User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Outstanding medical bills: Dealing with deadbeats
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
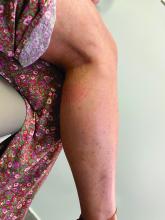
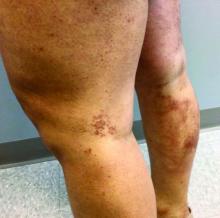
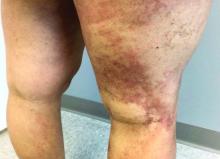
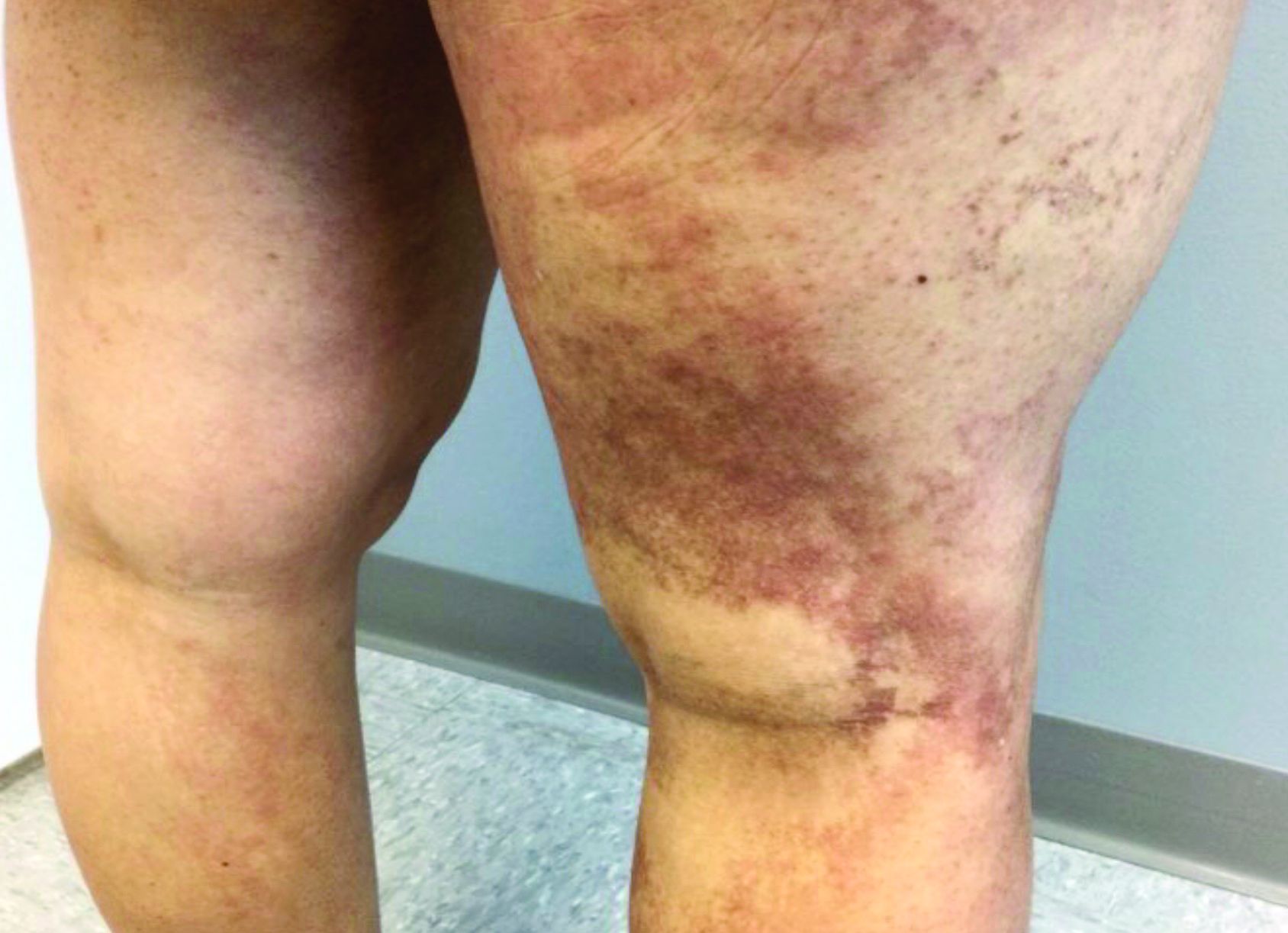
A 35-year-old with erythematous, dusky patches on both lower extremities
Zinc deficiency may be inherited or acquired. Acrodermatitis enteropathica is an autosomal recessive genetic disorder caused by a mutation in the gene that encodes a zinc transporter. It presents in infancy with the classic triad of diarrhea, dermatitis, and alopecia. Acquired zinc deficiency is due to causes such as alcoholism, malabsorption disorders like cystic fibrosis, inflammatory disease, gastrointestinal surgery, metabolic stress following general surgery, eating disorders, infections, malignancy, or occasionally in pregnancy. Classically, the face, groin, and extremities are affected (often acral), with erythematous, scaly patches. Pustules and bullae may be present. Angular cheilitis is often seen.
Necrolytic migratory erythema, or glucagonoma syndrome, is a very rare syndrome that presents as annular, erythematous patches with blisters that erode on the lower extremities and groin. The condition results from a cancerous tumor in the alpha cells of the pancreas called a glucagonoma, which secretes the hormone glucagon. It is often associated with diabetes and hyperglycemia.
Necrolytic acral erythema resembles acrodermatitis enteropathica and necrolytic migratory erythema clinically, however, it is associated with hepatitis C infection. Lesions are plaques with well defined borders distributed acrally. Treatment of the hepatitis C often improves the dermatitis.
Our patient’s blood work was consistent with nutritional deficiency and revealed low levels of zinc, vitamin A, ceruloplasmin, albumin and prealbumin, total protein, calcium, selenium, vitamin E, vitamin K, and vitamin C. Her hemoglobin A1C was under 4. Her hepatitis serologies were negative. The patient received total parenteral nutrition with subsequent complete resolution of her rash. Follow up for gastric bypass patients should be performed long term as they are at risk for nutritional deficiencies.
Dr. Bilu Martin, and Andrew Harris, DO, Mount Sinai Medical Center, Aventura, Fla., provided the case and photos.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Dermatol Online J. 2016 Nov 15; 22(11):13030.
Andrews’ Disease of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
Bolognia et al. Dermatology. St. Louis: Mosby/Elsevier, 2008.
