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Mecta Corp., Merck and Co., Neurocrine Biosciences, Neurogen Corp., Neuronetics, Organon, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon Pharmaceuticals, Takeda Pharmaceuticals North America, and TransOral Pharmaceuticals.
In clinical trials, ramelteon has helped patients fall asleep more quickly. Whereas other sleep-promoting medications sedate through effects on gamma-butyric acid (GABA) receptors, ramelteon interacts with melatonin receptors to regulate sleep patterns. It is FDA-approved for treating insomnia characterized by sleep-onset difficulty (Table 1)
Table 1
Ramelteon: Fast facts
| Brand name: |
| Rozerem |
| Class: |
| Nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia characterized by sleep-onset difficulty |
| Approval date: |
| August 18, 2005 |
| Manufacturer: |
| Takeda Pharmaceuticals North America |
| Dosing form: |
| 8-mg tablets |
| Recommended dosage: |
| 8 mg within 30 minutes of going to bed |
| Additional prescribing information: |
| www.rozerem.com |
How it works
Ramelteon, a melatonin receptor agonist, has high affinity for the MT1 and MT2 (melatonin) receptors. Although the precise mechanism by which ramelteon affects sleep remains unknown, its effect on sleep is hypothesized to be similar to that of the neurohormone melatonin.
Melatonin is important to maintaining the circadian rhythm that underlies the sleep-wake cycle. Sunlight influences neurohormones that mediate daytime-specific physiologic events. An increase in melatonin—a change that accompanies darkness—is believed to mediate changes in physiology that are characteristic of nighttime. Melatonin thus may be more of a circadian “clock” regulator than a sedative.
Ramelteon shows some features of melatonin that differentiate it from the GABA-related sedating agents. Both ramelteon and melatonin lack abuse potential and a dose-response relationship.
Pharmacokinetics
Ramelteon is absorbed rapidly from the GI tract and reaches median peak concentrations within 30 to 90 minutes of dosing. Taking ramelteon with a high-fat meal reduces its maximum concentration by 22% and slows hypnotic onset by approximately 45 minutes.
The drug is metabolized mostly through the 1A2 isoenzyme of the cytochrome P (CYP)-450 system, although CYP 2C and 3A4 isoenzymes are also involved. About 90% of the dose is excreted.
Ramelteon’s elimination half-life averages 1 to 2.6 hours, so blood levels upon awakening will likely be too low to cause residual effects. Interestingly, in one placebo-controlled study,1 subjects who received a single 64-mg dose reported significantly reduced alertness and diminished ability to concentrate upon awakening. Subjects who took a 16-mg dose did not report this effect. Whether this finding is clinically relevant or relates to a residual effect, sedation, or cognitive impairment is unclear.
Efficacy
In a randomized, double-blind, placebo-controlled trial, ramelteon shortened sleep latency (time between going to bed and falling asleep) among patients with transient insomnia.
Roth et al1 studied 375 healthy adults ages 35 to 60 who reported sleeping 6.5 to 8.5 hours nightly and usually taking ≥30 minutes to fall asleep. In sleep research centers, subjects received one dose of ramelteon, 16 or 64 mg, or placebo 30 minutes before bedtime.
Mean latency to persistent sleep, measured with polysomnography, was 10 minutes shorter among both ramelteon dosage groups than among the placebo group. Mean total sleep time was 11 to 14 minutes longer among both ramelteon groups based on polysomnography, although subjective sleep estimates the next morning were similar among all three groups.
Roth et al2 also assessed efficacy of ramelteon across 5 weeks among 829 older patients (mean age 72) with insomnia (as defined by DSM-IV-TR) for ≥3 months, total nightly sleep time ≤6.5 hours for 3 nights, and self-reported sleep latency ≥45 minutes nightly for ≥3 nights.
Mean sleep latency decreased 25 to 30 minutes among subjects taking ramelteon, 4 or 8 mg nightly, compared with a mean 15-minute decrease among the placebo group. Average total sleep time was 5 to 8 minutes longer among both ramelteon groups compared with placebo.
Subjects in both ramelteon groups then received placebo for 1 week, during which time their mean latency to persistent sleep improved further or stayed the same. This suggests that ramelteon did not cause rebound insomnia.
Safety and tolerability
Ramelteon was generally well tolerated in clinical and preclinical trials. Headaches (7% of subjects), somnolence (5%), dizziness (5%), fatigue (4%), nausea (3%), exacerbated insomnia (3%), and upper respiratory tract infection (3%) were most commonly reported.3 Less-common effects included diarrhea, myalgia, depression, dysgeusia, arthralgia, influenza, and blood cortisol decrease.
The most common side effects among subjects age ≥65 were dizziness, dysgeusia, headaches, myalgia, and somnolence (Table 2). These occurred less frequently over 5 weeks among patients taking 4 mg/d than among those who took 8 mg/d, the FDA-approved dosage.
Ramelteon also showed no abuse potential compared with triazolam and placebo in a trial of 14 patients with a history of anxiolytic or sedative/hypnotic abuse.4
Contraindications
Do not give ramelteon to patients taking fluvoxamine. The antidepressant has been shown to raise serum ramelteon approximately 70-fold, thus substantially increasing the risk of ramelteon-associated adverse events.3
Ramelteon has shown teratogenicity in animals, though at doses far exceeding human levels. Still, as with other sleep-promoting medications, avoid prescribing ramelteon to expectant mothers.
Concomitant use of a strong CYP enzyme inducer such as rifampin may increase ramelteon metabolism and reduce serum ramelteon, which might decrease its efficacy in some cases. Whether increasing the ramelteon dosage counters this interaction is unknown.
Strong CYP 2C9 inhibitors such as fluconazole or strong CYP 3A4 inhibitors such as ketoconazole can raise serum ramelteon and might increase the risk of adverse events in some persons.
Dosing
Start ramelteon at 8 mg nightly, and tell patients to take it within 30 minutes of going to bed.
Because high-fat food slows its absorption, advise patients not to take ramelteon within 1 hour of eating a high-fat meal.
Ramelteon’s efficacy and side effects do not appear to be dose-dependent when given at 8 to 64 mg/d. Whether dosages >64 mg/d increase side-effect risk or therapeutic effect is unknown.
As with other hypnotics, supplement ramelteon therapy with sleep hygiene education and relaxation techniques.
Clinical implications
Ramelteon appears to help patients who have trouble falling asleep.
Because no other prescription medication targets melatonin neurotransmitters, no precedent and little data exist to guide patient choice, dosing, and treatment duration. Effects of ramelteon use >5 weeks are unknown. Clinical use and future research should uncover more information about ramelteon’s properties.
Related resources
- Ramelteon Web site. www.rozerem.com.
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9:25-39.
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev 2005;9:5-9.
Drug brand names
- Fluconazole • Diflucan
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Ramelteon • Rozerem
- Rifampin • Rifadin
- Triazolam • Halcion
Disclosures
Dr. Krystal receives research/grant support, is a consultant to, or is a speaker for Cephalon, Cyberonics, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Mecta Corp., Merck and Co., Neurocrine Biosciences, Neurogen Corp., Neuronetics, Organon, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon Pharmaceuticals, Takeda Pharmaceuticals North America, and TransOral Pharmaceuticals.
1. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
2. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomnia in elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
3. Rozerem prescribing information. Takeda Pharmaceuticals North America, 2005.
4. Griffiths R, Seuss P. Ramelteon and triazolam in humans: behavioral effects and abuse potential (poster). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
1. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
2. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomnia in elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
3. Rozerem prescribing information. Takeda Pharmaceuticals North America, 2005.
4. Griffiths R, Seuss P. Ramelteon and triazolam in humans: behavioral effects and abuse potential (poster). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
PRN medications: Are you using them safely?
Prescribing PRN medications to manage agitation and psychotic behavior may expose psychiatric inpatients to unnecessary psychotropics or overmedication.1 Moreover, nurses’ documentation of “as needed” administration is often inadequate2 because of the volume of required documentation, nursing shortages in inpatient settings, and nurses’ differing opinions on how to pharmacologically manage difficult behaviors and persistent pain.3
In the absence of evidence-based guidelines,4 the following recommendations can help you ensure that PRNs are used safely and documented thoroughly.
Document the rationale for ordering a PRN in your progress notes. Note the behaviors you wish to target, and specify when nurses should administer the drug.
Review nursing progress notes to determine whether the drug rectified the targeted behavior(s). For partial response, consider increasing the dosage. For nonresponse after 2 doses of one agent, try switching to another.
Review medication administration sheets to determine how often a drug is being used. Frequent PRN use suggests a need to re-evaluate the patient and drug regimen. You may need to increase or add to the patient’s standing medications. Consider combining similar medications into a standing order for more-reliable administration.
Request staff education. One study suggests that PRN orders can benefit staff more than patients.1 Advocating for the education of direct-care staff may be psychiatrists’ most effective method of combating PRN medication overuse.
Improper charting or misestimation of PRN use could result in liability. For example, medication that is not immediately documented may be re-administered by another nurse, resulting in overdose. A physician’s clinical judgment is impaired if he or she relies on inaccurate documentation or verbal report when evaluating a patient’s PRN use and standing order.
Education, auditing of records, and corrective action can help maintain charting accuracy and reduce the risk of litigation.3
1. Thapa P, Palmer SL, Owen RR, et al. PRN (as-needed) orders and exposure of psychiatric inpatients to unnecessary psychotropic medications. Psychiatr Serv 2003;54(9):1282-6.
2. Curtis J, Capp K. Administration of “as needed” psychotropic medication: a retrospective study. Int J Ment Health Nurs 2003;12(3):229-34.
3. LaFerney M. To give or not to give: challenging the use of PRN medication for pain and behaviors in long-term care. Advance Nurses 205;7(9):35.
4. Usher K, Holmes C, Lindsay D, Luck L. PRN psychotropic medications: The need for nursing research. Contemp Nurse 2003;14(3):248-57.
Michael C. LaFerney is an advanced practice nurse at Arbour SeniorCare, Rockland, MA.
Prescribing PRN medications to manage agitation and psychotic behavior may expose psychiatric inpatients to unnecessary psychotropics or overmedication.1 Moreover, nurses’ documentation of “as needed” administration is often inadequate2 because of the volume of required documentation, nursing shortages in inpatient settings, and nurses’ differing opinions on how to pharmacologically manage difficult behaviors and persistent pain.3
In the absence of evidence-based guidelines,4 the following recommendations can help you ensure that PRNs are used safely and documented thoroughly.
Document the rationale for ordering a PRN in your progress notes. Note the behaviors you wish to target, and specify when nurses should administer the drug.
Review nursing progress notes to determine whether the drug rectified the targeted behavior(s). For partial response, consider increasing the dosage. For nonresponse after 2 doses of one agent, try switching to another.
Review medication administration sheets to determine how often a drug is being used. Frequent PRN use suggests a need to re-evaluate the patient and drug regimen. You may need to increase or add to the patient’s standing medications. Consider combining similar medications into a standing order for more-reliable administration.
Request staff education. One study suggests that PRN orders can benefit staff more than patients.1 Advocating for the education of direct-care staff may be psychiatrists’ most effective method of combating PRN medication overuse.
Improper charting or misestimation of PRN use could result in liability. For example, medication that is not immediately documented may be re-administered by another nurse, resulting in overdose. A physician’s clinical judgment is impaired if he or she relies on inaccurate documentation or verbal report when evaluating a patient’s PRN use and standing order.
Education, auditing of records, and corrective action can help maintain charting accuracy and reduce the risk of litigation.3
Prescribing PRN medications to manage agitation and psychotic behavior may expose psychiatric inpatients to unnecessary psychotropics or overmedication.1 Moreover, nurses’ documentation of “as needed” administration is often inadequate2 because of the volume of required documentation, nursing shortages in inpatient settings, and nurses’ differing opinions on how to pharmacologically manage difficult behaviors and persistent pain.3
In the absence of evidence-based guidelines,4 the following recommendations can help you ensure that PRNs are used safely and documented thoroughly.
Document the rationale for ordering a PRN in your progress notes. Note the behaviors you wish to target, and specify when nurses should administer the drug.
Review nursing progress notes to determine whether the drug rectified the targeted behavior(s). For partial response, consider increasing the dosage. For nonresponse after 2 doses of one agent, try switching to another.
Review medication administration sheets to determine how often a drug is being used. Frequent PRN use suggests a need to re-evaluate the patient and drug regimen. You may need to increase or add to the patient’s standing medications. Consider combining similar medications into a standing order for more-reliable administration.
Request staff education. One study suggests that PRN orders can benefit staff more than patients.1 Advocating for the education of direct-care staff may be psychiatrists’ most effective method of combating PRN medication overuse.
Improper charting or misestimation of PRN use could result in liability. For example, medication that is not immediately documented may be re-administered by another nurse, resulting in overdose. A physician’s clinical judgment is impaired if he or she relies on inaccurate documentation or verbal report when evaluating a patient’s PRN use and standing order.
Education, auditing of records, and corrective action can help maintain charting accuracy and reduce the risk of litigation.3
1. Thapa P, Palmer SL, Owen RR, et al. PRN (as-needed) orders and exposure of psychiatric inpatients to unnecessary psychotropic medications. Psychiatr Serv 2003;54(9):1282-6.
2. Curtis J, Capp K. Administration of “as needed” psychotropic medication: a retrospective study. Int J Ment Health Nurs 2003;12(3):229-34.
3. LaFerney M. To give or not to give: challenging the use of PRN medication for pain and behaviors in long-term care. Advance Nurses 205;7(9):35.
4. Usher K, Holmes C, Lindsay D, Luck L. PRN psychotropic medications: The need for nursing research. Contemp Nurse 2003;14(3):248-57.
Michael C. LaFerney is an advanced practice nurse at Arbour SeniorCare, Rockland, MA.
1. Thapa P, Palmer SL, Owen RR, et al. PRN (as-needed) orders and exposure of psychiatric inpatients to unnecessary psychotropic medications. Psychiatr Serv 2003;54(9):1282-6.
2. Curtis J, Capp K. Administration of “as needed” psychotropic medication: a retrospective study. Int J Ment Health Nurs 2003;12(3):229-34.
3. LaFerney M. To give or not to give: challenging the use of PRN medication for pain and behaviors in long-term care. Advance Nurses 205;7(9):35.
4. Usher K, Holmes C, Lindsay D, Luck L. PRN psychotropic medications: The need for nursing research. Contemp Nurse 2003;14(3):248-57.
Michael C. LaFerney is an advanced practice nurse at Arbour SeniorCare, Rockland, MA.
Restraint and monitoring of psychotic or suicidal patients
Vague laws and debate over use of physical restraint complicate management of dangerous patients. Restraints have historically been over-used in psychiatry, even contributing to patients’ deaths. Still, many psychiatric facilities grapple with a reluctance to use restraint versus a need to protect patients from themselves and from harming others.
The law requires use of “least-restrictive interventions” to manage patients, but clinicians cannot agree on what this term means. This article offers tips to maximize patient safety when using restraints and advice on when to use them.
Psychotic man breaks neck jumping into window
Dane County (WI) Circuit Court
A 40-year-old man was hospitalized during a psychotic episode, in which he acted out aural hallucinations.
The man—who was previously diagnosed with schizophrenia—received a dose of haloperidol, and at least two guards escorted him to a room in the psychiatric unit. While left with a nurse, he tried to smash a window. The nurse hit a panic button to summon help, but the patient climbed on top of his bed and dove headfirst into a shatterproof glass. He fractured his neck and became quadriplegic.
In court, the patient’s attorney argued that the hospital was negligent in its failure to restrain him from harming himself. The patient died shortly after the trial from complications of quadriplegia.
- The jury’s verdict, $13 million, was reduced to approximately $7 million because of a statutory capitation.
Dr. Grant’s observations
The legal issue here is not simply whether the staff failed to prevent the patient from harming himself. Instead, the jury believed a reasonable person could have foreseen danger to the patient, thereby deeming the hospital negligent.
I’m not suggesting that all psychotic patients be restrained to prevent litigation. This case, however, illustrates the importance of assessing patients for dangerousness and intervening appropriately. Because the patient acted out his hallucinations and required two guards to escort him to his room, one could argue that one nurse could not adequately manage this patient.
When restraints are necessary, assess and document the patient’s behavior and the reasons that necessitate restraints. In this case, for example, record that medication alone did not sufficiently calm this patient.
One-on-one verbal and behavioral interventions can be effective alternatives to seclusion and restraint (Table 1).1,2 Predictably, patients respond negatively to restraints, preferring medication instead.4 When less-restrictive, behavioral, or pharmacologic measures fail, consider restraints to protect aggressive, assaultive patients.
Table 1
Possible alternatives to restraints
| Allow the patient to vent his or her feelings one-on-one with staff |
| Offer use of a quiet area or provide privacy if patient is upset |
| Provide alternate activities such as relaxation therapy or art therapy |
| Set firm, clear limits |
| Offer medication |
| Source: Reference 3 |
Security personnel asphyxiate woman
Pima County (AZ) Superior Court
A 32-year-old woman with a history of psychiatric disorders was admitted to a county hospital’s psychiatric department. Several guards and security technicians held her face down on the floor for 15 to 30 minutes. The patient struggled to breathe, turned blue, then stopped breathing. She died of asphyxiation.
The estate sued both the county and the security technicians’ employer, claiming the guards were not properly trained on patient restraint.
- A $105,000 settlement with the county was reached; a confidential settlement was reached with the security employer.
Dr. Grant’s observations
This case shows how improper use of restraints may result in a successful lawsuit.
In 1998, the Hartford Courant ran a series of articles alleging that seclusion and restraint in a psychiatric setting led to 142 deaths across 10 years.5 State and federal legislation passed after the newspaper’s report has focused on protecting patients from improper use of restraints. Be aware of your state’s and hospital’s regulations. The guidelines in Table 2 reflect general policies for using restraints suggested by the Joint Commission on Accreditation of Health-care Organizations.6
Restraints should be used only by trained staff and for only as long as the patient is dangerous to self or others. Also assess patients who may be at increased risk for physical or psychological difficulties if restrained or secluded and consider alternate interventions. Generally, restraints should be avoided in patients with the following relative contraindications:
- pregnant
- history of breathing problems
- head or spinal injuries
- history of recent fractures or surgeries
- seizure disorder
- history of sexual or physical abuse.
Table 2
Guidelines for proper restraint use
| Ensure the restrained patient’s safety and observe him or her continuously: |
|
| Keep the patient as comfortable as possible |
| Provide frequent opportunities for eating, drinking, and elimination, and continually assess physical comfort |
| Assess the continuing need for restraint, and consider alternatives when possible |
| Source: Reference 6 |
Unmonitored suicidal man suffocates himself
Tarrant County (TX) District Court
A 26-year-old man in the suicide prevention unit of a community hospital suffocated himself using a vinyl pillowcase from his room and cellophane wrap from the hospital’s kitchen.
For more than 40 minutes before finding the patient dead, staff had not documented checking the patient’s room, which was required every 15 minutes. Paramedics documented the beginning of rigor mortis.
The estate claimed the hospital had not adequately monitored the patient despite clear indications of suicidality. In the days preceding his death, records showed a deteriorating condition related to problems with his companion, who had told him she was leaving the home they shared. He previously attempted suicide when she threatened to move out and had injured himself on similar occasions.
At the time of his death, four staff members were on duty; one claimed to have seen the patient 5 minutes before he was found. The estate contended that more than 1 hour would have been required for rigor mortis to develop.
- A settlement of $1.1 million was reached.
Dr. Grant’s observations
Immediately assess suicidal patients and their environment to reduce the risk of self-harm. One-on-one observation has been found to be most effective7 and should be required for patients with severe suicide risk. All suicidal patients should (at minimum) be visible to staff members at all times to maintain safety standards.7 Frequently document the patient’s location, activities, and behavior.
To ensure a safe environment for suicidal patients, identify and minimize risk factors associated with hospital settings.8 For example, access to cellophane wrap in this case should have been blocked. Ensure that suicidal patients cannot reach materials they could use to harm themselves such as pillowcases, drapery cords, ingestible cleaning supplies, shower curtains and rods, and breakable objects.
1. Richmond I, Trujillo D, Schmelzer J, et al. Least restrictive alternatives: do they really work? J Nurs Care Qual 1996;11:29-37.
2. Donat DC. Encouraging alternatives to seclusion, restraint, and reliance on PRN drugs in a public psychiatric hospital. Psychiatr Serv 2005;56:1105-8.
3. American Psychiatric Association, American Psychiatric Nurses Association, National Association of Psychiatric Health Systems. Learning from each other: Success stories and ideas for reducing restraint/seclusion in behavioral health 2003. Available at: http://www.psych.org/psych_pract/patient_safety/sandr.cfm. Accessed September 27, 2005.
4. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within a psychiatric setting. Psychiatr Serv 2005;56:1123-33.
5. Appelbaum PS. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv 1999;50:881-2.
6. Joint Commission on Accreditation of Healthcare Organizations. Behavioral Healthcare Standards FAQs on special interventions. Available at: http://www.jcaho.org/. Accessed September 27, 2005.
7. Sullivan AM, Barron CT, Bezmen J, et al. The safe treatment of the suicidal patient in an adult inpatient setting: a proactive approach. Psychiatr Q 2005;76:67-83.
