User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
When a patient threatens harm to a presidential candidate
Mr. K, age 52, has Asperger’s disorder and attention-deficit/hyperactivity disorder. Recently he sent an e-mail to President Bush, Vice President Cheney, Homeland Security Secretary Tom Ridge, television news commentator Wolf Blitzer, and numerous government agencies. Mr. K’s psychiatrist also received a copy.
In his message, Mr. K expressed intense personal offense at his belief that U.S. Sen. John Kerry had called his “beloved president” a liar, and challenged the presidential candidate to a duel. If Kerry refused, Mr. K wrote, he would “take other effective measures” to avenge this “insult to me, my family, and all loyal Americans.”
Immediately after seeing the note, the psychiatrist called the Secret Service. Ninety minutes later, agents interviewed Mr. K, searched his apartment, and found weapons and travel documents that strongly suggested Mr. K planned to follow Kerry. The patient was taken into custody and admitted to a secure psychiatric facility.
Patients with Asperger’s disorder often become fixated on a person or incident. Such patients’ social judgment is severely impaired, and they tend to view the world in absolute terms with no gray areas. In a presidential election year, that fixation can manifest as a verbal or written threat against the president, vice president, or a presidential candidate.
As doctors, we have both a civic duty and sworn obligation under state standard-of-practice codes to immediately inform the Secret Service of such a threat. Call the Secret Service even if you are unsure whether the patient will carry it out.
How to reach the Secret Service
- Find the phone number for the local Secret Service headquarters in the phone book’s U.S. government listings—usually under “frequently called numbers.”
- Tell the operator you are a psychiatrist reporting an imminent threat to the president’s or a candidate’s life. An agent will come on the line immediately.
If there is no Secret Service office in your area, contact the regional long-distance operator and demand to be connected with the nearest Secret Service headquarters.
When reporting a threat, insist on speaking to a live agent immediately. If you cannot reach the Secret Service, call the FBI at once.
Do not contact the patient once you have called authorities. The Secret Service will direct the investigation independent of your point of view.
Dr. Clark is a practicing psychiatrist and medical director, ADD Clinic Inc., Las Vegas, NV
Mr. K, age 52, has Asperger’s disorder and attention-deficit/hyperactivity disorder. Recently he sent an e-mail to President Bush, Vice President Cheney, Homeland Security Secretary Tom Ridge, television news commentator Wolf Blitzer, and numerous government agencies. Mr. K’s psychiatrist also received a copy.
In his message, Mr. K expressed intense personal offense at his belief that U.S. Sen. John Kerry had called his “beloved president” a liar, and challenged the presidential candidate to a duel. If Kerry refused, Mr. K wrote, he would “take other effective measures” to avenge this “insult to me, my family, and all loyal Americans.”
Immediately after seeing the note, the psychiatrist called the Secret Service. Ninety minutes later, agents interviewed Mr. K, searched his apartment, and found weapons and travel documents that strongly suggested Mr. K planned to follow Kerry. The patient was taken into custody and admitted to a secure psychiatric facility.
Patients with Asperger’s disorder often become fixated on a person or incident. Such patients’ social judgment is severely impaired, and they tend to view the world in absolute terms with no gray areas. In a presidential election year, that fixation can manifest as a verbal or written threat against the president, vice president, or a presidential candidate.
As doctors, we have both a civic duty and sworn obligation under state standard-of-practice codes to immediately inform the Secret Service of such a threat. Call the Secret Service even if you are unsure whether the patient will carry it out.
How to reach the Secret Service
- Find the phone number for the local Secret Service headquarters in the phone book’s U.S. government listings—usually under “frequently called numbers.”
- Tell the operator you are a psychiatrist reporting an imminent threat to the president’s or a candidate’s life. An agent will come on the line immediately.
If there is no Secret Service office in your area, contact the regional long-distance operator and demand to be connected with the nearest Secret Service headquarters.
When reporting a threat, insist on speaking to a live agent immediately. If you cannot reach the Secret Service, call the FBI at once.
Do not contact the patient once you have called authorities. The Secret Service will direct the investigation independent of your point of view.
Mr. K, age 52, has Asperger’s disorder and attention-deficit/hyperactivity disorder. Recently he sent an e-mail to President Bush, Vice President Cheney, Homeland Security Secretary Tom Ridge, television news commentator Wolf Blitzer, and numerous government agencies. Mr. K’s psychiatrist also received a copy.
In his message, Mr. K expressed intense personal offense at his belief that U.S. Sen. John Kerry had called his “beloved president” a liar, and challenged the presidential candidate to a duel. If Kerry refused, Mr. K wrote, he would “take other effective measures” to avenge this “insult to me, my family, and all loyal Americans.”
Immediately after seeing the note, the psychiatrist called the Secret Service. Ninety minutes later, agents interviewed Mr. K, searched his apartment, and found weapons and travel documents that strongly suggested Mr. K planned to follow Kerry. The patient was taken into custody and admitted to a secure psychiatric facility.
Patients with Asperger’s disorder often become fixated on a person or incident. Such patients’ social judgment is severely impaired, and they tend to view the world in absolute terms with no gray areas. In a presidential election year, that fixation can manifest as a verbal or written threat against the president, vice president, or a presidential candidate.
As doctors, we have both a civic duty and sworn obligation under state standard-of-practice codes to immediately inform the Secret Service of such a threat. Call the Secret Service even if you are unsure whether the patient will carry it out.
How to reach the Secret Service
- Find the phone number for the local Secret Service headquarters in the phone book’s U.S. government listings—usually under “frequently called numbers.”
- Tell the operator you are a psychiatrist reporting an imminent threat to the president’s or a candidate’s life. An agent will come on the line immediately.
If there is no Secret Service office in your area, contact the regional long-distance operator and demand to be connected with the nearest Secret Service headquarters.
When reporting a threat, insist on speaking to a live agent immediately. If you cannot reach the Secret Service, call the FBI at once.
Do not contact the patient once you have called authorities. The Secret Service will direct the investigation independent of your point of view.
Dr. Clark is a practicing psychiatrist and medical director, ADD Clinic Inc., Las Vegas, NV
Dr. Clark is a practicing psychiatrist and medical director, ADD Clinic Inc., Las Vegas, NV
Exercise and mood: Our parents were right
When I started residency, I promised myself I would never pass along to patients the medical and psychiatric advice my parents gave me. Not that my parents gave uniformly bad advice; some of it has helped me—a fact that has taken me decades to admit. The issue was that I wanted to be a scientific practitioner, rather than a purveyor of conventional wisdom.
This stance, of course, has created problems for me. I’m sure much of what my parents told me is true, even though we don’t have much supporting data. So whenever a paper confirms what seems like common knowledge, I feel happy.
That’s why I appreciate the article on exercise and mental health in this month’s issue. It validates what my parents told me and what I have always believed: exercise really does improve psychological well-being. The evidence has been in the literature, but until Sheila M. Dowd, PhD, Kristin S. Vickers, PhD, and Dean Krahn, MD, reviewed it for me, I was not sure I could believe it.
Now, if I can just get myself to start some sort of exercise, I will be really happy.
When I started residency, I promised myself I would never pass along to patients the medical and psychiatric advice my parents gave me. Not that my parents gave uniformly bad advice; some of it has helped me—a fact that has taken me decades to admit. The issue was that I wanted to be a scientific practitioner, rather than a purveyor of conventional wisdom.
This stance, of course, has created problems for me. I’m sure much of what my parents told me is true, even though we don’t have much supporting data. So whenever a paper confirms what seems like common knowledge, I feel happy.
That’s why I appreciate the article on exercise and mental health in this month’s issue. It validates what my parents told me and what I have always believed: exercise really does improve psychological well-being. The evidence has been in the literature, but until Sheila M. Dowd, PhD, Kristin S. Vickers, PhD, and Dean Krahn, MD, reviewed it for me, I was not sure I could believe it.
