User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Medicinal liquor and edited mosquitoes
Drink to your health?
Whether you drink or not, most of us can agree that liquor is not the first thing that comes to mind when looking to make health improvements. But researchers have found a small exception in something traditional.
We’ve added buckwheat to pancakes, bread, and other baked goodies we made during the height of quarantine, but it’s also used to create a traditional liquor in some East Asian countries, where it is used medicinally.
Investigators have found that extracts in the Tartary buckwheat used to make the liquor induce autophagy, a process cells go through to remove proteins that are damaged or not needed anymore – sort of like a cellular spring cleaning.
To test this, the researchers treated liver and skin cells with Tartary buckwheat extract and looked to see how the cells responded with fluorescent markers. The results were clear.
“Treating cells with the extract stimulated the formation of autophagosomes, specialized cellular structures that carry out autophagy, and altered the location of proteins involved in regulating autophagy,” said senior author Takeshi Noda of Osaka (Japan) University.
Looking deeper, the researchers found that quercetin, a component of the buckwheat extract, had the same autophagic effect. And both the buckwheat and the quercetin gave the green light for liver cells to induce aggrephagy, the process of cleaning up protein aggregates.
Those protein aggregates in liver cells are closely linked to alcoholic liver disease, suggesting that quercetin could be a game changer in its treatment. In other words, liquor could help fix the problem that liquor started. Go figure.
From hospital bills to X-rated
Ralph Puglisi was an accounting manager for the University Medical Service Association (UMSA), a nonprofit that supports the massive University of South Florida health system. The association took in over $300 million in revenue in the 2019-2020 fiscal year, which is a rather large sum of money, but we’ll glide over the ethics of a “nonprofit” making a few hundred million for now.
Mr. Puglisi was in very close proximity to the money, generated from patient care, and he pled guilty to stealing it using UMSA credit cards. Now, that wouldn’t be LOTME worthy on its own, but what elevates this above garden-variety embezzlement is how the intrepid Mr. Puglisi chose to spend the millions he stole from the university health system: Adult entertainment.
And before you ask, he didn’t spend $11.5 million on something most people so inclined can find for free with judicious Google searches. What Mr. Puglisi actually did was invest in a website providing adult content through individual user profiles, one of which is believed to belong to his stepson’s fiancée, which brings a whole new level of sleaze to this enterprise. Over the course of 2 years, he visited her profile 2,800 times, an amount some might view as excessive.
While the vast majority of the embezzled money went to the adult website, Mr. Puglisi also used thousands of UMSA dollars to pay for travel, household improvements, rent, the works. Almost $44,000 was spent at a resort sometimes known as the happiest place on earth.
Then there’s Mr. Puglisi’s wife. Oh yes, this guy is married. He poured over $600,000 into a company he and his wife owned, which is a lot, but how much do you think went to the woman he married? Probably quite a bit. Go ahead, try to think of a number. It’s not like it was his money.
Did you guess $100 went into his wife’s PayPal account? No? Clearly you don’t understand the criminal mind. His stepson’s fiancée got millions, and his wife got a hundred. Now there are some priorities.
Step 1: Sit at desk. Step 2: Get in shape
Being a physician is not really a desk job, but doctors must spend a fair share of their time sitting, yes? Dealing with recalcitrant EHRs or talking on the phone to insurers or PBMs? If you are one of these physicians, or if you have patients who spend a lot of time sitting at their desks and might need to get a bit of exercise, then we’ve got a multitasking tip for you.
It came to us via one of our favorite websites, Sad and Useless. It’s the site that declares itself “the most depressive humor site on the Internet” and they’re offering up the “12 Best Exercises To Do At Your Desk.” It may not sound like much, but we think that the gang at Dunder-Mifflin would approve. And besides, who couldn’t stand to burn a few calories without having to leave the chair?
We won’t spoil your fun by going through all 12 – each one comes with step-by-step instructions and a helpful illustration or GIF – but here are just a few:
- Bending over backwards: “Agree to do something you don’t want to do. Spend twice as long as expected doing that thing. Hate yourself.”
- Fake laughter: “Hear a joke that isn’t even remotely funny. Open your mouth and make laughing sounds.”
- Bang your head: Feel the “pointlessness of your job overwhelm you” and then “bring your head forcefully down to your desk.”
Now, we here at LOTME are, of course [Bang!], highly skilled, professional wordsmithing humorists [Bang!], so when we tell you that this is a great workout [Bang!] … that this is a great workout [Bang!] … it’s great … uggh.
Wooooo. Feel the burn.
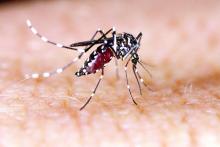
One order of mosquitoes, extra Crispr
What would it be like to have a barbecue in your backyard on a humid summer night and not get eaten alive by mosquitoes? If you’re like us, you probably thought you’d never see that day.
Mosquitoes cause itchy bites, but, more importantly, they can carry dengue, malaria, yellow fever, and Zika virus. New research shows that protection from these diseases may be possible with use of the Crispr-Cas9 gene-editing tool, which could make humans invisible to mosquitoes by taking away their light-sensing abilities and, thus, their ability to find us.
“The better we understand how they sense the human, the better we can control the mosquito in an eco-friendly manner,” Yinpeng Zhan, a postdoctoral researcher at the University of California, Santa Barbara, and the study’s lead author, told the New York Times.
After studying the mosquitoes and figuring out their hunting patterns, the researchers found that mosquitoes are attracted to dark spots more than white spots and used this to their advantage. After knocking out two of the proteins that mosquitoes need for vision – via Crispr – the little suckers could not distinguish the difference between the white and dark spots.
We’re sure mosquitoes don’t mean any harm – they’re just trying to survive and reproduce like any other species – but thanks to this new tool, gone might be the days of having to douse yourself in bug spray that smells like a mix of chemicals and melon.
Drink to your health?
Whether you drink or not, most of us can agree that liquor is not the first thing that comes to mind when looking to make health improvements. But researchers have found a small exception in something traditional.
We’ve added buckwheat to pancakes, bread, and other baked goodies we made during the height of quarantine, but it’s also used to create a traditional liquor in some East Asian countries, where it is used medicinally.
Investigators have found that extracts in the Tartary buckwheat used to make the liquor induce autophagy, a process cells go through to remove proteins that are damaged or not needed anymore – sort of like a cellular spring cleaning.
To test this, the researchers treated liver and skin cells with Tartary buckwheat extract and looked to see how the cells responded with fluorescent markers. The results were clear.
“Treating cells with the extract stimulated the formation of autophagosomes, specialized cellular structures that carry out autophagy, and altered the location of proteins involved in regulating autophagy,” said senior author Takeshi Noda of Osaka (Japan) University.
Looking deeper, the researchers found that quercetin, a component of the buckwheat extract, had the same autophagic effect. And both the buckwheat and the quercetin gave the green light for liver cells to induce aggrephagy, the process of cleaning up protein aggregates.
Those protein aggregates in liver cells are closely linked to alcoholic liver disease, suggesting that quercetin could be a game changer in its treatment. In other words, liquor could help fix the problem that liquor started. Go figure.
From hospital bills to X-rated
Ralph Puglisi was an accounting manager for the University Medical Service Association (UMSA), a nonprofit that supports the massive University of South Florida health system. The association took in over $300 million in revenue in the 2019-2020 fiscal year, which is a rather large sum of money, but we’ll glide over the ethics of a “nonprofit” making a few hundred million for now.
Mr. Puglisi was in very close proximity to the money, generated from patient care, and he pled guilty to stealing it using UMSA credit cards. Now, that wouldn’t be LOTME worthy on its own, but what elevates this above garden-variety embezzlement is how the intrepid Mr. Puglisi chose to spend the millions he stole from the university health system: Adult entertainment.
And before you ask, he didn’t spend $11.5 million on something most people so inclined can find for free with judicious Google searches. What Mr. Puglisi actually did was invest in a website providing adult content through individual user profiles, one of which is believed to belong to his stepson’s fiancée, which brings a whole new level of sleaze to this enterprise. Over the course of 2 years, he visited her profile 2,800 times, an amount some might view as excessive.
While the vast majority of the embezzled money went to the adult website, Mr. Puglisi also used thousands of UMSA dollars to pay for travel, household improvements, rent, the works. Almost $44,000 was spent at a resort sometimes known as the happiest place on earth.
Then there’s Mr. Puglisi’s wife. Oh yes, this guy is married. He poured over $600,000 into a company he and his wife owned, which is a lot, but how much do you think went to the woman he married? Probably quite a bit. Go ahead, try to think of a number. It’s not like it was his money.
Did you guess $100 went into his wife’s PayPal account? No? Clearly you don’t understand the criminal mind. His stepson’s fiancée got millions, and his wife got a hundred. Now there are some priorities.
Step 1: Sit at desk. Step 2: Get in shape
Being a physician is not really a desk job, but doctors must spend a fair share of their time sitting, yes? Dealing with recalcitrant EHRs or talking on the phone to insurers or PBMs? If you are one of these physicians, or if you have patients who spend a lot of time sitting at their desks and might need to get a bit of exercise, then we’ve got a multitasking tip for you.