8. Lieberman DZ, Resnik HL, Holder-Perkins V. Environmental risk factors in hospital suicide. Suicide Life Threat Behav 2004;34:448-53.
Vague laws and debate over use of physical restraint complicate management of dangerous patients. Restraints have historically been over-used in psychiatry, even contributing to patients’ deaths. Still, many psychiatric facilities grapple with a reluctance to use restraint versus a need to protect patients from themselves and from harming others.
The law requires use of “least-restrictive interventions” to manage patients, but clinicians cannot agree on what this term means. This article offers tips to maximize patient safety when using restraints and advice on when to use them.
Psychotic man breaks neck jumping into window
Dane County (WI) Circuit Court
A 40-year-old man was hospitalized during a psychotic episode, in which he acted out aural hallucinations.
The man—who was previously diagnosed with schizophrenia—received a dose of haloperidol, and at least two guards escorted him to a room in the psychiatric unit. While left with a nurse, he tried to smash a window. The nurse hit a panic button to summon help, but the patient climbed on top of his bed and dove headfirst into a shatterproof glass. He fractured his neck and became quadriplegic.
In court, the patient’s attorney argued that the hospital was negligent in its failure to restrain him from harming himself. The patient died shortly after the trial from complications of quadriplegia.
- The jury’s verdict, $13 million, was reduced to approximately $7 million because of a statutory capitation.
Dr. Grant’s observations
The legal issue here is not simply whether the staff failed to prevent the patient from harming himself. Instead, the jury believed a reasonable person could have foreseen danger to the patient, thereby deeming the hospital negligent.
I’m not suggesting that all psychotic patients be restrained to prevent litigation. This case, however, illustrates the importance of assessing patients for dangerousness and intervening appropriately. Because the patient acted out his hallucinations and required two guards to escort him to his room, one could argue that one nurse could not adequately manage this patient.
When restraints are necessary, assess and document the patient’s behavior and the reasons that necessitate restraints. In this case, for example, record that medication alone did not sufficiently calm this patient.
One-on-one verbal and behavioral interventions can be effective alternatives to seclusion and restraint (Table 1).1,2 Predictably, patients respond negatively to restraints, preferring medication instead.4 When less-restrictive, behavioral, or pharmacologic measures fail, consider restraints to protect aggressive, assaultive patients.
Table 1
Possible alternatives to restraints
| Allow the patient to vent his or her feelings one-on-one with staff |
| Offer use of a quiet area or provide privacy if patient is upset |
| Provide alternate activities such as relaxation therapy or art therapy |
| Set firm, clear limits |
| Offer medication |
| Source: Reference 3 |
Security personnel asphyxiate woman
Pima County (AZ) Superior Court
A 32-year-old woman with a history of psychiatric disorders was admitted to a county hospital’s psychiatric department. Several guards and security technicians held her face down on the floor for 15 to 30 minutes. The patient struggled to breathe, turned blue, then stopped breathing. She died of asphyxiation.
The estate sued both the county and the security technicians’ employer, claiming the guards were not properly trained on patient restraint.
- A $105,000 settlement with the county was reached; a confidential settlement was reached with the security employer.
Dr. Grant’s observations
This case shows how improper use of restraints may result in a successful lawsuit.
In 1998, the Hartford Courant ran a series of articles alleging that seclusion and restraint in a psychiatric setting led to 142 deaths across 10 years.5 State and federal legislation passed after the newspaper’s report has focused on protecting patients from improper use of restraints. Be aware of your state’s and hospital’s regulations. The guidelines in Table 2 reflect general policies for using restraints suggested by the Joint Commission on Accreditation of Health-care Organizations.6
Restraints should be used only by trained staff and for only as long as the patient is dangerous to self or others. Also assess patients who may be at increased risk for physical or psychological difficulties if restrained or secluded and consider alternate interventions. Generally, restraints should be avoided in patients with the following relative contraindications:
- pregnant
- history of breathing problems
- head or spinal injuries
- history of recent fractures or surgeries
- seizure disorder
- history of sexual or physical abuse.
Table 2
Guidelines for proper restraint use
| Ensure the restrained patient’s safety and observe him or her continuously: |
|
| Keep the patient as comfortable as possible |
| Provide frequent opportunities for eating, drinking, and elimination, and continually assess physical comfort |
| Assess the continuing need for restraint, and consider alternatives when possible |
| Source: Reference 6 |
Unmonitored suicidal man suffocates himself
Tarrant County (TX) District Court
A 26-year-old man in the suicide prevention unit of a community hospital suffocated himself using a vinyl pillowcase from his room and cellophane wrap from the hospital’s kitchen.
For more than 40 minutes before finding the patient dead, staff had not documented checking the patient’s room, which was required every 15 minutes. Paramedics documented the beginning of rigor mortis.
The estate claimed the hospital had not adequately monitored the patient despite clear indications of suicidality. In the days preceding his death, records showed a deteriorating condition related to problems with his companion, who had told him she was leaving the home they shared. He previously attempted suicide when she threatened to move out and had injured himself on similar occasions.
At the time of his death, four staff members were on duty; one claimed to have seen the patient 5 minutes before he was found. The estate contended that more than 1 hour would have been required for rigor mortis to develop.
- A settlement of $1.1 million was reached.
Dr. Grant’s observations
Immediately assess suicidal patients and their environment to reduce the risk of self-harm. One-on-one observation has been found to be most effective7 and should be required for patients with severe suicide risk. All suicidal patients should (at minimum) be visible to staff members at all times to maintain safety standards.7 Frequently document the patient’s location, activities, and behavior.
To ensure a safe environment for suicidal patients, identify and minimize risk factors associated with hospital settings.8 For example, access to cellophane wrap in this case should have been blocked. Ensure that suicidal patients cannot reach materials they could use to harm themselves such as pillowcases, drapery cords, ingestible cleaning supplies, shower curtains and rods, and breakable objects.
Vague laws and debate over use of physical restraint complicate management of dangerous patients. Restraints have historically been over-used in psychiatry, even contributing to patients’ deaths. Still, many psychiatric facilities grapple with a reluctance to use restraint versus a need to protect patients from themselves and from harming others.
The law requires use of “least-restrictive interventions” to manage patients, but clinicians cannot agree on what this term means. This article offers tips to maximize patient safety when using restraints and advice on when to use them.
Psychotic man breaks neck jumping into window
Dane County (WI) Circuit Court
A 40-year-old man was hospitalized during a psychotic episode, in which he acted out aural hallucinations.
The man—who was previously diagnosed with schizophrenia—received a dose of haloperidol, and at least two guards escorted him to a room in the psychiatric unit. While left with a nurse, he tried to smash a window. The nurse hit a panic button to summon help, but the patient climbed on top of his bed and dove headfirst into a shatterproof glass. He fractured his neck and became quadriplegic.
In court, the patient’s attorney argued that the hospital was negligent in its failure to restrain him from harming himself. The patient died shortly after the trial from complications of quadriplegia.
- The jury’s verdict, $13 million, was reduced to approximately $7 million because of a statutory capitation.
Dr. Grant’s observations
The legal issue here is not simply whether the staff failed to prevent the patient from harming himself. Instead, the jury believed a reasonable person could have foreseen danger to the patient, thereby deeming the hospital negligent.
I’m not suggesting that all psychotic patients be restrained to prevent litigation. This case, however, illustrates the importance of assessing patients for dangerousness and intervening appropriately. Because the patient acted out his hallucinations and required two guards to escort him to his room, one could argue that one nurse could not adequately manage this patient.
When restraints are necessary, assess and document the patient’s behavior and the reasons that necessitate restraints. In this case, for example, record that medication alone did not sufficiently calm this patient.
One-on-one verbal and behavioral interventions can be effective alternatives to seclusion and restraint (Table 1).1,2 Predictably, patients respond negatively to restraints, preferring medication instead.4 When less-restrictive, behavioral, or pharmacologic measures fail, consider restraints to protect aggressive, assaultive patients.
Table 1
Possible alternatives to restraints
| Allow the patient to vent his or her feelings one-on-one with staff |
| Offer use of a quiet area or provide privacy if patient is upset |
| Provide alternate activities such as relaxation therapy or art therapy |
| Set firm, clear limits |
| Offer medication |
| Source: Reference 3 |
Security personnel asphyxiate woman
Pima County (AZ) Superior Court
A 32-year-old woman with a history of psychiatric disorders was admitted to a county hospital’s psychiatric department. Several guards and security technicians held her face down on the floor for 15 to 30 minutes. The patient struggled to breathe, turned blue, then stopped breathing. She died of asphyxiation.
The estate sued both the county and the security technicians’ employer, claiming the guards were not properly trained on patient restraint.
- A $105,000 settlement with the county was reached; a confidential settlement was reached with the security employer.
Dr. Grant’s observations
This case shows how improper use of restraints may result in a successful lawsuit.
In 1998, the Hartford Courant ran a series of articles alleging that seclusion and restraint in a psychiatric setting led to 142 deaths across 10 years.5 State and federal legislation passed after the newspaper’s report has focused on protecting patients from improper use of restraints. Be aware of your state’s and hospital’s regulations. The guidelines in Table 2 reflect general policies for using restraints suggested by the Joint Commission on Accreditation of Health-care Organizations.6
Restraints should be used only by trained staff and for only as long as the patient is dangerous to self or others. Also assess patients who may be at increased risk for physical or psychological difficulties if restrained or secluded and consider alternate interventions. Generally, restraints should be avoided in patients with the following relative contraindications:
- pregnant
- history of breathing problems
- head or spinal injuries
- history of recent fractures or surgeries
- seizure disorder
- history of sexual or physical abuse.
Table 2
Guidelines for proper restraint use
| Ensure the restrained patient’s safety and observe him or her continuously: |
|
| Keep the patient as comfortable as possible |
| Provide frequent opportunities for eating, drinking, and elimination, and continually assess physical comfort |
| Assess the continuing need for restraint, and consider alternatives when possible |
| Source: Reference 6 |
Unmonitored suicidal man suffocates himself
Tarrant County (TX) District Court
A 26-year-old man in the suicide prevention unit of a community hospital suffocated himself using a vinyl pillowcase from his room and cellophane wrap from the hospital’s kitchen.
For more than 40 minutes before finding the patient dead, staff had not documented checking the patient’s room, which was required every 15 minutes. Paramedics documented the beginning of rigor mortis.
The estate claimed the hospital had not adequately monitored the patient despite clear indications of suicidality. In the days preceding his death, records showed a deteriorating condition related to problems with his companion, who had told him she was leaving the home they shared. He previously attempted suicide when she threatened to move out and had injured himself on similar occasions.
At the time of his death, four staff members were on duty; one claimed to have seen the patient 5 minutes before he was found. The estate contended that more than 1 hour would have been required for rigor mortis to develop.
- A settlement of $1.1 million was reached.
Dr. Grant’s observations
Immediately assess suicidal patients and their environment to reduce the risk of self-harm. One-on-one observation has been found to be most effective7 and should be required for patients with severe suicide risk. All suicidal patients should (at minimum) be visible to staff members at all times to maintain safety standards.7 Frequently document the patient’s location, activities, and behavior.
To ensure a safe environment for suicidal patients, identify and minimize risk factors associated with hospital settings.8 For example, access to cellophane wrap in this case should have been blocked. Ensure that suicidal patients cannot reach materials they could use to harm themselves such as pillowcases, drapery cords, ingestible cleaning supplies, shower curtains and rods, and breakable objects.
1. Richmond I, Trujillo D, Schmelzer J, et al. Least restrictive alternatives: do they really work? J Nurs Care Qual 1996;11:29-37.
2. Donat DC. Encouraging alternatives to seclusion, restraint, and reliance on PRN drugs in a public psychiatric hospital. Psychiatr Serv 2005;56:1105-8.
3. American Psychiatric Association, American Psychiatric Nurses Association, National Association of Psychiatric Health Systems. Learning from each other: Success stories and ideas for reducing restraint/seclusion in behavioral health 2003. Available at: http://www.psych.org/psych_pract/patient_safety/sandr.cfm. Accessed September 27, 2005.
4. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within a psychiatric setting. Psychiatr Serv 2005;56:1123-33.
5. Appelbaum PS. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv 1999;50:881-2.
6. Joint Commission on Accreditation of Healthcare Organizations. Behavioral Healthcare Standards FAQs on special interventions. Available at: http://www.jcaho.org/. Accessed September 27, 2005.
7. Sullivan AM, Barron CT, Bezmen J, et al. The safe treatment of the suicidal patient in an adult inpatient setting: a proactive approach. Psychiatr Q 2005;76:67-83.
8. Lieberman DZ, Resnik HL, Holder-Perkins V. Environmental risk factors in hospital suicide. Suicide Life Threat Behav 2004;34:448-53.
1. Richmond I, Trujillo D, Schmelzer J, et al. Least restrictive alternatives: do they really work? J Nurs Care Qual 1996;11:29-37.
2. Donat DC. Encouraging alternatives to seclusion, restraint, and reliance on PRN drugs in a public psychiatric hospital. Psychiatr Serv 2005;56:1105-8.
3. American Psychiatric Association, American Psychiatric Nurses Association, National Association of Psychiatric Health Systems. Learning from each other: Success stories and ideas for reducing restraint/seclusion in behavioral health 2003. Available at: http://www.psych.org/psych_pract/patient_safety/sandr.cfm. Accessed September 27, 2005.
4. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within a psychiatric setting. Psychiatr Serv 2005;56:1123-33.
5. Appelbaum PS. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv 1999;50:881-2.
6. Joint Commission on Accreditation of Healthcare Organizations. Behavioral Healthcare Standards FAQs on special interventions. Available at: http://www.jcaho.org/. Accessed September 27, 2005.
7. Sullivan AM, Barron CT, Bezmen J, et al. The safe treatment of the suicidal patient in an adult inpatient setting: a proactive approach. Psychiatr Q 2005;76:67-83.
8. Lieberman DZ, Resnik HL, Holder-Perkins V. Environmental risk factors in hospital suicide. Suicide Life Threat Behav 2004;34:448-53.
CORRECTION
In the article “Yoga: A breath of relief for Hurricane Katrina refugees” (Current Psychiatry October 2005), e-mail addresses for the International Association for Human Values (IAHV) and the Art of Living Foundation (AOLF) International Research Committee were listed as Web page addresses. The correct e-mail addresses are:
- IAHV: [email protected]
- AOLF International Research Committee: [email protected]
In the article “Yoga: A breath of relief for Hurricane Katrina refugees” (Current Psychiatry October 2005), e-mail addresses for the International Association for Human Values (IAHV) and the Art of Living Foundation (AOLF) International Research Committee were listed as Web page addresses. The correct e-mail addresses are:
- IAHV: [email protected]
- AOLF International Research Committee: [email protected]
In the article “Yoga: A breath of relief for Hurricane Katrina refugees” (Current Psychiatry October 2005), e-mail addresses for the International Association for Human Values (IAHV) and the Art of Living Foundation (AOLF) International Research Committee were listed as Web page addresses. The correct e-mail addresses are:
- IAHV: [email protected]
- AOLF International Research Committee: [email protected]
Neurobiology and medication adherence
Deborah S. Finnell, APRN, advises promoting medication adherence one stage at a time (Current Psychiatry, August 2005). Research suggests that neurobiologic interventions can help achieve this objective.
Blood pressure reduction is associated with longer, less-recurrent speech hesitation pauses (SHPs) of approximately 2 seconds.1 This supports the hypothesis that promoting neuroplasticity in small steps may have a lasting benefit in adults.2
SHPs are linked to rhythmic and prefrontal cortical modulation of dopamine lateralized to the right hemisphere, regulating brainstem cardiovascular control and coping behavior.1 Matching SHPs in spontaneous dialogues is a joint, mutually responsive rhythm with prelinguistic origins.
These findings suggest that interventions can be tailored to match the clinician’s communication style with that of the patient.1 This may influence the patient’s knowledge and beliefs about medication, help him/her become more engaged in and satisfied with treatment, and promote adherence.3
Ernest H. Friedman, MD
East Cleveland, OH
- Friedman EH. Neurobiology of managing perceived stress (letter). J Natl Med Assoc 2005;97:583-4.
- Hayward P. Small steps to neuroplasticity in adults. Lancet Neurology 2002;1:401.
- Friedman EH. Non-adherence research design in bipolar disorder (letter). Clin Approach Bipolar Disord 2005;4:19.
Dr. Finnell responds
In concert with Dr. Friedman’s point about congruent communications, clinicians should be ready to teach patients about a medication’s pharmaco-dynamics. Helping patients discover the neurobiological basis for mental disorders and psychotropics empowers them, eases their defenses, and reduces the stigma they experience.1
While this instruction is important during the precontemplation stage described in my article, clinicians should continue educating patients as they adopt medication-taking behavior. As patients gain experience with taking medications and understand more about them, additional evidence-based information should be provided.
Deborah S. Finnell, DNS, APRN
Assistant professor of nursing
State University of New York, Buffalo
- Finnell D. The case for teaching patients about the neurobiological basis of addictions. J Addict Nurs 2000;3/4:149-58.
Deborah S. Finnell, APRN, advises promoting medication adherence one stage at a time (Current Psychiatry, August 2005). Research suggests that neurobiologic interventions can help achieve this objective.
Blood pressure reduction is associated with longer, less-recurrent speech hesitation pauses (SHPs) of approximately 2 seconds.1 This supports the hypothesis that promoting neuroplasticity in small steps may have a lasting benefit in adults.2
SHPs are linked to rhythmic and prefrontal cortical modulation of dopamine lateralized to the right hemisphere, regulating brainstem cardiovascular control and coping behavior.1 Matching SHPs in spontaneous dialogues is a joint, mutually responsive rhythm with prelinguistic origins.
These findings suggest that interventions can be tailored to match the clinician’s communication style with that of the patient.1 This may influence the patient’s knowledge and beliefs about medication, help him/her become more engaged in and satisfied with treatment, and promote adherence.3
Ernest H. Friedman, MD
East Cleveland, OH
- Friedman EH. Neurobiology of managing perceived stress (letter). J Natl Med Assoc 2005;97:583-4.
- Hayward P. Small steps to neuroplasticity in adults. Lancet Neurology 2002;1:401.
- Friedman EH. Non-adherence research design in bipolar disorder (letter). Clin Approach Bipolar Disord 2005;4:19.
Dr. Finnell responds
In concert with Dr. Friedman’s point about congruent communications, clinicians should be ready to teach patients about a medication’s pharmaco-dynamics. Helping patients discover the neurobiological basis for mental disorders and psychotropics empowers them, eases their defenses, and reduces the stigma they experience.1
While this instruction is important during the precontemplation stage described in my article, clinicians should continue educating patients as they adopt medication-taking behavior. As patients gain experience with taking medications and understand more about them, additional evidence-based information should be provided.
Deborah S. Finnell, DNS, APRN
Assistant professor of nursing
State University of New York, Buffalo
- Finnell D. The case for teaching patients about the neurobiological basis of addictions. J Addict Nurs 2000;3/4:149-58.
Deborah S. Finnell, APRN, advises promoting medication adherence one stage at a time (Current Psychiatry, August 2005). Research suggests that neurobiologic interventions can help achieve this objective.
Blood pressure reduction is associated with longer, less-recurrent speech hesitation pauses (SHPs) of approximately 2 seconds.1 This supports the hypothesis that promoting neuroplasticity in small steps may have a lasting benefit in adults.2
SHPs are linked to rhythmic and prefrontal cortical modulation of dopamine lateralized to the right hemisphere, regulating brainstem cardiovascular control and coping behavior.1 Matching SHPs in spontaneous dialogues is a joint, mutually responsive rhythm with prelinguistic origins.
These findings suggest that interventions can be tailored to match the clinician’s communication style with that of the patient.1 This may influence the patient’s knowledge and beliefs about medication, help him/her become more engaged in and satisfied with treatment, and promote adherence.3
Ernest H. Friedman, MD
East Cleveland, OH
- Friedman EH. Neurobiology of managing perceived stress (letter). J Natl Med Assoc 2005;97:583-4.
- Hayward P. Small steps to neuroplasticity in adults. Lancet Neurology 2002;1:401.
- Friedman EH. Non-adherence research design in bipolar disorder (letter). Clin Approach Bipolar Disord 2005;4:19.
Dr. Finnell responds
In concert with Dr. Friedman’s point about congruent communications, clinicians should be ready to teach patients about a medication’s pharmaco-dynamics. Helping patients discover the neurobiological basis for mental disorders and psychotropics empowers them, eases their defenses, and reduces the stigma they experience.1
While this instruction is important during the precontemplation stage described in my article, clinicians should continue educating patients as they adopt medication-taking behavior. As patients gain experience with taking medications and understand more about them, additional evidence-based information should be provided.
Deborah S. Finnell, DNS, APRN
Assistant professor of nursing
State University of New York, Buffalo
- Finnell D. The case for teaching patients about the neurobiological basis of addictions. J Addict Nurs 2000;3/4:149-58.
‘Distracting’ patients from anxiety
Drs. Narsimha Pinninti and Rajnish Mago offer a brief, easy-to-use intervention to teach patients to control their anxiety. (“In-session anxiety: 5 steps to help patients relax,” Current Psychiatry, August 2005).
