User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
‘No energy, no hope,’ and no clear diagnosis
Case submitted by: Mary Ann Barnovitz, MD, psychiatry and internal medicine resident in northern California
Psychiatric consultant: Jaesu Han, MD, assistant clinical professor, departments of psychiatry and family medicine, University of California, Davis.
Starting as a regional problem in the southwestern states, methamphetamine abuse has spread to the rest of the country.1 Commonly known as “ice,” “speed,” “crank,” or “crystal”—to name a few—this synthetically derived stimulant can be used intranasally, swallowed, injected, smoked, or mixed with other drugs.
This case illustrates the type of diagnostic dilemma primary care physicians can see in chronic methamphetamine users. We offer this dialogue to help generalist psychiatrists asked for consultation to unravel the manic, depressive, and substance abuse symptoms that characterize long-term methamphetamine addiction.
Dr. Barnovitz’ patient: Addicted to ‘meth’
Mr. N, age 26, presented at the county outpatient clinic with a history of daily intranasal methamphetamine use. He told me, “I feel like I’m losing my mind and see no reason to go on.” He reported feeling depressed for 7 years and said his primary problem is, “no energy and no hope that things will improve.”
Mr. N complained of decreased concentration, and appetite as well as chronic insomnia. He takes no medications and denies drug allergies. He has no history of tobacco, alcohol, or other illicit drug use. He lives with his ex-girlfriend and has been unemployed for 1 month.
For the past 2 weeks he has been unable to speak slowly, and his thoughts have been “racing too fast to focus.” When I contacted his family (with his permission), they said he is paranoid and persistently claims the FBI is “monitoring his every move.”
Medical history. Mr. N has never been treated for psychiatric conditions. He denies auditory, visual, or tactile hallucinations and has no other medical problems. He has used methamphetamines for 5 years, with no period of sobriety. He said, “I don’t think I have a problem with drugs and see no reason to stop using.” Because of escalating relationship problems with his ex-girlfriend, he reported more-frequent methamphetamine use during the weeks before this assessment.
Physical exam. Mr. N was anxious and hypervigilant, with marked psychomotor agitation. He was otherwise well-related and described his mood as “really down,” with a corresponding labile affect. Vital signs were stable with a pulse of 114 bpm. Neurologic, cardiovascular, pulmonary, and abdominal exam results were normal.
Initial treatment. Despite his depression, I did not think Mr. N warranted psychiatric admission. I started him on paroxetine, 20 mg/d, and referred him for drug rehabilitation.
One week later, Mr. N’s family called me with concerns about his worsening insomnia, depression, paranoid thoughts, and suicidal ideation. I would like help determining if this patient should be admitted for psychiatric evaluation and if symptoms are likely caused by methamphetamine abuse or suggest other psychiatric disorders.
Dr. Han’s consultation
Because of Mr. N’s worsening symptoms and suicidal ideation, I strongly recommend evaluation for inpatient psychiatric hospitalization to allow for methamphetamine detoxification in a safe environment. Ideally, psychotropics would be held and the patient’s mood and psychotic symptoms would improve in a few days, confirming the diagnosis of methamphetamine intoxication.
Acute methamphetamine intoxication (Table 1) is associated with euphoria, talkativeness, and psychomotor agitation that can resemble the manic or mixed phase of bipolar disorder. Methamphetamine withdrawal (Table 2) is associated with dysphoric mood, disturbed sleep, and psychomotor changes that can resemble depression.
Close examination shows Mr. N fulfills criteria for:
- a major depressive episode (feeling depressed for 7 years, decreased energy, decreased concentration, insomnia)
- a manic episode (irritability, pressured speech, racing thoughts, psychomotor agitation, possibly decreased need for sleep).
His contention that the FBI is “monitoring his every move” also introduces a psychotic element.
Mr. N’s presenting diagnosis is amphetamine dependence with a provisional diagnosis of methamphetamine intoxication. Because of ongoing “meth” use, remaining diagnoses to rule out include bipolar disorder, mixed episode with psychotic features, and severe major depression with psychotic features. The drug-induced changes could also be aggravating a primary mood disorder. Obtaining a urine toxicology screen would help document recent intoxication.
Table 1
Symptoms of amphetamine intoxication
| Behavioral |
| Euphoria or affective blunting |
| Changes in sociability |
| Hypervigilance or interpersonal sensitivity |
| Anxiety, tension, or anger |
| Stereotyped behaviors |
| Impaired judgment |
| Impaired social or occupational functioning |
| Physiologic |
| Tachycardia or bradycardia |
| Pupillary dilation |
| Elevated or lowered blood pressure |
| Perspiration or chills |
| Nausea or vomiting |
| Evidence of weight loss |
| Psychomotor agitation or retardation |
| Muscular weakness, respiratory depression, chest pain, or cardiac arrythmias |
| Confusion, seizures, dyskinesias, dystonias, or coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed., text rev. |
Table 2
Symptoms of amphetamine withdrawal
| Dysphoric mood |
| Fatigue |
| Vivid, unpleasant dreams |
| Insomnia or hypersomnia |
| Increased appetite |
| Psychomotor retardation or agitation |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed., text rev. |
Addressing addiction. Diagnostic uncertainty because of continued methamphetamine use makes pharmacologic treatment of mood symptoms problematic. Mr. N’s denial of a drug problem complicates the physician’s job; nevertheless explaining to him how methamphetamine use can contribute to his symptoms is essential. Motivational interviewing (Box)2-4 may help you override resistance and motivate behavior change in a patient who is not yet contemplating the need to change.
Keep in mind that methamphetamine use can be linked to other risky behavior such as having multiple sexual partners. Thus, consider screening for sexually transmitted diseases, including HIV.5 Likewise, despite patients’ claim of only intranasal methamphetamine use, consider assessing for hepatitis C if the physical exam reveals evidence of intravenous drug use.
Mr. N’s symptoms escalated from depressed to suicidal before these interventions could be tried, however. Short-term goals for him now include safety and detoxification. Long-term goals include diagnosis and treatment of a possible underlying mood disorder and continued abstinence from methamphetamine use.
Motivational interviewing aims to change behavior by helping patients explore and resolve ambivalence. Rather than a set of techniques that are “used on” people, it is an interpersonal style not restricted to formal counseling settings.2
When consulting, recommend the following principles during typical office appointments (5 to 15 minutes) to encourage patients to change destructive behaviors over the long-term:
- Seek to understand the person’s frame of reference, particularly through reflective listening
- Express acceptance and affirmation
- Elicit self-motivational statements and expressions of problem recognition from the patient, and selectively reinforce his or her concerns and desires
- Monitor the patient’s degree of readiness to change, and avoid generating resistance by jumping ahead of the patient
- Affirm the patient’s freedom of choice and self-direction.
Self-training manuals and videotapes of motivational interviewing are available, although 1- or 2-day workshops may be more effective.3 Using the techniques alone or to prepare for more intensive treatment has shown favorable outcomes.4 Visit http://www.motivationalinterview.org for more information.
Deciphering mood disorders. Consider the diagnosis of methamphetamine-induced mood disorder only if symptoms persist or are more severe than would be expected from the pattern of use. Anhedonia and depressed mood usually present in these patients well beyond the typical withdrawal period, but these symptoms persist <1 month. Mr. N’s paranoid symptoms are likely related to methamphetamine intoxication and should resolve within 1 week of detoxification. In some chronic “meth” users, delusions and hallucinations persist for months or even years and are very difficult to distinguish from chronic schizophrenia. These patients require long-term antipsychotic treatment.
Mr. N does not have a substantial period of abstinence from methamphetamine use for us to evaluate symptom resolution. However, we do have a 7-year history of what he calls “depression” and only 5 years of methamphetamine use. This hints that a primary mood disorder existed before substance use, but even here we must be cautious. His description of depression resembles a mixed episode of bipolar disorder, with both manic and depressive elements.
Further exploring early symptoms and family history with Mr. N and his family might suggest major depression or bipolar disorder preceding methamphetamine use.
Comorbid bipolar disorder. If his pre-drug use history suggests bipolar disorder or he continues to show mixed mood symptoms despite sustained abstinence, adding a bipolar diagnosis would be reasonable. For his depressive symptoms, avoid using an antidepressant alone because of the risk of “switching” to mania. All antidepressants can cause switching—paroxetine in Mr. N’s case—but tricyclic antidepressants are most often implicated.
Try a mood stabilizer such as valproate, 20 to 30 mg/kg/d, or olanzapine, 7.5 to 10 mg qhs. After a therapeutic dose is attained, reconsider adding an antidepressant if depressive symptoms still predominate.
Comorbid depression. If, on the other hand, Mr. N’s pre-drug history suggests major depression and he shows essentially depressive symptoms after abstinence, adding a diagnosis of major depression would be reasonable. In that case, retry an antidepressant such as an SSRI.
The sobriety challenge. After Mr. N is discharged from the hospital, continued abstinence from methamphetamines will be a priority, whether his mood disorder was drug-induced or primary.
No specific, well-established treatments exist for methamphetamine dependence. Formal treatment programs use cognitive behavioral therapy, contingency management, and a community reinforcement approach. These techniques have been shown to achieve abstinence and prevent relapse in patients with alcohol, cocaine, and opiate dependence but are not more effective than 12-step community support groups.6,7
Success with 12-step programs requires at least weekly participation. Daily attendance during early recovery may be particularly helpful for Mr. N, who may have excessive unstructured time during his unemployment. The treatment setting depends on where services are available and the patient’s ability to pay.
Many methamphetamine users relapse within 1 year; thus view drug treatment in the context of a chronic illness rather than a “cure.” A comprehensive approach with continuing treatment and periodic monitoring appointments is essential.
1. The DASIS Report: Primary Methamphetamine/Amphetamine Treatment Admissions, 1992-2002. Office of Applied Studies, Substance Abuse and Mental Health Services Administration, September 14, 2004.
2. Rollnick S, Miller WR. What is motivational interviewing? Behavioural and Cognitive Psychotherapy 1995;23:325-34.
3. Miller WR, Yahne CE, Moyers TB, et al. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72(6):1050-62.
4. Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725-42.
5. Molitor F, Truax SR, Ruiz JD, Sun RK. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med 1998;168(2):93-7.
6. Morgenstern J, Longabaugh R. Cognitive behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction 2000;95:1475-90.
7. Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol 1996;4:46-54.
Case submitted by: Mary Ann Barnovitz, MD, psychiatry and internal medicine resident in northern California
Psychiatric consultant: Jaesu Han, MD, assistant clinical professor, departments of psychiatry and family medicine, University of California, Davis.
Starting as a regional problem in the southwestern states, methamphetamine abuse has spread to the rest of the country.1 Commonly known as “ice,” “speed,” “crank,” or “crystal”—to name a few—this synthetically derived stimulant can be used intranasally, swallowed, injected, smoked, or mixed with other drugs.
This case illustrates the type of diagnostic dilemma primary care physicians can see in chronic methamphetamine users. We offer this dialogue to help generalist psychiatrists asked for consultation to unravel the manic, depressive, and substance abuse symptoms that characterize long-term methamphetamine addiction.
Dr. Barnovitz’ patient: Addicted to ‘meth’
Mr. N, age 26, presented at the county outpatient clinic with a history of daily intranasal methamphetamine use. He told me, “I feel like I’m losing my mind and see no reason to go on.” He reported feeling depressed for 7 years and said his primary problem is, “no energy and no hope that things will improve.”
Mr. N complained of decreased concentration, and appetite as well as chronic insomnia. He takes no medications and denies drug allergies. He has no history of tobacco, alcohol, or other illicit drug use. He lives with his ex-girlfriend and has been unemployed for 1 month.
For the past 2 weeks he has been unable to speak slowly, and his thoughts have been “racing too fast to focus.” When I contacted his family (with his permission), they said he is paranoid and persistently claims the FBI is “monitoring his every move.”
Medical history. Mr. N has never been treated for psychiatric conditions. He denies auditory, visual, or tactile hallucinations and has no other medical problems. He has used methamphetamines for 5 years, with no period of sobriety. He said, “I don’t think I have a problem with drugs and see no reason to stop using.” Because of escalating relationship problems with his ex-girlfriend, he reported more-frequent methamphetamine use during the weeks before this assessment.
Physical exam. Mr. N was anxious and hypervigilant, with marked psychomotor agitation. He was otherwise well-related and described his mood as “really down,” with a corresponding labile affect. Vital signs were stable with a pulse of 114 bpm. Neurologic, cardiovascular, pulmonary, and abdominal exam results were normal.
Initial treatment. Despite his depression, I did not think Mr. N warranted psychiatric admission. I started him on paroxetine, 20 mg/d, and referred him for drug rehabilitation.
One week later, Mr. N’s family called me with concerns about his worsening insomnia, depression, paranoid thoughts, and suicidal ideation. I would like help determining if this patient should be admitted for psychiatric evaluation and if symptoms are likely caused by methamphetamine abuse or suggest other psychiatric disorders.
Dr. Han’s consultation
Because of Mr. N’s worsening symptoms and suicidal ideation, I strongly recommend evaluation for inpatient psychiatric hospitalization to allow for methamphetamine detoxification in a safe environment. Ideally, psychotropics would be held and the patient’s mood and psychotic symptoms would improve in a few days, confirming the diagnosis of methamphetamine intoxication.
Acute methamphetamine intoxication (Table 1) is associated with euphoria, talkativeness, and psychomotor agitation that can resemble the manic or mixed phase of bipolar disorder. Methamphetamine withdrawal (Table 2) is associated with dysphoric mood, disturbed sleep, and psychomotor changes that can resemble depression.
Close examination shows Mr. N fulfills criteria for:
- a major depressive episode (feeling depressed for 7 years, decreased energy, decreased concentration, insomnia)
- a manic episode (irritability, pressured speech, racing thoughts, psychomotor agitation, possibly decreased need for sleep).
His contention that the FBI is “monitoring his every move” also introduces a psychotic element.
Mr. N’s presenting diagnosis is amphetamine dependence with a provisional diagnosis of methamphetamine intoxication. Because of ongoing “meth” use, remaining diagnoses to rule out include bipolar disorder, mixed episode with psychotic features, and severe major depression with psychotic features. The drug-induced changes could also be aggravating a primary mood disorder. Obtaining a urine toxicology screen would help document recent intoxication.
Table 1
Symptoms of amphetamine intoxication
| Behavioral |
| Euphoria or affective blunting |
| Changes in sociability |
| Hypervigilance or interpersonal sensitivity |
| Anxiety, tension, or anger |
| Stereotyped behaviors |
| Impaired judgment |
| Impaired social or occupational functioning |
| Physiologic |
| Tachycardia or bradycardia |
| Pupillary dilation |
| Elevated or lowered blood pressure |
| Perspiration or chills |
| Nausea or vomiting |
| Evidence of weight loss |
| Psychomotor agitation or retardation |
| Muscular weakness, respiratory depression, chest pain, or cardiac arrythmias |
| Confusion, seizures, dyskinesias, dystonias, or coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed., text rev. |
Table 2
Symptoms of amphetamine withdrawal
| Dysphoric mood |
| Fatigue |
| Vivid, unpleasant dreams |
| Insomnia or hypersomnia |
| Increased appetite |
| Psychomotor retardation or agitation |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed., text rev. |
Addressing addiction. Diagnostic uncertainty because of continued methamphetamine use makes pharmacologic treatment of mood symptoms problematic. Mr. N’s denial of a drug problem complicates the physician’s job; nevertheless explaining to him how methamphetamine use can contribute to his symptoms is essential. Motivational interviewing (Box)2-4 may help you override resistance and motivate behavior change in a patient who is not yet contemplating the need to change.
Keep in mind that methamphetamine use can be linked to other risky behavior such as having multiple sexual partners. Thus, consider screening for sexually transmitted diseases, including HIV.5 Likewise, despite patients’ claim of only intranasal methamphetamine use, consider assessing for hepatitis C if the physical exam reveals evidence of intravenous drug use.
Mr. N’s symptoms escalated from depressed to suicidal before these interventions could be tried, however. Short-term goals for him now include safety and detoxification. Long-term goals include diagnosis and treatment of a possible underlying mood disorder and continued abstinence from methamphetamine use.
Motivational interviewing aims to change behavior by helping patients explore and resolve ambivalence. Rather than a set of techniques that are “used on” people, it is an interpersonal style not restricted to formal counseling settings.2
When consulting, recommend the following principles during typical office appointments (5 to 15 minutes) to encourage patients to change destructive behaviors over the long-term:
- Seek to understand the person’s frame of reference, particularly through reflective listening
- Express acceptance and affirmation
- Elicit self-motivational statements and expressions of problem recognition from the patient, and selectively reinforce his or her concerns and desires
- Monitor the patient’s degree of readiness to change, and avoid generating resistance by jumping ahead of the patient
- Affirm the patient’s freedom of choice and self-direction.
Self-training manuals and videotapes of motivational interviewing are available, although 1- or 2-day workshops may be more effective.3 Using the techniques alone or to prepare for more intensive treatment has shown favorable outcomes.4 Visit http://www.motivationalinterview.org for more information.
Deciphering mood disorders. Consider the diagnosis of methamphetamine-induced mood disorder only if symptoms persist or are more severe than would be expected from the pattern of use. Anhedonia and depressed mood usually present in these patients well beyond the typical withdrawal period, but these symptoms persist <1 month. Mr. N’s paranoid symptoms are likely related to methamphetamine intoxication and should resolve within 1 week of detoxification. In some chronic “meth” users, delusions and hallucinations persist for months or even years and are very difficult to distinguish from chronic schizophrenia. These patients require long-term antipsychotic treatment.
Mr. N does not have a substantial period of abstinence from methamphetamine use for us to evaluate symptom resolution. However, we do have a 7-year history of what he calls “depression” and only 5 years of methamphetamine use. This hints that a primary mood disorder existed before substance use, but even here we must be cautious. His description of depression resembles a mixed episode of bipolar disorder, with both manic and depressive elements.
Further exploring early symptoms and family history with Mr. N and his family might suggest major depression or bipolar disorder preceding methamphetamine use.
Comorbid bipolar disorder. If his pre-drug use history suggests bipolar disorder or he continues to show mixed mood symptoms despite sustained abstinence, adding a bipolar diagnosis would be reasonable. For his depressive symptoms, avoid using an antidepressant alone because of the risk of “switching” to mania. All antidepressants can cause switching—paroxetine in Mr. N’s case—but tricyclic antidepressants are most often implicated.
Try a mood stabilizer such as valproate, 20 to 30 mg/kg/d, or olanzapine, 7.5 to 10 mg qhs. After a therapeutic dose is attained, reconsider adding an antidepressant if depressive symptoms still predominate.
Comorbid depression. If, on the other hand, Mr. N’s pre-drug history suggests major depression and he shows essentially depressive symptoms after abstinence, adding a diagnosis of major depression would be reasonable. In that case, retry an antidepressant such as an SSRI.
The sobriety challenge. After Mr. N is discharged from the hospital, continued abstinence from methamphetamines will be a priority, whether his mood disorder was drug-induced or primary.
No specific, well-established treatments exist for methamphetamine dependence. Formal treatment programs use cognitive behavioral therapy, contingency management, and a community reinforcement approach. These techniques have been shown to achieve abstinence and prevent relapse in patients with alcohol, cocaine, and opiate dependence but are not more effective than 12-step community support groups.6,7
Success with 12-step programs requires at least weekly participation. Daily attendance during early recovery may be particularly helpful for Mr. N, who may have excessive unstructured time during his unemployment. The treatment setting depends on where services are available and the patient’s ability to pay.
Many methamphetamine users relapse within 1 year; thus view drug treatment in the context of a chronic illness rather than a “cure.” A comprehensive approach with continuing treatment and periodic monitoring appointments is essential.
Case submitted by: Mary Ann Barnovitz, MD, psychiatry and internal medicine resident in northern California
Psychiatric consultant: Jaesu Han, MD, assistant clinical professor, departments of psychiatry and family medicine, University of California, Davis.
Starting as a regional problem in the southwestern states, methamphetamine abuse has spread to the rest of the country.1 Commonly known as “ice,” “speed,” “crank,” or “crystal”—to name a few—this synthetically derived stimulant can be used intranasally, swallowed, injected, smoked, or mixed with other drugs.
This case illustrates the type of diagnostic dilemma primary care physicians can see in chronic methamphetamine users. We offer this dialogue to help generalist psychiatrists asked for consultation to unravel the manic, depressive, and substance abuse symptoms that characterize long-term methamphetamine addiction.
Dr. Barnovitz’ patient: Addicted to ‘meth’
Mr. N, age 26, presented at the county outpatient clinic with a history of daily intranasal methamphetamine use. He told me, “I feel like I’m losing my mind and see no reason to go on.” He reported feeling depressed for 7 years and said his primary problem is, “no energy and no hope that things will improve.”
Mr. N complained of decreased concentration, and appetite as well as chronic insomnia. He takes no medications and denies drug allergies. He has no history of tobacco, alcohol, or other illicit drug use. He lives with his ex-girlfriend and has been unemployed for 1 month.
For the past 2 weeks he has been unable to speak slowly, and his thoughts have been “racing too fast to focus.” When I contacted his family (with his permission), they said he is paranoid and persistently claims the FBI is “monitoring his every move.”
Medical history. Mr. N has never been treated for psychiatric conditions. He denies auditory, visual, or tactile hallucinations and has no other medical problems. He has used methamphetamines for 5 years, with no period of sobriety. He said, “I don’t think I have a problem with drugs and see no reason to stop using.” Because of escalating relationship problems with his ex-girlfriend, he reported more-frequent methamphetamine use during the weeks before this assessment.
Physical exam. Mr. N was anxious and hypervigilant, with marked psychomotor agitation. He was otherwise well-related and described his mood as “really down,” with a corresponding labile affect. Vital signs were stable with a pulse of 114 bpm. Neurologic, cardiovascular, pulmonary, and abdominal exam results were normal.
Initial treatment. Despite his depression, I did not think Mr. N warranted psychiatric admission. I started him on paroxetine, 20 mg/d, and referred him for drug rehabilitation.
One week later, Mr. N’s family called me with concerns about his worsening insomnia, depression, paranoid thoughts, and suicidal ideation. I would like help determining if this patient should be admitted for psychiatric evaluation and if symptoms are likely caused by methamphetamine abuse or suggest other psychiatric disorders.
Dr. Han’s consultation
Because of Mr. N’s worsening symptoms and suicidal ideation, I strongly recommend evaluation for inpatient psychiatric hospitalization to allow for methamphetamine detoxification in a safe environment. Ideally, psychotropics would be held and the patient’s mood and psychotic symptoms would improve in a few days, confirming the diagnosis of methamphetamine intoxication.
Acute methamphetamine intoxication (Table 1) is associated with euphoria, talkativeness, and psychomotor agitation that can resemble the manic or mixed phase of bipolar disorder. Methamphetamine withdrawal (Table 2) is associated with dysphoric mood, disturbed sleep, and psychomotor changes that can resemble depression.
Close examination shows Mr. N fulfills criteria for:
- a major depressive episode (feeling depressed for 7 years, decreased energy, decreased concentration, insomnia)
- a manic episode (irritability, pressured speech, racing thoughts, psychomotor agitation, possibly decreased need for sleep).
His contention that the FBI is “monitoring his every move” also introduces a psychotic element.
Mr. N’s presenting diagnosis is amphetamine dependence with a provisional diagnosis of methamphetamine intoxication. Because of ongoing “meth” use, remaining diagnoses to rule out include bipolar disorder, mixed episode with psychotic features, and severe major depression with psychotic features. The drug-induced changes could also be aggravating a primary mood disorder. Obtaining a urine toxicology screen would help document recent intoxication.
Table 1
Symptoms of amphetamine intoxication
| Behavioral |
| Euphoria or affective blunting |
| Changes in sociability |
| Hypervigilance or interpersonal sensitivity |
| Anxiety, tension, or anger |
| Stereotyped behaviors |
| Impaired judgment |
| Impaired social or occupational functioning |
| Physiologic |
| Tachycardia or bradycardia |
| Pupillary dilation |
| Elevated or lowered blood pressure |
| Perspiration or chills |
| Nausea or vomiting |
| Evidence of weight loss |
| Psychomotor agitation or retardation |
| Muscular weakness, respiratory depression, chest pain, or cardiac arrythmias |
| Confusion, seizures, dyskinesias, dystonias, or coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed., text rev. |
Table 2
Symptoms of amphetamine withdrawal
| Dysphoric mood |
| Fatigue |
| Vivid, unpleasant dreams |
| Insomnia or hypersomnia |
| Increased appetite |
| Psychomotor retardation or agitation |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed., text rev. |
Addressing addiction. Diagnostic uncertainty because of continued methamphetamine use makes pharmacologic treatment of mood symptoms problematic. Mr. N’s denial of a drug problem complicates the physician’s job; nevertheless explaining to him how methamphetamine use can contribute to his symptoms is essential. Motivational interviewing (Box)2-4 may help you override resistance and motivate behavior change in a patient who is not yet contemplating the need to change.
Keep in mind that methamphetamine use can be linked to other risky behavior such as having multiple sexual partners. Thus, consider screening for sexually transmitted diseases, including HIV.5 Likewise, despite patients’ claim of only intranasal methamphetamine use, consider assessing for hepatitis C if the physical exam reveals evidence of intravenous drug use.
Mr. N’s symptoms escalated from depressed to suicidal before these interventions could be tried, however. Short-term goals for him now include safety and detoxification. Long-term goals include diagnosis and treatment of a possible underlying mood disorder and continued abstinence from methamphetamine use.
Motivational interviewing aims to change behavior by helping patients explore and resolve ambivalence. Rather than a set of techniques that are “used on” people, it is an interpersonal style not restricted to formal counseling settings.2
When consulting, recommend the following principles during typical office appointments (5 to 15 minutes) to encourage patients to change destructive behaviors over the long-term:
- Seek to understand the person’s frame of reference, particularly through reflective listening
- Express acceptance and affirmation
- Elicit self-motivational statements and expressions of problem recognition from the patient, and selectively reinforce his or her concerns and desires
- Monitor the patient’s degree of readiness to change, and avoid generating resistance by jumping ahead of the patient
- Affirm the patient’s freedom of choice and self-direction.
Self-training manuals and videotapes of motivational interviewing are available, although 1- or 2-day workshops may be more effective.3 Using the techniques alone or to prepare for more intensive treatment has shown favorable outcomes.4 Visit http://www.motivationalinterview.org for more information.
Deciphering mood disorders. Consider the diagnosis of methamphetamine-induced mood disorder only if symptoms persist or are more severe than would be expected from the pattern of use. Anhedonia and depressed mood usually present in these patients well beyond the typical withdrawal period, but these symptoms persist <1 month. Mr. N’s paranoid symptoms are likely related to methamphetamine intoxication and should resolve within 1 week of detoxification. In some chronic “meth” users, delusions and hallucinations persist for months or even years and are very difficult to distinguish from chronic schizophrenia. These patients require long-term antipsychotic treatment.
Mr. N does not have a substantial period of abstinence from methamphetamine use for us to evaluate symptom resolution. However, we do have a 7-year history of what he calls “depression” and only 5 years of methamphetamine use. This hints that a primary mood disorder existed before substance use, but even here we must be cautious. His description of depression resembles a mixed episode of bipolar disorder, with both manic and depressive elements.
Further exploring early symptoms and family history with Mr. N and his family might suggest major depression or bipolar disorder preceding methamphetamine use.
Comorbid bipolar disorder. If his pre-drug use history suggests bipolar disorder or he continues to show mixed mood symptoms despite sustained abstinence, adding a bipolar diagnosis would be reasonable. For his depressive symptoms, avoid using an antidepressant alone because of the risk of “switching” to mania. All antidepressants can cause switching—paroxetine in Mr. N’s case—but tricyclic antidepressants are most often implicated.
Try a mood stabilizer such as valproate, 20 to 30 mg/kg/d, or olanzapine, 7.5 to 10 mg qhs. After a therapeutic dose is attained, reconsider adding an antidepressant if depressive symptoms still predominate.
Comorbid depression. If, on the other hand, Mr. N’s pre-drug history suggests major depression and he shows essentially depressive symptoms after abstinence, adding a diagnosis of major depression would be reasonable. In that case, retry an antidepressant such as an SSRI.
The sobriety challenge. After Mr. N is discharged from the hospital, continued abstinence from methamphetamines will be a priority, whether his mood disorder was drug-induced or primary.
No specific, well-established treatments exist for methamphetamine dependence. Formal treatment programs use cognitive behavioral therapy, contingency management, and a community reinforcement approach. These techniques have been shown to achieve abstinence and prevent relapse in patients with alcohol, cocaine, and opiate dependence but are not more effective than 12-step community support groups.6,7
Success with 12-step programs requires at least weekly participation. Daily attendance during early recovery may be particularly helpful for Mr. N, who may have excessive unstructured time during his unemployment. The treatment setting depends on where services are available and the patient’s ability to pay.
Many methamphetamine users relapse within 1 year; thus view drug treatment in the context of a chronic illness rather than a “cure.” A comprehensive approach with continuing treatment and periodic monitoring appointments is essential.
1. The DASIS Report: Primary Methamphetamine/Amphetamine Treatment Admissions, 1992-2002. Office of Applied Studies, Substance Abuse and Mental Health Services Administration, September 14, 2004.
2. Rollnick S, Miller WR. What is motivational interviewing? Behavioural and Cognitive Psychotherapy 1995;23:325-34.
3. Miller WR, Yahne CE, Moyers TB, et al. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72(6):1050-62.
4. Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725-42.
5. Molitor F, Truax SR, Ruiz JD, Sun RK. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med 1998;168(2):93-7.
6. Morgenstern J, Longabaugh R. Cognitive behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction 2000;95:1475-90.
7. Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol 1996;4:46-54.
1. The DASIS Report: Primary Methamphetamine/Amphetamine Treatment Admissions, 1992-2002. Office of Applied Studies, Substance Abuse and Mental Health Services Administration, September 14, 2004.
2. Rollnick S, Miller WR. What is motivational interviewing? Behavioural and Cognitive Psychotherapy 1995;23:325-34.
3. Miller WR, Yahne CE, Moyers TB, et al. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72(6):1050-62.
4. Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725-42.
5. Molitor F, Truax SR, Ruiz JD, Sun RK. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med 1998;168(2):93-7.
6. Morgenstern J, Longabaugh R. Cognitive behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction 2000;95:1475-90.
7. Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol 1996;4:46-54.
Beyond the mirror: Treating body dysmorphic disorder
Identifying which came first—body dysmorphic disorder (BDD) or comorbid anxiety or depressive disorders—can be as complex as treating the disorder’s delusional thinking and high suicide risk. To help you when working alone or with a psychotherapist, we offer strategies we have found useful for:
- diagnosing BDD
- educating patients and families about it
- choosing and dosing medications
- addressing inaccurate perceptions with targeted cognitive-behavioral therapies.
Though many recommendations are based on published data, we also draw on our clinical experience because research on effective BDD treatments is limited.
Body dysmorphic disorder (BDD) is preoccupation with an imagined defect in physical appearance or excessive concern about a slight physical anomaly that causes significant distress or impairs social, occupational, or other functioning.1 BDD patients have obsessive thoughts about their “flaws” and engage in compulsive behaviors and avoidances related to how they perceive their appearance, similar to behavior seen in obsessive-compulsive disorder. BDD causes great distress and disability, often accompanied by depression and suicidality.2
BDD occurs in an estimated 0.7% of the general population3 and in 6 to 14% of persons receiving treatment for anxiety or depressive disorders.4,5 These estimates may be low, however, as persons with BDD often do not seek treatment. Men and women are equally affected.6 Average age of onset is 16, although diagnosis often doesn’t occur for another 10 to 15 years.7
Assessment
BDD causes patients great distress and disability—often accompanied by major depression—but is easy to miss or misdiagnose (Box).1-7 Even when suicidal, BDD patients often do not reveal their symptoms to clinicians,2 probably because of poor insight or shame about their appearance. When a patient describes being unable to stop thinking about specific aspects of his or her appearance, assess further for BDD.
BDD patients’ conviction that their appearance is defective ranges from good insight to mildly overvalued ideation to frankly delusional.8 They often have ideas of reference (such as thinking others may be looking at their “defective” body part) and delusions of reference (such as being convinced others are talking about their “defective” body part). Asking a patient the questions in Table 1 can help establish the diagnosis. BDD also is included in the Structured Clinical Interview for DSM-IV (SCID). Useful assessment tools include:
- Body Dysmorphic Disorder Questionnaire,9 a 5-minute, patient-rated scale for screening
- Body Dysmorphic Disorder Examination,10 to diagnose BDD, survey BDD symptoms, and measure severity
- Yale-Brown Obsessive-Compulsive Scale modified for Body Dysmorphic Disorder (BDD-YBOCS),11 for measuring symptom severity and changes over time.
Comorbidity. Psychiatric comorbidity is common in BDD (Table 2),6,7,12-14 and deciding which disorder to address first can be difficult. If there is acute mania or non-BDD psychosis, we suggest that you stabilize these before treating BDD. Suicidality or severe substance dependence or abuse may result from BDD and therefore needs to be treated in conjunction with BDD.
If comorbid obsessive-compulsive disorder (OCD) or social phobia symptoms are interconnected with the patient’s BDD, treat concurrently; if not, address sequentially, starting with the more-severe symptoms. For example, symptoms that suggest social phobia (such as fear of public speaking) may be related to BDD, and treatment should focus on BDD. A patient with obsessive fears about how “contaminants” will affect her skin’s appearance may need to have the OCD and BDD addressed concurrently.
For other comorbidities, the treatment hierarchy is less clear. Major depression, for example, may be caused by severe BDD and might not improve until BDD improves. Even when a patient has several concurrent Axis I disorders, don’t over-look treating BDD; otherwise, the patient may remain quite impaired.
Assess suicide risk, as ≥ 25% of BDD patients may attempt suicide in their lifetimes.2 Safety measures include frequent monitoring, medication, family involvement, and—if necessary—hospitalization.
Table 1
Patient interview: Questions to help diagnose BDD
| Are you concerned about specific parts of your appearance that you believe are ugly or defective? |
| Do you find it difficult to stop thinking about parts of your appearance? |
| Do you avoid certain situations, places, or being seen in general because of your appearance? |
| Do you feel anxious, ashamed, disgusted, or depressed by specific aspects of your appearance? |
| Are any of your behaviors influenced by your appearance, such as trying to hide parts of your appearance or taking a long time getting ready to leave your residence? |
| Does your preoccupation cause you a lot of distress, anxiety, disgust, and/or shame? |
| Is preoccupation with your appearance interfering with your social life, ability to work, job performance, or other important areas of your life? |
| Do you tend to use mirrors very often or avoid them? |
| Does what you see in the mirror determine your mood that day? |
| How important do you think appearance is in life? |
| Do you use any oral or topical medications for dermatologic reasons or to prevent hair loss? |
| Have you ever had cosmetic surgery? If so, how satisfied were you with the outcome? Did you have any revisions? |
Table 2
Lifetime prevalence (%) of comorbid Axis I disorders in BDD
| Study | N | Major depression | Social phobia | OCD | Substance use disorders |
|---|---|---|---|---|---|
| Gunstad and Phillips (2003)*12 | 175 | 75 | 37 | 30 | 30 |
| Zimmerman and Mattia (1998)14 | 16 | 69 | 69 | 38 | 6 |
| Perugi et al (1997)13 | 58 | 41 | 12 | 41 | † |
| Veale et al (1996)7 | 50 | 8 | 16 | 6 | 2 |
| Hollander et al (1993)6 | 50 | 68 | 12 | 78 | 22 |
| N: number of study subjects | |||||
| OCD: obsessive-compulsive disorder | |||||
| * Phenomenology group | |||||
| † not reported | |||||
| Source: Adapted and reprinted with permission from reference 12. | |||||
Patient education
Improving insight. Educate patients that BDD is a brain disorder that creates faulty, inaccurate thoughts and perceptions about appearance. Many patients initially resist a BDD diagnosis; delusional thinking and poor insight lead them to assume the “flaw” they see is an accurate perception. They may need to hear about other persons with similar concerns to realize that a psychiatric disorder is causing their distress.
Other helpful resources for improving insight include:
- group therapy
- The Broken Mirror, by Katharine A. Phillips, MD,15 which contains case examples to which BDD sufferers may relate
- Websites and online forums (see Related resources).
Explaining BDD. Discuss possible causes of BDD, giving patients alternate explanations for the physical defects they perceive. Contributing factors may include:
- neurobiological abnormalities and genetic factors16
- a history since childhood of shyness, perfectionism, or anxious temperament
- being teased, abused, or in poor family and peer relationships.17
Emphasize that multiple, different, converging factors cause BDD for each individual.
The obsessive-compulsive cycle. Explain that thoughts create distressing emotions, and that persons with BDD try to relieve or prevent these emotions by performing compulsive behaviors. Compulsions then strengthen the association between intrusive thoughts about appearance “defects” and negative feelings about appearance.
Review a list of common compulsions (Table 3) with BDD patients, as many have engaged in these behaviors for years without realizing they are compulsions.
Table 3
Common BDD compulsions and avoidances
| Excessive grooming |
| Excessive checking or avoidance of mirrors and other reflective surfaces |
| Asking for reassurance about appearance |
| “Camouflaging” (hiding or covering up) supposed defects |
| Scrutinizing the appearance of other people and comparing to oneself |
| Avoiding social interactions |
| Avoidance of certain lighting conditions |
| Skin-picking to “fix” perceived flaws |
| Having repeated cosmetic or dermatological procedures, such as dermabrasion, cosmetic surgery, etc. |
Pharmacotherapy
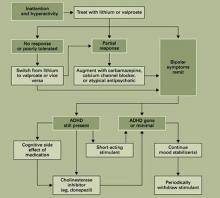
BDD is a severe and complex disorder that often requires multimodal treatment using cognitive-behavioral therapy (CBT) and medication (algorithm).18 In our experience, most BDD patients need medication for the disorder and for common comorbidities. We recommend starting medications before or when beginning CBT for patients with moderate to severe BDD (BDD YBOCs ≥ 20).