It came to us via one of our favorite websites, Sad and Useless. It’s the site that declares itself “the most depressive humor site on the Internet” and they’re offering up the “12 Best Exercises To Do At Your Desk.” It may not sound like much, but we think that the gang at Dunder-Mifflin would approve. And besides, who couldn’t stand to burn a few calories without having to leave the chair?
We won’t spoil your fun by going through all 12 – each one comes with step-by-step instructions and a helpful illustration or GIF – but here are just a few:
- Bending over backwards: “Agree to do something you don’t want to do. Spend twice as long as expected doing that thing. Hate yourself.”
- Fake laughter: “Hear a joke that isn’t even remotely funny. Open your mouth and make laughing sounds.”
- Bang your head: Feel the “pointlessness of your job overwhelm you” and then “bring your head forcefully down to your desk.”
Now, we here at LOTME are, of course [Bang!], highly skilled, professional wordsmithing humorists [Bang!], so when we tell you that this is a great workout [Bang!] … that this is a great workout [Bang!] … it’s great … uggh.
Wooooo. Feel the burn.
One order of mosquitoes, extra Crispr
What would it be like to have a barbecue in your backyard on a humid summer night and not get eaten alive by mosquitoes? If you’re like us, you probably thought you’d never see that day.
Mosquitoes cause itchy bites, but, more importantly, they can carry dengue, malaria, yellow fever, and Zika virus. New research shows that protection from these diseases may be possible with use of the Crispr-Cas9 gene-editing tool, which could make humans invisible to mosquitoes by taking away their light-sensing abilities and, thus, their ability to find us.
“The better we understand how they sense the human, the better we can control the mosquito in an eco-friendly manner,” Yinpeng Zhan, a postdoctoral researcher at the University of California, Santa Barbara, and the study’s lead author, told the New York Times.
After studying the mosquitoes and figuring out their hunting patterns, the researchers found that mosquitoes are attracted to dark spots more than white spots and used this to their advantage. After knocking out two of the proteins that mosquitoes need for vision – via Crispr – the little suckers could not distinguish the difference between the white and dark spots.
We’re sure mosquitoes don’t mean any harm – they’re just trying to survive and reproduce like any other species – but thanks to this new tool, gone might be the days of having to douse yourself in bug spray that smells like a mix of chemicals and melon.
Drink to your health?
Whether you drink or not, most of us can agree that liquor is not the first thing that comes to mind when looking to make health improvements. But researchers have found a small exception in something traditional.
We’ve added buckwheat to pancakes, bread, and other baked goodies we made during the height of quarantine, but it’s also used to create a traditional liquor in some East Asian countries, where it is used medicinally.
Investigators have found that extracts in the Tartary buckwheat used to make the liquor induce autophagy, a process cells go through to remove proteins that are damaged or not needed anymore – sort of like a cellular spring cleaning.
To test this, the researchers treated liver and skin cells with Tartary buckwheat extract and looked to see how the cells responded with fluorescent markers. The results were clear.
“Treating cells with the extract stimulated the formation of autophagosomes, specialized cellular structures that carry out autophagy, and altered the location of proteins involved in regulating autophagy,” said senior author Takeshi Noda of Osaka (Japan) University.
Looking deeper, the researchers found that quercetin, a component of the buckwheat extract, had the same autophagic effect. And both the buckwheat and the quercetin gave the green light for liver cells to induce aggrephagy, the process of cleaning up protein aggregates.
Those protein aggregates in liver cells are closely linked to alcoholic liver disease, suggesting that quercetin could be a game changer in its treatment. In other words, liquor could help fix the problem that liquor started. Go figure.
From hospital bills to X-rated
Ralph Puglisi was an accounting manager for the University Medical Service Association (UMSA), a nonprofit that supports the massive University of South Florida health system. The association took in over $300 million in revenue in the 2019-2020 fiscal year, which is a rather large sum of money, but we’ll glide over the ethics of a “nonprofit” making a few hundred million for now.
Mr. Puglisi was in very close proximity to the money, generated from patient care, and he pled guilty to stealing it using UMSA credit cards. Now, that wouldn’t be LOTME worthy on its own, but what elevates this above garden-variety embezzlement is how the intrepid Mr. Puglisi chose to spend the millions he stole from the university health system: Adult entertainment.
And before you ask, he didn’t spend $11.5 million on something most people so inclined can find for free with judicious Google searches. What Mr. Puglisi actually did was invest in a website providing adult content through individual user profiles, one of which is believed to belong to his stepson’s fiancée, which brings a whole new level of sleaze to this enterprise. Over the course of 2 years, he visited her profile 2,800 times, an amount some might view as excessive.
While the vast majority of the embezzled money went to the adult website, Mr. Puglisi also used thousands of UMSA dollars to pay for travel, household improvements, rent, the works. Almost $44,000 was spent at a resort sometimes known as the happiest place on earth.
Then there’s Mr. Puglisi’s wife. Oh yes, this guy is married. He poured over $600,000 into a company he and his wife owned, which is a lot, but how much do you think went to the woman he married? Probably quite a bit. Go ahead, try to think of a number. It’s not like it was his money.
Did you guess $100 went into his wife’s PayPal account? No? Clearly you don’t understand the criminal mind. His stepson’s fiancée got millions, and his wife got a hundred. Now there are some priorities.
Step 1: Sit at desk. Step 2: Get in shape
Being a physician is not really a desk job, but doctors must spend a fair share of their time sitting, yes? Dealing with recalcitrant EHRs or talking on the phone to insurers or PBMs? If you are one of these physicians, or if you have patients who spend a lot of time sitting at their desks and might need to get a bit of exercise, then we’ve got a multitasking tip for you.
It came to us via one of our favorite websites, Sad and Useless. It’s the site that declares itself “the most depressive humor site on the Internet” and they’re offering up the “12 Best Exercises To Do At Your Desk.” It may not sound like much, but we think that the gang at Dunder-Mifflin would approve. And besides, who couldn’t stand to burn a few calories without having to leave the chair?
We won’t spoil your fun by going through all 12 – each one comes with step-by-step instructions and a helpful illustration or GIF – but here are just a few:
- Bending over backwards: “Agree to do something you don’t want to do. Spend twice as long as expected doing that thing. Hate yourself.”
- Fake laughter: “Hear a joke that isn’t even remotely funny. Open your mouth and make laughing sounds.”
- Bang your head: Feel the “pointlessness of your job overwhelm you” and then “bring your head forcefully down to your desk.”
Now, we here at LOTME are, of course [Bang!], highly skilled, professional wordsmithing humorists [Bang!], so when we tell you that this is a great workout [Bang!] … that this is a great workout [Bang!] … it’s great … uggh.
Wooooo. Feel the burn.
One order of mosquitoes, extra Crispr
What would it be like to have a barbecue in your backyard on a humid summer night and not get eaten alive by mosquitoes? If you’re like us, you probably thought you’d never see that day.
Mosquitoes cause itchy bites, but, more importantly, they can carry dengue, malaria, yellow fever, and Zika virus. New research shows that protection from these diseases may be possible with use of the Crispr-Cas9 gene-editing tool, which could make humans invisible to mosquitoes by taking away their light-sensing abilities and, thus, their ability to find us.
“The better we understand how they sense the human, the better we can control the mosquito in an eco-friendly manner,” Yinpeng Zhan, a postdoctoral researcher at the University of California, Santa Barbara, and the study’s lead author, told the New York Times.
After studying the mosquitoes and figuring out their hunting patterns, the researchers found that mosquitoes are attracted to dark spots more than white spots and used this to their advantage. After knocking out two of the proteins that mosquitoes need for vision – via Crispr – the little suckers could not distinguish the difference between the white and dark spots.
We’re sure mosquitoes don’t mean any harm – they’re just trying to survive and reproduce like any other species – but thanks to this new tool, gone might be the days of having to douse yourself in bug spray that smells like a mix of chemicals and melon.
The hateful patient
A 64-year-old White woman with very few medical problems complains of bug bites. She had seen no bugs and had no visible bites. There is no rash. “So what bit me?” she asked, pulling her mask down for emphasis. How should I know? I thought, but didn’t say. She and I have been through this many times.
Before I could respond, she filled the pause with her usual complaints including how hard it is to get an appointment with me and how every appointment with me is a waste of her time. Ignoring the contradistinction of her charges, I took some satisfaction realizing she has just given me a topic to write about: The hateful patient.
They are frustrating, troublesome, rude, sometimes racist, misogynistic, depressing, hopeless, and disheartening. They call you, email you, and come to see you just to annoy you (so it seems). And they’re everywhere. According to one study, nearly one in six are “difficult patients.” It feels like more lately because the vaccine has brought haters back into clinic, just to get us.
But hateful patients aren’t new. In 1978, James E. Groves, MD, a Harvard psychiatrist, wrote a now-classic New England Journal of Medicine article about them called: Taking Care of the Hateful Patient. Even Osler, back in 1889, covered these patients in his lecture to University of Pennsylvania students, advising us to “deal gently with this deliciously credulous old human nature in which we work ... restrain your indignation.” But like much of Osler’s advice, it is easier said than done.
Dr. Groves is more helpful, and presents a model to understand them. Difficult patients, as we’d now call them, fall into four stereotypes: dependent clingers, entitled demanders, manipulative help-rejectors, and self-destructive deniers. It’s Dr. Groves’s bottom line I found insightful. He says that, when patients create negative feelings in us, we’re more likely to make errors. He then gives sound advice: Set firm boundaries and learn to counter the countertransference these patients provoke. Don’t disavow or discharge, Dr. Groves advises, redirect these emotions to motivate you to dig deeper. There you’ll find clinical data that will facilitate understanding and enable better patient management. Yes, easier said.