Zinc deficiency may be inherited or acquired. Acrodermatitis enteropathica is an autosomal recessive genetic disorder caused by a mutation in the gene that encodes a zinc transporter. It presents in infancy with the classic triad of diarrhea, dermatitis, and alopecia. Acquired zinc deficiency is due to causes such as alcoholism, malabsorption disorders like cystic fibrosis, inflammatory disease, gastrointestinal surgery, metabolic stress following general surgery, eating disorders, infections, malignancy, or occasionally in pregnancy. Classically, the face, groin, and extremities are affected (often acral), with erythematous, scaly patches. Pustules and bullae may be present. Angular cheilitis is often seen.
Necrolytic migratory erythema, or glucagonoma syndrome, is a very rare syndrome that presents as annular, erythematous patches with blisters that erode on the lower extremities and groin. The condition results from a cancerous tumor in the alpha cells of the pancreas called a glucagonoma, which secretes the hormone glucagon. It is often associated with diabetes and hyperglycemia.
Necrolytic acral erythema resembles acrodermatitis enteropathica and necrolytic migratory erythema clinically, however, it is associated with hepatitis C infection. Lesions are plaques with well defined borders distributed acrally. Treatment of the hepatitis C often improves the dermatitis.
Our patient’s blood work was consistent with nutritional deficiency and revealed low levels of zinc, vitamin A, ceruloplasmin, albumin and prealbumin, total protein, calcium, selenium, vitamin E, vitamin K, and vitamin C. Her hemoglobin A1C was under 4. Her hepatitis serologies were negative. The patient received total parenteral nutrition with subsequent complete resolution of her rash. Follow up for gastric bypass patients should be performed long term as they are at risk for nutritional deficiencies.
Dr. Bilu Martin, and Andrew Harris, DO, Mount Sinai Medical Center, Aventura, Fla., provided the case and photos.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Dermatol Online J. 2016 Nov 15; 22(11):13030.
Andrews’ Disease of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
Bolognia et al. Dermatology. St. Louis: Mosby/Elsevier, 2008.
Zinc deficiency may be inherited or acquired. Acrodermatitis enteropathica is an autosomal recessive genetic disorder caused by a mutation in the gene that encodes a zinc transporter. It presents in infancy with the classic triad of diarrhea, dermatitis, and alopecia. Acquired zinc deficiency is due to causes such as alcoholism, malabsorption disorders like cystic fibrosis, inflammatory disease, gastrointestinal surgery, metabolic stress following general surgery, eating disorders, infections, malignancy, or occasionally in pregnancy. Classically, the face, groin, and extremities are affected (often acral), with erythematous, scaly patches. Pustules and bullae may be present. Angular cheilitis is often seen.
Necrolytic migratory erythema, or glucagonoma syndrome, is a very rare syndrome that presents as annular, erythematous patches with blisters that erode on the lower extremities and groin. The condition results from a cancerous tumor in the alpha cells of the pancreas called a glucagonoma, which secretes the hormone glucagon. It is often associated with diabetes and hyperglycemia.
Necrolytic acral erythema resembles acrodermatitis enteropathica and necrolytic migratory erythema clinically, however, it is associated with hepatitis C infection. Lesions are plaques with well defined borders distributed acrally. Treatment of the hepatitis C often improves the dermatitis.
Our patient’s blood work was consistent with nutritional deficiency and revealed low levels of zinc, vitamin A, ceruloplasmin, albumin and prealbumin, total protein, calcium, selenium, vitamin E, vitamin K, and vitamin C. Her hemoglobin A1C was under 4. Her hepatitis serologies were negative. The patient received total parenteral nutrition with subsequent complete resolution of her rash. Follow up for gastric bypass patients should be performed long term as they are at risk for nutritional deficiencies.
Dr. Bilu Martin, and Andrew Harris, DO, Mount Sinai Medical Center, Aventura, Fla., provided the case and photos.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Dermatol Online J. 2016 Nov 15; 22(11):13030.
Andrews’ Disease of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
Bolognia et al. Dermatology. St. Louis: Mosby/Elsevier, 2008.
Western diet promoted skin, joint inflammation in preclinical study
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
CDC officially endorses third dose of mRNA vaccines for immunocompromised
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Heparin’s COVID-19 benefit greatest in moderately ill patients
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Critically ill derive no benefit
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
FDA authorizes booster shot for immunocompromised Americans
The decision, which came late on Aug. 12, was not unexpected and a Centers for Disease Control and Prevention (CDC) panel meeting Aug. 13 is expected to approve directions to doctors and health care providers on who should receive the booster shot.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” acting FDA Commissioner Janet Woodcock, MD, said in a statement.
Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
Meanwhile, White House officials said Aug. 12 they “have supply and are prepared” to give all U.S. residents COVID-19 boosters -- which, as of now, are likely to be authorized first only for immunocompromised people.
“We believe sooner or later you will need a booster,” Anthony Fauci, MD, said at a news briefing Aug. 12. “Right now, we are evaluating this on a day-by-day, week-by-week, month-by-month basis.”
He added: “Right at this moment, apart from the immunocompromised -- elderly or not elderly -- people do not need a booster.” But, he said, “We’re preparing for the eventuality of doing that.”
White House COVID-19 Response Coordinator Jeff Zients said officials “have supply and are prepared” to at some point provide widespread access to boosters.
The immunocompromised population is very small -- less than 3% of adults, said CDC Director Rochelle Walensky, MD.
Meanwhile, COVID-19 rates continue to rise. Dr. Walensky reported that the 7-day average of daily cases is 132,384 -- an increase of 24% from the previous week. Average daily hospitalizations are up 31%, at 9,700, and deaths are up to 452 -- an increase of 22%.
In the past week, Florida has had more COVID-19 cases than the 30 states with the lowest case rates combined, Mr. Zients said. Florida and Texas alone have accounted for nearly 40% of new hospitalizations across the country.
A version of this article first appeared on WebMD.com.
The decision, which came late on Aug. 12, was not unexpected and a Centers for Disease Control and Prevention (CDC) panel meeting Aug. 13 is expected to approve directions to doctors and health care providers on who should receive the booster shot.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” acting FDA Commissioner Janet Woodcock, MD, said in a statement.
Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
Meanwhile, White House officials said Aug. 12 they “have supply and are prepared” to give all U.S. residents COVID-19 boosters -- which, as of now, are likely to be authorized first only for immunocompromised people.
“We believe sooner or later you will need a booster,” Anthony Fauci, MD, said at a news briefing Aug. 12. “Right now, we are evaluating this on a day-by-day, week-by-week, month-by-month basis.”
He added: “Right at this moment, apart from the immunocompromised -- elderly or not elderly -- people do not need a booster.” But, he said, “We’re preparing for the eventuality of doing that.”
White House COVID-19 Response Coordinator Jeff Zients said officials “have supply and are prepared” to at some point provide widespread access to boosters.
The immunocompromised population is very small -- less than 3% of adults, said CDC Director Rochelle Walensky, MD.