Now, if I can just get myself to start some sort of exercise, I will be really happy.
When I started residency, I promised myself I would never pass along to patients the medical and psychiatric advice my parents gave me. Not that my parents gave uniformly bad advice; some of it has helped me—a fact that has taken me decades to admit. The issue was that I wanted to be a scientific practitioner, rather than a purveyor of conventional wisdom.
This stance, of course, has created problems for me. I’m sure much of what my parents told me is true, even though we don’t have much supporting data. So whenever a paper confirms what seems like common knowledge, I feel happy.
That’s why I appreciate the article on exercise and mental health in this month’s issue. It validates what my parents told me and what I have always believed: exercise really does improve psychological well-being. The evidence has been in the literature, but until Sheila M. Dowd, PhD, Kristin S. Vickers, PhD, and Dean Krahn, MD, reviewed it for me, I was not sure I could believe it.
Now, if I can just get myself to start some sort of exercise, I will be really happy.
Posttraumatic stress disorder: Nature and nurture?
Posttraumatic stress disorder (PTSD) can be one of the most frustrating anxiety disorders for both the patient and clinician. Asymptomatic persons become haunted by an experience they can’t forget. Their resulting anxiety can sour what were once healthy relationships or disable someone who previously was productive.
In some cases, despite aggressive psychopharmacology and psychotherapy, the patient remains incapacitated by inappropriate and unremitting fear. The trauma seems to have broken something—changed something inside the brain—that can’t be fixed.
Brain imaging studies of patients with PTSD—combat veterans and women with histories of childhood sexual abuse—have shown smaller hippocampal volumes compared with patients without PTSD.1,2 This finding has led to speculation that stress hormones (glucocorticoids) adversely affect the hippocampus (Figure 1).
This line of reasoning suggests that prolonged stress causes increased production of glucocorticoids that are neurotoxic to the hippocampus, resulting in hippocampal atrophy.3 Studies of rodents and patients with Cushing’s syndrome support this hypothesis. The hippocampus, therefore, may have been irreversibly damaged in patients with severe PTSD.
Figure 1
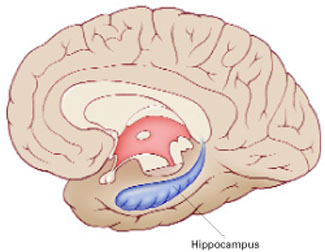
The hippocampus, a specialized type of cortex, is key to memory and emotion. As this medial view shows, it extends along the lateral ventricle floor on each side of the brain.
Illustration for Current Psychiatry by Marcia Hartsock, CMI Hippocampus
Intuitively, this theory makes sense, as the hippocampus is crucial for memory and emotion. However, a recent study of identical twins raises doubts.
Surprising evidence
Gilbertson et al recruited 40 pairs of twins, in which one was a Vietnam combat veteran and the other stayed home.4 Using MRI, the researchers measured hippocampal volume in each twin and assessed the presence and severity of PTSD in the combat-exposed twin.
Consistent with earlier reports, the authors found smaller hippocampal volumes in combat-exposed individuals diagnosed with PTSD. However, they found an almost identical correlation between the noncombat-exposed twin’s hippocampal volume and the combat-exposed twin’s PTSD score (Figure 2). In other words, the twin’s hippocampus size was a better predictor of the veteran’s hippocampus size than was the veteran’s trauma exposure or PTSD symptoms.
This finding puts a new spin on the association between small hippocampal volume and PTSD. The authors stated, “these data indicate that smaller hippocampi in PTSD represents a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se.” Put another way, the small hippocampus is not created by stress and trauma but is a preexisting condition. Further, this study suggests that a larger hippocampus may protect a person from developing PTSD.
This study may help explain why different individuals exposed to the same trauma are frequently left with different symptoms.5,6 PTSD would seem to be an excellent example of the combined effects of nature (small hippocampus) and nurture (traumatic experience).
Figure 2 Hippocampal volume correlates with posttraumatic symptoms
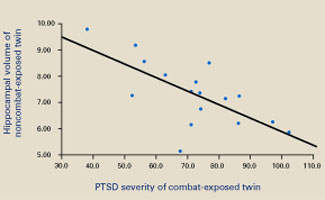
Smaller hippocampal volume in identical twins not exposed to combat was related to more-severe PTSD symptoms in their combat-exposed brothers (P = 0.002). Symptom severity was measured using Clinician-Administered PTSD Scale (CAPS) total scores.
Source: Reprinted with permission from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002;5:1242-7.
1. Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81.
2. Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160:924-32.
3. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35.
4. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7.
5. Macklin ML, Metzger LJ, Litz BT, et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998;66:323-6.
6. Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans’ Reactions to September 11. JAMA 2002;288:581-8.
Posttraumatic stress disorder (PTSD) can be one of the most frustrating anxiety disorders for both the patient and clinician. Asymptomatic persons become haunted by an experience they can’t forget. Their resulting anxiety can sour what were once healthy relationships or disable someone who previously was productive.
In some cases, despite aggressive psychopharmacology and psychotherapy, the patient remains incapacitated by inappropriate and unremitting fear. The trauma seems to have broken something—changed something inside the brain—that can’t be fixed.
Brain imaging studies of patients with PTSD—combat veterans and women with histories of childhood sexual abuse—have shown smaller hippocampal volumes compared with patients without PTSD.1,2 This finding has led to speculation that stress hormones (glucocorticoids) adversely affect the hippocampus (Figure 1).
This line of reasoning suggests that prolonged stress causes increased production of glucocorticoids that are neurotoxic to the hippocampus, resulting in hippocampal atrophy.3 Studies of rodents and patients with Cushing’s syndrome support this hypothesis. The hippocampus, therefore, may have been irreversibly damaged in patients with severe PTSD.
Figure 1

The hippocampus, a specialized type of cortex, is key to memory and emotion. As this medial view shows, it extends along the lateral ventricle floor on each side of the brain.
Illustration for Current Psychiatry by Marcia Hartsock, CMI Hippocampus
Intuitively, this theory makes sense, as the hippocampus is crucial for memory and emotion. However, a recent study of identical twins raises doubts.
Surprising evidence
Gilbertson et al recruited 40 pairs of twins, in which one was a Vietnam combat veteran and the other stayed home.4 Using MRI, the researchers measured hippocampal volume in each twin and assessed the presence and severity of PTSD in the combat-exposed twin.
Consistent with earlier reports, the authors found smaller hippocampal volumes in combat-exposed individuals diagnosed with PTSD. However, they found an almost identical correlation between the noncombat-exposed twin’s hippocampal volume and the combat-exposed twin’s PTSD score (Figure 2). In other words, the twin’s hippocampus size was a better predictor of the veteran’s hippocampus size than was the veteran’s trauma exposure or PTSD symptoms.
This finding puts a new spin on the association between small hippocampal volume and PTSD. The authors stated, “these data indicate that smaller hippocampi in PTSD represents a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se.” Put another way, the small hippocampus is not created by stress and trauma but is a preexisting condition. Further, this study suggests that a larger hippocampus may protect a person from developing PTSD.
This study may help explain why different individuals exposed to the same trauma are frequently left with different symptoms.5,6 PTSD would seem to be an excellent example of the combined effects of nature (small hippocampus) and nurture (traumatic experience).
Figure 2 Hippocampal volume correlates with posttraumatic symptoms

Smaller hippocampal volume in identical twins not exposed to combat was related to more-severe PTSD symptoms in their combat-exposed brothers (P = 0.002). Symptom severity was measured using Clinician-Administered PTSD Scale (CAPS) total scores.
Source: Reprinted with permission from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002;5:1242-7.
Posttraumatic stress disorder (PTSD) can be one of the most frustrating anxiety disorders for both the patient and clinician. Asymptomatic persons become haunted by an experience they can’t forget. Their resulting anxiety can sour what were once healthy relationships or disable someone who previously was productive.