In addition to Dr. Groves’s analysis of how we harm these patients, I’d add that these disagreeable, malingering patients also harm us doctors. The hangover from a difficult patient encounter can linger for several appointments later or, worse, carryover to home. And now with patient emails proliferating, demanding patients behave as if we have an inexhaustible ability to engage them. We don’t. Many physicians are struggling to care at all; their low empathy battery warnings are blinking red, less than 1% remaining.
What is toxic to us doctors is the maelstrom of cognitive dissonance these patients create in us. Have you ever felt relief to learn a difficult patient has “finally” died? How could we think such a thing?! Didn’t we choose medicine instead of Wall Street because we care about people? But manipulative patients can make us care less. We even use secret language with each other to protect ourselves from them, those GOMERs (get out of my emergency room), bouncebacks, patients with status dramaticus, and those ornery FTDs (failure to die). Save yourself, we say to each other, this patient will kill you.
Caring for my somatizing 64-year-old patient has been difficult, but writing this has helped me reframe our interaction. Unsurprisingly, at the end of her failed visit she asked when she could see me again. “I need to schedule now because I have to find a neighbor to watch my dogs. It takes two buses to come here and I can’t take them with me.” Ah, there’s the clinical data Dr. Groves said I’d find – she’s not here to hurt me, she’s here because I’m all she’s got. At least for this difficult patient, I have a plan. At the bottom of my note I type “RTC 3 mo.”
Dr. Benabio is director of healthcare transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
A 64-year-old White woman with very few medical problems complains of bug bites. She had seen no bugs and had no visible bites. There is no rash. “So what bit me?” she asked, pulling her mask down for emphasis. How should I know? I thought, but didn’t say. She and I have been through this many times.
Before I could respond, she filled the pause with her usual complaints including how hard it is to get an appointment with me and how every appointment with me is a waste of her time. Ignoring the contradistinction of her charges, I took some satisfaction realizing she has just given me a topic to write about: The hateful patient.
They are frustrating, troublesome, rude, sometimes racist, misogynistic, depressing, hopeless, and disheartening. They call you, email you, and come to see you just to annoy you (so it seems). And they’re everywhere. According to one study, nearly one in six are “difficult patients.” It feels like more lately because the vaccine has brought haters back into clinic, just to get us.
But hateful patients aren’t new. In 1978, James E. Groves, MD, a Harvard psychiatrist, wrote a now-classic New England Journal of Medicine article about them called: Taking Care of the Hateful Patient. Even Osler, back in 1889, covered these patients in his lecture to University of Pennsylvania students, advising us to “deal gently with this deliciously credulous old human nature in which we work ... restrain your indignation.” But like much of Osler’s advice, it is easier said than done.
Dr. Groves is more helpful, and presents a model to understand them. Difficult patients, as we’d now call them, fall into four stereotypes: dependent clingers, entitled demanders, manipulative help-rejectors, and self-destructive deniers. It’s Dr. Groves’s bottom line I found insightful. He says that, when patients create negative feelings in us, we’re more likely to make errors. He then gives sound advice: Set firm boundaries and learn to counter the countertransference these patients provoke. Don’t disavow or discharge, Dr. Groves advises, redirect these emotions to motivate you to dig deeper. There you’ll find clinical data that will facilitate understanding and enable better patient management. Yes, easier said.
In addition to Dr. Groves’s analysis of how we harm these patients, I’d add that these disagreeable, malingering patients also harm us doctors. The hangover from a difficult patient encounter can linger for several appointments later or, worse, carryover to home. And now with patient emails proliferating, demanding patients behave as if we have an inexhaustible ability to engage them. We don’t. Many physicians are struggling to care at all; their low empathy battery warnings are blinking red, less than 1% remaining.
What is toxic to us doctors is the maelstrom of cognitive dissonance these patients create in us. Have you ever felt relief to learn a difficult patient has “finally” died? How could we think such a thing?! Didn’t we choose medicine instead of Wall Street because we care about people? But manipulative patients can make us care less. We even use secret language with each other to protect ourselves from them, those GOMERs (get out of my emergency room), bouncebacks, patients with status dramaticus, and those ornery FTDs (failure to die). Save yourself, we say to each other, this patient will kill you.
Caring for my somatizing 64-year-old patient has been difficult, but writing this has helped me reframe our interaction. Unsurprisingly, at the end of her failed visit she asked when she could see me again. “I need to schedule now because I have to find a neighbor to watch my dogs. It takes two buses to come here and I can’t take them with me.” Ah, there’s the clinical data Dr. Groves said I’d find – she’s not here to hurt me, she’s here because I’m all she’s got. At least for this difficult patient, I have a plan. At the bottom of my note I type “RTC 3 mo.”
Dr. Benabio is director of healthcare transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
A 64-year-old White woman with very few medical problems complains of bug bites. She had seen no bugs and had no visible bites. There is no rash. “So what bit me?” she asked, pulling her mask down for emphasis. How should I know? I thought, but didn’t say. She and I have been through this many times.
Before I could respond, she filled the pause with her usual complaints including how hard it is to get an appointment with me and how every appointment with me is a waste of her time. Ignoring the contradistinction of her charges, I took some satisfaction realizing she has just given me a topic to write about: The hateful patient.
They are frustrating, troublesome, rude, sometimes racist, misogynistic, depressing, hopeless, and disheartening. They call you, email you, and come to see you just to annoy you (so it seems). And they’re everywhere. According to one study, nearly one in six are “difficult patients.” It feels like more lately because the vaccine has brought haters back into clinic, just to get us.
But hateful patients aren’t new. In 1978, James E. Groves, MD, a Harvard psychiatrist, wrote a now-classic New England Journal of Medicine article about them called: Taking Care of the Hateful Patient. Even Osler, back in 1889, covered these patients in his lecture to University of Pennsylvania students, advising us to “deal gently with this deliciously credulous old human nature in which we work ... restrain your indignation.” But like much of Osler’s advice, it is easier said than done.
Dr. Groves is more helpful, and presents a model to understand them. Difficult patients, as we’d now call them, fall into four stereotypes: dependent clingers, entitled demanders, manipulative help-rejectors, and self-destructive deniers. It’s Dr. Groves’s bottom line I found insightful. He says that, when patients create negative feelings in us, we’re more likely to make errors. He then gives sound advice: Set firm boundaries and learn to counter the countertransference these patients provoke. Don’t disavow or discharge, Dr. Groves advises, redirect these emotions to motivate you to dig deeper. There you’ll find clinical data that will facilitate understanding and enable better patient management. Yes, easier said.
In addition to Dr. Groves’s analysis of how we harm these patients, I’d add that these disagreeable, malingering patients also harm us doctors. The hangover from a difficult patient encounter can linger for several appointments later or, worse, carryover to home. And now with patient emails proliferating, demanding patients behave as if we have an inexhaustible ability to engage them. We don’t. Many physicians are struggling to care at all; their low empathy battery warnings are blinking red, less than 1% remaining.
What is toxic to us doctors is the maelstrom of cognitive dissonance these patients create in us. Have you ever felt relief to learn a difficult patient has “finally” died? How could we think such a thing?! Didn’t we choose medicine instead of Wall Street because we care about people? But manipulative patients can make us care less. We even use secret language with each other to protect ourselves from them, those GOMERs (get out of my emergency room), bouncebacks, patients with status dramaticus, and those ornery FTDs (failure to die). Save yourself, we say to each other, this patient will kill you.
Caring for my somatizing 64-year-old patient has been difficult, but writing this has helped me reframe our interaction. Unsurprisingly, at the end of her failed visit she asked when she could see me again. “I need to schedule now because I have to find a neighbor to watch my dogs. It takes two buses to come here and I can’t take them with me.” Ah, there’s the clinical data Dr. Groves said I’d find – she’s not here to hurt me, she’s here because I’m all she’s got. At least for this difficult patient, I have a plan. At the bottom of my note I type “RTC 3 mo.”
Dr. Benabio is director of healthcare transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Microbiome: Gut dysbiosis linked to development of alopecia areata
presented at the annual meeting of the Society for Investigative Dermatology.
There have been reports of gut microbiome dysbiosis associated with autoimmune diseases such as rheumatoid arthritis, diabetes, and celiac disease. “It is now clear that these events not just shape the immune response in the gut, but also distant sites and immune-privileged organs,” Tanya Sezin, a doctor of natural science from the University of Lübeck (Germany) and Columbia University, New York, said in her presentation.
Whether the gut microbiome may also play a role as an environmental factor in alopecia areata, another T-cell–mediated autoimmune disease for which there are few available treatment options, is being evaluated at the Christiano Laboratory at Columbia University, Dr. Sezin noted. “Much of the difficulty underlying the lack of an effective treatment has been the incomplete understanding of the pathogenesis of AA.”
She also referred to several case reports describing hair growth in patients who received fecal microbiota transplantation (FMT), including a 20-year-old with alopecia universalis, who experienced hair growth after receiving FMT for Crohn’s disease.