Meanwhile, COVID-19 rates continue to rise. Dr. Walensky reported that the 7-day average of daily cases is 132,384 -- an increase of 24% from the previous week. Average daily hospitalizations are up 31%, at 9,700, and deaths are up to 452 -- an increase of 22%.
In the past week, Florida has had more COVID-19 cases than the 30 states with the lowest case rates combined, Mr. Zients said. Florida and Texas alone have accounted for nearly 40% of new hospitalizations across the country.
A version of this article first appeared on WebMD.com.
The decision, which came late on Aug. 12, was not unexpected and a Centers for Disease Control and Prevention (CDC) panel meeting Aug. 13 is expected to approve directions to doctors and health care providers on who should receive the booster shot.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” acting FDA Commissioner Janet Woodcock, MD, said in a statement.
Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
Meanwhile, White House officials said Aug. 12 they “have supply and are prepared” to give all U.S. residents COVID-19 boosters -- which, as of now, are likely to be authorized first only for immunocompromised people.
“We believe sooner or later you will need a booster,” Anthony Fauci, MD, said at a news briefing Aug. 12. “Right now, we are evaluating this on a day-by-day, week-by-week, month-by-month basis.”
He added: “Right at this moment, apart from the immunocompromised -- elderly or not elderly -- people do not need a booster.” But, he said, “We’re preparing for the eventuality of doing that.”
White House COVID-19 Response Coordinator Jeff Zients said officials “have supply and are prepared” to at some point provide widespread access to boosters.
The immunocompromised population is very small -- less than 3% of adults, said CDC Director Rochelle Walensky, MD.
Meanwhile, COVID-19 rates continue to rise. Dr. Walensky reported that the 7-day average of daily cases is 132,384 -- an increase of 24% from the previous week. Average daily hospitalizations are up 31%, at 9,700, and deaths are up to 452 -- an increase of 22%.
In the past week, Florida has had more COVID-19 cases than the 30 states with the lowest case rates combined, Mr. Zients said. Florida and Texas alone have accounted for nearly 40% of new hospitalizations across the country.
A version of this article first appeared on WebMD.com.
Hep B vaccine response varied among youth with inflammatory, autoimmune disorders
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
FROM SPD 2021
CAR T-cell therapy drives refractory SLE into remission
Chimeric antigen receptor T-cell (CAR T) therapy, a life-extending treatment for patients with advanced B-cell malignancies and multiple myeloma, has now been shown to be effective for treating refractory systemic lupus erythematosus (SLE) in at least one patient.
A 20-year-old woman with severe, refractory SLE, active lupus nephritis, pericarditis, and other serious symptoms had both serologic and clinical remission follow the infusion of a CAR T cell product directed against the B-cell surface antigen CD19, reported Georg Schett, MD, and colleagues from the German Center for Immunotherapy at Friedrich Alexander University Erlangen-Nuremberg in Erlangen, Germany.
“Given the role of B cells in a variety of severe autoimmune diseases, CAR T-cell therapy that targets B-cell antigens may have wider application,” they wrote in a letter to the editor of The New England Journal of Medicine.
Dr. Schett said in an email response to an interview request that the patient has remained healthy and asymptomatic without further treatment after 6 months of follow-up.
“The key question will be whether B cells return and whether these B cells will carry on to make antibodies against double-stranded DNA,” he said. “We think that the loss of B cells could be sustained given that CAR T cells are still present in the patient. The main question will be how long CAR T cells will be there and how long they deplete the B cells.”
Not just for cancer anymore
CAR T therapy involves harvesting autologous T cells and transducing them with a lentiviral vector to recognize CD19 or other B-cell surface antigens. The transduced cells are then expanded and reinfused into the patient following a lymphodepletion regimen.
There are currently five CAR T constructs approved by the Food and Drug Administration for the treatment of diffuse large B-cell lymphoma and other B-lineage lymphomas, acute lymphoblastic leukemia, multiple myeloma, and other hematologic malignancies.
For this patient, however, Dr. Schett and colleagues created their own CAR T construct rather than adapting an off-the-shelf product.
The use of this groundbreaking therapy to treat an autoimmune condition is novel, the investigators noted: “This technological breakthrough, together with recent convincing data on the role of B cells in disease pathogenesis derived from preclinical lupus models, provides a rationale for the use of CAR T-cell therapies in patients with SLE,” they wrote.
One such preclinical study was reported in Science Translational Medicine in 2019 by Marko Z. Radic, PhD, of the University of Tennessee Health Science Center in Memphis, and colleagues.
Those investigators generated CD19-targeted CAR T constructs and demonstrated that in mouse models of lupus, CD8-positive T cells from two different lupus strains could be successfully transfected, and that transfer of the CD19-targeting CAR T cells ablated both autoantibodies and CD19-positive cells.
“In both models, survival was remarkably extended, and target organs were spared. These exciting results could pave the way for using CD19-targeted T cells to treat patients with lupus,” they wrote.
Now, that prediction has come to fruition.
“It’s brilliant that the first case report has now been accomplished. I am fully convinced that this method will rid therapy refractory patients of their symptoms,” Dr. Radic said in an interview.
Anti-CD20 failures
B-cell depletion with the anti-CD20 monoclonal antibody rituximab has been shown to be an effective therapeutic strategy for patients with rheumatoid arthritis and multiple sclerosis, but was ineffective in two separate clinical trials for SLE.
“Incomplete B-cell depletion of tissue-resident B cells, or the transient nature of the treatment, may have contributed to the failure of the initial rituximab trials to attain satisfactory outcomes,” Dr. Radic and coauthors wrote.
In patients with severe lupus, autoreactive B cells may lurk in lymphatic organs and/or inflamed tissues. Alternatively, CD20-negative plasma cells, which are unaffected by rituximab, could also be a source of SLE autoantibodies, Dr. Schett and coinvestigators said.
Case details
As noted before, the 20-year-old patient described by Dr. Schett and colleagues presented with World Health Organization class IIIA active lupus nephritis, indicating focal proliferative disease. In addition, she also had nephritic syndrome, pericarditis, pleurisy, rash, and arthritis, and had a history of Libman-Sacks endocarditis.
Her disease was refractory to treatment with all the usual suspects, including hydroxychloroquine, high-dose glucocorticoids, cyclophosphamide, mycophenolate mofetil, tacrolimus, rituximab, and belimumab, another B-cell targeted agent.
The T cell collection, transduction, expansion, and infusion were all successfully performed. By day 9 following infusion, CAR T cells comprised nearly one-third of her total circulating T cells, and then began to decrease, but remained detectable in circulation for the ensuing 7 weeks.
Levels of anti–double-stranded DNA decreased from above 5,000 U/mL to 4 U/mL within 5 weeks, and her complement levels (C3 and C4) normalized.
