User login
In Case You Missed It: COVID
White House to end COVID vaccine mandate for federal workers
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
Long-COVID rate may be higher with rheumatic diseases
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
AT BSR 2023
Researchers seek to understand post-COVID autoimmune disease risk
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-COVID patients respond differently to COVID vaccines
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
Skin Diseases Associated With COVID-19: A Narrative Review
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
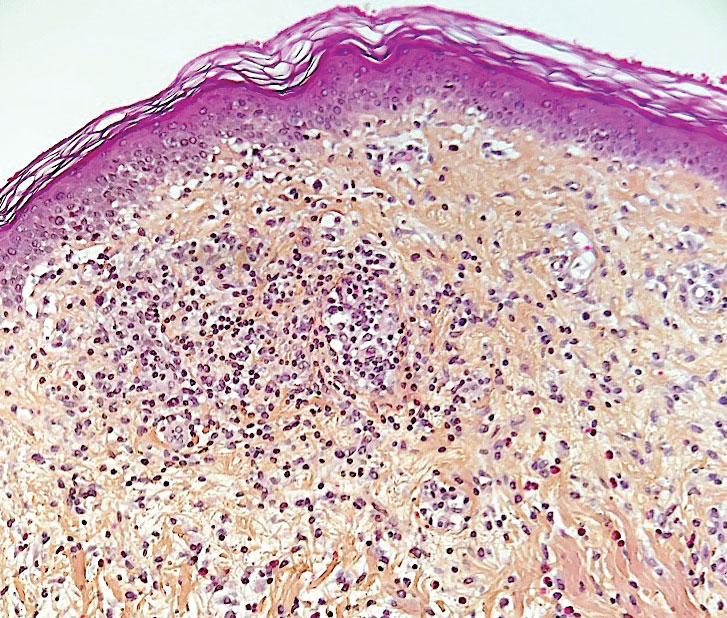
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.
Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
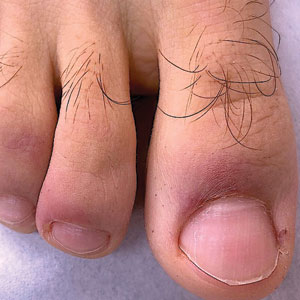
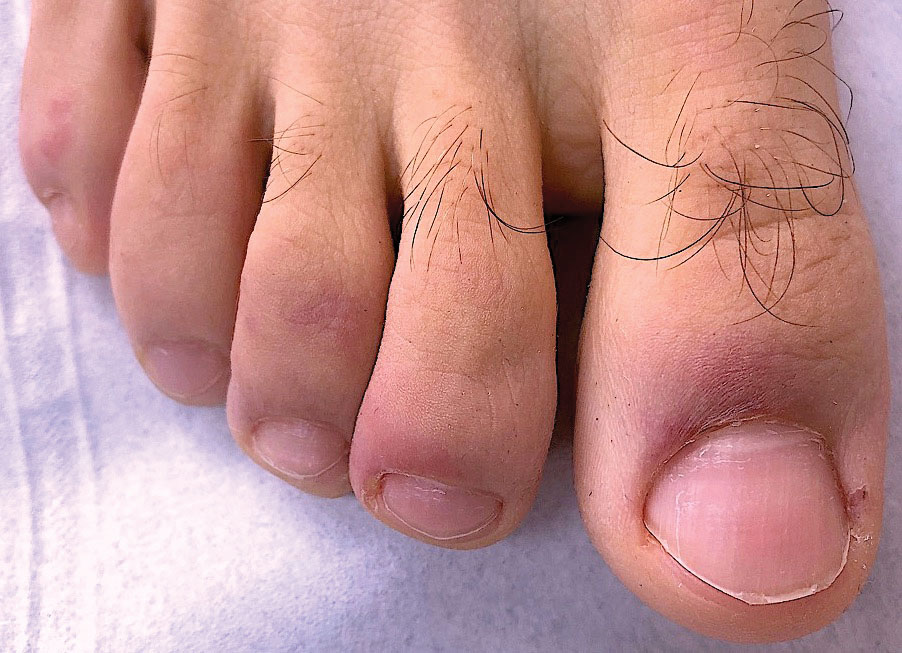
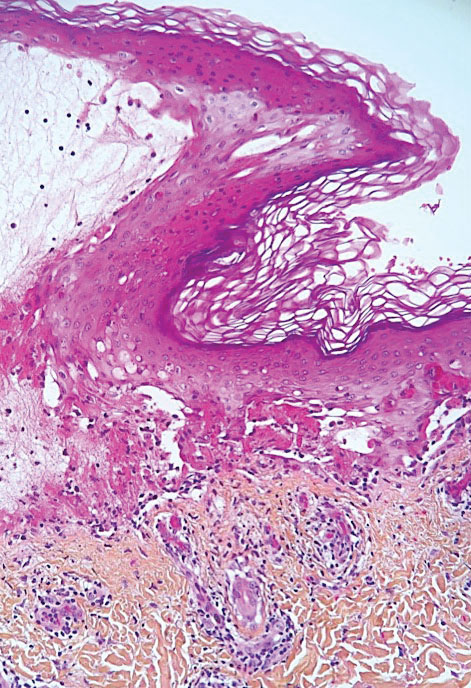
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30
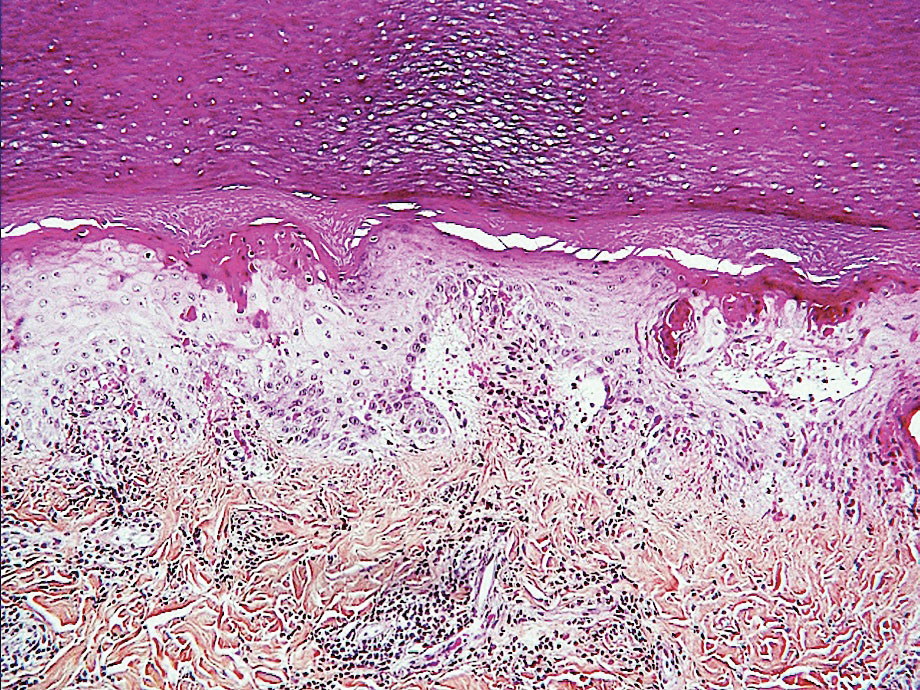
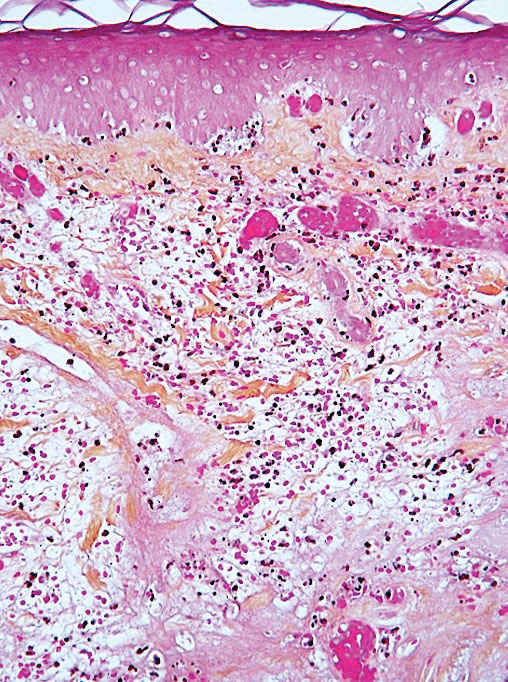
Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).
Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).
Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.
The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
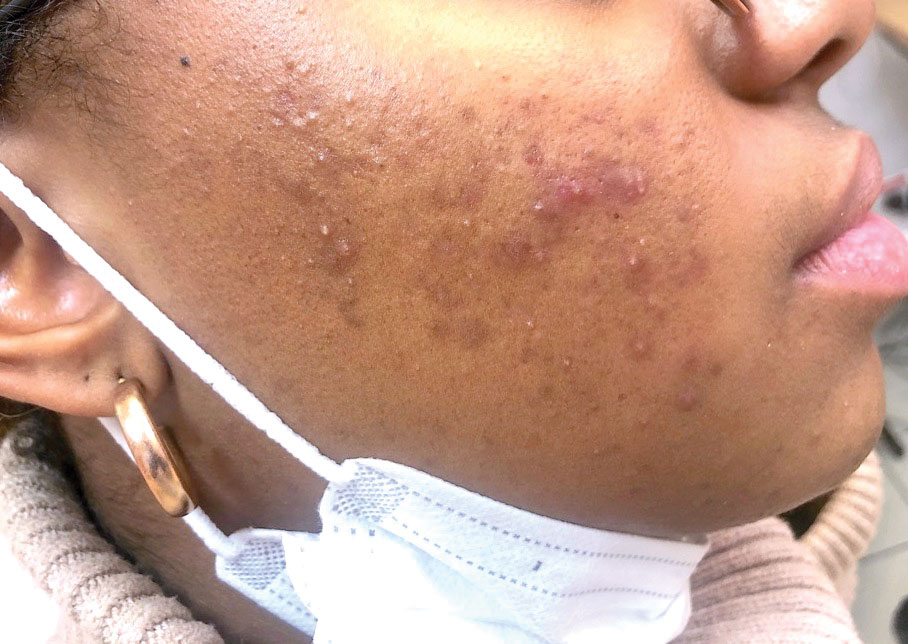
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54
DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.

Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30

Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).

Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).

Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.

The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54

DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.

Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30

Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).

Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).

Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.

The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54

DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
Practice Points
- During the COVID-19 pandemic, several skin diseases were reported in association with this new infectious disease and were classified mainly according to their morphologic aspect. However, the pathogenetic mechanisms often are unclear and the causal link of the culprit virus (SARS-CoV-2) not always well established.
- Currently, most skin manifestations related to COVID-19 are reported after vaccination against COVID-19; remarkably, many of them are similar to those attributed to the natural infection.
Diagnosis by dog: Canines detect COVID in schoolchildren with no symptoms
Scent-detecting dogs have long been used to sniff out medical conditions ranging from low blood sugar and cancer to malaria, impending seizures, and migraines – not to mention explosives and narcotics.
Recently, the sensitivity of the canine nose has been tested as a strategy for screening for SARS-CoV-2 infection in schoolchildren showing no outward symptoms of the virus. A pilot study led by Carol A. Glaser, DVM, MD, of the California Department of Public Health in Richmond, found that trained dogs had an accuracy of more than 95% for detecting the odor of volatile organic compounds, or VOCs, produced by COVID-infected individuals.
The authors believe that odor-based diagnosis with dogs could eventually provide a rapid, inexpensive, and noninvasive way to screen large groups for COVID-19 without the need for antigen testing.
“This is a new program with research ongoing, so it would be premature to consider it from a consumer’s perspective,” Dr. Glaser said in an interview. “However, the data look promising and we are hopeful we can continue to pilot various programs in various settings to see where, and if, dogs can be used for biomedical detection.”
In the lab and in the field
In a study published online in JAMA Pediatrics, Dr. Glaser’s group found that after 2 months’ training on COVID-19 scent samples in the laboratory, the dogs detected the presence of the virus more than 95% of the time. Antigen tests were used as a comparative reference.
In medical terms, the dogs achieved a greater than 95% accuracy on two important measures of effectiveness: sensitivity – a test’s ability to correctly detect the positive presence of disease – and specificity – the ability of a test to accurately rule out the presence of disease and identify as negative an uninfected person.
Next, the researchers piloted field tests in 50 visits at 27 schools from April 1 to May 25, 2022, to compare dogs’ detection ability with that of standard laboratory antigen testing. Participants in the completely voluntary screening numbered 1,558 and ranged in age from 9 to 17 years. Of these, 56% were girls and 89% were students. Almost 70% were screened at least twice.
Overall, the field test compared 3,897 paired antigen-vs.-dog screenings. The dogs accurately signaled the presence of 85 infections and ruled out 3,411 infections, for an overall accuracy of 90%. In 383 cases, however, they inaccurately signaled the presence of infection (false positives) and missed 18 actual infections (false negatives). That translated to a sensitivity in the field of 83%, considerably lower than that of their lab performance.
Direct screening of individuals with dogs outside of the lab involved circumstantial factors that likely contributed to decreased sensitivity and specificity, the authors acknowledged. These included such distractions as noise and the presence of excitable young children as well environmental conditions such as wind and other odors. What about dog phobia and dog hair allergy? “Dog screening takes only a few seconds per student and the dogs do not generally touch the participant as they run a line and sniff at ankles,” Dr. Glaser explained.
As for allergies, the rapid, ankle-level screening occurred in outdoor settings. “The chance of allergies is very low. This would be similar to someone who is out walking on the sidewalk and walks by a dog,” Dr. Glaser said.
Last year, a British trial of almost 4,000 adults tested six dogs trained to detect differences in VOCs between COVID-infected and uninfected individuals. Given samples from both groups, the dogs were able to distinguish between infected and uninfected samples with a sensitivity for detecting the virus ranging from 82% to 94% and a specificity for ruling it out of 76% to 92%. And they were able to smell the VOCs even when the viral load was low. The study also tested organic sensors, which proved even more accurate than the canines.