Dr. Sezin and colleagues at the lab first performed a study in mice to test whether the gut microbiome was involved in the pathogenesis of AA. Mice given an antibiotic cocktail of ampicillin, neomycin, and vancomycin prior to or at the time of a skin graft taken from a mouse model of AA to induce AA were protected from hair loss, while mice given the antibiotic cocktail after skin grafting were not protected from hair loss.
“16S rRNA sequencing analysis of the gut microbiota revealed a significant shift in gut microbiome composition in animals treated with antibiotics and protected from hair loss, as reflected by significant changes in alpha and beta diversity,” Dr. Sezin explained. “In AA mice, we also observed differential abundance of families from the Bacteroidetes and Firmicutes phyla.” Specifically, Lactobacillus murinus and Muribaculum intestinale were overrepresented in mice with AA.
The investigators then performed 16S rRNA sequencing on 26 patients with AA, who stopped treatment for 30 days beforehand, and 9 participants who did not have AA as controls. “Though we did not observe difference in alpha and beta diversity, we see changes in the relative abundance of several families belonging to the Firmicutes phyla,” in patients with AA, Dr. Sezin said.
In another cohort of 30 patients with AA and 20 participants without AA, who stopped treatment before the study, Dr. Sezin and colleagues found “differences in the relative abundance of members of the Firmicutes and Bacteroides phyla,” including Bacteroides caccae, Prevotella copri, Syntrophomonas wolfei, Blautia wexlerae, and Eubacterium eligens, she said. “Consistent with our findings, there are previous reports in the literature showing gut dysbiosis in several other autoimmune diseases associated with differential regulation of some of the top species we have identified.”
Dr. Sezin said her group is recruiting patients for a clinical trial evaluating FMT in patients with AA. “We plan to study the association between changes in the gut microbiome and immune cell composition in AA patients undergoing FMT,” she said. “Additionally, functional studies in mice are also currently [being conducted] to further pinpoint the contribution of gut microbiome to the pathogenesis of AA.”
When asked during the discussion session if there was any relationship between the skin microbiome and AA, Dr. Sezin said there was no connection found in mice studies, which she and her colleagues are investigating further. “In the human samples, we are currently recruiting more patients and healthy controls to try to get a better understanding of whether we see differences in the skin microbiome,” she added.
Dr. Sezin explained that how the gut microbiota “is really remediated in alopecia areata” is not well understood. “We think that it is possible that we see intestinal permeability in the gut due to the gut dysbiosis that we see in alopecia areata patients, and this might lead to systemic distribution of bacteria, which might cross-react or present cross reactivity with the antigens” identified in AA, which is also being investigated, she said.
FMT not a ‘simple fix’ for AA
Leslie Castelo-Soccio, MD, PhD, a dermatologist at the Children’s Hospital of Philadelphia, who was not involved with the research, said in an interview that the findings presented by Dr. Sezin show how AA shares similarities with other autoimmune diseases. “It does highlight how important the gut microbiome is to human disease, and that differences in relative abundance of bacteria play one part as a trigger in a genetically susceptible person.”
However, while some autoimmune diseases have a big difference in alpha and beta diversity, for example, “this has not been seen in people with alopecia areata,” Dr. Castelo-Soccio pointed out. “The differences are more subtle in terms of amounts of certain bacteria,” she said, noting that, in this study, the biggest differences were seen in the studies of mice.
Dr. Castelo-Soccio also said there may be also be differences in the gut microbiome in children and adults. “The gut microbiome shifts in very early childhood from a very diverse microbiome to a more ‘adult microbiome’ around age 4, which is the age we see the first peak of many autoimmune diseases, including alopecia areata. I think microbiome work in humans needs to focus on this transition point.”
As for the clinical trial at Columbia that is evaluating FMT in patients with AA, Dr. Castelo-Soccio said she is excited. “There is much to learn about fecal transplant for all diseases and about the role of the gut microbiome and environment. Most of what we know for fecal transplant centers on its use for Clostridium difficile infections.”
Patients and their families have been asking about the potential for FMT in alopecia area, Dr. Castelo-Soccio said, but some believe it is a “simple fix” when the reality is much more complex.
“When I speak to patients and families about this, I explain that currently the ‘active ingredient’ in fecal transplants is not definitively established. In any one donor, the community of bacteria is highly variable and can be from batch to batch. While the short-term risks are relatively low – cramping, diarrhea, discomfort, mode of delivery – there are reports of transmission of infectious bacteria from donors like [Escherichia] coli, which have led to severe infections.”
Long-term safety and durability of effects are also unclear, “so we do not know if a patient receiving one [FMT] will need many in the future. We do not know how changing the microbiome could affect the transplant recipient in terms of noninfectious diseases/disorders. We are learning about the role of microbiome in obesity, insulin resistance, mood disorders. We could be ‘fixing’ one trigger of alopecia but setting up [the] patient for other noninfectious conditions,” Dr. Castelo-Soccio said.
Dr. Sezin and Dr. Castelo-Soccio reported no relevant financial disclosures.
presented at the annual meeting of the Society for Investigative Dermatology.
There have been reports of gut microbiome dysbiosis associated with autoimmune diseases such as rheumatoid arthritis, diabetes, and celiac disease. “It is now clear that these events not just shape the immune response in the gut, but also distant sites and immune-privileged organs,” Tanya Sezin, a doctor of natural science from the University of Lübeck (Germany) and Columbia University, New York, said in her presentation.
Whether the gut microbiome may also play a role as an environmental factor in alopecia areata, another T-cell–mediated autoimmune disease for which there are few available treatment options, is being evaluated at the Christiano Laboratory at Columbia University, Dr. Sezin noted. “Much of the difficulty underlying the lack of an effective treatment has been the incomplete understanding of the pathogenesis of AA.”
She also referred to several case reports describing hair growth in patients who received fecal microbiota transplantation (FMT), including a 20-year-old with alopecia universalis, who experienced hair growth after receiving FMT for Crohn’s disease.
Dr. Sezin and colleagues at the lab first performed a study in mice to test whether the gut microbiome was involved in the pathogenesis of AA. Mice given an antibiotic cocktail of ampicillin, neomycin, and vancomycin prior to or at the time of a skin graft taken from a mouse model of AA to induce AA were protected from hair loss, while mice given the antibiotic cocktail after skin grafting were not protected from hair loss.
“16S rRNA sequencing analysis of the gut microbiota revealed a significant shift in gut microbiome composition in animals treated with antibiotics and protected from hair loss, as reflected by significant changes in alpha and beta diversity,” Dr. Sezin explained. “In AA mice, we also observed differential abundance of families from the Bacteroidetes and Firmicutes phyla.” Specifically, Lactobacillus murinus and Muribaculum intestinale were overrepresented in mice with AA.
The investigators then performed 16S rRNA sequencing on 26 patients with AA, who stopped treatment for 30 days beforehand, and 9 participants who did not have AA as controls. “Though we did not observe difference in alpha and beta diversity, we see changes in the relative abundance of several families belonging to the Firmicutes phyla,” in patients with AA, Dr. Sezin said.
In another cohort of 30 patients with AA and 20 participants without AA, who stopped treatment before the study, Dr. Sezin and colleagues found “differences in the relative abundance of members of the Firmicutes and Bacteroides phyla,” including Bacteroides caccae, Prevotella copri, Syntrophomonas wolfei, Blautia wexlerae, and Eubacterium eligens, she said. “Consistent with our findings, there are previous reports in the literature showing gut dysbiosis in several other autoimmune diseases associated with differential regulation of some of the top species we have identified.”
Dr. Sezin said her group is recruiting patients for a clinical trial evaluating FMT in patients with AA. “We plan to study the association between changes in the gut microbiome and immune cell composition in AA patients undergoing FMT,” she said. “Additionally, functional studies in mice are also currently [being conducted] to further pinpoint the contribution of gut microbiome to the pathogenesis of AA.”
When asked during the discussion session if there was any relationship between the skin microbiome and AA, Dr. Sezin said there was no connection found in mice studies, which she and her colleagues are investigating further. “In the human samples, we are currently recruiting more patients and healthy controls to try to get a better understanding of whether we see differences in the skin microbiome,” she added.
Dr. Sezin explained that how the gut microbiota “is really remediated in alopecia areata” is not well understood. “We think that it is possible that we see intestinal permeability in the gut due to the gut dysbiosis that we see in alopecia areata patients, and this might lead to systemic distribution of bacteria, which might cross-react or present cross reactivity with the antigens” identified in AA, which is also being investigated, she said.
FMT not a ‘simple fix’ for AA
Leslie Castelo-Soccio, MD, PhD, a dermatologist at the Children’s Hospital of Philadelphia, who was not involved with the research, said in an interview that the findings presented by Dr. Sezin show how AA shares similarities with other autoimmune diseases. “It does highlight how important the gut microbiome is to human disease, and that differences in relative abundance of bacteria play one part as a trigger in a genetically susceptible person.”
However, while some autoimmune diseases have a big difference in alpha and beta diversity, for example, “this has not been seen in people with alopecia areata,” Dr. Castelo-Soccio pointed out. “The differences are more subtle in terms of amounts of certain bacteria,” she said, noting that, in this study, the biggest differences were seen in the studies of mice.