In some cases, despite aggressive psychopharmacology and psychotherapy, the patient remains incapacitated by inappropriate and unremitting fear. The trauma seems to have broken something—changed something inside the brain—that can’t be fixed.
Brain imaging studies of patients with PTSD—combat veterans and women with histories of childhood sexual abuse—have shown smaller hippocampal volumes compared with patients without PTSD.1,2 This finding has led to speculation that stress hormones (glucocorticoids) adversely affect the hippocampus (Figure 1).
This line of reasoning suggests that prolonged stress causes increased production of glucocorticoids that are neurotoxic to the hippocampus, resulting in hippocampal atrophy.3 Studies of rodents and patients with Cushing’s syndrome support this hypothesis. The hippocampus, therefore, may have been irreversibly damaged in patients with severe PTSD.
Figure 1

The hippocampus, a specialized type of cortex, is key to memory and emotion. As this medial view shows, it extends along the lateral ventricle floor on each side of the brain.
Illustration for Current Psychiatry by Marcia Hartsock, CMI Hippocampus
Intuitively, this theory makes sense, as the hippocampus is crucial for memory and emotion. However, a recent study of identical twins raises doubts.
Surprising evidence
Gilbertson et al recruited 40 pairs of twins, in which one was a Vietnam combat veteran and the other stayed home.4 Using MRI, the researchers measured hippocampal volume in each twin and assessed the presence and severity of PTSD in the combat-exposed twin.
Consistent with earlier reports, the authors found smaller hippocampal volumes in combat-exposed individuals diagnosed with PTSD. However, they found an almost identical correlation between the noncombat-exposed twin’s hippocampal volume and the combat-exposed twin’s PTSD score (Figure 2). In other words, the twin’s hippocampus size was a better predictor of the veteran’s hippocampus size than was the veteran’s trauma exposure or PTSD symptoms.
This finding puts a new spin on the association between small hippocampal volume and PTSD. The authors stated, “these data indicate that smaller hippocampi in PTSD represents a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se.” Put another way, the small hippocampus is not created by stress and trauma but is a preexisting condition. Further, this study suggests that a larger hippocampus may protect a person from developing PTSD.
This study may help explain why different individuals exposed to the same trauma are frequently left with different symptoms.5,6 PTSD would seem to be an excellent example of the combined effects of nature (small hippocampus) and nurture (traumatic experience).
Figure 2 Hippocampal volume correlates with posttraumatic symptoms

Smaller hippocampal volume in identical twins not exposed to combat was related to more-severe PTSD symptoms in their combat-exposed brothers (P = 0.002). Symptom severity was measured using Clinician-Administered PTSD Scale (CAPS) total scores.
Source: Reprinted with permission from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002;5:1242-7.
1. Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81.
2. Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160:924-32.
3. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35.
4. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7.
5. Macklin ML, Metzger LJ, Litz BT, et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998;66:323-6.
6. Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans’ Reactions to September 11. JAMA 2002;288:581-8.
1. Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81.
2. Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160:924-32.
3. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35.
4. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7.
5. Macklin ML, Metzger LJ, Litz BT, et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998;66:323-6.
6. Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans’ Reactions to September 11. JAMA 2002;288:581-8.
Writing prescriptions on PDAs
Dr. John Luo’s article, “Handhelds: A cure for illegible prescriptions” (Current Psychiatry, April 2003 online edition), missed some important issues.
First, once a practice computerizes or installs a network—especially a wireless network—the physician needs to guard against HIPAA violations. Each violation could result in a fine ranging from $100,000 to $1 million. The switch to a Palm-, local area network-, or Internet-based program requires security procedures and operation controls.
Second, medical file management programs offer prescription writing, but as an accessory. Prescription writing should be the prominent component of any software title.
Third, during a power failure you need backup power and database recovery software to bring your system back up if it crashes. This not only protects your database, but also ensures that your patient files and formulary are available during an emergency, when risk is greatest.
Finally, let’s say 10 patient files fall to the floor. The contents are spilled, and you hastily pick up and re-collate the files. Incorrectly collating the files will not lead to a malpractice suit.
Now let’s say your system crashes, wiping out those same 10 Internet database files, and you cannot verify a new script’s contraindications because your program has no prescription component. Your ability to manage risk is lost.
Reid Schwabach
Internet technology systems manager/underwriter
Sarasota, FL
Dr. Luo responds
I appreciate Mr. Schwabach’s points about security issues regarding use of personal digital assistants (PDAs) for prescription writing. I mention these issues in my review article in the Canadian Journal of Psychiatry.1
Practicality and safety must always temper enthusiasm for technology. Prescription writing as a prominent feature of a medical file management program is desirable if the process is as easy and fail-proof as possible. However, any electronic system must have a back-up or redundancy system in case of data loss. Also, feedback from a fax- or Internet-based system is necessary to indicate that the pharmacy received the prescription.
Back-up systems are critical with technology to manage risk if data are lost, but technological failure should be anticipated. Paper- or CD-ROM-based drug interaction guides should be available, and documenting communication to other physicians is another reasonable method of managing risk.
These issues should not deter physicians from implementing an electronic prescription system because traditional paper-based or telephone prescriptions are also at risk for error or data loss. Technology should be carefully evaluated, much as we counsel our patients about the risks and benefits of prescription medications.
John S.Luo, MD
Assistant professor of psychiatry
UCLA Neuropsychiatric Institute and Hospital
Los Angeles, CA
- Luo J. Portable computing in psychiatry. Can J Psychiatry 2004;49:24-30
Dr. John Luo’s article, “Handhelds: A cure for illegible prescriptions” (Current Psychiatry, April 2003 online edition), missed some important issues.
First, once a practice computerizes or installs a network—especially a wireless network—the physician needs to guard against HIPAA violations. Each violation could result in a fine ranging from $100,000 to $1 million. The switch to a Palm-, local area network-, or Internet-based program requires security procedures and operation controls.
Second, medical file management programs offer prescription writing, but as an accessory. Prescription writing should be the prominent component of any software title.
Third, during a power failure you need backup power and database recovery software to bring your system back up if it crashes. This not only protects your database, but also ensures that your patient files and formulary are available during an emergency, when risk is greatest.
Finally, let’s say 10 patient files fall to the floor. The contents are spilled, and you hastily pick up and re-collate the files. Incorrectly collating the files will not lead to a malpractice suit.
Now let’s say your system crashes, wiping out those same 10 Internet database files, and you cannot verify a new script’s contraindications because your program has no prescription component. Your ability to manage risk is lost.
Reid Schwabach
Internet technology systems manager/underwriter
Sarasota, FL
Dr. Luo responds
I appreciate Mr. Schwabach’s points about security issues regarding use of personal digital assistants (PDAs) for prescription writing. I mention these issues in my review article in the Canadian Journal of Psychiatry.1
Practicality and safety must always temper enthusiasm for technology. Prescription writing as a prominent feature of a medical file management program is desirable if the process is as easy and fail-proof as possible. However, any electronic system must have a back-up or redundancy system in case of data loss. Also, feedback from a fax- or Internet-based system is necessary to indicate that the pharmacy received the prescription.
Back-up systems are critical with technology to manage risk if data are lost, but technological failure should be anticipated. Paper- or CD-ROM-based drug interaction guides should be available, and documenting communication to other physicians is another reasonable method of managing risk.
These issues should not deter physicians from implementing an electronic prescription system because traditional paper-based or telephone prescriptions are also at risk for error or data loss. Technology should be carefully evaluated, much as we counsel our patients about the risks and benefits of prescription medications.
John S.Luo, MD
Assistant professor of psychiatry
UCLA Neuropsychiatric Institute and Hospital
Los Angeles, CA
- Luo J. Portable computing in psychiatry. Can J Psychiatry 2004;49:24-30
Dr. John Luo’s article, “Handhelds: A cure for illegible prescriptions” (Current Psychiatry, April 2003 online edition), missed some important issues.