In step 4, the authors recommend having a patient with cognitive symptoms “look around the room and describe in detail what he sees” over 3 minutes. They also suggest having a patient with physiologic/affective symptoms “close his eyes and (remember) when he felt safe and content,” also known as the “safe-place technique.”
Distraction—the central ingredient in both interventions—is often used in cognitive-behavioral therapy (CBT), an empirically supported treatment for anxiety disorders. In CBT, however, the therapist first conceptualizes what is generating and maintaining the anxiety and hypothesizes what the intervention will teach the patient. For example, a patient who fears flying might use distraction to decrease pre-flight anxiety.
In other instances, such as during panic attacks, distraction may be a “safety behavior” that allows patients to control or avoid anxiety out of fear that the physical sensations they experience during panic are dangerous. While these behaviors may provide temporary relief (via negative reinforcement), they condition patients to rely on them to feel safe, thus perpetuating the anxiety.1 These patients should be encouraged to gradually and systematically experience anxiety symptoms and learn to manage or tolerate them.
Likewise, interventions such as those found in step 4 may help most anxious patients feel better during the session (via avoidance/distraction) but might maintain the anxiety that patients (and doctors) want to reduce.
Rather than applying a universal or “Procrustean” approach, psychiatrists should tailor interventions such as those suggested in step 4 to each patient’s anxiety.2 This way, they can be applied when appropriate with more durable and meaningful results.
Simon A. Rego, PsyD
Katherine L. Muller, PsyD
Colleen Jacobson, PhD
Cognitive-Behavioral Therapy Program Montefiore Medical Center
Bronx, NY
- Lazarus AA. Behavior therapy and beyond. New York: McGraw-Hill; 1971.
- Mowrer OH. Learning theory and behavior. New York: Wiley; 1960.
The authors respond
Dr. Rego et al raise some excellent points.
We agree that techniques based on distraction are among several that a clinician should consider. We do not advocate use of these interventions for long-term anxiety control or as complete cognitive-behavioral therapy.
Techniques based on distraction, however, can have unique advantages when used appropriately. First, distraction techniques are obviously more likely to work when in-session anxiety is pronounced. Also, as Dr. Rego et al note, distraction techniques can be valuable in acute situations.
Second, associated dysfunctional beliefs often fuel anxiety. For example, patients commonly believe that they cannot control their anxiety. Some also believe that they need PRN medications such as benzodiazepines to control the symptoms (safety behavior), leading in some cases to abuse of prescribed medications. The steps we suggest would help show patients that they don’t need PRN medication. Learning not to rely on these agents can improve their sense of self-efficacy and reduce their overall anxiety.
Third, patients engage in a range of “safety behaviors”—from simple distraction to substance abuse. In some instances, helping the patient change his or her safety behavior from medication reliance to reliance on self-regulated activities is a reasonable short-term therapeutic goal. We have found that these techniques have helped some patients reduce PRN medication use.
Narsimha R. Pinninti, MD
School of Osteopathic Medicine
University of Medicine and Dentistry of New Jersey, Camden
Rajnish Mago, MD
Thomas Jefferson University,
Philadelphia, PA
Drs. Narsimha Pinninti and Rajnish Mago offer a brief, easy-to-use intervention to teach patients to control their anxiety. (“In-session anxiety: 5 steps to help patients relax,” Current Psychiatry, August 2005).
In step 4, the authors recommend having a patient with cognitive symptoms “look around the room and describe in detail what he sees” over 3 minutes. They also suggest having a patient with physiologic/affective symptoms “close his eyes and (remember) when he felt safe and content,” also known as the “safe-place technique.”
Distraction—the central ingredient in both interventions—is often used in cognitive-behavioral therapy (CBT), an empirically supported treatment for anxiety disorders. In CBT, however, the therapist first conceptualizes what is generating and maintaining the anxiety and hypothesizes what the intervention will teach the patient. For example, a patient who fears flying might use distraction to decrease pre-flight anxiety.
In other instances, such as during panic attacks, distraction may be a “safety behavior” that allows patients to control or avoid anxiety out of fear that the physical sensations they experience during panic are dangerous. While these behaviors may provide temporary relief (via negative reinforcement), they condition patients to rely on them to feel safe, thus perpetuating the anxiety.1 These patients should be encouraged to gradually and systematically experience anxiety symptoms and learn to manage or tolerate them.
Likewise, interventions such as those found in step 4 may help most anxious patients feel better during the session (via avoidance/distraction) but might maintain the anxiety that patients (and doctors) want to reduce.
Rather than applying a universal or “Procrustean” approach, psychiatrists should tailor interventions such as those suggested in step 4 to each patient’s anxiety.2 This way, they can be applied when appropriate with more durable and meaningful results.
Simon A. Rego, PsyD
Katherine L. Muller, PsyD
Colleen Jacobson, PhD
Cognitive-Behavioral Therapy Program Montefiore Medical Center
Bronx, NY
- Lazarus AA. Behavior therapy and beyond. New York: McGraw-Hill; 1971.
- Mowrer OH. Learning theory and behavior. New York: Wiley; 1960.
The authors respond
Dr. Rego et al raise some excellent points.
We agree that techniques based on distraction are among several that a clinician should consider. We do not advocate use of these interventions for long-term anxiety control or as complete cognitive-behavioral therapy.
Techniques based on distraction, however, can have unique advantages when used appropriately. First, distraction techniques are obviously more likely to work when in-session anxiety is pronounced. Also, as Dr. Rego et al note, distraction techniques can be valuable in acute situations.
Second, associated dysfunctional beliefs often fuel anxiety. For example, patients commonly believe that they cannot control their anxiety. Some also believe that they need PRN medications such as benzodiazepines to control the symptoms (safety behavior), leading in some cases to abuse of prescribed medications. The steps we suggest would help show patients that they don’t need PRN medication. Learning not to rely on these agents can improve their sense of self-efficacy and reduce their overall anxiety.
Third, patients engage in a range of “safety behaviors”—from simple distraction to substance abuse. In some instances, helping the patient change his or her safety behavior from medication reliance to reliance on self-regulated activities is a reasonable short-term therapeutic goal. We have found that these techniques have helped some patients reduce PRN medication use.
Narsimha R. Pinninti, MD
School of Osteopathic Medicine
University of Medicine and Dentistry of New Jersey, Camden
Rajnish Mago, MD
Thomas Jefferson University,
Philadelphia, PA
Drs. Narsimha Pinninti and Rajnish Mago offer a brief, easy-to-use intervention to teach patients to control their anxiety. (“In-session anxiety: 5 steps to help patients relax,” Current Psychiatry, August 2005).
In step 4, the authors recommend having a patient with cognitive symptoms “look around the room and describe in detail what he sees” over 3 minutes. They also suggest having a patient with physiologic/affective symptoms “close his eyes and (remember) when he felt safe and content,” also known as the “safe-place technique.”
Distraction—the central ingredient in both interventions—is often used in cognitive-behavioral therapy (CBT), an empirically supported treatment for anxiety disorders. In CBT, however, the therapist first conceptualizes what is generating and maintaining the anxiety and hypothesizes what the intervention will teach the patient. For example, a patient who fears flying might use distraction to decrease pre-flight anxiety.
In other instances, such as during panic attacks, distraction may be a “safety behavior” that allows patients to control or avoid anxiety out of fear that the physical sensations they experience during panic are dangerous. While these behaviors may provide temporary relief (via negative reinforcement), they condition patients to rely on them to feel safe, thus perpetuating the anxiety.1 These patients should be encouraged to gradually and systematically experience anxiety symptoms and learn to manage or tolerate them.
Likewise, interventions such as those found in step 4 may help most anxious patients feel better during the session (via avoidance/distraction) but might maintain the anxiety that patients (and doctors) want to reduce.
Rather than applying a universal or “Procrustean” approach, psychiatrists should tailor interventions such as those suggested in step 4 to each patient’s anxiety.2 This way, they can be applied when appropriate with more durable and meaningful results.
Simon A. Rego, PsyD
Katherine L. Muller, PsyD
Colleen Jacobson, PhD
Cognitive-Behavioral Therapy Program Montefiore Medical Center
Bronx, NY
- Lazarus AA. Behavior therapy and beyond. New York: McGraw-Hill; 1971.
- Mowrer OH. Learning theory and behavior. New York: Wiley; 1960.
The authors respond
Dr. Rego et al raise some excellent points.
We agree that techniques based on distraction are among several that a clinician should consider. We do not advocate use of these interventions for long-term anxiety control or as complete cognitive-behavioral therapy.
Techniques based on distraction, however, can have unique advantages when used appropriately. First, distraction techniques are obviously more likely to work when in-session anxiety is pronounced. Also, as Dr. Rego et al note, distraction techniques can be valuable in acute situations.
Second, associated dysfunctional beliefs often fuel anxiety. For example, patients commonly believe that they cannot control their anxiety. Some also believe that they need PRN medications such as benzodiazepines to control the symptoms (safety behavior), leading in some cases to abuse of prescribed medications. The steps we suggest would help show patients that they don’t need PRN medication. Learning not to rely on these agents can improve their sense of self-efficacy and reduce their overall anxiety.
Third, patients engage in a range of “safety behaviors”—from simple distraction to substance abuse. In some instances, helping the patient change his or her safety behavior from medication reliance to reliance on self-regulated activities is a reasonable short-term therapeutic goal. We have found that these techniques have helped some patients reduce PRN medication use.
Narsimha R. Pinninti, MD
School of Osteopathic Medicine
University of Medicine and Dentistry of New Jersey, Camden
Rajnish Mago, MD
Thomas Jefferson University,
Philadelphia, PA
Is your ‘dream clinic’ worth $1,000?
The University of Cincinnati has received a once-in-a-lifetime opportunity to do something great for psychiatry, and we invite you to share our dream.
We have received a $30 million donation to build the Craig and Frances Lindner Center of HOPE, a state-of-the-science psychiatric treatment center, on a 100-acre site north of Cincinnati. The center—with adult and adolescent inpatient beds and integrated outpatient services—will be operated by the Health Alliance of Greater Cincinnati. Current Psychiatry Deputy Editor Paul E. Keck, Jr., MD, professor and vice chairman for research at the university’s department of psychiatry, has been named psychiatrist-in-chief.
Whom do we consult for the most practical, creative ideas to design the center? Current Psychiatry readers, of course!
Have you complained about programs or facilities you inherited and dreamed of things you might do differently if you could start from scratch? If so, please send us:
- suggestions for innovative programs we might implement
- ideas to make our center clinically and financially successful
- ways to improve the “usual” hospital and outpatient programs.
Please e-mail your ideas to [email protected]. We will award $1,000 for the best suggestion received by Dec. 1, 2005. Dr. Keck and I will determine the winner, with deputy editor Lois E. Krahn, MD, casting a tie-breaking vote if necessary. We will post all useful or thought-provoking suggestions on www.currentpsychiatry.com.
The University of Cincinnati has received a once-in-a-lifetime opportunity to do something great for psychiatry, and we invite you to share our dream.
We have received a $30 million donation to build the Craig and Frances Lindner Center of HOPE, a state-of-the-science psychiatric treatment center, on a 100-acre site north of Cincinnati. The center—with adult and adolescent inpatient beds and integrated outpatient services—will be operated by the Health Alliance of Greater Cincinnati. Current Psychiatry Deputy Editor Paul E. Keck, Jr., MD, professor and vice chairman for research at the university’s department of psychiatry, has been named psychiatrist-in-chief.
Whom do we consult for the most practical, creative ideas to design the center? Current Psychiatry readers, of course!
Have you complained about programs or facilities you inherited and dreamed of things you might do differently if you could start from scratch? If so, please send us:
- suggestions for innovative programs we might implement
- ideas to make our center clinically and financially successful
- ways to improve the “usual” hospital and outpatient programs.
Please e-mail your ideas to [email protected]. We will award $1,000 for the best suggestion received by Dec. 1, 2005. Dr. Keck and I will determine the winner, with deputy editor Lois E. Krahn, MD, casting a tie-breaking vote if necessary. We will post all useful or thought-provoking suggestions on www.currentpsychiatry.com.
The University of Cincinnati has received a once-in-a-lifetime opportunity to do something great for psychiatry, and we invite you to share our dream.
We have received a $30 million donation to build the Craig and Frances Lindner Center of HOPE, a state-of-the-science psychiatric treatment center, on a 100-acre site north of Cincinnati. The center—with adult and adolescent inpatient beds and integrated outpatient services—will be operated by the Health Alliance of Greater Cincinnati. Current Psychiatry Deputy Editor Paul E. Keck, Jr., MD, professor and vice chairman for research at the university’s department of psychiatry, has been named psychiatrist-in-chief.
Whom do we consult for the most practical, creative ideas to design the center? Current Psychiatry readers, of course!
Have you complained about programs or facilities you inherited and dreamed of things you might do differently if you could start from scratch? If so, please send us:
- suggestions for innovative programs we might implement
- ideas to make our center clinically and financially successful
- ways to improve the “usual” hospital and outpatient programs.
Please e-mail your ideas to [email protected]. We will award $1,000 for the best suggestion received by Dec. 1, 2005. Dr. Keck and I will determine the winner, with deputy editor Lois E. Krahn, MD, casting a tie-breaking vote if necessary. We will post all useful or thought-provoking suggestions on www.currentpsychiatry.com.
Antipsychotics in the elderly: Reducing risks of stroke and death
In early-stage Alzheimer’s disease, Mrs. P enters a nursing home because her daughter, who usually takes care of her, is hospitalized for cancer chemotherapy. Mrs. P promptly develops paranoid delusions and refuses her medications for high blood pressure and high cholesterol. What are the treatment options? Is any one antipsychotic safer than another?
Writing an antipsychotic prescription for patients such as Mrs. P is no longer a quick scribble. First we learned that atypicals may alter glucose and lipid metabolism in clinically troublesome ways.1,2 Then we learned that antipsychotics can triple the risk for sudden death in older patients with dementia. (See FDA advisory, Related resources.)
How great are the risks, who is at risk, and how strong is the evidence for these new risks? The debate is not yet settled, but the boundaries of good practice for antipsychotic use in older patients are being redrawn. This is particularly true for those with dementia, in whom antipsychotics’ risk/benefit ratio is higher than for older patients with schizophrenia or bipolar disorder.
Unproven effectiveness
Antipsychotics are not FDA-approved for treating dementia-related psychosis. Though antipsychotics are commonly used off-label to treat behavioral disturbances in the elderly with dementia, no standard of care exists for managing these symptoms with drugs. So far, the evidence for antipsychotics’ effectiveness for dementia’s behavioral and psychological symptoms is spotty at best.
Sink et al (Table 1)3 systematically reviewed 14 randomized, controlled trials and concluded “there is no clear evidence that typical antipsychotics are useful for treating neuropsychiatric symptoms [of dementia].” They concluded from 6 studies of atypicals that only olanzapine and risperidone had shown efficacy, but the effects were “modest and further complicated by risk of stroke.” When the benefits are modest, the risks are more difficult to justify.
When medication is necessary, on the other hand, the Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients reported in 2004 that “for agitated dementia with delusions, the experts’ first-line recommendation is an antipsychotic drug alone…. Risperidone (0.5 to 2.0 mg/day) was first line, followed by quetiapine (50 to 150 mg/day) and olanzapine (5.0 to 7.5 mg/day) as high second-line options.”4
CATIE studies. The definitive prospective study of antipsychotics’ effectiveness in dementia has not been completed. The National Institute of Mental Health is sponsoring CATIE—Clinical Antipsychotic Trials of Intervention Effectiveness—a multi-site research program comparing the effectiveness and outcomes of antipsychotics in treating schizophrenia and Alzheimer’s disease. Results of the Alzheimer disease arm5 are expected next year.
The schizophrenia arm comparing four atypical antipsychotics (quetiapine, risperidone, ziprasidone, and olanzapine) and one typical antipsychotic (perphenazine) found:
- Typical and atypical antipsychotics were similarly effective in 1,493 patients with chronic schizophrenia
- 74% of patients discontinued assigned medications before 18 months for lack of efficacy, intolerable side effects, or other reasons.6
Table 1
Timeline: Evidence on risks, efficacy of atypical antipsychotics
| Year | Summary of study findings, FDA warnings |
|---|---|
| 2002 | Higher incidence of stroke seen with risperidone than with placebo in two of four clinical trials (Wooltorton7) Health Canada and Janssen-Ortho warn Canadian physicians of possible link between risperidone and stroke |
| 2003 | FDA warns of increased risk of stroke with risperidone |
| 2004 | Threefold increased risk of sudden cardiac death associated with antipsychotic use in patients age >65, most without dementia (Straus et al11) |
| 2005 | Stroke risk reported no greater in older patients who took atypicals than in those who took typical antipsychotics (Gill et al10) Analysis of 14 controlled trials finds “no clear evidence” that typical antipsychotics are effective in dementia; atypicals’ effects seen as “modest” (Sink et al3) FDA issues warning after finding 60% increase in risk of sudden death in review of 17 trials in older patients receiving atypical antipsychotics for dementia Efficacy of conventional and atypical antipsychotics found similar in patients with chronic schizophrenia (Lieberman et al6) |
Stroke risk
How strong is the evidence for stroke risk among older patients who take antipsychotics? Wooltorton7 first raised concern about increased risk of stroke with risperidone in 2002 in a summary of four clinical trials. Though none was designed to examine stroke risk as the primary outcome, two showed significantly higher incidence of cerebrovascular events with risperidone than with placebo. The stroke rate with risperidone was double that with placebo (4% vs 2%) across the total 1,200 subjects in the four studies.
This preliminary report led to an FDA advisory but surprisingly no definitive studies or systematic reviews. No epidemiologic studies have examined stroke rates in those who take antipsychotics compared with those who don’t, while controlling for common stroke predictors. So we have a warning based on post hoc analyses in two positive and two negative studies, but no sound estimate of how much antipsychotic use in general adds to the risk of stroke.
Atypicals vs. typicals. Are atypicals worse than typicals in their effect on stroke risk?
Herrmann et al8,9 reviewed a health care database of 11,400 older persons and found no statistically significant increase in stroke rate with risperidone or olanzapine compared with typical antipsychotics. This study did not assess whether patients had dementia or primary psychotic disorders.
In a larger retrospective study of 32,710 older persons with dementia, Herrmann’s group10 found no greater stroke risk in those who took atypicals than in those who took typical antipsychotics.
So the evidence for the risk of stroke in older patients who take antipsychotics is based on a few reports and no definitive studies.
Risk of early death
What about the risk of early death? After reviewing 17 clinical trials of atypical antipsychotics in older patients with dementia, the FDA issued its warning in April 2005 about increased mortality risk (see Related resources). Fifteen of the trials showed an increased mortality risk, resulting in an estimated 1.6- to 1.7-fold increase in risk of death, mostly by cardiac or infectious causes.
A year earlier, Straus et al11 reported on risk of sudden cardiac death with antipsychotic use in a population-based, case-control study in the Netherlands. In this longitudinal, observational database of 250,000 patients, 75% were age >65 and <1% had dementia. Current use of antipsychotics was associated with a threefold increase in risk of sudden cardiac death (554 cases), after other predictors of sudden death were factored in. This risk increased with higher antipsychotic dosages and was similarly elevated for patients with and without schizophrenia-related disorders.
Mechanisms unknown
By what mechanisms could antipsychotics precipitate stroke, sudden cardiac death, or pneumonia? A clear biological mechanism has not been proposed, much less proven. The risks seen in clinical trials—usually lasting 12 weeks or less—suggest an acute effect rather than the more-gradual consequences of weight gain, altered lipid metabolism, or diabetes.
Speculations range widely. Possibilities awaiting study include postural hypotension, altered platelet aggregation, increased venous thromboembolism, peripheral vasodilation leading to cardiovascular collapse, acute dystonia, acute cardiomyopathy, arrhythmias related to QT prolongation, and other forms of cardiac toxicity.10,11
Remaining questions
The few studies plus two FDA advisories force clinicians to make complex treatment decisions on insufficient evidence. Here’s what we don’t know:
- The magnitude of stroke or sudden death risk in older patients who take antipsychotics for any diagnosis. (All studies have limitations, and public health policy should not rely on one or two studies, no matter how good they may be.)
- Who is at higher or lower risk with antipsychotic use—men vs women? blacks vs whites?etc.
- Biological mechanisms of an association between antipsychotics and risks of stroke and premature death.
Strategies for reducing risk
We can minimize patients’ clinical risk and our legal risk only by using the limited evidence, expert consensus, and sound clinical judgment. Suggested strategies are listed in Table 2.
Table 2
Strategies to minimize antipsychotic risk in patients age 65 and older
| Review and document risk factors for cardiovascular disease—including stroke—with physical examination, laboratory tests (lipid profile, fasting glucose), and ECG in consultation with a primary care physician or specialist |
| Try nonpharmacologic approaches first whenever possible to manage behavioral disturbances in patients with dementia; document results before trying an antipsychotic |
| Review antipsychotics’ risks and benefits with patient and family |
| Use low dosages and increase gradually, as sudden death risk is dose-related |
| Monitor antipsychotic effectiveness, and discontinue trials of questionable benefit |
| Monitor cardiovascular symptoms, heart rate, blood pressure and body mass index of patients with cardiovascular risk |
| Avoid using sedating antipsychotics for insomnia in patients without psychiatric disorders |

Dr. Wulsin treats psychiatric outpatients and has published reviews on depression and heart disease. He is training director, University of Cincinnati family medicine-psychiatry residency program.