“These signs of serologic remission were paralleled by clinical remission with proteinuria decreasing from above 2,000 mg of protein per gram of creatinine to less than 250 mg of protein per gram of creatinine,” the investigators wrote.
The patient’s SLE Disease Activity Index score with SELENA (Safety of Estrogens in Lupus National Assessment) modification dropped from 16 at baseline to 0 at follow-up.
The patient did not experience any of the adverse events that are commonly seen in patients treated with CAR T therapy, such as the cytokine release syndrome, neurotoxic adverse events, or prolonged cytopenias.
Unanswered questions
Dr. Radic said that it was unclear from the brief case report whether Dr. Schett and colleagues considered including a “kill switch” in their CAR T construct, which could be activated in the case of serious toxicities.
In addition, their use of both CD4-positive T cells in addition to CD8-positive cells in their construct raises some concern, because in patients with SLE there is evidence that CD4-positive helper T cells can be autoreactive, he noted.
The work by Dr. Schett and colleagues was supported by grants from the German government, European Union, and the Innovative Medicines Initiative. Dr. Schett reported having no conflicts of interest to disclose. Dr. Radic is listed as inventor on a patent for anti-CD19 CAR T cells in lupus.
Chimeric antigen receptor T-cell (CAR T) therapy, a life-extending treatment for patients with advanced B-cell malignancies and multiple myeloma, has now been shown to be effective for treating refractory systemic lupus erythematosus (SLE) in at least one patient.
A 20-year-old woman with severe, refractory SLE, active lupus nephritis, pericarditis, and other serious symptoms had both serologic and clinical remission follow the infusion of a CAR T cell product directed against the B-cell surface antigen CD19, reported Georg Schett, MD, and colleagues from the German Center for Immunotherapy at Friedrich Alexander University Erlangen-Nuremberg in Erlangen, Germany.
“Given the role of B cells in a variety of severe autoimmune diseases, CAR T-cell therapy that targets B-cell antigens may have wider application,” they wrote in a letter to the editor of The New England Journal of Medicine.
Dr. Schett said in an email response to an interview request that the patient has remained healthy and asymptomatic without further treatment after 6 months of follow-up.
“The key question will be whether B cells return and whether these B cells will carry on to make antibodies against double-stranded DNA,” he said. “We think that the loss of B cells could be sustained given that CAR T cells are still present in the patient. The main question will be how long CAR T cells will be there and how long they deplete the B cells.”
Not just for cancer anymore
CAR T therapy involves harvesting autologous T cells and transducing them with a lentiviral vector to recognize CD19 or other B-cell surface antigens. The transduced cells are then expanded and reinfused into the patient following a lymphodepletion regimen.
There are currently five CAR T constructs approved by the Food and Drug Administration for the treatment of diffuse large B-cell lymphoma and other B-lineage lymphomas, acute lymphoblastic leukemia, multiple myeloma, and other hematologic malignancies.
For this patient, however, Dr. Schett and colleagues created their own CAR T construct rather than adapting an off-the-shelf product.
The use of this groundbreaking therapy to treat an autoimmune condition is novel, the investigators noted: “This technological breakthrough, together with recent convincing data on the role of B cells in disease pathogenesis derived from preclinical lupus models, provides a rationale for the use of CAR T-cell therapies in patients with SLE,” they wrote.
One such preclinical study was reported in Science Translational Medicine in 2019 by Marko Z. Radic, PhD, of the University of Tennessee Health Science Center in Memphis, and colleagues.
Those investigators generated CD19-targeted CAR T constructs and demonstrated that in mouse models of lupus, CD8-positive T cells from two different lupus strains could be successfully transfected, and that transfer of the CD19-targeting CAR T cells ablated both autoantibodies and CD19-positive cells.
“In both models, survival was remarkably extended, and target organs were spared. These exciting results could pave the way for using CD19-targeted T cells to treat patients with lupus,” they wrote.
Now, that prediction has come to fruition.
“It’s brilliant that the first case report has now been accomplished. I am fully convinced that this method will rid therapy refractory patients of their symptoms,” Dr. Radic said in an interview.
Anti-CD20 failures
B-cell depletion with the anti-CD20 monoclonal antibody rituximab has been shown to be an effective therapeutic strategy for patients with rheumatoid arthritis and multiple sclerosis, but was ineffective in two separate clinical trials for SLE.
“Incomplete B-cell depletion of tissue-resident B cells, or the transient nature of the treatment, may have contributed to the failure of the initial rituximab trials to attain satisfactory outcomes,” Dr. Radic and coauthors wrote.
In patients with severe lupus, autoreactive B cells may lurk in lymphatic organs and/or inflamed tissues. Alternatively, CD20-negative plasma cells, which are unaffected by rituximab, could also be a source of SLE autoantibodies, Dr. Schett and coinvestigators said.
Case details
As noted before, the 20-year-old patient described by Dr. Schett and colleagues presented with World Health Organization class IIIA active lupus nephritis, indicating focal proliferative disease. In addition, she also had nephritic syndrome, pericarditis, pleurisy, rash, and arthritis, and had a history of Libman-Sacks endocarditis.
Her disease was refractory to treatment with all the usual suspects, including hydroxychloroquine, high-dose glucocorticoids, cyclophosphamide, mycophenolate mofetil, tacrolimus, rituximab, and belimumab, another B-cell targeted agent.
The T cell collection, transduction, expansion, and infusion were all successfully performed. By day 9 following infusion, CAR T cells comprised nearly one-third of her total circulating T cells, and then began to decrease, but remained detectable in circulation for the ensuing 7 weeks.
Levels of anti–double-stranded DNA decreased from above 5,000 U/mL to 4 U/mL within 5 weeks, and her complement levels (C3 and C4) normalized.
“These signs of serologic remission were paralleled by clinical remission with proteinuria decreasing from above 2,000 mg of protein per gram of creatinine to less than 250 mg of protein per gram of creatinine,” the investigators wrote.
The patient’s SLE Disease Activity Index score with SELENA (Safety of Estrogens in Lupus National Assessment) modification dropped from 16 at baseline to 0 at follow-up.
The patient did not experience any of the adverse events that are commonly seen in patients treated with CAR T therapy, such as the cytokine release syndrome, neurotoxic adverse events, or prolonged cytopenias.
Unanswered questions
Dr. Radic said that it was unclear from the brief case report whether Dr. Schett and colleagues considered including a “kill switch” in their CAR T construct, which could be activated in the case of serious toxicities.
In addition, their use of both CD4-positive T cells in addition to CD8-positive cells in their construct raises some concern, because in patients with SLE there is evidence that CD4-positive helper T cells can be autoreactive, he noted.