Dr. Castelo-Soccio also said there may be also be differences in the gut microbiome in children and adults. “The gut microbiome shifts in very early childhood from a very diverse microbiome to a more ‘adult microbiome’ around age 4, which is the age we see the first peak of many autoimmune diseases, including alopecia areata. I think microbiome work in humans needs to focus on this transition point.”
As for the clinical trial at Columbia that is evaluating FMT in patients with AA, Dr. Castelo-Soccio said she is excited. “There is much to learn about fecal transplant for all diseases and about the role of the gut microbiome and environment. Most of what we know for fecal transplant centers on its use for Clostridium difficile infections.”
Patients and their families have been asking about the potential for FMT in alopecia area, Dr. Castelo-Soccio said, but some believe it is a “simple fix” when the reality is much more complex.
“When I speak to patients and families about this, I explain that currently the ‘active ingredient’ in fecal transplants is not definitively established. In any one donor, the community of bacteria is highly variable and can be from batch to batch. While the short-term risks are relatively low – cramping, diarrhea, discomfort, mode of delivery – there are reports of transmission of infectious bacteria from donors like [Escherichia] coli, which have led to severe infections.”
Long-term safety and durability of effects are also unclear, “so we do not know if a patient receiving one [FMT] will need many in the future. We do not know how changing the microbiome could affect the transplant recipient in terms of noninfectious diseases/disorders. We are learning about the role of microbiome in obesity, insulin resistance, mood disorders. We could be ‘fixing’ one trigger of alopecia but setting up [the] patient for other noninfectious conditions,” Dr. Castelo-Soccio said.
Dr. Sezin and Dr. Castelo-Soccio reported no relevant financial disclosures.
presented at the annual meeting of the Society for Investigative Dermatology.
There have been reports of gut microbiome dysbiosis associated with autoimmune diseases such as rheumatoid arthritis, diabetes, and celiac disease. “It is now clear that these events not just shape the immune response in the gut, but also distant sites and immune-privileged organs,” Tanya Sezin, a doctor of natural science from the University of Lübeck (Germany) and Columbia University, New York, said in her presentation.
Whether the gut microbiome may also play a role as an environmental factor in alopecia areata, another T-cell–mediated autoimmune disease for which there are few available treatment options, is being evaluated at the Christiano Laboratory at Columbia University, Dr. Sezin noted. “Much of the difficulty underlying the lack of an effective treatment has been the incomplete understanding of the pathogenesis of AA.”
She also referred to several case reports describing hair growth in patients who received fecal microbiota transplantation (FMT), including a 20-year-old with alopecia universalis, who experienced hair growth after receiving FMT for Crohn’s disease.
Dr. Sezin and colleagues at the lab first performed a study in mice to test whether the gut microbiome was involved in the pathogenesis of AA. Mice given an antibiotic cocktail of ampicillin, neomycin, and vancomycin prior to or at the time of a skin graft taken from a mouse model of AA to induce AA were protected from hair loss, while mice given the antibiotic cocktail after skin grafting were not protected from hair loss.
“16S rRNA sequencing analysis of the gut microbiota revealed a significant shift in gut microbiome composition in animals treated with antibiotics and protected from hair loss, as reflected by significant changes in alpha and beta diversity,” Dr. Sezin explained. “In AA mice, we also observed differential abundance of families from the Bacteroidetes and Firmicutes phyla.” Specifically, Lactobacillus murinus and Muribaculum intestinale were overrepresented in mice with AA.
The investigators then performed 16S rRNA sequencing on 26 patients with AA, who stopped treatment for 30 days beforehand, and 9 participants who did not have AA as controls. “Though we did not observe difference in alpha and beta diversity, we see changes in the relative abundance of several families belonging to the Firmicutes phyla,” in patients with AA, Dr. Sezin said.
In another cohort of 30 patients with AA and 20 participants without AA, who stopped treatment before the study, Dr. Sezin and colleagues found “differences in the relative abundance of members of the Firmicutes and Bacteroides phyla,” including Bacteroides caccae, Prevotella copri, Syntrophomonas wolfei, Blautia wexlerae, and Eubacterium eligens, she said. “Consistent with our findings, there are previous reports in the literature showing gut dysbiosis in several other autoimmune diseases associated with differential regulation of some of the top species we have identified.”
Dr. Sezin said her group is recruiting patients for a clinical trial evaluating FMT in patients with AA. “We plan to study the association between changes in the gut microbiome and immune cell composition in AA patients undergoing FMT,” she said. “Additionally, functional studies in mice are also currently [being conducted] to further pinpoint the contribution of gut microbiome to the pathogenesis of AA.”
When asked during the discussion session if there was any relationship between the skin microbiome and AA, Dr. Sezin said there was no connection found in mice studies, which she and her colleagues are investigating further. “In the human samples, we are currently recruiting more patients and healthy controls to try to get a better understanding of whether we see differences in the skin microbiome,” she added.
Dr. Sezin explained that how the gut microbiota “is really remediated in alopecia areata” is not well understood. “We think that it is possible that we see intestinal permeability in the gut due to the gut dysbiosis that we see in alopecia areata patients, and this might lead to systemic distribution of bacteria, which might cross-react or present cross reactivity with the antigens” identified in AA, which is also being investigated, she said.
FMT not a ‘simple fix’ for AA
Leslie Castelo-Soccio, MD, PhD, a dermatologist at the Children’s Hospital of Philadelphia, who was not involved with the research, said in an interview that the findings presented by Dr. Sezin show how AA shares similarities with other autoimmune diseases. “It does highlight how important the gut microbiome is to human disease, and that differences in relative abundance of bacteria play one part as a trigger in a genetically susceptible person.”
However, while some autoimmune diseases have a big difference in alpha and beta diversity, for example, “this has not been seen in people with alopecia areata,” Dr. Castelo-Soccio pointed out. “The differences are more subtle in terms of amounts of certain bacteria,” she said, noting that, in this study, the biggest differences were seen in the studies of mice.
Dr. Castelo-Soccio also said there may be also be differences in the gut microbiome in children and adults. “The gut microbiome shifts in very early childhood from a very diverse microbiome to a more ‘adult microbiome’ around age 4, which is the age we see the first peak of many autoimmune diseases, including alopecia areata. I think microbiome work in humans needs to focus on this transition point.”
As for the clinical trial at Columbia that is evaluating FMT in patients with AA, Dr. Castelo-Soccio said she is excited. “There is much to learn about fecal transplant for all diseases and about the role of the gut microbiome and environment. Most of what we know for fecal transplant centers on its use for Clostridium difficile infections.”
Patients and their families have been asking about the potential for FMT in alopecia area, Dr. Castelo-Soccio said, but some believe it is a “simple fix” when the reality is much more complex.
“When I speak to patients and families about this, I explain that currently the ‘active ingredient’ in fecal transplants is not definitively established. In any one donor, the community of bacteria is highly variable and can be from batch to batch. While the short-term risks are relatively low – cramping, diarrhea, discomfort, mode of delivery – there are reports of transmission of infectious bacteria from donors like [Escherichia] coli, which have led to severe infections.”
Long-term safety and durability of effects are also unclear, “so we do not know if a patient receiving one [FMT] will need many in the future. We do not know how changing the microbiome could affect the transplant recipient in terms of noninfectious diseases/disorders. We are learning about the role of microbiome in obesity, insulin resistance, mood disorders. We could be ‘fixing’ one trigger of alopecia but setting up [the] patient for other noninfectious conditions,” Dr. Castelo-Soccio said.
Dr. Sezin and Dr. Castelo-Soccio reported no relevant financial disclosures.
FROM SID 2021
COVID-19 booster shots to start in September: Officials
at a press briefing August 18.
Those who received the Pfizer-BioNTech and Moderna vaccines would be eligible to get a booster shot 8 months after they received the second dose of those vaccines, officials said. Information on boosters for those who got the one-dose Johnson & Johnson vaccine will be forthcoming.
“We anticipate a booster will [also] likely be needed,” said U.S. Surgeon General Vivek Murthy, MD. The J&J vaccine was not available in the U.S. until March, he said, and ‘’we expect more data on J&J in the coming weeks, so that plan is coming.”
The plan for boosters for the two mRNA vaccines is pending the FDA’s conducting of an independent review and authorizing the third dose of the Moderna and Pfizer-BioNTech vaccines, as well as an advisory committee of the CDC making the recommendation.
“We know that even highly effective vaccines become less effective over time,” Dr. Murthy said. “Having reviewed the most current data, it is now our clinical judgment that the time to lay out a plan for the COVID-19 boosters is now.”
Research released Aug. 18 shows waning effectiveness of the two mRNA vaccines.
At the briefing, Dr. Murthy and others continually reassured listeners that while effectiveness against infection declines, the vaccines continue to protect against severe infections, hospitalizations, and death.
“If you are fully vaccinated, you still have a high degree of protection against the worst outcomes,” Dr. Murthy said.
Data driving the plan
CDC Director Rochelle Walensky, MD, cited three research studies published Aug. 18 in the CDC’s Morbidity and Mortality Weekly Report that helped to drive the decision to recommend boosters.
Analysis of nursing home COVID-19 data from the CDC’s National Healthcare Safety Network showed a significant decline in the effectiveness of the full mRNA vaccine against lab-confirmed COVID-19 infection, from 74.7% before the Delta variant (March 1-May 9, 2021) to 53% when the Delta variant became predominant in the United States. The analysis during the Delta dominant period included 85,000 weekly reports from nearly 15,000 facilities.