- Food and Drug Administration. Advisory on increased risk of mortality with use of atypical antipsychotics to treat behavioral disturbances in older patients with dementia. www.fda.gov/cder/drug/advisory/antipsychotics.htm.
Drug brand names
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zimmermann U, Kraus T, Himmerich H, et al. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 2003;37(3):193-220.
2. Ryan MC, Thakore JH. Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sci 2002;71(3):239-57.
3. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005;293(5):596-608.
4. Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry 2004;65(suppl 2):5-99.
5. Schneider LS, Ismail MS, Dagerman K, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophr Bull 2003;29(1):57-72.
6. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
7. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ 2002;167(11):1269-1270.
8. Herrmann N, Mamdani M, Lanctot KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry 2004;161(6):1113-1115.
9. Herrmann N, Lanctot KL. Do atypical antipsychotics cause stroke? CNS Drugs 2005;19(2):91-103.
10. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330(7489):445.-
11. Straus SM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004;164(12):1293-7.
In early-stage Alzheimer’s disease, Mrs. P enters a nursing home because her daughter, who usually takes care of her, is hospitalized for cancer chemotherapy. Mrs. P promptly develops paranoid delusions and refuses her medications for high blood pressure and high cholesterol. What are the treatment options? Is any one antipsychotic safer than another?
Writing an antipsychotic prescription for patients such as Mrs. P is no longer a quick scribble. First we learned that atypicals may alter glucose and lipid metabolism in clinically troublesome ways.1,2 Then we learned that antipsychotics can triple the risk for sudden death in older patients with dementia. (See FDA advisory, Related resources.)
How great are the risks, who is at risk, and how strong is the evidence for these new risks? The debate is not yet settled, but the boundaries of good practice for antipsychotic use in older patients are being redrawn. This is particularly true for those with dementia, in whom antipsychotics’ risk/benefit ratio is higher than for older patients with schizophrenia or bipolar disorder.
Unproven effectiveness
Antipsychotics are not FDA-approved for treating dementia-related psychosis. Though antipsychotics are commonly used off-label to treat behavioral disturbances in the elderly with dementia, no standard of care exists for managing these symptoms with drugs. So far, the evidence for antipsychotics’ effectiveness for dementia’s behavioral and psychological symptoms is spotty at best.
Sink et al (Table 1)3 systematically reviewed 14 randomized, controlled trials and concluded “there is no clear evidence that typical antipsychotics are useful for treating neuropsychiatric symptoms [of dementia].” They concluded from 6 studies of atypicals that only olanzapine and risperidone had shown efficacy, but the effects were “modest and further complicated by risk of stroke.” When the benefits are modest, the risks are more difficult to justify.
When medication is necessary, on the other hand, the Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients reported in 2004 that “for agitated dementia with delusions, the experts’ first-line recommendation is an antipsychotic drug alone…. Risperidone (0.5 to 2.0 mg/day) was first line, followed by quetiapine (50 to 150 mg/day) and olanzapine (5.0 to 7.5 mg/day) as high second-line options.”4
CATIE studies. The definitive prospective study of antipsychotics’ effectiveness in dementia has not been completed. The National Institute of Mental Health is sponsoring CATIE—Clinical Antipsychotic Trials of Intervention Effectiveness—a multi-site research program comparing the effectiveness and outcomes of antipsychotics in treating schizophrenia and Alzheimer’s disease. Results of the Alzheimer disease arm5 are expected next year.
The schizophrenia arm comparing four atypical antipsychotics (quetiapine, risperidone, ziprasidone, and olanzapine) and one typical antipsychotic (perphenazine) found:
- Typical and atypical antipsychotics were similarly effective in 1,493 patients with chronic schizophrenia
- 74% of patients discontinued assigned medications before 18 months for lack of efficacy, intolerable side effects, or other reasons.6
Table 1
Timeline: Evidence on risks, efficacy of atypical antipsychotics
| Year | Summary of study findings, FDA warnings |
|---|---|
| 2002 | Higher incidence of stroke seen with risperidone than with placebo in two of four clinical trials (Wooltorton7) Health Canada and Janssen-Ortho warn Canadian physicians of possible link between risperidone and stroke |
| 2003 | FDA warns of increased risk of stroke with risperidone |
| 2004 | Threefold increased risk of sudden cardiac death associated with antipsychotic use in patients age >65, most without dementia (Straus et al11) |
| 2005 | Stroke risk reported no greater in older patients who took atypicals than in those who took typical antipsychotics (Gill et al10) Analysis of 14 controlled trials finds “no clear evidence” that typical antipsychotics are effective in dementia; atypicals’ effects seen as “modest” (Sink et al3) FDA issues warning after finding 60% increase in risk of sudden death in review of 17 trials in older patients receiving atypical antipsychotics for dementia Efficacy of conventional and atypical antipsychotics found similar in patients with chronic schizophrenia (Lieberman et al6) |
Stroke risk
How strong is the evidence for stroke risk among older patients who take antipsychotics? Wooltorton7 first raised concern about increased risk of stroke with risperidone in 2002 in a summary of four clinical trials. Though none was designed to examine stroke risk as the primary outcome, two showed significantly higher incidence of cerebrovascular events with risperidone than with placebo. The stroke rate with risperidone was double that with placebo (4% vs 2%) across the total 1,200 subjects in the four studies.
This preliminary report led to an FDA advisory but surprisingly no definitive studies or systematic reviews. No epidemiologic studies have examined stroke rates in those who take antipsychotics compared with those who don’t, while controlling for common stroke predictors. So we have a warning based on post hoc analyses in two positive and two negative studies, but no sound estimate of how much antipsychotic use in general adds to the risk of stroke.
Atypicals vs. typicals. Are atypicals worse than typicals in their effect on stroke risk?
Herrmann et al8,9 reviewed a health care database of 11,400 older persons and found no statistically significant increase in stroke rate with risperidone or olanzapine compared with typical antipsychotics. This study did not assess whether patients had dementia or primary psychotic disorders.
In a larger retrospective study of 32,710 older persons with dementia, Herrmann’s group10 found no greater stroke risk in those who took atypicals than in those who took typical antipsychotics.
So the evidence for the risk of stroke in older patients who take antipsychotics is based on a few reports and no definitive studies.
Risk of early death
What about the risk of early death? After reviewing 17 clinical trials of atypical antipsychotics in older patients with dementia, the FDA issued its warning in April 2005 about increased mortality risk (see Related resources). Fifteen of the trials showed an increased mortality risk, resulting in an estimated 1.6- to 1.7-fold increase in risk of death, mostly by cardiac or infectious causes.
A year earlier, Straus et al11 reported on risk of sudden cardiac death with antipsychotic use in a population-based, case-control study in the Netherlands. In this longitudinal, observational database of 250,000 patients, 75% were age >65 and <1% had dementia. Current use of antipsychotics was associated with a threefold increase in risk of sudden cardiac death (554 cases), after other predictors of sudden death were factored in. This risk increased with higher antipsychotic dosages and was similarly elevated for patients with and without schizophrenia-related disorders.
Mechanisms unknown
By what mechanisms could antipsychotics precipitate stroke, sudden cardiac death, or pneumonia? A clear biological mechanism has not been proposed, much less proven. The risks seen in clinical trials—usually lasting 12 weeks or less—suggest an acute effect rather than the more-gradual consequences of weight gain, altered lipid metabolism, or diabetes.
Speculations range widely. Possibilities awaiting study include postural hypotension, altered platelet aggregation, increased venous thromboembolism, peripheral vasodilation leading to cardiovascular collapse, acute dystonia, acute cardiomyopathy, arrhythmias related to QT prolongation, and other forms of cardiac toxicity.10,11
Remaining questions
The few studies plus two FDA advisories force clinicians to make complex treatment decisions on insufficient evidence. Here’s what we don’t know:
- The magnitude of stroke or sudden death risk in older patients who take antipsychotics for any diagnosis. (All studies have limitations, and public health policy should not rely on one or two studies, no matter how good they may be.)
- Who is at higher or lower risk with antipsychotic use—men vs women? blacks vs whites?etc.
- Biological mechanisms of an association between antipsychotics and risks of stroke and premature death.
Strategies for reducing risk
We can minimize patients’ clinical risk and our legal risk only by using the limited evidence, expert consensus, and sound clinical judgment. Suggested strategies are listed in Table 2.
Table 2
Strategies to minimize antipsychotic risk in patients age 65 and older
| Review and document risk factors for cardiovascular disease—including stroke—with physical examination, laboratory tests (lipid profile, fasting glucose), and ECG in consultation with a primary care physician or specialist |
| Try nonpharmacologic approaches first whenever possible to manage behavioral disturbances in patients with dementia; document results before trying an antipsychotic |
| Review antipsychotics’ risks and benefits with patient and family |
| Use low dosages and increase gradually, as sudden death risk is dose-related |
| Monitor antipsychotic effectiveness, and discontinue trials of questionable benefit |
| Monitor cardiovascular symptoms, heart rate, blood pressure and body mass index of patients with cardiovascular risk |
| Avoid using sedating antipsychotics for insomnia in patients without psychiatric disorders |

Dr. Wulsin treats psychiatric outpatients and has published reviews on depression and heart disease. He is training director, University of Cincinnati family medicine-psychiatry residency program.
- Food and Drug Administration. Advisory on increased risk of mortality with use of atypical antipsychotics to treat behavioral disturbances in older patients with dementia. www.fda.gov/cder/drug/advisory/antipsychotics.htm.
Drug brand names
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In early-stage Alzheimer’s disease, Mrs. P enters a nursing home because her daughter, who usually takes care of her, is hospitalized for cancer chemotherapy. Mrs. P promptly develops paranoid delusions and refuses her medications for high blood pressure and high cholesterol. What are the treatment options? Is any one antipsychotic safer than another?
Writing an antipsychotic prescription for patients such as Mrs. P is no longer a quick scribble. First we learned that atypicals may alter glucose and lipid metabolism in clinically troublesome ways.1,2 Then we learned that antipsychotics can triple the risk for sudden death in older patients with dementia. (See FDA advisory, Related resources.)
How great are the risks, who is at risk, and how strong is the evidence for these new risks? The debate is not yet settled, but the boundaries of good practice for antipsychotic use in older patients are being redrawn. This is particularly true for those with dementia, in whom antipsychotics’ risk/benefit ratio is higher than for older patients with schizophrenia or bipolar disorder.
Unproven effectiveness
Antipsychotics are not FDA-approved for treating dementia-related psychosis. Though antipsychotics are commonly used off-label to treat behavioral disturbances in the elderly with dementia, no standard of care exists for managing these symptoms with drugs. So far, the evidence for antipsychotics’ effectiveness for dementia’s behavioral and psychological symptoms is spotty at best.
Sink et al (Table 1)3 systematically reviewed 14 randomized, controlled trials and concluded “there is no clear evidence that typical antipsychotics are useful for treating neuropsychiatric symptoms [of dementia].” They concluded from 6 studies of atypicals that only olanzapine and risperidone had shown efficacy, but the effects were “modest and further complicated by risk of stroke.” When the benefits are modest, the risks are more difficult to justify.
When medication is necessary, on the other hand, the Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients reported in 2004 that “for agitated dementia with delusions, the experts’ first-line recommendation is an antipsychotic drug alone…. Risperidone (0.5 to 2.0 mg/day) was first line, followed by quetiapine (50 to 150 mg/day) and olanzapine (5.0 to 7.5 mg/day) as high second-line options.”4
CATIE studies. The definitive prospective study of antipsychotics’ effectiveness in dementia has not been completed. The National Institute of Mental Health is sponsoring CATIE—Clinical Antipsychotic Trials of Intervention Effectiveness—a multi-site research program comparing the effectiveness and outcomes of antipsychotics in treating schizophrenia and Alzheimer’s disease. Results of the Alzheimer disease arm5 are expected next year.
The schizophrenia arm comparing four atypical antipsychotics (quetiapine, risperidone, ziprasidone, and olanzapine) and one typical antipsychotic (perphenazine) found:
- Typical and atypical antipsychotics were similarly effective in 1,493 patients with chronic schizophrenia
- 74% of patients discontinued assigned medications before 18 months for lack of efficacy, intolerable side effects, or other reasons.6
Table 1
Timeline: Evidence on risks, efficacy of atypical antipsychotics
| Year | Summary of study findings, FDA warnings |
|---|---|
| 2002 | Higher incidence of stroke seen with risperidone than with placebo in two of four clinical trials (Wooltorton7) Health Canada and Janssen-Ortho warn Canadian physicians of possible link between risperidone and stroke |
| 2003 | FDA warns of increased risk of stroke with risperidone |
| 2004 | Threefold increased risk of sudden cardiac death associated with antipsychotic use in patients age >65, most without dementia (Straus et al11) |
| 2005 | Stroke risk reported no greater in older patients who took atypicals than in those who took typical antipsychotics (Gill et al10) Analysis of 14 controlled trials finds “no clear evidence” that typical antipsychotics are effective in dementia; atypicals’ effects seen as “modest” (Sink et al3) FDA issues warning after finding 60% increase in risk of sudden death in review of 17 trials in older patients receiving atypical antipsychotics for dementia Efficacy of conventional and atypical antipsychotics found similar in patients with chronic schizophrenia (Lieberman et al6) |
Stroke risk
How strong is the evidence for stroke risk among older patients who take antipsychotics? Wooltorton7 first raised concern about increased risk of stroke with risperidone in 2002 in a summary of four clinical trials. Though none was designed to examine stroke risk as the primary outcome, two showed significantly higher incidence of cerebrovascular events with risperidone than with placebo. The stroke rate with risperidone was double that with placebo (4% vs 2%) across the total 1,200 subjects in the four studies.
This preliminary report led to an FDA advisory but surprisingly no definitive studies or systematic reviews. No epidemiologic studies have examined stroke rates in those who take antipsychotics compared with those who don’t, while controlling for common stroke predictors. So we have a warning based on post hoc analyses in two positive and two negative studies, but no sound estimate of how much antipsychotic use in general adds to the risk of stroke.
Atypicals vs. typicals. Are atypicals worse than typicals in their effect on stroke risk?
Herrmann et al8,9 reviewed a health care database of 11,400 older persons and found no statistically significant increase in stroke rate with risperidone or olanzapine compared with typical antipsychotics. This study did not assess whether patients had dementia or primary psychotic disorders.
In a larger retrospective study of 32,710 older persons with dementia, Herrmann’s group10 found no greater stroke risk in those who took atypicals than in those who took typical antipsychotics.
So the evidence for the risk of stroke in older patients who take antipsychotics is based on a few reports and no definitive studies.
Risk of early death
What about the risk of early death? After reviewing 17 clinical trials of atypical antipsychotics in older patients with dementia, the FDA issued its warning in April 2005 about increased mortality risk (see Related resources). Fifteen of the trials showed an increased mortality risk, resulting in an estimated 1.6- to 1.7-fold increase in risk of death, mostly by cardiac or infectious causes.
A year earlier, Straus et al11 reported on risk of sudden cardiac death with antipsychotic use in a population-based, case-control study in the Netherlands. In this longitudinal, observational database of 250,000 patients, 75% were age >65 and <1% had dementia. Current use of antipsychotics was associated with a threefold increase in risk of sudden cardiac death (554 cases), after other predictors of sudden death were factored in. This risk increased with higher antipsychotic dosages and was similarly elevated for patients with and without schizophrenia-related disorders.
Mechanisms unknown
By what mechanisms could antipsychotics precipitate stroke, sudden cardiac death, or pneumonia? A clear biological mechanism has not been proposed, much less proven. The risks seen in clinical trials—usually lasting 12 weeks or less—suggest an acute effect rather than the more-gradual consequences of weight gain, altered lipid metabolism, or diabetes.
Speculations range widely. Possibilities awaiting study include postural hypotension, altered platelet aggregation, increased venous thromboembolism, peripheral vasodilation leading to cardiovascular collapse, acute dystonia, acute cardiomyopathy, arrhythmias related to QT prolongation, and other forms of cardiac toxicity.10,11
Remaining questions
The few studies plus two FDA advisories force clinicians to make complex treatment decisions on insufficient evidence. Here’s what we don’t know:
- The magnitude of stroke or sudden death risk in older patients who take antipsychotics for any diagnosis. (All studies have limitations, and public health policy should not rely on one or two studies, no matter how good they may be.)
- Who is at higher or lower risk with antipsychotic use—men vs women? blacks vs whites?etc.
- Biological mechanisms of an association between antipsychotics and risks of stroke and premature death.
Strategies for reducing risk
We can minimize patients’ clinical risk and our legal risk only by using the limited evidence, expert consensus, and sound clinical judgment. Suggested strategies are listed in Table 2.
Table 2
Strategies to minimize antipsychotic risk in patients age 65 and older
| Review and document risk factors for cardiovascular disease—including stroke—with physical examination, laboratory tests (lipid profile, fasting glucose), and ECG in consultation with a primary care physician or specialist |
| Try nonpharmacologic approaches first whenever possible to manage behavioral disturbances in patients with dementia; document results before trying an antipsychotic |
| Review antipsychotics’ risks and benefits with patient and family |
| Use low dosages and increase gradually, as sudden death risk is dose-related |
| Monitor antipsychotic effectiveness, and discontinue trials of questionable benefit |
| Monitor cardiovascular symptoms, heart rate, blood pressure and body mass index of patients with cardiovascular risk |
| Avoid using sedating antipsychotics for insomnia in patients without psychiatric disorders |

Dr. Wulsin treats psychiatric outpatients and has published reviews on depression and heart disease. He is training director, University of Cincinnati family medicine-psychiatry residency program.
- Food and Drug Administration. Advisory on increased risk of mortality with use of atypical antipsychotics to treat behavioral disturbances in older patients with dementia. www.fda.gov/cder/drug/advisory/antipsychotics.htm.
Drug brand names
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zimmermann U, Kraus T, Himmerich H, et al. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 2003;37(3):193-220.
2. Ryan MC, Thakore JH. Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sci 2002;71(3):239-57.
3. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005;293(5):596-608.
4. Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry 2004;65(suppl 2):5-99.
5. Schneider LS, Ismail MS, Dagerman K, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophr Bull 2003;29(1):57-72.
6. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
7. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ 2002;167(11):1269-1270.
8. Herrmann N, Mamdani M, Lanctot KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry 2004;161(6):1113-1115.
9. Herrmann N, Lanctot KL. Do atypical antipsychotics cause stroke? CNS Drugs 2005;19(2):91-103.
10. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330(7489):445.-
11. Straus SM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004;164(12):1293-7.
1. Zimmermann U, Kraus T, Himmerich H, et al. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 2003;37(3):193-220.
2. Ryan MC, Thakore JH. Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sci 2002;71(3):239-57.
3. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005;293(5):596-608.
4. Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry 2004;65(suppl 2):5-99.
5. Schneider LS, Ismail MS, Dagerman K, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophr Bull 2003;29(1):57-72.
6. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
7. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ 2002;167(11):1269-1270.
8. Herrmann N, Mamdani M, Lanctot KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry 2004;161(6):1113-1115.
9. Herrmann N, Lanctot KL. Do atypical antipsychotics cause stroke? CNS Drugs 2005;19(2):91-103.
10. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330(7489):445.-
11. Straus SM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004;164(12):1293-7.
The skinny on one patient’s psychosis
Presentation: ‘they’re stalking me’
Ms. P, age 30, fears she is being stalked and is too terrified to be home alone. Worried, her ex-boyfriend calls police, who bring her to the emergency room
At the ER, Ms. P reports that surveillance cameras have been planted inside her house, that men often stand on her roof and watch her go to her car, and that men constantly are stalking her. She also hears voices and reports frightening peripheral visions of “outsiders.” The ER doctor consults the psychiatry service and orders laboratory tests, but all results—including urine drug screen findings—are negative.
Ms. P says she has been sleeping 3 to 4 hours nightly. She acknowledges depressed mood and decreased appetite, leading to a 10-lb weight loss over 1 month. She says she has felt depressed off and on for several years but has received no treatment for her mood symptoms. We admit her to the psychiatric unit to treat her acute-onset psychosis.
Lately, Ms. P’s life has been difficult. A college sophomore, she is failing all her classes. She was recently fired from her job as a case manager because of inappropriate behavior, such as buying gifts for the children she was managing and taking them for hair-cuts without their parents’ permission. Several months ago, she broke up with her boyfriend of 6 years. In addition to these stressors, she recently moved into an apartment and for the first time was living on her own.
Medical history. Ms. P has no major medical problems. Her mother has battled alcohol and drug dependence and depression but to Ms. P’s knowledge has never experienced psychosis. Ms. P, who admits that she binge drinks once or twice monthly, meets DSM-IV-TR criteria for alcohol abuse disorder. She denies using illicit drugs but admits that she regularly takes “energy pills” purchased over the Internet because she cannot wake up without them.
Physical exam is normal, but Ms. P’s body mass index (BMI) is 18 kg/m2, slightly below normal (height: 5 feet 8 inches; weight: 117.5 lb).
The authors’ observations
We diagnosed Ms. P as having recurrent and severe major depressive disorder with psychotic features because of her longstanding depressive symptoms. We considered substance-induced psychosis, but her urine drug screen is negative.