The work by Dr. Schett and colleagues was supported by grants from the German government, European Union, and the Innovative Medicines Initiative. Dr. Schett reported having no conflicts of interest to disclose. Dr. Radic is listed as inventor on a patent for anti-CD19 CAR T cells in lupus.
Chimeric antigen receptor T-cell (CAR T) therapy, a life-extending treatment for patients with advanced B-cell malignancies and multiple myeloma, has now been shown to be effective for treating refractory systemic lupus erythematosus (SLE) in at least one patient.
A 20-year-old woman with severe, refractory SLE, active lupus nephritis, pericarditis, and other serious symptoms had both serologic and clinical remission follow the infusion of a CAR T cell product directed against the B-cell surface antigen CD19, reported Georg Schett, MD, and colleagues from the German Center for Immunotherapy at Friedrich Alexander University Erlangen-Nuremberg in Erlangen, Germany.
“Given the role of B cells in a variety of severe autoimmune diseases, CAR T-cell therapy that targets B-cell antigens may have wider application,” they wrote in a letter to the editor of The New England Journal of Medicine.
Dr. Schett said in an email response to an interview request that the patient has remained healthy and asymptomatic without further treatment after 6 months of follow-up.
“The key question will be whether B cells return and whether these B cells will carry on to make antibodies against double-stranded DNA,” he said. “We think that the loss of B cells could be sustained given that CAR T cells are still present in the patient. The main question will be how long CAR T cells will be there and how long they deplete the B cells.”
Not just for cancer anymore
CAR T therapy involves harvesting autologous T cells and transducing them with a lentiviral vector to recognize CD19 or other B-cell surface antigens. The transduced cells are then expanded and reinfused into the patient following a lymphodepletion regimen.
There are currently five CAR T constructs approved by the Food and Drug Administration for the treatment of diffuse large B-cell lymphoma and other B-lineage lymphomas, acute lymphoblastic leukemia, multiple myeloma, and other hematologic malignancies.
For this patient, however, Dr. Schett and colleagues created their own CAR T construct rather than adapting an off-the-shelf product.
The use of this groundbreaking therapy to treat an autoimmune condition is novel, the investigators noted: “This technological breakthrough, together with recent convincing data on the role of B cells in disease pathogenesis derived from preclinical lupus models, provides a rationale for the use of CAR T-cell therapies in patients with SLE,” they wrote.
One such preclinical study was reported in Science Translational Medicine in 2019 by Marko Z. Radic, PhD, of the University of Tennessee Health Science Center in Memphis, and colleagues.
Those investigators generated CD19-targeted CAR T constructs and demonstrated that in mouse models of lupus, CD8-positive T cells from two different lupus strains could be successfully transfected, and that transfer of the CD19-targeting CAR T cells ablated both autoantibodies and CD19-positive cells.
“In both models, survival was remarkably extended, and target organs were spared. These exciting results could pave the way for using CD19-targeted T cells to treat patients with lupus,” they wrote.
Now, that prediction has come to fruition.
“It’s brilliant that the first case report has now been accomplished. I am fully convinced that this method will rid therapy refractory patients of their symptoms,” Dr. Radic said in an interview.
Anti-CD20 failures
B-cell depletion with the anti-CD20 monoclonal antibody rituximab has been shown to be an effective therapeutic strategy for patients with rheumatoid arthritis and multiple sclerosis, but was ineffective in two separate clinical trials for SLE.
“Incomplete B-cell depletion of tissue-resident B cells, or the transient nature of the treatment, may have contributed to the failure of the initial rituximab trials to attain satisfactory outcomes,” Dr. Radic and coauthors wrote.
In patients with severe lupus, autoreactive B cells may lurk in lymphatic organs and/or inflamed tissues. Alternatively, CD20-negative plasma cells, which are unaffected by rituximab, could also be a source of SLE autoantibodies, Dr. Schett and coinvestigators said.
Case details
As noted before, the 20-year-old patient described by Dr. Schett and colleagues presented with World Health Organization class IIIA active lupus nephritis, indicating focal proliferative disease. In addition, she also had nephritic syndrome, pericarditis, pleurisy, rash, and arthritis, and had a history of Libman-Sacks endocarditis.
Her disease was refractory to treatment with all the usual suspects, including hydroxychloroquine, high-dose glucocorticoids, cyclophosphamide, mycophenolate mofetil, tacrolimus, rituximab, and belimumab, another B-cell targeted agent.
The T cell collection, transduction, expansion, and infusion were all successfully performed. By day 9 following infusion, CAR T cells comprised nearly one-third of her total circulating T cells, and then began to decrease, but remained detectable in circulation for the ensuing 7 weeks.
Levels of anti–double-stranded DNA decreased from above 5,000 U/mL to 4 U/mL within 5 weeks, and her complement levels (C3 and C4) normalized.
“These signs of serologic remission were paralleled by clinical remission with proteinuria decreasing from above 2,000 mg of protein per gram of creatinine to less than 250 mg of protein per gram of creatinine,” the investigators wrote.
The patient’s SLE Disease Activity Index score with SELENA (Safety of Estrogens in Lupus National Assessment) modification dropped from 16 at baseline to 0 at follow-up.
The patient did not experience any of the adverse events that are commonly seen in patients treated with CAR T therapy, such as the cytokine release syndrome, neurotoxic adverse events, or prolonged cytopenias.
Unanswered questions
Dr. Radic said that it was unclear from the brief case report whether Dr. Schett and colleagues considered including a “kill switch” in their CAR T construct, which could be activated in the case of serious toxicities.
In addition, their use of both CD4-positive T cells in addition to CD8-positive cells in their construct raises some concern, because in patients with SLE there is evidence that CD4-positive helper T cells can be autoreactive, he noted.
The work by Dr. Schett and colleagues was supported by grants from the German government, European Union, and the Innovative Medicines Initiative. Dr. Schett reported having no conflicts of interest to disclose. Dr. Radic is listed as inventor on a patent for anti-CD19 CAR T cells in lupus.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Age, distance from dermatology clinic <p>predict number of melanomas diagnosed
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
FROM JAMA DERMATOLOGY
Hospitals struggle to find nurses, beds, even oxygen as Delta surges
The state of Mississippi is out of intensive care unit beds. The University of Mississippi Medical Center in Jackson – the state’s largest health system – is converting part of a parking garage into a field hospital to make more room.
“Hospitals are full from Memphis to Gulfport, Natchez to Meridian. Everything’s full,” said Alan Jones, MD, the hospital’s COVID-19 response leader, in a press briefing Aug. 11.
The state has requested the help of a federal disaster medical assistance team of physicians, nurses, respiratory therapists, pharmacists, and paramedics to staff the extra beds. The goal is to open the field hospital on Aug. 13.
Arkansas hospitals have as little as eight ICU beds left to serve a population of 3 million people. Alabama isn’t far behind.