According to lead author James G. Logan, PhD, a disease control expert at the London School of Hygiene & Tropical Medicine in London, “Odour-based diagnostics using dogs and/or sensors may prove a rapid and effective tool for screening large numbers of people. Mathematical modelling suggests that dog screening plus a confirmatory PCR test could detect up to 89% of SARS-CoV-2 infections, averting up to 2.2 times as much transmission compared to isolation of symptomatic individuals only.”
Funding was provided by the Centers for Disease Control and Prevention Foundation (CDCF) to Early Alert Canines for the purchase and care of the dogs and the support of the handlers and trainers. The CDCF had no other role in the study. Coauthor Carol A. Edwards of Early Alert Canines reported receiving grants from the CDCF.
Scent-detecting dogs have long been used to sniff out medical conditions ranging from low blood sugar and cancer to malaria, impending seizures, and migraines – not to mention explosives and narcotics.
Recently, the sensitivity of the canine nose has been tested as a strategy for screening for SARS-CoV-2 infection in schoolchildren showing no outward symptoms of the virus. A pilot study led by Carol A. Glaser, DVM, MD, of the California Department of Public Health in Richmond, found that trained dogs had an accuracy of more than 95% for detecting the odor of volatile organic compounds, or VOCs, produced by COVID-infected individuals.
The authors believe that odor-based diagnosis with dogs could eventually provide a rapid, inexpensive, and noninvasive way to screen large groups for COVID-19 without the need for antigen testing.
“This is a new program with research ongoing, so it would be premature to consider it from a consumer’s perspective,” Dr. Glaser said in an interview. “However, the data look promising and we are hopeful we can continue to pilot various programs in various settings to see where, and if, dogs can be used for biomedical detection.”
In the lab and in the field
In a study published online in JAMA Pediatrics, Dr. Glaser’s group found that after 2 months’ training on COVID-19 scent samples in the laboratory, the dogs detected the presence of the virus more than 95% of the time. Antigen tests were used as a comparative reference.
In medical terms, the dogs achieved a greater than 95% accuracy on two important measures of effectiveness: sensitivity – a test’s ability to correctly detect the positive presence of disease – and specificity – the ability of a test to accurately rule out the presence of disease and identify as negative an uninfected person.
Next, the researchers piloted field tests in 50 visits at 27 schools from April 1 to May 25, 2022, to compare dogs’ detection ability with that of standard laboratory antigen testing. Participants in the completely voluntary screening numbered 1,558 and ranged in age from 9 to 17 years. Of these, 56% were girls and 89% were students. Almost 70% were screened at least twice.
Overall, the field test compared 3,897 paired antigen-vs.-dog screenings. The dogs accurately signaled the presence of 85 infections and ruled out 3,411 infections, for an overall accuracy of 90%. In 383 cases, however, they inaccurately signaled the presence of infection (false positives) and missed 18 actual infections (false negatives). That translated to a sensitivity in the field of 83%, considerably lower than that of their lab performance.
Direct screening of individuals with dogs outside of the lab involved circumstantial factors that likely contributed to decreased sensitivity and specificity, the authors acknowledged. These included such distractions as noise and the presence of excitable young children as well environmental conditions such as wind and other odors. What about dog phobia and dog hair allergy? “Dog screening takes only a few seconds per student and the dogs do not generally touch the participant as they run a line and sniff at ankles,” Dr. Glaser explained.
As for allergies, the rapid, ankle-level screening occurred in outdoor settings. “The chance of allergies is very low. This would be similar to someone who is out walking on the sidewalk and walks by a dog,” Dr. Glaser said.
Last year, a British trial of almost 4,000 adults tested six dogs trained to detect differences in VOCs between COVID-infected and uninfected individuals. Given samples from both groups, the dogs were able to distinguish between infected and uninfected samples with a sensitivity for detecting the virus ranging from 82% to 94% and a specificity for ruling it out of 76% to 92%. And they were able to smell the VOCs even when the viral load was low. The study also tested organic sensors, which proved even more accurate than the canines.
According to lead author James G. Logan, PhD, a disease control expert at the London School of Hygiene & Tropical Medicine in London, “Odour-based diagnostics using dogs and/or sensors may prove a rapid and effective tool for screening large numbers of people. Mathematical modelling suggests that dog screening plus a confirmatory PCR test could detect up to 89% of SARS-CoV-2 infections, averting up to 2.2 times as much transmission compared to isolation of symptomatic individuals only.”
Funding was provided by the Centers for Disease Control and Prevention Foundation (CDCF) to Early Alert Canines for the purchase and care of the dogs and the support of the handlers and trainers. The CDCF had no other role in the study. Coauthor Carol A. Edwards of Early Alert Canines reported receiving grants from the CDCF.
Scent-detecting dogs have long been used to sniff out medical conditions ranging from low blood sugar and cancer to malaria, impending seizures, and migraines – not to mention explosives and narcotics.