First, once a practice computerizes or installs a network—especially a wireless network—the physician needs to guard against HIPAA violations. Each violation could result in a fine ranging from $100,000 to $1 million. The switch to a Palm-, local area network-, or Internet-based program requires security procedures and operation controls.
Second, medical file management programs offer prescription writing, but as an accessory. Prescription writing should be the prominent component of any software title.
Third, during a power failure you need backup power and database recovery software to bring your system back up if it crashes. This not only protects your database, but also ensures that your patient files and formulary are available during an emergency, when risk is greatest.
Finally, let’s say 10 patient files fall to the floor. The contents are spilled, and you hastily pick up and re-collate the files. Incorrectly collating the files will not lead to a malpractice suit.
Now let’s say your system crashes, wiping out those same 10 Internet database files, and you cannot verify a new script’s contraindications because your program has no prescription component. Your ability to manage risk is lost.
Reid Schwabach
Internet technology systems manager/underwriter
Sarasota, FL
Dr. Luo responds
I appreciate Mr. Schwabach’s points about security issues regarding use of personal digital assistants (PDAs) for prescription writing. I mention these issues in my review article in the Canadian Journal of Psychiatry.1
Practicality and safety must always temper enthusiasm for technology. Prescription writing as a prominent feature of a medical file management program is desirable if the process is as easy and fail-proof as possible. However, any electronic system must have a back-up or redundancy system in case of data loss. Also, feedback from a fax- or Internet-based system is necessary to indicate that the pharmacy received the prescription.
Back-up systems are critical with technology to manage risk if data are lost, but technological failure should be anticipated. Paper- or CD-ROM-based drug interaction guides should be available, and documenting communication to other physicians is another reasonable method of managing risk.
These issues should not deter physicians from implementing an electronic prescription system because traditional paper-based or telephone prescriptions are also at risk for error or data loss. Technology should be carefully evaluated, much as we counsel our patients about the risks and benefits of prescription medications.
John S.Luo, MD
Assistant professor of psychiatry
UCLA Neuropsychiatric Institute and Hospital
Los Angeles, CA
- Luo J. Portable computing in psychiatry. Can J Psychiatry 2004;49:24-30
Skin picking: one teenager’s struggle
I am most grateful for “Captive of the mirror” (Current Psychiatry, December 2003) by Drs. Jon Grant and Katharine Phillips.
I am a 19-year-old female college student who has been diagnosed with depression, anxiety, and trichotillomania. I have bitten my nails all my life, have been taking SSRIs since age 12, and have had numerous problems related to skin-picking. Since adolescence I have obsessively picked and squeezed at acne and miniscule bumps on my face, causing redness, bruising and—in some cases—bleeding. I would then hide from the world, sometimes for days, until the wounds healed.
Until now, I didn’t know skin picking was a recognized disorder, let alone common. Your article is a detailed, clear, evidence-based summary of the problem and possible treatments, most of which I never knew existed. After having tried many things with limited success, I am taking the article to a psychiatrist so that I can discuss options. For the first time in months, I am filled with hope.
I am most grateful for “Captive of the mirror” (Current Psychiatry, December 2003) by Drs. Jon Grant and Katharine Phillips.
I am a 19-year-old female college student who has been diagnosed with depression, anxiety, and trichotillomania. I have bitten my nails all my life, have been taking SSRIs since age 12, and have had numerous problems related to skin-picking. Since adolescence I have obsessively picked and squeezed at acne and miniscule bumps on my face, causing redness, bruising and—in some cases—bleeding. I would then hide from the world, sometimes for days, until the wounds healed.
Until now, I didn’t know skin picking was a recognized disorder, let alone common. Your article is a detailed, clear, evidence-based summary of the problem and possible treatments, most of which I never knew existed. After having tried many things with limited success, I am taking the article to a psychiatrist so that I can discuss options. For the first time in months, I am filled with hope.
I am most grateful for “Captive of the mirror” (Current Psychiatry, December 2003) by Drs. Jon Grant and Katharine Phillips.
I am a 19-year-old female college student who has been diagnosed with depression, anxiety, and trichotillomania. I have bitten my nails all my life, have been taking SSRIs since age 12, and have had numerous problems related to skin-picking. Since adolescence I have obsessively picked and squeezed at acne and miniscule bumps on my face, causing redness, bruising and—in some cases—bleeding. I would then hide from the world, sometimes for days, until the wounds healed.
Until now, I didn’t know skin picking was a recognized disorder, let alone common. Your article is a detailed, clear, evidence-based summary of the problem and possible treatments, most of which I never knew existed. After having tried many things with limited success, I am taking the article to a psychiatrist so that I can discuss options. For the first time in months, I am filled with hope.
SSRIs in pediatric patients
“SSRIs in children and adolescents: Where do we stand?” (Current Psychiatry, March 2004) is excellent and timely.
As a child psychiatrist working in inpatient and outpatient settings, I have often seen activation and dysphoria in depressed children taking selective serotonin reuptake inhibitors (SSRIs). Drs. A. Bela Sood, Elizabeth Weller, and Ronald Weller reflect my view that “bipolar illness may be a possible explanation.” Without a high index of suspicion for bipolarity and a thorough family history, physicians are likely to be surprised when suicidality emerges after starting an antidepressant.
SSRIs clearly have contributed to the wellbeing of children with mood and anxiety disorders and are safe and effective in clinical practice. Unfortunately, the article does not address the dangers of using SSRIs in youths with bipolar disorder.
Stephen J. Wieder MD
Newburyport, MA
The authors respond
Dr. Wieder raises a pertinent clinical question regarding use of SSRIs in children at risk for bipolar disorder. SSRIs could cause hypomania or mania in depressed children with a clear history of bipolar disorder. When the clinical picture is not as clear, however, keep the following data in mind.
Follow-up studies have shown that 20% to 40% of adolescents with major depression develop bipolar type I disorder within 5 years after onset of depression.1 The clinician must strongly consider using prophylactic mood stabilizers along with SSRIs in depressed adolescents who present with psychomotor retardation, psychosis, family history of bipolar illness, and previous hypomanic disinhibition secondary to SSRI use, as these predict future bipolar disorder.2,3 Baseline irritability and aggression should also contraindicate SSRI monotherapy in unipolar depression, as exacerbation of rage and impulsivity with SSRIs seems to be high in this population.
A. Bela Sood, MD
Virginia Commonwealth University Health Systems
Elizabeth B. Weller, MD
Children’s Hospital of Philadelphia, University of Pennsylvania
Ronald Weller, MD
University of Pennsylvania
- Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry 1995;34:562–78.
- Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year old depressed children. J Am Acad Child Adolesc Psychiatry 1994;33:461–8.
- Strober M, Carlson G. Bipolar illness in adolescents with major depression. Arch Gen Psychiatry 1982;39:549–55.
“SSRIs in children and adolescents: Where do we stand?” (Current Psychiatry, March 2004) is excellent and timely.
As a child psychiatrist working in inpatient and outpatient settings, I have often seen activation and dysphoria in depressed children taking selective serotonin reuptake inhibitors (SSRIs). Drs. A. Bela Sood, Elizabeth Weller, and Ronald Weller reflect my view that “bipolar illness may be a possible explanation.” Without a high index of suspicion for bipolarity and a thorough family history, physicians are likely to be surprised when suicidality emerges after starting an antidepressant.
SSRIs clearly have contributed to the wellbeing of children with mood and anxiety disorders and are safe and effective in clinical practice. Unfortunately, the article does not address the dangers of using SSRIs in youths with bipolar disorder.
Stephen J. Wieder MD
Newburyport, MA
The authors respond
Dr. Wieder raises a pertinent clinical question regarding use of SSRIs in children at risk for bipolar disorder. SSRIs could cause hypomania or mania in depressed children with a clear history of bipolar disorder. When the clinical picture is not as clear, however, keep the following data in mind.