Serotonin reuptake inhibitors (SRIs) have reduced BDD symptoms in open-label19,20 and controlled trials.21,22 As first-line treatments, SRIs decrease distress, compulsions, and frequency and intensity of obsessions about perceived defects; they also can improve insight.21-24 SRIs appear equally effective for delusional and nondelusional patients;21,23 whether CBT is similarly effective is unclear.
Relatively high dosages are usually necessary, according to published flexible-dosing trials in BDD,19-23 a retrospective chart review24 and our experience. Try dosages similar to those used for OCD (Table 4) as tolerated, and monitor for side effects. Twelve to 16 weeks of treatment are often needed for a full therapeutic effect.20-21
Augmentation. Consider adding another agent if a full SRI trial achieves partial symptom relief. One open-label trial of 13 BDD patients found that 6 (46%) improved when buspirone (mean dosage 48.3 mg/d) was added to SRI therapy.25 In a chart review, Phillips et al24 reported variable response rates of BDD patients treated with augmentation trials of clomipramine (4/9), buspirone (12/36), lithium (1/5), methylphenidate (1/6), and antipsychotics (2/13).
Very few studies have examined antipsychotic use in BDD. Placebo-controlled data are available only for pimozide.27 Conventional antipsychotics are unlikely to be effective, either as monotherapy26 or augmentation.27 As for the atypicals, olanzapine augmentation showed little to no efficacy in one small trial, although the average dosage used was low (4.6 mg/d).28 In our experience, atypicals—such as aripiprazole, 5 to 30 mg/d; quetiapine 100 to 300 mg/d; olanzapine, 7.5 to 15 mg/d; or risperidone, 1 to 3 mg/d—can improve BDD core symptoms and improve insight.
Benzodiazepines can be useful for acute anxiety or agitation. Carefully monitor benzodiazepine use, however, as substance abuse is relatively common in BDD patients.29
Table 4
Recommended SRI dosages for treating BDD*†
| Drug | Dosage range (mg/d) |
|---|---|
| Citalopram | 40 to 100 |
| Clomipramine | 150 to 250 |
| Escitalopram | 20 to 50 |
| Fluoxetine | 40 to 100 |
| Fluvoxamine | 200 to 400 |
| Paroxetine | 40 to 100 |
| Sertraline | 150 to 400 |
| * Off-label use. | |
| † May exceed FDA-recommended maximum dosages. | |
Specialized cbt techniques
Cognitive restructuring. Trying to convince BDD patients there is nothing wrong with their appearance will not be successful. Instead, we use cognitive restructuring to challenge the rationality of their thoughts and beliefs and to find alternate, more rational ones:
Therapist: “I know I cannot convince you that your (body area) is not defective, but can you give me evidence of how this ‘defect’ has affected your life?”
BDD patient: “Well, I haven’t had a date for a long time. I think this is evidence that my (body part) must be ugly, and that no one wants to date me because of it.”
Therapist: “What are some other possible reasons why you haven’t had a date in a long time? You admitted that you have barely left your house for many months. Is it possible that you have not had a date for a long time because you rarely go outside?”
With cognitive restructuring, patients learn to:
- identify automatic thoughts and beliefs that provoke distress
- examine evidence supporting or refuting these beliefs
- de-catastrophize (such as “What is the worst thing that could happen if you left the house today without checking your [body part]? Do you think you would eventually be able to cope with that?”)
- learn to more accurately assess the probability of feared negative consequences
- arrive at rational responses.
In our experience—which is supported by OCD literature—participating in CBT is very hard for patients with frank delusions, and insight determines how effective cognitive restructuring can be.30 If a patient is convinced a body part is defective, she is unlikely to stay in treatment—much less be open to restructuring her thoughts. Even unsuccessful attempts can help you gauge the intensity of patients’ beliefs, however.
During cognitive restructuring, it is important to uncover patients’ core beliefs (underlying, organizing principles they hold about themselves, others, and the world). BDD patients commonly believe that appearance is of utmost importance and that no one could love them because of their “defect.” The therapist can then help the patient challenge the rationality of those core beliefs.
Behavioral therapy. Basic behavioral therapy attempts to normalize excessive response to appearance concerns and to prepare patients for exposure and response prevention therapy (ERP). Having identified their compulsions, the next step is to guide patients in changing these behaviors, such as by:
- decreasing reassurance-seeking
- reducing avoidance of social situations
- decreasing opportunities to use the mirror
- reducing time spent on the Internet seeking cosmetic solutions
- increasing eye contact in social situations
- decreasing scanning of others’ physical features.
For example, suggest that BDD patients stand at least an arm’s length away when using a mirror for normal grooming. Then, instead of focusing on their body part, they will view it within the context of their entire face and body.
Exposure and response prevention
ERP exposes the patient to situations that evoke negative emotions—primarily shame and anxiety in BDD—so that they gradually habituate to these feelings. Individualize exposure exercises, targeting the body parts each person believes are defective. Because these exercises are intended to induce the discomfort patients usually experience, do not attempt ERP until the patient has had extensive education, developed insight, and has consented to treatment.
Create a hierarchy of ERP tasks (Table 5), ranking situations from low- to high-distress. Address items lower on the hierarchy first, and progress to higher items as the lower ones become easier to perform. Do not attempt the highest-distress items until the patient has improved insight and is not severely ill and suicidal.
During exposures, patients must remain in distress-provoking situations—without performing compulsive behaviors—until their negative feelings decrease by at least 50% of the initial subjective, self-rated distress level. Leaving the situation before stress diminishes may reinforce shame and discomfort. Performing compulsive behaviors during or after an exposure will negate the exposure’s effect.
Mirrors and ERP. Some therapists use mirrors for exposure exercises, but this is a complex issue. Mirror-checking is a common BDD compulsion that provides temporary relief but ultimately reinforces negative, intrusive thoughts about the disliked body area. How BDD patients perceive themselves changes from moment to moment; they may stare at and analyze any reflective surface in hopes that their “defect” will not appear as deformed or ugly that day. Thus, one cannot predict whether looking in the mirror at any one time is an exposure or a compulsion.
ERP exercises for BDD need to emphasize behaviors that involve interactions with the outside world, rather than reinforcing the importance of appearance. Using the mirror for ERP could promote checking compulsions and may send the message that appearance is the focal point of treatment. On the other hand, for patients with persistent mirror avoidance, gradual mirror exposures may be useful. A technique called mirror retraining helps patients objectively view their appearance and has been used with success in some individuals.
Table 5
Exposure and response therapy: a BDD patient’s sample hierarchy
| High-distress tasks | Subjective distress rating (scale of 0 to 100) |
| 1. Purposely creating the appearance of acne/skin defects | 100 |
| 2. Intentionally messing up my hair before going in public | 100 |
| 3. Standing under bright or fluorescent lighting in public | 90 |
| 4. Sitting in a position where others can directly see my face for an extended period | 85 |
| 5. Highlighting my face with a flashlight or bright light, while sitting in front of my therapist or another person. | 80 |
| Lower-distress tasks | |
| 6. Intentionally going outside in daylight hours, instead of only after dark | 70 |
| 7. Not turning away from others in an attempt to prevent them from seeing my face | 65 |
| 8. Standing close to people when talking to them, rather than standing at a distance | 50 |
| 9. Going out in public without camouflaging my hair with hats or scarves | 40 |
Psychosocial development
BDD therapy challenges the disorder’s core theme—that appearance is one’s only important attribute—and helps patients identify and develop qualities not related to appearance. Through social interactions, the BDD patient can:
- develop a multidimensional sense of self
- receive nonappearance-related positive feedback from the outside world.
Explore psychosocial development during the assessment phase and when a patient shows little progress in CBT. In some patients, for example, BDD onset in childhood or adolescence interferes with developmental transition to adulthood.
In our experience, some patients may resist treatment because of conscious and unconscious fears of adult responsibilities and relationships. We focus therapy on making them aware of these phenomena, exploring fears of development, and encouraging them to seek new relationships and responsibilities.
Because a BDD patient’s symptoms often create conflict and distress at home, offer the family support and education about the disorder. Occasionally, forces within the family seem to be working against the individual’s recovery and/or independence.
In some families, an individual with BDD may become the “identified patient,” diverting attention from other dysfunctional family members or relationships. During therapy, the BDD patient’s goal to develop a sense of self that is not appearance-based may run counter to the family’s need to keep him or her in the “sick” role.
If therapy is to succeed, talk to the patient about these dynamics. Consider family therapy if resistance to change is strong. When a patient is not progressing well with CBT, we find understanding the family system can be useful to comprehensive BDD treatment, although this observation remains to be validated.
Preventing and treating relapse
Educate patients that BDD is usually chronic, even when treated with psychotherapy and medication.31 Relapse often occurs, especially when patients discontinue medications on their own24 or drop out of therapy early. No guidelines exist, but based on our experience:
- we continue medication for at least 1 year after a patient improves
- psychotherapy is more variable but may need to last 6 to 12 months or more.
When therapy ends, we encourage patients to practice and reinforce everything they learned during treatment. Casting BDD resurgence as normal—and not as failure—will help patients who relapse to resist the downward spiral of low self-esteem, shame, and turning to the mirror for reassurance. Identifying BDD symptom triggers and developing plans to cope with them may also prevent relapse. CBT “booster sessions” scheduled monthly for 3 to 6 months may help patients who have completed therapy.
FOR CLINICIANS:
- Phillips KA. “I’m as ugly as the elephant man:” How to recognize and treat body dysmorphic disorder. Current Psychiatry. 2002;1(1):58-65.
- Cororve MB, Gleaves DH. Body dysmorphic disorder: a review of conceptualizations, assessment, and treatment strategies. Clin Psychol Rev. 2001;21(6):949-70.
FOR PATIENTS AND FAMILIES:
- Phillips KA. The broken mirror. New York: Oxford University Press; 2005.
- BDD and body image program, Butler Hospital, Providence, RI. BDD education and support. www.BDDcentral.com.
- Winograd A. Director, Accurate Reflections, Los Angeles, CA. Support group and information on BDD and obsessive compulsive spectrum disorders. www.AccurateReflections.com
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Methylphenidate • Ritalin, Concerta
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders (4th ed. text rev.). Washington, DC: American Psychiatric Association; 2000.
2. Phillips KA, Coles ME, Menard W, et al. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry 2005;66(6):717-25.
3. Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. Am J Psychiatry 2001;158(12):2061-3.
4. Wilhelm S, Otto MW, Zucker BG, Pollack MH. Prevalence of body dysmorphic disorder in patients with anxiety disorders. J Anxiety Disord 1997;11(5):499-502.
5. Phillips KA, Nierenberg AA, Brendel G, Fava M. Prevalence and clinical features of body dysmorphic disorder in atypical major depression. J Nerv Ment Dis 1996;184(2):125-9.
6. Hollander E, Cohen L, Simeon D. Body dysmorphic disorder. Psychiatr Ann 1993;23:359-64.
7. Veale D, Boocock A, Gournay K, et al. Body dysmorphic disorder. survey of fifty cases. Br J Psychiatry 1996;169(2):196-201.
8. Phillips KA. Psychosis in body dysmorphic disorder. J Psychiatr Res 2004;38(1):63-72.
9. Dufresne RG, Phillips KA, Vittorio CC, Wilkel CS. A screening questionnaire for body dysmorphic disorder in a cosmetic dermatologic surgery practice. Dermatol Surg 2001;27(5):457-62.
10. Rosen JC, Reiter J. Development of the body dysmorphic disorder examination. Behav Res Ther 1996;34(9):755-66.
11. Phillips KA, Hollander E, Rasmussen SA, et al. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull 1997;33(1):17-22.
12. Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Compr Psychiatry 2003;44(4):270-6.
13. Perugi G, Akiskal HS, Giannotti D, et al. Gender-related differences in body dysmorphic disorder (dysmorphophobia). J Nerv Ment Dis 1997;185(9):578-82.
14. Zimmerman M, Mattia JI. Body dysmorphic disorder in psychiatric outpatients: recognition, prevalence, comorbidity, demographic, and clinical correlates. Compr Psychiatry 1998;39(5):265-70.
15. Phillips KA. The broken mirror. New York: Oxford University Press; 2005.
16. Rauch SL, Phillips KA, Segal E, et al. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Res 2003;122(1):13-19.
17. Veale D. Body dysmorphic disorder. Postgrad Med J 2004;80(940):67-71.
18. Saxena S, Winograd A, Dunkin JJ, et al. A retrospective review of clinical characteristics and treatment response in body dysmorphic disorder versus obsessive-compulsive disorder. J Clin Psychiatry 2001;62:67-72.
19. Phillips KA, Najjar F. An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry 2003;64(6):715-20.
20. Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 1998;59(4):165-71.
21. Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry 2002;59(4):381-8.
22. Hollander E, Allen A, Kwon J, et al. Clomipramine vs desipramine crossover trial in body dysmorphic disorder: Selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry 1999;56(11):1033-9.
23. Phillips KA, McElroy SL, Dwight MM, et al. Delusionality and response to open-label fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 2001;62(2):87-91.
24. Phillips KA, Albertini RS, Siniscalchi JM, et al. Effectiveness of pharmacotherapy for body dysmorphic disorder: a chart-review study. J Clin Psychiatry 2001;62(9):721-7.
25. Phillips KA. An open study of buspirone augmentation of serotonin-reuptake inhibitors in body dysmorphic disorder. Psychopharmacol Bull 1996;32(1):175-80.
26. Phillips KA, McElroy SL, Keck PE, Jr, et al. A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacol Bull 1994;30(2):179-86.
27. Phillips KA. Placebo-controlled study of pimozide augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005;162(2):377-9.
28. Phillips KA. Olanzapine augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005;162(5):1022-3.
29. Grant JE, Menard W, Pagano ME, et al. Substance use disorders in individuals with body dysmorphic disorder. J Clin Psychiatry 2005;66(3):309-16.
30. Foa EB. Failures in treating obsessive-compulsives. Behav Res Ther 1979;17:169-76.
31. Phillips KA, McElroy SL, Keck PE, Jr, et al. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry 1993;150(2):302-8.
Identifying which came first—body dysmorphic disorder (BDD) or comorbid anxiety or depressive disorders—can be as complex as treating the disorder’s delusional thinking and high suicide risk. To help you when working alone or with a psychotherapist, we offer strategies we have found useful for:
- diagnosing BDD
- educating patients and families about it
- choosing and dosing medications
- addressing inaccurate perceptions with targeted cognitive-behavioral therapies.
Though many recommendations are based on published data, we also draw on our clinical experience because research on effective BDD treatments is limited.
Body dysmorphic disorder (BDD) is preoccupation with an imagined defect in physical appearance or excessive concern about a slight physical anomaly that causes significant distress or impairs social, occupational, or other functioning.1 BDD patients have obsessive thoughts about their “flaws” and engage in compulsive behaviors and avoidances related to how they perceive their appearance, similar to behavior seen in obsessive-compulsive disorder. BDD causes great distress and disability, often accompanied by depression and suicidality.2
BDD occurs in an estimated 0.7% of the general population3 and in 6 to 14% of persons receiving treatment for anxiety or depressive disorders.4,5 These estimates may be low, however, as persons with BDD often do not seek treatment. Men and women are equally affected.6 Average age of onset is 16, although diagnosis often doesn’t occur for another 10 to 15 years.7
Assessment
BDD causes patients great distress and disability—often accompanied by major depression—but is easy to miss or misdiagnose (Box).1-7 Even when suicidal, BDD patients often do not reveal their symptoms to clinicians,2 probably because of poor insight or shame about their appearance. When a patient describes being unable to stop thinking about specific aspects of his or her appearance, assess further for BDD.
BDD patients’ conviction that their appearance is defective ranges from good insight to mildly overvalued ideation to frankly delusional.8 They often have ideas of reference (such as thinking others may be looking at their “defective” body part) and delusions of reference (such as being convinced others are talking about their “defective” body part). Asking a patient the questions in Table 1 can help establish the diagnosis. BDD also is included in the Structured Clinical Interview for DSM-IV (SCID). Useful assessment tools include:
- Body Dysmorphic Disorder Questionnaire,9 a 5-minute, patient-rated scale for screening
- Body Dysmorphic Disorder Examination,10 to diagnose BDD, survey BDD symptoms, and measure severity
- Yale-Brown Obsessive-Compulsive Scale modified for Body Dysmorphic Disorder (BDD-YBOCS),11 for measuring symptom severity and changes over time.
Comorbidity. Psychiatric comorbidity is common in BDD (Table 2),6,7,12-14 and deciding which disorder to address first can be difficult. If there is acute mania or non-BDD psychosis, we suggest that you stabilize these before treating BDD. Suicidality or severe substance dependence or abuse may result from BDD and therefore needs to be treated in conjunction with BDD.
If comorbid obsessive-compulsive disorder (OCD) or social phobia symptoms are interconnected with the patient’s BDD, treat concurrently; if not, address sequentially, starting with the more-severe symptoms. For example, symptoms that suggest social phobia (such as fear of public speaking) may be related to BDD, and treatment should focus on BDD. A patient with obsessive fears about how “contaminants” will affect her skin’s appearance may need to have the OCD and BDD addressed concurrently.
For other comorbidities, the treatment hierarchy is less clear. Major depression, for example, may be caused by severe BDD and might not improve until BDD improves. Even when a patient has several concurrent Axis I disorders, don’t over-look treating BDD; otherwise, the patient may remain quite impaired.
Assess suicide risk, as ≥ 25% of BDD patients may attempt suicide in their lifetimes.2 Safety measures include frequent monitoring, medication, family involvement, and—if necessary—hospitalization.
Table 1
Patient interview: Questions to help diagnose BDD
| Are you concerned about specific parts of your appearance that you believe are ugly or defective? |
| Do you find it difficult to stop thinking about parts of your appearance? |
| Do you avoid certain situations, places, or being seen in general because of your appearance? |
| Do you feel anxious, ashamed, disgusted, or depressed by specific aspects of your appearance? |
| Are any of your behaviors influenced by your appearance, such as trying to hide parts of your appearance or taking a long time getting ready to leave your residence? |
| Does your preoccupation cause you a lot of distress, anxiety, disgust, and/or shame? |
| Is preoccupation with your appearance interfering with your social life, ability to work, job performance, or other important areas of your life? |
| Do you tend to use mirrors very often or avoid them? |
| Does what you see in the mirror determine your mood that day? |
| How important do you think appearance is in life? |
| Do you use any oral or topical medications for dermatologic reasons or to prevent hair loss? |
| Have you ever had cosmetic surgery? If so, how satisfied were you with the outcome? Did you have any revisions? |
Table 2
Lifetime prevalence (%) of comorbid Axis I disorders in BDD
| Study | N | Major depression | Social phobia | OCD | Substance use disorders |
|---|---|---|---|---|---|
| Gunstad and Phillips (2003)*12 | 175 | 75 | 37 | 30 | 30 |
| Zimmerman and Mattia (1998)14 | 16 | 69 | 69 | 38 | 6 |
| Perugi et al (1997)13 | 58 | 41 | 12 | 41 | † |
| Veale et al (1996)7 | 50 | 8 | 16 | 6 | 2 |
| Hollander et al (1993)6 | 50 | 68 | 12 | 78 | 22 |
| N: number of study subjects | |||||
| OCD: obsessive-compulsive disorder | |||||
| * Phenomenology group | |||||
| † not reported | |||||
| Source: Adapted and reprinted with permission from reference 12. | |||||
Patient education
Improving insight. Educate patients that BDD is a brain disorder that creates faulty, inaccurate thoughts and perceptions about appearance. Many patients initially resist a BDD diagnosis; delusional thinking and poor insight lead them to assume the “flaw” they see is an accurate perception. They may need to hear about other persons with similar concerns to realize that a psychiatric disorder is causing their distress.
Other helpful resources for improving insight include:
- group therapy
- The Broken Mirror, by Katharine A. Phillips, MD,15 which contains case examples to which BDD sufferers may relate
- Websites and online forums (see Related resources).
Explaining BDD. Discuss possible causes of BDD, giving patients alternate explanations for the physical defects they perceive. Contributing factors may include:
- neurobiological abnormalities and genetic factors16
- a history since childhood of shyness, perfectionism, or anxious temperament
- being teased, abused, or in poor family and peer relationships.17
Emphasize that multiple, different, converging factors cause BDD for each individual.
The obsessive-compulsive cycle. Explain that thoughts create distressing emotions, and that persons with BDD try to relieve or prevent these emotions by performing compulsive behaviors. Compulsions then strengthen the association between intrusive thoughts about appearance “defects” and negative feelings about appearance.
Review a list of common compulsions (Table 3) with BDD patients, as many have engaged in these behaviors for years without realizing they are compulsions.
Table 3
Common BDD compulsions and avoidances
| Excessive grooming |
| Excessive checking or avoidance of mirrors and other reflective surfaces |
| Asking for reassurance about appearance |
| “Camouflaging” (hiding or covering up) supposed defects |
| Scrutinizing the appearance of other people and comparing to oneself |
| Avoiding social interactions |
| Avoidance of certain lighting conditions |
| Skin-picking to “fix” perceived flaws |
| Having repeated cosmetic or dermatological procedures, such as dermabrasion, cosmetic surgery, etc. |
Pharmacotherapy
BDD is a severe and complex disorder that often requires multimodal treatment using cognitive-behavioral therapy (CBT) and medication (algorithm).18 In our experience, most BDD patients need medication for the disorder and for common comorbidities. We recommend starting medications before or when beginning CBT for patients with moderate to severe BDD (BDD YBOCs ≥ 20).
Serotonin reuptake inhibitors (SRIs) have reduced BDD symptoms in open-label19,20 and controlled trials.21,22 As first-line treatments, SRIs decrease distress, compulsions, and frequency and intensity of obsessions about perceived defects; they also can improve insight.21-24 SRIs appear equally effective for delusional and nondelusional patients;21,23 whether CBT is similarly effective is unclear.
Relatively high dosages are usually necessary, according to published flexible-dosing trials in BDD,19-23 a retrospective chart review24 and our experience. Try dosages similar to those used for OCD (Table 4) as tolerated, and monitor for side effects. Twelve to 16 weeks of treatment are often needed for a full therapeutic effect.20-21
Augmentation. Consider adding another agent if a full SRI trial achieves partial symptom relief. One open-label trial of 13 BDD patients found that 6 (46%) improved when buspirone (mean dosage 48.3 mg/d) was added to SRI therapy.25 In a chart review, Phillips et al24 reported variable response rates of BDD patients treated with augmentation trials of clomipramine (4/9), buspirone (12/36), lithium (1/5), methylphenidate (1/6), and antipsychotics (2/13).
Very few studies have examined antipsychotic use in BDD. Placebo-controlled data are available only for pimozide.27 Conventional antipsychotics are unlikely to be effective, either as monotherapy26 or augmentation.27 As for the atypicals, olanzapine augmentation showed little to no efficacy in one small trial, although the average dosage used was low (4.6 mg/d).28 In our experience, atypicals—such as aripiprazole, 5 to 30 mg/d; quetiapine 100 to 300 mg/d; olanzapine, 7.5 to 15 mg/d; or risperidone, 1 to 3 mg/d—can improve BDD core symptoms and improve insight.
Benzodiazepines can be useful for acute anxiety or agitation. Carefully monitor benzodiazepine use, however, as substance abuse is relatively common in BDD patients.29
Table 4
Recommended SRI dosages for treating BDD*†
| Drug | Dosage range (mg/d) |
|---|---|
| Citalopram | 40 to 100 |
| Clomipramine | 150 to 250 |
| Escitalopram | 20 to 50 |
| Fluoxetine | 40 to 100 |
| Fluvoxamine | 200 to 400 |
| Paroxetine | 40 to 100 |
| Sertraline | 150 to 400 |
| * Off-label use. | |
| † May exceed FDA-recommended maximum dosages. | |
Specialized cbt techniques
Cognitive restructuring. Trying to convince BDD patients there is nothing wrong with their appearance will not be successful. Instead, we use cognitive restructuring to challenge the rationality of their thoughts and beliefs and to find alternate, more rational ones:
Therapist: “I know I cannot convince you that your (body area) is not defective, but can you give me evidence of how this ‘defect’ has affected your life?”
BDD patient: “Well, I haven’t had a date for a long time. I think this is evidence that my (body part) must be ugly, and that no one wants to date me because of it.”
Therapist: “What are some other possible reasons why you haven’t had a date in a long time? You admitted that you have barely left your house for many months. Is it possible that you have not had a date for a long time because you rarely go outside?”
With cognitive restructuring, patients learn to:
- identify automatic thoughts and beliefs that provoke distress
- examine evidence supporting or refuting these beliefs
- de-catastrophize (such as “What is the worst thing that could happen if you left the house today without checking your [body part]? Do you think you would eventually be able to cope with that?”)
- learn to more accurately assess the probability of feared negative consequences
- arrive at rational responses.
In our experience—which is supported by OCD literature—participating in CBT is very hard for patients with frank delusions, and insight determines how effective cognitive restructuring can be.30 If a patient is convinced a body part is defective, she is unlikely to stay in treatment—much less be open to restructuring her thoughts. Even unsuccessful attempts can help you gauge the intensity of patients’ beliefs, however.
During cognitive restructuring, it is important to uncover patients’ core beliefs (underlying, organizing principles they hold about themselves, others, and the world). BDD patients commonly believe that appearance is of utmost importance and that no one could love them because of their “defect.” The therapist can then help the patient challenge the rationality of those core beliefs.
Behavioral therapy. Basic behavioral therapy attempts to normalize excessive response to appearance concerns and to prepare patients for exposure and response prevention therapy (ERP). Having identified their compulsions, the next step is to guide patients in changing these behaviors, such as by:
- decreasing reassurance-seeking
- reducing avoidance of social situations
- decreasing opportunities to use the mirror
- reducing time spent on the Internet seeking cosmetic solutions
- increasing eye contact in social situations
- decreasing scanning of others’ physical features.
For example, suggest that BDD patients stand at least an arm’s length away when using a mirror for normal grooming. Then, instead of focusing on their body part, they will view it within the context of their entire face and body.
Exposure and response prevention
ERP exposes the patient to situations that evoke negative emotions—primarily shame and anxiety in BDD—so that they gradually habituate to these feelings. Individualize exposure exercises, targeting the body parts each person believes are defective. Because these exercises are intended to induce the discomfort patients usually experience, do not attempt ERP until the patient has had extensive education, developed insight, and has consented to treatment.
Create a hierarchy of ERP tasks (Table 5), ranking situations from low- to high-distress. Address items lower on the hierarchy first, and progress to higher items as the lower ones become easier to perform. Do not attempt the highest-distress items until the patient has improved insight and is not severely ill and suicidal.
During exposures, patients must remain in distress-provoking situations—without performing compulsive behaviors—until their negative feelings decrease by at least 50% of the initial subjective, self-rated distress level. Leaving the situation before stress diminishes may reinforce shame and discomfort. Performing compulsive behaviors during or after an exposure will negate the exposure’s effect.
Mirrors and ERP. Some therapists use mirrors for exposure exercises, but this is a complex issue. Mirror-checking is a common BDD compulsion that provides temporary relief but ultimately reinforces negative, intrusive thoughts about the disliked body area. How BDD patients perceive themselves changes from moment to moment; they may stare at and analyze any reflective surface in hopes that their “defect” will not appear as deformed or ugly that day. Thus, one cannot predict whether looking in the mirror at any one time is an exposure or a compulsion.
ERP exercises for BDD need to emphasize behaviors that involve interactions with the outside world, rather than reinforcing the importance of appearance. Using the mirror for ERP could promote checking compulsions and may send the message that appearance is the focal point of treatment. On the other hand, for patients with persistent mirror avoidance, gradual mirror exposures may be useful. A technique called mirror retraining helps patients objectively view their appearance and has been used with success in some individuals.
Table 5
Exposure and response therapy: a BDD patient’s sample hierarchy
| High-distress tasks | Subjective distress rating (scale of 0 to 100) |
| 1. Purposely creating the appearance of acne/skin defects | 100 |
| 2. Intentionally messing up my hair before going in public | 100 |
| 3. Standing under bright or fluorescent lighting in public | 90 |
| 4. Sitting in a position where others can directly see my face for an extended period | 85 |
| 5. Highlighting my face with a flashlight or bright light, while sitting in front of my therapist or another person. | 80 |
| Lower-distress tasks | |
| 6. Intentionally going outside in daylight hours, instead of only after dark | 70 |
| 7. Not turning away from others in an attempt to prevent them from seeing my face | 65 |
| 8. Standing close to people when talking to them, rather than standing at a distance | 50 |
| 9. Going out in public without camouflaging my hair with hats or scarves | 40 |
Psychosocial development
BDD therapy challenges the disorder’s core theme—that appearance is one’s only important attribute—and helps patients identify and develop qualities not related to appearance. Through social interactions, the BDD patient can:
- develop a multidimensional sense of self
- receive nonappearance-related positive feedback from the outside world.
Explore psychosocial development during the assessment phase and when a patient shows little progress in CBT. In some patients, for example, BDD onset in childhood or adolescence interferes with developmental transition to adulthood.
In our experience, some patients may resist treatment because of conscious and unconscious fears of adult responsibilities and relationships. We focus therapy on making them aware of these phenomena, exploring fears of development, and encouraging them to seek new relationships and responsibilities.
Because a BDD patient’s symptoms often create conflict and distress at home, offer the family support and education about the disorder. Occasionally, forces within the family seem to be working against the individual’s recovery and/or independence.
In some families, an individual with BDD may become the “identified patient,” diverting attention from other dysfunctional family members or relationships. During therapy, the BDD patient’s goal to develop a sense of self that is not appearance-based may run counter to the family’s need to keep him or her in the “sick” role.
If therapy is to succeed, talk to the patient about these dynamics. Consider family therapy if resistance to change is strong. When a patient is not progressing well with CBT, we find understanding the family system can be useful to comprehensive BDD treatment, although this observation remains to be validated.
Preventing and treating relapse
Educate patients that BDD is usually chronic, even when treated with psychotherapy and medication.31 Relapse often occurs, especially when patients discontinue medications on their own24 or drop out of therapy early. No guidelines exist, but based on our experience:
- we continue medication for at least 1 year after a patient improves
- psychotherapy is more variable but may need to last 6 to 12 months or more.
When therapy ends, we encourage patients to practice and reinforce everything they learned during treatment. Casting BDD resurgence as normal—and not as failure—will help patients who relapse to resist the downward spiral of low self-esteem, shame, and turning to the mirror for reassurance. Identifying BDD symptom triggers and developing plans to cope with them may also prevent relapse. CBT “booster sessions” scheduled monthly for 3 to 6 months may help patients who have completed therapy.
FOR CLINICIANS:
- Phillips KA. “I’m as ugly as the elephant man:” How to recognize and treat body dysmorphic disorder. Current Psychiatry. 2002;1(1):58-65.
- Cororve MB, Gleaves DH. Body dysmorphic disorder: a review of conceptualizations, assessment, and treatment strategies. Clin Psychol Rev. 2001;21(6):949-70.
FOR PATIENTS AND FAMILIES:
- Phillips KA. The broken mirror. New York: Oxford University Press; 2005.
- BDD and body image program, Butler Hospital, Providence, RI. BDD education and support. www.BDDcentral.com.
- Winograd A. Director, Accurate Reflections, Los Angeles, CA. Support group and information on BDD and obsessive compulsive spectrum disorders. www.AccurateReflections.com
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Methylphenidate • Ritalin, Concerta
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Identifying which came first—body dysmorphic disorder (BDD) or comorbid anxiety or depressive disorders—can be as complex as treating the disorder’s delusional thinking and high suicide risk. To help you when working alone or with a psychotherapist, we offer strategies we have found useful for:
- diagnosing BDD
- educating patients and families about it
- choosing and dosing medications
- addressing inaccurate perceptions with targeted cognitive-behavioral therapies.
Though many recommendations are based on published data, we also draw on our clinical experience because research on effective BDD treatments is limited.
Body dysmorphic disorder (BDD) is preoccupation with an imagined defect in physical appearance or excessive concern about a slight physical anomaly that causes significant distress or impairs social, occupational, or other functioning.1 BDD patients have obsessive thoughts about their “flaws” and engage in compulsive behaviors and avoidances related to how they perceive their appearance, similar to behavior seen in obsessive-compulsive disorder. BDD causes great distress and disability, often accompanied by depression and suicidality.2
BDD occurs in an estimated 0.7% of the general population3 and in 6 to 14% of persons receiving treatment for anxiety or depressive disorders.4,5 These estimates may be low, however, as persons with BDD often do not seek treatment. Men and women are equally affected.6 Average age of onset is 16, although diagnosis often doesn’t occur for another 10 to 15 years.7
Assessment
BDD causes patients great distress and disability—often accompanied by major depression—but is easy to miss or misdiagnose (Box).1-7 Even when suicidal, BDD patients often do not reveal their symptoms to clinicians,2 probably because of poor insight or shame about their appearance. When a patient describes being unable to stop thinking about specific aspects of his or her appearance, assess further for BDD.
BDD patients’ conviction that their appearance is defective ranges from good insight to mildly overvalued ideation to frankly delusional.8 They often have ideas of reference (such as thinking others may be looking at their “defective” body part) and delusions of reference (such as being convinced others are talking about their “defective” body part). Asking a patient the questions in Table 1 can help establish the diagnosis. BDD also is included in the Structured Clinical Interview for DSM-IV (SCID). Useful assessment tools include:
- Body Dysmorphic Disorder Questionnaire,9 a 5-minute, patient-rated scale for screening
- Body Dysmorphic Disorder Examination,10 to diagnose BDD, survey BDD symptoms, and measure severity
- Yale-Brown Obsessive-Compulsive Scale modified for Body Dysmorphic Disorder (BDD-YBOCS),11 for measuring symptom severity and changes over time.
Comorbidity. Psychiatric comorbidity is common in BDD (Table 2),6,7,12-14 and deciding which disorder to address first can be difficult. If there is acute mania or non-BDD psychosis, we suggest that you stabilize these before treating BDD. Suicidality or severe substance dependence or abuse may result from BDD and therefore needs to be treated in conjunction with BDD.
If comorbid obsessive-compulsive disorder (OCD) or social phobia symptoms are interconnected with the patient’s BDD, treat concurrently; if not, address sequentially, starting with the more-severe symptoms. For example, symptoms that suggest social phobia (such as fear of public speaking) may be related to BDD, and treatment should focus on BDD. A patient with obsessive fears about how “contaminants” will affect her skin’s appearance may need to have the OCD and BDD addressed concurrently.
For other comorbidities, the treatment hierarchy is less clear. Major depression, for example, may be caused by severe BDD and might not improve until BDD improves. Even when a patient has several concurrent Axis I disorders, don’t over-look treating BDD; otherwise, the patient may remain quite impaired.
Assess suicide risk, as ≥ 25% of BDD patients may attempt suicide in their lifetimes.2 Safety measures include frequent monitoring, medication, family involvement, and—if necessary—hospitalization.
Table 1
Patient interview: Questions to help diagnose BDD
| Are you concerned about specific parts of your appearance that you believe are ugly or defective? |
| Do you find it difficult to stop thinking about parts of your appearance? |
| Do you avoid certain situations, places, or being seen in general because of your appearance? |
| Do you feel anxious, ashamed, disgusted, or depressed by specific aspects of your appearance? |
| Are any of your behaviors influenced by your appearance, such as trying to hide parts of your appearance or taking a long time getting ready to leave your residence? |
| Does your preoccupation cause you a lot of distress, anxiety, disgust, and/or shame? |
| Is preoccupation with your appearance interfering with your social life, ability to work, job performance, or other important areas of your life? |
| Do you tend to use mirrors very often or avoid them? |
| Does what you see in the mirror determine your mood that day? |
| How important do you think appearance is in life? |
| Do you use any oral or topical medications for dermatologic reasons or to prevent hair loss? |
| Have you ever had cosmetic surgery? If so, how satisfied were you with the outcome? Did you have any revisions? |
Table 2
Lifetime prevalence (%) of comorbid Axis I disorders in BDD
| Study | N | Major depression | Social phobia | OCD | Substance use disorders |
|---|---|---|---|---|---|
| Gunstad and Phillips (2003)*12 | 175 | 75 | 37 | 30 | 30 |
| Zimmerman and Mattia (1998)14 | 16 | 69 | 69 | 38 | 6 |
| Perugi et al (1997)13 | 58 | 41 | 12 | 41 | † |
| Veale et al (1996)7 | 50 | 8 | 16 | 6 | 2 |
| Hollander et al (1993)6 | 50 | 68 | 12 | 78 | 22 |
| N: number of study subjects | |||||
| OCD: obsessive-compulsive disorder | |||||
| * Phenomenology group | |||||
| † not reported | |||||
| Source: Adapted and reprinted with permission from reference 12. | |||||
Patient education
Improving insight. Educate patients that BDD is a brain disorder that creates faulty, inaccurate thoughts and perceptions about appearance. Many patients initially resist a BDD diagnosis; delusional thinking and poor insight lead them to assume the “flaw” they see is an accurate perception. They may need to hear about other persons with similar concerns to realize that a psychiatric disorder is causing their distress.
Other helpful resources for improving insight include:
- group therapy
- The Broken Mirror, by Katharine A. Phillips, MD,15 which contains case examples to which BDD sufferers may relate
- Websites and online forums (see Related resources).
Explaining BDD. Discuss possible causes of BDD, giving patients alternate explanations for the physical defects they perceive. Contributing factors may include:
- neurobiological abnormalities and genetic factors16
- a history since childhood of shyness, perfectionism, or anxious temperament
- being teased, abused, or in poor family and peer relationships.17
Emphasize that multiple, different, converging factors cause BDD for each individual.
The obsessive-compulsive cycle. Explain that thoughts create distressing emotions, and that persons with BDD try to relieve or prevent these emotions by performing compulsive behaviors. Compulsions then strengthen the association between intrusive thoughts about appearance “defects” and negative feelings about appearance.
Review a list of common compulsions (Table 3) with BDD patients, as many have engaged in these behaviors for years without realizing they are compulsions.