Recently, the sensitivity of the canine nose has been tested as a strategy for screening for SARS-CoV-2 infection in schoolchildren showing no outward symptoms of the virus. A pilot study led by Carol A. Glaser, DVM, MD, of the California Department of Public Health in Richmond, found that trained dogs had an accuracy of more than 95% for detecting the odor of volatile organic compounds, or VOCs, produced by COVID-infected individuals.
The authors believe that odor-based diagnosis with dogs could eventually provide a rapid, inexpensive, and noninvasive way to screen large groups for COVID-19 without the need for antigen testing.
“This is a new program with research ongoing, so it would be premature to consider it from a consumer’s perspective,” Dr. Glaser said in an interview. “However, the data look promising and we are hopeful we can continue to pilot various programs in various settings to see where, and if, dogs can be used for biomedical detection.”
In the lab and in the field
In a study published online in JAMA Pediatrics, Dr. Glaser’s group found that after 2 months’ training on COVID-19 scent samples in the laboratory, the dogs detected the presence of the virus more than 95% of the time. Antigen tests were used as a comparative reference.
In medical terms, the dogs achieved a greater than 95% accuracy on two important measures of effectiveness: sensitivity – a test’s ability to correctly detect the positive presence of disease – and specificity – the ability of a test to accurately rule out the presence of disease and identify as negative an uninfected person.
Next, the researchers piloted field tests in 50 visits at 27 schools from April 1 to May 25, 2022, to compare dogs’ detection ability with that of standard laboratory antigen testing. Participants in the completely voluntary screening numbered 1,558 and ranged in age from 9 to 17 years. Of these, 56% were girls and 89% were students. Almost 70% were screened at least twice.
Overall, the field test compared 3,897 paired antigen-vs.-dog screenings. The dogs accurately signaled the presence of 85 infections and ruled out 3,411 infections, for an overall accuracy of 90%. In 383 cases, however, they inaccurately signaled the presence of infection (false positives) and missed 18 actual infections (false negatives). That translated to a sensitivity in the field of 83%, considerably lower than that of their lab performance.
Direct screening of individuals with dogs outside of the lab involved circumstantial factors that likely contributed to decreased sensitivity and specificity, the authors acknowledged. These included such distractions as noise and the presence of excitable young children as well environmental conditions such as wind and other odors. What about dog phobia and dog hair allergy? “Dog screening takes only a few seconds per student and the dogs do not generally touch the participant as they run a line and sniff at ankles,” Dr. Glaser explained.
As for allergies, the rapid, ankle-level screening occurred in outdoor settings. “The chance of allergies is very low. This would be similar to someone who is out walking on the sidewalk and walks by a dog,” Dr. Glaser said.
Last year, a British trial of almost 4,000 adults tested six dogs trained to detect differences in VOCs between COVID-infected and uninfected individuals. Given samples from both groups, the dogs were able to distinguish between infected and uninfected samples with a sensitivity for detecting the virus ranging from 82% to 94% and a specificity for ruling it out of 76% to 92%. And they were able to smell the VOCs even when the viral load was low. The study also tested organic sensors, which proved even more accurate than the canines.
According to lead author James G. Logan, PhD, a disease control expert at the London School of Hygiene & Tropical Medicine in London, “Odour-based diagnostics using dogs and/or sensors may prove a rapid and effective tool for screening large numbers of people. Mathematical modelling suggests that dog screening plus a confirmatory PCR test could detect up to 89% of SARS-CoV-2 infections, averting up to 2.2 times as much transmission compared to isolation of symptomatic individuals only.”
Funding was provided by the Centers for Disease Control and Prevention Foundation (CDCF) to Early Alert Canines for the purchase and care of the dogs and the support of the handlers and trainers. The CDCF had no other role in the study. Coauthor Carol A. Edwards of Early Alert Canines reported receiving grants from the CDCF.
FROM JAMA PEDIATRICS
Explanation proposed for long-COVID symptoms in the CNS
BOSTON – , according to a collaborative study presented at the 2023 annual meeting of the American Academy of Neurology.
Already documented in several other viral infections, such as influenza and human immunodeficiency virus, antigenic imprinting results in production of antibodies to previously encountered viral infections rather than to the immediate threat, according to Marianna Spatola, MD, PhD, a research fellow at the Ragon Institute, Harvard University, Cambridge, Mass.
Original antigenic sin
In the case of persistent neurologic symptoms after COVID, a condition known as neuroPASC (neurological postacute sequelae of SARS-CoV2 infection), antibodies produced for previously encountered coronaviruses rather than for SARS-CoV2 might explain most or all cases, according to the data Dr. Spatola presented.
The evidence for this explanation was drawn from a study of 112 patients evaluated months after an acute episode of COVID-19. Of these, 18 patients had persistent neurologic dysfunction. When compared with the 94 whose infection resolved without sequelae, the patients with prolonged neurologic impairments had relatively low systemic antibody response to SARS-CoV2. However, they showed relatively high antibody responses against other coronaviruses.