Treatment at this point should address both the paranoid delusions and depressive symptoms.
Treatment: starved for energy
We start haloperidol, 1 mg nightly, to treat Ms. P’s paranoid delusions, and mirtazapine, 15 mg nightly, to improve her sleep. We choose mirtazapine—which can increase appetite and lead to weight gain—because Ms. P is underweight. We also choose haloperidol because Ms. P is unemployed and cannot afford a second-generation antipsychotic.
Shortly afterward, we interview Ms. P’s ex-boyfriend. He tells us that she has been using diet pills regularly for 3 to 4 years, and that her chronic use has been escalating by the month. Lately, he says, she has been “popping the pills like candy.”
When we ask Ms. P about her diet pill use, she says she had mainly been using Xenadrine, an over-the-counter weight-loss supplement. Five months ago, she also started taking prescription phentermine, which she purchases over the Internet. She says that before her hospitalization, she was taking three phentermine tablets daily to boost her energy.
According to her ex-boyfriend, Ms. P began showing signs of psychosis 3 to 4 weeks after starting phentermine, and Ms. P notes that her initial paranoia and gustatory hallucinations have worsened. She now fears her bathroom is rigged with cameras. She showers with her swimsuit on.
We change Ms. P’s diagnosis to diet pill-induced psychosis. Because she had discarded the pill packaging before admission, we could not examine it for dosing information or ingredients.
The authors’ observations
Differentiating drug-induced psychosis from other psychoses often is difficult. Mood disorder with psychosis, schizophrenia, and substance-induced psychosis have similar characteristics (Table).
Ms. P has no personal or family history of psychosis that would suggest a thought disorder. She had good pre-morbid functioning (going to college, steady employment, long-term relationship with boyfriend) before her psychosis onset. She did, however, have a personal and family history of depression and was confronting many stressors (losing her job, failing grades at school, breaking up with her longtime boyfriend) that would suggest a primary mood disorder with psychosis.
We suspected an eating disorder and asked Ms. P more than once about her eating habits, but she insists she does not take the pills to lose weight. Also, her ex-boyfriend believes she is eating normally. Her low BMI and suspected obsession with weight loss could have signaled anorexia nervosa, but no other signs were present and her history does not support the diagnosis.
Table
Three causes of psychosis—and different characteristics of each presentation
| Characteristic | Mood disorder with psychosis | Schizophrenia | Substance-induced psychosis |
|---|---|---|---|
| Acute onset | x | - | x |
| Delusions | x | x | x |
| Disorganized or catatonic behavior | x | x | x |
| Family history of psychosis | x | x | _ |
| Good premorbid function | x | _ | x |
| Hallucinations | x | x | x |
| Negative symptoms | x | x | x |
| Personal history of psychosis | x | x | _ |
| Prodromal and residual symptoms | _ | x | _ |
Relapse: cameras ‘off’ for 1 week
Five days after admission, we discharge Ms. P as her psychosis has improved significantly.
Later that day at the outpatient clinic, Ms. P requests a medication change, voicing fears about haloperidol’s long term side effects and mirtazapine-induced weight gain. Risperidone, 2 mg nightly, and citalopram, 20 mg/d, are started instead.
One week later, Ms. P’s parents again bring her to the ER after police find her sitting in her car, confused and paranoid. She complains that cameras have been set up in her car, and she responds to voices when alone.
On the way to the ER, Ms. P tries to jump from the moving car. She assaults her mother as she stops her from jumping.
Blood pressure is 155/92, heart rate is 82 beats per minute, respiratory rate is 18 breaths per minute, and temperature is 96°F.
On interview, Ms. P admits that she stopped risperidone and citalopram and restarted Xenadrine and phentermine. She also reports orthostasis from risperidone. We again admit her to the acute-care psychiatric unit and restart haloperidol, 1 mg/d, and citalopram, 20 mg/d.
The authors’ observations
Although we knew Ms. P was abusing diet pills, we could have easily ruled out drug-induced psychosis based on her three negative urine drug screens.
The clinical course of Ms. P’s psychosis, however, closely followed her diet pill use—emerging soon after starting phentermine and remitting soon after stopping it. Also:
- she was taking 2 to 3 times the recommended dosage of phentermine for several months. Phentermine is indicated for short-term (a few weeks) treatment of exogenous obesity (BMI ≥27 kg/m2 in persons with hypertension, diabetes, or hyperlipidemia; BMI ≥30 kg/m2 in persons without these risk factors)1
- her BMI was below normal
- her psychosis remains in remission without use of an antipsychotic.
Stimulant medications such as amphetamines and stimulant drugs such as cocaine can produce psychotic symptoms including paranoid delusions, hallucinations, and bizarre behavior. Farrell and colleagues5 found that cannabis and psychostimulants increase the risk of psychosis.
Genetic load could have influenced Ms. P’s response to diet pills, but we have no information to support a genetic predisposition. Also, we saw no clear family history of a formal thought disorder.
The authors’ observations
Urine drug screens can pick up the main drug classes and often their derivatives, but this testing method is limited.2
Urine tests employ assays with semi-quantitative results. A urine sample may contain an abused substance but at levels below the cutoff. Also, because no correlation exists between cutoff levels and drug effect, a patient can have drug-induced symptoms but a negative urine drug screen. This makes detecting a suspected but unknown drug of abuse extremely difficult.
A routine urine screen can detect phentermine and other stimulants, but the phentermine level needed for a positive assay is 50 times that of pure amphetamine.2 Ms. P’s last urine drug screen showed an amphetamine level just under the cutoff.
Use of cocaine—undetectable in urine 3 to 4 days after use—could be considered when drug-induced psychosis is suspected. Ms. P’s psychosis correlated with her phentermine relapse, however, and both she and her ex-boyfriend denied that she uses street drugs.
Obtain specific drug levels when you suspect medication abuse. Request gas chromatography or mass spectrometry to provide a quantitative result and confirm medication abuse.2,3 These tests would have been appropriate for Ms. P once her ex-boyfriend revealed the diet pill abuse.
Detecting diet pill abuse
Use of weight-loss supplements and appetite suppressants is alarmingly common (Box). Many patients suffer adverse effects from diet pills but do not tell their doctors they are using them because they:
- fear the physician will scold them for circumventing his or her advice by obtaining medications online
- sense that obtaining diet pills over the Internet might be illegal
- do not realize the doctor needs to know about nonprescription drug use
- or fear the physician will tell them to stop taking the drug.
Rapid or unexplained weight loss, hypertension, tachycardia, tremors, psychomotor agitation, and hyperalertness could signal diet pill abuse. Emotional lability, such as euphoria during a high and fatigue and dysphoria during withdrawal, also could be indicative. Collateral information from family members or significant others can narrow the differential diagnosis.
Cognitive-behavioral therapy (CBT) can help Ms. P, who claimed she used diet pills to boost her energy. CBT would challenge her unrealistically high goals, teach and explain the consequences of drug use, and offer support to reinforce abstinence from diet pills. Educating patients about potential adverse drug effects also is essential.
Use of prescription and over-the-counter weight-loss products is alarmingly common. American culture values the “perfect body,” and the Internet has made appetite suppressants and weight-loss agents more available. Users can conveniently purchase large quantities of OTC weight-loss aids online.
In one multi-state survey,4 18% of women and 8% of men who were trying to lose weight reported using nonprescription weight loss products. Also:
- 28.4% of obese women (defined as BMI ≥30 kg/m2) reported using OTC diet pills, as did nearly 8% of women at normal weight (BMI 2)
- concomitant nonprescription and prescription pill use was often reported.
Conclusion: back to baseline
After 10 weeks, Ms. P’s condition returns to baseline. She starts a new job and abstains from diet pills. Her thought process and cognition improve significantly, and she reports no depressive symptoms at her most-recent visit. She maintains her weight at 139 lb. BMI is 21.1kg/m2 (normal).
Haloperidol is slowly tapered across 2 weeks with no return of psychosis. Although Ms. P wants to stop haloperidol, we taper instead to guard against psychotic relapse. She continues to take citalopram, 20 mg/d, to prevent depressive symptom re-emergence and is receiving supportive psychotherapy to aid her relapse prevention.
Related resources
- Supplement Research Foundation. Supplement reviews. www.tsrf.com/supplements.htm
- Devan GS. Phentermine and Psychosis. Br J Psychiatry 1990;156:442-3.
- Cleare AJ. Phentermine, psychosis, and family history. J Clin Psychopharmacol 1996;16:470-1.
- Hoffman BF. Diet pill psychosis. CMAJ 1977;116:351-5.
- Citalopram • Celexa
- Haloperidol • Haldol
- Mirtazapine • Remeron
- Phenteramine • Adipex
- Risperidone • Risperdal
Dr. Khan is a speaker for Pfizer and Wyeth Pharmaceuticals.
Drs. Tan and Williamson report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 2003;289:1537-45.
2. Shindelman J, Mahal J, Hemphill G, et al. Development and evaluation of an improved method for screening of amphetamines. J Anal Toxicol 1999;23:506-10.
3. Crosby RD, Carlson GA, Specker SM. Simulation of drug use and urine screening patterns. J Addict Dis 2003;22:89-98.
4. Blanck HM, Khan LK, Serdula Mk. Use of nonprescription weight loss products: results from a multistate survey. JAMA 2001;286:930-5.
5. Farrell M, Boys A, Bebbington P, et al. Psychosis and drug dependence: results from a national survey of prisoners. Br J Psychiatry 2002;181:393-8.
Presentation: ‘they’re stalking me’
Ms. P, age 30, fears she is being stalked and is too terrified to be home alone. Worried, her ex-boyfriend calls police, who bring her to the emergency room
At the ER, Ms. P reports that surveillance cameras have been planted inside her house, that men often stand on her roof and watch her go to her car, and that men constantly are stalking her. She also hears voices and reports frightening peripheral visions of “outsiders.” The ER doctor consults the psychiatry service and orders laboratory tests, but all results—including urine drug screen findings—are negative.
Ms. P says she has been sleeping 3 to 4 hours nightly. She acknowledges depressed mood and decreased appetite, leading to a 10-lb weight loss over 1 month. She says she has felt depressed off and on for several years but has received no treatment for her mood symptoms. We admit her to the psychiatric unit to treat her acute-onset psychosis.
Lately, Ms. P’s life has been difficult. A college sophomore, she is failing all her classes. She was recently fired from her job as a case manager because of inappropriate behavior, such as buying gifts for the children she was managing and taking them for hair-cuts without their parents’ permission. Several months ago, she broke up with her boyfriend of 6 years. In addition to these stressors, she recently moved into an apartment and for the first time was living on her own.
Medical history. Ms. P has no major medical problems. Her mother has battled alcohol and drug dependence and depression but to Ms. P’s knowledge has never experienced psychosis. Ms. P, who admits that she binge drinks once or twice monthly, meets DSM-IV-TR criteria for alcohol abuse disorder. She denies using illicit drugs but admits that she regularly takes “energy pills” purchased over the Internet because she cannot wake up without them.
Physical exam is normal, but Ms. P’s body mass index (BMI) is 18 kg/m2, slightly below normal (height: 5 feet 8 inches; weight: 117.5 lb).
The authors’ observations
We diagnosed Ms. P as having recurrent and severe major depressive disorder with psychotic features because of her longstanding depressive symptoms. We considered substance-induced psychosis, but her urine drug screen is negative.
Treatment at this point should address both the paranoid delusions and depressive symptoms.
Treatment: starved for energy
We start haloperidol, 1 mg nightly, to treat Ms. P’s paranoid delusions, and mirtazapine, 15 mg nightly, to improve her sleep. We choose mirtazapine—which can increase appetite and lead to weight gain—because Ms. P is underweight. We also choose haloperidol because Ms. P is unemployed and cannot afford a second-generation antipsychotic.
Shortly afterward, we interview Ms. P’s ex-boyfriend. He tells us that she has been using diet pills regularly for 3 to 4 years, and that her chronic use has been escalating by the month. Lately, he says, she has been “popping the pills like candy.”
When we ask Ms. P about her diet pill use, she says she had mainly been using Xenadrine, an over-the-counter weight-loss supplement. Five months ago, she also started taking prescription phentermine, which she purchases over the Internet. She says that before her hospitalization, she was taking three phentermine tablets daily to boost her energy.
According to her ex-boyfriend, Ms. P began showing signs of psychosis 3 to 4 weeks after starting phentermine, and Ms. P notes that her initial paranoia and gustatory hallucinations have worsened. She now fears her bathroom is rigged with cameras. She showers with her swimsuit on.
We change Ms. P’s diagnosis to diet pill-induced psychosis. Because she had discarded the pill packaging before admission, we could not examine it for dosing information or ingredients.
The authors’ observations
Differentiating drug-induced psychosis from other psychoses often is difficult. Mood disorder with psychosis, schizophrenia, and substance-induced psychosis have similar characteristics (Table).
Ms. P has no personal or family history of psychosis that would suggest a thought disorder. She had good pre-morbid functioning (going to college, steady employment, long-term relationship with boyfriend) before her psychosis onset. She did, however, have a personal and family history of depression and was confronting many stressors (losing her job, failing grades at school, breaking up with her longtime boyfriend) that would suggest a primary mood disorder with psychosis.
We suspected an eating disorder and asked Ms. P more than once about her eating habits, but she insists she does not take the pills to lose weight. Also, her ex-boyfriend believes she is eating normally. Her low BMI and suspected obsession with weight loss could have signaled anorexia nervosa, but no other signs were present and her history does not support the diagnosis.
Table
Three causes of psychosis—and different characteristics of each presentation
| Characteristic | Mood disorder with psychosis | Schizophrenia | Substance-induced psychosis |
|---|---|---|---|
| Acute onset | x | - | x |
| Delusions | x | x | x |
| Disorganized or catatonic behavior | x | x | x |
| Family history of psychosis | x | x | _ |
| Good premorbid function | x | _ | x |
| Hallucinations | x | x | x |
| Negative symptoms | x | x | x |
| Personal history of psychosis | x | x | _ |
| Prodromal and residual symptoms | _ | x | _ |
Relapse: cameras ‘off’ for 1 week
Five days after admission, we discharge Ms. P as her psychosis has improved significantly.
Later that day at the outpatient clinic, Ms. P requests a medication change, voicing fears about haloperidol’s long term side effects and mirtazapine-induced weight gain. Risperidone, 2 mg nightly, and citalopram, 20 mg/d, are started instead.
One week later, Ms. P’s parents again bring her to the ER after police find her sitting in her car, confused and paranoid. She complains that cameras have been set up in her car, and she responds to voices when alone.
On the way to the ER, Ms. P tries to jump from the moving car. She assaults her mother as she stops her from jumping.
Blood pressure is 155/92, heart rate is 82 beats per minute, respiratory rate is 18 breaths per minute, and temperature is 96°F.
On interview, Ms. P admits that she stopped risperidone and citalopram and restarted Xenadrine and phentermine. She also reports orthostasis from risperidone. We again admit her to the acute-care psychiatric unit and restart haloperidol, 1 mg/d, and citalopram, 20 mg/d.
The authors’ observations
Although we knew Ms. P was abusing diet pills, we could have easily ruled out drug-induced psychosis based on her three negative urine drug screens.
The clinical course of Ms. P’s psychosis, however, closely followed her diet pill use—emerging soon after starting phentermine and remitting soon after stopping it. Also:
- she was taking 2 to 3 times the recommended dosage of phentermine for several months. Phentermine is indicated for short-term (a few weeks) treatment of exogenous obesity (BMI ≥27 kg/m2 in persons with hypertension, diabetes, or hyperlipidemia; BMI ≥30 kg/m2 in persons without these risk factors)1
- her BMI was below normal
- her psychosis remains in remission without use of an antipsychotic.
Stimulant medications such as amphetamines and stimulant drugs such as cocaine can produce psychotic symptoms including paranoid delusions, hallucinations, and bizarre behavior. Farrell and colleagues5 found that cannabis and psychostimulants increase the risk of psychosis.
Genetic load could have influenced Ms. P’s response to diet pills, but we have no information to support a genetic predisposition. Also, we saw no clear family history of a formal thought disorder.
The authors’ observations
Urine drug screens can pick up the main drug classes and often their derivatives, but this testing method is limited.2
Urine tests employ assays with semi-quantitative results. A urine sample may contain an abused substance but at levels below the cutoff. Also, because no correlation exists between cutoff levels and drug effect, a patient can have drug-induced symptoms but a negative urine drug screen. This makes detecting a suspected but unknown drug of abuse extremely difficult.
A routine urine screen can detect phentermine and other stimulants, but the phentermine level needed for a positive assay is 50 times that of pure amphetamine.2 Ms. P’s last urine drug screen showed an amphetamine level just under the cutoff.
Use of cocaine—undetectable in urine 3 to 4 days after use—could be considered when drug-induced psychosis is suspected. Ms. P’s psychosis correlated with her phentermine relapse, however, and both she and her ex-boyfriend denied that she uses street drugs.
Obtain specific drug levels when you suspect medication abuse. Request gas chromatography or mass spectrometry to provide a quantitative result and confirm medication abuse.2,3 These tests would have been appropriate for Ms. P once her ex-boyfriend revealed the diet pill abuse.
Detecting diet pill abuse
Use of weight-loss supplements and appetite suppressants is alarmingly common (Box). Many patients suffer adverse effects from diet pills but do not tell their doctors they are using them because they:
- fear the physician will scold them for circumventing his or her advice by obtaining medications online
- sense that obtaining diet pills over the Internet might be illegal
- do not realize the doctor needs to know about nonprescription drug use
- or fear the physician will tell them to stop taking the drug.
Rapid or unexplained weight loss, hypertension, tachycardia, tremors, psychomotor agitation, and hyperalertness could signal diet pill abuse. Emotional lability, such as euphoria during a high and fatigue and dysphoria during withdrawal, also could be indicative. Collateral information from family members or significant others can narrow the differential diagnosis.
Cognitive-behavioral therapy (CBT) can help Ms. P, who claimed she used diet pills to boost her energy. CBT would challenge her unrealistically high goals, teach and explain the consequences of drug use, and offer support to reinforce abstinence from diet pills. Educating patients about potential adverse drug effects also is essential.
Use of prescription and over-the-counter weight-loss products is alarmingly common. American culture values the “perfect body,” and the Internet has made appetite suppressants and weight-loss agents more available. Users can conveniently purchase large quantities of OTC weight-loss aids online.
In one multi-state survey,4 18% of women and 8% of men who were trying to lose weight reported using nonprescription weight loss products. Also:
- 28.4% of obese women (defined as BMI ≥30 kg/m2) reported using OTC diet pills, as did nearly 8% of women at normal weight (BMI 2)
- concomitant nonprescription and prescription pill use was often reported.
Conclusion: back to baseline
After 10 weeks, Ms. P’s condition returns to baseline. She starts a new job and abstains from diet pills. Her thought process and cognition improve significantly, and she reports no depressive symptoms at her most-recent visit. She maintains her weight at 139 lb. BMI is 21.1kg/m2 (normal).
Haloperidol is slowly tapered across 2 weeks with no return of psychosis. Although Ms. P wants to stop haloperidol, we taper instead to guard against psychotic relapse. She continues to take citalopram, 20 mg/d, to prevent depressive symptom re-emergence and is receiving supportive psychotherapy to aid her relapse prevention.
Related resources
- Supplement Research Foundation. Supplement reviews. www.tsrf.com/supplements.htm
- Devan GS. Phentermine and Psychosis. Br J Psychiatry 1990;156:442-3.
- Cleare AJ. Phentermine, psychosis, and family history. J Clin Psychopharmacol 1996;16:470-1.
- Hoffman BF. Diet pill psychosis. CMAJ 1977;116:351-5.
- Citalopram • Celexa
- Haloperidol • Haldol
- Mirtazapine • Remeron
- Phenteramine • Adipex
- Risperidone • Risperdal
Dr. Khan is a speaker for Pfizer and Wyeth Pharmaceuticals.
Drs. Tan and Williamson report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Presentation: ‘they’re stalking me’
Ms. P, age 30, fears she is being stalked and is too terrified to be home alone. Worried, her ex-boyfriend calls police, who bring her to the emergency room
At the ER, Ms. P reports that surveillance cameras have been planted inside her house, that men often stand on her roof and watch her go to her car, and that men constantly are stalking her. She also hears voices and reports frightening peripheral visions of “outsiders.” The ER doctor consults the psychiatry service and orders laboratory tests, but all results—including urine drug screen findings—are negative.
Ms. P says she has been sleeping 3 to 4 hours nightly. She acknowledges depressed mood and decreased appetite, leading to a 10-lb weight loss over 1 month. She says she has felt depressed off and on for several years but has received no treatment for her mood symptoms. We admit her to the psychiatric unit to treat her acute-onset psychosis.
Lately, Ms. P’s life has been difficult. A college sophomore, she is failing all her classes. She was recently fired from her job as a case manager because of inappropriate behavior, such as buying gifts for the children she was managing and taking them for hair-cuts without their parents’ permission. Several months ago, she broke up with her boyfriend of 6 years. In addition to these stressors, she recently moved into an apartment and for the first time was living on her own.