Table 3
Common BDD compulsions and avoidances
| Excessive grooming |
| Excessive checking or avoidance of mirrors and other reflective surfaces |
| Asking for reassurance about appearance |
| “Camouflaging” (hiding or covering up) supposed defects |
| Scrutinizing the appearance of other people and comparing to oneself |
| Avoiding social interactions |
| Avoidance of certain lighting conditions |
| Skin-picking to “fix” perceived flaws |
| Having repeated cosmetic or dermatological procedures, such as dermabrasion, cosmetic surgery, etc. |
Pharmacotherapy
BDD is a severe and complex disorder that often requires multimodal treatment using cognitive-behavioral therapy (CBT) and medication (algorithm).18 In our experience, most BDD patients need medication for the disorder and for common comorbidities. We recommend starting medications before or when beginning CBT for patients with moderate to severe BDD (BDD YBOCs ≥ 20).
Serotonin reuptake inhibitors (SRIs) have reduced BDD symptoms in open-label19,20 and controlled trials.21,22 As first-line treatments, SRIs decrease distress, compulsions, and frequency and intensity of obsessions about perceived defects; they also can improve insight.21-24 SRIs appear equally effective for delusional and nondelusional patients;21,23 whether CBT is similarly effective is unclear.
Relatively high dosages are usually necessary, according to published flexible-dosing trials in BDD,19-23 a retrospective chart review24 and our experience. Try dosages similar to those used for OCD (Table 4) as tolerated, and monitor for side effects. Twelve to 16 weeks of treatment are often needed for a full therapeutic effect.20-21
Augmentation. Consider adding another agent if a full SRI trial achieves partial symptom relief. One open-label trial of 13 BDD patients found that 6 (46%) improved when buspirone (mean dosage 48.3 mg/d) was added to SRI therapy.25 In a chart review, Phillips et al24 reported variable response rates of BDD patients treated with augmentation trials of clomipramine (4/9), buspirone (12/36), lithium (1/5), methylphenidate (1/6), and antipsychotics (2/13).
Very few studies have examined antipsychotic use in BDD. Placebo-controlled data are available only for pimozide.27 Conventional antipsychotics are unlikely to be effective, either as monotherapy26 or augmentation.27 As for the atypicals, olanzapine augmentation showed little to no efficacy in one small trial, although the average dosage used was low (4.6 mg/d).28 In our experience, atypicals—such as aripiprazole, 5 to 30 mg/d; quetiapine 100 to 300 mg/d; olanzapine, 7.5 to 15 mg/d; or risperidone, 1 to 3 mg/d—can improve BDD core symptoms and improve insight.
Benzodiazepines can be useful for acute anxiety or agitation. Carefully monitor benzodiazepine use, however, as substance abuse is relatively common in BDD patients.29
Table 4
Recommended SRI dosages for treating BDD*†
| Drug | Dosage range (mg/d) |
|---|---|
| Citalopram | 40 to 100 |
| Clomipramine | 150 to 250 |
| Escitalopram | 20 to 50 |
| Fluoxetine | 40 to 100 |
| Fluvoxamine | 200 to 400 |
| Paroxetine | 40 to 100 |
| Sertraline | 150 to 400 |
| * Off-label use. | |
| † May exceed FDA-recommended maximum dosages. | |
Specialized cbt techniques
Cognitive restructuring. Trying to convince BDD patients there is nothing wrong with their appearance will not be successful. Instead, we use cognitive restructuring to challenge the rationality of their thoughts and beliefs and to find alternate, more rational ones:
Therapist: “I know I cannot convince you that your (body area) is not defective, but can you give me evidence of how this ‘defect’ has affected your life?”
BDD patient: “Well, I haven’t had a date for a long time. I think this is evidence that my (body part) must be ugly, and that no one wants to date me because of it.”
Therapist: “What are some other possible reasons why you haven’t had a date in a long time? You admitted that you have barely left your house for many months. Is it possible that you have not had a date for a long time because you rarely go outside?”
With cognitive restructuring, patients learn to:
- identify automatic thoughts and beliefs that provoke distress
- examine evidence supporting or refuting these beliefs
- de-catastrophize (such as “What is the worst thing that could happen if you left the house today without checking your [body part]? Do you think you would eventually be able to cope with that?”)
- learn to more accurately assess the probability of feared negative consequences
- arrive at rational responses.
In our experience—which is supported by OCD literature—participating in CBT is very hard for patients with frank delusions, and insight determines how effective cognitive restructuring can be.30 If a patient is convinced a body part is defective, she is unlikely to stay in treatment—much less be open to restructuring her thoughts. Even unsuccessful attempts can help you gauge the intensity of patients’ beliefs, however.
During cognitive restructuring, it is important to uncover patients’ core beliefs (underlying, organizing principles they hold about themselves, others, and the world). BDD patients commonly believe that appearance is of utmost importance and that no one could love them because of their “defect.” The therapist can then help the patient challenge the rationality of those core beliefs.
Behavioral therapy. Basic behavioral therapy attempts to normalize excessive response to appearance concerns and to prepare patients for exposure and response prevention therapy (ERP). Having identified their compulsions, the next step is to guide patients in changing these behaviors, such as by:
- decreasing reassurance-seeking
- reducing avoidance of social situations
- decreasing opportunities to use the mirror
- reducing time spent on the Internet seeking cosmetic solutions
- increasing eye contact in social situations
- decreasing scanning of others’ physical features.
For example, suggest that BDD patients stand at least an arm’s length away when using a mirror for normal grooming. Then, instead of focusing on their body part, they will view it within the context of their entire face and body.
Exposure and response prevention
ERP exposes the patient to situations that evoke negative emotions—primarily shame and anxiety in BDD—so that they gradually habituate to these feelings. Individualize exposure exercises, targeting the body parts each person believes are defective. Because these exercises are intended to induce the discomfort patients usually experience, do not attempt ERP until the patient has had extensive education, developed insight, and has consented to treatment.
Create a hierarchy of ERP tasks (Table 5), ranking situations from low- to high-distress. Address items lower on the hierarchy first, and progress to higher items as the lower ones become easier to perform. Do not attempt the highest-distress items until the patient has improved insight and is not severely ill and suicidal.
During exposures, patients must remain in distress-provoking situations—without performing compulsive behaviors—until their negative feelings decrease by at least 50% of the initial subjective, self-rated distress level. Leaving the situation before stress diminishes may reinforce shame and discomfort. Performing compulsive behaviors during or after an exposure will negate the exposure’s effect.
Mirrors and ERP. Some therapists use mirrors for exposure exercises, but this is a complex issue. Mirror-checking is a common BDD compulsion that provides temporary relief but ultimately reinforces negative, intrusive thoughts about the disliked body area. How BDD patients perceive themselves changes from moment to moment; they may stare at and analyze any reflective surface in hopes that their “defect” will not appear as deformed or ugly that day. Thus, one cannot predict whether looking in the mirror at any one time is an exposure or a compulsion.
ERP exercises for BDD need to emphasize behaviors that involve interactions with the outside world, rather than reinforcing the importance of appearance. Using the mirror for ERP could promote checking compulsions and may send the message that appearance is the focal point of treatment. On the other hand, for patients with persistent mirror avoidance, gradual mirror exposures may be useful. A technique called mirror retraining helps patients objectively view their appearance and has been used with success in some individuals.
Table 5
Exposure and response therapy: a BDD patient’s sample hierarchy
| High-distress tasks | Subjective distress rating (scale of 0 to 100) |
| 1. Purposely creating the appearance of acne/skin defects | 100 |
| 2. Intentionally messing up my hair before going in public | 100 |
| 3. Standing under bright or fluorescent lighting in public | 90 |
| 4. Sitting in a position where others can directly see my face for an extended period | 85 |
| 5. Highlighting my face with a flashlight or bright light, while sitting in front of my therapist or another person. | 80 |
| Lower-distress tasks | |
| 6. Intentionally going outside in daylight hours, instead of only after dark | 70 |
| 7. Not turning away from others in an attempt to prevent them from seeing my face | 65 |
| 8. Standing close to people when talking to them, rather than standing at a distance | 50 |
| 9. Going out in public without camouflaging my hair with hats or scarves | 40 |
Psychosocial development
BDD therapy challenges the disorder’s core theme—that appearance is one’s only important attribute—and helps patients identify and develop qualities not related to appearance. Through social interactions, the BDD patient can:
- develop a multidimensional sense of self
- receive nonappearance-related positive feedback from the outside world.
Explore psychosocial development during the assessment phase and when a patient shows little progress in CBT. In some patients, for example, BDD onset in childhood or adolescence interferes with developmental transition to adulthood.
In our experience, some patients may resist treatment because of conscious and unconscious fears of adult responsibilities and relationships. We focus therapy on making them aware of these phenomena, exploring fears of development, and encouraging them to seek new relationships and responsibilities.
Because a BDD patient’s symptoms often create conflict and distress at home, offer the family support and education about the disorder. Occasionally, forces within the family seem to be working against the individual’s recovery and/or independence.
In some families, an individual with BDD may become the “identified patient,” diverting attention from other dysfunctional family members or relationships. During therapy, the BDD patient’s goal to develop a sense of self that is not appearance-based may run counter to the family’s need to keep him or her in the “sick” role.
If therapy is to succeed, talk to the patient about these dynamics. Consider family therapy if resistance to change is strong. When a patient is not progressing well with CBT, we find understanding the family system can be useful to comprehensive BDD treatment, although this observation remains to be validated.
Preventing and treating relapse
Educate patients that BDD is usually chronic, even when treated with psychotherapy and medication.31 Relapse often occurs, especially when patients discontinue medications on their own24 or drop out of therapy early. No guidelines exist, but based on our experience:
- we continue medication for at least 1 year after a patient improves
- psychotherapy is more variable but may need to last 6 to 12 months or more.
When therapy ends, we encourage patients to practice and reinforce everything they learned during treatment. Casting BDD resurgence as normal—and not as failure—will help patients who relapse to resist the downward spiral of low self-esteem, shame, and turning to the mirror for reassurance. Identifying BDD symptom triggers and developing plans to cope with them may also prevent relapse. CBT “booster sessions” scheduled monthly for 3 to 6 months may help patients who have completed therapy.
FOR CLINICIANS:
- Phillips KA. “I’m as ugly as the elephant man:” How to recognize and treat body dysmorphic disorder. Current Psychiatry. 2002;1(1):58-65.
- Cororve MB, Gleaves DH. Body dysmorphic disorder: a review of conceptualizations, assessment, and treatment strategies. Clin Psychol Rev. 2001;21(6):949-70.
FOR PATIENTS AND FAMILIES:
- Phillips KA. The broken mirror. New York: Oxford University Press; 2005.
- BDD and body image program, Butler Hospital, Providence, RI. BDD education and support. www.BDDcentral.com.
- Winograd A. Director, Accurate Reflections, Los Angeles, CA. Support group and information on BDD and obsessive compulsive spectrum disorders. www.AccurateReflections.com
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lithium • Lithobid, others
- Methylphenidate • Ritalin, Concerta
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders (4th ed. text rev.). Washington, DC: American Psychiatric Association; 2000.
2. Phillips KA, Coles ME, Menard W, et al. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry 2005;66(6):717-25.
3. Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. Am J Psychiatry 2001;158(12):2061-3.
4. Wilhelm S, Otto MW, Zucker BG, Pollack MH. Prevalence of body dysmorphic disorder in patients with anxiety disorders. J Anxiety Disord 1997;11(5):499-502.
5. Phillips KA, Nierenberg AA, Brendel G, Fava M. Prevalence and clinical features of body dysmorphic disorder in atypical major depression. J Nerv Ment Dis 1996;184(2):125-9.
6. Hollander E, Cohen L, Simeon D. Body dysmorphic disorder. Psychiatr Ann 1993;23:359-64.
7. Veale D, Boocock A, Gournay K, et al. Body dysmorphic disorder. survey of fifty cases. Br J Psychiatry 1996;169(2):196-201.
8. Phillips KA. Psychosis in body dysmorphic disorder. J Psychiatr Res 2004;38(1):63-72.
9. Dufresne RG, Phillips KA, Vittorio CC, Wilkel CS. A screening questionnaire for body dysmorphic disorder in a cosmetic dermatologic surgery practice. Dermatol Surg 2001;27(5):457-62.
10. Rosen JC, Reiter J. Development of the body dysmorphic disorder examination. Behav Res Ther 1996;34(9):755-66.
11. Phillips KA, Hollander E, Rasmussen SA, et al. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull 1997;33(1):17-22.
12. Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Compr Psychiatry 2003;44(4):270-6.
13. Perugi G, Akiskal HS, Giannotti D, et al. Gender-related differences in body dysmorphic disorder (dysmorphophobia). J Nerv Ment Dis 1997;185(9):578-82.
14. Zimmerman M, Mattia JI. Body dysmorphic disorder in psychiatric outpatients: recognition, prevalence, comorbidity, demographic, and clinical correlates. Compr Psychiatry 1998;39(5):265-70.
15. Phillips KA. The broken mirror. New York: Oxford University Press; 2005.
16. Rauch SL, Phillips KA, Segal E, et al. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Res 2003;122(1):13-19.
17. Veale D. Body dysmorphic disorder. Postgrad Med J 2004;80(940):67-71.
18. Saxena S, Winograd A, Dunkin JJ, et al. A retrospective review of clinical characteristics and treatment response in body dysmorphic disorder versus obsessive-compulsive disorder. J Clin Psychiatry 2001;62:67-72.
19. Phillips KA, Najjar F. An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry 2003;64(6):715-20.
20. Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 1998;59(4):165-71.
21. Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry 2002;59(4):381-8.
22. Hollander E, Allen A, Kwon J, et al. Clomipramine vs desipramine crossover trial in body dysmorphic disorder: Selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry 1999;56(11):1033-9.
23. Phillips KA, McElroy SL, Dwight MM, et al. Delusionality and response to open-label fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 2001;62(2):87-91.
24. Phillips KA, Albertini RS, Siniscalchi JM, et al. Effectiveness of pharmacotherapy for body dysmorphic disorder: a chart-review study. J Clin Psychiatry 2001;62(9):721-7.
25. Phillips KA. An open study of buspirone augmentation of serotonin-reuptake inhibitors in body dysmorphic disorder. Psychopharmacol Bull 1996;32(1):175-80.
26. Phillips KA, McElroy SL, Keck PE, Jr, et al. A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacol Bull 1994;30(2):179-86.
27. Phillips KA. Placebo-controlled study of pimozide augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005;162(2):377-9.
28. Phillips KA. Olanzapine augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005;162(5):1022-3.
29. Grant JE, Menard W, Pagano ME, et al. Substance use disorders in individuals with body dysmorphic disorder. J Clin Psychiatry 2005;66(3):309-16.
30. Foa EB. Failures in treating obsessive-compulsives. Behav Res Ther 1979;17:169-76.
31. Phillips KA, McElroy SL, Keck PE, Jr, et al. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry 1993;150(2):302-8.
1. Diagnostic and statistical manual of mental disorders (4th ed. text rev.). Washington, DC: American Psychiatric Association; 2000.
2. Phillips KA, Coles ME, Menard W, et al. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry 2005;66(6):717-25.
3. Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. Am J Psychiatry 2001;158(12):2061-3.
4. Wilhelm S, Otto MW, Zucker BG, Pollack MH. Prevalence of body dysmorphic disorder in patients with anxiety disorders. J Anxiety Disord 1997;11(5):499-502.
5. Phillips KA, Nierenberg AA, Brendel G, Fava M. Prevalence and clinical features of body dysmorphic disorder in atypical major depression. J Nerv Ment Dis 1996;184(2):125-9.
6. Hollander E, Cohen L, Simeon D. Body dysmorphic disorder. Psychiatr Ann 1993;23:359-64.
7. Veale D, Boocock A, Gournay K, et al. Body dysmorphic disorder. survey of fifty cases. Br J Psychiatry 1996;169(2):196-201.
8. Phillips KA. Psychosis in body dysmorphic disorder. J Psychiatr Res 2004;38(1):63-72.
9. Dufresne RG, Phillips KA, Vittorio CC, Wilkel CS. A screening questionnaire for body dysmorphic disorder in a cosmetic dermatologic surgery practice. Dermatol Surg 2001;27(5):457-62.
10. Rosen JC, Reiter J. Development of the body dysmorphic disorder examination. Behav Res Ther 1996;34(9):755-66.
11. Phillips KA, Hollander E, Rasmussen SA, et al. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull 1997;33(1):17-22.
12. Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Compr Psychiatry 2003;44(4):270-6.
13. Perugi G, Akiskal HS, Giannotti D, et al. Gender-related differences in body dysmorphic disorder (dysmorphophobia). J Nerv Ment Dis 1997;185(9):578-82.
14. Zimmerman M, Mattia JI. Body dysmorphic disorder in psychiatric outpatients: recognition, prevalence, comorbidity, demographic, and clinical correlates. Compr Psychiatry 1998;39(5):265-70.
15. Phillips KA. The broken mirror. New York: Oxford University Press; 2005.
16. Rauch SL, Phillips KA, Segal E, et al. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Res 2003;122(1):13-19.
17. Veale D. Body dysmorphic disorder. Postgrad Med J 2004;80(940):67-71.
18. Saxena S, Winograd A, Dunkin JJ, et al. A retrospective review of clinical characteristics and treatment response in body dysmorphic disorder versus obsessive-compulsive disorder. J Clin Psychiatry 2001;62:67-72.
19. Phillips KA, Najjar F. An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry 2003;64(6):715-20.
20. Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 1998;59(4):165-71.
21. Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry 2002;59(4):381-8.
22. Hollander E, Allen A, Kwon J, et al. Clomipramine vs desipramine crossover trial in body dysmorphic disorder: Selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry 1999;56(11):1033-9.
23. Phillips KA, McElroy SL, Dwight MM, et al. Delusionality and response to open-label fluvoxamine in body dysmorphic disorder. J Clin Psychiatry 2001;62(2):87-91.
24. Phillips KA, Albertini RS, Siniscalchi JM, et al. Effectiveness of pharmacotherapy for body dysmorphic disorder: a chart-review study. J Clin Psychiatry 2001;62(9):721-7.
25. Phillips KA. An open study of buspirone augmentation of serotonin-reuptake inhibitors in body dysmorphic disorder. Psychopharmacol Bull 1996;32(1):175-80.
26. Phillips KA, McElroy SL, Keck PE, Jr, et al. A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacol Bull 1994;30(2):179-86.
27. Phillips KA. Placebo-controlled study of pimozide augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005;162(2):377-9.
28. Phillips KA. Olanzapine augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005;162(5):1022-3.
29. Grant JE, Menard W, Pagano ME, et al. Substance use disorders in individuals with body dysmorphic disorder. J Clin Psychiatry 2005;66(3):309-16.
30. Foa EB. Failures in treating obsessive-compulsives. Behav Res Ther 1979;17:169-76.
31. Phillips KA, McElroy SL, Keck PE, Jr, et al. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry 1993;150(2):302-8.
How to reduce mania risk when prescribing stimulants
Stimulants are most effective for childhood attention-deficit/hyperactivity disorder (ADHD),1 but they may induce mania or trigger a treatment-resistant course in children with comorbid bipolar disorder. To help you safely manage these complicated symptoms, this article offers a treatment algorithm and tips to:
- differentiate bipolar and ADHD symptoms
- identify patients at risk for stimulantinduced mania
- choose medications by a hierarchythat may reduce the risk of mood destabilization.
Bipolar mood symptoms emerge before age 20 in about 25% of persons with bipolar disorder (BP).3 Early-onset BP may be more severe than the adult-onset form, with more-affected family members and greater comorbidity with other disorders, especially ADHD.4
In one study, 91% of children with BP also met criteria for ADHD, and 19% of patients with ADHD also received a diagnosis of BP.5 Among 31 children ages 2 to 5 with BP, 80% met criteria for concurrent ADHD.6
Of 40 children age <5 presenting consecutively to a mental health clinic, 11 (28%) met criteria for mania, which was usually associated with euphoria.7 These 11 children also met criteria for ADHD.
A comparison study8 of children (mean age 12) found greater impairment, suicidality, irritability, and sadness in 43 with ADHD plus bipolar depression than in:
- 109 with ADHD plus major depressive disorder
- 128 without depression or mania.
Family prevalence of bipolar disorder and major depression was highest in the bipolar-ADHD group, which also had the highest rates of comorbid conduct disorder, oppositional defiant disorder, alcohol abuse, and agoraphobia. Average age of bipolar diagnosis was 6.3 years.
Adhd and/or bipolar disorder?
Some 70% to 90% of bipolar children and at least 30% to 40% of bipolar adolescents also have ADHD.2 This high comorbidity (Box 1)3-8 might mean that:
- one disorder predisposes to the other
- one is a precursor of the other
- they share common vulnerabilities or causes
- their symptoms overlap so much that patients with one disorder appear to meet criteria for the other.
Some experts contend that bipolar disorder and ADHD usually can be differentiated. Bipolar children score higher than those with ADHD on measures of anxiety/depression, aggression, and attention problems on the Child Behavior Checklist.9 Others believe ADHD symptoms that occur with bipolar disorder are a dimension of bipolar illness rather than a separate disorder.10
For every DSM-IV-TR diagnostic criterion for ADHD, a corresponding diagnostic criterion or common feature of bipolar disorder can be identified (Table 1). Mania and hypomania are obviously associated with hyperactivity and impulsivity, and tangential thinking and distractibility interfere with attention in many patients with bipolar disorder.
Though most ADHD symptoms can occur in bipolar patients, some features of bipolar illness are not characteristic of ADHD (Table 2). Children with ADHD can become hyper-focused on video games and television, for example, but they usually do not become engrossed in long, complicated books or preoccupied with other people, as can occur in bipolar disorder.
Table 1
How ADHD, bipolar symptoms overlap in three domains
| ADHD | Bipolar disorder |
|---|---|
| Inattention | |
| Fails to pay attention | Racing and tangential thoughts |
| Difficulty sustaining attention | Attention driven by racing thoughts, affective themes, and psychosis |
| Does not follow through | Direction of activity shifts with shifting mood |
| Difficulty organizing tasks | Disorganization, psychosis, excessive energy |
| Easily distracted | Distractibility |
| Hyperactivity | |
| Fidgets or squirms | Increased energy and activity |
| Runs about or climbs excessively | Hyperactivity, thrill-seeking |
| Difficulty engaging quietly in leisure activities | Increased energy, boredom |
| Often on the go | Increased energy, hyperactivity |
| Talks excessively | Rapid, pressured speech |
| Impulsivity | |
| Blurts out answers | Rapid, pressured, impulsive speech |
| Difficulty awaiting turn | Hyperactivity, increased energy, impatience, grandiosity |
| Interrupts or intrudes on others | Grandiosity, impatience, pressured speech, increased mental content |
Table 2
Bipolar features not seen in ADHD
|
A treatment hierarchy
Whether a bipolar patient’s attention problems are features of the primary condition or caused by comorbid ADHD may be unclear, but the treatment implications are important. All antidepressants can induce mania/hypomania and increase the risk of mixed states and mood cycling. Because stimulants have antidepressant properties and because some antidepressants are used to treat ADHD, a systematic approach is necessary when treating inattention in juvenile bipolar disorder.
A treatment hierarchy developed by the American Academy of Child and Adolescent Psychiatry Workgroup on Bipolar Disorder recommends beginning psychosocial approaches, such as training parents in behavior management techniques, and:
- treating bipolar disorder first in children who clearly have both ADHD and bipolar disorder
- adding ADHD treatment if ADHD symptoms persist and impair functioning.2
Who’s at risk for mood destabilization?
No data address differences between bipolar patients whose mood disorders deteriorate with stimulant use and those who remain stable. However, risk factors for mood destabilization that have been reported with antidepressants likely also apply to stimulants (Table 3) because stimulants’ adverse effects in bipolar disorder are probably related to their antidepressant properties.
For example, depressed patients who report that an antidepressant worked within hours to days may have bipolar disorder and be at risk for mood destabilization leading to treatment resistance.11 Antidepressant-induced mania also may be more likely:
- when depression is mixed with hypomanic symptoms such as racing thoughts, excessive talkativeness, aggression, irritability, distractibility, and increased drive12
- in patients with a history of antidepressant-induced mania, family history of bipolar disorder, or multiple antidepressant trials.13
Similarly, patients who report feeling better immediately after starting a stimulant—especially if they have evidence of elation, increased irritability, more aggression or impulsivity, decreased sleep, or related symptoms—may be developing stimulant-induced hypomania.
Table 3
Risk factors that may increase risk of stimulant-induced mania
|
| Source: Reference 25 |
Antidepressant-induced mania
Most studies of antidepressant-induced mania have examined outright mania, but hypomania and subsyndromal hypomanic syndromes also may cause significant morbidity and may worsen bipolar disorder’s course. A change in polarity may worsen a patient’s prognosis, but how do we know that antidepressants (or stimulants) caused it?
One suggested criterion is that mania or hypomania develops within 8 weeks of starting an antidepressant for the first time. A chart review of 51 bipolar patients who had extensive life charting found that 82% developed mania while taking an antidepressant—35% of them within 8 weeks.14 The authors attributed 50% of the risk of a first manic episode and/or cycle acceleration to antidepressants and 50% to spontaneous mood swings. They also noted that:
- an initial manic episode appeared to sensitize patients to subsequent manic episodes and rapid cycling
- mood stabilizers did not seem to prevent these outcomes.
A meta-analysis of 12 randomized, controlled, 4-to 12-week trials among 1,088 patients found antidepressants no more likely than placebo to induce mania in the short term.15 These trials did not, however, consider less-severe forms of overstimulation and were not designed to determine mania risk in bipolar depressed patients.
Post-mania cycling. Rapid and ultradian cycling and other forms of deterioration are more likely to occur after a manic or hypomanic episode than after a depressive episode.16
A longitudinal study17 indicated that antidepressant use did not predictably predate rapid cycling when depression was controlled. The authors, however, looked at the correlation between taking an antidepressant at study entry and rapid cycling over 1 year but did not examine whether antidepressants were started or stopped during the study.18 Rapid cycling prevalence declined from 19% to 5% during the study, but they did not determine whether withdrawing antidepressants was associated with this change.
In an earlier prospective study, rapid cycling was more severe while patients were taking antidepressants—despite the use of mood stabilizers—and cycling duration decreased when antidepressants were withdrawn.19
TCAs vs. newer agents. Tricyclic antidepressants (TCAs) are perceived as more likely to induce mania than are selective serotonin reuptake inhibitors (SSRIs) or bupropion. Comparing TCAs’ and newer antidepressants’ switch rates is difficult, however. Most antidepressant trials were designed to show efficacy and safety in unipolar, not bipolar, depression. Moreover, as exclusion criteria have improved with greater awareness of bipolar illness’ polymorphic manifestations, recent studies likely have enrolled fewer bipolar patients—who are most at risk to develop a manic switch—than did earlier TCA trials.
Bupropion, which has been used to treat ADHD, has been thought to have a low risk of inducing mania. In open observation, however, >50% of 11 patients with a history of developing mania with other antidepressants also had a manic switch on bupropion, even though they were taking mood stabilizers.20
Analysis of 155 antidepressant trials in 41 depressed patients found mania risk to be similar with bupropion, SSRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and other newer antidepressants.21 Mania risk doubled when patients were not also taking mood stabilizers.
Going without mood stabilizers. Reports have emerged of patients with bipolar depression taking antidepressants such as fluoxetine and venlafaxine without a mood stabilizer for extended periods, without high rates of mania or mood cycling.22-24 These reports suggest that some bipolar depressed patients can tolerate antidepressants without a mood stabilizer, although we have no way to identify such patients in advance.
Cycle acceleration and treatment resistance may follow antidepressant-induced mania.25 In DSM-IV field trials, antidepressants appeared to have triggered rapid cycling in some 20% of bipolar patients.26 Mood stabilizers were not particularly effective in patients with treatment-resistant ultradian cycling, but withdrawing antidepressants improved outcome.27
Stimulant-induced mania
Compared with antidepressants, less information is available about stimulant-induced mania and rapid cycling.
Some carefully selected bipolar patients may tolerate ongoing stimulant treatment. For example, in 2 years of open experience with 5 bipolar type I and 3 bipolar type II adults, adding methylphenidate or amphetamine for residual depression or sedation was moderately helpful and did not lead to manic switching or drug misuse.28
On the other hand, affective symptoms worsened in nearly two-thirds of 31 children ages 2 to 5 when treated with stimulants or antidepressants without mood stabilizers. Most of the children also had ADHD, and valproate usually helped.6
In 40 patients, mean age 10, who entered the open-label phase of an 8-week trial of divalproex for manic and ADHD symptoms:
- Young Mania Rating Scale (YMRS) scores declined by≥50% in 32 (80%) by week 8, a greater initial response than usually reported in pediatric bipolar disorder with comorbid ADHD.
- ADHD symptoms, measured by Clinical Global Impressions (CGI) scores, did not change significantly.29
Thirty divalproex responders then received mixed amphetamine salts, 10 mg/d, or placebo plus divalproex, crossing over to the other treatment in a 4-week, double-blind trial. ADHD symptoms improved twice as much with the stimulant as with placebo, as measured by CGI scores, whereas YMRS scores did not differ significantly. Among 23 patients who continued the stimulant and divalproex for 12 more weeks, 45% required an increase in stimulant dosage and 1 relapsed into mania.
In this study, ADHD symptoms did not respond to mania treatment but did improve when a stimulant was added. This suggests either that patients had two disorders or that not all bipolar features remit at the same time. The trial’s low stimulant dosage and short duration provide insufficient evidence to support using stimulants over long periods in bipolar children.
LOng-term stimulant effects
Without long-term observations, some investigators have inferred stimulants’ impact on bipolar disorder. A poll of pediatric psychiatrists in the Netherlands, for example, found bipolar disorder in 39 children ages <13 (0.001%) in the previous year, compared with a prevalence of at least 1% in the United States.3 The authors concluded:
- Bipolar disorder emerges at younger ages in the United States than in the Netherlands.
- One reason may be that U.S. psychiatrists have a lower threshold for treating pediatric depression and hyperactivity with antidepressants and stimulants than Dutch psychiatrists do, evoking more-obvious bipolar symptoms at an earlier age.
Observations of 30 U.S. children with a manic episode and ADHD suggested that stimulants can induce manic symptoms:
- Mean age of ADHD onset was 5.5 years.
- Mean age of starting stimulants was 6.9 years.
- Mean age of hypomanic or manic symptom onset was 7.1 years.30
Similarly, in a survey of 34 adolescent manic inpatients, those who had taken stimulants had earlier mania onset (mean age 10.7) than did those who had not taken stimulants (mean age 13.9). Exposure to two stimulants was associated with earlier onset than exposure to one, but comorbid ADHD alone did not affect age of bipolar disorder onset.31
The same group10 reviewed charts of 80 consecutively hospitalized adolescents with a manic or mixed bipolar episode and found stimulant exposure was associated with relatively worse inpatient course, longer length of stay, more emergency medications, and more seclusion and restraint orders. Comorbid ADHD, mixed versus manic episode, and prior antidepressant exposure did not worsen the inpatient course.
A chart review by El-Mallakh et al32 found bipolar disorder was diagnosed at mean age 10.7 in 49 children exposed to antidepressants or stimulants, compared with mean age 12.7 in 44 unexposed children. The exposed group appeared to have tolerated stimulants longer than antidepressants before mania or hypomania emerged.33
In contrast, a retrospective review by Carlson et al34 of data from a longitudinal study of 75 boys with “hyperkinetic reaction of childhood” found that methylphenidate treatment did not appear more common in boys later diagnosed with bipolar disorder than in those without a bipolar diagnosis. This study had obvious methodologic limitations, lacking a hypothesis and focusing on a population with “minimal brain dysfunction.”
In a reanalysis of data from a 1-month methylphenidate titration trial, Galanter et al35 examined whether some 300 children ages 5 to 12 experienced manic symptoms, using the Diagnostic Interview Schedule for Children or the Child Behavior Checklist. At least during this brief trial, patients with and without manic symptoms showed no differences in response rates or adverse effects with stimulant therapy.
Drug treatment hierarchy
Mood stabilizers. Evidence supports starting all bipolar children with a mood stabilizer such as lithium or valproate (Algorithm). A few patients may tolerate stimulants without mood stabilizers, but the risk is high of inducing mania and precipitating a more complex and treatment-resistant disorder.
Carbamazepine can be effective, but it makes some youths aggressive or disorganized. Antipsychotics have not been tested in controlled trials in bipolar children and are not considered first-line treatments, especially as mood stabilizers. They can be effective for childhood mania, but outpatients needing ADHD treatment usually do not have severe manic syndromes.
Algorithm Reducing mania risk: Using stimulants in children with bipolar disorder
Combination therapy. Like many adults, bipolar children often require combinations of mood stabilizers. Kowatch et al36 found that 16 of 20 acutely ill bipolar children (mean age 11) responded to a combination of mood stabilizers after not responding to 8 weeks of a single mood stabilizer. Because bipolar disorder with comorbid ADHD suggests a complex pathophysiology, patients with both disorders may be more likely to require mood-stabilizer combinations than those with bipolar disorder alone.
The goal in treating bipolar disorder is to eliminate symptoms as completely as possible. In bipolar children with comorbid ADHD, be certain that subtle hypomanic symptoms—irritability, decreased sleep, hypersensitivity to interactions, psychosis—have remitted, as they could account for continued inattention. Persistent mood lability may indicate incomplete treatment of the mood disorder, which can increase sensitivity to destabilization by a stimulant.
If a child remains inattentive after the mood disorder is controlled, consider whether medications for the mood disorder are to blame. If medications are working well but causing cognitive side effects, a cholinesterase inhibitor may help.
Adding stimulants. If attention problems persist, consider cautiously adding a stimulant. Informed consent includes telling patients and families about the risks of mood destabilization with stimulants, even when used with mood stabilizers.
Increase stimulant dosage very slowly, and monitor the patient closely for emerging mood instability or subtle evidence of dysphoric hypomania. Address hypersensitivity to sounds, increased irritability, or other signs of activation with more-aggressive mood stabilization before assuming that these are ADHD symptoms that require a higher stimulant dosage.
Sustained-release stimulant preparations are probably second-line choices in patients with concomitant bipolar disorder. With long-acting stimulants, any worsening of the mood disorder will take longer to wear off. Antidepressants such as bupropion are potential alternatives to stimulants but are as likely to induce hypomania and mood cycling and may not be as effective.
Compared with stimulants, atomoxetine has a less-potent antidepressant effect and may be somewhat safer, but it is not as effective for ADHD and is longer-acting. Thus, atomoxetine could be a first-line alternative for comorbid ADHD, with stimulants being added if it is not effective. Clonidine can reduce hyperactivity but does not stabilize mood or improve attention.
When an antidepressant has brought bipolar depression into remission, discontinue it slowly to reduce the risk of rebound while continuing mood stabilizers to prevent recurrence. Because ADHD is not cyclical like bipolar depression, inattention returns for many patients when stimulants are withdrawn.
We do not yet know whether the risk of mood destabilization increases with long-term stimulant use, but discontinuation-induced refractoriness has not been reported with stimulants as it has with mood stabilizers and antidepressants. Thus, trying to withdraw stimulants once ADHD symptoms have remitted is prudent, while supplementing the regimen with behavioral treatments. If managing ADHD symptoms requires continued stimulant treatment, monitor the patient closely for mood destabilization.
Related resources
- American Academy of Child and Adolescent Psychiatry. Facts for families: Bipolar disorder in children and teens.
www.aacap.org/publications/factsFam/bipolar.htm. - National Institute of Mental Health. Database on ADHD.
www.nimh.nih.gov/publicat/adhd.cfm.
Drug brand names
- Amphetamine salts • Adderall
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Clonidine • Catapres
- Dexmethylphenidate • Focalin
- Fluoxetine • Prozac
- Lithium • Lithobid, others
- Methylphenidate • Concerta,
- Ritalin, others
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
Disclosures
Dr. Dubovsky receives research support from UCB Pharma, Forest Laboratories, and Solvay Pharmaceuticals, and is a speaker for Janssen Pharmaceutica and Forest Laboratories.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. AACAP. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(suppl 2):26S-49S.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder: child psychiatric workgroup on bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Reichart CG, Nolen W. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004;78:81-4.
4. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003;53:970-7.
5. Geller B, Zimmerman B, Williams MB, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001;158:125-7.
6. Scheffer RE, Niskala Apps JA. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J Affect Disord. 2004;82(suppl 1):S25-S34.
7. Dilsaver SC, Akiskal HS. Preschool-onset mania: incidence, phenomenology and family history. J Affect Disord 2004;82(suppl 1):S35-S43.
8. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82(suppl 1):S59-S69.
9. Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the Child Behavior Checklist in pediatric bipolar disorder. Biol Psychiatry 2003;53:1021-7.
10. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70:323-7.
11. Piver A. Ultrarapid response to an antidepressant: A clue to bipolarity? Can J Psychiatry 2003;48:427-8.
12. Bottlender R, Sato T, Kleindienst N, et al. Mixed depressive features predict maniform switch during treatment of depression in bipolar I disorder. J Affect Disord 2004;78:149-52.
13. Goldberg J, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003;5:407-20.
14. Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152(8):1130-8.
15. Gijsman HJ, Geddes J, Rendell J, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004;161:1537-47.
16. Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988;145:844-8.
17. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder: demographics, diagnosis, family history and course. Arch Gen Psychiatry 1992;49:126-31.
18. Wehr TA. Can antidepressants induce rapid cycling? Arch Gen Psychiatry 1993;50(6):495-6.
19. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145:179-84.
20. Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story. J Clin Psychiatry 1992;53:443-6.
21. Goldberg J, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry 2002;63:985-91.
22. Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression—low manic switch rate. Bipolar Disord 2004;6:75-81.
23. Simpson SG, DePaulo JR. Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991;11:52-4.
24. Amsterdam JD, Garcia-Espana F. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000;59:225-9.
25. Goldberg J. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract 2003;9:181-94.
26. Bauer M, Calabrese JR, Dunner DL. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry 1994;151:506-15.
27. Prien RF, Kupfer DJ, Mansky PA. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984;41:1096-1104.
28. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6:416-20.
29. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
30. Kowatch RA, Suppes T, Carmody T, et al. Effect size of lithium, divalproex sodium, and carbamezepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000;39:713-20.
31. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
32. El-Mallakh RS, Cicero D, Holman J, Robertson J. Antidepressant exposure in children diagnosed with bipolar disorder. Bipolar Disord 2001;3(suppl 1):35-9.
33. Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003;66:317-22.
34. Carlson G, Loney J, Salisbury H, et al. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol 2000;10:175-84.
35. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13:123-36.
36. Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978-84.
Stimulants are most effective for childhood attention-deficit/hyperactivity disorder (ADHD),1 but they may induce mania or trigger a treatment-resistant course in children with comorbid bipolar disorder. To help you safely manage these complicated symptoms, this article offers a treatment algorithm and tips to:
- differentiate bipolar and ADHD symptoms
- identify patients at risk for stimulantinduced mania
- choose medications by a hierarchythat may reduce the risk of mood destabilization.
Bipolar mood symptoms emerge before age 20 in about 25% of persons with bipolar disorder (BP).3 Early-onset BP may be more severe than the adult-onset form, with more-affected family members and greater comorbidity with other disorders, especially ADHD.4
In one study, 91% of children with BP also met criteria for ADHD, and 19% of patients with ADHD also received a diagnosis of BP.5 Among 31 children ages 2 to 5 with BP, 80% met criteria for concurrent ADHD.6
Of 40 children age <5 presenting consecutively to a mental health clinic, 11 (28%) met criteria for mania, which was usually associated with euphoria.7 These 11 children also met criteria for ADHD.
A comparison study8 of children (mean age 12) found greater impairment, suicidality, irritability, and sadness in 43 with ADHD plus bipolar depression than in:
- 109 with ADHD plus major depressive disorder
- 128 without depression or mania.
Family prevalence of bipolar disorder and major depression was highest in the bipolar-ADHD group, which also had the highest rates of comorbid conduct disorder, oppositional defiant disorder, alcohol abuse, and agoraphobia. Average age of bipolar diagnosis was 6.3 years.
Adhd and/or bipolar disorder?
Some 70% to 90% of bipolar children and at least 30% to 40% of bipolar adolescents also have ADHD.2 This high comorbidity (Box 1)3-8 might mean that:
- one disorder predisposes to the other
- one is a precursor of the other
- they share common vulnerabilities or causes
- their symptoms overlap so much that patients with one disorder appear to meet criteria for the other.
Some experts contend that bipolar disorder and ADHD usually can be differentiated. Bipolar children score higher than those with ADHD on measures of anxiety/depression, aggression, and attention problems on the Child Behavior Checklist.9 Others believe ADHD symptoms that occur with bipolar disorder are a dimension of bipolar illness rather than a separate disorder.10
For every DSM-IV-TR diagnostic criterion for ADHD, a corresponding diagnostic criterion or common feature of bipolar disorder can be identified (Table 1). Mania and hypomania are obviously associated with hyperactivity and impulsivity, and tangential thinking and distractibility interfere with attention in many patients with bipolar disorder.
Though most ADHD symptoms can occur in bipolar patients, some features of bipolar illness are not characteristic of ADHD (Table 2). Children with ADHD can become hyper-focused on video games and television, for example, but they usually do not become engrossed in long, complicated books or preoccupied with other people, as can occur in bipolar disorder.
Table 1
How ADHD, bipolar symptoms overlap in three domains
| ADHD | Bipolar disorder |
|---|---|
| Inattention | |
| Fails to pay attention | Racing and tangential thoughts |
| Difficulty sustaining attention | Attention driven by racing thoughts, affective themes, and psychosis |
| Does not follow through | Direction of activity shifts with shifting mood |
| Difficulty organizing tasks | Disorganization, psychosis, excessive energy |
| Easily distracted | Distractibility |
| Hyperactivity | |
| Fidgets or squirms | Increased energy and activity |
| Runs about or climbs excessively | Hyperactivity, thrill-seeking |
| Difficulty engaging quietly in leisure activities | Increased energy, boredom |
| Often on the go | Increased energy, hyperactivity |
| Talks excessively | Rapid, pressured speech |
| Impulsivity | |
| Blurts out answers | Rapid, pressured, impulsive speech |
| Difficulty awaiting turn | Hyperactivity, increased energy, impatience, grandiosity |
| Interrupts or intrudes on others | Grandiosity, impatience, pressured speech, increased mental content |
Table 2
Bipolar features not seen in ADHD
|
A treatment hierarchy
Whether a bipolar patient’s attention problems are features of the primary condition or caused by comorbid ADHD may be unclear, but the treatment implications are important. All antidepressants can induce mania/hypomania and increase the risk of mixed states and mood cycling. Because stimulants have antidepressant properties and because some antidepressants are used to treat ADHD, a systematic approach is necessary when treating inattention in juvenile bipolar disorder.
A treatment hierarchy developed by the American Academy of Child and Adolescent Psychiatry Workgroup on Bipolar Disorder recommends beginning psychosocial approaches, such as training parents in behavior management techniques, and:
- treating bipolar disorder first in children who clearly have both ADHD and bipolar disorder
- adding ADHD treatment if ADHD symptoms persist and impair functioning.2
Who’s at risk for mood destabilization?
No data address differences between bipolar patients whose mood disorders deteriorate with stimulant use and those who remain stable. However, risk factors for mood destabilization that have been reported with antidepressants likely also apply to stimulants (Table 3) because stimulants’ adverse effects in bipolar disorder are probably related to their antidepressant properties.
For example, depressed patients who report that an antidepressant worked within hours to days may have bipolar disorder and be at risk for mood destabilization leading to treatment resistance.11 Antidepressant-induced mania also may be more likely:
- when depression is mixed with hypomanic symptoms such as racing thoughts, excessive talkativeness, aggression, irritability, distractibility, and increased drive12
- in patients with a history of antidepressant-induced mania, family history of bipolar disorder, or multiple antidepressant trials.13
Similarly, patients who report feeling better immediately after starting a stimulant—especially if they have evidence of elation, increased irritability, more aggression or impulsivity, decreased sleep, or related symptoms—may be developing stimulant-induced hypomania.
Table 3
Risk factors that may increase risk of stimulant-induced mania
|
| Source: Reference 25 |
Antidepressant-induced mania
Most studies of antidepressant-induced mania have examined outright mania, but hypomania and subsyndromal hypomanic syndromes also may cause significant morbidity and may worsen bipolar disorder’s course. A change in polarity may worsen a patient’s prognosis, but how do we know that antidepressants (or stimulants) caused it?
One suggested criterion is that mania or hypomania develops within 8 weeks of starting an antidepressant for the first time. A chart review of 51 bipolar patients who had extensive life charting found that 82% developed mania while taking an antidepressant—35% of them within 8 weeks.14 The authors attributed 50% of the risk of a first manic episode and/or cycle acceleration to antidepressants and 50% to spontaneous mood swings. They also noted that:
- an initial manic episode appeared to sensitize patients to subsequent manic episodes and rapid cycling
- mood stabilizers did not seem to prevent these outcomes.
A meta-analysis of 12 randomized, controlled, 4-to 12-week trials among 1,088 patients found antidepressants no more likely than placebo to induce mania in the short term.15 These trials did not, however, consider less-severe forms of overstimulation and were not designed to determine mania risk in bipolar depressed patients.
Post-mania cycling. Rapid and ultradian cycling and other forms of deterioration are more likely to occur after a manic or hypomanic episode than after a depressive episode.16
A longitudinal study17 indicated that antidepressant use did not predictably predate rapid cycling when depression was controlled. The authors, however, looked at the correlation between taking an antidepressant at study entry and rapid cycling over 1 year but did not examine whether antidepressants were started or stopped during the study.18 Rapid cycling prevalence declined from 19% to 5% during the study, but they did not determine whether withdrawing antidepressants was associated with this change.
In an earlier prospective study, rapid cycling was more severe while patients were taking antidepressants—despite the use of mood stabilizers—and cycling duration decreased when antidepressants were withdrawn.19
TCAs vs. newer agents. Tricyclic antidepressants (TCAs) are perceived as more likely to induce mania than are selective serotonin reuptake inhibitors (SSRIs) or bupropion. Comparing TCAs’ and newer antidepressants’ switch rates is difficult, however. Most antidepressant trials were designed to show efficacy and safety in unipolar, not bipolar, depression. Moreover, as exclusion criteria have improved with greater awareness of bipolar illness’ polymorphic manifestations, recent studies likely have enrolled fewer bipolar patients—who are most at risk to develop a manic switch—than did earlier TCA trials.
Bupropion, which has been used to treat ADHD, has been thought to have a low risk of inducing mania. In open observation, however, >50% of 11 patients with a history of developing mania with other antidepressants also had a manic switch on bupropion, even though they were taking mood stabilizers.20
Analysis of 155 antidepressant trials in 41 depressed patients found mania risk to be similar with bupropion, SSRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and other newer antidepressants.21 Mania risk doubled when patients were not also taking mood stabilizers.
Going without mood stabilizers. Reports have emerged of patients with bipolar depression taking antidepressants such as fluoxetine and venlafaxine without a mood stabilizer for extended periods, without high rates of mania or mood cycling.22-24 These reports suggest that some bipolar depressed patients can tolerate antidepressants without a mood stabilizer, although we have no way to identify such patients in advance.
Cycle acceleration and treatment resistance may follow antidepressant-induced mania.25 In DSM-IV field trials, antidepressants appeared to have triggered rapid cycling in some 20% of bipolar patients.26 Mood stabilizers were not particularly effective in patients with treatment-resistant ultradian cycling, but withdrawing antidepressants improved outcome.27
Stimulant-induced mania
Compared with antidepressants, less information is available about stimulant-induced mania and rapid cycling.
Some carefully selected bipolar patients may tolerate ongoing stimulant treatment. For example, in 2 years of open experience with 5 bipolar type I and 3 bipolar type II adults, adding methylphenidate or amphetamine for residual depression or sedation was moderately helpful and did not lead to manic switching or drug misuse.28
On the other hand, affective symptoms worsened in nearly two-thirds of 31 children ages 2 to 5 when treated with stimulants or antidepressants without mood stabilizers. Most of the children also had ADHD, and valproate usually helped.6
In 40 patients, mean age 10, who entered the open-label phase of an 8-week trial of divalproex for manic and ADHD symptoms:
- Young Mania Rating Scale (YMRS) scores declined by≥50% in 32 (80%) by week 8, a greater initial response than usually reported in pediatric bipolar disorder with comorbid ADHD.
- ADHD symptoms, measured by Clinical Global Impressions (CGI) scores, did not change significantly.29
Thirty divalproex responders then received mixed amphetamine salts, 10 mg/d, or placebo plus divalproex, crossing over to the other treatment in a 4-week, double-blind trial. ADHD symptoms improved twice as much with the stimulant as with placebo, as measured by CGI scores, whereas YMRS scores did not differ significantly. Among 23 patients who continued the stimulant and divalproex for 12 more weeks, 45% required an increase in stimulant dosage and 1 relapsed into mania.
In this study, ADHD symptoms did not respond to mania treatment but did improve when a stimulant was added. This suggests either that patients had two disorders or that not all bipolar features remit at the same time. The trial’s low stimulant dosage and short duration provide insufficient evidence to support using stimulants over long periods in bipolar children.
LOng-term stimulant effects
Without long-term observations, some investigators have inferred stimulants’ impact on bipolar disorder. A poll of pediatric psychiatrists in the Netherlands, for example, found bipolar disorder in 39 children ages <13 (0.001%) in the previous year, compared with a prevalence of at least 1% in the United States.3 The authors concluded:
- Bipolar disorder emerges at younger ages in the United States than in the Netherlands.
- One reason may be that U.S. psychiatrists have a lower threshold for treating pediatric depression and hyperactivity with antidepressants and stimulants than Dutch psychiatrists do, evoking more-obvious bipolar symptoms at an earlier age.
Observations of 30 U.S. children with a manic episode and ADHD suggested that stimulants can induce manic symptoms:
- Mean age of ADHD onset was 5.5 years.
- Mean age of starting stimulants was 6.9 years.
- Mean age of hypomanic or manic symptom onset was 7.1 years.30
Similarly, in a survey of 34 adolescent manic inpatients, those who had taken stimulants had earlier mania onset (mean age 10.7) than did those who had not taken stimulants (mean age 13.9). Exposure to two stimulants was associated with earlier onset than exposure to one, but comorbid ADHD alone did not affect age of bipolar disorder onset.31
The same group10 reviewed charts of 80 consecutively hospitalized adolescents with a manic or mixed bipolar episode and found stimulant exposure was associated with relatively worse inpatient course, longer length of stay, more emergency medications, and more seclusion and restraint orders. Comorbid ADHD, mixed versus manic episode, and prior antidepressant exposure did not worsen the inpatient course.
A chart review by El-Mallakh et al32 found bipolar disorder was diagnosed at mean age 10.7 in 49 children exposed to antidepressants or stimulants, compared with mean age 12.7 in 44 unexposed children. The exposed group appeared to have tolerated stimulants longer than antidepressants before mania or hypomania emerged.33
In contrast, a retrospective review by Carlson et al34 of data from a longitudinal study of 75 boys with “hyperkinetic reaction of childhood” found that methylphenidate treatment did not appear more common in boys later diagnosed with bipolar disorder than in those without a bipolar diagnosis. This study had obvious methodologic limitations, lacking a hypothesis and focusing on a population with “minimal brain dysfunction.”
In a reanalysis of data from a 1-month methylphenidate titration trial, Galanter et al35 examined whether some 300 children ages 5 to 12 experienced manic symptoms, using the Diagnostic Interview Schedule for Children or the Child Behavior Checklist. At least during this brief trial, patients with and without manic symptoms showed no differences in response rates or adverse effects with stimulant therapy.
Drug treatment hierarchy
Mood stabilizers. Evidence supports starting all bipolar children with a mood stabilizer such as lithium or valproate (Algorithm). A few patients may tolerate stimulants without mood stabilizers, but the risk is high of inducing mania and precipitating a more complex and treatment-resistant disorder.
Carbamazepine can be effective, but it makes some youths aggressive or disorganized. Antipsychotics have not been tested in controlled trials in bipolar children and are not considered first-line treatments, especially as mood stabilizers. They can be effective for childhood mania, but outpatients needing ADHD treatment usually do not have severe manic syndromes.
Algorithm Reducing mania risk: Using stimulants in children with bipolar disorder
Combination therapy. Like many adults, bipolar children often require combinations of mood stabilizers. Kowatch et al36 found that 16 of 20 acutely ill bipolar children (mean age 11) responded to a combination of mood stabilizers after not responding to 8 weeks of a single mood stabilizer. Because bipolar disorder with comorbid ADHD suggests a complex pathophysiology, patients with both disorders may be more likely to require mood-stabilizer combinations than those with bipolar disorder alone.
The goal in treating bipolar disorder is to eliminate symptoms as completely as possible. In bipolar children with comorbid ADHD, be certain that subtle hypomanic symptoms—irritability, decreased sleep, hypersensitivity to interactions, psychosis—have remitted, as they could account for continued inattention. Persistent mood lability may indicate incomplete treatment of the mood disorder, which can increase sensitivity to destabilization by a stimulant.
If a child remains inattentive after the mood disorder is controlled, consider whether medications for the mood disorder are to blame. If medications are working well but causing cognitive side effects, a cholinesterase inhibitor may help.
Adding stimulants. If attention problems persist, consider cautiously adding a stimulant. Informed consent includes telling patients and families about the risks of mood destabilization with stimulants, even when used with mood stabilizers.
Increase stimulant dosage very slowly, and monitor the patient closely for emerging mood instability or subtle evidence of dysphoric hypomania. Address hypersensitivity to sounds, increased irritability, or other signs of activation with more-aggressive mood stabilization before assuming that these are ADHD symptoms that require a higher stimulant dosage.
Sustained-release stimulant preparations are probably second-line choices in patients with concomitant bipolar disorder. With long-acting stimulants, any worsening of the mood disorder will take longer to wear off. Antidepressants such as bupropion are potential alternatives to stimulants but are as likely to induce hypomania and mood cycling and may not be as effective.
Compared with stimulants, atomoxetine has a less-potent antidepressant effect and may be somewhat safer, but it is not as effective for ADHD and is longer-acting. Thus, atomoxetine could be a first-line alternative for comorbid ADHD, with stimulants being added if it is not effective. Clonidine can reduce hyperactivity but does not stabilize mood or improve attention.
When an antidepressant has brought bipolar depression into remission, discontinue it slowly to reduce the risk of rebound while continuing mood stabilizers to prevent recurrence. Because ADHD is not cyclical like bipolar depression, inattention returns for many patients when stimulants are withdrawn.
We do not yet know whether the risk of mood destabilization increases with long-term stimulant use, but discontinuation-induced refractoriness has not been reported with stimulants as it has with mood stabilizers and antidepressants. Thus, trying to withdraw stimulants once ADHD symptoms have remitted is prudent, while supplementing the regimen with behavioral treatments. If managing ADHD symptoms requires continued stimulant treatment, monitor the patient closely for mood destabilization.
Related resources
- American Academy of Child and Adolescent Psychiatry. Facts for families: Bipolar disorder in children and teens.
www.aacap.org/publications/factsFam/bipolar.htm. - National Institute of Mental Health. Database on ADHD.
www.nimh.nih.gov/publicat/adhd.cfm.
Drug brand names
- Amphetamine salts • Adderall
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Clonidine • Catapres
- Dexmethylphenidate • Focalin
- Fluoxetine • Prozac
- Lithium • Lithobid, others
- Methylphenidate • Concerta,
- Ritalin, others
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
Disclosures
Dr. Dubovsky receives research support from UCB Pharma, Forest Laboratories, and Solvay Pharmaceuticals, and is a speaker for Janssen Pharmaceutica and Forest Laboratories.
Stimulants are most effective for childhood attention-deficit/hyperactivity disorder (ADHD),1 but they may induce mania or trigger a treatment-resistant course in children with comorbid bipolar disorder. To help you safely manage these complicated symptoms, this article offers a treatment algorithm and tips to:
- differentiate bipolar and ADHD symptoms
- identify patients at risk for stimulantinduced mania
- choose medications by a hierarchythat may reduce the risk of mood destabilization.
Bipolar mood symptoms emerge before age 20 in about 25% of persons with bipolar disorder (BP).3 Early-onset BP may be more severe than the adult-onset form, with more-affected family members and greater comorbidity with other disorders, especially ADHD.4
In one study, 91% of children with BP also met criteria for ADHD, and 19% of patients with ADHD also received a diagnosis of BP.5 Among 31 children ages 2 to 5 with BP, 80% met criteria for concurrent ADHD.6
Of 40 children age <5 presenting consecutively to a mental health clinic, 11 (28%) met criteria for mania, which was usually associated with euphoria.7 These 11 children also met criteria for ADHD.
A comparison study8 of children (mean age 12) found greater impairment, suicidality, irritability, and sadness in 43 with ADHD plus bipolar depression than in:
- 109 with ADHD plus major depressive disorder
- 128 without depression or mania.
Family prevalence of bipolar disorder and major depression was highest in the bipolar-ADHD group, which also had the highest rates of comorbid conduct disorder, oppositional defiant disorder, alcohol abuse, and agoraphobia. Average age of bipolar diagnosis was 6.3 years.
Adhd and/or bipolar disorder?
Some 70% to 90% of bipolar children and at least 30% to 40% of bipolar adolescents also have ADHD.2 This high comorbidity (Box 1)3-8 might mean that:
- one disorder predisposes to the other
- one is a precursor of the other
- they share common vulnerabilities or causes
- their symptoms overlap so much that patients with one disorder appear to meet criteria for the other.
Some experts contend that bipolar disorder and ADHD usually can be differentiated. Bipolar children score higher than those with ADHD on measures of anxiety/depression, aggression, and attention problems on the Child Behavior Checklist.9 Others believe ADHD symptoms that occur with bipolar disorder are a dimension of bipolar illness rather than a separate disorder.10
For every DSM-IV-TR diagnostic criterion for ADHD, a corresponding diagnostic criterion or common feature of bipolar disorder can be identified (Table 1). Mania and hypomania are obviously associated with hyperactivity and impulsivity, and tangential thinking and distractibility interfere with attention in many patients with bipolar disorder.
Though most ADHD symptoms can occur in bipolar patients, some features of bipolar illness are not characteristic of ADHD (Table 2). Children with ADHD can become hyper-focused on video games and television, for example, but they usually do not become engrossed in long, complicated books or preoccupied with other people, as can occur in bipolar disorder.
Table 1
How ADHD, bipolar symptoms overlap in three domains
| ADHD | Bipolar disorder |
|---|---|
| Inattention | |
| Fails to pay attention | Racing and tangential thoughts |
| Difficulty sustaining attention | Attention driven by racing thoughts, affective themes, and psychosis |
| Does not follow through | Direction of activity shifts with shifting mood |
| Difficulty organizing tasks | Disorganization, psychosis, excessive energy |
| Easily distracted | Distractibility |
| Hyperactivity | |
| Fidgets or squirms | Increased energy and activity |
| Runs about or climbs excessively | Hyperactivity, thrill-seeking |
| Difficulty engaging quietly in leisure activities | Increased energy, boredom |
| Often on the go | Increased energy, hyperactivity |
| Talks excessively | Rapid, pressured speech |
| Impulsivity | |
| Blurts out answers | Rapid, pressured, impulsive speech |
| Difficulty awaiting turn | Hyperactivity, increased energy, impatience, grandiosity |
| Interrupts or intrudes on others | Grandiosity, impatience, pressured speech, increased mental content |
Table 2
Bipolar features not seen in ADHD
|
A treatment hierarchy
Whether a bipolar patient’s attention problems are features of the primary condition or caused by comorbid ADHD may be unclear, but the treatment implications are important. All antidepressants can induce mania/hypomania and increase the risk of mixed states and mood cycling. Because stimulants have antidepressant properties and because some antidepressants are used to treat ADHD, a systematic approach is necessary when treating inattention in juvenile bipolar disorder.
A treatment hierarchy developed by the American Academy of Child and Adolescent Psychiatry Workgroup on Bipolar Disorder recommends beginning psychosocial approaches, such as training parents in behavior management techniques, and:
- treating bipolar disorder first in children who clearly have both ADHD and bipolar disorder
- adding ADHD treatment if ADHD symptoms persist and impair functioning.2
Who’s at risk for mood destabilization?
No data address differences between bipolar patients whose mood disorders deteriorate with stimulant use and those who remain stable. However, risk factors for mood destabilization that have been reported with antidepressants likely also apply to stimulants (Table 3) because stimulants’ adverse effects in bipolar disorder are probably related to their antidepressant properties.
For example, depressed patients who report that an antidepressant worked within hours to days may have bipolar disorder and be at risk for mood destabilization leading to treatment resistance.11 Antidepressant-induced mania also may be more likely:
- when depression is mixed with hypomanic symptoms such as racing thoughts, excessive talkativeness, aggression, irritability, distractibility, and increased drive12
- in patients with a history of antidepressant-induced mania, family history of bipolar disorder, or multiple antidepressant trials.13
Similarly, patients who report feeling better immediately after starting a stimulant—especially if they have evidence of elation, increased irritability, more aggression or impulsivity, decreased sleep, or related symptoms—may be developing stimulant-induced hypomania.
Table 3
Risk factors that may increase risk of stimulant-induced mania
|
| Source: Reference 25 |
Antidepressant-induced mania
Most studies of antidepressant-induced mania have examined outright mania, but hypomania and subsyndromal hypomanic syndromes also may cause significant morbidity and may worsen bipolar disorder’s course. A change in polarity may worsen a patient’s prognosis, but how do we know that antidepressants (or stimulants) caused it?
One suggested criterion is that mania or hypomania develops within 8 weeks of starting an antidepressant for the first time. A chart review of 51 bipolar patients who had extensive life charting found that 82% developed mania while taking an antidepressant—35% of them within 8 weeks.14 The authors attributed 50% of the risk of a first manic episode and/or cycle acceleration to antidepressants and 50% to spontaneous mood swings. They also noted that:
- an initial manic episode appeared to sensitize patients to subsequent manic episodes and rapid cycling
- mood stabilizers did not seem to prevent these outcomes.
A meta-analysis of 12 randomized, controlled, 4-to 12-week trials among 1,088 patients found antidepressants no more likely than placebo to induce mania in the short term.15 These trials did not, however, consider less-severe forms of overstimulation and were not designed to determine mania risk in bipolar depressed patients.
Post-mania cycling. Rapid and ultradian cycling and other forms of deterioration are more likely to occur after a manic or hypomanic episode than after a depressive episode.16
A longitudinal study17 indicated that antidepressant use did not predictably predate rapid cycling when depression was controlled. The authors, however, looked at the correlation between taking an antidepressant at study entry and rapid cycling over 1 year but did not examine whether antidepressants were started or stopped during the study.18 Rapid cycling prevalence declined from 19% to 5% during the study, but they did not determine whether withdrawing antidepressants was associated with this change.
In an earlier prospective study, rapid cycling was more severe while patients were taking antidepressants—despite the use of mood stabilizers—and cycling duration decreased when antidepressants were withdrawn.19
TCAs vs. newer agents. Tricyclic antidepressants (TCAs) are perceived as more likely to induce mania than are selective serotonin reuptake inhibitors (SSRIs) or bupropion. Comparing TCAs’ and newer antidepressants’ switch rates is difficult, however. Most antidepressant trials were designed to show efficacy and safety in unipolar, not bipolar, depression. Moreover, as exclusion criteria have improved with greater awareness of bipolar illness’ polymorphic manifestations, recent studies likely have enrolled fewer bipolar patients—who are most at risk to develop a manic switch—than did earlier TCA trials.
Bupropion, which has been used to treat ADHD, has been thought to have a low risk of inducing mania. In open observation, however, >50% of 11 patients with a history of developing mania with other antidepressants also had a manic switch on bupropion, even though they were taking mood stabilizers.20
Analysis of 155 antidepressant trials in 41 depressed patients found mania risk to be similar with bupropion, SSRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and other newer antidepressants.21 Mania risk doubled when patients were not also taking mood stabilizers.
Going without mood stabilizers. Reports have emerged of patients with bipolar depression taking antidepressants such as fluoxetine and venlafaxine without a mood stabilizer for extended periods, without high rates of mania or mood cycling.22-24 These reports suggest that some bipolar depressed patients can tolerate antidepressants without a mood stabilizer, although we have no way to identify such patients in advance.
Cycle acceleration and treatment resistance may follow antidepressant-induced mania.25 In DSM-IV field trials, antidepressants appeared to have triggered rapid cycling in some 20% of bipolar patients.26 Mood stabilizers were not particularly effective in patients with treatment-resistant ultradian cycling, but withdrawing antidepressants improved outcome.27
Stimulant-induced mania
Compared with antidepressants, less information is available about stimulant-induced mania and rapid cycling.
Some carefully selected bipolar patients may tolerate ongoing stimulant treatment. For example, in 2 years of open experience with 5 bipolar type I and 3 bipolar type II adults, adding methylphenidate or amphetamine for residual depression or sedation was moderately helpful and did not lead to manic switching or drug misuse.28
On the other hand, affective symptoms worsened in nearly two-thirds of 31 children ages 2 to 5 when treated with stimulants or antidepressants without mood stabilizers. Most of the children also had ADHD, and valproate usually helped.6
In 40 patients, mean age 10, who entered the open-label phase of an 8-week trial of divalproex for manic and ADHD symptoms:
- Young Mania Rating Scale (YMRS) scores declined by≥50% in 32 (80%) by week 8, a greater initial response than usually reported in pediatric bipolar disorder with comorbid ADHD.
- ADHD symptoms, measured by Clinical Global Impressions (CGI) scores, did not change significantly.29
Thirty divalproex responders then received mixed amphetamine salts, 10 mg/d, or placebo plus divalproex, crossing over to the other treatment in a 4-week, double-blind trial. ADHD symptoms improved twice as much with the stimulant as with placebo, as measured by CGI scores, whereas YMRS scores did not differ significantly. Among 23 patients who continued the stimulant and divalproex for 12 more weeks, 45% required an increase in stimulant dosage and 1 relapsed into mania.
In this study, ADHD symptoms did not respond to mania treatment but did improve when a stimulant was added. This suggests either that patients had two disorders or that not all bipolar features remit at the same time. The trial’s low stimulant dosage and short duration provide insufficient evidence to support using stimulants over long periods in bipolar children.
LOng-term stimulant effects
Without long-term observations, some investigators have inferred stimulants’ impact on bipolar disorder. A poll of pediatric psychiatrists in the Netherlands, for example, found bipolar disorder in 39 children ages <13 (0.001%) in the previous year, compared with a prevalence of at least 1% in the United States.3 The authors concluded:
- Bipolar disorder emerges at younger ages in the United States than in the Netherlands.
- One reason may be that U.S. psychiatrists have a lower threshold for treating pediatric depression and hyperactivity with antidepressants and stimulants than Dutch psychiatrists do, evoking more-obvious bipolar symptoms at an earlier age.
Observations of 30 U.S. children with a manic episode and ADHD suggested that stimulants can induce manic symptoms:
- Mean age of ADHD onset was 5.5 years.
- Mean age of starting stimulants was 6.9 years.
- Mean age of hypomanic or manic symptom onset was 7.1 years.30
Similarly, in a survey of 34 adolescent manic inpatients, those who had taken stimulants had earlier mania onset (mean age 10.7) than did those who had not taken stimulants (mean age 13.9). Exposure to two stimulants was associated with earlier onset than exposure to one, but comorbid ADHD alone did not affect age of bipolar disorder onset.31
The same group10 reviewed charts of 80 consecutively hospitalized adolescents with a manic or mixed bipolar episode and found stimulant exposure was associated with relatively worse inpatient course, longer length of stay, more emergency medications, and more seclusion and restraint orders. Comorbid ADHD, mixed versus manic episode, and prior antidepressant exposure did not worsen the inpatient course.
A chart review by El-Mallakh et al32 found bipolar disorder was diagnosed at mean age 10.7 in 49 children exposed to antidepressants or stimulants, compared with mean age 12.7 in 44 unexposed children. The exposed group appeared to have tolerated stimulants longer than antidepressants before mania or hypomania emerged.33
In contrast, a retrospective review by Carlson et al34 of data from a longitudinal study of 75 boys with “hyperkinetic reaction of childhood” found that methylphenidate treatment did not appear more common in boys later diagnosed with bipolar disorder than in those without a bipolar diagnosis. This study had obvious methodologic limitations, lacking a hypothesis and focusing on a population with “minimal brain dysfunction.”
In a reanalysis of data from a 1-month methylphenidate titration trial, Galanter et al35 examined whether some 300 children ages 5 to 12 experienced manic symptoms, using the Diagnostic Interview Schedule for Children or the Child Behavior Checklist. At least during this brief trial, patients with and without manic symptoms showed no differences in response rates or adverse effects with stimulant therapy.
Drug treatment hierarchy
Mood stabilizers. Evidence supports starting all bipolar children with a mood stabilizer such as lithium or valproate (Algorithm). A few patients may tolerate stimulants without mood stabilizers, but the risk is high of inducing mania and precipitating a more complex and treatment-resistant disorder.
Carbamazepine can be effective, but it makes some youths aggressive or disorganized. Antipsychotics have not been tested in controlled trials in bipolar children and are not considered first-line treatments, especially as mood stabilizers. They can be effective for childhood mania, but outpatients needing ADHD treatment usually do not have severe manic syndromes.
Algorithm Reducing mania risk: Using stimulants in children with bipolar disorder
Combination therapy. Like many adults, bipolar children often require combinations of mood stabilizers. Kowatch et al36 found that 16 of 20 acutely ill bipolar children (mean age 11) responded to a combination of mood stabilizers after not responding to 8 weeks of a single mood stabilizer. Because bipolar disorder with comorbid ADHD suggests a complex pathophysiology, patients with both disorders may be more likely to require mood-stabilizer combinations than those with bipolar disorder alone.
The goal in treating bipolar disorder is to eliminate symptoms as completely as possible. In bipolar children with comorbid ADHD, be certain that subtle hypomanic symptoms—irritability, decreased sleep, hypersensitivity to interactions, psychosis—have remitted, as they could account for continued inattention. Persistent mood lability may indicate incomplete treatment of the mood disorder, which can increase sensitivity to destabilization by a stimulant.
If a child remains inattentive after the mood disorder is controlled, consider whether medications for the mood disorder are to blame. If medications are working well but causing cognitive side effects, a cholinesterase inhibitor may help.
Adding stimulants. If attention problems persist, consider cautiously adding a stimulant. Informed consent includes telling patients and families about the risks of mood destabilization with stimulants, even when used with mood stabilizers.
Increase stimulant dosage very slowly, and monitor the patient closely for emerging mood instability or subtle evidence of dysphoric hypomania. Address hypersensitivity to sounds, increased irritability, or other signs of activation with more-aggressive mood stabilization before assuming that these are ADHD symptoms that require a higher stimulant dosage.
Sustained-release stimulant preparations are probably second-line choices in patients with concomitant bipolar disorder. With long-acting stimulants, any worsening of the mood disorder will take longer to wear off. Antidepressants such as bupropion are potential alternatives to stimulants but are as likely to induce hypomania and mood cycling and may not be as effective.
Compared with stimulants, atomoxetine has a less-potent antidepressant effect and may be somewhat safer, but it is not as effective for ADHD and is longer-acting. Thus, atomoxetine could be a first-line alternative for comorbid ADHD, with stimulants being added if it is not effective. Clonidine can reduce hyperactivity but does not stabilize mood or improve attention.
When an antidepressant has brought bipolar depression into remission, discontinue it slowly to reduce the risk of rebound while continuing mood stabilizers to prevent recurrence. Because ADHD is not cyclical like bipolar depression, inattention returns for many patients when stimulants are withdrawn.
We do not yet know whether the risk of mood destabilization increases with long-term stimulant use, but discontinuation-induced refractoriness has not been reported with stimulants as it has with mood stabilizers and antidepressants. Thus, trying to withdraw stimulants once ADHD symptoms have remitted is prudent, while supplementing the regimen with behavioral treatments. If managing ADHD symptoms requires continued stimulant treatment, monitor the patient closely for mood destabilization.
Related resources
- American Academy of Child and Adolescent Psychiatry. Facts for families: Bipolar disorder in children and teens.
www.aacap.org/publications/factsFam/bipolar.htm. - National Institute of Mental Health. Database on ADHD.
www.nimh.nih.gov/publicat/adhd.cfm.
Drug brand names
- Amphetamine salts • Adderall
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Clonidine • Catapres
- Dexmethylphenidate • Focalin
- Fluoxetine • Prozac
- Lithium • Lithobid, others
- Methylphenidate • Concerta,
- Ritalin, others
- Valproate • Depakene, Depakote
- Venlafaxine • Effexor
Disclosures
Dr. Dubovsky receives research support from UCB Pharma, Forest Laboratories, and Solvay Pharmaceuticals, and is a speaker for Janssen Pharmaceutica and Forest Laboratories.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. AACAP. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(suppl 2):26S-49S.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder: child psychiatric workgroup on bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Reichart CG, Nolen W. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004;78:81-4.
4. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003;53:970-7.
5. Geller B, Zimmerman B, Williams MB, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001;158:125-7.
6. Scheffer RE, Niskala Apps JA. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J Affect Disord. 2004;82(suppl 1):S25-S34.
7. Dilsaver SC, Akiskal HS. Preschool-onset mania: incidence, phenomenology and family history. J Affect Disord 2004;82(suppl 1):S35-S43.
8. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82(suppl 1):S59-S69.
9. Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the Child Behavior Checklist in pediatric bipolar disorder. Biol Psychiatry 2003;53:1021-7.
10. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70:323-7.
11. Piver A. Ultrarapid response to an antidepressant: A clue to bipolarity? Can J Psychiatry 2003;48:427-8.
12. Bottlender R, Sato T, Kleindienst N, et al. Mixed depressive features predict maniform switch during treatment of depression in bipolar I disorder. J Affect Disord 2004;78:149-52.
13. Goldberg J, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003;5:407-20.
14. Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152(8):1130-8.
15. Gijsman HJ, Geddes J, Rendell J, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004;161:1537-47.
16. Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988;145:844-8.
17. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder: demographics, diagnosis, family history and course. Arch Gen Psychiatry 1992;49:126-31.
18. Wehr TA. Can antidepressants induce rapid cycling? Arch Gen Psychiatry 1993;50(6):495-6.
19. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145:179-84.
20. Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story. J Clin Psychiatry 1992;53:443-6.
21. Goldberg J, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry 2002;63:985-91.
22. Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression—low manic switch rate. Bipolar Disord 2004;6:75-81.
23. Simpson SG, DePaulo JR. Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991;11:52-4.
24. Amsterdam JD, Garcia-Espana F. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000;59:225-9.
25. Goldberg J. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract 2003;9:181-94.
26. Bauer M, Calabrese JR, Dunner DL. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry 1994;151:506-15.
27. Prien RF, Kupfer DJ, Mansky PA. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984;41:1096-1104.
28. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6:416-20.
29. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
30. Kowatch RA, Suppes T, Carmody T, et al. Effect size of lithium, divalproex sodium, and carbamezepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000;39:713-20.
31. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
32. El-Mallakh RS, Cicero D, Holman J, Robertson J. Antidepressant exposure in children diagnosed with bipolar disorder. Bipolar Disord 2001;3(suppl 1):35-9.
33. Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003;66:317-22.
34. Carlson G, Loney J, Salisbury H, et al. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol 2000;10:175-84.
35. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13:123-36.
36. Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978-84.
1. Greenhill LL, Pliszka S, Dulcan MK, et al. AACAP. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41(suppl 2):26S-49S.
2. Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder: child psychiatric workgroup on bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:213-35.
3. Reichart CG, Nolen W. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004;78:81-4.
4. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry 2003;53:970-7.
5. Geller B, Zimmerman B, Williams MB, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001;158:125-7.
6. Scheffer RE, Niskala Apps JA. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J Affect Disord. 2004;82(suppl 1):S25-S34.