This is a pattern consistent with antigenic imprinting, a concept first described more than 60 years ago as original antigenic sin. When the immune system becomes imprinted with an antigen from the first encountered virus from a family of pathogens, it governs all subsequent antibody responses, according to several published studies that have described and evaluated this concept.
Additional evidence
In Dr. Spatola’s study, other differences, particularly in regard to the cerebrospinal fluid (CSF), further supported the role of antigenic imprinting as a cause of neuroPASC. For one, those with elevated immune responses to other common coronaviruses rather than SARS-CoV2 in the CSF relative to the periphery were more likely to have a bad outcome in regard to neurologic symptoms.
Moreover, the CSF in neuroPASC patients “was characterized by increased IgG1 and absence of IgM, suggesting compartmentalized humoral responses within the CSF through selective transfer of antibodies from the serum to the CSF across the blood-brain barrier rather than through intrathecal synthesis,” Dr. Spatola reported.
In the case of COVID-19, the propensity for antigenic imprinting is not difficult to understand.
“The common cold coronaviruses are pretty similar to SARS-CoV2, but they are not exactly the same,” Dr. Spatola said. Her work and studies by others suggest that when antigenic imprinting occurs, “it prevents full maturation of the antibody response.”
NeuroPASC is one of many manifestations of long COVID, but Dr. Spatola pointed out that the immune response in the CSF is unique and the causes of prolonged neurologic impairment after COVID-19 are likely to involve different mechanisms than other long-COVID symptoms.
“Antibodies in the brain are functionally different,” said Dr. Spatola, noting for example that antibody-directed defenses against viral threats show a greater relative reliance on phagocytosis. This might become important in the development of therapeutics for neurologic symptoms of long COVID.
A different phenomenon
The manifestations of neuroPASC are heterogeneous and can include confusion, cognitive dysfunction, headache, encephalitis, and other impairments. Neurologic symptoms occur during acute SARS-CoV2 infections, but neuroPASC appears to be a different phenomenon. These symptoms, which develop after the initial respiratory disease has resolved, were attributed by Dr. Spatola to persistent inflammation that is not necessarily directly related to ongoing infection.
“The reason why some patients develop neuroPASC is unknown, but I think the evidence has pointed to a role for the immune system rather than the virus itself,” Dr. Spatola said.
Currently, neuroPASC is a clinical diagnosis but Dr. Spatola and her coinvestigators are conducting research to identify biomarkers. A viable diagnostic test is not expected imminently. They have identified 150 different features with potential relevance to neuroPASC.
In their comparison of those who did relative to those who did not develop neuroPASC, the initial studies were undertaken 2-4 months after the acute COVID-19 symptoms had resolved. The patients with neuroPASC and those without neurologic sequelae have now been followed for 6-8 months, which Dr. Spatola said was too short to draw firm conclusions about outcomes.
An evolving concept
Despite the small sample size of this study, these are “very interesting data” for considering the pathogenesis of neuroPASC, which is “a concept that is still evolving,” according to Natalia S. Rost, MD, chief of the stroke division, department of neurology, Massachusetts General Hospital, Boston.
Applied to SARS-CoV2, the concept of original antigenic sin “is new” but Dr. Rost said that it might help differentiate neuroPASC from acute neurologic symptoms of COVID-19, which include stroke. She indicated that the work performed by Dr. Spatola and others might eventually explain the pathology while leading to treatment strategies. She cautioned that the concepts explored in this study “need to be further developed” through larger sample sizes and the exploration of other variables that support the hypothesis.
Dr. Spatola and Dr. Rost report no potential conflicts of interest.
BOSTON – , according to a collaborative study presented at the 2023 annual meeting of the American Academy of Neurology.
Already documented in several other viral infections, such as influenza and human immunodeficiency virus, antigenic imprinting results in production of antibodies to previously encountered viral infections rather than to the immediate threat, according to Marianna Spatola, MD, PhD, a research fellow at the Ragon Institute, Harvard University, Cambridge, Mass.
Original antigenic sin
In the case of persistent neurologic symptoms after COVID, a condition known as neuroPASC (neurological postacute sequelae of SARS-CoV2 infection), antibodies produced for previously encountered coronaviruses rather than for SARS-CoV2 might explain most or all cases, according to the data Dr. Spatola presented.
The evidence for this explanation was drawn from a study of 112 patients evaluated months after an acute episode of COVID-19. Of these, 18 patients had persistent neurologic dysfunction. When compared with the 94 whose infection resolved without sequelae, the patients with prolonged neurologic impairments had relatively low systemic antibody response to SARS-CoV2. However, they showed relatively high antibody responses against other coronaviruses.
This is a pattern consistent with antigenic imprinting, a concept first described more than 60 years ago as original antigenic sin. When the immune system becomes imprinted with an antigen from the first encountered virus from a family of pathogens, it governs all subsequent antibody responses, according to several published studies that have described and evaluated this concept.