Another study looked at more than 10 million New York adults who had been fully vaccinated with either the Moderna, Pfizer, or J&J vaccine by July 25. During the period from May 3 to July 25, overall, the age-adjusted vaccine effectiveness against infection decreased from 91.7% to 79.8%.
Vaccine effectiveness against hospitalization remains high, another study found. An analysis of 1,129 patients who had gotten two doses of an mRNA vaccine showed vaccine effectiveness against hospitalization after 24 weeks. It was 86% at weeks 2-12 and 84% at weeks 13-24.
Immunologic facts
Immunologic information also points to the need for a booster, said Anthony Fauci, MD, the chief medical advisor to the president and director of the National Institute of Allergy and Infectious Diseases.
“Antibody levels decline over time,” he said, “and higher antibody levels are associated with higher efficacy of the vaccine. Higher levels of antibody may be needed to protect against Delta.”
A booster increased antibody levels by ‘’at least tenfold and possibly more,” he said. And higher levels of antibody may be required to protect against Delta. Taken together, he said, the data support the use of a booster to increase the overall level of protection.
Booster details
“We will make sure it is convenient and easy to get the booster shot,” said Jeff Zients, the White House COVID-19 response coordinator. As with the previous immunization, he said, the booster will be free, and no one will be asked about immigration status.
The plan for booster shots is an attempt to stay ahead of the virus, officials stressed
Big picture
Not everyone agrees with the booster dose idea. At a World Health Organization briefing Aug. 18, WHO’s Chief Scientist Soumya Swaminathan, MD, an Indian pediatrician, said that the right thing to do right now ‘’is to wait for the science to tell us when boosters, which groups of people, and which vaccines need boosters.”
Like others, she also broached the ‘’moral and ethical argument of giving people third doses, when they’re already well protected and while the rest of the world is waiting for their primary immunization.”
Dr. Swaminathan does see a role for boosters to protect immunocompromised people but noted that ‘’that’s a small number of people.” Widespread boosters ‘’will only lead to more variants, to more escape variants, and perhaps we’re heading into more dire situations.”
A version of this article first appeared on WebMD.com.
at a press briefing August 18.
Those who received the Pfizer-BioNTech and Moderna vaccines would be eligible to get a booster shot 8 months after they received the second dose of those vaccines, officials said. Information on boosters for those who got the one-dose Johnson & Johnson vaccine will be forthcoming.
“We anticipate a booster will [also] likely be needed,” said U.S. Surgeon General Vivek Murthy, MD. The J&J vaccine was not available in the U.S. until March, he said, and ‘’we expect more data on J&J in the coming weeks, so that plan is coming.”
The plan for boosters for the two mRNA vaccines is pending the FDA’s conducting of an independent review and authorizing the third dose of the Moderna and Pfizer-BioNTech vaccines, as well as an advisory committee of the CDC making the recommendation.
“We know that even highly effective vaccines become less effective over time,” Dr. Murthy said. “Having reviewed the most current data, it is now our clinical judgment that the time to lay out a plan for the COVID-19 boosters is now.”
Research released Aug. 18 shows waning effectiveness of the two mRNA vaccines.
At the briefing, Dr. Murthy and others continually reassured listeners that while effectiveness against infection declines, the vaccines continue to protect against severe infections, hospitalizations, and death.
“If you are fully vaccinated, you still have a high degree of protection against the worst outcomes,” Dr. Murthy said.
Data driving the plan
CDC Director Rochelle Walensky, MD, cited three research studies published Aug. 18 in the CDC’s Morbidity and Mortality Weekly Report that helped to drive the decision to recommend boosters.
Analysis of nursing home COVID-19 data from the CDC’s National Healthcare Safety Network showed a significant decline in the effectiveness of the full mRNA vaccine against lab-confirmed COVID-19 infection, from 74.7% before the Delta variant (March 1-May 9, 2021) to 53% when the Delta variant became predominant in the United States. The analysis during the Delta dominant period included 85,000 weekly reports from nearly 15,000 facilities.
Another study looked at more than 10 million New York adults who had been fully vaccinated with either the Moderna, Pfizer, or J&J vaccine by July 25. During the period from May 3 to July 25, overall, the age-adjusted vaccine effectiveness against infection decreased from 91.7% to 79.8%.
Vaccine effectiveness against hospitalization remains high, another study found. An analysis of 1,129 patients who had gotten two doses of an mRNA vaccine showed vaccine effectiveness against hospitalization after 24 weeks. It was 86% at weeks 2-12 and 84% at weeks 13-24.
Immunologic facts
Immunologic information also points to the need for a booster, said Anthony Fauci, MD, the chief medical advisor to the president and director of the National Institute of Allergy and Infectious Diseases.
“Antibody levels decline over time,” he said, “and higher antibody levels are associated with higher efficacy of the vaccine. Higher levels of antibody may be needed to protect against Delta.”
A booster increased antibody levels by ‘’at least tenfold and possibly more,” he said. And higher levels of antibody may be required to protect against Delta. Taken together, he said, the data support the use of a booster to increase the overall level of protection.
Booster details
“We will make sure it is convenient and easy to get the booster shot,” said Jeff Zients, the White House COVID-19 response coordinator. As with the previous immunization, he said, the booster will be free, and no one will be asked about immigration status.
The plan for booster shots is an attempt to stay ahead of the virus, officials stressed
Big picture
Not everyone agrees with the booster dose idea. At a World Health Organization briefing Aug. 18, WHO’s Chief Scientist Soumya Swaminathan, MD, an Indian pediatrician, said that the right thing to do right now ‘’is to wait for the science to tell us when boosters, which groups of people, and which vaccines need boosters.”
Like others, she also broached the ‘’moral and ethical argument of giving people third doses, when they’re already well protected and while the rest of the world is waiting for their primary immunization.”
Dr. Swaminathan does see a role for boosters to protect immunocompromised people but noted that ‘’that’s a small number of people.” Widespread boosters ‘’will only lead to more variants, to more escape variants, and perhaps we’re heading into more dire situations.”
A version of this article first appeared on WebMD.com.
at a press briefing August 18.
Those who received the Pfizer-BioNTech and Moderna vaccines would be eligible to get a booster shot 8 months after they received the second dose of those vaccines, officials said. Information on boosters for those who got the one-dose Johnson & Johnson vaccine will be forthcoming.
“We anticipate a booster will [also] likely be needed,” said U.S. Surgeon General Vivek Murthy, MD. The J&J vaccine was not available in the U.S. until March, he said, and ‘’we expect more data on J&J in the coming weeks, so that plan is coming.”
The plan for boosters for the two mRNA vaccines is pending the FDA’s conducting of an independent review and authorizing the third dose of the Moderna and Pfizer-BioNTech vaccines, as well as an advisory committee of the CDC making the recommendation.
“We know that even highly effective vaccines become less effective over time,” Dr. Murthy said. “Having reviewed the most current data, it is now our clinical judgment that the time to lay out a plan for the COVID-19 boosters is now.”
Research released Aug. 18 shows waning effectiveness of the two mRNA vaccines.
At the briefing, Dr. Murthy and others continually reassured listeners that while effectiveness against infection declines, the vaccines continue to protect against severe infections, hospitalizations, and death.
“If you are fully vaccinated, you still have a high degree of protection against the worst outcomes,” Dr. Murthy said.
Data driving the plan
CDC Director Rochelle Walensky, MD, cited three research studies published Aug. 18 in the CDC’s Morbidity and Mortality Weekly Report that helped to drive the decision to recommend boosters.
Analysis of nursing home COVID-19 data from the CDC’s National Healthcare Safety Network showed a significant decline in the effectiveness of the full mRNA vaccine against lab-confirmed COVID-19 infection, from 74.7% before the Delta variant (March 1-May 9, 2021) to 53% when the Delta variant became predominant in the United States. The analysis during the Delta dominant period included 85,000 weekly reports from nearly 15,000 facilities.
Another study looked at more than 10 million New York adults who had been fully vaccinated with either the Moderna, Pfizer, or J&J vaccine by July 25. During the period from May 3 to July 25, overall, the age-adjusted vaccine effectiveness against infection decreased from 91.7% to 79.8%.
Vaccine effectiveness against hospitalization remains high, another study found. An analysis of 1,129 patients who had gotten two doses of an mRNA vaccine showed vaccine effectiveness against hospitalization after 24 weeks. It was 86% at weeks 2-12 and 84% at weeks 13-24.
Immunologic facts
Immunologic information also points to the need for a booster, said Anthony Fauci, MD, the chief medical advisor to the president and director of the National Institute of Allergy and Infectious Diseases.
“Antibody levels decline over time,” he said, “and higher antibody levels are associated with higher efficacy of the vaccine. Higher levels of antibody may be needed to protect against Delta.”
A booster increased antibody levels by ‘’at least tenfold and possibly more,” he said. And higher levels of antibody may be required to protect against Delta. Taken together, he said, the data support the use of a booster to increase the overall level of protection.
Booster details
“We will make sure it is convenient and easy to get the booster shot,” said Jeff Zients, the White House COVID-19 response coordinator. As with the previous immunization, he said, the booster will be free, and no one will be asked about immigration status.