Medical history. Ms. P has no major medical problems. Her mother has battled alcohol and drug dependence and depression but to Ms. P’s knowledge has never experienced psychosis. Ms. P, who admits that she binge drinks once or twice monthly, meets DSM-IV-TR criteria for alcohol abuse disorder. She denies using illicit drugs but admits that she regularly takes “energy pills” purchased over the Internet because she cannot wake up without them.
Physical exam is normal, but Ms. P’s body mass index (BMI) is 18 kg/m2, slightly below normal (height: 5 feet 8 inches; weight: 117.5 lb).
The authors’ observations
We diagnosed Ms. P as having recurrent and severe major depressive disorder with psychotic features because of her longstanding depressive symptoms. We considered substance-induced psychosis, but her urine drug screen is negative.
Treatment at this point should address both the paranoid delusions and depressive symptoms.
Treatment: starved for energy
We start haloperidol, 1 mg nightly, to treat Ms. P’s paranoid delusions, and mirtazapine, 15 mg nightly, to improve her sleep. We choose mirtazapine—which can increase appetite and lead to weight gain—because Ms. P is underweight. We also choose haloperidol because Ms. P is unemployed and cannot afford a second-generation antipsychotic.
Shortly afterward, we interview Ms. P’s ex-boyfriend. He tells us that she has been using diet pills regularly for 3 to 4 years, and that her chronic use has been escalating by the month. Lately, he says, she has been “popping the pills like candy.”
When we ask Ms. P about her diet pill use, she says she had mainly been using Xenadrine, an over-the-counter weight-loss supplement. Five months ago, she also started taking prescription phentermine, which she purchases over the Internet. She says that before her hospitalization, she was taking three phentermine tablets daily to boost her energy.
According to her ex-boyfriend, Ms. P began showing signs of psychosis 3 to 4 weeks after starting phentermine, and Ms. P notes that her initial paranoia and gustatory hallucinations have worsened. She now fears her bathroom is rigged with cameras. She showers with her swimsuit on.
We change Ms. P’s diagnosis to diet pill-induced psychosis. Because she had discarded the pill packaging before admission, we could not examine it for dosing information or ingredients.
The authors’ observations
Differentiating drug-induced psychosis from other psychoses often is difficult. Mood disorder with psychosis, schizophrenia, and substance-induced psychosis have similar characteristics (Table).
Ms. P has no personal or family history of psychosis that would suggest a thought disorder. She had good pre-morbid functioning (going to college, steady employment, long-term relationship with boyfriend) before her psychosis onset. She did, however, have a personal and family history of depression and was confronting many stressors (losing her job, failing grades at school, breaking up with her longtime boyfriend) that would suggest a primary mood disorder with psychosis.
We suspected an eating disorder and asked Ms. P more than once about her eating habits, but she insists she does not take the pills to lose weight. Also, her ex-boyfriend believes she is eating normally. Her low BMI and suspected obsession with weight loss could have signaled anorexia nervosa, but no other signs were present and her history does not support the diagnosis.
Table
Three causes of psychosis—and different characteristics of each presentation
| Characteristic | Mood disorder with psychosis | Schizophrenia | Substance-induced psychosis |
|---|---|---|---|
| Acute onset | x | - | x |
| Delusions | x | x | x |
| Disorganized or catatonic behavior | x | x | x |
| Family history of psychosis | x | x | _ |
| Good premorbid function | x | _ | x |
| Hallucinations | x | x | x |
| Negative symptoms | x | x | x |
| Personal history of psychosis | x | x | _ |
| Prodromal and residual symptoms | _ | x | _ |
Relapse: cameras ‘off’ for 1 week
Five days after admission, we discharge Ms. P as her psychosis has improved significantly.
Later that day at the outpatient clinic, Ms. P requests a medication change, voicing fears about haloperidol’s long term side effects and mirtazapine-induced weight gain. Risperidone, 2 mg nightly, and citalopram, 20 mg/d, are started instead.
One week later, Ms. P’s parents again bring her to the ER after police find her sitting in her car, confused and paranoid. She complains that cameras have been set up in her car, and she responds to voices when alone.
On the way to the ER, Ms. P tries to jump from the moving car. She assaults her mother as she stops her from jumping.
Blood pressure is 155/92, heart rate is 82 beats per minute, respiratory rate is 18 breaths per minute, and temperature is 96°F.
On interview, Ms. P admits that she stopped risperidone and citalopram and restarted Xenadrine and phentermine. She also reports orthostasis from risperidone. We again admit her to the acute-care psychiatric unit and restart haloperidol, 1 mg/d, and citalopram, 20 mg/d.
The authors’ observations
Although we knew Ms. P was abusing diet pills, we could have easily ruled out drug-induced psychosis based on her three negative urine drug screens.
The clinical course of Ms. P’s psychosis, however, closely followed her diet pill use—emerging soon after starting phentermine and remitting soon after stopping it. Also:
- she was taking 2 to 3 times the recommended dosage of phentermine for several months. Phentermine is indicated for short-term (a few weeks) treatment of exogenous obesity (BMI ≥27 kg/m2 in persons with hypertension, diabetes, or hyperlipidemia; BMI ≥30 kg/m2 in persons without these risk factors)1
- her BMI was below normal
- her psychosis remains in remission without use of an antipsychotic.
Stimulant medications such as amphetamines and stimulant drugs such as cocaine can produce psychotic symptoms including paranoid delusions, hallucinations, and bizarre behavior. Farrell and colleagues5 found that cannabis and psychostimulants increase the risk of psychosis.
Genetic load could have influenced Ms. P’s response to diet pills, but we have no information to support a genetic predisposition. Also, we saw no clear family history of a formal thought disorder.
The authors’ observations
Urine drug screens can pick up the main drug classes and often their derivatives, but this testing method is limited.2
Urine tests employ assays with semi-quantitative results. A urine sample may contain an abused substance but at levels below the cutoff. Also, because no correlation exists between cutoff levels and drug effect, a patient can have drug-induced symptoms but a negative urine drug screen. This makes detecting a suspected but unknown drug of abuse extremely difficult.
A routine urine screen can detect phentermine and other stimulants, but the phentermine level needed for a positive assay is 50 times that of pure amphetamine.2 Ms. P’s last urine drug screen showed an amphetamine level just under the cutoff.
Use of cocaine—undetectable in urine 3 to 4 days after use—could be considered when drug-induced psychosis is suspected. Ms. P’s psychosis correlated with her phentermine relapse, however, and both she and her ex-boyfriend denied that she uses street drugs.
Obtain specific drug levels when you suspect medication abuse. Request gas chromatography or mass spectrometry to provide a quantitative result and confirm medication abuse.2,3 These tests would have been appropriate for Ms. P once her ex-boyfriend revealed the diet pill abuse.
Detecting diet pill abuse
Use of weight-loss supplements and appetite suppressants is alarmingly common (Box). Many patients suffer adverse effects from diet pills but do not tell their doctors they are using them because they:
- fear the physician will scold them for circumventing his or her advice by obtaining medications online
- sense that obtaining diet pills over the Internet might be illegal
- do not realize the doctor needs to know about nonprescription drug use
- or fear the physician will tell them to stop taking the drug.
Rapid or unexplained weight loss, hypertension, tachycardia, tremors, psychomotor agitation, and hyperalertness could signal diet pill abuse. Emotional lability, such as euphoria during a high and fatigue and dysphoria during withdrawal, also could be indicative. Collateral information from family members or significant others can narrow the differential diagnosis.
Cognitive-behavioral therapy (CBT) can help Ms. P, who claimed she used diet pills to boost her energy. CBT would challenge her unrealistically high goals, teach and explain the consequences of drug use, and offer support to reinforce abstinence from diet pills. Educating patients about potential adverse drug effects also is essential.
Use of prescription and over-the-counter weight-loss products is alarmingly common. American culture values the “perfect body,” and the Internet has made appetite suppressants and weight-loss agents more available. Users can conveniently purchase large quantities of OTC weight-loss aids online.
In one multi-state survey,4 18% of women and 8% of men who were trying to lose weight reported using nonprescription weight loss products. Also:
- 28.4% of obese women (defined as BMI ≥30 kg/m2) reported using OTC diet pills, as did nearly 8% of women at normal weight (BMI 2)
- concomitant nonprescription and prescription pill use was often reported.
Conclusion: back to baseline
After 10 weeks, Ms. P’s condition returns to baseline. She starts a new job and abstains from diet pills. Her thought process and cognition improve significantly, and she reports no depressive symptoms at her most-recent visit. She maintains her weight at 139 lb. BMI is 21.1kg/m2 (normal).
Haloperidol is slowly tapered across 2 weeks with no return of psychosis. Although Ms. P wants to stop haloperidol, we taper instead to guard against psychotic relapse. She continues to take citalopram, 20 mg/d, to prevent depressive symptom re-emergence and is receiving supportive psychotherapy to aid her relapse prevention.
Related resources
- Supplement Research Foundation. Supplement reviews. www.tsrf.com/supplements.htm
- Devan GS. Phentermine and Psychosis. Br J Psychiatry 1990;156:442-3.
- Cleare AJ. Phentermine, psychosis, and family history. J Clin Psychopharmacol 1996;16:470-1.
- Hoffman BF. Diet pill psychosis. CMAJ 1977;116:351-5.
- Citalopram • Celexa
- Haloperidol • Haldol
- Mirtazapine • Remeron
- Phenteramine • Adipex
- Risperidone • Risperdal
Dr. Khan is a speaker for Pfizer and Wyeth Pharmaceuticals.
Drs. Tan and Williamson report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 2003;289:1537-45.
2. Shindelman J, Mahal J, Hemphill G, et al. Development and evaluation of an improved method for screening of amphetamines. J Anal Toxicol 1999;23:506-10.
3. Crosby RD, Carlson GA, Specker SM. Simulation of drug use and urine screening patterns. J Addict Dis 2003;22:89-98.
4. Blanck HM, Khan LK, Serdula Mk. Use of nonprescription weight loss products: results from a multistate survey. JAMA 2001;286:930-5.
5. Farrell M, Boys A, Bebbington P, et al. Psychosis and drug dependence: results from a national survey of prisoners. Br J Psychiatry 2002;181:393-8.
1. Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 2003;289:1537-45.
2. Shindelman J, Mahal J, Hemphill G, et al. Development and evaluation of an improved method for screening of amphetamines. J Anal Toxicol 1999;23:506-10.
3. Crosby RD, Carlson GA, Specker SM. Simulation of drug use and urine screening patterns. J Addict Dis 2003;22:89-98.
4. Blanck HM, Khan LK, Serdula Mk. Use of nonprescription weight loss products: results from a multistate survey. JAMA 2001;286:930-5.
5. Farrell M, Boys A, Bebbington P, et al. Psychosis and drug dependence: results from a national survey of prisoners. Br J Psychiatry 2002;181:393-8.
Which cholinesterase inhibitor for early dementia?
Using a cholinesterase inhibitor (ChEI) makes sense for any disorder with a significant cholinergic deficit, such as Alzheimer’s disease (AD) and other forms of mild-to-moderate dementia (Box 1).1-3 Yet the ChEIs tacrine, donepezil, rivastigmine, and galantamine have pharmacologic differences, and individual patients respond differently to them.
To help you choose the safest, most effective treatment for each patient, we discuss:
- three cases that show how ChEIs differ in mechanism of action, administration, and side effects
- evidence of ChEIs’ efficacy in AD—for which they are approved—and in other dementias for which they have been tried
- when to switch agents, and how long to continue treatment.
Probable Alzheimer’s disease (AD) accounts for 64% of all dementias in the United States. Less-common causes include:
- vascular dementia (5%)
- combined vascular dementia and AD (10%)
- probable dementia with Lewy bodies, Parkinson’s dementia, or diffuse Lewy body disease (9%)
- Lewy body variant of AD, or AD and dementia with Lewy bodies (6%)
- frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, or Creutzfeldt-Jakob disease (6%).2,3
In our experience, many primary care physicians choose to follow their patients with dementia, even when clinical features are atypical or suggest unusual causes. Psychiatrists are asked most often to assist in diagnosis and management of patients with:
- uncommon dementias, including frontotemporal dementia or dementia with Lewy bodies
- rapidly progressive dementia
- dementia in a patient age
- dementia with psychiatric comorbidities or severe behavior disturbances.4
How Cheis Differ
Although dementia remains incurable, recognizing cognitive decline early allows you to start ChEI therapy before substantial neuronal loss occurs (Box 2).3,4 The goal of early treatment is to improve or stabilize cognition, behavior, and activities of daily living for as long as possible.
In comparison studies,5,6 ChEIs have shown differences in tolerability but not consistent differences in efficacy for mild to moderate AD—though these studies had methodologic limitations. Because the agents appear similarly effective, the initial ChEI choice often depends on how their differences might benefit your patient (Table 1). Consider the following cases:
An early dementia diagnosis enables you educate the patient and family (Box 3) and begin the most effective treatment for the person with cognitive decline. Although dementia remains incurable, early recognition presents the opportunity to start cholinesterase inhibitors before substantial neuronal loss occurs.3,4
Patient workup. The Alzheimer’s Association offers online information for health care professionals on AD diagnosis and treatment protocols (see Related resources). A detailed history, physical examination, and Mini-Mental State Examination (MMSE) are necessary if you suspect Alzheimer’s or a related dementia.
Also recommended are a comprehensive metabolic screen, complete blood counts with differential, urine analysis, serum B12 and folate studies, homocysteine levels, thyroid studies, chest radiography, ECG, lipid profile, and brain scan (MRI or CT). Perform studies such as the rapid plasma reagin test for syphilis and HIV testing as appropriate.
Similarities and differences among cholinesterase inhibitors
| Tacrine | Donepezil | Rivastigmine | Galantamine | |
|---|---|---|---|---|
| Administration | Four times daily | Once daily | Twice daily with full meals | Once daily (extended-release formulation) |
| AChE inhibitor | Yes | Yes | Yes | Yes |
| BuChE inhibitor | Yes | No | Yes | No |
| Allosteric modulation of nicotinic receptor | No | Yes | No | Yes |
| Pharmacodynamic nicotinic/muscarinic effect | Yes | Yes | Yes | Yes |
| GI side effects | Present | Present | Present | Present |
| Hepatotoxicity | Present | Absent | Absent | Absent |
| Metabolism | CYP-450 | CYP-450 | Autohydrolysis | CYP-450 |
| Drug–drug interactions | Yes | Yes | None reported | Yes |
| AChE: acetylcholinesterase | ||||
| BuChE: butyrylcholinesterase | ||||
| CYP-450: cytochrome P-450 hepatic isoenzymes | ||||
Case 1: Gradual Memory Loss
Mrs. J, age 76, has experienced a slow, insidious memory decline across 5 years. She has become socially withdrawn and shows some language difficulties. She has had peptic ulcer disease and often does not take medications as prescribed.
Her psychiatrist diagnoses probable AD and chooses donepezil with its easy dosing schedule because of Mrs. J’s history of nonadherence. Donepezil’s GI tolerability is also a factor in this choice because of the patient’s peptic ulcer disease.
Case 2: Dementia And Motor Deficits
Mr. L, age 82, has gradually developed memory loss and parkinsonian symptoms, including slowness of movement and shuffling gait. He has visual hallucinations of people and episodic confusion. His medications include warfarin and digoxin for atrial fibrillation and congestive heart failure.
Mr. L is diagnosed with probable dementia with Lewy bodies. His psychiatrist chooses rivastigmine because it has shown efficacy in this type of dementia and is not known to interact significantly with cardiovascular medications.
Case 3: Stroke, Then Rapid Decline
Mrs. D, age 68, has a history of hypertension and suffered a stroke in the past. Her family says her memory and behavior—anger outbursts and excessive irritability—have worsened rapidly across 2 years. Examination reveals some focal neurologic deficits.
Her psychiatrist diagnoses probable vascular dementia and chooses galantamine for its efficacy in patients with this dementia type. Mrs. D has no history of GI illness and will likely tolerate the drug’s GI side effects. Follow-up care will include monitoring for tolerability.
Mechanism. Donepezil inhibits the enzyme acetylcholinesterase, and rivastigmine inhibits acetylcholinesterase and butyrylcholinesterase. Galantamine inhibits acetylcholinesterase and shows allosteric modulation of the presynaptic nicotinic receptor.
Data indicating that rivastigmine is particularly effective in patients with rapidly progressive illness is consistent with the possible advantage of inhibiting both butyrylcholinesterase and acetylcholinesterase. It has been argued that galantamine’s binding to nicotinic receptors modulates their function, which may enhance acetylcholine release.
Among the three agents, only rivastigmine shows a consistent, linear dose-response relationship. It is rapidly and extensively metabolized, primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite (autohydrolysis). Minimal metabolism occurs via the major cytochrome P (CYP)-450 isoenzymes. Donepezil and galantamine are metabolized by isoenzymes 2D6 and 3A4 and undergo glucuronidation.7
Drug interactions. Because rivastigmine avoids hepatic metabolism, interactions with drugs metabolized by CYP-450 isoenzymes have not been reported.8
Donepezil interacts with ketoconazole and quinidine, which inhibit donepezil metabolism and increase mean donepezil concentrations. Galantamine interacts with ketoconazole, paroxetine, and erythromycin, which increase mean galantamine concentrations.9
Administration. Donepezil and extended-release galantamine are given once daily because of their long half-lives, whereas regular galantamine and rivastigmine are taken twice daily with meals to minimize GI effects (Table 2). Nausea and vomiting can occur with any of the ChEIs but are more common and troublesome with rivastigmine and galantamine.
Table 2
How to use cholinesterase inhibitors for patients with dementia
| Drug | Recommended dosing | Possible side effects | Titration | Administration |
|---|---|---|---|---|
| Tacrine | Initial: 40 mg/d Maximum: 160 mg/d | Liver damage causing increase in ALT levels, GI effects (nausea, indigestion, vomiting, diarrhea, abdominal pain), skin rash | Dosage can be increased every 4 weeks | Divide into four doses; take on empty stomach |
| Donepezil | Initial: 5 mg/d Maximum: 10 mg/d | GI effects (nausea, diarrhea, vomiting, loss of appetite), insomnia, muscle cramps, fatigue | Increase dosage after 4 weeks | Once daily in morning or at bedtime |
| Rivastigmine | Initial: 3 mg/d Maximum: 12 mg/d | GI effects (nausea, vomiting, loss of appetite, weight loss, diarrhea, heartburn) | Increase dosage every 4 weeks | Twice daily after meals |
| Galantamine (regular, ER) | Initial: 8 mg/d Maximum: 24 mg/d | GI effects (nausea, vomiting, diarrhea, weight loss), possible increased mortality risk in patients with MCI | Increase dosage every 4 weeks | Regular: Twice daily after meals ER: Once daily after a meal |
| ALT: alanine transferase | ||||
| ER: extended-release formulation | ||||
| MCI: mild cognitive impairment | ||||
Efficacy In Early AD
In controlled clinical trials, all four ChEIs have significantly improved cognition, behavior, and activities of daily living in patients with mild-to-moderate AD.10-12 Tacrine—the first FDA-approved ChEI—is rarely used because its associated hepatoxicity requires ongoing liver enzyme monitoring.13 Among the other three:
Donepezil. A review of 16 trials involving 4,365 participants10 found significant benefits in cognitive functioning, activities of daily living, and behavior in persons with mild, moderate, or severe AD who were treated with donepezil for 12, 24, or 52 weeks.
Rivastigmine improved or maintained cognitive function, activities of daily living, and behavior for up to 52 weeks in patients with mild to moderate AD, according to a review of studies from 1995 to 2002.11 GI irritation was the most common adverse effect. Giving rivastigmine for up to 2 years may reduce the cost of caring for patients with AD, mostly by delaying nursing home placement.
Galantamine has beneficial effects on cognition, global function, activities of daily living, and behavior in patients with AD, vascular dementia, and AD with cerebrovascular components, according to a review of clinical studies.12 Adverse events are generally mild to moderate, transient, and gastrointestinal.
Efficacy In Other Dementias
In addition to their FDA-approved use for mildto-moderate AD, ChEIs also have been studied in persons with other types of dementia and mild cognitive impairment (MCI).
Dementia with Lewy bodies. Rivastigmine given with flexible titration from 6 to 12 mg/d improved behavior in 120 patients with Lewy body dementia.14 In the double-blind, multicenter study, patients taking rivastigmine, mean 9.7 mg/d for 20 weeks, were less apathetic and anxious and had fewer delusions and hallucinations than did those taking placebo. The drug was judged to be safe and well tolerated.
Vascular dementia. Patients with vascular dementia showed improved cognition and global function when treated with donepezil, 5 or 10 mg/d, for up to 24 weeks. Donepezil was well tolerated in this combined analysis of two randomized, placebo-controlled trials.15
Kumar et al16 compared two rivastigmine dosages in patients with mild-to-moderate AD, some of whom also had vascular dementia risk factors. Patients were randomly assigned to placebo, low-dose rivastigmine (1 to 4 mg/d), or high-dose rivastigmine (6 to 12 mg/d) for 26 weeks. Cognition, activities of daily living, and disease severity improved with rivastigmine in patients with or without vascular risk factors. Greater benefit was seen with high-dose than low-dose rivastigmine and in patients with AD plus vascular risk factors than in those with AD alone.