As of Aug. 10, several large metro Atlanta hospitals were diverting patients because they were full.
Hospitals in Alabama, Florida, Tennessee, and Texas are canceling elective surgeries, as they are flooded with COVID patients.
Florida has ordered more ventilators from the federal government. Some hospitals in that state have so many patients on high-flow medical oxygen that it is taxing the building supply lines.
“Most hospitals were not designed for this type of volume distribution in their facilities,” said Mary Mayhew, president of the Florida Hospital Association.
That’s when they can get it. Oxygen deliveries have been disrupted because of a shortage of drivers who are trained to transport it.
“Any disruption in the timing of a delivery can be hugely problematic because of the volume of oxygen they’re going through,” Ms. Mayhew said.
Hospitals ‘under great stress’
Over the month of June, the number of COVID patients in Florida hospitals soared from 2,000 to 10,000. Ms. Mayhew says it took twice as long during the last surge for the state to reach those numbers. And they’re still climbing. The state had 15,000 hospitalized COVID patients as of Aug. 11.
COVID hospitalizations tripled in 3 weeks in South Carolina, said state epidemiologist Linda Bell, MD, in a news conference Aug. 11.
“These hospitals are under great stress,” says Eric Toner, MD, a senior scientist at the Johns Hopkins Center for Health Security in Baltimore
The Delta variant has swept through the unvaccinated South with such veracity that hospitals in the region are unable to keep up. Patients with non-COVID health conditions are in jeopardy too.
Lee Owens, age 56, said he was supposed to have triple bypass surgery on Aug. 12 at St. Thomas West Hospital in Nashville, Tenn. Three of the arteries around his heart are 100%, 90%, and 70% blocked. Mr. Owens said the hospital called him Aug. 10 to postpone his surgery because they’ve cut back elective procedures to just one each day because the ICU beds there are full.
“I’m okay with having to wait a few days (my family isn’t!), especially if there are people worse than me, but so much anger at the reason,” he said. “These idiots that refused health care are now taking up my slot for heart surgery. It’s really aggravating.”
Anjali Bright, a spokesperson for St. Thomas West, provided a statement to this news organization saying they are not suspending elective procedures, but they are reviewing those “requiring an inpatient stay on a case-by-case basis.”
She emphasized, though, that “we will never delay care if the patient’s status changes to ‘urgent.’ ”
“Because of how infectious this variant is, this has the potential to be so much worse than what we saw in January,” said Donald Williamson, MD, president of the Alabama Hospital Association.
Dr. Williamson said they have modeled three possible scenarios for spread in the state, which ranks dead last in the United States for vaccination, with just 35% of its population fully protected. If the Delta variant spreads as it did in the United Kingdom, Alabama could see it hospitalize up to 3,000 people.
“That’s the best scenario,” he said.
If it sweeps through the state as it did in India, Alabama is looking at up to 4,500 patients hospitalized, a number that would require more beds and more staff to care for patients.
Then, there is what Dr. Williamson calls his “nightmare scenario.” If the entire state begins to see transmission rates as high as they’re currently seeing in coastal Mobile and Baldwin counties, that could mean up to 8,000 people in the hospital.
“If we see R-naughts of 5-8 statewide, we’re in real trouble,” he said. The R-naught is the basic rate of reproduction, and it means that each infected person would go on to infect 5-8 others. Dr. Williamson said the federal government would have to send them more staff to handle that kind of a surge.
‘Sense of betrayal’
Unlike the surges of last winter and spring, which sent hospitals scrambling for beds and supplies, the biggest pain point for hospitals now is staffing.
In Mississippi, where 200 patients are parked in emergency departments waiting for available and staffed ICU beds, the state is facing Delta with 2,000 fewer registered nurses than it had during its winter surge.
Some have left because of stress and burnout. Others have taken higher-paying jobs with travel nursing companies. To stop the exodus, hospitals are offering better pay, easier schedules, and sign-on and stay-on bonuses.
Doctors say the incentives are nice, but they don’t help with the anguish and anger many feel after months of battling COVID.
“There’s a big sense of betrayal,” said Sarah Nafziger, MD, vice president of clinical support services at the University of Alabama at Birmingham Hospital. “Our staff and health care workers, in general, feel like we’ve been betrayed by the community.”
“We have a vaccine, which is the key to ending this pandemic and people just refuse to take it, and so I think we’re very frustrated. We feel that our communities have let us down by not taking advantage of the vaccine,” Dr. Nafziger said. “It’s just baffling to me and it’s broken my heart every single day.”
Dr. Nafziger said she met with several surgeons at UAB on Aug. 11 and began making decisions about which surgeries would need to be canceled the following week. “We’re talking about cancer surgery. We’re talking about heart surgery. We’re talking about things that are critical to people.”
Compounding the staffing problems, about half of hospital workers in Alabama are still unvaccinated. Dr. Williamson says they’re now starting to see these unvaccinated health care workers come down with COVID too. He says that will exacerbate their surge even further as health care workers become too sick to help care for patients and some will end up needing hospital beds themselves.
At the University of Mississippi Medical Center, 70 hospital employees and another 20 clinic employees are now being quarantined or have COVID, Dr. Jones said.
“The situation is bleak for Mississippi hospitals,” said Timothy Moore, president and CEO of the Mississippi Hospital Association. He said he doesn’t expect it to get better anytime soon.
Mississippi has more patients hospitalized now than at any other point in the pandemic, said Thomas Dobbs, MD, MPH, the state epidemiologist.
“If we look at the rapidity of this rise, it’s really kind of terrifying and awe-inspiring,” Dr. Dobbs said in a news conference Aug. 11.
Schools are just starting back, and, in many parts of the South, districts are operating under a patchwork of policies – some require masks, while others have made them voluntary. Physicians say they are bracing for what these half measures could mean for pediatric cases and community transmission.
The only sure way for people to help themselves and their hospitals and schools, experts said, is vaccination.
“State data show that in this latest COVID surge, 97% of new COVID-19 infections, 89% of hospitalizations, and 82% of deaths occur in unvaccinated residents,” Mr. Moore said.
“To relieve pressure on hospitals, we need Mississippians – even those who have previously had COVID – to get vaccinated and wear a mask in public. The Delta variant is highly contagious and we need to do all we can to stop the spread,” he said.
A version of this article first appeared on Medscape.com.
The state of Mississippi is out of intensive care unit beds. The University of Mississippi Medical Center in Jackson – the state’s largest health system – is converting part of a parking garage into a field hospital to make more room.
“Hospitals are full from Memphis to Gulfport, Natchez to Meridian. Everything’s full,” said Alan Jones, MD, the hospital’s COVID-19 response leader, in a press briefing Aug. 11.
The state has requested the help of a federal disaster medical assistance team of physicians, nurses, respiratory therapists, pharmacists, and paramedics to staff the extra beds. The goal is to open the field hospital on Aug. 13.