Follow-up studies have shown that 20% to 40% of adolescents with major depression develop bipolar type I disorder within 5 years after onset of depression.1 The clinician must strongly consider using prophylactic mood stabilizers along with SSRIs in depressed adolescents who present with psychomotor retardation, psychosis, family history of bipolar illness, and previous hypomanic disinhibition secondary to SSRI use, as these predict future bipolar disorder.2,3 Baseline irritability and aggression should also contraindicate SSRI monotherapy in unipolar depression, as exacerbation of rage and impulsivity with SSRIs seems to be high in this population.
A. Bela Sood, MD
Virginia Commonwealth University Health Systems
Elizabeth B. Weller, MD
Children’s Hospital of Philadelphia, University of Pennsylvania
Ronald Weller, MD
University of Pennsylvania
- Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry 1995;34:562–78.
- Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year old depressed children. J Am Acad Child Adolesc Psychiatry 1994;33:461–8.
- Strober M, Carlson G. Bipolar illness in adolescents with major depression. Arch Gen Psychiatry 1982;39:549–55.
“SSRIs in children and adolescents: Where do we stand?” (Current Psychiatry, March 2004) is excellent and timely.
As a child psychiatrist working in inpatient and outpatient settings, I have often seen activation and dysphoria in depressed children taking selective serotonin reuptake inhibitors (SSRIs). Drs. A. Bela Sood, Elizabeth Weller, and Ronald Weller reflect my view that “bipolar illness may be a possible explanation.” Without a high index of suspicion for bipolarity and a thorough family history, physicians are likely to be surprised when suicidality emerges after starting an antidepressant.
SSRIs clearly have contributed to the wellbeing of children with mood and anxiety disorders and are safe and effective in clinical practice. Unfortunately, the article does not address the dangers of using SSRIs in youths with bipolar disorder.
Stephen J. Wieder MD
Newburyport, MA
The authors respond
Dr. Wieder raises a pertinent clinical question regarding use of SSRIs in children at risk for bipolar disorder. SSRIs could cause hypomania or mania in depressed children with a clear history of bipolar disorder. When the clinical picture is not as clear, however, keep the following data in mind.
Follow-up studies have shown that 20% to 40% of adolescents with major depression develop bipolar type I disorder within 5 years after onset of depression.1 The clinician must strongly consider using prophylactic mood stabilizers along with SSRIs in depressed adolescents who present with psychomotor retardation, psychosis, family history of bipolar illness, and previous hypomanic disinhibition secondary to SSRI use, as these predict future bipolar disorder.2,3 Baseline irritability and aggression should also contraindicate SSRI monotherapy in unipolar depression, as exacerbation of rage and impulsivity with SSRIs seems to be high in this population.
A. Bela Sood, MD
Virginia Commonwealth University Health Systems
Elizabeth B. Weller, MD
Children’s Hospital of Philadelphia, University of Pennsylvania
Ronald Weller, MD
University of Pennsylvania
- Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry 1995;34:562–78.
- Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year old depressed children. J Am Acad Child Adolesc Psychiatry 1994;33:461–8.
- Strober M, Carlson G. Bipolar illness in adolescents with major depression. Arch Gen Psychiatry 1982;39:549–55.
Premenstrual moods or depression? Use logs to track monthly cycles
Differentiating premenstrual dysphoric disorder (PMDD) from depression or premenstrual syndrome (PMS) is crucial to restoring the patient’s well-being. PMS is relatively mild; its symptoms range from bloating to breast tenderness to irritability. PMDD is more severe and is characterized by marked and persistent irritability, depressed mood, anxiety, or affective lability.
Symptoms of clinical depression are present throughout the month, but PMDD symptoms emerge only during the luteal phase of most menstrual cycles. Having the patient keep a menstruation log can provide valuable clues to diagnosis.
Choosing the right tool
PMDD diagnosis requires confirmation of symptoms by prospective daily ratings over at least two menstrual cycles.
Available monitoring tools include:
- Daily Record of Severity of Problems (DRSP), the only scale keyed to DSM-IV criteria for PMDD. The patient rates symptoms daily from 1 (not present) to 6 (extreme).
- Premenstrual Symptom Diary (PSD), which uses a 4-point scale. It includes common psychological and physical symptoms and allows the patient to add others.
- Calendar of Premenstrual Experiences (COPE). One of the best-validated scales, COPE contains many more symptoms (10 physical and 12 psychological) than the others, making it both more thorough and more difficult to use.
How to use the log
Although many symptoms point to PMDD, a woman with this disorder tends to have the same symptoms across cycles. Common symptoms include irritability, anxiety, mood swings, sadness, crying spells, fatigue, lethargy, insomnia or hypersomnia, bloating, headaches, breast tenderness, poor concentration, and food cravings. The patient can rate each symptom or choose three or four that bother her most, then rate them daily from absent to severe.
Have the patient begin charting on the first day of her period. Few or no symptoms during the follicular phase (days 7-14) and an increase in symptoms during the luteal phase (days 14-28) may indicate PMDD.
Compare postmenstrual follicular phase and luteal phase scores. A luteal phase symptom increase of 30% to 50% (depending on which scale is used) confirms the PMDD diagnosis. The log can also help distinguish PMDD from pre-menstrual worsening of major depression or other disorders.
By keeping a menstruation log, a patient can predict and manage days when she will be most symptomatic. For example, the patient can adjust her diet, maximize sleep and exercise, and—where possible—avoid stressful events the week before her period. In severe cases, the log can help determine when antidepressants are warranted.
Dr. Rasminsky is assistant professor of clinical psychiatry, Women’s Mental Health Program, University of Illinois at Chicago.
Differentiating premenstrual dysphoric disorder (PMDD) from depression or premenstrual syndrome (PMS) is crucial to restoring the patient’s well-being. PMS is relatively mild; its symptoms range from bloating to breast tenderness to irritability. PMDD is more severe and is characterized by marked and persistent irritability, depressed mood, anxiety, or affective lability.
Symptoms of clinical depression are present throughout the month, but PMDD symptoms emerge only during the luteal phase of most menstrual cycles. Having the patient keep a menstruation log can provide valuable clues to diagnosis.
Choosing the right tool
PMDD diagnosis requires confirmation of symptoms by prospective daily ratings over at least two menstrual cycles.
Available monitoring tools include:
- Daily Record of Severity of Problems (DRSP), the only scale keyed to DSM-IV criteria for PMDD. The patient rates symptoms daily from 1 (not present) to 6 (extreme).
- Premenstrual Symptom Diary (PSD), which uses a 4-point scale. It includes common psychological and physical symptoms and allows the patient to add others.
- Calendar of Premenstrual Experiences (COPE). One of the best-validated scales, COPE contains many more symptoms (10 physical and 12 psychological) than the others, making it both more thorough and more difficult to use.
How to use the log
Although many symptoms point to PMDD, a woman with this disorder tends to have the same symptoms across cycles. Common symptoms include irritability, anxiety, mood swings, sadness, crying spells, fatigue, lethargy, insomnia or hypersomnia, bloating, headaches, breast tenderness, poor concentration, and food cravings. The patient can rate each symptom or choose three or four that bother her most, then rate them daily from absent to severe.
Have the patient begin charting on the first day of her period. Few or no symptoms during the follicular phase (days 7-14) and an increase in symptoms during the luteal phase (days 14-28) may indicate PMDD.
Compare postmenstrual follicular phase and luteal phase scores. A luteal phase symptom increase of 30% to 50% (depending on which scale is used) confirms the PMDD diagnosis. The log can also help distinguish PMDD from pre-menstrual worsening of major depression or other disorders.
By keeping a menstruation log, a patient can predict and manage days when she will be most symptomatic. For example, the patient can adjust her diet, maximize sleep and exercise, and—where possible—avoid stressful events the week before her period. In severe cases, the log can help determine when antidepressants are warranted.
Differentiating premenstrual dysphoric disorder (PMDD) from depression or premenstrual syndrome (PMS) is crucial to restoring the patient’s well-being. PMS is relatively mild; its symptoms range from bloating to breast tenderness to irritability. PMDD is more severe and is characterized by marked and persistent irritability, depressed mood, anxiety, or affective lability.