7. Dilsaver SC, Akiskal HS. Preschool-onset mania: incidence, phenomenology and family history. J Affect Disord 2004;82(suppl 1):S35-S43.
8. Wozniak J, Spencer T, Biederman J, et al. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord 2004;82(suppl 1):S59-S69.
9. Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the Child Behavior Checklist in pediatric bipolar disorder. Biol Psychiatry 2003;53:1021-7.
10. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70:323-7.
11. Piver A. Ultrarapid response to an antidepressant: A clue to bipolarity? Can J Psychiatry 2003;48:427-8.
12. Bottlender R, Sato T, Kleindienst N, et al. Mixed depressive features predict maniform switch during treatment of depression in bipolar I disorder. J Affect Disord 2004;78:149-52.
13. Goldberg J, Truman CJ. Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003;5:407-20.
14. Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995;152(8):1130-8.
15. Gijsman HJ, Geddes J, Rendell J, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004;161:1537-47.
16. Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988;145:844-8.
17. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder: demographics, diagnosis, family history and course. Arch Gen Psychiatry 1992;49:126-31.
18. Wehr TA. Can antidepressants induce rapid cycling? Arch Gen Psychiatry 1993;50(6):495-6.
19. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145:179-84.
20. Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story. J Clin Psychiatry 1992;53:443-6.
21. Goldberg J, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry 2002;63:985-91.
22. Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression—low manic switch rate. Bipolar Disord 2004;6:75-81.
23. Simpson SG, DePaulo JR. Fluoxetine treatment of bipolar II depression. J Clin Psychopharmacol 1991;11:52-4.
24. Amsterdam JD, Garcia-Espana F. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000;59:225-9.
25. Goldberg J. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract 2003;9:181-94.
26. Bauer M, Calabrese JR, Dunner DL. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry 1994;151:506-15.
27. Prien RF, Kupfer DJ, Mansky PA. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984;41:1096-1104.
28. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6:416-20.
29. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162:58-64.
30. Kowatch RA, Suppes T, Carmody T, et al. Effect size of lithium, divalproex sodium, and carbamezepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000;39:713-20.
31. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3:53-7.
32. El-Mallakh RS, Cicero D, Holman J, Robertson J. Antidepressant exposure in children diagnosed with bipolar disorder. Bipolar Disord 2001;3(suppl 1):35-9.
33. Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003;66:317-22.
34. Carlson G, Loney J, Salisbury H, et al. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol 2000;10:175-84.
35. Galanter CA, Carlson GA, Jensen PS, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol 2003;13:123-36.
36. Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978-84.
EARLY LIFE STRESS AND DEPRESSION Childhood trauma may lead to neurobiologically unique mood disorders
Sadly, parental neglect and child abuse are very common in the United States and worldwide. Patients abused or exploited in childhood, who experience neglect or the loss of a parent during childhood, are haunted by these experiences.
Considerable evidence from laboratory animal and clinical studies indicate that stressful or traumatic events early in development have long-lasting effects on brain development. In particular, the neural and endocrine systems mediating the response to stress exhibit persistent alterations after adverse childhood events.
Clinically, patients with a history of childhood trauma often struggle with variable symptom complexes including both depression and anxiety. In this article, we review evidence that depression in patients with a history of early life stress (ELS) is biologically and clinically distinct from depression in patients without childhood abuse or neglect.
Data linking ELS to depression
Conservative estimates suggest that every year in the United States more than 1 million children are exposed to sexual or physical abuse or severe neglect.1 Unfortunately, this is only the tip of the iceberg. Emotional abuse is by definition comorbid with sexual and physical abuse and may occur alone at even higher rates.
Psychiatric sequelae of child abuse have been studied in adult survivors in considerable detail (Table). Women abused as children report greater numbers of depression, anxiety, somatic, and substance abuse symptoms compared with women without such a history.2 Not only are these women at increased risk for attempted suicide, but they attempt suicide at a rate that is proportional to the number of early life traumatic events that occurred during childhood.3 Men also are at increased risk for depression in the wake of child abuse.4
Sexual abuse in particular is a marker of especially severe childhood trauma. Depressed women who were sexually abused as children report more childhood physical abuse, childhood emotional abuse, parental conflict, and an earlier onset of depression than depressed women without a history of sexual abuse.5
Finally, recent data drawn from the National Comorbidity Survey suggest that child abuse and neglect may independently elevate risk for several stress-related diseases including cardiac disease, peptic ulcer, autoimmune disease, diabetes mellitus, and lung disease.6
Table
Early life stress as a risk factor for mood and anxiety disorders
| Child abuse and neglect are predictors of episodes of major depression in identical twins |
| Women with a history of childhood abuse are more than twice as likely to develop depression as non-abused women |
| Childhood abuse is related to the development of anxiety disorders in adulthood |
| Childhood physical abuse predisposes for combat-related posttraumatic stress disorder (PTSD) |
| Stress early in life may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders |
Depression and the biology of stress
Preclinical research using laboratory animals and clinical research with humans has provided significant insight into the relationship between the pathophysiology of depression and the neurobiology of stress. A burgeoning database suggests that disruption of the neural systems mediating the stress response plays a significant role in the etiology of certain forms of depression and anxiety.7 Much of this work has focused on the preeminent role of corticotropin-releasing factor (CRF) in this process (Figure).
CRF is one of the principal mediators of the mammalian stress response. One CRF system is composed of neurons of the paraventricular nuclei of the hypothalamus that project nerve terminals to the median eminence, where they secrete CRF into the hypophyseal portal system. CRF is then transported within the portal system to the anterior pituitary where it acts on corticotrophs to increase adrenocorticotrophic hormone (ACTH) secretion, thereby controlling hypothalamic-pituitary-adrenal (HPA) axis activity.8 CRF is also found in extrahypothalamic brain areas where it functions, in concert with the hypothalamic CRF system, as a neurotransmitter in coordinating the behavioral, autonomic, and immune responses to stress.9
Direct central nervous system (CNS) administration of CRF in laboratory animals, typically rodents or nonhuman primates, results in activation of the autonomic nervous system leading to elevation of peripheral catecholamines, modification of gastrointestinal activity, increased heart rate and increased blood pressure. In addition, changes in behavior similar to those observed in human depression occur, including disturbed sleep patterns, reduced food intake, decreased reproductive behavior, and enhanced fear conditioning.10,11
In humans, elevated CRF concentrations are found in the cerebrospinal fluid (CSF) of patients with depression12,13 and of combat veterans with posttraumatic stress disorder (PTSD).14,15 Further, postmortem studies of suicide victims have revealed decreased density of CRF receptors in the frontal cortex,16 decreased expression of CRF receptor mRNA and increased CRF concentrations in the frontal cortex when compared with controls,17 and increased concentrations of cisternal CSF CRF.18 Collectively, these clinical data are consistent with the hypothesis that CRF is chronically hypersecreted in patients with depression or PTSD.
Figure Biological and behavioral effects of chronic CRF hypersecretion
A distinct ‘ELS depression’?
Depression has a complex etiology based on interacting contributions from genes and the environment19 that may ultimately result in biologically and clinically distinct forms of depression.20 Exposure to stress, particularly during neurobiologically vulnerable periods of development, may be one means whereby the environment influences the development of depression in genetically susceptible individuals.21
Heredity. Kendler and colleagues22 studied 1,404 female adult twins and observed that childhood sexual abuse was associated with both an increased risk for major depression and a marked increased sensitivity to the depressogenic effects of stressful life events. Moreover, research in human gene-environment interactions has identified a functional polymorphism in the promoter region of the gene for the serotonin transporter which appears to moderate the influence of stressful life events on the development of depression and potential for suicide.23,24
Environment. Similarly, a key variable in determining the clinical outcome of childhood trauma may be the developmental timing of the abuse. Women abused before age 13 are at equivalent risk for developing PTSD or major depressive disorder (MDD), whereas women abused after age 13 are more likely to develop PTSD.25
Thus a major challenge in depression research is to understand the biological mechanisms that mediate the effects of trauma during development through the genetic windows of vulnerability and resilience.
Animal models of ELS have been studied to elucidate the neurobiological consequences of early life trauma in adult humans. This work has largely been performed in rodents and nonhuman primates using a variety of experimental paradigms.
Although a comprehensive review of these data is beyond the scope of this article, ELS in laboratory animals has consistently been found to produce both short- and long-term adverse neurobiological and endocrine effects as well as cognitive dysfunction and abnormal behavior.21 One possible mechanism mediating these effects is a persistent hyperresponsiveness of different components of the HPA axis following exposure to stress.
Studies in adult women have sought to uncover the long-term effects of ELS (prepubertal physical or sexual abuse) on reactivity of the HPA axis in response to the Trier Social Stress Test, a standardized psychosocial stress test.26 It consists of the subject giving a 10-minute speech and performing a mental arithmetic task in front of a panel of stern-appearing evaluators. Variables measured include heart rate, plasma ACTH, and cortisol concentration at intervals before and after the performance component of the test. The four groups in this study included:
- women without psychiatric illness or history of ELS serving as a control group (CON)
- depressed women without a history of ELS (non-ELS/MDD)
- depressed women with a history of ELS (ELS/MDD)
- non-depressed women with a history of ELS (ELS/non-MDD).
The largest ACTH and cortisol responses and increases in heart rate following this stress exposure were seen in the ELS/MDD group. In fact, the ACTH response of these women was more than 6 times greater than that observed in the control group. The ELS/MDD group of women also had greater rates of comorbid PTSD (85%) in comparison to the other experimental groups as well.
These data are consistent with the hypothesis that ELS produces enduring sensitization of the HPA axis and autonomic nervous system response to stress. This phenomenon may constitute an important etiological element in the development of stress-related adult psychiatric illnesses such as depression or PTSD.
To further explore the hypothesis that ELS alters set points of the HPA axis, we sought to characterize the effects of standard HPA axis challenge tests (CRF stimulation test and ACTH1-24 stimulation test) in a similar population of women.27 Depressed women with a history of ELS and depressed women without a history of ELS both exhibited a blunted ACTH response to infusion of exogenous CRF. Conversely, women with a history of ELS but without current depression had an increased ACTH response following CRF infusion.
With respect to the ACTH1-24 stimulation test, abused women who were not depressed had lower plasma cortisol levels at baseline and after administration of ACTH1-24. Similar to the findings of our previous study,26 women with MDD and a history of ELS were more likely to report current life stress and to also have comorbid PTSD than women with ELS who were not depressed. Blunting of the ACTH response to exogenous CRF in depressed women with a history of ELS may in part be secondary to acute downregulation of pituitary CRF receptors as a result of chronic CRF hypersecretion.
More recently, Carpenter and colleagues28 evaluated the relationship between the perception of ELS and CSF CRF in patients with depression and healthy control subjects. The perception of ELS predicted CSF CRF concentration independent of the presence or absence of depression. Further, and most interestingly, the developmental timing of the stress exposure was predictive of either relatively increased or decreased CSF CRF. ELS before age 6 was associated with elevated CSF CRF, whereas perinatal and preteen exposure to stressful events was associated with decreased CSF CRF.
Brain structure changes? In addition to the neuroendocrine changes observed in patients with ELS, there is evidence that ELS may also alter brain structure. Reduced hippocampal volume is found in some but not all patients with unipolar depression.29 In patients with a history of depression who also have hippocampal atrophy, the extent of atrophy is greater in patients with higher total lifetime duration of depression.30,31
Patients with ELS also have been found to have decreased hippocampal volume.32,33 However, previous structural imaging studies have not controlled for the presence of ELS when attempting to determine the relationship between depression and structural changes in the hippocampus, and this methodologic confound may explain in part the inconsistent relationship between altered hippocampal volume and depression.
To evaluate this hypothesis, hippocampal volume was measured in depressed women with and without a history of ELS and in a control group of women. Reduced hippocampal volume was found to occur solely in depressed women with a history of ELS. Depressed women without ELS and women from the control group had similar hippocampal volumes.34 These data suggest that previous reports of reduced hippocampal size in patients with depression may in fact be related to a history of ELS rather than depression.
Treatment implications
The data discussed in this paper indicate that patients with depression and a history of ELS may constitute a unique subgroup among depressed patients as a whole. A growing body of evidence suggests that depressed patients with ELS may also be unique with respect to their response to treatment.
ELS has been found to impact the clinical response of patients to pharmacotherapy with either dysthymia or depression.35,36 Further, patients with depression and a history of ELS have been reported to exhibit increased rates of relapse following treatment of depression.37 The course of depression in individuals with ELS is often characterized by chronicity.
ELS and therapeutic response, Recently, our group has sought to determine whether ELS in patients with chronic depression moderates their response to pharmacotherapy or psychotherapy.38 In this study, data from a large multicenter trial39 originally designed to compare the relative efficacy of pharmacotherapy (nefazodone), psychotherapy (Cognitive Behavioral Analysis System of Psychotherapy), or their combination in the treatment of chronic depression was reanalyzed by stratifying patients based on the presence or absence of ELS. In the overall sample of patients with chronic depression, psychotherapy and pharmacotherapy were comparable in efficacy but significantly less effective than their combination.
ELS in chronically depressed patients was highly prevalent. Approximately one-third experienced loss of a parent before age 15, 45% experienced childhood physical abuse, 16% experienced childhood sexual abuse, and 10% experienced neglect. Most significantly, depressed patients with a history of ELS had a superior response to psychotherapy alone compared with antidepressant monotherapy. In addition, combination therapy was only slightly more effective than psychotherapy alone in the group of depressed patients with ELS.
These data suggest that ELS is common in the population of patients with chronic depression and that psychotherapy is a critical element in the treatment of depressed patients with ELS40 (Box). However, it will be important in future studies to ascertain whether the differences in treatment response for psychotherapy compared with antidepressant in patients with ELS and depression are able to be replicated with the SSRI class of antidepressants.
Assessment of trauma and neglect should be a standard component of the diagnostic interview. Patients with a history of early life stress (ELS) may present for treatment with complaints that represent depression, anxiety, or substance abuse, but they may also have complicated presentations involving psychotic or dissociative symptoms, reflecting the diagnostic comorbidity in this population.
How to identify ELS. No standardized office-based screening tools exist for ELS, and clinical interviewing is the primary means of assessing exposure to ELS. A common error in history-taking with this population, particularly in high-volume settings, is to merely ask patients whether they were abused or neglected as children or to elaborate only very slightly on this aspect of the history. We risk not finding information that is critical to understanding our patients if we assume they share a common definition of abuse and neglect with us, can recognize such events in their personal history, and are willing to share that information with us.
When framing questions about abuse or neglect, it is important to remember that our own sense of what constitutes neglect or abuse may be very different from what a patient thinks of as neglect or abuse. For example, some patients may not consider their experience as a child abusive because of a distorted sense of responsibility, possibly further exaggerated by comorbid depression (ie, “My parents locked me in the closet overnight all the time when I was a child because I deserved it.”)
Other patients may try to minimize the impact of the experience or the responsibility of the perpetrator and attempt to normalize it (ie, “My uncle used to touch me between my legs in the swimming pool but he didn’t mean anything by it; he did it to everybody.”)
Avoiding ‘false memories.’ As important as it is to identify abuse or neglect when it has occurred, it is equally important to avoid intensifying the impact of an incident of abuse or, worse, creating a “false memory” of abuse in suggestible patients (bearing in mind that there is no definitive way to exclude the presence of “suggestibility” in patients). Our task as clinicians is to help patients correctly identify experiences of abuse and neglect and understand their response to these experiences clinically to facilitate case formulation and a treatment plan.
Creating a therapeutic alliance. Abuse and neglect during early life fundamentally alter the core assumptions that patients have about trust and safety in their relationships with others. Not only does this potentially impact the disclosure by patients of the nature and extent of trauma they have experienced, but it can also slow the formation of an effective therapeutic alliance.
To that end, asking open-ended questions about neglect and specific forms of abuse, creating an atmosphere of safety and trust, and a warm, empathic, nonjudgmental manner are central to the accurate assessment of ELS and provide the foundation for treatment by establishing an effective therapeutic alliance.
Optimal treatment. No published clinical trials have specifically compared the relative efficacy of particular forms of psychotherapy or pharmacotherapy for depressed patients with a history of ELS. However, it is clear from the available data that psychotherapy should be considered a core component of treatment for these patients.
Because psychiatric comorbidity is common in these patients, their treatment should be individualized in a manner that accounts for and addresses depression as well as associated diagnoses such as panic disorder or posttraumatic stress disorder (PTSD) with disorder-specific psychotherapy. Pharmacotherapy in combination with psychotherapy may also be helpful to patients with ELS and depression, though definitive data are lacking.
Judicious combination of medications such as antidepressants and benzodiazepines, particularly in patients with comorbid panic or PTSD, in concert with psychotherapy probably constitutes optimal treatment.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- National Clearinghouse on Child Abuse and Neglect. http://nccanch.acf.hhs.gov.
- Charney DS, Nemeroff CB. The peace of mind prescription: An authoritative guide to finding the most effective treatment for anxiety and depression. Boston: Houghton Mifflin, 2004.
Drug brand name
- Nefazodone • Serzone
Disclosure
Dr. Gillespie reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nemeroff receives research grants from or is a consultant/speaker for Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Corcept Therapeutics, Cyberonics, Cypress Biosciences, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Merck & Co., Neurocrine Biosciences, Otsuka Inc., Pfizer Inc., Sanofi Aventis, and Wyeth.
Acknowledgement
Supported by NIH MH-42088, MH-52899 (CBN), and NIH NCRRM01-RR00039 to Emory University.
1. Sedlack AJ, Broadhurst DD. Third National Incidence Study of Child Abuse and Neglect. Washington, DC: US Department of Health and Human Services; 1996.
2. McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of child abuse: unhealed wounds. JAMA 1997;277(17):1362-8.
3. Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA 2001;286(24):3089-96.
4. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82(2):217-25.
5. Gladstone GL, Parker GB, Mitchell PB, et al. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry 2004;161(8):1417-25.
6. Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34(3):509-20.
7. Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004;29(4):641-8.
8. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983;36(3):165-86.
9. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160(1):1-12.
10. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 1990;15(2):71-100.
11. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev 1991;43(4):425-73.
12. Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotrophin-releasing factor-like immunoreactivity in depressed patients. Science 1984;226(4680):1342-4.
13. Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996;780:96-105.
14. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997;154(5):624-9.
15. Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999;156(4):585-8.
16. Nemeroff CB, Owens MJ, Bissette G, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988;45(6):577-9.
17. Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004;24(6):1478-85.
18. Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 1989;25(3):355-9.
19. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157(10):1552-62.
20. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004;29(10):1765-81.
21. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79(3):471-8.
22. Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004;34(8):1475-82.
23. Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-9.
24. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA 2004;101(49):17316-21.
25. Maercker A, Michael T, Fehm L, et al. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004;184:482-7.
26. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284(5):592-7.
27. Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001;158(4):575-81.
28. Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004;29(4):777-84.
29. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417-26.
30. Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996;93(9):3908-13.
31. Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19(12):5034-43.
32. Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27(4):951-9.
33. Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000;57(12):1115-22.
34. Vythilingam M, Heim C, Newport DJ, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159(12):2072-80.
35. Hayden EP, Klein DM. Outcome of dysthymic disorder at 5-year follow-up: the effect of familial psychopathology, early adversity, personality, comorbidity, and chronic stress. Am J Psychiatry 2001;158(11):1864-70.
36. Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596-601.
37. Lara ME, Klein DN, Kasch KL. Psychosocial predictors of the short-term course and outcome of major depression: a longitudinal study of a nonclinical sample with recent-onset episodes. J Abnorm Psychol 2000;109(4):644-50.
38. Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100(24):14293-6.
39. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342(20):1462-70.
40. Craighead WE, Nemeroff CB. The impact of early trauma on response to psychotherapy. Clin Neurosci Res 2005;4(5-6):405-11.
Sadly, parental neglect and child abuse are very common in the United States and worldwide. Patients abused or exploited in childhood, who experience neglect or the loss of a parent during childhood, are haunted by these experiences.
Considerable evidence from laboratory animal and clinical studies indicate that stressful or traumatic events early in development have long-lasting effects on brain development. In particular, the neural and endocrine systems mediating the response to stress exhibit persistent alterations after adverse childhood events.
Clinically, patients with a history of childhood trauma often struggle with variable symptom complexes including both depression and anxiety. In this article, we review evidence that depression in patients with a history of early life stress (ELS) is biologically and clinically distinct from depression in patients without childhood abuse or neglect.
Data linking ELS to depression
Conservative estimates suggest that every year in the United States more than 1 million children are exposed to sexual or physical abuse or severe neglect.1 Unfortunately, this is only the tip of the iceberg. Emotional abuse is by definition comorbid with sexual and physical abuse and may occur alone at even higher rates.
Psychiatric sequelae of child abuse have been studied in adult survivors in considerable detail (Table). Women abused as children report greater numbers of depression, anxiety, somatic, and substance abuse symptoms compared with women without such a history.2 Not only are these women at increased risk for attempted suicide, but they attempt suicide at a rate that is proportional to the number of early life traumatic events that occurred during childhood.3 Men also are at increased risk for depression in the wake of child abuse.4
Sexual abuse in particular is a marker of especially severe childhood trauma. Depressed women who were sexually abused as children report more childhood physical abuse, childhood emotional abuse, parental conflict, and an earlier onset of depression than depressed women without a history of sexual abuse.5
Finally, recent data drawn from the National Comorbidity Survey suggest that child abuse and neglect may independently elevate risk for several stress-related diseases including cardiac disease, peptic ulcer, autoimmune disease, diabetes mellitus, and lung disease.6
Table
Early life stress as a risk factor for mood and anxiety disorders
| Child abuse and neglect are predictors of episodes of major depression in identical twins |
| Women with a history of childhood abuse are more than twice as likely to develop depression as non-abused women |
| Childhood abuse is related to the development of anxiety disorders in adulthood |
| Childhood physical abuse predisposes for combat-related posttraumatic stress disorder (PTSD) |
| Stress early in life may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders |
Depression and the biology of stress
Preclinical research using laboratory animals and clinical research with humans has provided significant insight into the relationship between the pathophysiology of depression and the neurobiology of stress. A burgeoning database suggests that disruption of the neural systems mediating the stress response plays a significant role in the etiology of certain forms of depression and anxiety.7 Much of this work has focused on the preeminent role of corticotropin-releasing factor (CRF) in this process (Figure).
CRF is one of the principal mediators of the mammalian stress response. One CRF system is composed of neurons of the paraventricular nuclei of the hypothalamus that project nerve terminals to the median eminence, where they secrete CRF into the hypophyseal portal system. CRF is then transported within the portal system to the anterior pituitary where it acts on corticotrophs to increase adrenocorticotrophic hormone (ACTH) secretion, thereby controlling hypothalamic-pituitary-adrenal (HPA) axis activity.8 CRF is also found in extrahypothalamic brain areas where it functions, in concert with the hypothalamic CRF system, as a neurotransmitter in coordinating the behavioral, autonomic, and immune responses to stress.9
Direct central nervous system (CNS) administration of CRF in laboratory animals, typically rodents or nonhuman primates, results in activation of the autonomic nervous system leading to elevation of peripheral catecholamines, modification of gastrointestinal activity, increased heart rate and increased blood pressure. In addition, changes in behavior similar to those observed in human depression occur, including disturbed sleep patterns, reduced food intake, decreased reproductive behavior, and enhanced fear conditioning.10,11
In humans, elevated CRF concentrations are found in the cerebrospinal fluid (CSF) of patients with depression12,13 and of combat veterans with posttraumatic stress disorder (PTSD).14,15 Further, postmortem studies of suicide victims have revealed decreased density of CRF receptors in the frontal cortex,16 decreased expression of CRF receptor mRNA and increased CRF concentrations in the frontal cortex when compared with controls,17 and increased concentrations of cisternal CSF CRF.18 Collectively, these clinical data are consistent with the hypothesis that CRF is chronically hypersecreted in patients with depression or PTSD.
Figure Biological and behavioral effects of chronic CRF hypersecretion
A distinct ‘ELS depression’?
Depression has a complex etiology based on interacting contributions from genes and the environment19 that may ultimately result in biologically and clinically distinct forms of depression.20 Exposure to stress, particularly during neurobiologically vulnerable periods of development, may be one means whereby the environment influences the development of depression in genetically susceptible individuals.21
Heredity. Kendler and colleagues22 studied 1,404 female adult twins and observed that childhood sexual abuse was associated with both an increased risk for major depression and a marked increased sensitivity to the depressogenic effects of stressful life events. Moreover, research in human gene-environment interactions has identified a functional polymorphism in the promoter region of the gene for the serotonin transporter which appears to moderate the influence of stressful life events on the development of depression and potential for suicide.23,24
Environment. Similarly, a key variable in determining the clinical outcome of childhood trauma may be the developmental timing of the abuse. Women abused before age 13 are at equivalent risk for developing PTSD or major depressive disorder (MDD), whereas women abused after age 13 are more likely to develop PTSD.25
Thus a major challenge in depression research is to understand the biological mechanisms that mediate the effects of trauma during development through the genetic windows of vulnerability and resilience.
Animal models of ELS have been studied to elucidate the neurobiological consequences of early life trauma in adult humans. This work has largely been performed in rodents and nonhuman primates using a variety of experimental paradigms.
Although a comprehensive review of these data is beyond the scope of this article, ELS in laboratory animals has consistently been found to produce both short- and long-term adverse neurobiological and endocrine effects as well as cognitive dysfunction and abnormal behavior.21 One possible mechanism mediating these effects is a persistent hyperresponsiveness of different components of the HPA axis following exposure to stress.
Studies in adult women have sought to uncover the long-term effects of ELS (prepubertal physical or sexual abuse) on reactivity of the HPA axis in response to the Trier Social Stress Test, a standardized psychosocial stress test.26 It consists of the subject giving a 10-minute speech and performing a mental arithmetic task in front of a panel of stern-appearing evaluators. Variables measured include heart rate, plasma ACTH, and cortisol concentration at intervals before and after the performance component of the test. The four groups in this study included:
- women without psychiatric illness or history of ELS serving as a control group (CON)
- depressed women without a history of ELS (non-ELS/MDD)
- depressed women with a history of ELS (ELS/MDD)
- non-depressed women with a history of ELS (ELS/non-MDD).
The largest ACTH and cortisol responses and increases in heart rate following this stress exposure were seen in the ELS/MDD group. In fact, the ACTH response of these women was more than 6 times greater than that observed in the control group. The ELS/MDD group of women also had greater rates of comorbid PTSD (85%) in comparison to the other experimental groups as well.
These data are consistent with the hypothesis that ELS produces enduring sensitization of the HPA axis and autonomic nervous system response to stress. This phenomenon may constitute an important etiological element in the development of stress-related adult psychiatric illnesses such as depression or PTSD.
To further explore the hypothesis that ELS alters set points of the HPA axis, we sought to characterize the effects of standard HPA axis challenge tests (CRF stimulation test and ACTH1-24 stimulation test) in a similar population of women.27 Depressed women with a history of ELS and depressed women without a history of ELS both exhibited a blunted ACTH response to infusion of exogenous CRF. Conversely, women with a history of ELS but without current depression had an increased ACTH response following CRF infusion.
With respect to the ACTH1-24 stimulation test, abused women who were not depressed had lower plasma cortisol levels at baseline and after administration of ACTH1-24. Similar to the findings of our previous study,26 women with MDD and a history of ELS were more likely to report current life stress and to also have comorbid PTSD than women with ELS who were not depressed. Blunting of the ACTH response to exogenous CRF in depressed women with a history of ELS may in part be secondary to acute downregulation of pituitary CRF receptors as a result of chronic CRF hypersecretion.
More recently, Carpenter and colleagues28 evaluated the relationship between the perception of ELS and CSF CRF in patients with depression and healthy control subjects. The perception of ELS predicted CSF CRF concentration independent of the presence or absence of depression. Further, and most interestingly, the developmental timing of the stress exposure was predictive of either relatively increased or decreased CSF CRF. ELS before age 6 was associated with elevated CSF CRF, whereas perinatal and preteen exposure to stressful events was associated with decreased CSF CRF.
Brain structure changes? In addition to the neuroendocrine changes observed in patients with ELS, there is evidence that ELS may also alter brain structure. Reduced hippocampal volume is found in some but not all patients with unipolar depression.29 In patients with a history of depression who also have hippocampal atrophy, the extent of atrophy is greater in patients with higher total lifetime duration of depression.30,31
Patients with ELS also have been found to have decreased hippocampal volume.32,33 However, previous structural imaging studies have not controlled for the presence of ELS when attempting to determine the relationship between depression and structural changes in the hippocampus, and this methodologic confound may explain in part the inconsistent relationship between altered hippocampal volume and depression.
To evaluate this hypothesis, hippocampal volume was measured in depressed women with and without a history of ELS and in a control group of women. Reduced hippocampal volume was found to occur solely in depressed women with a history of ELS. Depressed women without ELS and women from the control group had similar hippocampal volumes.34 These data suggest that previous reports of reduced hippocampal size in patients with depression may in fact be related to a history of ELS rather than depression.
Treatment implications
The data discussed in this paper indicate that patients with depression and a history of ELS may constitute a unique subgroup among depressed patients as a whole. A growing body of evidence suggests that depressed patients with ELS may also be unique with respect to their response to treatment.
ELS has been found to impact the clinical response of patients to pharmacotherapy with either dysthymia or depression.35,36 Further, patients with depression and a history of ELS have been reported to exhibit increased rates of relapse following treatment of depression.37 The course of depression in individuals with ELS is often characterized by chronicity.
ELS and therapeutic response, Recently, our group has sought to determine whether ELS in patients with chronic depression moderates their response to pharmacotherapy or psychotherapy.38 In this study, data from a large multicenter trial39 originally designed to compare the relative efficacy of pharmacotherapy (nefazodone), psychotherapy (Cognitive Behavioral Analysis System of Psychotherapy), or their combination in the treatment of chronic depression was reanalyzed by stratifying patients based on the presence or absence of ELS. In the overall sample of patients with chronic depression, psychotherapy and pharmacotherapy were comparable in efficacy but significantly less effective than their combination.
ELS in chronically depressed patients was highly prevalent. Approximately one-third experienced loss of a parent before age 15, 45% experienced childhood physical abuse, 16% experienced childhood sexual abuse, and 10% experienced neglect. Most significantly, depressed patients with a history of ELS had a superior response to psychotherapy alone compared with antidepressant monotherapy. In addition, combination therapy was only slightly more effective than psychotherapy alone in the group of depressed patients with ELS.
These data suggest that ELS is common in the population of patients with chronic depression and that psychotherapy is a critical element in the treatment of depressed patients with ELS40 (Box). However, it will be important in future studies to ascertain whether the differences in treatment response for psychotherapy compared with antidepressant in patients with ELS and depression are able to be replicated with the SSRI class of antidepressants.
Assessment of trauma and neglect should be a standard component of the diagnostic interview. Patients with a history of early life stress (ELS) may present for treatment with complaints that represent depression, anxiety, or substance abuse, but they may also have complicated presentations involving psychotic or dissociative symptoms, reflecting the diagnostic comorbidity in this population.
How to identify ELS. No standardized office-based screening tools exist for ELS, and clinical interviewing is the primary means of assessing exposure to ELS. A common error in history-taking with this population, particularly in high-volume settings, is to merely ask patients whether they were abused or neglected as children or to elaborate only very slightly on this aspect of the history. We risk not finding information that is critical to understanding our patients if we assume they share a common definition of abuse and neglect with us, can recognize such events in their personal history, and are willing to share that information with us.
When framing questions about abuse or neglect, it is important to remember that our own sense of what constitutes neglect or abuse may be very different from what a patient thinks of as neglect or abuse. For example, some patients may not consider their experience as a child abusive because of a distorted sense of responsibility, possibly further exaggerated by comorbid depression (ie, “My parents locked me in the closet overnight all the time when I was a child because I deserved it.”)
Other patients may try to minimize the impact of the experience or the responsibility of the perpetrator and attempt to normalize it (ie, “My uncle used to touch me between my legs in the swimming pool but he didn’t mean anything by it; he did it to everybody.”)
Avoiding ‘false memories.’ As important as it is to identify abuse or neglect when it has occurred, it is equally important to avoid intensifying the impact of an incident of abuse or, worse, creating a “false memory” of abuse in suggestible patients (bearing in mind that there is no definitive way to exclude the presence of “suggestibility” in patients). Our task as clinicians is to help patients correctly identify experiences of abuse and neglect and understand their response to these experiences clinically to facilitate case formulation and a treatment plan.
Creating a therapeutic alliance. Abuse and neglect during early life fundamentally alter the core assumptions that patients have about trust and safety in their relationships with others. Not only does this potentially impact the disclosure by patients of the nature and extent of trauma they have experienced, but it can also slow the formation of an effective therapeutic alliance.
To that end, asking open-ended questions about neglect and specific forms of abuse, creating an atmosphere of safety and trust, and a warm, empathic, nonjudgmental manner are central to the accurate assessment of ELS and provide the foundation for treatment by establishing an effective therapeutic alliance.
Optimal treatment. No published clinical trials have specifically compared the relative efficacy of particular forms of psychotherapy or pharmacotherapy for depressed patients with a history of ELS. However, it is clear from the available data that psychotherapy should be considered a core component of treatment for these patients.
Because psychiatric comorbidity is common in these patients, their treatment should be individualized in a manner that accounts for and addresses depression as well as associated diagnoses such as panic disorder or posttraumatic stress disorder (PTSD) with disorder-specific psychotherapy. Pharmacotherapy in combination with psychotherapy may also be helpful to patients with ELS and depression, though definitive data are lacking.
Judicious combination of medications such as antidepressants and benzodiazepines, particularly in patients with comorbid panic or PTSD, in concert with psychotherapy probably constitutes optimal treatment.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- National Clearinghouse on Child Abuse and Neglect. http://nccanch.acf.hhs.gov.
- Charney DS, Nemeroff CB. The peace of mind prescription: An authoritative guide to finding the most effective treatment for anxiety and depression. Boston: Houghton Mifflin, 2004.
Drug brand name
- Nefazodone • Serzone
Disclosure
Dr. Gillespie reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nemeroff receives research grants from or is a consultant/speaker for Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Corcept Therapeutics, Cyberonics, Cypress Biosciences, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Merck & Co., Neurocrine Biosciences, Otsuka Inc., Pfizer Inc., Sanofi Aventis, and Wyeth.
Acknowledgement
Supported by NIH MH-42088, MH-52899 (CBN), and NIH NCRRM01-RR00039 to Emory University.
Sadly, parental neglect and child abuse are very common in the United States and worldwide. Patients abused or exploited in childhood, who experience neglect or the loss of a parent during childhood, are haunted by these experiences.
Considerable evidence from laboratory animal and clinical studies indicate that stressful or traumatic events early in development have long-lasting effects on brain development. In particular, the neural and endocrine systems mediating the response to stress exhibit persistent alterations after adverse childhood events.
Clinically, patients with a history of childhood trauma often struggle with variable symptom complexes including both depression and anxiety. In this article, we review evidence that depression in patients with a history of early life stress (ELS) is biologically and clinically distinct from depression in patients without childhood abuse or neglect.
Data linking ELS to depression
Conservative estimates suggest that every year in the United States more than 1 million children are exposed to sexual or physical abuse or severe neglect.1 Unfortunately, this is only the tip of the iceberg. Emotional abuse is by definition comorbid with sexual and physical abuse and may occur alone at even higher rates.
Psychiatric sequelae of child abuse have been studied in adult survivors in considerable detail (Table). Women abused as children report greater numbers of depression, anxiety, somatic, and substance abuse symptoms compared with women without such a history.2 Not only are these women at increased risk for attempted suicide, but they attempt suicide at a rate that is proportional to the number of early life traumatic events that occurred during childhood.3 Men also are at increased risk for depression in the wake of child abuse.4
Sexual abuse in particular is a marker of especially severe childhood trauma. Depressed women who were sexually abused as children report more childhood physical abuse, childhood emotional abuse, parental conflict, and an earlier onset of depression than depressed women without a history of sexual abuse.5
Finally, recent data drawn from the National Comorbidity Survey suggest that child abuse and neglect may independently elevate risk for several stress-related diseases including cardiac disease, peptic ulcer, autoimmune disease, diabetes mellitus, and lung disease.6
Table
Early life stress as a risk factor for mood and anxiety disorders
| Child abuse and neglect are predictors of episodes of major depression in identical twins |
| Women with a history of childhood abuse are more than twice as likely to develop depression as non-abused women |
| Childhood abuse is related to the development of anxiety disorders in adulthood |
| Childhood physical abuse predisposes for combat-related posttraumatic stress disorder (PTSD) |
| Stress early in life may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders |
Depression and the biology of stress
Preclinical research using laboratory animals and clinical research with humans has provided significant insight into the relationship between the pathophysiology of depression and the neurobiology of stress. A burgeoning database suggests that disruption of the neural systems mediating the stress response plays a significant role in the etiology of certain forms of depression and anxiety.7 Much of this work has focused on the preeminent role of corticotropin-releasing factor (CRF) in this process (Figure).
CRF is one of the principal mediators of the mammalian stress response. One CRF system is composed of neurons of the paraventricular nuclei of the hypothalamus that project nerve terminals to the median eminence, where they secrete CRF into the hypophyseal portal system. CRF is then transported within the portal system to the anterior pituitary where it acts on corticotrophs to increase adrenocorticotrophic hormone (ACTH) secretion, thereby controlling hypothalamic-pituitary-adrenal (HPA) axis activity.8 CRF is also found in extrahypothalamic brain areas where it functions, in concert with the hypothalamic CRF system, as a neurotransmitter in coordinating the behavioral, autonomic, and immune responses to stress.9
Direct central nervous system (CNS) administration of CRF in laboratory animals, typically rodents or nonhuman primates, results in activation of the autonomic nervous system leading to elevation of peripheral catecholamines, modification of gastrointestinal activity, increased heart rate and increased blood pressure. In addition, changes in behavior similar to those observed in human depression occur, including disturbed sleep patterns, reduced food intake, decreased reproductive behavior, and enhanced fear conditioning.10,11
In humans, elevated CRF concentrations are found in the cerebrospinal fluid (CSF) of patients with depression12,13 and of combat veterans with posttraumatic stress disorder (PTSD).14,15 Further, postmortem studies of suicide victims have revealed decreased density of CRF receptors in the frontal cortex,16 decreased expression of CRF receptor mRNA and increased CRF concentrations in the frontal cortex when compared with controls,17 and increased concentrations of cisternal CSF CRF.18 Collectively, these clinical data are consistent with the hypothesis that CRF is chronically hypersecreted in patients with depression or PTSD.