Arkansas hospitals have as little as eight ICU beds left to serve a population of 3 million people. Alabama isn’t far behind.
As of Aug. 10, several large metro Atlanta hospitals were diverting patients because they were full.
Hospitals in Alabama, Florida, Tennessee, and Texas are canceling elective surgeries, as they are flooded with COVID patients.
Florida has ordered more ventilators from the federal government. Some hospitals in that state have so many patients on high-flow medical oxygen that it is taxing the building supply lines.
“Most hospitals were not designed for this type of volume distribution in their facilities,” said Mary Mayhew, president of the Florida Hospital Association.
That’s when they can get it. Oxygen deliveries have been disrupted because of a shortage of drivers who are trained to transport it.
“Any disruption in the timing of a delivery can be hugely problematic because of the volume of oxygen they’re going through,” Ms. Mayhew said.
Hospitals ‘under great stress’
Over the month of June, the number of COVID patients in Florida hospitals soared from 2,000 to 10,000. Ms. Mayhew says it took twice as long during the last surge for the state to reach those numbers. And they’re still climbing. The state had 15,000 hospitalized COVID patients as of Aug. 11.
COVID hospitalizations tripled in 3 weeks in South Carolina, said state epidemiologist Linda Bell, MD, in a news conference Aug. 11.
“These hospitals are under great stress,” says Eric Toner, MD, a senior scientist at the Johns Hopkins Center for Health Security in Baltimore
The Delta variant has swept through the unvaccinated South with such veracity that hospitals in the region are unable to keep up. Patients with non-COVID health conditions are in jeopardy too.
Lee Owens, age 56, said he was supposed to have triple bypass surgery on Aug. 12 at St. Thomas West Hospital in Nashville, Tenn. Three of the arteries around his heart are 100%, 90%, and 70% blocked. Mr. Owens said the hospital called him Aug. 10 to postpone his surgery because they’ve cut back elective procedures to just one each day because the ICU beds there are full.
“I’m okay with having to wait a few days (my family isn’t!), especially if there are people worse than me, but so much anger at the reason,” he said. “These idiots that refused health care are now taking up my slot for heart surgery. It’s really aggravating.”
Anjali Bright, a spokesperson for St. Thomas West, provided a statement to this news organization saying they are not suspending elective procedures, but they are reviewing those “requiring an inpatient stay on a case-by-case basis.”
She emphasized, though, that “we will never delay care if the patient’s status changes to ‘urgent.’ ”
“Because of how infectious this variant is, this has the potential to be so much worse than what we saw in January,” said Donald Williamson, MD, president of the Alabama Hospital Association.
Dr. Williamson said they have modeled three possible scenarios for spread in the state, which ranks dead last in the United States for vaccination, with just 35% of its population fully protected. If the Delta variant spreads as it did in the United Kingdom, Alabama could see it hospitalize up to 3,000 people.
“That’s the best scenario,” he said.
If it sweeps through the state as it did in India, Alabama is looking at up to 4,500 patients hospitalized, a number that would require more beds and more staff to care for patients.
Then, there is what Dr. Williamson calls his “nightmare scenario.” If the entire state begins to see transmission rates as high as they’re currently seeing in coastal Mobile and Baldwin counties, that could mean up to 8,000 people in the hospital.
“If we see R-naughts of 5-8 statewide, we’re in real trouble,” he said. The R-naught is the basic rate of reproduction, and it means that each infected person would go on to infect 5-8 others. Dr. Williamson said the federal government would have to send them more staff to handle that kind of a surge.
‘Sense of betrayal’
Unlike the surges of last winter and spring, which sent hospitals scrambling for beds and supplies, the biggest pain point for hospitals now is staffing.
In Mississippi, where 200 patients are parked in emergency departments waiting for available and staffed ICU beds, the state is facing Delta with 2,000 fewer registered nurses than it had during its winter surge.
Some have left because of stress and burnout. Others have taken higher-paying jobs with travel nursing companies. To stop the exodus, hospitals are offering better pay, easier schedules, and sign-on and stay-on bonuses.
Doctors say the incentives are nice, but they don’t help with the anguish and anger many feel after months of battling COVID.
“There’s a big sense of betrayal,” said Sarah Nafziger, MD, vice president of clinical support services at the University of Alabama at Birmingham Hospital. “Our staff and health care workers, in general, feel like we’ve been betrayed by the community.”
“We have a vaccine, which is the key to ending this pandemic and people just refuse to take it, and so I think we’re very frustrated. We feel that our communities have let us down by not taking advantage of the vaccine,” Dr. Nafziger said. “It’s just baffling to me and it’s broken my heart every single day.”
Dr. Nafziger said she met with several surgeons at UAB on Aug. 11 and began making decisions about which surgeries would need to be canceled the following week. “We’re talking about cancer surgery. We’re talking about heart surgery. We’re talking about things that are critical to people.”
Compounding the staffing problems, about half of hospital workers in Alabama are still unvaccinated. Dr. Williamson says they’re now starting to see these unvaccinated health care workers come down with COVID too. He says that will exacerbate their surge even further as health care workers become too sick to help care for patients and some will end up needing hospital beds themselves.
At the University of Mississippi Medical Center, 70 hospital employees and another 20 clinic employees are now being quarantined or have COVID, Dr. Jones said.
“The situation is bleak for Mississippi hospitals,” said Timothy Moore, president and CEO of the Mississippi Hospital Association. He said he doesn’t expect it to get better anytime soon.
Mississippi has more patients hospitalized now than at any other point in the pandemic, said Thomas Dobbs, MD, MPH, the state epidemiologist.
“If we look at the rapidity of this rise, it’s really kind of terrifying and awe-inspiring,” Dr. Dobbs said in a news conference Aug. 11.
Schools are just starting back, and, in many parts of the South, districts are operating under a patchwork of policies – some require masks, while others have made them voluntary. Physicians say they are bracing for what these half measures could mean for pediatric cases and community transmission.
The only sure way for people to help themselves and their hospitals and schools, experts said, is vaccination.
“State data show that in this latest COVID surge, 97% of new COVID-19 infections, 89% of hospitalizations, and 82% of deaths occur in unvaccinated residents,” Mr. Moore said.
“To relieve pressure on hospitals, we need Mississippians – even those who have previously had COVID – to get vaccinated and wear a mask in public. The Delta variant is highly contagious and we need to do all we can to stop the spread,” he said.
A version of this article first appeared on Medscape.com.
The state of Mississippi is out of intensive care unit beds. The University of Mississippi Medical Center in Jackson – the state’s largest health system – is converting part of a parking garage into a field hospital to make more room.