Additional evidence
In Dr. Spatola’s study, other differences, particularly in regard to the cerebrospinal fluid (CSF), further supported the role of antigenic imprinting as a cause of neuroPASC. For one, those with elevated immune responses to other common coronaviruses rather than SARS-CoV2 in the CSF relative to the periphery were more likely to have a bad outcome in regard to neurologic symptoms.
Moreover, the CSF in neuroPASC patients “was characterized by increased IgG1 and absence of IgM, suggesting compartmentalized humoral responses within the CSF through selective transfer of antibodies from the serum to the CSF across the blood-brain barrier rather than through intrathecal synthesis,” Dr. Spatola reported.
In the case of COVID-19, the propensity for antigenic imprinting is not difficult to understand.
“The common cold coronaviruses are pretty similar to SARS-CoV2, but they are not exactly the same,” Dr. Spatola said. Her work and studies by others suggest that when antigenic imprinting occurs, “it prevents full maturation of the antibody response.”
NeuroPASC is one of many manifestations of long COVID, but Dr. Spatola pointed out that the immune response in the CSF is unique and the causes of prolonged neurologic impairment after COVID-19 are likely to involve different mechanisms than other long-COVID symptoms.
“Antibodies in the brain are functionally different,” said Dr. Spatola, noting for example that antibody-directed defenses against viral threats show a greater relative reliance on phagocytosis. This might become important in the development of therapeutics for neurologic symptoms of long COVID.
A different phenomenon
The manifestations of neuroPASC are heterogeneous and can include confusion, cognitive dysfunction, headache, encephalitis, and other impairments. Neurologic symptoms occur during acute SARS-CoV2 infections, but neuroPASC appears to be a different phenomenon. These symptoms, which develop after the initial respiratory disease has resolved, were attributed by Dr. Spatola to persistent inflammation that is not necessarily directly related to ongoing infection.
“The reason why some patients develop neuroPASC is unknown, but I think the evidence has pointed to a role for the immune system rather than the virus itself,” Dr. Spatola said.
Currently, neuroPASC is a clinical diagnosis but Dr. Spatola and her coinvestigators are conducting research to identify biomarkers. A viable diagnostic test is not expected imminently. They have identified 150 different features with potential relevance to neuroPASC.
In their comparison of those who did relative to those who did not develop neuroPASC, the initial studies were undertaken 2-4 months after the acute COVID-19 symptoms had resolved. The patients with neuroPASC and those without neurologic sequelae have now been followed for 6-8 months, which Dr. Spatola said was too short to draw firm conclusions about outcomes.
An evolving concept
Despite the small sample size of this study, these are “very interesting data” for considering the pathogenesis of neuroPASC, which is “a concept that is still evolving,” according to Natalia S. Rost, MD, chief of the stroke division, department of neurology, Massachusetts General Hospital, Boston.
Applied to SARS-CoV2, the concept of original antigenic sin “is new” but Dr. Rost said that it might help differentiate neuroPASC from acute neurologic symptoms of COVID-19, which include stroke. She indicated that the work performed by Dr. Spatola and others might eventually explain the pathology while leading to treatment strategies. She cautioned that the concepts explored in this study “need to be further developed” through larger sample sizes and the exploration of other variables that support the hypothesis.
Dr. Spatola and Dr. Rost report no potential conflicts of interest.
BOSTON – , according to a collaborative study presented at the 2023 annual meeting of the American Academy of Neurology.
Already documented in several other viral infections, such as influenza and human immunodeficiency virus, antigenic imprinting results in production of antibodies to previously encountered viral infections rather than to the immediate threat, according to Marianna Spatola, MD, PhD, a research fellow at the Ragon Institute, Harvard University, Cambridge, Mass.
Original antigenic sin
In the case of persistent neurologic symptoms after COVID, a condition known as neuroPASC (neurological postacute sequelae of SARS-CoV2 infection), antibodies produced for previously encountered coronaviruses rather than for SARS-CoV2 might explain most or all cases, according to the data Dr. Spatola presented.
The evidence for this explanation was drawn from a study of 112 patients evaluated months after an acute episode of COVID-19. Of these, 18 patients had persistent neurologic dysfunction. When compared with the 94 whose infection resolved without sequelae, the patients with prolonged neurologic impairments had relatively low systemic antibody response to SARS-CoV2. However, they showed relatively high antibody responses against other coronaviruses.
This is a pattern consistent with antigenic imprinting, a concept first described more than 60 years ago as original antigenic sin. When the immune system becomes imprinted with an antigen from the first encountered virus from a family of pathogens, it governs all subsequent antibody responses, according to several published studies that have described and evaluated this concept.
Additional evidence
In Dr. Spatola’s study, other differences, particularly in regard to the cerebrospinal fluid (CSF), further supported the role of antigenic imprinting as a cause of neuroPASC. For one, those with elevated immune responses to other common coronaviruses rather than SARS-CoV2 in the CSF relative to the periphery were more likely to have a bad outcome in regard to neurologic symptoms.