Symptoms of clinical depression are present throughout the month, but PMDD symptoms emerge only during the luteal phase of most menstrual cycles. Having the patient keep a menstruation log can provide valuable clues to diagnosis.
Choosing the right tool
PMDD diagnosis requires confirmation of symptoms by prospective daily ratings over at least two menstrual cycles.
Available monitoring tools include:
- Daily Record of Severity of Problems (DRSP), the only scale keyed to DSM-IV criteria for PMDD. The patient rates symptoms daily from 1 (not present) to 6 (extreme).
- Premenstrual Symptom Diary (PSD), which uses a 4-point scale. It includes common psychological and physical symptoms and allows the patient to add others.
- Calendar of Premenstrual Experiences (COPE). One of the best-validated scales, COPE contains many more symptoms (10 physical and 12 psychological) than the others, making it both more thorough and more difficult to use.
How to use the log
Although many symptoms point to PMDD, a woman with this disorder tends to have the same symptoms across cycles. Common symptoms include irritability, anxiety, mood swings, sadness, crying spells, fatigue, lethargy, insomnia or hypersomnia, bloating, headaches, breast tenderness, poor concentration, and food cravings. The patient can rate each symptom or choose three or four that bother her most, then rate them daily from absent to severe.
Have the patient begin charting on the first day of her period. Few or no symptoms during the follicular phase (days 7-14) and an increase in symptoms during the luteal phase (days 14-28) may indicate PMDD.
Compare postmenstrual follicular phase and luteal phase scores. A luteal phase symptom increase of 30% to 50% (depending on which scale is used) confirms the PMDD diagnosis. The log can also help distinguish PMDD from pre-menstrual worsening of major depression or other disorders.
By keeping a menstruation log, a patient can predict and manage days when she will be most symptomatic. For example, the patient can adjust her diet, maximize sleep and exercise, and—where possible—avoid stressful events the week before her period. In severe cases, the log can help determine when antidepressants are warranted.
Dr. Rasminsky is assistant professor of clinical psychiatry, Women’s Mental Health Program, University of Illinois at Chicago.
Dr. Rasminsky is assistant professor of clinical psychiatry, Women’s Mental Health Program, University of Illinois at Chicago.
Intramuscular olanzapine: Treating acute agitation in psychosis and bipolar mania
Oral atypical antipsychotics are given to treat a variety of psychiatric illnesses. Intramuscular (IM) preparations of atypicals are increasingly becoming available for emergency use, such as treating acute agitation.
The FDA has approved IM olanzapine for treating acute agitation associated with schizophrenia and bipolar type I mania.
How it works
As with the agent’s oral formulations (tablets, capsules, wafers), IM olanzapine is primarily an antagonist at serotonergic (5-HT2A) and dopaminergic (D2) receptors. Olanzapine is about twice as active at 5-HT2A compared with D2 receptors, which may underlie the agent’s efficacy as an antipsychotic and mood stabilizer without significant extrapyramidal effects.
Olanzapine also shows primarily antagonistic binding affinity at the 5-HT2B/2C, D1/D3/D4/D5, muscarinic, histamine H1 and alpha1-adrenergic receptors.1 This binding profile is comparable to that of clozapine and predicts a similar clinical response.
Pharmacokinetics
On most pharmacokinetic measures, IM olanzapine is nearly identical to its oral formulations, allowing easy comparison when switching to oral dosing as the patient improves.2
Plasma clearance (linear pharmacokinetics), half-life (approximately 30 hours), and volume of distribution are similar for IM and oral olanzapine. Maximum plasma concentrations after one, two, or three 10-mg injections given over 24 hours were similar to steady-state concentrations after daily administration of oral olanzapine, 20 mg.
The one key difference between IM and oral olanzapine is rate of absorption, which influences onset of action. IM olanzapine generally reaches maximum concentration in 15 to 45 minutes, compared with 4 hours after an oral dose. This rapid peak absorption could prove valuable in the first hour of a psychiatric emergency.
Efficacy
Three double-blind, randomized, placebo and active comparator-controlled studies demonstrated IM olanzapine’s safety and efficacy for treating acute agitation in patients with schizophrenia and bipolar type I mania. A fourth study gauged its efficacy in treating acute agitation in dementia.
Schizophrenia. In a study of 285 patients,3 IM olanzapine, 10 mg, was significantly more effective in reducing agitation than IM haloperidol, 7.5 mg, and IM placebo 15, 30, and 45 minutes after injection. Agitation was measured with the Positive and Negative Symptom Scale-Excited Component (PANSS-EC), Agitated Behavior Scale, and Agitation-Calmness Evaluation scale. Olanzapine and haloperidol were similar in efficacy 1 and 2 hours after injection, and both were more effective than placebo.
In another study,4 270 acutely agitated inpatients with schizophrenia received 1 to 3 IM injections of olanzapine (2.5, 5, 7.5, or 10 mg), haloperidol (7.5 mg), or placebo. The higher the olanzapine dose, the greater the PANSS-EC score reduction 2 hours after the first injection. Olanzapine was more effective than haloperidol on some agitation measures at 7.5 and 10 mg, and olanzapine was significantly more effective than haloperidol 24 hours post-injection, based on Agitated Behavior Scale scores.4 Both agents were similarly effective 2 hours after injection.
Bipolar type I mania. Agitated patients (N = 201) received 1 to 3 IM injections of olanzapine (10 mg for the first two injections, 5 mg for the third), lorazepam (2 mg first two, 1 mg third), or placebo.
Two hours after the first injection, agitation was more greatly reduced within the olanzapine group than in the lorazepam or placebo groups based on PANSS-EC, Agitated Behavior Scale, and Agitation-Calmness Evaluation Scale scores. At 24 hours, olanzapine was more effective than placebo but similar in efficacy to lorazepam.5
Table
IM olanzapine: Fast facts
| Drug brand name: Zyprexa IntraMuscular |
| Class Atypical antipsychotic |
| FDA-approved indication: Acute agitation associated with bipolar type I mania and schizophrenia |
| Approval date: March 29, 2004 |
| Manufacturer: Eli Lilly and Co. |
| Dosing form: 10 mg |
| Dosing recommendations: 10 mg for adults with schizophrenia and bipolar type I mania (5 mg ages 65 and older); 2.5 mg for patients who are debilitated, predisposed to hypotensive reactions, or sensitive to olanzapine. Consider 5- or 7.5-mg doses if clinical factors warrant, such as reduced clearance/slower metabolism in older, nonsmoking women. |
Dementia. A total of 272 patients with Alzheimer’s dementia, mixed dementia, or both received IM olanzapine (2.5 mg or 5 mg), IM lorazepam (1 mg), or IM placebo. The 5-mg olanzapine dose significantly reduced agitation 30 minutes post-injection, whereas lorazepam separated from placebo 60 minutes post-injection based on PANSS-EC scores. At 24 hours, both olanzapine doses were more effective than lorazepam or placebo.6
Tolerability
No clinically significant side effects have been reported with IM olanzapine. Incidence of extrapyramidal symptoms and QTc interval changes has been similar to that reported with placebo. Most studies have reported little change in vital signs, although a 7-bpm increase in heart rate and 5- to 7-mm Hg decrease in systolic blood pressure have been noted (Eli Lilly and Co., data on file).
Differences in treatment-emergent somnolence rates among patients receiving IM olanzapine (4% to 13%) and placebo (3% to 6%) were not statistically significant. Analyses of patients without treatment-emergent somnolence suggest that IM olanzapine retains a specific calming effect (as opposed to nonspecific sedation).7
Clinical implications
IM olanzapine offers psychiatrists a fast-acting option for treating agitation in patients with schizophrenia and bipolar type I mania. Its onset of action, measurable at 15 minutes post-injection, should prove valuable in the critical first hour of emergency psychiatric treatment. IM olanzapine’s efficacy and safety profile compare favorably with those of IM haloperidol and IM lorazepam.