Figure Biological and behavioral effects of chronic CRF hypersecretion
A distinct ‘ELS depression’?
Depression has a complex etiology based on interacting contributions from genes and the environment19 that may ultimately result in biologically and clinically distinct forms of depression.20 Exposure to stress, particularly during neurobiologically vulnerable periods of development, may be one means whereby the environment influences the development of depression in genetically susceptible individuals.21
Heredity. Kendler and colleagues22 studied 1,404 female adult twins and observed that childhood sexual abuse was associated with both an increased risk for major depression and a marked increased sensitivity to the depressogenic effects of stressful life events. Moreover, research in human gene-environment interactions has identified a functional polymorphism in the promoter region of the gene for the serotonin transporter which appears to moderate the influence of stressful life events on the development of depression and potential for suicide.23,24
Environment. Similarly, a key variable in determining the clinical outcome of childhood trauma may be the developmental timing of the abuse. Women abused before age 13 are at equivalent risk for developing PTSD or major depressive disorder (MDD), whereas women abused after age 13 are more likely to develop PTSD.25
Thus a major challenge in depression research is to understand the biological mechanisms that mediate the effects of trauma during development through the genetic windows of vulnerability and resilience.
Animal models of ELS have been studied to elucidate the neurobiological consequences of early life trauma in adult humans. This work has largely been performed in rodents and nonhuman primates using a variety of experimental paradigms.
Although a comprehensive review of these data is beyond the scope of this article, ELS in laboratory animals has consistently been found to produce both short- and long-term adverse neurobiological and endocrine effects as well as cognitive dysfunction and abnormal behavior.21 One possible mechanism mediating these effects is a persistent hyperresponsiveness of different components of the HPA axis following exposure to stress.
Studies in adult women have sought to uncover the long-term effects of ELS (prepubertal physical or sexual abuse) on reactivity of the HPA axis in response to the Trier Social Stress Test, a standardized psychosocial stress test.26 It consists of the subject giving a 10-minute speech and performing a mental arithmetic task in front of a panel of stern-appearing evaluators. Variables measured include heart rate, plasma ACTH, and cortisol concentration at intervals before and after the performance component of the test. The four groups in this study included:
- women without psychiatric illness or history of ELS serving as a control group (CON)
- depressed women without a history of ELS (non-ELS/MDD)
- depressed women with a history of ELS (ELS/MDD)
- non-depressed women with a history of ELS (ELS/non-MDD).
The largest ACTH and cortisol responses and increases in heart rate following this stress exposure were seen in the ELS/MDD group. In fact, the ACTH response of these women was more than 6 times greater than that observed in the control group. The ELS/MDD group of women also had greater rates of comorbid PTSD (85%) in comparison to the other experimental groups as well.
These data are consistent with the hypothesis that ELS produces enduring sensitization of the HPA axis and autonomic nervous system response to stress. This phenomenon may constitute an important etiological element in the development of stress-related adult psychiatric illnesses such as depression or PTSD.
To further explore the hypothesis that ELS alters set points of the HPA axis, we sought to characterize the effects of standard HPA axis challenge tests (CRF stimulation test and ACTH1-24 stimulation test) in a similar population of women.27 Depressed women with a history of ELS and depressed women without a history of ELS both exhibited a blunted ACTH response to infusion of exogenous CRF. Conversely, women with a history of ELS but without current depression had an increased ACTH response following CRF infusion.
With respect to the ACTH1-24 stimulation test, abused women who were not depressed had lower plasma cortisol levels at baseline and after administration of ACTH1-24. Similar to the findings of our previous study,26 women with MDD and a history of ELS were more likely to report current life stress and to also have comorbid PTSD than women with ELS who were not depressed. Blunting of the ACTH response to exogenous CRF in depressed women with a history of ELS may in part be secondary to acute downregulation of pituitary CRF receptors as a result of chronic CRF hypersecretion.
More recently, Carpenter and colleagues28 evaluated the relationship between the perception of ELS and CSF CRF in patients with depression and healthy control subjects. The perception of ELS predicted CSF CRF concentration independent of the presence or absence of depression. Further, and most interestingly, the developmental timing of the stress exposure was predictive of either relatively increased or decreased CSF CRF. ELS before age 6 was associated with elevated CSF CRF, whereas perinatal and preteen exposure to stressful events was associated with decreased CSF CRF.
Brain structure changes? In addition to the neuroendocrine changes observed in patients with ELS, there is evidence that ELS may also alter brain structure. Reduced hippocampal volume is found in some but not all patients with unipolar depression.29 In patients with a history of depression who also have hippocampal atrophy, the extent of atrophy is greater in patients with higher total lifetime duration of depression.30,31
Patients with ELS also have been found to have decreased hippocampal volume.32,33 However, previous structural imaging studies have not controlled for the presence of ELS when attempting to determine the relationship between depression and structural changes in the hippocampus, and this methodologic confound may explain in part the inconsistent relationship between altered hippocampal volume and depression.
To evaluate this hypothesis, hippocampal volume was measured in depressed women with and without a history of ELS and in a control group of women. Reduced hippocampal volume was found to occur solely in depressed women with a history of ELS. Depressed women without ELS and women from the control group had similar hippocampal volumes.34 These data suggest that previous reports of reduced hippocampal size in patients with depression may in fact be related to a history of ELS rather than depression.
Treatment implications
The data discussed in this paper indicate that patients with depression and a history of ELS may constitute a unique subgroup among depressed patients as a whole. A growing body of evidence suggests that depressed patients with ELS may also be unique with respect to their response to treatment.
ELS has been found to impact the clinical response of patients to pharmacotherapy with either dysthymia or depression.35,36 Further, patients with depression and a history of ELS have been reported to exhibit increased rates of relapse following treatment of depression.37 The course of depression in individuals with ELS is often characterized by chronicity.
ELS and therapeutic response, Recently, our group has sought to determine whether ELS in patients with chronic depression moderates their response to pharmacotherapy or psychotherapy.38 In this study, data from a large multicenter trial39 originally designed to compare the relative efficacy of pharmacotherapy (nefazodone), psychotherapy (Cognitive Behavioral Analysis System of Psychotherapy), or their combination in the treatment of chronic depression was reanalyzed by stratifying patients based on the presence or absence of ELS. In the overall sample of patients with chronic depression, psychotherapy and pharmacotherapy were comparable in efficacy but significantly less effective than their combination.
ELS in chronically depressed patients was highly prevalent. Approximately one-third experienced loss of a parent before age 15, 45% experienced childhood physical abuse, 16% experienced childhood sexual abuse, and 10% experienced neglect. Most significantly, depressed patients with a history of ELS had a superior response to psychotherapy alone compared with antidepressant monotherapy. In addition, combination therapy was only slightly more effective than psychotherapy alone in the group of depressed patients with ELS.
These data suggest that ELS is common in the population of patients with chronic depression and that psychotherapy is a critical element in the treatment of depressed patients with ELS40 (Box). However, it will be important in future studies to ascertain whether the differences in treatment response for psychotherapy compared with antidepressant in patients with ELS and depression are able to be replicated with the SSRI class of antidepressants.
Assessment of trauma and neglect should be a standard component of the diagnostic interview. Patients with a history of early life stress (ELS) may present for treatment with complaints that represent depression, anxiety, or substance abuse, but they may also have complicated presentations involving psychotic or dissociative symptoms, reflecting the diagnostic comorbidity in this population.
How to identify ELS. No standardized office-based screening tools exist for ELS, and clinical interviewing is the primary means of assessing exposure to ELS. A common error in history-taking with this population, particularly in high-volume settings, is to merely ask patients whether they were abused or neglected as children or to elaborate only very slightly on this aspect of the history. We risk not finding information that is critical to understanding our patients if we assume they share a common definition of abuse and neglect with us, can recognize such events in their personal history, and are willing to share that information with us.
When framing questions about abuse or neglect, it is important to remember that our own sense of what constitutes neglect or abuse may be very different from what a patient thinks of as neglect or abuse. For example, some patients may not consider their experience as a child abusive because of a distorted sense of responsibility, possibly further exaggerated by comorbid depression (ie, “My parents locked me in the closet overnight all the time when I was a child because I deserved it.”)
Other patients may try to minimize the impact of the experience or the responsibility of the perpetrator and attempt to normalize it (ie, “My uncle used to touch me between my legs in the swimming pool but he didn’t mean anything by it; he did it to everybody.”)
Avoiding ‘false memories.’ As important as it is to identify abuse or neglect when it has occurred, it is equally important to avoid intensifying the impact of an incident of abuse or, worse, creating a “false memory” of abuse in suggestible patients (bearing in mind that there is no definitive way to exclude the presence of “suggestibility” in patients). Our task as clinicians is to help patients correctly identify experiences of abuse and neglect and understand their response to these experiences clinically to facilitate case formulation and a treatment plan.
Creating a therapeutic alliance. Abuse and neglect during early life fundamentally alter the core assumptions that patients have about trust and safety in their relationships with others. Not only does this potentially impact the disclosure by patients of the nature and extent of trauma they have experienced, but it can also slow the formation of an effective therapeutic alliance.
To that end, asking open-ended questions about neglect and specific forms of abuse, creating an atmosphere of safety and trust, and a warm, empathic, nonjudgmental manner are central to the accurate assessment of ELS and provide the foundation for treatment by establishing an effective therapeutic alliance.
Optimal treatment. No published clinical trials have specifically compared the relative efficacy of particular forms of psychotherapy or pharmacotherapy for depressed patients with a history of ELS. However, it is clear from the available data that psychotherapy should be considered a core component of treatment for these patients.
Because psychiatric comorbidity is common in these patients, their treatment should be individualized in a manner that accounts for and addresses depression as well as associated diagnoses such as panic disorder or posttraumatic stress disorder (PTSD) with disorder-specific psychotherapy. Pharmacotherapy in combination with psychotherapy may also be helpful to patients with ELS and depression, though definitive data are lacking.
Judicious combination of medications such as antidepressants and benzodiazepines, particularly in patients with comorbid panic or PTSD, in concert with psychotherapy probably constitutes optimal treatment.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- National Clearinghouse on Child Abuse and Neglect. http://nccanch.acf.hhs.gov.
- Charney DS, Nemeroff CB. The peace of mind prescription: An authoritative guide to finding the most effective treatment for anxiety and depression. Boston: Houghton Mifflin, 2004.
Drug brand name
- Nefazodone • Serzone
Disclosure
Dr. Gillespie reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nemeroff receives research grants from or is a consultant/speaker for Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Corcept Therapeutics, Cyberonics, Cypress Biosciences, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Merck & Co., Neurocrine Biosciences, Otsuka Inc., Pfizer Inc., Sanofi Aventis, and Wyeth.
Acknowledgement
Supported by NIH MH-42088, MH-52899 (CBN), and NIH NCRRM01-RR00039 to Emory University.
1. Sedlack AJ, Broadhurst DD. Third National Incidence Study of Child Abuse and Neglect. Washington, DC: US Department of Health and Human Services; 1996.
2. McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of child abuse: unhealed wounds. JAMA 1997;277(17):1362-8.
3. Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA 2001;286(24):3089-96.
4. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82(2):217-25.
5. Gladstone GL, Parker GB, Mitchell PB, et al. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry 2004;161(8):1417-25.
6. Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34(3):509-20.
7. Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004;29(4):641-8.
8. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983;36(3):165-86.
9. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160(1):1-12.
10. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 1990;15(2):71-100.
11. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev 1991;43(4):425-73.
12. Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotrophin-releasing factor-like immunoreactivity in depressed patients. Science 1984;226(4680):1342-4.
13. Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996;780:96-105.
14. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997;154(5):624-9.
15. Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999;156(4):585-8.
16. Nemeroff CB, Owens MJ, Bissette G, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988;45(6):577-9.
17. Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004;24(6):1478-85.
18. Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 1989;25(3):355-9.
19. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157(10):1552-62.
20. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004;29(10):1765-81.
21. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79(3):471-8.
22. Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004;34(8):1475-82.
23. Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-9.
24. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA 2004;101(49):17316-21.
25. Maercker A, Michael T, Fehm L, et al. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004;184:482-7.
26. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284(5):592-7.
27. Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001;158(4):575-81.
28. Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004;29(4):777-84.
29. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417-26.
30. Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996;93(9):3908-13.
31. Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19(12):5034-43.
32. Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27(4):951-9.
33. Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000;57(12):1115-22.
34. Vythilingam M, Heim C, Newport DJ, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159(12):2072-80.
35. Hayden EP, Klein DM. Outcome of dysthymic disorder at 5-year follow-up: the effect of familial psychopathology, early adversity, personality, comorbidity, and chronic stress. Am J Psychiatry 2001;158(11):1864-70.
36. Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596-601.
37. Lara ME, Klein DN, Kasch KL. Psychosocial predictors of the short-term course and outcome of major depression: a longitudinal study of a nonclinical sample with recent-onset episodes. J Abnorm Psychol 2000;109(4):644-50.
38. Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100(24):14293-6.
39. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342(20):1462-70.
40. Craighead WE, Nemeroff CB. The impact of early trauma on response to psychotherapy. Clin Neurosci Res 2005;4(5-6):405-11.
1. Sedlack AJ, Broadhurst DD. Third National Incidence Study of Child Abuse and Neglect. Washington, DC: US Department of Health and Human Services; 1996.
2. McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of child abuse: unhealed wounds. JAMA 1997;277(17):1362-8.
3. Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA 2001;286(24):3089-96.
4. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82(2):217-25.
5. Gladstone GL, Parker GB, Mitchell PB, et al. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry 2004;161(8):1417-25.
6. Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34(3):509-20.
7. Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004;29(4):641-8.
8. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983;36(3):165-86.
9. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160(1):1-12.
10. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 1990;15(2):71-100.
11. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev 1991;43(4):425-73.
12. Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotrophin-releasing factor-like immunoreactivity in depressed patients. Science 1984;226(4680):1342-4.
13. Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996;780:96-105.
14. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997;154(5):624-9.
15. Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999;156(4):585-8.
16. Nemeroff CB, Owens MJ, Bissette G, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988;45(6):577-9.
17. Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004;24(6):1478-85.
18. Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry 1989;25(3):355-9.
19. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157(10):1552-62.
20. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004;29(10):1765-81.
21. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79(3):471-8.
22. Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004;34(8):1475-82.
23. Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-9.
24. Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA 2004;101(49):17316-21.
25. Maercker A, Michael T, Fehm L, et al. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004;184:482-7.
26. Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000;284(5):592-7.
27. Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001;158(4):575-81.
28. Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004;29(4):777-84.
29. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417-26.
30. Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996;93(9):3908-13.
31. Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19(12):5034-43.
32. Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27(4):951-9.
33. Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000;57(12):1115-22.
34. Vythilingam M, Heim C, Newport DJ, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159(12):2072-80.
35. Hayden EP, Klein DM. Outcome of dysthymic disorder at 5-year follow-up: the effect of familial psychopathology, early adversity, personality, comorbidity, and chronic stress. Am J Psychiatry 2001;158(11):1864-70.
36. Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596-601.
37. Lara ME, Klein DN, Kasch KL. Psychosocial predictors of the short-term course and outcome of major depression: a longitudinal study of a nonclinical sample with recent-onset episodes. J Abnorm Psychol 2000;109(4):644-50.
38. Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 2003;100(24):14293-6.
39. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342(20):1462-70.
40. Craighead WE, Nemeroff CB. The impact of early trauma on response to psychotherapy. Clin Neurosci Res 2005;4(5-6):405-11.
Is Tom Cruise acting out?
Several years ago, actor Tom Cruise denounced psychiatrists’ use of antidepressants and methylphenidate in children and adults. I took his comments as innocent expressions of ignorance about a specialty that takes years of study to understand.
Recently, however, Cruise criticized actress Brooke Shields for taking antidepressants for her postpartum depression. He then verbally attacked NBC television’s Today show host Matt Lauer, who said he knows people who have benefited from using methylphenidate.
I think Cruise is venting his psychological issues in public but using the wrong venue. A well-known country music singer interviewed by CNN’s Anderson Cooper also suggested that Cruise discuss his own emotional issues instead of chastising the people who could help him. The singer told Cooper how antidepressants saved her career and possibly her life.
If your patients won’t try medication after Cruise’s diatribes, I offer these two examples:
- A 76-year-old woman refused to eat for fear of being poisoned by her relatives. She spent all day in bed and demanded to see her lawyer to change her power of attorney. I started her on olanzapine, 5 mg/d, which increases appetite as a side effect. Three days later, she was eating and sleeping normally and attending physical therapy sessions.
- An 8-year-old boy with obsessive-compulsive disorder was using three or four rolls of toilet paper per day and taking 1-hour showers to make sure he didn’t smell. After 2 weeks of sertraline, 50 mg/d, and behavior modification by a psychotherapist, his behavior improved dramatically.
I would love to hear how Cruise and others who stigmatize psychotropic use dispute how the medications improved these patients’ quality of life. Shields offered this testimony:
“To suggest that I was wrong to take drugs to deal with my depression, and that instead I should have taken vitamins and exercised, shows an utter lack of understanding about postpartum depression and childbirth in general,” Shields wrote in The New York Times. “If any good can come of (Cruise’s comments), let’s hope (they call) much-needed attention to a serious disease.”
Manuel Mota-Castillo, MD
Medical director
The Grove Academy, Sanford, FL
and Lake Mary Psychiatric Services, Lake Mary, FL
Several years ago, actor Tom Cruise denounced psychiatrists’ use of antidepressants and methylphenidate in children and adults. I took his comments as innocent expressions of ignorance about a specialty that takes years of study to understand.
Recently, however, Cruise criticized actress Brooke Shields for taking antidepressants for her postpartum depression. He then verbally attacked NBC television’s Today show host Matt Lauer, who said he knows people who have benefited from using methylphenidate.
I think Cruise is venting his psychological issues in public but using the wrong venue. A well-known country music singer interviewed by CNN’s Anderson Cooper also suggested that Cruise discuss his own emotional issues instead of chastising the people who could help him. The singer told Cooper how antidepressants saved her career and possibly her life.
If your patients won’t try medication after Cruise’s diatribes, I offer these two examples:
- A 76-year-old woman refused to eat for fear of being poisoned by her relatives. She spent all day in bed and demanded to see her lawyer to change her power of attorney. I started her on olanzapine, 5 mg/d, which increases appetite as a side effect. Three days later, she was eating and sleeping normally and attending physical therapy sessions.
- An 8-year-old boy with obsessive-compulsive disorder was using three or four rolls of toilet paper per day and taking 1-hour showers to make sure he didn’t smell. After 2 weeks of sertraline, 50 mg/d, and behavior modification by a psychotherapist, his behavior improved dramatically.
I would love to hear how Cruise and others who stigmatize psychotropic use dispute how the medications improved these patients’ quality of life. Shields offered this testimony:
“To suggest that I was wrong to take drugs to deal with my depression, and that instead I should have taken vitamins and exercised, shows an utter lack of understanding about postpartum depression and childbirth in general,” Shields wrote in The New York Times. “If any good can come of (Cruise’s comments), let’s hope (they call) much-needed attention to a serious disease.”
Manuel Mota-Castillo, MD
Medical director
The Grove Academy, Sanford, FL
and Lake Mary Psychiatric Services, Lake Mary, FL
Several years ago, actor Tom Cruise denounced psychiatrists’ use of antidepressants and methylphenidate in children and adults. I took his comments as innocent expressions of ignorance about a specialty that takes years of study to understand.
Recently, however, Cruise criticized actress Brooke Shields for taking antidepressants for her postpartum depression. He then verbally attacked NBC television’s Today show host Matt Lauer, who said he knows people who have benefited from using methylphenidate.
I think Cruise is venting his psychological issues in public but using the wrong venue. A well-known country music singer interviewed by CNN’s Anderson Cooper also suggested that Cruise discuss his own emotional issues instead of chastising the people who could help him. The singer told Cooper how antidepressants saved her career and possibly her life.
If your patients won’t try medication after Cruise’s diatribes, I offer these two examples:
- A 76-year-old woman refused to eat for fear of being poisoned by her relatives. She spent all day in bed and demanded to see her lawyer to change her power of attorney. I started her on olanzapine, 5 mg/d, which increases appetite as a side effect. Three days later, she was eating and sleeping normally and attending physical therapy sessions.
- An 8-year-old boy with obsessive-compulsive disorder was using three or four rolls of toilet paper per day and taking 1-hour showers to make sure he didn’t smell. After 2 weeks of sertraline, 50 mg/d, and behavior modification by a psychotherapist, his behavior improved dramatically.
I would love to hear how Cruise and others who stigmatize psychotropic use dispute how the medications improved these patients’ quality of life. Shields offered this testimony:
“To suggest that I was wrong to take drugs to deal with my depression, and that instead I should have taken vitamins and exercised, shows an utter lack of understanding about postpartum depression and childbirth in general,” Shields wrote in The New York Times. “If any good can come of (Cruise’s comments), let’s hope (they call) much-needed attention to a serious disease.”
Manuel Mota-Castillo, MD
Medical director
The Grove Academy, Sanford, FL
and Lake Mary Psychiatric Services, Lake Mary, FL
Involuntary admission: Weighing patient rights vs. appropriate care
Widower denies suicidal thoughts in hospital, but acts on them at home
DuPage County (IL) Circuit Court
A 77-year-old man was hospitalized after complaining of chest pain. He reported attempting suicide the night before by taking pills. His wife had died 5 months previously.
When the psychiatrist evaluated the patient the next day, the patient assured him that he was no longer suicidal, refused inpatient admission, but agreed to enter outpatient therapy. The patient repeated this intent to the hospital social worker.
The psychiatrist arranged visits by a home health care nurse. The patient was discharged after a 2-day stay, and the nurse visited the following day. The patient assured the nurse that he was not suicidal and called the psychiatrist to make an appointment for the next week. Two days later, the patient stabbed himself to death at home.
The estate claimed the psychiatrist should have kept the patient hospitalized. The psychiatrist claimed that involuntary admission was not possible because the patient was not dangerous to himself or others. The patient’s toxicology screen was negative except for his prescription drugs.
- The jury decided for the defense
Alcoholic promises to attend AA, but takes his life on Christmas Day
Davidson County (TN) Circuit Court
A 44-year-old man with a long history of alcohol abuse and failed rehabilitation was involuntarily admitted to a hospital after threatening suicide. His blood alcohol level was 0.393, and he had threatened suicide at the same facility 8 months before. A court order gave the hospital authority to involuntarily detain him until a hearing the following week.
The next day, the patient was transferred from the detoxification center to the psychiatric unit and evaluated by the psychiatrist. The patient disavowed suicidal thoughts, and the psychiatrist discharged the patient the following day (Christmas Eve, 48 hours after admission). The psychiatrist based this decision partially on the patient’s promise to enter inpatient alcohol treatment and attend an Alcoholics Anonymous meeting within 2 days.
On Christmas Day, the patient shot himself and died. His blood alcohol content at the time of death was 0.303.
The patient’s estate charged that the final discharge was negligent, the discharge instructions were inadequate, and the psychiatrist and hospital’s assessments were inaccurate.
The hospital argued that it deferred to the psychiatrist in the discharge decision. The psychiatrist argued that state law defined holding an individual without “immediate risk of substantial harm” as a felony.
- The jury decided in favor of the defendant psychiatrist. A directed verdict was granted for the hospital.
Plaintiff: Discharge led to hemiplegia
Broward County (FL) Circuit Court
Police took into custody a 27-year-old woman who had been wandering a public road, apparently under the influence of illegal substances. The officers transported her to a hospital, where the emergency room staff admitted her for psychiatric evaluation.
The psychiatrist determined that involuntary admission was not appropriate. When the patient refused the psychiatrist’s recommendation for voluntary admission, she was discharged.
The patient then went to her mother’s house, began drinking, and became combative. She started brandishing a rifle. The next day, the weapon discharged and a bullet lodged in her spine at the L2 vertebra. The patient is now hemiplegic and has no bladder or bowel control. She alleged that the hospital and psychiatrist were negligent in not admitting her.
- The hospital reached a $50,000 settlement before trial; the jury returned a $190,007 award, with 90% of fault apportioned to the plaintiff and 10% to the psychiatrist. After setoffs, the plaintiff’s net award was $80.
Dr. Grant’s observations
These cases illustrate suicide risk factors psychiatrists must consider even when a patient denies suicidal thoughts or intent. Suicide risk factors these patients showed include:
- recent discharge from psychiatric facilities1
- recent suicide attempt with fairly high lethality potential (overdosing on pills)
- depressive turmoil and psychological isolation (recent loss of spouse)
- older widowed male2-3
- history of dangerous behavior when intoxicated4
- possible “holiday effect.”5
As the verdicts in these cases suggest, the legal system recognizes that psychiatrists cannot predict suicide.8 Mistakes in clinical judgment are not the same as negligence, however, and failure to assess suicide risk or intervene appropriately for the level of risk may result in successful negligence claims.
Standards for emergency short-term hospitalization vary from state to state, so familiarize yourself with your state’s standards. Although one standard for involuntary admission is often imminent threat of harm to self, do not base the threat of danger only on a patient’s self-report. One study of patients who committed suicide while hospitalized found that 78% denied suicidal thoughts at their last communication.9 However, “locking up” suicidal patients to prevent a malpractice suit is equally inappropriate.
Assess suicide risk during a thoroughly documented psychiatric examination with particular attention to the patient’s history of suicidal behavior. Record details of the assessment in the patient’s chart (Table) at the time of evaluation, and document how these clinical factors influence your final decision.
Involuntary hospitalization provides the immediate benefit of supervision in a safe environment, and patients can gain short-term therapeutic benefits from inpatient treatment whether or not the admission was voluntary.10 Patients may eventually recognize admission was helpful, but their attitudes about the process often do not become more positive. To ease the stress of involuntary admission:
- acknowledge the patient’s disapproval
- tell the patient why he’s being hospitalized
- inform the patient about his or her legal rights.
Table
Documenting suicide risk assessment
| Include in patient’s chart… | Examples… |
|---|---|
| Short-term factors | Current suicidal ideation/plan, lethality potential, current stressors (bereavement, illness, loss of job), recent discharge from a psychiatric facility, time of year (holiday effect, anniversaries) |
| Long-term factors | History of suicidal behavior/attempts, personality factors (agitation, hopelessness), gender, age, marital status, substance abuse history, psychiatric illness (depression, bipolar disorder, schizophrenia) |
| Appropriate psychiatric interventions based on the assessed degree of risk | Involuntary admission, intensive monitoring, outpatient visits, home healthcare nursing, residential placement, substance abuse treatment |
| Sources of information used | Medical records, patient self-report, family report, observation |
1. Qin P, Nordentoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry 2005;62(4):427-32.
2. Fawcett J, Scheftner W, Clark D, et al. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry 1987;144(1):35-40.
3. Fawcett J, Clark DC, Busch KA. Assessing and treating the patient at risk for suicide. Psychiatr Ann 1993;23:244-55.
4. Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 1990;147(9):1189-94.
5. Jessen G, Jensen BF, Arensman E, et al. Attempted suicide and major public holidays in Europe: findings from the WHO/EURO Multicentre Study on Parasuicide. Acta Psychiatr Scand 1999;99(6):412-8.
6. Carpenter WT, Jr. The challenge to psychiatry as society’s agent for mental illness treatment and research. Am J Psychiatry 1999;156(9):1307-10.
7. Lavoie FW. Consent, involuntary treatment, and the use of force in an urban emergency department. Ann Emerg Med 1992;21:25-32.
8. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
9. Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry 2003;64(1):14-9.
10. Steinert T, Schmid P. Effect of voluntariness of participation in treatment on short-term outcome of inpatients with schizophrenia. Psychiatr Serv 2004;55(7):786-91.
11. Gardner W, Lidz CW, Hoge SK, et al. Patients’ revisions of their belief about the need for hospitalization. Am J Psychiatry 1999;156(9):1385-91.
Widower denies suicidal thoughts in hospital, but acts on them at home
DuPage County (IL) Circuit Court
A 77-year-old man was hospitalized after complaining of chest pain. He reported attempting suicide the night before by taking pills. His wife had died 5 months previously.
When the psychiatrist evaluated the patient the next day, the patient assured him that he was no longer suicidal, refused inpatient admission, but agreed to enter outpatient therapy. The patient repeated this intent to the hospital social worker.
The psychiatrist arranged visits by a home health care nurse. The patient was discharged after a 2-day stay, and the nurse visited the following day. The patient assured the nurse that he was not suicidal and called the psychiatrist to make an appointment for the next week. Two days later, the patient stabbed himself to death at home.
The estate claimed the psychiatrist should have kept the patient hospitalized. The psychiatrist claimed that involuntary admission was not possible because the patient was not dangerous to himself or others. The patient’s toxicology screen was negative except for his prescription drugs.
- The jury decided for the defense
Alcoholic promises to attend AA, but takes his life on Christmas Day
Davidson County (TN) Circuit Court
A 44-year-old man with a long history of alcohol abuse and failed rehabilitation was involuntarily admitted to a hospital after threatening suicide. His blood alcohol level was 0.393, and he had threatened suicide at the same facility 8 months before. A court order gave the hospital authority to involuntarily detain him until a hearing the following week.
The next day, the patient was transferred from the detoxification center to the psychiatric unit and evaluated by the psychiatrist. The patient disavowed suicidal thoughts, and the psychiatrist discharged the patient the following day (Christmas Eve, 48 hours after admission). The psychiatrist based this decision partially on the patient’s promise to enter inpatient alcohol treatment and attend an Alcoholics Anonymous meeting within 2 days.
On Christmas Day, the patient shot himself and died. His blood alcohol content at the time of death was 0.303.
The patient’s estate charged that the final discharge was negligent, the discharge instructions were inadequate, and the psychiatrist and hospital’s assessments were inaccurate.
The hospital argued that it deferred to the psychiatrist in the discharge decision. The psychiatrist argued that state law defined holding an individual without “immediate risk of substantial harm” as a felony.
- The jury decided in favor of the defendant psychiatrist. A directed verdict was granted for the hospital.
Plaintiff: Discharge led to hemiplegia
Broward County (FL) Circuit Court
Police took into custody a 27-year-old woman who had been wandering a public road, apparently under the influence of illegal substances. The officers transported her to a hospital, where the emergency room staff admitted her for psychiatric evaluation.
The psychiatrist determined that involuntary admission was not appropriate. When the patient refused the psychiatrist’s recommendation for voluntary admission, she was discharged.
The patient then went to her mother’s house, began drinking, and became combative. She started brandishing a rifle. The next day, the weapon discharged and a bullet lodged in her spine at the L2 vertebra. The patient is now hemiplegic and has no bladder or bowel control. She alleged that the hospital and psychiatrist were negligent in not admitting her.
- The hospital reached a $50,000 settlement before trial; the jury returned a $190,007 award, with 90% of fault apportioned to the plaintiff and 10% to the psychiatrist. After setoffs, the plaintiff’s net award was $80.
Dr. Grant’s observations
These cases illustrate suicide risk factors psychiatrists must consider even when a patient denies suicidal thoughts or intent. Suicide risk factors these patients showed include:
- recent discharge from psychiatric facilities1
- recent suicide attempt with fairly high lethality potential (overdosing on pills)
- depressive turmoil and psychological isolation (recent loss of spouse)
- older widowed male2-3
- history of dangerous behavior when intoxicated4
- possible “holiday effect.”5
As the verdicts in these cases suggest, the legal system recognizes that psychiatrists cannot predict suicide.8 Mistakes in clinical judgment are not the same as negligence, however, and failure to assess suicide risk or intervene appropriately for the level of risk may result in successful negligence claims.
Standards for emergency short-term hospitalization vary from state to state, so familiarize yourself with your state’s standards. Although one standard for involuntary admission is often imminent threat of harm to self, do not base the threat of danger only on a patient’s self-report. One study of patients who committed suicide while hospitalized found that 78% denied suicidal thoughts at their last communication.9 However, “locking up” suicidal patients to prevent a malpractice suit is equally inappropriate.
Assess suicide risk during a thoroughly documented psychiatric examination with particular attention to the patient’s history of suicidal behavior. Record details of the assessment in the patient’s chart (Table) at the time of evaluation, and document how these clinical factors influence your final decision.
Involuntary hospitalization provides the immediate benefit of supervision in a safe environment, and patients can gain short-term therapeutic benefits from inpatient treatment whether or not the admission was voluntary.10 Patients may eventually recognize admission was helpful, but their attitudes about the process often do not become more positive. To ease the stress of involuntary admission:
- acknowledge the patient’s disapproval
- tell the patient why he’s being hospitalized
- inform the patient about his or her legal rights.
Table
Documenting suicide risk assessment
| Include in patient’s chart… | Examples… |
|---|---|
| Short-term factors | Current suicidal ideation/plan, lethality potential, current stressors (bereavement, illness, loss of job), recent discharge from a psychiatric facility, time of year (holiday effect, anniversaries) |
| Long-term factors | History of suicidal behavior/attempts, personality factors (agitation, hopelessness), gender, age, marital status, substance abuse history, psychiatric illness (depression, bipolar disorder, schizophrenia) |
| Appropriate psychiatric interventions based on the assessed degree of risk | Involuntary admission, intensive monitoring, outpatient visits, home healthcare nursing, residential placement, substance abuse treatment |
| Sources of information used | Medical records, patient self-report, family report, observation |
Widower denies suicidal thoughts in hospital, but acts on them at home
DuPage County (IL) Circuit Court
A 77-year-old man was hospitalized after complaining of chest pain. He reported attempting suicide the night before by taking pills. His wife had died 5 months previously.
When the psychiatrist evaluated the patient the next day, the patient assured him that he was no longer suicidal, refused inpatient admission, but agreed to enter outpatient therapy. The patient repeated this intent to the hospital social worker.
The psychiatrist arranged visits by a home health care nurse. The patient was discharged after a 2-day stay, and the nurse visited the following day. The patient assured the nurse that he was not suicidal and called the psychiatrist to make an appointment for the next week. Two days later, the patient stabbed himself to death at home.
The estate claimed the psychiatrist should have kept the patient hospitalized. The psychiatrist claimed that involuntary admission was not possible because the patient was not dangerous to himself or others. The patient’s toxicology screen was negative except for his prescription drugs.
- The jury decided for the defense
Alcoholic promises to attend AA, but takes his life on Christmas Day
Davidson County (TN) Circuit Court
A 44-year-old man with a long history of alcohol abuse and failed rehabilitation was involuntarily admitted to a hospital after threatening suicide. His blood alcohol level was 0.393, and he had threatened suicide at the same facility 8 months before. A court order gave the hospital authority to involuntarily detain him until a hearing the following week.
The next day, the patient was transferred from the detoxification center to the psychiatric unit and evaluated by the psychiatrist. The patient disavowed suicidal thoughts, and the psychiatrist discharged the patient the following day (Christmas Eve, 48 hours after admission). The psychiatrist based this decision partially on the patient’s promise to enter inpatient alcohol treatment and attend an Alcoholics Anonymous meeting within 2 days.
On Christmas Day, the patient shot himself and died. His blood alcohol content at the time of death was 0.303.
The patient’s estate charged that the final discharge was negligent, the discharge instructions were inadequate, and the psychiatrist and hospital’s assessments were inaccurate.
The hospital argued that it deferred to the psychiatrist in the discharge decision. The psychiatrist argued that state law defined holding an individual without “immediate risk of substantial harm” as a felony.
- The jury decided in favor of the defendant psychiatrist. A directed verdict was granted for the hospital.
Plaintiff: Discharge led to hemiplegia
Broward County (FL) Circuit Court
Police took into custody a 27-year-old woman who had been wandering a public road, apparently under the influence of illegal substances. The officers transported her to a hospital, where the emergency room staff admitted her for psychiatric evaluation.
The psychiatrist determined that involuntary admission was not appropriate. When the patient refused the psychiatrist’s recommendation for voluntary admission, she was discharged.
The patient then went to her mother’s house, began drinking, and became combative. She started brandishing a rifle. The next day, the weapon discharged and a bullet lodged in her spine at the L2 vertebra. The patient is now hemiplegic and has no bladder or bowel control. She alleged that the hospital and psychiatrist were negligent in not admitting her.
- The hospital reached a $50,000 settlement before trial; the jury returned a $190,007 award, with 90% of fault apportioned to the plaintiff and 10% to the psychiatrist. After setoffs, the plaintiff’s net award was $80.
Dr. Grant’s observations
These cases illustrate suicide risk factors psychiatrists must consider even when a patient denies suicidal thoughts or intent. Suicide risk factors these patients showed include:
- recent discharge from psychiatric facilities1
- recent suicide attempt with fairly high lethality potential (overdosing on pills)
- depressive turmoil and psychological isolation (recent loss of spouse)
- older widowed male2-3
- history of dangerous behavior when intoxicated4
- possible “holiday effect.”5
As the verdicts in these cases suggest, the legal system recognizes that psychiatrists cannot predict suicide.8 Mistakes in clinical judgment are not the same as negligence, however, and failure to assess suicide risk or intervene appropriately for the level of risk may result in successful negligence claims.
Standards for emergency short-term hospitalization vary from state to state, so familiarize yourself with your state’s standards. Although one standard for involuntary admission is often imminent threat of harm to self, do not base the threat of danger only on a patient’s self-report. One study of patients who committed suicide while hospitalized found that 78% denied suicidal thoughts at their last communication.9 However, “locking up” suicidal patients to prevent a malpractice suit is equally inappropriate.
Assess suicide risk during a thoroughly documented psychiatric examination with particular attention to the patient’s history of suicidal behavior. Record details of the assessment in the patient’s chart (Table) at the time of evaluation, and document how these clinical factors influence your final decision.