“Hospitals are full from Memphis to Gulfport, Natchez to Meridian. Everything’s full,” said Alan Jones, MD, the hospital’s COVID-19 response leader, in a press briefing Aug. 11.
The state has requested the help of a federal disaster medical assistance team of physicians, nurses, respiratory therapists, pharmacists, and paramedics to staff the extra beds. The goal is to open the field hospital on Aug. 13.
Arkansas hospitals have as little as eight ICU beds left to serve a population of 3 million people. Alabama isn’t far behind.
As of Aug. 10, several large metro Atlanta hospitals were diverting patients because they were full.
Hospitals in Alabama, Florida, Tennessee, and Texas are canceling elective surgeries, as they are flooded with COVID patients.
Florida has ordered more ventilators from the federal government. Some hospitals in that state have so many patients on high-flow medical oxygen that it is taxing the building supply lines.
“Most hospitals were not designed for this type of volume distribution in their facilities,” said Mary Mayhew, president of the Florida Hospital Association.
That’s when they can get it. Oxygen deliveries have been disrupted because of a shortage of drivers who are trained to transport it.
“Any disruption in the timing of a delivery can be hugely problematic because of the volume of oxygen they’re going through,” Ms. Mayhew said.
Hospitals ‘under great stress’
Over the month of June, the number of COVID patients in Florida hospitals soared from 2,000 to 10,000. Ms. Mayhew says it took twice as long during the last surge for the state to reach those numbers. And they’re still climbing. The state had 15,000 hospitalized COVID patients as of Aug. 11.
COVID hospitalizations tripled in 3 weeks in South Carolina, said state epidemiologist Linda Bell, MD, in a news conference Aug. 11.
“These hospitals are under great stress,” says Eric Toner, MD, a senior scientist at the Johns Hopkins Center for Health Security in Baltimore
The Delta variant has swept through the unvaccinated South with such veracity that hospitals in the region are unable to keep up. Patients with non-COVID health conditions are in jeopardy too.
Lee Owens, age 56, said he was supposed to have triple bypass surgery on Aug. 12 at St. Thomas West Hospital in Nashville, Tenn. Three of the arteries around his heart are 100%, 90%, and 70% blocked. Mr. Owens said the hospital called him Aug. 10 to postpone his surgery because they’ve cut back elective procedures to just one each day because the ICU beds there are full.
“I’m okay with having to wait a few days (my family isn’t!), especially if there are people worse than me, but so much anger at the reason,” he said. “These idiots that refused health care are now taking up my slot for heart surgery. It’s really aggravating.”
Anjali Bright, a spokesperson for St. Thomas West, provided a statement to this news organization saying they are not suspending elective procedures, but they are reviewing those “requiring an inpatient stay on a case-by-case basis.”
She emphasized, though, that “we will never delay care if the patient’s status changes to ‘urgent.’ ”
“Because of how infectious this variant is, this has the potential to be so much worse than what we saw in January,” said Donald Williamson, MD, president of the Alabama Hospital Association.
Dr. Williamson said they have modeled three possible scenarios for spread in the state, which ranks dead last in the United States for vaccination, with just 35% of its population fully protected. If the Delta variant spreads as it did in the United Kingdom, Alabama could see it hospitalize up to 3,000 people.
“That’s the best scenario,” he said.
If it sweeps through the state as it did in India, Alabama is looking at up to 4,500 patients hospitalized, a number that would require more beds and more staff to care for patients.
Then, there is what Dr. Williamson calls his “nightmare scenario.” If the entire state begins to see transmission rates as high as they’re currently seeing in coastal Mobile and Baldwin counties, that could mean up to 8,000 people in the hospital.
“If we see R-naughts of 5-8 statewide, we’re in real trouble,” he said. The R-naught is the basic rate of reproduction, and it means that each infected person would go on to infect 5-8 others. Dr. Williamson said the federal government would have to send them more staff to handle that kind of a surge.
‘Sense of betrayal’
Unlike the surges of last winter and spring, which sent hospitals scrambling for beds and supplies, the biggest pain point for hospitals now is staffing.
In Mississippi, where 200 patients are parked in emergency departments waiting for available and staffed ICU beds, the state is facing Delta with 2,000 fewer registered nurses than it had during its winter surge.
Some have left because of stress and burnout. Others have taken higher-paying jobs with travel nursing companies. To stop the exodus, hospitals are offering better pay, easier schedules, and sign-on and stay-on bonuses.
Doctors say the incentives are nice, but they don’t help with the anguish and anger many feel after months of battling COVID.
“There’s a big sense of betrayal,” said Sarah Nafziger, MD, vice president of clinical support services at the University of Alabama at Birmingham Hospital. “Our staff and health care workers, in general, feel like we’ve been betrayed by the community.”
“We have a vaccine, which is the key to ending this pandemic and people just refuse to take it, and so I think we’re very frustrated. We feel that our communities have let us down by not taking advantage of the vaccine,” Dr. Nafziger said. “It’s just baffling to me and it’s broken my heart every single day.”
Dr. Nafziger said she met with several surgeons at UAB on Aug. 11 and began making decisions about which surgeries would need to be canceled the following week. “We’re talking about cancer surgery. We’re talking about heart surgery. We’re talking about things that are critical to people.”
Compounding the staffing problems, about half of hospital workers in Alabama are still unvaccinated. Dr. Williamson says they’re now starting to see these unvaccinated health care workers come down with COVID too. He says that will exacerbate their surge even further as health care workers become too sick to help care for patients and some will end up needing hospital beds themselves.
At the University of Mississippi Medical Center, 70 hospital employees and another 20 clinic employees are now being quarantined or have COVID, Dr. Jones said.
“The situation is bleak for Mississippi hospitals,” said Timothy Moore, president and CEO of the Mississippi Hospital Association. He said he doesn’t expect it to get better anytime soon.
Mississippi has more patients hospitalized now than at any other point in the pandemic, said Thomas Dobbs, MD, MPH, the state epidemiologist.
“If we look at the rapidity of this rise, it’s really kind of terrifying and awe-inspiring,” Dr. Dobbs said in a news conference Aug. 11.
Schools are just starting back, and, in many parts of the South, districts are operating under a patchwork of policies – some require masks, while others have made them voluntary. Physicians say they are bracing for what these half measures could mean for pediatric cases and community transmission.
The only sure way for people to help themselves and their hospitals and schools, experts said, is vaccination.
“State data show that in this latest COVID surge, 97% of new COVID-19 infections, 89% of hospitalizations, and 82% of deaths occur in unvaccinated residents,” Mr. Moore said.
“To relieve pressure on hospitals, we need Mississippians – even those who have previously had COVID – to get vaccinated and wear a mask in public. The Delta variant is highly contagious and we need to do all we can to stop the spread,” he said.
A version of this article first appeared on Medscape.com.