In a multicenter, double-blind trial,17 patients with vascular dementia or AD with vascular risk factors received galantamine, up to 24 mg/d, or placebo for 6 months. Compared with controls, those taking galantamine showed improved cognition, behavior, and function. The drug overall was well tolerated, with nausea and vomiting the most common side effects.
Parkinson’s dementia. Emre et al18 evaluated rivastigmine’s efficacy and safety in patients whose mild-to-moderate dementia developed at least 2 years after a clinical diagnosis of Parkinson’s disease (PD). Patients were randomly assigned to placebo or rivastigmine, 3 to 12 mg/d, for 24 weeks, and 410 of 541 enrollees completed the study. Compared with placebo, rivastigmine was associated with statistically significant improvements in cognition and global measures in dementia associated with PD but also with higher rates of nausea, vomiting, and tremor. PD’s motor symptoms did not change significantly in either group.
Mixed dementia states. As mentioned, galantamine improved cognitive and noncognitive abilities in patients with vascular dementia or AD with vascular risk factors in a 6-month, double-blind trial.17 Patients who received galantamine or placebo could then continue open-label galantamine, 24 mg/d, for another 6 months. In patients treated the full 12 months, galantamine continued to improve or maintain:
- cognition, based on Alzheimer’s Disease Assessment Scale-cognitive subscale scores
- functional ability, measured by the 40-item Disability Assessment for Dementia
- behavior, measured by the Neuro-psychiatric Inventory.19
After 12 months, the rivastigmine-treated patients were less behaviorally impaired than the matched patients, and their caregivers reported reduced stress. Rivastigmine did not prevent cognitive deterioration, as assessed with the Mini-Mental State Examination (MMSE).
Mild cognitive impairment. Persons with MCI have objective psychometric evidence of memory loss compared with their peers, but they are not significantly impaired in activities of daily living or other cognitive functions (language, abstract thinking, or problem-solving).
At this time, we do not recommend using ChEIs to treat MCI. These agents have shown little benefit and potential risk in patients who do not meet diagnostic criteria for dementia:
- Salloway et al21 tested donepezil’s efficacy and safety in 270 patients with MCI in a 24-week, double-blind, placebo-controlled trial. Donepezil was started at 5 mg/d for 42 days, then escalated to 10 mg/d. Compared with placebo, donepezil showed no significant effects on recall, but some improvements were seen in attention and psychomotor speed.
- In two unpublished placebo-controlled trials, galantamine did not improve memory when given for 2 years to elderly patients with MCI. A precaution was added to the drug’s prescribing information because 13 of the 1,026 patients taking galantamine died, compared with 1 of 1,022 taking placebo. Vascular disease caused one-half of the galantamine group deaths. No evidence of increased mortality risk has been seen in studies of galantamine in patients with mild-to-moderate AD, for which it is indicated.
Getting The Greatest Response
To gauge response to ChEI therapy, family reports about the patient are helpful—such as that cognition has improved or cognitive decline has not progressed as rapidly as before. Assessment tools such as the MMSE can document improvement or stabilization.
We recommend trying an initial ChEI for at least 6 months to determine its efficacy. If your patient cannot tolerate one ChEI or fails to respond to initial treatment, two consensus panels22,23 recommend that you consider changing ChEIs:
- If switching because of intolerable side effects, wait at least 2 to 3 days after stopping the first ChEI before starting another.
- If switching because of poor response, you can start a different ChEI immediately after the first one is stopped.
- Cholinesterase inhibitors may help improve or stabilize cognition, behavior, and/or activities of daily living
- Persons receiving these agents may decline more slowly than those who have not been treated
- Common side effects include nausea, vomiting, diarrhea, and loss of appetite
- Other less-common side effects are muscle cramps, slowed heart rate, dizziness, and fainting
- Because of differences in these agents, it may make sense to switch to another cholinesterase inhibitor if the patient has intolerable side effects or does not improve with the first one tried
Related resources
- Alzheimer’s Association. Information for health care professionals:
- • Diagnosing Alzheimer’s www.alz.org/Health/Diagnose/overview.asp
- • Treating cognitive symptoms www.alz.org/Health/Treating/symptoms.asp
- • Diagnosing Alzheimer’s www.alz.org/Health/Diagnose/overview.asp
- American Association for Geriatric Psychiatry. Information for older patients and their families on Alzheimer’s disease, other dementias. www.gmhfonline.org/gmhf/consumer/alzheimers.html.
- Tacrine • Cognex
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Razadyne (was Reminyl)
Drs. Kamat and LeFevre report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg receives grant/research support from Abbott Laboratories, Boehringer Ingelheim Pharmaceuticals, Cyberonics, Eli Lilly and Co., Eunoe, Forest Pharmaceuticals, Novartis Pharmaceuticals Corp., Pfizer, and Wyeth Pharmaceuticals. He is a consultant to AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutica, KV Pharma, Novartis Pharmaceuticals Corp., Organon International, and Sanofi-Synthelabo.
Acknowledgment
The authors thank Anjali Baliga, MD, for her contribution and help in preparing this article
1. Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997;278(16):1363-71.
2. Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54(11 suppl 5):S4-S9.
3. Grossberg GT, Lake JT. The role of the psychiatrist in Alzheimer’s disease. J Clin Psychiatry 1998;59(suppl 9):3-6.
4. Doraiswamy PM, Steffens DC, Pitchumoni S, Tabrizi S. Early recognition of Alzheimer’s disease: what is consensual? What is controversial? What is practical? J Clin Psychiatry 1998;59(suppl 13):6-18.
5. Wilkinson DG, Passmore AP, Bullock R, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 2002;56(6):441-6
6. Jones RW, Soininen H, Hager K, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19(1):58-67.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twentytwo classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. U. S. Bureau of the Census. 2004 International database: Midyear population, by age and sex. Table 094. U.S. Bureau of the Census; 2004.
9. Reminyl (galantamine HBr). Physicians’ desk reference (59th ed). Montvale, NJ: Thomson PDR; 2005:1739.
10. Birks JS, Harvey R. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 2003;(3):CD001190.-
11. Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther 2003;25(6):1634-53.
12. Corey-Bloom J. Galantamine: a review of its use in Alzheimer’s disease and vascular dementia. Int J Clin Pract 2003;57(3):219-23.
13. Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994;271(13):992-8.
14. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000;356(9247):2031-6.
15. Passmore AP, Bayer AJ, Steinhagen-Thiessen E. Cognitive, global, and functional benefits of donepezil in Alzheimer’s disease and vascular dementia: results from large-scale clinical trials. J Neurol Sci 2005;229-30:141-6.
16. Kumar V, Anand R, Messina J, et al. An efficacy and safety analysis of rivastigmine in Alzheimer’s disease patients with concurrent vascular risk factors. Eur J Neurol 2000;7(2):159-69.
17. Kurz AF, Erkinjuntti T, Gauthier S, et al. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359(9314):1283-90.
18. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004;351(24):2509-18.
19. Erkinjuntti T, Kurz A, Small GW, et al. An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clin Ther 2003;25(6):1765-82.
20. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging 2004;21(14):931-7.
21. Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004;63(4):651-7.
22. Emre M. Switching cholinesterase inhibitors in patients with Alzheimer’s disease. Int J Clin Pract Suppl 2002;(127):64-72.
23. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl 2002;(127):45-63.
Using a cholinesterase inhibitor (ChEI) makes sense for any disorder with a significant cholinergic deficit, such as Alzheimer’s disease (AD) and other forms of mild-to-moderate dementia (Box 1).1-3 Yet the ChEIs tacrine, donepezil, rivastigmine, and galantamine have pharmacologic differences, and individual patients respond differently to them.
To help you choose the safest, most effective treatment for each patient, we discuss:
- three cases that show how ChEIs differ in mechanism of action, administration, and side effects
- evidence of ChEIs’ efficacy in AD—for which they are approved—and in other dementias for which they have been tried
- when to switch agents, and how long to continue treatment.
Probable Alzheimer’s disease (AD) accounts for 64% of all dementias in the United States. Less-common causes include:
- vascular dementia (5%)
- combined vascular dementia and AD (10%)
- probable dementia with Lewy bodies, Parkinson’s dementia, or diffuse Lewy body disease (9%)
- Lewy body variant of AD, or AD and dementia with Lewy bodies (6%)
- frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, or Creutzfeldt-Jakob disease (6%).2,3
In our experience, many primary care physicians choose to follow their patients with dementia, even when clinical features are atypical or suggest unusual causes. Psychiatrists are asked most often to assist in diagnosis and management of patients with:
- uncommon dementias, including frontotemporal dementia or dementia with Lewy bodies
- rapidly progressive dementia
- dementia in a patient age
- dementia with psychiatric comorbidities or severe behavior disturbances.4
How Cheis Differ
Although dementia remains incurable, recognizing cognitive decline early allows you to start ChEI therapy before substantial neuronal loss occurs (Box 2).3,4 The goal of early treatment is to improve or stabilize cognition, behavior, and activities of daily living for as long as possible.
In comparison studies,5,6 ChEIs have shown differences in tolerability but not consistent differences in efficacy for mild to moderate AD—though these studies had methodologic limitations. Because the agents appear similarly effective, the initial ChEI choice often depends on how their differences might benefit your patient (Table 1). Consider the following cases:
An early dementia diagnosis enables you educate the patient and family (Box 3) and begin the most effective treatment for the person with cognitive decline. Although dementia remains incurable, early recognition presents the opportunity to start cholinesterase inhibitors before substantial neuronal loss occurs.3,4
Patient workup. The Alzheimer’s Association offers online information for health care professionals on AD diagnosis and treatment protocols (see Related resources). A detailed history, physical examination, and Mini-Mental State Examination (MMSE) are necessary if you suspect Alzheimer’s or a related dementia.
Also recommended are a comprehensive metabolic screen, complete blood counts with differential, urine analysis, serum B12 and folate studies, homocysteine levels, thyroid studies, chest radiography, ECG, lipid profile, and brain scan (MRI or CT). Perform studies such as the rapid plasma reagin test for syphilis and HIV testing as appropriate.
Similarities and differences among cholinesterase inhibitors
| Tacrine | Donepezil | Rivastigmine | Galantamine | |
|---|---|---|---|---|
| Administration | Four times daily | Once daily | Twice daily with full meals | Once daily (extended-release formulation) |
| AChE inhibitor | Yes | Yes | Yes | Yes |
| BuChE inhibitor | Yes | No | Yes | No |
| Allosteric modulation of nicotinic receptor | No | Yes | No | Yes |
| Pharmacodynamic nicotinic/muscarinic effect | Yes | Yes | Yes | Yes |
| GI side effects | Present | Present | Present | Present |
| Hepatotoxicity | Present | Absent | Absent | Absent |
| Metabolism | CYP-450 | CYP-450 | Autohydrolysis | CYP-450 |
| Drug–drug interactions | Yes | Yes | None reported | Yes |
| AChE: acetylcholinesterase | ||||
| BuChE: butyrylcholinesterase | ||||
| CYP-450: cytochrome P-450 hepatic isoenzymes | ||||
Case 1: Gradual Memory Loss
Mrs. J, age 76, has experienced a slow, insidious memory decline across 5 years. She has become socially withdrawn and shows some language difficulties. She has had peptic ulcer disease and often does not take medications as prescribed.
Her psychiatrist diagnoses probable AD and chooses donepezil with its easy dosing schedule because of Mrs. J’s history of nonadherence. Donepezil’s GI tolerability is also a factor in this choice because of the patient’s peptic ulcer disease.
Case 2: Dementia And Motor Deficits
Mr. L, age 82, has gradually developed memory loss and parkinsonian symptoms, including slowness of movement and shuffling gait. He has visual hallucinations of people and episodic confusion. His medications include warfarin and digoxin for atrial fibrillation and congestive heart failure.
Mr. L is diagnosed with probable dementia with Lewy bodies. His psychiatrist chooses rivastigmine because it has shown efficacy in this type of dementia and is not known to interact significantly with cardiovascular medications.
Case 3: Stroke, Then Rapid Decline
Mrs. D, age 68, has a history of hypertension and suffered a stroke in the past. Her family says her memory and behavior—anger outbursts and excessive irritability—have worsened rapidly across 2 years. Examination reveals some focal neurologic deficits.
Her psychiatrist diagnoses probable vascular dementia and chooses galantamine for its efficacy in patients with this dementia type. Mrs. D has no history of GI illness and will likely tolerate the drug’s GI side effects. Follow-up care will include monitoring for tolerability.
Mechanism. Donepezil inhibits the enzyme acetylcholinesterase, and rivastigmine inhibits acetylcholinesterase and butyrylcholinesterase. Galantamine inhibits acetylcholinesterase and shows allosteric modulation of the presynaptic nicotinic receptor.
Data indicating that rivastigmine is particularly effective in patients with rapidly progressive illness is consistent with the possible advantage of inhibiting both butyrylcholinesterase and acetylcholinesterase. It has been argued that galantamine’s binding to nicotinic receptors modulates their function, which may enhance acetylcholine release.
Among the three agents, only rivastigmine shows a consistent, linear dose-response relationship. It is rapidly and extensively metabolized, primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite (autohydrolysis). Minimal metabolism occurs via the major cytochrome P (CYP)-450 isoenzymes. Donepezil and galantamine are metabolized by isoenzymes 2D6 and 3A4 and undergo glucuronidation.7
Drug interactions. Because rivastigmine avoids hepatic metabolism, interactions with drugs metabolized by CYP-450 isoenzymes have not been reported.8
Donepezil interacts with ketoconazole and quinidine, which inhibit donepezil metabolism and increase mean donepezil concentrations. Galantamine interacts with ketoconazole, paroxetine, and erythromycin, which increase mean galantamine concentrations.9
Administration. Donepezil and extended-release galantamine are given once daily because of their long half-lives, whereas regular galantamine and rivastigmine are taken twice daily with meals to minimize GI effects (Table 2). Nausea and vomiting can occur with any of the ChEIs but are more common and troublesome with rivastigmine and galantamine.
Table 2
How to use cholinesterase inhibitors for patients with dementia
| Drug | Recommended dosing | Possible side effects | Titration | Administration |
|---|---|---|---|---|
| Tacrine | Initial: 40 mg/d Maximum: 160 mg/d | Liver damage causing increase in ALT levels, GI effects (nausea, indigestion, vomiting, diarrhea, abdominal pain), skin rash | Dosage can be increased every 4 weeks | Divide into four doses; take on empty stomach |
| Donepezil | Initial: 5 mg/d Maximum: 10 mg/d | GI effects (nausea, diarrhea, vomiting, loss of appetite), insomnia, muscle cramps, fatigue | Increase dosage after 4 weeks | Once daily in morning or at bedtime |
| Rivastigmine | Initial: 3 mg/d Maximum: 12 mg/d | GI effects (nausea, vomiting, loss of appetite, weight loss, diarrhea, heartburn) | Increase dosage every 4 weeks | Twice daily after meals |
| Galantamine (regular, ER) | Initial: 8 mg/d Maximum: 24 mg/d | GI effects (nausea, vomiting, diarrhea, weight loss), possible increased mortality risk in patients with MCI | Increase dosage every 4 weeks | Regular: Twice daily after meals ER: Once daily after a meal |
| ALT: alanine transferase | ||||
| ER: extended-release formulation | ||||
| MCI: mild cognitive impairment | ||||
Efficacy In Early AD
In controlled clinical trials, all four ChEIs have significantly improved cognition, behavior, and activities of daily living in patients with mild-to-moderate AD.10-12 Tacrine—the first FDA-approved ChEI—is rarely used because its associated hepatoxicity requires ongoing liver enzyme monitoring.13 Among the other three:
Donepezil. A review of 16 trials involving 4,365 participants10 found significant benefits in cognitive functioning, activities of daily living, and behavior in persons with mild, moderate, or severe AD who were treated with donepezil for 12, 24, or 52 weeks.
Rivastigmine improved or maintained cognitive function, activities of daily living, and behavior for up to 52 weeks in patients with mild to moderate AD, according to a review of studies from 1995 to 2002.11 GI irritation was the most common adverse effect. Giving rivastigmine for up to 2 years may reduce the cost of caring for patients with AD, mostly by delaying nursing home placement.
Galantamine has beneficial effects on cognition, global function, activities of daily living, and behavior in patients with AD, vascular dementia, and AD with cerebrovascular components, according to a review of clinical studies.12 Adverse events are generally mild to moderate, transient, and gastrointestinal.
Efficacy In Other Dementias
In addition to their FDA-approved use for mildto-moderate AD, ChEIs also have been studied in persons with other types of dementia and mild cognitive impairment (MCI).
Dementia with Lewy bodies. Rivastigmine given with flexible titration from 6 to 12 mg/d improved behavior in 120 patients with Lewy body dementia.14 In the double-blind, multicenter study, patients taking rivastigmine, mean 9.7 mg/d for 20 weeks, were less apathetic and anxious and had fewer delusions and hallucinations than did those taking placebo. The drug was judged to be safe and well tolerated.
Vascular dementia. Patients with vascular dementia showed improved cognition and global function when treated with donepezil, 5 or 10 mg/d, for up to 24 weeks. Donepezil was well tolerated in this combined analysis of two randomized, placebo-controlled trials.15
Kumar et al16 compared two rivastigmine dosages in patients with mild-to-moderate AD, some of whom also had vascular dementia risk factors. Patients were randomly assigned to placebo, low-dose rivastigmine (1 to 4 mg/d), or high-dose rivastigmine (6 to 12 mg/d) for 26 weeks. Cognition, activities of daily living, and disease severity improved with rivastigmine in patients with or without vascular risk factors. Greater benefit was seen with high-dose than low-dose rivastigmine and in patients with AD plus vascular risk factors than in those with AD alone.
In a multicenter, double-blind trial,17 patients with vascular dementia or AD with vascular risk factors received galantamine, up to 24 mg/d, or placebo for 6 months. Compared with controls, those taking galantamine showed improved cognition, behavior, and function. The drug overall was well tolerated, with nausea and vomiting the most common side effects.
Parkinson’s dementia. Emre et al18 evaluated rivastigmine’s efficacy and safety in patients whose mild-to-moderate dementia developed at least 2 years after a clinical diagnosis of Parkinson’s disease (PD). Patients were randomly assigned to placebo or rivastigmine, 3 to 12 mg/d, for 24 weeks, and 410 of 541 enrollees completed the study. Compared with placebo, rivastigmine was associated with statistically significant improvements in cognition and global measures in dementia associated with PD but also with higher rates of nausea, vomiting, and tremor. PD’s motor symptoms did not change significantly in either group.
Mixed dementia states. As mentioned, galantamine improved cognitive and noncognitive abilities in patients with vascular dementia or AD with vascular risk factors in a 6-month, double-blind trial.17 Patients who received galantamine or placebo could then continue open-label galantamine, 24 mg/d, for another 6 months. In patients treated the full 12 months, galantamine continued to improve or maintain:
- cognition, based on Alzheimer’s Disease Assessment Scale-cognitive subscale scores
- functional ability, measured by the 40-item Disability Assessment for Dementia
- behavior, measured by the Neuro-psychiatric Inventory.19
After 12 months, the rivastigmine-treated patients were less behaviorally impaired than the matched patients, and their caregivers reported reduced stress. Rivastigmine did not prevent cognitive deterioration, as assessed with the Mini-Mental State Examination (MMSE).
Mild cognitive impairment. Persons with MCI have objective psychometric evidence of memory loss compared with their peers, but they are not significantly impaired in activities of daily living or other cognitive functions (language, abstract thinking, or problem-solving).
At this time, we do not recommend using ChEIs to treat MCI. These agents have shown little benefit and potential risk in patients who do not meet diagnostic criteria for dementia:
- Salloway et al21 tested donepezil’s efficacy and safety in 270 patients with MCI in a 24-week, double-blind, placebo-controlled trial. Donepezil was started at 5 mg/d for 42 days, then escalated to 10 mg/d. Compared with placebo, donepezil showed no significant effects on recall, but some improvements were seen in attention and psychomotor speed.
- In two unpublished placebo-controlled trials, galantamine did not improve memory when given for 2 years to elderly patients with MCI. A precaution was added to the drug’s prescribing information because 13 of the 1,026 patients taking galantamine died, compared with 1 of 1,022 taking placebo. Vascular disease caused one-half of the galantamine group deaths. No evidence of increased mortality risk has been seen in studies of galantamine in patients with mild-to-moderate AD, for which it is indicated.
Getting The Greatest Response
To gauge response to ChEI therapy, family reports about the patient are helpful—such as that cognition has improved or cognitive decline has not progressed as rapidly as before. Assessment tools such as the MMSE can document improvement or stabilization.
We recommend trying an initial ChEI for at least 6 months to determine its efficacy. If your patient cannot tolerate one ChEI or fails to respond to initial treatment, two consensus panels22,23 recommend that you consider changing ChEIs:
- If switching because of intolerable side effects, wait at least 2 to 3 days after stopping the first ChEI before starting another.
- If switching because of poor response, you can start a different ChEI immediately after the first one is stopped.