Involuntary hospitalization provides the immediate benefit of supervision in a safe environment, and patients can gain short-term therapeutic benefits from inpatient treatment whether or not the admission was voluntary.10 Patients may eventually recognize admission was helpful, but their attitudes about the process often do not become more positive. To ease the stress of involuntary admission:
- acknowledge the patient’s disapproval
- tell the patient why he’s being hospitalized
- inform the patient about his or her legal rights.
Table
Documenting suicide risk assessment
| Include in patient’s chart… | Examples… |
|---|---|
| Short-term factors | Current suicidal ideation/plan, lethality potential, current stressors (bereavement, illness, loss of job), recent discharge from a psychiatric facility, time of year (holiday effect, anniversaries) |
| Long-term factors | History of suicidal behavior/attempts, personality factors (agitation, hopelessness), gender, age, marital status, substance abuse history, psychiatric illness (depression, bipolar disorder, schizophrenia) |
| Appropriate psychiatric interventions based on the assessed degree of risk | Involuntary admission, intensive monitoring, outpatient visits, home healthcare nursing, residential placement, substance abuse treatment |
| Sources of information used | Medical records, patient self-report, family report, observation |
1. Qin P, Nordentoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry 2005;62(4):427-32.
2. Fawcett J, Scheftner W, Clark D, et al. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry 1987;144(1):35-40.
3. Fawcett J, Clark DC, Busch KA. Assessing and treating the patient at risk for suicide. Psychiatr Ann 1993;23:244-55.
4. Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 1990;147(9):1189-94.
5. Jessen G, Jensen BF, Arensman E, et al. Attempted suicide and major public holidays in Europe: findings from the WHO/EURO Multicentre Study on Parasuicide. Acta Psychiatr Scand 1999;99(6):412-8.
6. Carpenter WT, Jr. The challenge to psychiatry as society’s agent for mental illness treatment and research. Am J Psychiatry 1999;156(9):1307-10.
7. Lavoie FW. Consent, involuntary treatment, and the use of force in an urban emergency department. Ann Emerg Med 1992;21:25-32.
8. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
9. Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry 2003;64(1):14-9.
10. Steinert T, Schmid P. Effect of voluntariness of participation in treatment on short-term outcome of inpatients with schizophrenia. Psychiatr Serv 2004;55(7):786-91.
11. Gardner W, Lidz CW, Hoge SK, et al. Patients’ revisions of their belief about the need for hospitalization. Am J Psychiatry 1999;156(9):1385-91.
1. Qin P, Nordentoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry 2005;62(4):427-32.
2. Fawcett J, Scheftner W, Clark D, et al. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry 1987;144(1):35-40.
3. Fawcett J, Clark DC, Busch KA. Assessing and treating the patient at risk for suicide. Psychiatr Ann 1993;23:244-55.
4. Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 1990;147(9):1189-94.
5. Jessen G, Jensen BF, Arensman E, et al. Attempted suicide and major public holidays in Europe: findings from the WHO/EURO Multicentre Study on Parasuicide. Acta Psychiatr Scand 1999;99(6):412-8.
6. Carpenter WT, Jr. The challenge to psychiatry as society’s agent for mental illness treatment and research. Am J Psychiatry 1999;156(9):1307-10.
7. Lavoie FW. Consent, involuntary treatment, and the use of force in an urban emergency department. Ann Emerg Med 1992;21:25-32.
8. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
9. Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry 2003;64(1):14-9.
10. Steinert T, Schmid P. Effect of voluntariness of participation in treatment on short-term outcome of inpatients with schizophrenia. Psychiatr Serv 2004;55(7):786-91.
11. Gardner W, Lidz CW, Hoge SK, et al. Patients’ revisions of their belief about the need for hospitalization. Am J Psychiatry 1999;156(9):1385-91.
Acute MI risk? Protecting your patients’ heart health
One in five of your patients could suffer a heart attack in the near future—unless you take steps to ensure their heart health.
Psychiatric patients have more modifiable risk factors for coronary artery disease (CAD) compared with the general population.When depression treatment goes nowhere,” Current Psychiatry, August 2005.)
To help keep you abreast of constantly changing guidelines and strategies for recognizing and minimizing CAD risk, this article discusses:
- preventive and diagnostic guidelines for managing hypertension, diabetes, and dyslipidemia
- practical advice on convincing at-risk patients to adopt a healthier lifestyle and have a primary care doctor monitor their health.
Case: cigarettes and supersizing
Mr. H, age 54, is receiving cognitive-behavioral therapy for mild depression. He has been smoking one pack of cigarettes per day for 20 years and has never seriously considered quitting.
The patient, a school teacher, says his “busy schedule” keeps him from exercising and eating properly; he eats fast-food hamburgers and fries approximately five times per week. His father had a heart attack at age 52 and died in his sleep 10 years later.
Mr. H says he feels fine and has never seen a physician other than his psychiatrist. He is reluctant to see a primary care physician for a check-up and, because he is asymptomatic, has no incentive to do so. The psychiatrist thus decides to do a routine examination.
Blood pressure is 148/86; other vital signs are normal. Mr. H’s waist size is 42 inches, he weighs 242 lbs, and his body mass index (BMI) is 34 kg/m2, indicating clinical obesity. Cardiovascular, pulmonary, and abdominal exams are unremarkable.
Discussion. Mr. H is at high risk of a myocardial ischemic event in the near future. He has six risk factors for CAD (Table 1)—four of which are modifiable:
- family history
- age
- current cigarette use
- provisional hypertension diagnosis
- obesity
- physical inactivity.
Table 1
Risk factors for coronary artery disease
| Core risk factors |
| Age ≥45 for men* |
| Age ≥55 for women or premature menopause without estrogen-replacement therapy* |
Family history: premature coronary artery disease with myocardial infarction or sudden death before:
|
| Current cigarette smoking |
| Hypertension or antihypertensive treatment* |
| Elevated LDL cholesterol (>130 mg/dL in patients with low cardiac risk) |
| HDL cholesterol |
| Triglycerides >150 mg/dL |
| Total cholesterol >200 mg/dL* |
| Obesity (BMI >30 kg/m2)† |
| Sedentary lifestyle |
| Other risk factors |
| Elevated C-reactive protein |
| Elevated homocysteine |
| Chronic renal failure |
| Depression |
| Negative (cardio-protective) risk factors |
| HDL >60 mg/dL |
| Moderate alcohol use—no more than 1 to 2 drinks per day (1 drink = 12 oz beer or 5 oz of wine) |
| If >1 risk factor, refer to primary care doctor or quantify 10-year risk by using the Framingham/ATP III point system scale (www.nhlbi.nih.gov). |
| * Framingham/ATP III point system scale variables |
| † Use BMI calculator (http://www.nhlbisupport.com/bmi/bmicalc.htm) to determine body mass index. |
| HDL: High-density lipoprotein |
| LDL: Low-density lipoprotein |
| Source: References 9,10 |
Tools for assessing risk
The lifetime risk at age 40 for developing CAD is 49% and 32% in men and women, respectively.6
The National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel) has focused on decreasing heart disease incidence by educating patients and providers. Preventive strategies and standards of care have changed several times over the past decade; the Adult Treatment Panel (ATP III) was last revised in July 2004.7
The American College of Cardiology and American Heart Association both endorse the modified Framingham/ATP III scale to measure CAD risk (see Related resources). Although this somewhat tedious point system has limitations, it can precisely calculate coronary risk across 10 years.8 Variables not included in the scoring system—such as C-reactive protein, homocysteine, and postmenopausal state—may be clinically significant and should be gauged separately.
An easier-to-use alternative, the ATP III “core risk factors” scale, estimates hypertension, hypercholesterolemia, family history, current cigarette smoking, and age as low, intermediate, or high (“risk equivalent”) risks (Table 1).8 Psychiatrists can quickly obtain this information from a brief history, blood pressure assessment, and relatively inexpensive lab studies.
Generally, the more risk factors present, the higher the risk of having a major coronary event. Presence of ≥ 2 risk factors signals intermediate or high risk and necessitates referral to a primary care doctor for monitoring.
Patients with a cardiac “risk equivalent” face a >20% risk of having a cardiac ischemic event within 10 years8 (Table 2). Examples of risk equivalents include diabetes or significant vascular disease in any artery.
“Non-core” variables. Also consider certain “non-core” variables—such as pre-existing psychiatric illness—when estimating clinical risk for heart disease. Depression, anxiety, and stress are correlated with an increase in pro-inflammatory markers such as C-reactive protein and predispose patients to CAD.11,12 Depression has repeatedly been shown to increase morbidity and mortality two- to four-fold after myocardial infarction (MI).9,13,14 Interestingly, however, depression treatment after an acute coronary event does not clearly decrease mortality.15 Although prospective, randomized studies are lacking, mood and anxiety disorder treatment is presumed to help prevent CAD development.16
Table 2
Risk equivalents for CAD*
| Established coronary artery disease |
| Symptomatic carotid artery disease |
| Peripheral vascular disease |
| Abdominal aortic aneurism |
| Diabetes mellitus |
| *Risk equivalent: Patient is assumed to have coronary artery disease (CAD). |
Recognizing cad risk
At what point do hypertension and dyslipidemia become risk factors for CAD? When and how often should patients be screened for diabetes mellitus?
Hypertension is one of the most common and deadly CAD risk factors, affecting 50 million Americans.10 Although hypertension awareness and treatment have improved, only 35% of adults have “controlled” blood pressure (
According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), normalizing blood pressure can reduce stroke incidence by 35% and MI by 25%, respectively. JNC 7, however, also found that 90% of persons who are normotensive at age 55 eventually develop hypertension.10
Based on these findings, JNC 7 in 2003 drastically changed the standard of care for diagnosing hypertension. JNC 7 defines normal blood pressure as
- systolic blood pressure 120 to 139 mm Hg
- diastolic blood pressure 80 to 89 mm Hg (Table 3).
Patients with diabetes mellitus or chronic kidney disease are considered hypertensive with blood pressure >130 mm Hg systolic and/or >80 mm Hg diastolic.
As with Mr. H, a blood pressure check is imperative for patients who have rarely or never seen a primary care physician in recent years. The U.S. Preventive Services Task Force strongly recommends measuring blood pressure during a routine medical evaluation at least every 2 years. A second abnormal reading at a separate visit at any time should prompt a hypertension diagnosis. Once diagnosed with hypertension, patients should be treated and checked monthly until stable, then monitored every 3 to 6 months indefinitely.10
If you cannot measure blood pressure in the office, urge patients to use an over-the-counter blood pressure measuring device and refer them to a primary care physician. Check the patient’s self-test reading for accuracy against a clinician’s measurement.
Diabetes is now considered a risk equivalent for CAD development.8 Patients diagnosed with diabetes are extremely likely to have established vascular disease,8 which predisposes them to MI, stroke, kidney disease, blindness, and lower-extremity amputations.17 Those with type 1 diabetes usually present with acute symptoms—including polyuria, polydipsia, weight loss, malaise, dry mouth, and blurred vision—and are readily diagnosed with elevated plasma glucose.
Screening for diabetes is critical because one-third of patients with the disease are undiagnosed. Also, more than 90% of patients with diabetes are non-insulin-dependent (type 2) and are asymptomatic early in the disease course.
No data definitively show benefits from screening asymptomatic adults. Recently revised diagnostic criteria for diabetes, however, call for re-testing asymptomatic patients who were found to have normal fasting plasma glucose (FPG) levels and were considered “free” of diabetes. The American Diabetes Association recommends measuring FPG after no caloric intake for ≥ 8 hours for asymptomatic patients.
FPG measurement is cost-effective and generally more convenient than other diabetes tests.17 Expert consensus strongly suggests checking FPG every 3 years beginning at age 45:17
- FPG
- FPG 100 to 125 mg/dL suggests prediabetes or impaired fasting glucose
- FPG ≥ 126 mg/dL demands a provisional diabetes diagnosis and a follow-up test on another day to confirm the diagnosis.
- comorbid cardiac risk factors
- history of polycystic ovary disease
- a first-degree relative with diabetes
- habitual inactivity
- or FPG 100 to 125 mg/dL.
Do not base diabetes diagnosis on glycosylated hemoglobin measurements, as this test can produce false-negative results in patients with new-onset diabetes.
Dyslipidemia. Every 10% reduction in serum cholesterol reduces cardiovascular mortality by 10% to 15%.19 Data from the large, prospective Framingham heart study show a 25% increase in MIs with each 5-mg/dL decrease in high-density lipoprotein cholesterol (HDL) below the age-based median for men and women.20 Serum triglycerides >150 mg/dL clearly predict future CAD and increase the likelihood of abnormally low HDL.
Every 30-mg/dL increase in low-density lipoprotein cholesterol (LDL) raises the relative risk for CAD by 30%.7 ATP III classifies LDL as the “primary target of cholesterol-lowering therapy.”8Table 4 lists LDL target levels based on other CAD risk factors.
Check fasting lipid profile or serum cholesterol, LDL, HDL, and triglycerides beginning at age 20 and about every 5 years thereafter.8 Total cholesterol
Table 3
JNC 7: What blood pressure readings mean
| Category | Systolic BP (mm Hg) | Diastolic BP (mm Hg) |
|---|---|---|
| Normal | and | |
| Prehypertension | 120-139 | or 80-89 |
| Stage 1 hypertension* | 140-159 | or 90-99 |
| Stage 2 hypertension | 160 | or ≥100 |
| JNC 7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure | ||
| *Patients with diabetes mellitus or chronic kidney disease have stage 1 hypertension at >130/80 mm Hg. | ||
| Source: Reference 10 | ||
Acceptable LDL cholesterol levels for adults based on CAD risk
| Risk category | Existing CAD risk factors | LDL goal |
|---|---|---|
| High risk (10-year risk > 20%) | History of diabetes, CAD, symptomatic carotid artery disease, peripheral vascular disease, or abdominal aortic aneurysm | |
| Moderate high risk* (10-year risk 10 to 20%) | >2 risk factors | |
| Moderate risk* (10-year risk | >2 risk factors | |
| Low risk | 0 to 1 risk factor | ≤160 mg/dL |
| CAD: Coronary artery disease | ||
| *Same goals apply to managing moderate high and moderate risk. Find 10-year risk calculations at nhlbi.nih.gov/guidelines/cholesterol. | ||
| Source: Reference 7 | ||
Addressing smoking, obesity
Smoking. Before trying nicotine patches or bupropion, Mr. H should realistically contemplate his risks with continued smoking; if he doesn’t want to stop, periodically encourage him to reconsider.10 Most people know the dangers of smoking but few understand that complete cessation for 1 to 2 years often nearly reverses cardiovascular disease.21
Obesity and lack of exercise go hand in hand. Reducing Mr. H’s waist size to 2 is a reasonable short-term goal. To that end, encourage him to:
- decrease his number of weekly fast-food meals from five to three, with an eventual goal of one per week. As an alternative, microwaveable, low-calorie meals—each with at least two servings of fruits or vegetables—can be prepared at home or work.
- walk 30 minutes three times weekly and progress to 1 hour five times weekly over 6 months. As with any exercise program, remind Mr. H to “start low and go slow.”
The patient’s role in treatment. Patients often feel overwhelmed after getting large amounts of information on CAD risk and may feel hopeless and unenthusiastic about improving their physical health. Work with the primary care doctor to emphasize a patient care plan that clearly defines easily attainable, step-by-step goals. Make sure the patient agrees to these goals.
Case continued: no more supersizing
Mr. H now understands the importance of minimizing his CAD risk and realizes that CAD and many associated risk factors are asymptomatic in the early stages of development.
With help from his doctors, Mr. H quit smoking. He also became more mindful of his caloric intake and the types of foods he was eating. He advanced from briskly walking 30 minutes three times per week to slow jogging 40 minutes five times weekly. He still eats at fast-food restaurants but usually orders broiled chicken, salads, or the occasional burger.
- National Cholesterol Education Program. CAD risk assessment tool and ATP III guidelines. www.nhlbi.nih.gov/guidelines/cholesterol/.
- U.S. Preventive Services Task Force preventive guidelines. www.ahrq.gov/clinic/uspstfix.htm.
- National Heart, Lung, and Blood Institute. Calculate your body mass index. http://nhlbisupport.com/bmi/.
- American Heart Association. www.americanheart.org.
1. Holt RI, Peveler RC, Byrne CD. Schizophrenia, the metabolic syndrome and diabetes. Diabetes Med 2004;21:515-23.
2. Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
3. Weiser M, Reichenberg A, Grotto I, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry 2004;161:1219-23.
4. Druss B, Rosenheck R. Mental disorders and access to medical care in the United States. Am J Psychiatry 1998;155:1775-7.
5. Druss B, Rosenheck R, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care 2002;40:129-36.
6. Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999;353(9147):89-92.
7. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227-9.
8. Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
9. Frasure-Smith N, Lesperance F, Talajic M, et al. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72.
11. Ford DE, Erlinger TP. Depression and C-reactive protein in U.S. adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2004;164:1010-14.
12. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Inflammation, coagulation and depressive symptomatology in cardiovascular disease-free people: the ATTICA study. Eur Heart J 2004;25:492-9.
13. Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819-25.
14. Ladwig KH, Kieser M, Konig J, et al. Affective disorders and survival after acute myocardial infarction: results from the post-infarction late potential study. Eur Heart J 1991;12:959-64.
15. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Artery Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003;289:3106-16.
16. Rosengren A, Hawken S, Ounpuu S, et al. Association of psychological risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study). Lancet 2004;364:953-62.
17. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care 2004;27(suppl 1):11-14.
18. American Diabetes Association; American Psychiatric Association. American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
19. Gould AL, Rossouw JE, Santanello NC, et al. Cholesterol reduction yields clinical benefit; impact of statin trials. Circulation 1998;97:946-52.
20. Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary artery disease. The Framingham Study. Am J Med 1977;62:707-14.
21. Rigotti N, Pasternak L. Changing the natural history of coronary artery disease. Cardiology Clinics 1996;14:51-68.
One in five of your patients could suffer a heart attack in the near future—unless you take steps to ensure their heart health.
Psychiatric patients have more modifiable risk factors for coronary artery disease (CAD) compared with the general population.When depression treatment goes nowhere,” Current Psychiatry, August 2005.)
To help keep you abreast of constantly changing guidelines and strategies for recognizing and minimizing CAD risk, this article discusses:
- preventive and diagnostic guidelines for managing hypertension, diabetes, and dyslipidemia
- practical advice on convincing at-risk patients to adopt a healthier lifestyle and have a primary care doctor monitor their health.
Case: cigarettes and supersizing
Mr. H, age 54, is receiving cognitive-behavioral therapy for mild depression. He has been smoking one pack of cigarettes per day for 20 years and has never seriously considered quitting.
The patient, a school teacher, says his “busy schedule” keeps him from exercising and eating properly; he eats fast-food hamburgers and fries approximately five times per week. His father had a heart attack at age 52 and died in his sleep 10 years later.
Mr. H says he feels fine and has never seen a physician other than his psychiatrist. He is reluctant to see a primary care physician for a check-up and, because he is asymptomatic, has no incentive to do so. The psychiatrist thus decides to do a routine examination.
Blood pressure is 148/86; other vital signs are normal. Mr. H’s waist size is 42 inches, he weighs 242 lbs, and his body mass index (BMI) is 34 kg/m2, indicating clinical obesity. Cardiovascular, pulmonary, and abdominal exams are unremarkable.
Discussion. Mr. H is at high risk of a myocardial ischemic event in the near future. He has six risk factors for CAD (Table 1)—four of which are modifiable:
- family history
- age
- current cigarette use
- provisional hypertension diagnosis
- obesity
- physical inactivity.
Table 1
Risk factors for coronary artery disease
| Core risk factors |
| Age ≥45 for men* |
| Age ≥55 for women or premature menopause without estrogen-replacement therapy* |
Family history: premature coronary artery disease with myocardial infarction or sudden death before:
|
| Current cigarette smoking |
| Hypertension or antihypertensive treatment* |
| Elevated LDL cholesterol (>130 mg/dL in patients with low cardiac risk) |
| HDL cholesterol |
| Triglycerides >150 mg/dL |
| Total cholesterol >200 mg/dL* |
| Obesity (BMI >30 kg/m2)† |
| Sedentary lifestyle |
| Other risk factors |
| Elevated C-reactive protein |
| Elevated homocysteine |
| Chronic renal failure |
| Depression |
| Negative (cardio-protective) risk factors |
| HDL >60 mg/dL |
| Moderate alcohol use—no more than 1 to 2 drinks per day (1 drink = 12 oz beer or 5 oz of wine) |
| If >1 risk factor, refer to primary care doctor or quantify 10-year risk by using the Framingham/ATP III point system scale (www.nhlbi.nih.gov). |
| * Framingham/ATP III point system scale variables |
| † Use BMI calculator (http://www.nhlbisupport.com/bmi/bmicalc.htm) to determine body mass index. |
| HDL: High-density lipoprotein |
| LDL: Low-density lipoprotein |
| Source: References 9,10 |
Tools for assessing risk
The lifetime risk at age 40 for developing CAD is 49% and 32% in men and women, respectively.6
The National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel) has focused on decreasing heart disease incidence by educating patients and providers. Preventive strategies and standards of care have changed several times over the past decade; the Adult Treatment Panel (ATP III) was last revised in July 2004.7
The American College of Cardiology and American Heart Association both endorse the modified Framingham/ATP III scale to measure CAD risk (see Related resources). Although this somewhat tedious point system has limitations, it can precisely calculate coronary risk across 10 years.8 Variables not included in the scoring system—such as C-reactive protein, homocysteine, and postmenopausal state—may be clinically significant and should be gauged separately.
An easier-to-use alternative, the ATP III “core risk factors” scale, estimates hypertension, hypercholesterolemia, family history, current cigarette smoking, and age as low, intermediate, or high (“risk equivalent”) risks (Table 1).8 Psychiatrists can quickly obtain this information from a brief history, blood pressure assessment, and relatively inexpensive lab studies.
Generally, the more risk factors present, the higher the risk of having a major coronary event. Presence of ≥ 2 risk factors signals intermediate or high risk and necessitates referral to a primary care doctor for monitoring.
Patients with a cardiac “risk equivalent” face a >20% risk of having a cardiac ischemic event within 10 years8 (Table 2). Examples of risk equivalents include diabetes or significant vascular disease in any artery.
“Non-core” variables. Also consider certain “non-core” variables—such as pre-existing psychiatric illness—when estimating clinical risk for heart disease. Depression, anxiety, and stress are correlated with an increase in pro-inflammatory markers such as C-reactive protein and predispose patients to CAD.11,12 Depression has repeatedly been shown to increase morbidity and mortality two- to four-fold after myocardial infarction (MI).9,13,14 Interestingly, however, depression treatment after an acute coronary event does not clearly decrease mortality.15 Although prospective, randomized studies are lacking, mood and anxiety disorder treatment is presumed to help prevent CAD development.16
Table 2
Risk equivalents for CAD*
| Established coronary artery disease |
| Symptomatic carotid artery disease |
| Peripheral vascular disease |
| Abdominal aortic aneurism |
| Diabetes mellitus |
| *Risk equivalent: Patient is assumed to have coronary artery disease (CAD). |
Recognizing cad risk
At what point do hypertension and dyslipidemia become risk factors for CAD? When and how often should patients be screened for diabetes mellitus?
Hypertension is one of the most common and deadly CAD risk factors, affecting 50 million Americans.10 Although hypertension awareness and treatment have improved, only 35% of adults have “controlled” blood pressure (
According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), normalizing blood pressure can reduce stroke incidence by 35% and MI by 25%, respectively. JNC 7, however, also found that 90% of persons who are normotensive at age 55 eventually develop hypertension.10
Based on these findings, JNC 7 in 2003 drastically changed the standard of care for diagnosing hypertension. JNC 7 defines normal blood pressure as
- systolic blood pressure 120 to 139 mm Hg
- diastolic blood pressure 80 to 89 mm Hg (Table 3).
Patients with diabetes mellitus or chronic kidney disease are considered hypertensive with blood pressure >130 mm Hg systolic and/or >80 mm Hg diastolic.
As with Mr. H, a blood pressure check is imperative for patients who have rarely or never seen a primary care physician in recent years. The U.S. Preventive Services Task Force strongly recommends measuring blood pressure during a routine medical evaluation at least every 2 years. A second abnormal reading at a separate visit at any time should prompt a hypertension diagnosis. Once diagnosed with hypertension, patients should be treated and checked monthly until stable, then monitored every 3 to 6 months indefinitely.10
If you cannot measure blood pressure in the office, urge patients to use an over-the-counter blood pressure measuring device and refer them to a primary care physician. Check the patient’s self-test reading for accuracy against a clinician’s measurement.
Diabetes is now considered a risk equivalent for CAD development.8 Patients diagnosed with diabetes are extremely likely to have established vascular disease,8 which predisposes them to MI, stroke, kidney disease, blindness, and lower-extremity amputations.17 Those with type 1 diabetes usually present with acute symptoms—including polyuria, polydipsia, weight loss, malaise, dry mouth, and blurred vision—and are readily diagnosed with elevated plasma glucose.
Screening for diabetes is critical because one-third of patients with the disease are undiagnosed. Also, more than 90% of patients with diabetes are non-insulin-dependent (type 2) and are asymptomatic early in the disease course.
No data definitively show benefits from screening asymptomatic adults. Recently revised diagnostic criteria for diabetes, however, call for re-testing asymptomatic patients who were found to have normal fasting plasma glucose (FPG) levels and were considered “free” of diabetes. The American Diabetes Association recommends measuring FPG after no caloric intake for ≥ 8 hours for asymptomatic patients.
FPG measurement is cost-effective and generally more convenient than other diabetes tests.17 Expert consensus strongly suggests checking FPG every 3 years beginning at age 45:17
- FPG
- FPG 100 to 125 mg/dL suggests prediabetes or impaired fasting glucose
- FPG ≥ 126 mg/dL demands a provisional diabetes diagnosis and a follow-up test on another day to confirm the diagnosis.
- comorbid cardiac risk factors
- history of polycystic ovary disease
- a first-degree relative with diabetes
- habitual inactivity
- or FPG 100 to 125 mg/dL.
Do not base diabetes diagnosis on glycosylated hemoglobin measurements, as this test can produce false-negative results in patients with new-onset diabetes.
Dyslipidemia. Every 10% reduction in serum cholesterol reduces cardiovascular mortality by 10% to 15%.19 Data from the large, prospective Framingham heart study show a 25% increase in MIs with each 5-mg/dL decrease in high-density lipoprotein cholesterol (HDL) below the age-based median for men and women.20 Serum triglycerides >150 mg/dL clearly predict future CAD and increase the likelihood of abnormally low HDL.
Every 30-mg/dL increase in low-density lipoprotein cholesterol (LDL) raises the relative risk for CAD by 30%.7 ATP III classifies LDL as the “primary target of cholesterol-lowering therapy.”8Table 4 lists LDL target levels based on other CAD risk factors.
Check fasting lipid profile or serum cholesterol, LDL, HDL, and triglycerides beginning at age 20 and about every 5 years thereafter.8 Total cholesterol
Table 3
JNC 7: What blood pressure readings mean
| Category | Systolic BP (mm Hg) | Diastolic BP (mm Hg) |
|---|---|---|
| Normal | and | |
| Prehypertension | 120-139 | or 80-89 |
| Stage 1 hypertension* | 140-159 | or 90-99 |
| Stage 2 hypertension | 160 | or ≥100 |
| JNC 7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure | ||
| *Patients with diabetes mellitus or chronic kidney disease have stage 1 hypertension at >130/80 mm Hg. | ||
| Source: Reference 10 | ||
Acceptable LDL cholesterol levels for adults based on CAD risk
| Risk category | Existing CAD risk factors | LDL goal |
|---|---|---|
| High risk (10-year risk > 20%) | History of diabetes, CAD, symptomatic carotid artery disease, peripheral vascular disease, or abdominal aortic aneurysm | |
| Moderate high risk* (10-year risk 10 to 20%) | >2 risk factors | |
| Moderate risk* (10-year risk | >2 risk factors | |
| Low risk | 0 to 1 risk factor | ≤160 mg/dL |
| CAD: Coronary artery disease | ||
| *Same goals apply to managing moderate high and moderate risk. Find 10-year risk calculations at nhlbi.nih.gov/guidelines/cholesterol. | ||
| Source: Reference 7 | ||
Addressing smoking, obesity
Smoking. Before trying nicotine patches or bupropion, Mr. H should realistically contemplate his risks with continued smoking; if he doesn’t want to stop, periodically encourage him to reconsider.10 Most people know the dangers of smoking but few understand that complete cessation for 1 to 2 years often nearly reverses cardiovascular disease.21
Obesity and lack of exercise go hand in hand. Reducing Mr. H’s waist size to 2 is a reasonable short-term goal. To that end, encourage him to:
- decrease his number of weekly fast-food meals from five to three, with an eventual goal of one per week. As an alternative, microwaveable, low-calorie meals—each with at least two servings of fruits or vegetables—can be prepared at home or work.
- walk 30 minutes three times weekly and progress to 1 hour five times weekly over 6 months. As with any exercise program, remind Mr. H to “start low and go slow.”
The patient’s role in treatment. Patients often feel overwhelmed after getting large amounts of information on CAD risk and may feel hopeless and unenthusiastic about improving their physical health. Work with the primary care doctor to emphasize a patient care plan that clearly defines easily attainable, step-by-step goals. Make sure the patient agrees to these goals.
Case continued: no more supersizing
Mr. H now understands the importance of minimizing his CAD risk and realizes that CAD and many associated risk factors are asymptomatic in the early stages of development.
With help from his doctors, Mr. H quit smoking. He also became more mindful of his caloric intake and the types of foods he was eating. He advanced from briskly walking 30 minutes three times per week to slow jogging 40 minutes five times weekly. He still eats at fast-food restaurants but usually orders broiled chicken, salads, or the occasional burger.
- National Cholesterol Education Program. CAD risk assessment tool and ATP III guidelines. www.nhlbi.nih.gov/guidelines/cholesterol/.
- U.S. Preventive Services Task Force preventive guidelines. www.ahrq.gov/clinic/uspstfix.htm.
- National Heart, Lung, and Blood Institute. Calculate your body mass index. http://nhlbisupport.com/bmi/.
- American Heart Association. www.americanheart.org.
One in five of your patients could suffer a heart attack in the near future—unless you take steps to ensure their heart health.
Psychiatric patients have more modifiable risk factors for coronary artery disease (CAD) compared with the general population.When depression treatment goes nowhere,” Current Psychiatry, August 2005.)
To help keep you abreast of constantly changing guidelines and strategies for recognizing and minimizing CAD risk, this article discusses:
- preventive and diagnostic guidelines for managing hypertension, diabetes, and dyslipidemia
- practical advice on convincing at-risk patients to adopt a healthier lifestyle and have a primary care doctor monitor their health.
Case: cigarettes and supersizing
Mr. H, age 54, is receiving cognitive-behavioral therapy for mild depression. He has been smoking one pack of cigarettes per day for 20 years and has never seriously considered quitting.
The patient, a school teacher, says his “busy schedule” keeps him from exercising and eating properly; he eats fast-food hamburgers and fries approximately five times per week. His father had a heart attack at age 52 and died in his sleep 10 years later.
Mr. H says he feels fine and has never seen a physician other than his psychiatrist. He is reluctant to see a primary care physician for a check-up and, because he is asymptomatic, has no incentive to do so. The psychiatrist thus decides to do a routine examination.
Blood pressure is 148/86; other vital signs are normal. Mr. H’s waist size is 42 inches, he weighs 242 lbs, and his body mass index (BMI) is 34 kg/m2, indicating clinical obesity. Cardiovascular, pulmonary, and abdominal exams are unremarkable.
Discussion. Mr. H is at high risk of a myocardial ischemic event in the near future. He has six risk factors for CAD (Table 1)—four of which are modifiable:
- family history
- age
- current cigarette use
- provisional hypertension diagnosis
- obesity
- physical inactivity.
Table 1
Risk factors for coronary artery disease
| Core risk factors |
| Age ≥45 for men* |
| Age ≥55 for women or premature menopause without estrogen-replacement therapy* |
Family history: premature coronary artery disease with myocardial infarction or sudden death before:
|
| Current cigarette smoking |
| Hypertension or antihypertensive treatment* |
| Elevated LDL cholesterol (>130 mg/dL in patients with low cardiac risk) |
| HDL cholesterol |
| Triglycerides >150 mg/dL |
| Total cholesterol >200 mg/dL* |
| Obesity (BMI >30 kg/m2)† |
| Sedentary lifestyle |
| Other risk factors |
| Elevated C-reactive protein |
| Elevated homocysteine |
| Chronic renal failure |
| Depression |
| Negative (cardio-protective) risk factors |
| HDL >60 mg/dL |
| Moderate alcohol use—no more than 1 to 2 drinks per day (1 drink = 12 oz beer or 5 oz of wine) |
| If >1 risk factor, refer to primary care doctor or quantify 10-year risk by using the Framingham/ATP III point system scale (www.nhlbi.nih.gov). |
| * Framingham/ATP III point system scale variables |
| † Use BMI calculator (http://www.nhlbisupport.com/bmi/bmicalc.htm) to determine body mass index. |
| HDL: High-density lipoprotein |
| LDL: Low-density lipoprotein |
| Source: References 9,10 |
Tools for assessing risk
The lifetime risk at age 40 for developing CAD is 49% and 32% in men and women, respectively.6
The National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel) has focused on decreasing heart disease incidence by educating patients and providers. Preventive strategies and standards of care have changed several times over the past decade; the Adult Treatment Panel (ATP III) was last revised in July 2004.7
The American College of Cardiology and American Heart Association both endorse the modified Framingham/ATP III scale to measure CAD risk (see Related resources). Although this somewhat tedious point system has limitations, it can precisely calculate coronary risk across 10 years.8 Variables not included in the scoring system—such as C-reactive protein, homocysteine, and postmenopausal state—may be clinically significant and should be gauged separately.
An easier-to-use alternative, the ATP III “core risk factors” scale, estimates hypertension, hypercholesterolemia, family history, current cigarette smoking, and age as low, intermediate, or high (“risk equivalent”) risks (Table 1).8 Psychiatrists can quickly obtain this information from a brief history, blood pressure assessment, and relatively inexpensive lab studies.
Generally, the more risk factors present, the higher the risk of having a major coronary event. Presence of ≥ 2 risk factors signals intermediate or high risk and necessitates referral to a primary care doctor for monitoring.
Patients with a cardiac “risk equivalent” face a >20% risk of having a cardiac ischemic event within 10 years8 (Table 2). Examples of risk equivalents include diabetes or significant vascular disease in any artery.
“Non-core” variables. Also consider certain “non-core” variables—such as pre-existing psychiatric illness—when estimating clinical risk for heart disease. Depression, anxiety, and stress are correlated with an increase in pro-inflammatory markers such as C-reactive protein and predispose patients to CAD.11,12 Depression has repeatedly been shown to increase morbidity and mortality two- to four-fold after myocardial infarction (MI).9,13,14 Interestingly, however, depression treatment after an acute coronary event does not clearly decrease mortality.15 Although prospective, randomized studies are lacking, mood and anxiety disorder treatment is presumed to help prevent CAD development.16
Table 2
Risk equivalents for CAD*
| Established coronary artery disease |
| Symptomatic carotid artery disease |
| Peripheral vascular disease |
| Abdominal aortic aneurism |
| Diabetes mellitus |
| *Risk equivalent: Patient is assumed to have coronary artery disease (CAD). |
Recognizing cad risk
At what point do hypertension and dyslipidemia become risk factors for CAD? When and how often should patients be screened for diabetes mellitus?
Hypertension is one of the most common and deadly CAD risk factors, affecting 50 million Americans.10 Although hypertension awareness and treatment have improved, only 35% of adults have “controlled” blood pressure (
According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), normalizing blood pressure can reduce stroke incidence by 35% and MI by 25%, respectively. JNC 7, however, also found that 90% of persons who are normotensive at age 55 eventually develop hypertension.10
Based on these findings, JNC 7 in 2003 drastically changed the standard of care for diagnosing hypertension. JNC 7 defines normal blood pressure as
- systolic blood pressure 120 to 139 mm Hg
- diastolic blood pressure 80 to 89 mm Hg (Table 3).
Patients with diabetes mellitus or chronic kidney disease are considered hypertensive with blood pressure >130 mm Hg systolic and/or >80 mm Hg diastolic.
As with Mr. H, a blood pressure check is imperative for patients who have rarely or never seen a primary care physician in recent years. The U.S. Preventive Services Task Force strongly recommends measuring blood pressure during a routine medical evaluation at least every 2 years. A second abnormal reading at a separate visit at any time should prompt a hypertension diagnosis. Once diagnosed with hypertension, patients should be treated and checked monthly until stable, then monitored every 3 to 6 months indefinitely.10
If you cannot measure blood pressure in the office, urge patients to use an over-the-counter blood pressure measuring device and refer them to a primary care physician. Check the patient’s self-test reading for accuracy against a clinician’s measurement.
Diabetes is now considered a risk equivalent for CAD development.8 Patients diagnosed with diabetes are extremely likely to have established vascular disease,8 which predisposes them to MI, stroke, kidney disease, blindness, and lower-extremity amputations.17 Those with type 1 diabetes usually present with acute symptoms—including polyuria, polydipsia, weight loss, malaise, dry mouth, and blurred vision—and are readily diagnosed with elevated plasma glucose.
Screening for diabetes is critical because one-third of patients with the disease are undiagnosed. Also, more than 90% of patients with diabetes are non-insulin-dependent (type 2) and are asymptomatic early in the disease course.
No data definitively show benefits from screening asymptomatic adults. Recently revised diagnostic criteria for diabetes, however, call for re-testing asymptomatic patients who were found to have normal fasting plasma glucose (FPG) levels and were considered “free” of diabetes. The American Diabetes Association recommends measuring FPG after no caloric intake for ≥ 8 hours for asymptomatic patients.