The plan for booster shots is an attempt to stay ahead of the virus, officials stressed
Big picture
Not everyone agrees with the booster dose idea. At a World Health Organization briefing Aug. 18, WHO’s Chief Scientist Soumya Swaminathan, MD, an Indian pediatrician, said that the right thing to do right now ‘’is to wait for the science to tell us when boosters, which groups of people, and which vaccines need boosters.”
Like others, she also broached the ‘’moral and ethical argument of giving people third doses, when they’re already well protected and while the rest of the world is waiting for their primary immunization.”
Dr. Swaminathan does see a role for boosters to protect immunocompromised people but noted that ‘’that’s a small number of people.” Widespread boosters ‘’will only lead to more variants, to more escape variants, and perhaps we’re heading into more dire situations.”
A version of this article first appeared on WebMD.com.
Latest data show increase in breakthrough COVID-19 cases
Breakthrough cases accounted for about one in five newly diagnosed cases in six of the states, according to the New York Times. Hospitalizations and deaths among vaccinated people may be higher than previously thought as well.
“Remember when the early vaccine studies came out, it was like nobody gets hospitalized, nobody dies,” Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, said in an interview. “That clearly is not true.”
The New York Times analyzed data in seven states – California, Colorado, Massachusetts, Oregon, Utah, Vermont, and Virginia – that are tracking the most detailed information. The trends in these states may not reflect the numbers throughout the country, the newspaper reported.
Even still, the numbers back up the idea that vaccinated people may need booster shots this fall to support their earlier vaccine doses. Federal health officials are scheduled to approve the extra shots in coming weeks, potentially in September. The first people to receive booster shots will likely be health care workers and nursing home residents who took the first vaccines in December and January.
“If the chances of a breakthrough infection have gone up considerably, and I think the evidence is clear that they have, and the level of protection against severe illness is no longer as robust as it was, I think the case for boosters goes up pretty quickly,” Dr. Wachter said.
Previous analyses of breakthrough cases included data from June and earlier, the newspaper reported. But since July, COVID-19 cases have soared again because of the Delta variant, and the most recent numbers show an uptick among vaccinated people. In Los Angeles County, for instance, fully vaccinated people account for 20% of new COVID-19 cases, which is up from 11% in May, 5% in April, and 2% in March, according to a late July report from the Los Angeles County Department of Public Health.
What’s more, breakthrough infections in the seven states accounted for 12%-24% of COVID-19 hospitalizations in those states. About 8,000 breakthrough hospitalizations have been reported to the CDC. Still, the overall numbers remain low – in California, for instance, about 1,615 people have been hospitalized with breakthrough infections, which accounts for 0.007% of the state’s 22 million vaccinated people, the Times reported.
The breakthrough infections appear to be more severe among vaccinated people who are older or have weakened immune systems. About 74% of breakthrough cases are among adults 65 or older, the CDC reported.
The increase may shift how vaccinated people see their risks for infection and interact with loved ones. Public health officials have suggested that people follow some COVID-19 safety protocols again, such as wearing masks in public indoor spaces regardless of vaccination status.
As the Delta variant continues to circulate this fall, public health researchers will be researching more about breakthrough cases among vaccinated people, including whether they have prolonged symptoms and how easily they may pass the virus to others.
“I think some of us have been challenged by the numbers of clusters that we’ve seen,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told this news organization.
“I think that really needs to be examined more,” he said.
A version of this article first appeared on WebMD.com.
Breakthrough cases accounted for about one in five newly diagnosed cases in six of the states, according to the New York Times. Hospitalizations and deaths among vaccinated people may be higher than previously thought as well.
“Remember when the early vaccine studies came out, it was like nobody gets hospitalized, nobody dies,” Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, said in an interview. “That clearly is not true.”
The New York Times analyzed data in seven states – California, Colorado, Massachusetts, Oregon, Utah, Vermont, and Virginia – that are tracking the most detailed information. The trends in these states may not reflect the numbers throughout the country, the newspaper reported.
Even still, the numbers back up the idea that vaccinated people may need booster shots this fall to support their earlier vaccine doses. Federal health officials are scheduled to approve the extra shots in coming weeks, potentially in September. The first people to receive booster shots will likely be health care workers and nursing home residents who took the first vaccines in December and January.
“If the chances of a breakthrough infection have gone up considerably, and I think the evidence is clear that they have, and the level of protection against severe illness is no longer as robust as it was, I think the case for boosters goes up pretty quickly,” Dr. Wachter said.
Previous analyses of breakthrough cases included data from June and earlier, the newspaper reported. But since July, COVID-19 cases have soared again because of the Delta variant, and the most recent numbers show an uptick among vaccinated people. In Los Angeles County, for instance, fully vaccinated people account for 20% of new COVID-19 cases, which is up from 11% in May, 5% in April, and 2% in March, according to a late July report from the Los Angeles County Department of Public Health.
What’s more, breakthrough infections in the seven states accounted for 12%-24% of COVID-19 hospitalizations in those states. About 8,000 breakthrough hospitalizations have been reported to the CDC. Still, the overall numbers remain low – in California, for instance, about 1,615 people have been hospitalized with breakthrough infections, which accounts for 0.007% of the state’s 22 million vaccinated people, the Times reported.
The breakthrough infections appear to be more severe among vaccinated people who are older or have weakened immune systems. About 74% of breakthrough cases are among adults 65 or older, the CDC reported.
The increase may shift how vaccinated people see their risks for infection and interact with loved ones. Public health officials have suggested that people follow some COVID-19 safety protocols again, such as wearing masks in public indoor spaces regardless of vaccination status.
As the Delta variant continues to circulate this fall, public health researchers will be researching more about breakthrough cases among vaccinated people, including whether they have prolonged symptoms and how easily they may pass the virus to others.
“I think some of us have been challenged by the numbers of clusters that we’ve seen,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told this news organization.
“I think that really needs to be examined more,” he said.
A version of this article first appeared on WebMD.com.
Breakthrough cases accounted for about one in five newly diagnosed cases in six of the states, according to the New York Times. Hospitalizations and deaths among vaccinated people may be higher than previously thought as well.
“Remember when the early vaccine studies came out, it was like nobody gets hospitalized, nobody dies,” Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, said in an interview. “That clearly is not true.”
The New York Times analyzed data in seven states – California, Colorado, Massachusetts, Oregon, Utah, Vermont, and Virginia – that are tracking the most detailed information. The trends in these states may not reflect the numbers throughout the country, the newspaper reported.
Even still, the numbers back up the idea that vaccinated people may need booster shots this fall to support their earlier vaccine doses. Federal health officials are scheduled to approve the extra shots in coming weeks, potentially in September. The first people to receive booster shots will likely be health care workers and nursing home residents who took the first vaccines in December and January.
“If the chances of a breakthrough infection have gone up considerably, and I think the evidence is clear that they have, and the level of protection against severe illness is no longer as robust as it was, I think the case for boosters goes up pretty quickly,” Dr. Wachter said.
Previous analyses of breakthrough cases included data from June and earlier, the newspaper reported. But since July, COVID-19 cases have soared again because of the Delta variant, and the most recent numbers show an uptick among vaccinated people. In Los Angeles County, for instance, fully vaccinated people account for 20% of new COVID-19 cases, which is up from 11% in May, 5% in April, and 2% in March, according to a late July report from the Los Angeles County Department of Public Health.
What’s more, breakthrough infections in the seven states accounted for 12%-24% of COVID-19 hospitalizations in those states. About 8,000 breakthrough hospitalizations have been reported to the CDC. Still, the overall numbers remain low – in California, for instance, about 1,615 people have been hospitalized with breakthrough infections, which accounts for 0.007% of the state’s 22 million vaccinated people, the Times reported.
The breakthrough infections appear to be more severe among vaccinated people who are older or have weakened immune systems. About 74% of breakthrough cases are among adults 65 or older, the CDC reported.
The increase may shift how vaccinated people see their risks for infection and interact with loved ones. Public health officials have suggested that people follow some COVID-19 safety protocols again, such as wearing masks in public indoor spaces regardless of vaccination status.
As the Delta variant continues to circulate this fall, public health researchers will be researching more about breakthrough cases among vaccinated people, including whether they have prolonged symptoms and how easily they may pass the virus to others.
“I think some of us have been challenged by the numbers of clusters that we’ve seen,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told this news organization.
“I think that really needs to be examined more,” he said.
A version of this article first appeared on WebMD.com.
Exsanguinating the truth about dragon’s blood in cosmeceuticals
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
What’s under my toenail?
After the teledermatology consultation, an x-ray was recommended. The x-ray showed an elongated irregular radiopaque mass projecting from the anterior medial aspect of the midshaft of the distal phalanx of the great toe (Picture 3). With these findings, subungual exostosis was suspected, and she was referred to orthopedic surgery for excision of the lesion. Histopathology showed a stack of trabecular bone with a fibrocartilaginous cap, confirming the diagnosis of subungual exostosis.