IM olanzapine has shown safety and efficacy in treating agitation associated with dementia. Though the FDA has not approved this indication, the agent will likely be used for this purpose.
The only other fast-acting, injectable atypical antipsychotic—IM ziprasidone—is indicated for treatment of acute agitation in schizophrenia. Head-to-head comparisons between IM olanzapine and IM ziprasidone have not been conducted.
Clinical use and research will determine IM olanzapine’s role in treating patients with severe agitation (such as nonconsenting patients), those who are medically compromised, or patients in drug-induced psychotic states.
Related resources
- Zyprexa Web site. www.zyprexa.com.
- American Association for Emergency Psychiatry. http://www.emergencypsychiatry.org
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
Dr. Battaglia is a consultant to and speaker for Eli Lilly and Co.
1. Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996;14:87-96.
2. FDA Psychopharmacological Drugs Advisory Committee. Briefing document for Zyprexa (intramuscular olanzapine), February 13, 2001.
3. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry 2001;158:1149-51.
4. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry 2002;59:441-8.
5. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating patients diagnosed with bipolar mania. J Clin Psychopharmacol 2001;21:389-97.
6. Meehan KM, Wang J, David S, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: A double blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26:494-504.
7. Battaglia J, Lindborg S, Alaka K, et al. To sleep or not to sleep? Calming versus sedative effects of intramuscular olanzapine in agitated patients. Am J Emerg Med 2003;21:192-8.
Oral atypical antipsychotics are given to treat a variety of psychiatric illnesses. Intramuscular (IM) preparations of atypicals are increasingly becoming available for emergency use, such as treating acute agitation.
The FDA has approved IM olanzapine for treating acute agitation associated with schizophrenia and bipolar type I mania.
How it works
As with the agent’s oral formulations (tablets, capsules, wafers), IM olanzapine is primarily an antagonist at serotonergic (5-HT2A) and dopaminergic (D2) receptors. Olanzapine is about twice as active at 5-HT2A compared with D2 receptors, which may underlie the agent’s efficacy as an antipsychotic and mood stabilizer without significant extrapyramidal effects.
Olanzapine also shows primarily antagonistic binding affinity at the 5-HT2B/2C, D1/D3/D4/D5, muscarinic, histamine H1 and alpha1-adrenergic receptors.1 This binding profile is comparable to that of clozapine and predicts a similar clinical response.
Pharmacokinetics
On most pharmacokinetic measures, IM olanzapine is nearly identical to its oral formulations, allowing easy comparison when switching to oral dosing as the patient improves.2
Plasma clearance (linear pharmacokinetics), half-life (approximately 30 hours), and volume of distribution are similar for IM and oral olanzapine. Maximum plasma concentrations after one, two, or three 10-mg injections given over 24 hours were similar to steady-state concentrations after daily administration of oral olanzapine, 20 mg.
The one key difference between IM and oral olanzapine is rate of absorption, which influences onset of action. IM olanzapine generally reaches maximum concentration in 15 to 45 minutes, compared with 4 hours after an oral dose. This rapid peak absorption could prove valuable in the first hour of a psychiatric emergency.
Efficacy
Three double-blind, randomized, placebo and active comparator-controlled studies demonstrated IM olanzapine’s safety and efficacy for treating acute agitation in patients with schizophrenia and bipolar type I mania. A fourth study gauged its efficacy in treating acute agitation in dementia.
Schizophrenia. In a study of 285 patients,3 IM olanzapine, 10 mg, was significantly more effective in reducing agitation than IM haloperidol, 7.5 mg, and IM placebo 15, 30, and 45 minutes after injection. Agitation was measured with the Positive and Negative Symptom Scale-Excited Component (PANSS-EC), Agitated Behavior Scale, and Agitation-Calmness Evaluation scale. Olanzapine and haloperidol were similar in efficacy 1 and 2 hours after injection, and both were more effective than placebo.
In another study,4 270 acutely agitated inpatients with schizophrenia received 1 to 3 IM injections of olanzapine (2.5, 5, 7.5, or 10 mg), haloperidol (7.5 mg), or placebo. The higher the olanzapine dose, the greater the PANSS-EC score reduction 2 hours after the first injection. Olanzapine was more effective than haloperidol on some agitation measures at 7.5 and 10 mg, and olanzapine was significantly more effective than haloperidol 24 hours post-injection, based on Agitated Behavior Scale scores.4 Both agents were similarly effective 2 hours after injection.
Bipolar type I mania. Agitated patients (N = 201) received 1 to 3 IM injections of olanzapine (10 mg for the first two injections, 5 mg for the third), lorazepam (2 mg first two, 1 mg third), or placebo.
Two hours after the first injection, agitation was more greatly reduced within the olanzapine group than in the lorazepam or placebo groups based on PANSS-EC, Agitated Behavior Scale, and Agitation-Calmness Evaluation Scale scores. At 24 hours, olanzapine was more effective than placebo but similar in efficacy to lorazepam.5
Table
IM olanzapine: Fast facts
| Drug brand name: Zyprexa IntraMuscular |
| Class Atypical antipsychotic |
| FDA-approved indication: Acute agitation associated with bipolar type I mania and schizophrenia |
| Approval date: March 29, 2004 |
| Manufacturer: Eli Lilly and Co. |
| Dosing form: 10 mg |
| Dosing recommendations: 10 mg for adults with schizophrenia and bipolar type I mania (5 mg ages 65 and older); 2.5 mg for patients who are debilitated, predisposed to hypotensive reactions, or sensitive to olanzapine. Consider 5- or 7.5-mg doses if clinical factors warrant, such as reduced clearance/slower metabolism in older, nonsmoking women. |
Dementia. A total of 272 patients with Alzheimer’s dementia, mixed dementia, or both received IM olanzapine (2.5 mg or 5 mg), IM lorazepam (1 mg), or IM placebo. The 5-mg olanzapine dose significantly reduced agitation 30 minutes post-injection, whereas lorazepam separated from placebo 60 minutes post-injection based on PANSS-EC scores. At 24 hours, both olanzapine doses were more effective than lorazepam or placebo.6
Tolerability
No clinically significant side effects have been reported with IM olanzapine. Incidence of extrapyramidal symptoms and QTc interval changes has been similar to that reported with placebo. Most studies have reported little change in vital signs, although a 7-bpm increase in heart rate and 5- to 7-mm Hg decrease in systolic blood pressure have been noted (Eli Lilly and Co., data on file).
Differences in treatment-emergent somnolence rates among patients receiving IM olanzapine (4% to 13%) and placebo (3% to 6%) were not statistically significant. Analyses of patients without treatment-emergent somnolence suggest that IM olanzapine retains a specific calming effect (as opposed to nonspecific sedation).7
Clinical implications
IM olanzapine offers psychiatrists a fast-acting option for treating agitation in patients with schizophrenia and bipolar type I mania. Its onset of action, measurable at 15 minutes post-injection, should prove valuable in the critical first hour of emergency psychiatric treatment. IM olanzapine’s efficacy and safety profile compare favorably with those of IM haloperidol and IM lorazepam.
IM olanzapine has shown safety and efficacy in treating agitation associated with dementia. Though the FDA has not approved this indication, the agent will likely be used for this purpose.
The only other fast-acting, injectable atypical antipsychotic—IM ziprasidone—is indicated for treatment of acute agitation in schizophrenia. Head-to-head comparisons between IM olanzapine and IM ziprasidone have not been conducted.
Clinical use and research will determine IM olanzapine’s role in treating patients with severe agitation (such as nonconsenting patients), those who are medically compromised, or patients in drug-induced psychotic states.
Related resources
- Zyprexa Web site. www.zyprexa.com.
- American Association for Emergency Psychiatry. http://www.emergencypsychiatry.org
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
Dr. Battaglia is a consultant to and speaker for Eli Lilly and Co.