FPG measurement is cost-effective and generally more convenient than other diabetes tests.17 Expert consensus strongly suggests checking FPG every 3 years beginning at age 45:17
- FPG
- FPG 100 to 125 mg/dL suggests prediabetes or impaired fasting glucose
- FPG ≥ 126 mg/dL demands a provisional diabetes diagnosis and a follow-up test on another day to confirm the diagnosis.
- comorbid cardiac risk factors
- history of polycystic ovary disease
- a first-degree relative with diabetes
- habitual inactivity
- or FPG 100 to 125 mg/dL.
Do not base diabetes diagnosis on glycosylated hemoglobin measurements, as this test can produce false-negative results in patients with new-onset diabetes.
Dyslipidemia. Every 10% reduction in serum cholesterol reduces cardiovascular mortality by 10% to 15%.19 Data from the large, prospective Framingham heart study show a 25% increase in MIs with each 5-mg/dL decrease in high-density lipoprotein cholesterol (HDL) below the age-based median for men and women.20 Serum triglycerides >150 mg/dL clearly predict future CAD and increase the likelihood of abnormally low HDL.
Every 30-mg/dL increase in low-density lipoprotein cholesterol (LDL) raises the relative risk for CAD by 30%.7 ATP III classifies LDL as the “primary target of cholesterol-lowering therapy.”8Table 4 lists LDL target levels based on other CAD risk factors.
Check fasting lipid profile or serum cholesterol, LDL, HDL, and triglycerides beginning at age 20 and about every 5 years thereafter.8 Total cholesterol
Table 3
JNC 7: What blood pressure readings mean
| Category | Systolic BP (mm Hg) | Diastolic BP (mm Hg) |
|---|---|---|
| Normal | and | |
| Prehypertension | 120-139 | or 80-89 |
| Stage 1 hypertension* | 140-159 | or 90-99 |
| Stage 2 hypertension | 160 | or ≥100 |
| JNC 7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure | ||
| *Patients with diabetes mellitus or chronic kidney disease have stage 1 hypertension at >130/80 mm Hg. | ||
| Source: Reference 10 | ||
Acceptable LDL cholesterol levels for adults based on CAD risk
| Risk category | Existing CAD risk factors | LDL goal |
|---|---|---|
| High risk (10-year risk > 20%) | History of diabetes, CAD, symptomatic carotid artery disease, peripheral vascular disease, or abdominal aortic aneurysm | |
| Moderate high risk* (10-year risk 10 to 20%) | >2 risk factors | |
| Moderate risk* (10-year risk | >2 risk factors | |
| Low risk | 0 to 1 risk factor | ≤160 mg/dL |
| CAD: Coronary artery disease | ||
| *Same goals apply to managing moderate high and moderate risk. Find 10-year risk calculations at nhlbi.nih.gov/guidelines/cholesterol. | ||
| Source: Reference 7 | ||
Addressing smoking, obesity
Smoking. Before trying nicotine patches or bupropion, Mr. H should realistically contemplate his risks with continued smoking; if he doesn’t want to stop, periodically encourage him to reconsider.10 Most people know the dangers of smoking but few understand that complete cessation for 1 to 2 years often nearly reverses cardiovascular disease.21
Obesity and lack of exercise go hand in hand. Reducing Mr. H’s waist size to 2 is a reasonable short-term goal. To that end, encourage him to:
- decrease his number of weekly fast-food meals from five to three, with an eventual goal of one per week. As an alternative, microwaveable, low-calorie meals—each with at least two servings of fruits or vegetables—can be prepared at home or work.
- walk 30 minutes three times weekly and progress to 1 hour five times weekly over 6 months. As with any exercise program, remind Mr. H to “start low and go slow.”
The patient’s role in treatment. Patients often feel overwhelmed after getting large amounts of information on CAD risk and may feel hopeless and unenthusiastic about improving their physical health. Work with the primary care doctor to emphasize a patient care plan that clearly defines easily attainable, step-by-step goals. Make sure the patient agrees to these goals.
Case continued: no more supersizing
Mr. H now understands the importance of minimizing his CAD risk and realizes that CAD and many associated risk factors are asymptomatic in the early stages of development.
With help from his doctors, Mr. H quit smoking. He also became more mindful of his caloric intake and the types of foods he was eating. He advanced from briskly walking 30 minutes three times per week to slow jogging 40 minutes five times weekly. He still eats at fast-food restaurants but usually orders broiled chicken, salads, or the occasional burger.
- National Cholesterol Education Program. CAD risk assessment tool and ATP III guidelines. www.nhlbi.nih.gov/guidelines/cholesterol/.
- U.S. Preventive Services Task Force preventive guidelines. www.ahrq.gov/clinic/uspstfix.htm.
- National Heart, Lung, and Blood Institute. Calculate your body mass index. http://nhlbisupport.com/bmi/.
- American Heart Association. www.americanheart.org.
1. Holt RI, Peveler RC, Byrne CD. Schizophrenia, the metabolic syndrome and diabetes. Diabetes Med 2004;21:515-23.
2. Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
3. Weiser M, Reichenberg A, Grotto I, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry 2004;161:1219-23.
4. Druss B, Rosenheck R. Mental disorders and access to medical care in the United States. Am J Psychiatry 1998;155:1775-7.
5. Druss B, Rosenheck R, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care 2002;40:129-36.
6. Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999;353(9147):89-92.
7. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227-9.
8. Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
9. Frasure-Smith N, Lesperance F, Talajic M, et al. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72.
11. Ford DE, Erlinger TP. Depression and C-reactive protein in U.S. adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2004;164:1010-14.
12. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Inflammation, coagulation and depressive symptomatology in cardiovascular disease-free people: the ATTICA study. Eur Heart J 2004;25:492-9.
13. Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819-25.
14. Ladwig KH, Kieser M, Konig J, et al. Affective disorders and survival after acute myocardial infarction: results from the post-infarction late potential study. Eur Heart J 1991;12:959-64.
15. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Artery Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003;289:3106-16.
16. Rosengren A, Hawken S, Ounpuu S, et al. Association of psychological risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study). Lancet 2004;364:953-62.
17. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care 2004;27(suppl 1):11-14.
18. American Diabetes Association; American Psychiatric Association. American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
19. Gould AL, Rossouw JE, Santanello NC, et al. Cholesterol reduction yields clinical benefit; impact of statin trials. Circulation 1998;97:946-52.
20. Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary artery disease. The Framingham Study. Am J Med 1977;62:707-14.
21. Rigotti N, Pasternak L. Changing the natural history of coronary artery disease. Cardiology Clinics 1996;14:51-68.
1. Holt RI, Peveler RC, Byrne CD. Schizophrenia, the metabolic syndrome and diabetes. Diabetes Med 2004;21:515-23.
2. Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res 2002;53:897-902.
3. Weiser M, Reichenberg A, Grotto I, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry 2004;161:1219-23.
4. Druss B, Rosenheck R. Mental disorders and access to medical care in the United States. Am J Psychiatry 1998;155:1775-7.
5. Druss B, Rosenheck R, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care 2002;40:129-36.
6. Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999;353(9147):89-92.
7. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227-9.
8. Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
9. Frasure-Smith N, Lesperance F, Talajic M, et al. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72.
11. Ford DE, Erlinger TP. Depression and C-reactive protein in U.S. adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2004;164:1010-14.
12. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Inflammation, coagulation and depressive symptomatology in cardiovascular disease-free people: the ATTICA study. Eur Heart J 2004;25:492-9.
13. Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819-25.
14. Ladwig KH, Kieser M, Konig J, et al. Affective disorders and survival after acute myocardial infarction: results from the post-infarction late potential study. Eur Heart J 1991;12:959-64.
15. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Artery Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003;289:3106-16.
16. Rosengren A, Hawken S, Ounpuu S, et al. Association of psychological risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study). Lancet 2004;364:953-62.
17. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care 2004;27(suppl 1):11-14.
18. American Diabetes Association; American Psychiatric Association. American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
19. Gould AL, Rossouw JE, Santanello NC, et al. Cholesterol reduction yields clinical benefit; impact of statin trials. Circulation 1998;97:946-52.
20. Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary artery disease. The Framingham Study. Am J Med 1977;62:707-14.
21. Rigotti N, Pasternak L. Changing the natural history of coronary artery disease. Cardiology Clinics 1996;14:51-68.
Vagus nerve stimulation
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
What is vagus nerve stimulation’s (VNS) role in treating chronic or recurrent depression? Which patients would benefit from this implant, now FDA-approved for depression as well as epilepsy?
Drawing from the evidence, this article discusses which patients with depression may be candidates for VNS, how it works, and its potential benefits and side effects.
Clinical Applicability
VNS is indicated for patients with chronic or recurrent treatment-resistant depression during an episode that has not responded to ≥4 adequate antidepressant treatment trials (defined as ≥3 on the Antidepressant Treatment History Form [ATHF]) (Table 1). Implantation theoretically promotes 100% adherence and reduces drug-drug interaction risk. Interactions between VNS and nonpsychotropics are possible but unlikely.
Paradoxically, data suggest that patients with low to moderate resistance to antidepressant treatment (≤3 antidepressant trial failures) are most likely to benefit from VNS.1 Patients who had never received electroconvulsive therapy (ECT) (indicating relatively low treatment resistance) were nearly four times more likely than ECT-treated patients to respond to VNS.2 Conversely, 13 subjects who had not responded to ≥ 7 adequate treatment trials (indicating relatively severe treatment resistance) did not respond to VNS.2
Table 1
Vagus nerve stimulation device: Fast facts
| Brand name: Cyberonics Vagus Nerve Stimulation (VNS) Therapy System |
| FDA-approved indications: Treatment-resistant depression (previously approved for treatment-refractory epilepsy) |
| Manufacturer: Cyberonics |
| Recommended use: Treating depressive episode that has not responded to ≥4 antidepressant trials or electroconvulsive therapy in a patient with chronic or recurrent depression |
| Information on VNS remote device training: 1-877-NOW-4-VNS (669-4867) or www.vnstherapy.com |
How VNS Works
The vagus (10th cranial) nerve is a main efferent outflow tract for parasympathetic innervation of the abdomen and chest, regulating heart rate, acid secretion, and bowel motility.
The largest component of the left vagus nerve—approximately 80%—conducts information about pain, hunger, and satiety. These fibers are also believed to contribute to VNS’ antidepressant effects by carrying information to the solitary nucleus of the medulla. From there, fibers project to the median raphe nucleus and locus coeruleus, key areas of serotonergic and noradrenergic innervation relevant to depression.
Positron emission tomography studies suggest that VNS also increases blood flow to the thalamus, hypothalamus, and insula—brain areas considered relevant to mood disorders.3
VNS requires subcutaneous implantation of a pacemaker-like pulse generator into the upper left chest. The generator is 6.9 mm thick and weighs 25 grams. Wires extend from the device into the left vagus nerve in the neck (Figure). A neurosurgeon usually performs the 1- to 2-hour outpatient procedure, although ENT, vascular, and general surgeons may also do the implant.
The device sends electric pulses to the left vagus nerve every few seconds (Table 2). Using an accompanying hand-held device and a computer, the clinician programs the implant and adjusts stimulation parameters to ensure the correct amount of stimulation.
FDA approved VNS in 1997 for refractory epilepsy. Clinical observations that VNS improved epilepsy patients’ mood spurred interest in its antidepressant effects.4 Preliminary data suggest VNS also could help manage anxiety disorders, obesity, pain syndromes, and Alzheimer’s disease.5
Figure How VNS device works
Pacemaker-like VNSdevice is implanted into the upper left chest. Wires extending from the device transport electric pulses into the left vagus nerve in the neck, which carries information to areas of serotonergic and noradrenergic innervation relevant to depression.Table 2
VNS stimulation parameters
| Frequency: 20 to 30 Hz |
| Intensity: 0.25 mA (0.25 to 3.0 mA) |
| Pulse width: 250 to 500 μs |
| Duty cycle: 30 seconds on/5 minutes off |
Cost
VNS implantation costs approximately $25,000, including the device, surgeon’s fee, and facility charge. Psychiatrists generally would initiate the referral process.
Follow-up management fees for epilepsy are $150 to $250 per visit. Several follow-up visits are required after stimulation is started to verify the device is working, evaluate treatment response and tolerability, and adjust stimulation as needed. Thereafter, periodic visits are appropriate.
Generally, insurers cover VNS as an epilepsy treatment; whether private insurers and Medicare will cover VNS for depression remains to be seen. Case mangers at Cyberonics, the device’s manufacturer, are on call to assist with VNS coverage, coding, and reimbursement issues (see Related resources).
Because the internal implant’s battery life is 6 to 11 years, VNS therapy will likely be cost-effective for many patients, although follow-up surgery would be required to replace the battery. Costs of using VNS have not been compared with other antidepressant modalities.
VNS’ Efficacy In Depression
In an open-label trial, 60 patients ages 20 to 63 received VNS with no placebo or active comparator.2 Thirty had completed an open-label pilot study that showed VNS’ potential antidepressant effects.6 Before implantation, all subjects had:
- a major depressive episode lasting >2 years or >4 lifetime major depressive episodes
- nonresponse to ECT or ≥2 adequate antidepressant trials (ATHF scores >3) during their current major depressive episode (median duration: 4.7 years)
- DSM-IV diagnosis of major depressive disorder or bipolar type I or II disorder depressed phase.
- baseline scores ≥20 on the 28-item Hamilton Rating Scale for Depression (HRSD-28) and ≤50 on the Global Assessment of Functioning (GAF) scale.
Two weeks after implantation, the stimulator was turned on and adjusted for another 2 weeks to the maximum tolerable dose. Patients then received 8 weeks of fixed-dose stimulation. Participants who had been taking an antidepressant, mood stabilizer, second-generation antipsychotic, or other psychotropic at the same dosages for ≥4 weeks before the study could continue their medications during the VNS trial (median concurrent treatments: 4).
Three months after implantation, 18 of 59 subjects (30.5%) showed clinical response (≥50% improvement in HRSD-28 scores over baseline). Nine patients (15.3%) showed depression remission (HRSD-28 score ≤10). Median time to first response was 45.5 days.
Twenty participants (34%) showed a ≥50% reduction in baseline Montgomery-Asberg Depression Rating Scale (MADRS) scores, and 22 (37%) showed Clinical Global Impression-Improvement Scale (CGI-I) scores improving to 1 or 2.
Therapeutic effects did not differ among patients with unipolar and bipolar depression. Participants with mild to moderate depression (defined as 2 to 3 failed adequate trials) showed higher response rates (50% vs. 29.1%) than did those with more-severe depression (defined as ≥4 failed adequate trials).2
Among 28 patients followed for 1 year, 13 (46%) met HRSD-28 response criteria (≥ 50% score reduction) and 8 (29%) met remission criteria (score ≤ 10), showing gradual improvement.1 After 2 years, 44% of patients met HDRS-28 response criteria, and 22% met remission criteria, showing sustained benefit.7 How many subjects were taking one or more concomitant psychotropics is unknown.
In a double-blind controlled trial, 235 subjects ages 18 to 80 received VNS or a sham comparator.8 Treatment response and remission were defined as ≥50% reduction from baseline and ≤9, respectively, on the 24-item HRSD (HRSD-24). Patient selection criteria were similar to those of the open-label study.
All patients received VNS implants, which were inactive the first 2 weeks. Patients were then randomly assigned to active treatment (stimulator turned on) or sham control (stimulator left off). After 10 weeks of treatment, HRSD-24, CGI-I, and MADRS scores were similar between the VNS and sham groups, but Inventory of Depressive Symptomatology Self Report (IDS-SR) scores improved much more in the active treatment group (P<0.03). Patients in the sham group then had their stimulators turned on.
After 1 year of active treatment for both groups, response and remission rates more than doubled among 205 evaluable subjects (response: 14.4% to 29.8%; remission: 7.3% to 17.1%). MADRS and IDS-SR scores also improved. Three percent of subjects dropped out because of adverse events.
Another analysis of these data revealed significant improvement among the VNS treatment group vs. a comparator-matched control group of treatment-resistant patients across 2 years.8
Depression treatment among patients in the comparator group followed standard clinical practice.
Side Effects
Voice alteration or hoarseness was most commonly reported after 12 weeks in the open-label trial (55% of subjects). Headache (22%), cough (17%), shortness of breath (15%), neck pain (17%), dysphagia (20%), and pain (15%) were also reported.2 These effects emerge or increase with stimulation intensity and may be ameliorated by reducing the dose.
Small risks of infection (1%) and nerve damage (1%) were reported. Leaving the stimulator off for 14 days after implantation decreases nerve damage risk. Pain at the incision site (experienced by 30%) resolved after 1 to 2 weeks.2 Other adverse events included:
- hypomania in one bipolar patient; this was resolved by adjusting medication and reducing stimulation
- leg pain in 2 subjects
- worsened depression in 5 patients (2 of these may have been related to stimulation)
- emesis and diarrhea in 1 subject.
One patient with multiple cardiac risk factors developed a myocardial infarction but completed the trial after angioplasty and stent placement.2
After 1 year in the open-label trial, no subjects dropped out because of adverse events. Common side events included voice alteration (21%), shortness of breath (7%), and neck pain (7%). More-serious adverse events reported between the acute trial and 12-month follow-up included hypomania (2 episodes), one deep venous thrombophlebitits episode, and one episode each of back pain and appendicitis.1 No cognitive effects have been reported.
In the double-blind controlled trial, 31 of 235 subjects (13%) experienced worsening of depression, and 25 of the 31 depressed subjects attempted suicide.9 Whether these effects were related to the depression or VNS stimulation is unclear. Side effects reported more frequently in the active treatment group than in the sham control group included voice alteration (68% vs. 38%), cough (29% vs. 9%), shortness of breath (23% vs. 14%), dysphagia (21% vs. 10%), and neck pain (21% vs. 10%).
If VNS Is Intolerable
Patients may deactivate the device with a magnet if they are uncomfortable. Pulse stimulation stops when a magnet is held against the left upper chest and resumes when the magnet is removed.
Training
Cyberonics plans to offer free VNS training to psychiatrists who practice at selected centers that accept treatment-resistant depression case referrals from primary care physicians, community psychiatrists, and other providers. Community psychiatrists who see treatment-resistant patients also are eligible for free training. For information, see Related resources.
- Cyberonics VNS therapy Web site. www.vnstherapy.com.
- Cyberonics reimbursement and case management support services. www.vnstherapy.com/depression/hcp/ReimbursementIns/default.aspx.
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 2000;1:93-9.
Disclosure
The authors receive grant support from Neuronetics. They report no proprietary interest in the technology discussed in this article.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
1. Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002;51:280-7.
2. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25(5):713-28.
3. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced in partial epilepsy I: acute effects at high and low levels of stimulation. Epilepsia 1998;39(9):983-90.
4. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 2000;42(2):203-10.
5. George MS, Nahas Z, Bohning DE, et al. Vagus nerve stimulation therapy: a research update. Neurology 2002;59(6 suppl 4):S56-61.
6. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol Psychiatry 2000;47:276-86.
7. Rush AJ, George MS, Sackeim HA, et al. Continuing benefit of VNS therapy over 2 years for treatment-resistant depression. San Juan, Puerto Rico: American College of Neuropsychopharmacology annual meeting, 2002.
8. Cyberonics premarket approval application supplement (D-02/D-04 clinical report, PMA-S), submitted to FDA October 2003.
9. Zwillich T. FDA panel recommends device for depression. WebMD Medical News June 17, 2004. Available at: http://my.webmd.com/content/article/89/100114.htm. Accessed August 9, 2005.
Treatment via telephone: Tips for handling patient calls
Patient phone calls—if handled appropriately—can eliminate unnecessary office visits, prevent relapse, and help you monitor treatment adherence. We offer strategies for turning potential disruptions into opportunities.
Develop a protocol for handling distress calls (and communicate it to staff) to minimize frustration for you and patients who might struggle to reach you. Your protocol could include:
- instructions for patients on how to contact you
- a 24-hour answering service and/or voice mail option during business hours
- how and when staff should connect you to patients during clinic hours
- how staff verifies your office is authorized to communicate with family members who call
- how you or your staff verifies callers are your patients. (In some states you establish a doctor-patient relationship and assume responsibility for care simply by answering a call and identifying yourself as a doctor.)
Some psychiatrists give patients their cell or home phone numbers and/or e-mail addresses. They say most patients find this reassuring and rarely contact them. We recommend offering your personal information on a case-by-case basis.
For billing and medicolegal protection, purchase software that documents patient phone calls and messages and their outcomes and decisions.
‘911’. Train staff to triage phone calls from patients with dangerous intentions. Instruct staff to immediately notify you or a covering provider or refer the patient to the emergency room. Check your voice mail several times daily for such calls.
If a patient is in crisis and you are in an appointment, have staff page you a ‘911.’ Avoid talking with one patient in the presence of another.
Scheduled phone calls can help monitor treatment. Decide whether you or your patient will initiate planned calls. Aim to return all messages the same day or at least within 24 hours.
Although a patient providing callback information implies consent, use caution when identifying yourself or leaving messages, especially if patients give you their work numbers.
Patients with personality disorders tend to consume substantial mental health resources.1 Scheduling phone calls with such patients, even if they are doing well, provides positive support. If you treat their frequent calls as disruptive, they may feel rejected and seek treatment elsewhere.
Prescribing. Use caution when patients—especially those with addiction problems and/or a history of nonadherence—call in prescription requests. Prescribe opioids and stimulants in person.
Reference
1. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry 2001;158(2):295-302.
Dr. Ramaswamy is instructor of psychiatry, Creighton University, Omaha, NE, and staff psychiatrist, Omaha VA Medical Center.
Dr. Fernandes is assistant professor of psychiatry, Creighton University, and staff psychiatrist, Omaha VA Medical Center.
Patient phone calls—if handled appropriately—can eliminate unnecessary office visits, prevent relapse, and help you monitor treatment adherence. We offer strategies for turning potential disruptions into opportunities.
Develop a protocol for handling distress calls (and communicate it to staff) to minimize frustration for you and patients who might struggle to reach you. Your protocol could include:
- instructions for patients on how to contact you
- a 24-hour answering service and/or voice mail option during business hours
- how and when staff should connect you to patients during clinic hours
- how staff verifies your office is authorized to communicate with family members who call
- how you or your staff verifies callers are your patients. (In some states you establish a doctor-patient relationship and assume responsibility for care simply by answering a call and identifying yourself as a doctor.)
Some psychiatrists give patients their cell or home phone numbers and/or e-mail addresses. They say most patients find this reassuring and rarely contact them. We recommend offering your personal information on a case-by-case basis.
For billing and medicolegal protection, purchase software that documents patient phone calls and messages and their outcomes and decisions.
‘911’. Train staff to triage phone calls from patients with dangerous intentions. Instruct staff to immediately notify you or a covering provider or refer the patient to the emergency room. Check your voice mail several times daily for such calls.
If a patient is in crisis and you are in an appointment, have staff page you a ‘911.’ Avoid talking with one patient in the presence of another.
Scheduled phone calls can help monitor treatment. Decide whether you or your patient will initiate planned calls. Aim to return all messages the same day or at least within 24 hours.
Although a patient providing callback information implies consent, use caution when identifying yourself or leaving messages, especially if patients give you their work numbers.
Patients with personality disorders tend to consume substantial mental health resources.1 Scheduling phone calls with such patients, even if they are doing well, provides positive support. If you treat their frequent calls as disruptive, they may feel rejected and seek treatment elsewhere.
Prescribing. Use caution when patients—especially those with addiction problems and/or a history of nonadherence—call in prescription requests. Prescribe opioids and stimulants in person.
Patient phone calls—if handled appropriately—can eliminate unnecessary office visits, prevent relapse, and help you monitor treatment adherence. We offer strategies for turning potential disruptions into opportunities.
Develop a protocol for handling distress calls (and communicate it to staff) to minimize frustration for you and patients who might struggle to reach you. Your protocol could include:
- instructions for patients on how to contact you
- a 24-hour answering service and/or voice mail option during business hours
- how and when staff should connect you to patients during clinic hours
- how staff verifies your office is authorized to communicate with family members who call
- how you or your staff verifies callers are your patients. (In some states you establish a doctor-patient relationship and assume responsibility for care simply by answering a call and identifying yourself as a doctor.)
Some psychiatrists give patients their cell or home phone numbers and/or e-mail addresses. They say most patients find this reassuring and rarely contact them. We recommend offering your personal information on a case-by-case basis.
For billing and medicolegal protection, purchase software that documents patient phone calls and messages and their outcomes and decisions.
‘911’. Train staff to triage phone calls from patients with dangerous intentions. Instruct staff to immediately notify you or a covering provider or refer the patient to the emergency room. Check your voice mail several times daily for such calls.
If a patient is in crisis and you are in an appointment, have staff page you a ‘911.’ Avoid talking with one patient in the presence of another.
Scheduled phone calls can help monitor treatment. Decide whether you or your patient will initiate planned calls. Aim to return all messages the same day or at least within 24 hours.
Although a patient providing callback information implies consent, use caution when identifying yourself or leaving messages, especially if patients give you their work numbers.
Patients with personality disorders tend to consume substantial mental health resources.1 Scheduling phone calls with such patients, even if they are doing well, provides positive support. If you treat their frequent calls as disruptive, they may feel rejected and seek treatment elsewhere.
Prescribing. Use caution when patients—especially those with addiction problems and/or a history of nonadherence—call in prescription requests. Prescribe opioids and stimulants in person.
Reference
1. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry 2001;158(2):295-302.
Dr. Ramaswamy is instructor of psychiatry, Creighton University, Omaha, NE, and staff psychiatrist, Omaha VA Medical Center.
Dr. Fernandes is assistant professor of psychiatry, Creighton University, and staff psychiatrist, Omaha VA Medical Center.
Reference
1. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry 2001;158(2):295-302.
Dr. Ramaswamy is instructor of psychiatry, Creighton University, Omaha, NE, and staff psychiatrist, Omaha VA Medical Center.
Dr. Fernandes is assistant professor of psychiatry, Creighton University, and staff psychiatrist, Omaha VA Medical Center.
Medication concerns during Ramadan fasting
On October 4, Muslims begin the month-long Ramadan, during which they refrain from all food and drink (even water) in the daytime. Clinical concerns that may arise from fasting are:
- inability to take medications during the day
- dehydration and other somatic changes that necessitate dosing modification
- psychiatric symptom exacerbation.
Based on our experience, we offer these guidelines to address possible health risks in these patients.
Symptom risks. During Ramadan, even persons without mental disorders have reported irritability, decreased sleep, difficulty concentrating, and anxiety.1 In patients with bipolar disorder, one study described a high rate (45%) of breakthrough manic or depressive episodes during Ramadan, despite stable lithium levels.2 Fasting-related changes in circadian rhythms and insomnia are thought to contribute to psychiatric symptom exacerbation.
Religious sensitivity. The best way to identify a Muslim patient who intends to fast during Ramadan is to ask about religious or spiritual backgrounds as part of a thorough psychiatric intake. Ramadan serves as a reminder of the suffering of the poor and signifies an opportunity for self-restraint, prayer, and charity. Common interpretation of the Quran requires fasting unless it is medically harmful.1
If you anticipate a clinical problem, inform your patient of the risks of fasting. For patients with severe mental illness, fasting may not be reasonable. Be aware, however, that patients may ignore your advice because of the stigma of mental illness coupled with family or community pressure to fast.
Do not underestimate the importance Muslims place on fasting. Although the consequences of psychiatric symptom exacerbation can be disastrous, your advice must be balanced by your patient’s religious beliefs. Patients may be reluctant to broach this topic, but discussing it will strengthen your therapeutic alliance.
Allay natural guilt by focusing on medical necessity. Consider increased psychotherapy as a transitional possibility during Ramadan.
Modifying pharmacotherapy. Fasting may cause dehydration and decrease glomerular filtration, which may increase serum levels of renally dependent psychotropics such as lithium.3 Fasting also changes gastric pH and phase II liver metabolism, altering the pharmacokinetics of valproic acid.4
For patients taking antidepressants and antipsychotics, watch for anticholinergic side effects—dry mouth, dehydration, and confusion—especially in the elderly. To avoid having patients alter their treatment regimens without consulting you, consider a temporary switch before Ramadan to longer-acting medications or medications with once- or twice-daily dosing. Increased monitoring may also be necessary during this period.
Substance abstinence. Fasting mandates abstinence from alcohol, caffeine, and tobacco. For users of these substances, discuss a tapering approach to preempt possible withdrawal symptoms. Changes in intake of caffeine and tobacco—inducers of cytochrome P-450 isoenzymes, especially 1A2—can alter (usually by increasing) drug levels of antidepressants and antipsychotics metabolized by this pathway.5
Disclaimer
The opinions expressed in this article are the authors’ and do not reflect those of the Department of Defense or the Department of Veterans Affairs.
Acknowledgments
The authors thank Hashib Faruque, MD, for his comments and suggestions.
1. Toda M, Morimoto K. Ramadan fasting—effect on healthy Muslims. Soc Beh Pers 2004;32:13-18.
2. Kadri N, Mouchtaq N, Hakkou F, Moussaoui D. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol 2000;3:45-9.
3. Aadil I, Houti I, Moussamih S. Drug intake during Ramadan. BMJ 2004;329:778-82.
4. Aadil N, Fassi-Fihri I, Houti B, et al. Influence of Ramadan on the pharmacokinetics of a single oral dose of valproic acid administered at two different times. Methods Find Exp Clin Pharmacol 2000;22(2):109-14.
5. Kadri N, Tilane A, Batal M, et al. Irritability during the month of Ramadan. Psychosom Med 2000;62:280-5.
Dr. Benjamin is a staff psychiatrist, Oklahoma City Veteran’s Administration Center and clinical assistant professor, department of psychiatry and behavioral sciences, University of Oklahoma Health Sciences Center, Oklahoma City.
Dr. Dennis is director of consultation-liaison service, Oklahoma City Veteran’s Administration Center, and clinical associate professor, department of psychiatry and behavioral sciences, University of Oklahoma Health Sciences Center.
Dr. Mosallaei-Benjamin is a first-year fellow in the hematology-oncology department, University of Oklahoma Health Sciences Center.
On October 4, Muslims begin the month-long Ramadan, during which they refrain from all food and drink (even water) in the daytime. Clinical concerns that may arise from fasting are:
- inability to take medications during the day
- dehydration and other somatic changes that necessitate dosing modification
- psychiatric symptom exacerbation.
Based on our experience, we offer these guidelines to address possible health risks in these patients.
Symptom risks. During Ramadan, even persons without mental disorders have reported irritability, decreased sleep, difficulty concentrating, and anxiety.1 In patients with bipolar disorder, one study described a high rate (45%) of breakthrough manic or depressive episodes during Ramadan, despite stable lithium levels.2 Fasting-related changes in circadian rhythms and insomnia are thought to contribute to psychiatric symptom exacerbation.
Religious sensitivity. The best way to identify a Muslim patient who intends to fast during Ramadan is to ask about religious or spiritual backgrounds as part of a thorough psychiatric intake. Ramadan serves as a reminder of the suffering of the poor and signifies an opportunity for self-restraint, prayer, and charity. Common interpretation of the Quran requires fasting unless it is medically harmful.1
If you anticipate a clinical problem, inform your patient of the risks of fasting. For patients with severe mental illness, fasting may not be reasonable. Be aware, however, that patients may ignore your advice because of the stigma of mental illness coupled with family or community pressure to fast.
Do not underestimate the importance Muslims place on fasting. Although the consequences of psychiatric symptom exacerbation can be disastrous, your advice must be balanced by your patient’s religious beliefs. Patients may be reluctant to broach this topic, but discussing it will strengthen your therapeutic alliance.
Allay natural guilt by focusing on medical necessity. Consider increased psychotherapy as a transitional possibility during Ramadan.
Modifying pharmacotherapy. Fasting may cause dehydration and decrease glomerular filtration, which may increase serum levels of renally dependent psychotropics such as lithium.3 Fasting also changes gastric pH and phase II liver metabolism, altering the pharmacokinetics of valproic acid.4
For patients taking antidepressants and antipsychotics, watch for anticholinergic side effects—dry mouth, dehydration, and confusion—especially in the elderly. To avoid having patients alter their treatment regimens without consulting you, consider a temporary switch before Ramadan to longer-acting medications or medications with once- or twice-daily dosing. Increased monitoring may also be necessary during this period.
Substance abstinence. Fasting mandates abstinence from alcohol, caffeine, and tobacco. For users of these substances, discuss a tapering approach to preempt possible withdrawal symptoms. Changes in intake of caffeine and tobacco—inducers of cytochrome P-450 isoenzymes, especially 1A2—can alter (usually by increasing) drug levels of antidepressants and antipsychotics metabolized by this pathway.5
Disclaimer
The opinions expressed in this article are the authors’ and do not reflect those of the Department of Defense or the Department of Veterans Affairs.
Acknowledgments
The authors thank Hashib Faruque, MD, for his comments and suggestions.
On October 4, Muslims begin the month-long Ramadan, during which they refrain from all food and drink (even water) in the daytime. Clinical concerns that may arise from fasting are:
- inability to take medications during the day
- dehydration and other somatic changes that necessitate dosing modification
- psychiatric symptom exacerbation.
Based on our experience, we offer these guidelines to address possible health risks in these patients.
Symptom risks. During Ramadan, even persons without mental disorders have reported irritability, decreased sleep, difficulty concentrating, and anxiety.1 In patients with bipolar disorder, one study described a high rate (45%) of breakthrough manic or depressive episodes during Ramadan, despite stable lithium levels.2 Fasting-related changes in circadian rhythms and insomnia are thought to contribute to psychiatric symptom exacerbation.
Religious sensitivity. The best way to identify a Muslim patient who intends to fast during Ramadan is to ask about religious or spiritual backgrounds as part of a thorough psychiatric intake. Ramadan serves as a reminder of the suffering of the poor and signifies an opportunity for self-restraint, prayer, and charity. Common interpretation of the Quran requires fasting unless it is medically harmful.1
If you anticipate a clinical problem, inform your patient of the risks of fasting. For patients with severe mental illness, fasting may not be reasonable. Be aware, however, that patients may ignore your advice because of the stigma of mental illness coupled with family or community pressure to fast.
Do not underestimate the importance Muslims place on fasting. Although the consequences of psychiatric symptom exacerbation can be disastrous, your advice must be balanced by your patient’s religious beliefs. Patients may be reluctant to broach this topic, but discussing it will strengthen your therapeutic alliance.
Allay natural guilt by focusing on medical necessity. Consider increased psychotherapy as a transitional possibility during Ramadan.
Modifying pharmacotherapy. Fasting may cause dehydration and decrease glomerular filtration, which may increase serum levels of renally dependent psychotropics such as lithium.3 Fasting also changes gastric pH and phase II liver metabolism, altering the pharmacokinetics of valproic acid.4
For patients taking antidepressants and antipsychotics, watch for anticholinergic side effects—dry mouth, dehydration, and confusion—especially in the elderly. To avoid having patients alter their treatment regimens without consulting you, consider a temporary switch before Ramadan to longer-acting medications or medications with once- or twice-daily dosing. Increased monitoring may also be necessary during this period.
Substance abstinence. Fasting mandates abstinence from alcohol, caffeine, and tobacco. For users of these substances, discuss a tapering approach to preempt possible withdrawal symptoms. Changes in intake of caffeine and tobacco—inducers of cytochrome P-450 isoenzymes, especially 1A2—can alter (usually by increasing) drug levels of antidepressants and antipsychotics metabolized by this pathway.5
Disclaimer
The opinions expressed in this article are the authors’ and do not reflect those of the Department of Defense or the Department of Veterans Affairs.
Acknowledgments
The authors thank Hashib Faruque, MD, for his comments and suggestions.
1. Toda M, Morimoto K. Ramadan fasting—effect on healthy Muslims. Soc Beh Pers 2004;32:13-18.
2. Kadri N, Mouchtaq N, Hakkou F, Moussaoui D. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol 2000;3:45-9.
3. Aadil I, Houti I, Moussamih S. Drug intake during Ramadan. BMJ 2004;329:778-82.
4. Aadil N, Fassi-Fihri I, Houti B, et al. Influence of Ramadan on the pharmacokinetics of a single oral dose of valproic acid administered at two different times. Methods Find Exp Clin Pharmacol 2000;22(2):109-14.
5. Kadri N, Tilane A, Batal M, et al. Irritability during the month of Ramadan. Psychosom Med 2000;62:280-5.
Dr. Benjamin is a staff psychiatrist, Oklahoma City Veteran’s Administration Center and clinical assistant professor, department of psychiatry and behavioral sciences, University of Oklahoma Health Sciences Center, Oklahoma City.
Dr. Dennis is director of consultation-liaison service, Oklahoma City Veteran’s Administration Center, and clinical associate professor, department of psychiatry and behavioral sciences, University of Oklahoma Health Sciences Center.
Dr. Mosallaei-Benjamin is a first-year fellow in the hematology-oncology department, University of Oklahoma Health Sciences Center.
1. Toda M, Morimoto K. Ramadan fasting—effect on healthy Muslims. Soc Beh Pers 2004;32:13-18.
2. Kadri N, Mouchtaq N, Hakkou F, Moussaoui D. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol 2000;3:45-9.
3. Aadil I, Houti I, Moussamih S. Drug intake during Ramadan. BMJ 2004;329:778-82.
4. Aadil N, Fassi-Fihri I, Houti B, et al. Influence of Ramadan on the pharmacokinetics of a single oral dose of valproic acid administered at two different times. Methods Find Exp Clin Pharmacol 2000;22(2):109-14.
5. Kadri N, Tilane A, Batal M, et al. Irritability during the month of Ramadan. Psychosom Med 2000;62:280-5.
Dr. Benjamin is a staff psychiatrist, Oklahoma City Veteran’s Administration Center and clinical assistant professor, department of psychiatry and behavioral sciences, University of Oklahoma Health Sciences Center, Oklahoma City.
Dr. Dennis is director of consultation-liaison service, Oklahoma City Veteran’s Administration Center, and clinical associate professor, department of psychiatry and behavioral sciences, University of Oklahoma Health Sciences Center.
Dr. Mosallaei-Benjamin is a first-year fellow in the hematology-oncology department, University of Oklahoma Health Sciences Center.