- Cholinesterase inhibitors may help improve or stabilize cognition, behavior, and/or activities of daily living
- Persons receiving these agents may decline more slowly than those who have not been treated
- Common side effects include nausea, vomiting, diarrhea, and loss of appetite
- Other less-common side effects are muscle cramps, slowed heart rate, dizziness, and fainting
- Because of differences in these agents, it may make sense to switch to another cholinesterase inhibitor if the patient has intolerable side effects or does not improve with the first one tried
Related resources
- Alzheimer’s Association. Information for health care professionals:
- • Diagnosing Alzheimer’s www.alz.org/Health/Diagnose/overview.asp
- • Treating cognitive symptoms www.alz.org/Health/Treating/symptoms.asp
- • Diagnosing Alzheimer’s www.alz.org/Health/Diagnose/overview.asp
- American Association for Geriatric Psychiatry. Information for older patients and their families on Alzheimer’s disease, other dementias. www.gmhfonline.org/gmhf/consumer/alzheimers.html.
- Tacrine • Cognex
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Razadyne (was Reminyl)
Drs. Kamat and LeFevre report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg receives grant/research support from Abbott Laboratories, Boehringer Ingelheim Pharmaceuticals, Cyberonics, Eli Lilly and Co., Eunoe, Forest Pharmaceuticals, Novartis Pharmaceuticals Corp., Pfizer, and Wyeth Pharmaceuticals. He is a consultant to AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutica, KV Pharma, Novartis Pharmaceuticals Corp., Organon International, and Sanofi-Synthelabo.
Acknowledgment
The authors thank Anjali Baliga, MD, for her contribution and help in preparing this article
Using a cholinesterase inhibitor (ChEI) makes sense for any disorder with a significant cholinergic deficit, such as Alzheimer’s disease (AD) and other forms of mild-to-moderate dementia (Box 1).1-3 Yet the ChEIs tacrine, donepezil, rivastigmine, and galantamine have pharmacologic differences, and individual patients respond differently to them.
To help you choose the safest, most effective treatment for each patient, we discuss:
- three cases that show how ChEIs differ in mechanism of action, administration, and side effects
- evidence of ChEIs’ efficacy in AD—for which they are approved—and in other dementias for which they have been tried
- when to switch agents, and how long to continue treatment.
Probable Alzheimer’s disease (AD) accounts for 64% of all dementias in the United States. Less-common causes include:
- vascular dementia (5%)
- combined vascular dementia and AD (10%)
- probable dementia with Lewy bodies, Parkinson’s dementia, or diffuse Lewy body disease (9%)
- Lewy body variant of AD, or AD and dementia with Lewy bodies (6%)
- frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, or Creutzfeldt-Jakob disease (6%).2,3
In our experience, many primary care physicians choose to follow their patients with dementia, even when clinical features are atypical or suggest unusual causes. Psychiatrists are asked most often to assist in diagnosis and management of patients with:
- uncommon dementias, including frontotemporal dementia or dementia with Lewy bodies
- rapidly progressive dementia
- dementia in a patient age
- dementia with psychiatric comorbidities or severe behavior disturbances.4
How Cheis Differ
Although dementia remains incurable, recognizing cognitive decline early allows you to start ChEI therapy before substantial neuronal loss occurs (Box 2).3,4 The goal of early treatment is to improve or stabilize cognition, behavior, and activities of daily living for as long as possible.
In comparison studies,5,6 ChEIs have shown differences in tolerability but not consistent differences in efficacy for mild to moderate AD—though these studies had methodologic limitations. Because the agents appear similarly effective, the initial ChEI choice often depends on how their differences might benefit your patient (Table 1). Consider the following cases:
An early dementia diagnosis enables you educate the patient and family (Box 3) and begin the most effective treatment for the person with cognitive decline. Although dementia remains incurable, early recognition presents the opportunity to start cholinesterase inhibitors before substantial neuronal loss occurs.3,4
Patient workup. The Alzheimer’s Association offers online information for health care professionals on AD diagnosis and treatment protocols (see Related resources). A detailed history, physical examination, and Mini-Mental State Examination (MMSE) are necessary if you suspect Alzheimer’s or a related dementia.
Also recommended are a comprehensive metabolic screen, complete blood counts with differential, urine analysis, serum B12 and folate studies, homocysteine levels, thyroid studies, chest radiography, ECG, lipid profile, and brain scan (MRI or CT). Perform studies such as the rapid plasma reagin test for syphilis and HIV testing as appropriate.
Similarities and differences among cholinesterase inhibitors
| Tacrine | Donepezil | Rivastigmine | Galantamine | |
|---|---|---|---|---|
| Administration | Four times daily | Once daily | Twice daily with full meals | Once daily (extended-release formulation) |
| AChE inhibitor | Yes | Yes | Yes | Yes |
| BuChE inhibitor | Yes | No | Yes | No |
| Allosteric modulation of nicotinic receptor | No | Yes | No | Yes |
| Pharmacodynamic nicotinic/muscarinic effect | Yes | Yes | Yes | Yes |
| GI side effects | Present | Present | Present | Present |
| Hepatotoxicity | Present | Absent | Absent | Absent |
| Metabolism | CYP-450 | CYP-450 | Autohydrolysis | CYP-450 |
| Drug–drug interactions | Yes | Yes | None reported | Yes |
| AChE: acetylcholinesterase | ||||
| BuChE: butyrylcholinesterase | ||||
| CYP-450: cytochrome P-450 hepatic isoenzymes | ||||
Case 1: Gradual Memory Loss
Mrs. J, age 76, has experienced a slow, insidious memory decline across 5 years. She has become socially withdrawn and shows some language difficulties. She has had peptic ulcer disease and often does not take medications as prescribed.
Her psychiatrist diagnoses probable AD and chooses donepezil with its easy dosing schedule because of Mrs. J’s history of nonadherence. Donepezil’s GI tolerability is also a factor in this choice because of the patient’s peptic ulcer disease.
Case 2: Dementia And Motor Deficits
Mr. L, age 82, has gradually developed memory loss and parkinsonian symptoms, including slowness of movement and shuffling gait. He has visual hallucinations of people and episodic confusion. His medications include warfarin and digoxin for atrial fibrillation and congestive heart failure.
Mr. L is diagnosed with probable dementia with Lewy bodies. His psychiatrist chooses rivastigmine because it has shown efficacy in this type of dementia and is not known to interact significantly with cardiovascular medications.
Case 3: Stroke, Then Rapid Decline
Mrs. D, age 68, has a history of hypertension and suffered a stroke in the past. Her family says her memory and behavior—anger outbursts and excessive irritability—have worsened rapidly across 2 years. Examination reveals some focal neurologic deficits.
Her psychiatrist diagnoses probable vascular dementia and chooses galantamine for its efficacy in patients with this dementia type. Mrs. D has no history of GI illness and will likely tolerate the drug’s GI side effects. Follow-up care will include monitoring for tolerability.
Mechanism. Donepezil inhibits the enzyme acetylcholinesterase, and rivastigmine inhibits acetylcholinesterase and butyrylcholinesterase. Galantamine inhibits acetylcholinesterase and shows allosteric modulation of the presynaptic nicotinic receptor.
Data indicating that rivastigmine is particularly effective in patients with rapidly progressive illness is consistent with the possible advantage of inhibiting both butyrylcholinesterase and acetylcholinesterase. It has been argued that galantamine’s binding to nicotinic receptors modulates their function, which may enhance acetylcholine release.
Among the three agents, only rivastigmine shows a consistent, linear dose-response relationship. It is rapidly and extensively metabolized, primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite (autohydrolysis). Minimal metabolism occurs via the major cytochrome P (CYP)-450 isoenzymes. Donepezil and galantamine are metabolized by isoenzymes 2D6 and 3A4 and undergo glucuronidation.7
Drug interactions. Because rivastigmine avoids hepatic metabolism, interactions with drugs metabolized by CYP-450 isoenzymes have not been reported.8
Donepezil interacts with ketoconazole and quinidine, which inhibit donepezil metabolism and increase mean donepezil concentrations. Galantamine interacts with ketoconazole, paroxetine, and erythromycin, which increase mean galantamine concentrations.9
Administration. Donepezil and extended-release galantamine are given once daily because of their long half-lives, whereas regular galantamine and rivastigmine are taken twice daily with meals to minimize GI effects (Table 2). Nausea and vomiting can occur with any of the ChEIs but are more common and troublesome with rivastigmine and galantamine.
Table 2
How to use cholinesterase inhibitors for patients with dementia
| Drug | Recommended dosing | Possible side effects | Titration | Administration |
|---|---|---|---|---|
| Tacrine | Initial: 40 mg/d Maximum: 160 mg/d | Liver damage causing increase in ALT levels, GI effects (nausea, indigestion, vomiting, diarrhea, abdominal pain), skin rash | Dosage can be increased every 4 weeks | Divide into four doses; take on empty stomach |
| Donepezil | Initial: 5 mg/d Maximum: 10 mg/d | GI effects (nausea, diarrhea, vomiting, loss of appetite), insomnia, muscle cramps, fatigue | Increase dosage after 4 weeks | Once daily in morning or at bedtime |
| Rivastigmine | Initial: 3 mg/d Maximum: 12 mg/d | GI effects (nausea, vomiting, loss of appetite, weight loss, diarrhea, heartburn) | Increase dosage every 4 weeks | Twice daily after meals |
| Galantamine (regular, ER) | Initial: 8 mg/d Maximum: 24 mg/d | GI effects (nausea, vomiting, diarrhea, weight loss), possible increased mortality risk in patients with MCI | Increase dosage every 4 weeks | Regular: Twice daily after meals ER: Once daily after a meal |
| ALT: alanine transferase | ||||
| ER: extended-release formulation | ||||
| MCI: mild cognitive impairment | ||||
Efficacy In Early AD
In controlled clinical trials, all four ChEIs have significantly improved cognition, behavior, and activities of daily living in patients with mild-to-moderate AD.10-12 Tacrine—the first FDA-approved ChEI—is rarely used because its associated hepatoxicity requires ongoing liver enzyme monitoring.13 Among the other three:
Donepezil. A review of 16 trials involving 4,365 participants10 found significant benefits in cognitive functioning, activities of daily living, and behavior in persons with mild, moderate, or severe AD who were treated with donepezil for 12, 24, or 52 weeks.
Rivastigmine improved or maintained cognitive function, activities of daily living, and behavior for up to 52 weeks in patients with mild to moderate AD, according to a review of studies from 1995 to 2002.11 GI irritation was the most common adverse effect. Giving rivastigmine for up to 2 years may reduce the cost of caring for patients with AD, mostly by delaying nursing home placement.
Galantamine has beneficial effects on cognition, global function, activities of daily living, and behavior in patients with AD, vascular dementia, and AD with cerebrovascular components, according to a review of clinical studies.12 Adverse events are generally mild to moderate, transient, and gastrointestinal.
Efficacy In Other Dementias
In addition to their FDA-approved use for mildto-moderate AD, ChEIs also have been studied in persons with other types of dementia and mild cognitive impairment (MCI).
Dementia with Lewy bodies. Rivastigmine given with flexible titration from 6 to 12 mg/d improved behavior in 120 patients with Lewy body dementia.14 In the double-blind, multicenter study, patients taking rivastigmine, mean 9.7 mg/d for 20 weeks, were less apathetic and anxious and had fewer delusions and hallucinations than did those taking placebo. The drug was judged to be safe and well tolerated.
Vascular dementia. Patients with vascular dementia showed improved cognition and global function when treated with donepezil, 5 or 10 mg/d, for up to 24 weeks. Donepezil was well tolerated in this combined analysis of two randomized, placebo-controlled trials.15
Kumar et al16 compared two rivastigmine dosages in patients with mild-to-moderate AD, some of whom also had vascular dementia risk factors. Patients were randomly assigned to placebo, low-dose rivastigmine (1 to 4 mg/d), or high-dose rivastigmine (6 to 12 mg/d) for 26 weeks. Cognition, activities of daily living, and disease severity improved with rivastigmine in patients with or without vascular risk factors. Greater benefit was seen with high-dose than low-dose rivastigmine and in patients with AD plus vascular risk factors than in those with AD alone.
In a multicenter, double-blind trial,17 patients with vascular dementia or AD with vascular risk factors received galantamine, up to 24 mg/d, or placebo for 6 months. Compared with controls, those taking galantamine showed improved cognition, behavior, and function. The drug overall was well tolerated, with nausea and vomiting the most common side effects.
Parkinson’s dementia. Emre et al18 evaluated rivastigmine’s efficacy and safety in patients whose mild-to-moderate dementia developed at least 2 years after a clinical diagnosis of Parkinson’s disease (PD). Patients were randomly assigned to placebo or rivastigmine, 3 to 12 mg/d, for 24 weeks, and 410 of 541 enrollees completed the study. Compared with placebo, rivastigmine was associated with statistically significant improvements in cognition and global measures in dementia associated with PD but also with higher rates of nausea, vomiting, and tremor. PD’s motor symptoms did not change significantly in either group.
Mixed dementia states. As mentioned, galantamine improved cognitive and noncognitive abilities in patients with vascular dementia or AD with vascular risk factors in a 6-month, double-blind trial.17 Patients who received galantamine or placebo could then continue open-label galantamine, 24 mg/d, for another 6 months. In patients treated the full 12 months, galantamine continued to improve or maintain:
- cognition, based on Alzheimer’s Disease Assessment Scale-cognitive subscale scores
- functional ability, measured by the 40-item Disability Assessment for Dementia
- behavior, measured by the Neuro-psychiatric Inventory.19
After 12 months, the rivastigmine-treated patients were less behaviorally impaired than the matched patients, and their caregivers reported reduced stress. Rivastigmine did not prevent cognitive deterioration, as assessed with the Mini-Mental State Examination (MMSE).
Mild cognitive impairment. Persons with MCI have objective psychometric evidence of memory loss compared with their peers, but they are not significantly impaired in activities of daily living or other cognitive functions (language, abstract thinking, or problem-solving).
At this time, we do not recommend using ChEIs to treat MCI. These agents have shown little benefit and potential risk in patients who do not meet diagnostic criteria for dementia:
- Salloway et al21 tested donepezil’s efficacy and safety in 270 patients with MCI in a 24-week, double-blind, placebo-controlled trial. Donepezil was started at 5 mg/d for 42 days, then escalated to 10 mg/d. Compared with placebo, donepezil showed no significant effects on recall, but some improvements were seen in attention and psychomotor speed.
- In two unpublished placebo-controlled trials, galantamine did not improve memory when given for 2 years to elderly patients with MCI. A precaution was added to the drug’s prescribing information because 13 of the 1,026 patients taking galantamine died, compared with 1 of 1,022 taking placebo. Vascular disease caused one-half of the galantamine group deaths. No evidence of increased mortality risk has been seen in studies of galantamine in patients with mild-to-moderate AD, for which it is indicated.
Getting The Greatest Response
To gauge response to ChEI therapy, family reports about the patient are helpful—such as that cognition has improved or cognitive decline has not progressed as rapidly as before. Assessment tools such as the MMSE can document improvement or stabilization.
We recommend trying an initial ChEI for at least 6 months to determine its efficacy. If your patient cannot tolerate one ChEI or fails to respond to initial treatment, two consensus panels22,23 recommend that you consider changing ChEIs:
- If switching because of intolerable side effects, wait at least 2 to 3 days after stopping the first ChEI before starting another.
- If switching because of poor response, you can start a different ChEI immediately after the first one is stopped.
- Cholinesterase inhibitors may help improve or stabilize cognition, behavior, and/or activities of daily living
- Persons receiving these agents may decline more slowly than those who have not been treated
- Common side effects include nausea, vomiting, diarrhea, and loss of appetite
- Other less-common side effects are muscle cramps, slowed heart rate, dizziness, and fainting
- Because of differences in these agents, it may make sense to switch to another cholinesterase inhibitor if the patient has intolerable side effects or does not improve with the first one tried
Related resources
- Alzheimer’s Association. Information for health care professionals:
- • Diagnosing Alzheimer’s www.alz.org/Health/Diagnose/overview.asp
- • Treating cognitive symptoms www.alz.org/Health/Treating/symptoms.asp
- • Diagnosing Alzheimer’s www.alz.org/Health/Diagnose/overview.asp
- American Association for Geriatric Psychiatry. Information for older patients and their families on Alzheimer’s disease, other dementias. www.gmhfonline.org/gmhf/consumer/alzheimers.html.
- Tacrine • Cognex
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Razadyne (was Reminyl)
Drs. Kamat and LeFevre report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg receives grant/research support from Abbott Laboratories, Boehringer Ingelheim Pharmaceuticals, Cyberonics, Eli Lilly and Co., Eunoe, Forest Pharmaceuticals, Novartis Pharmaceuticals Corp., Pfizer, and Wyeth Pharmaceuticals. He is a consultant to AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutica, KV Pharma, Novartis Pharmaceuticals Corp., Organon International, and Sanofi-Synthelabo.
Acknowledgment
The authors thank Anjali Baliga, MD, for her contribution and help in preparing this article
1. Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997;278(16):1363-71.
2. Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54(11 suppl 5):S4-S9.
3. Grossberg GT, Lake JT. The role of the psychiatrist in Alzheimer’s disease. J Clin Psychiatry 1998;59(suppl 9):3-6.
4. Doraiswamy PM, Steffens DC, Pitchumoni S, Tabrizi S. Early recognition of Alzheimer’s disease: what is consensual? What is controversial? What is practical? J Clin Psychiatry 1998;59(suppl 13):6-18.
5. Wilkinson DG, Passmore AP, Bullock R, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 2002;56(6):441-6
6. Jones RW, Soininen H, Hager K, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19(1):58-67.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twentytwo classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. U. S. Bureau of the Census. 2004 International database: Midyear population, by age and sex. Table 094. U.S. Bureau of the Census; 2004.
9. Reminyl (galantamine HBr). Physicians’ desk reference (59th ed). Montvale, NJ: Thomson PDR; 2005:1739.
10. Birks JS, Harvey R. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 2003;(3):CD001190.-
11. Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther 2003;25(6):1634-53.
12. Corey-Bloom J. Galantamine: a review of its use in Alzheimer’s disease and vascular dementia. Int J Clin Pract 2003;57(3):219-23.
13. Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994;271(13):992-8.
14. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000;356(9247):2031-6.
15. Passmore AP, Bayer AJ, Steinhagen-Thiessen E. Cognitive, global, and functional benefits of donepezil in Alzheimer’s disease and vascular dementia: results from large-scale clinical trials. J Neurol Sci 2005;229-30:141-6.
16. Kumar V, Anand R, Messina J, et al. An efficacy and safety analysis of rivastigmine in Alzheimer’s disease patients with concurrent vascular risk factors. Eur J Neurol 2000;7(2):159-69.
17. Kurz AF, Erkinjuntti T, Gauthier S, et al. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359(9314):1283-90.
18. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004;351(24):2509-18.
19. Erkinjuntti T, Kurz A, Small GW, et al. An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clin Ther 2003;25(6):1765-82.
20. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging 2004;21(14):931-7.
21. Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004;63(4):651-7.
22. Emre M. Switching cholinesterase inhibitors in patients with Alzheimer’s disease. Int J Clin Pract Suppl 2002;(127):64-72.
23. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl 2002;(127):45-63.
1. Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997;278(16):1363-71.
2. Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54(11 suppl 5):S4-S9.
3. Grossberg GT, Lake JT. The role of the psychiatrist in Alzheimer’s disease. J Clin Psychiatry 1998;59(suppl 9):3-6.
4. Doraiswamy PM, Steffens DC, Pitchumoni S, Tabrizi S. Early recognition of Alzheimer’s disease: what is consensual? What is controversial? What is practical? J Clin Psychiatry 1998;59(suppl 13):6-18.
5. Wilkinson DG, Passmore AP, Bullock R, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 2002;56(6):441-6
6. Jones RW, Soininen H, Hager K, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19(1):58-67.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twentytwo classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. U. S. Bureau of the Census. 2004 International database: Midyear population, by age and sex. Table 094. U.S. Bureau of the Census; 2004.
9. Reminyl (galantamine HBr). Physicians’ desk reference (59th ed). Montvale, NJ: Thomson PDR; 2005:1739.
10. Birks JS, Harvey R. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 2003;(3):CD001190.-
11. Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther 2003;25(6):1634-53.
12. Corey-Bloom J. Galantamine: a review of its use in Alzheimer’s disease and vascular dementia. Int J Clin Pract 2003;57(3):219-23.
13. Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994;271(13):992-8.
14. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000;356(9247):2031-6.
15. Passmore AP, Bayer AJ, Steinhagen-Thiessen E. Cognitive, global, and functional benefits of donepezil in Alzheimer’s disease and vascular dementia: results from large-scale clinical trials. J Neurol Sci 2005;229-30:141-6.
16. Kumar V, Anand R, Messina J, et al. An efficacy and safety analysis of rivastigmine in Alzheimer’s disease patients with concurrent vascular risk factors. Eur J Neurol 2000;7(2):159-69.
17. Kurz AF, Erkinjuntti T, Gauthier S, et al. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359(9314):1283-90.
18. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004;351(24):2509-18.
19. Erkinjuntti T, Kurz A, Small GW, et al. An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clin Ther 2003;25(6):1765-82.
20. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging 2004;21(14):931-7.
21. Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004;63(4):651-7.
22. Emre M. Switching cholinesterase inhibitors in patients with Alzheimer’s disease. Int J Clin Pract Suppl 2002;(127):64-72.
23. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl 2002;(127):45-63.