Subungual exostosis is a benign osteocartilaginous tumor, first described by Dupuytren in 1874. These lesions are rare and are seen mainly in children and young adults. Females appear to be affected more often than males.1 In a systematic review by DaCambra and colleagues, 55% of the cases occur in patients aged younger than 18 years, and the hallux was the most commonly affected digit, though any finger or toe can be affected.2 There are reported case of congenital multiple exostosis delineated to translocation t(X;6)(q22;q13-14).3
The exact cause of these lesions is unknown, but there are multiple theories, which include a reactive process secondary to trauma, infection, or genetic causes. Pathologic examination of the lesions shows an osseous center covered by a fibrocartilaginous cap. There is proliferation of spindle cells that generate cartilage, which later forms trabecular bone.4
On physical examination, subungual exostosis appear like a firm, fixed nodule with a hyperkeratotic smooth surface at the distal end of the nail bed, that slowly grows and can distort and lift up the nail. Dermoscopy features of these lesions include vascular ectasia, hyperkeratosis, onycholysis, and ulceration.
The differential diagnosis of subungual growths includes osteochondromas, which can present in a similar way but are rarer. Pathologic examination is usually required to differentiate between both lesions.5 In exostoses, bone is formed directly from fibrous tissue, whereas in osteochondromas they derive from enchondral ossification.6 The cartilaginous cap of this lesion is what helps to differentiate it in histopathology. In subungual exostosis, the cap is composed of fibrocartilage, while in osteochondromas it is made of hyaline cartilage similar to what is seen in normal growing epiphysis.5 Subungual exostosis can be confused with pyogenic granulomas and verruca, and often are treated as such, which delays appropriate surgical management.
Firm, slow-growing tumors in the fingers or toes of children should raise suspicion for underlying bony lesions like subungual exostosis and osteochondromas. X-rays of the lesion should be performed in order to clarify the diagnosis. Referral to orthopedic surgery is needed for definitive surgical management.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Zhang W et al. JAAD Case Rep. 2020 Jun 1;6(8):725-6.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9.
3. Torlazzi C et al. Int J Cancer. 2006;118:1972-6.
4. Calonje E et al. McKee’s pathology of the skin: With clinical correlations. (4th ed.) Philadelphia: Elsevier/Saunders, 2012.
5. Lee SK et al. Foot Ankle Int. 2007 May;28(5):595-601.
6. Mavrogenis A et al. Orthopedics. 2008 Oct;31(10).
After the teledermatology consultation, an x-ray was recommended. The x-ray showed an elongated irregular radiopaque mass projecting from the anterior medial aspect of the midshaft of the distal phalanx of the great toe (Picture 3). With these findings, subungual exostosis was suspected, and she was referred to orthopedic surgery for excision of the lesion. Histopathology showed a stack of trabecular bone with a fibrocartilaginous cap, confirming the diagnosis of subungual exostosis.
Subungual exostosis is a benign osteocartilaginous tumor, first described by Dupuytren in 1874. These lesions are rare and are seen mainly in children and young adults. Females appear to be affected more often than males.1 In a systematic review by DaCambra and colleagues, 55% of the cases occur in patients aged younger than 18 years, and the hallux was the most commonly affected digit, though any finger or toe can be affected.2 There are reported case of congenital multiple exostosis delineated to translocation t(X;6)(q22;q13-14).3
The exact cause of these lesions is unknown, but there are multiple theories, which include a reactive process secondary to trauma, infection, or genetic causes. Pathologic examination of the lesions shows an osseous center covered by a fibrocartilaginous cap. There is proliferation of spindle cells that generate cartilage, which later forms trabecular bone.4
On physical examination, subungual exostosis appear like a firm, fixed nodule with a hyperkeratotic smooth surface at the distal end of the nail bed, that slowly grows and can distort and lift up the nail. Dermoscopy features of these lesions include vascular ectasia, hyperkeratosis, onycholysis, and ulceration.
The differential diagnosis of subungual growths includes osteochondromas, which can present in a similar way but are rarer. Pathologic examination is usually required to differentiate between both lesions.5 In exostoses, bone is formed directly from fibrous tissue, whereas in osteochondromas they derive from enchondral ossification.6 The cartilaginous cap of this lesion is what helps to differentiate it in histopathology. In subungual exostosis, the cap is composed of fibrocartilage, while in osteochondromas it is made of hyaline cartilage similar to what is seen in normal growing epiphysis.5 Subungual exostosis can be confused with pyogenic granulomas and verruca, and often are treated as such, which delays appropriate surgical management.
Firm, slow-growing tumors in the fingers or toes of children should raise suspicion for underlying bony lesions like subungual exostosis and osteochondromas. X-rays of the lesion should be performed in order to clarify the diagnosis. Referral to orthopedic surgery is needed for definitive surgical management.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Zhang W et al. JAAD Case Rep. 2020 Jun 1;6(8):725-6.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9.
3. Torlazzi C et al. Int J Cancer. 2006;118:1972-6.
4. Calonje E et al. McKee’s pathology of the skin: With clinical correlations. (4th ed.) Philadelphia: Elsevier/Saunders, 2012.
5. Lee SK et al. Foot Ankle Int. 2007 May;28(5):595-601.
6. Mavrogenis A et al. Orthopedics. 2008 Oct;31(10).
After the teledermatology consultation, an x-ray was recommended. The x-ray showed an elongated irregular radiopaque mass projecting from the anterior medial aspect of the midshaft of the distal phalanx of the great toe (Picture 3). With these findings, subungual exostosis was suspected, and she was referred to orthopedic surgery for excision of the lesion. Histopathology showed a stack of trabecular bone with a fibrocartilaginous cap, confirming the diagnosis of subungual exostosis.
Subungual exostosis is a benign osteocartilaginous tumor, first described by Dupuytren in 1874. These lesions are rare and are seen mainly in children and young adults. Females appear to be affected more often than males.1 In a systematic review by DaCambra and colleagues, 55% of the cases occur in patients aged younger than 18 years, and the hallux was the most commonly affected digit, though any finger or toe can be affected.2 There are reported case of congenital multiple exostosis delineated to translocation t(X;6)(q22;q13-14).3
The exact cause of these lesions is unknown, but there are multiple theories, which include a reactive process secondary to trauma, infection, or genetic causes. Pathologic examination of the lesions shows an osseous center covered by a fibrocartilaginous cap. There is proliferation of spindle cells that generate cartilage, which later forms trabecular bone.4
On physical examination, subungual exostosis appear like a firm, fixed nodule with a hyperkeratotic smooth surface at the distal end of the nail bed, that slowly grows and can distort and lift up the nail. Dermoscopy features of these lesions include vascular ectasia, hyperkeratosis, onycholysis, and ulceration.
The differential diagnosis of subungual growths includes osteochondromas, which can present in a similar way but are rarer. Pathologic examination is usually required to differentiate between both lesions.5 In exostoses, bone is formed directly from fibrous tissue, whereas in osteochondromas they derive from enchondral ossification.6 The cartilaginous cap of this lesion is what helps to differentiate it in histopathology. In subungual exostosis, the cap is composed of fibrocartilage, while in osteochondromas it is made of hyaline cartilage similar to what is seen in normal growing epiphysis.5 Subungual exostosis can be confused with pyogenic granulomas and verruca, and often are treated as such, which delays appropriate surgical management.
Firm, slow-growing tumors in the fingers or toes of children should raise suspicion for underlying bony lesions like subungual exostosis and osteochondromas. X-rays of the lesion should be performed in order to clarify the diagnosis. Referral to orthopedic surgery is needed for definitive surgical management.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Zhang W et al. JAAD Case Rep. 2020 Jun 1;6(8):725-6.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9.
3. Torlazzi C et al. Int J Cancer. 2006;118:1972-6.
4. Calonje E et al. McKee’s pathology of the skin: With clinical correlations. (4th ed.) Philadelphia: Elsevier/Saunders, 2012.
5. Lee SK et al. Foot Ankle Int. 2007 May;28(5):595-601.
6. Mavrogenis A et al. Orthopedics. 2008 Oct;31(10).
A 13-year-old female was seen by her pediatrician for a lesion that had been on her right toe for about 6 months. She is unaware of any trauma to the area. The lesion has been growing slowly and recently it started lifting up the nail, became tender, and was bleeding, which is the reason why she sought care.
At the pediatrician's office, he noted a pink crusted papule under the nail. The nail was lifting up and was tender to the touch. She is a healthy girl who is not taking any medications and has no allergies. There is no family history of similar lesions.
The pediatrician took a picture of the lesion and he send it to our pediatric teledermatology service for consultation.
Ulcerated and Verrucous Plaque on the Chest
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
A 36-year-old man presented to an emergency department in the southwestern United States with a cough, fatigue, and worsening back pain associated with night sweats of 1 month’s duration. He experienced a 9.07-kg weight loss, as well as development of a rough, nontender, nonpruritic rash along the left upper chest over the prior month. The patient was born in West Africa and reported that he had moved to the southwestern United States from the eastern United States approximately 6 years prior to presentation. Physical examination on admission revealed a 5×3-cm, purple-brown, verrucous plaque with a central pink cobblestone appearance and ulceration. Chest radiography was notable for perihilar adenopathy with no focal infiltrates or cavitary lesions. Computed tomography and magnetic resonance imaging of the chest were notable for miliary nodules throughout the lungs; extensive lytic spine lesions of cervical, thoracic, and lumbar vertebral bodies and left twelfth rib; and a left paraspinal thoracic epidural soft tissue phlegmon. Initial laboratory investigations revealed peripheral eosinophilia without absolute leukocytosis and a microcytic anemia.
Shedding the super-doctor myth requires an honest look at systemic racism
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Outstanding medical bills: Dealing with deadbeats
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].