Oral atypical antipsychotics are given to treat a variety of psychiatric illnesses. Intramuscular (IM) preparations of atypicals are increasingly becoming available for emergency use, such as treating acute agitation.
The FDA has approved IM olanzapine for treating acute agitation associated with schizophrenia and bipolar type I mania.
How it works
As with the agent’s oral formulations (tablets, capsules, wafers), IM olanzapine is primarily an antagonist at serotonergic (5-HT2A) and dopaminergic (D2) receptors. Olanzapine is about twice as active at 5-HT2A compared with D2 receptors, which may underlie the agent’s efficacy as an antipsychotic and mood stabilizer without significant extrapyramidal effects.
Olanzapine also shows primarily antagonistic binding affinity at the 5-HT2B/2C, D1/D3/D4/D5, muscarinic, histamine H1 and alpha1-adrenergic receptors.1 This binding profile is comparable to that of clozapine and predicts a similar clinical response.
Pharmacokinetics
On most pharmacokinetic measures, IM olanzapine is nearly identical to its oral formulations, allowing easy comparison when switching to oral dosing as the patient improves.2
Plasma clearance (linear pharmacokinetics), half-life (approximately 30 hours), and volume of distribution are similar for IM and oral olanzapine. Maximum plasma concentrations after one, two, or three 10-mg injections given over 24 hours were similar to steady-state concentrations after daily administration of oral olanzapine, 20 mg.
The one key difference between IM and oral olanzapine is rate of absorption, which influences onset of action. IM olanzapine generally reaches maximum concentration in 15 to 45 minutes, compared with 4 hours after an oral dose. This rapid peak absorption could prove valuable in the first hour of a psychiatric emergency.
Efficacy
Three double-blind, randomized, placebo and active comparator-controlled studies demonstrated IM olanzapine’s safety and efficacy for treating acute agitation in patients with schizophrenia and bipolar type I mania. A fourth study gauged its efficacy in treating acute agitation in dementia.
Schizophrenia. In a study of 285 patients,3 IM olanzapine, 10 mg, was significantly more effective in reducing agitation than IM haloperidol, 7.5 mg, and IM placebo 15, 30, and 45 minutes after injection. Agitation was measured with the Positive and Negative Symptom Scale-Excited Component (PANSS-EC), Agitated Behavior Scale, and Agitation-Calmness Evaluation scale. Olanzapine and haloperidol were similar in efficacy 1 and 2 hours after injection, and both were more effective than placebo.
In another study,4 270 acutely agitated inpatients with schizophrenia received 1 to 3 IM injections of olanzapine (2.5, 5, 7.5, or 10 mg), haloperidol (7.5 mg), or placebo. The higher the olanzapine dose, the greater the PANSS-EC score reduction 2 hours after the first injection. Olanzapine was more effective than haloperidol on some agitation measures at 7.5 and 10 mg, and olanzapine was significantly more effective than haloperidol 24 hours post-injection, based on Agitated Behavior Scale scores.4 Both agents were similarly effective 2 hours after injection.
Bipolar type I mania. Agitated patients (N = 201) received 1 to 3 IM injections of olanzapine (10 mg for the first two injections, 5 mg for the third), lorazepam (2 mg first two, 1 mg third), or placebo.
Two hours after the first injection, agitation was more greatly reduced within the olanzapine group than in the lorazepam or placebo groups based on PANSS-EC, Agitated Behavior Scale, and Agitation-Calmness Evaluation Scale scores. At 24 hours, olanzapine was more effective than placebo but similar in efficacy to lorazepam.5
Table
IM olanzapine: Fast facts
| Drug brand name: Zyprexa IntraMuscular |
| Class Atypical antipsychotic |
| FDA-approved indication: Acute agitation associated with bipolar type I mania and schizophrenia |
| Approval date: March 29, 2004 |
| Manufacturer: Eli Lilly and Co. |
| Dosing form: 10 mg |
| Dosing recommendations: 10 mg for adults with schizophrenia and bipolar type I mania (5 mg ages 65 and older); 2.5 mg for patients who are debilitated, predisposed to hypotensive reactions, or sensitive to olanzapine. Consider 5- or 7.5-mg doses if clinical factors warrant, such as reduced clearance/slower metabolism in older, nonsmoking women. |
Dementia. A total of 272 patients with Alzheimer’s dementia, mixed dementia, or both received IM olanzapine (2.5 mg or 5 mg), IM lorazepam (1 mg), or IM placebo. The 5-mg olanzapine dose significantly reduced agitation 30 minutes post-injection, whereas lorazepam separated from placebo 60 minutes post-injection based on PANSS-EC scores. At 24 hours, both olanzapine doses were more effective than lorazepam or placebo.6
Tolerability
No clinically significant side effects have been reported with IM olanzapine. Incidence of extrapyramidal symptoms and QTc interval changes has been similar to that reported with placebo. Most studies have reported little change in vital signs, although a 7-bpm increase in heart rate and 5- to 7-mm Hg decrease in systolic blood pressure have been noted (Eli Lilly and Co., data on file).
Differences in treatment-emergent somnolence rates among patients receiving IM olanzapine (4% to 13%) and placebo (3% to 6%) were not statistically significant. Analyses of patients without treatment-emergent somnolence suggest that IM olanzapine retains a specific calming effect (as opposed to nonspecific sedation).7
Clinical implications
IM olanzapine offers psychiatrists a fast-acting option for treating agitation in patients with schizophrenia and bipolar type I mania. Its onset of action, measurable at 15 minutes post-injection, should prove valuable in the critical first hour of emergency psychiatric treatment. IM olanzapine’s efficacy and safety profile compare favorably with those of IM haloperidol and IM lorazepam.
IM olanzapine has shown safety and efficacy in treating agitation associated with dementia. Though the FDA has not approved this indication, the agent will likely be used for this purpose.
The only other fast-acting, injectable atypical antipsychotic—IM ziprasidone—is indicated for treatment of acute agitation in schizophrenia. Head-to-head comparisons between IM olanzapine and IM ziprasidone have not been conducted.
Clinical use and research will determine IM olanzapine’s role in treating patients with severe agitation (such as nonconsenting patients), those who are medically compromised, or patients in drug-induced psychotic states.
Related resources
- Zyprexa Web site. www.zyprexa.com.
- American Association for Emergency Psychiatry. http://www.emergencypsychiatry.org
Drug brand names
- Clozapine • Clozaril
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
Dr. Battaglia is a consultant to and speaker for Eli Lilly and Co.
1. Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996;14:87-96.
2. FDA Psychopharmacological Drugs Advisory Committee. Briefing document for Zyprexa (intramuscular olanzapine), February 13, 2001.
3. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry 2001;158:1149-51.
4. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry 2002;59:441-8.
5. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating patients diagnosed with bipolar mania. J Clin Psychopharmacol 2001;21:389-97.
6. Meehan KM, Wang J, David S, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: A double blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26:494-504.
7. Battaglia J, Lindborg S, Alaka K, et al. To sleep or not to sleep? Calming versus sedative effects of intramuscular olanzapine in agitated patients. Am J Emerg Med 2003;21:192-8.
1. Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996;14:87-96.
2. FDA Psychopharmacological Drugs Advisory Committee. Briefing document for Zyprexa (intramuscular olanzapine), February 13, 2001.
3. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry 2001;158:1149-51.
4. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry 2002;59:441-8.
5. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating patients diagnosed with bipolar mania. J Clin Psychopharmacol 2001;21:389-97.
6. Meehan KM, Wang J, David S, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: A double blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26:494-504.
7. Battaglia J, Lindborg S, Alaka K, et al. To sleep or not to sleep? Calming versus sedative effects of intramuscular olanzapine in agitated patients. Am J Emerg Med 2003;21:192-8.
Treating affective illness in patients with chronic pain
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
Persistent depression? Low libido? Androgen decline may be to blame
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.