Moreover, the CSF in neuroPASC patients “was characterized by increased IgG1 and absence of IgM, suggesting compartmentalized humoral responses within the CSF through selective transfer of antibodies from the serum to the CSF across the blood-brain barrier rather than through intrathecal synthesis,” Dr. Spatola reported.
In the case of COVID-19, the propensity for antigenic imprinting is not difficult to understand.
“The common cold coronaviruses are pretty similar to SARS-CoV2, but they are not exactly the same,” Dr. Spatola said. Her work and studies by others suggest that when antigenic imprinting occurs, “it prevents full maturation of the antibody response.”
NeuroPASC is one of many manifestations of long COVID, but Dr. Spatola pointed out that the immune response in the CSF is unique and the causes of prolonged neurologic impairment after COVID-19 are likely to involve different mechanisms than other long-COVID symptoms.
“Antibodies in the brain are functionally different,” said Dr. Spatola, noting for example that antibody-directed defenses against viral threats show a greater relative reliance on phagocytosis. This might become important in the development of therapeutics for neurologic symptoms of long COVID.
A different phenomenon
The manifestations of neuroPASC are heterogeneous and can include confusion, cognitive dysfunction, headache, encephalitis, and other impairments. Neurologic symptoms occur during acute SARS-CoV2 infections, but neuroPASC appears to be a different phenomenon. These symptoms, which develop after the initial respiratory disease has resolved, were attributed by Dr. Spatola to persistent inflammation that is not necessarily directly related to ongoing infection.
“The reason why some patients develop neuroPASC is unknown, but I think the evidence has pointed to a role for the immune system rather than the virus itself,” Dr. Spatola said.
Currently, neuroPASC is a clinical diagnosis but Dr. Spatola and her coinvestigators are conducting research to identify biomarkers. A viable diagnostic test is not expected imminently. They have identified 150 different features with potential relevance to neuroPASC.
In their comparison of those who did relative to those who did not develop neuroPASC, the initial studies were undertaken 2-4 months after the acute COVID-19 symptoms had resolved. The patients with neuroPASC and those without neurologic sequelae have now been followed for 6-8 months, which Dr. Spatola said was too short to draw firm conclusions about outcomes.
An evolving concept
Despite the small sample size of this study, these are “very interesting data” for considering the pathogenesis of neuroPASC, which is “a concept that is still evolving,” according to Natalia S. Rost, MD, chief of the stroke division, department of neurology, Massachusetts General Hospital, Boston.
Applied to SARS-CoV2, the concept of original antigenic sin “is new” but Dr. Rost said that it might help differentiate neuroPASC from acute neurologic symptoms of COVID-19, which include stroke. She indicated that the work performed by Dr. Spatola and others might eventually explain the pathology while leading to treatment strategies. She cautioned that the concepts explored in this study “need to be further developed” through larger sample sizes and the exploration of other variables that support the hypothesis.
Dr. Spatola and Dr. Rost report no potential conflicts of interest.
FROM AAN 2023
Trial shows some relief for long COVID fatigue, researchers say
In a phase 2 clinical trial of a potential treatment for fatigue associated with long COVID-19, people who received the medicine reported positive results over those receiving a placebo.
The study was conducted by researchers at the University of Oxford, England, and published in eClinical Medicine.
It was one of the first randomized double-blind placebo controlled trial for a possible treatment for long COVID, according to a press release from the university.
the university reported.
Forty-one people participated. They had fatigue for 18 months beforehand. All completed the study, and none reported serious side effects.
AXA1125 was developed by U.S. pharmaceutical company Axcella Therapeutics.
“Potential causes [of long COVID fatigue] include reduced mitochondrial function and cellular bioenergetics,” the researchers reported.
“AXA1125 was tested in long COVID fatigue as previous data from Axcella showed effects on cellular energetics and inflammation. Emerging data on long COVID suggests that the virus targets the mitochondrial, which are essential to normal energy generation and control of inflammation,” the university noted in its press release. “AXA1125 may improve energy generation and reduce the amount of inflammation in the body.”
The study’s authors wrote that AXA1125 was tied to a “significant reduction in 28-day Chalder Fatigue Questionnaire score relative to placebo.” They said participants who reported less fatigue also had better mitochondrial health and walked farther in a 6-minute test.
A version of this article first appeared on WebMD.com.
In a phase 2 clinical trial of a potential treatment for fatigue associated with long COVID-19, people who received the medicine reported positive results over those receiving a placebo.
The study was conducted by researchers at the University of Oxford, England, and published in eClinical Medicine.
It was one of the first randomized double-blind placebo controlled trial for a possible treatment for long COVID, according to a press release from the university.
the university reported.
Forty-one people participated. They had fatigue for 18 months beforehand. All completed the study, and none reported serious side effects.
AXA1125 was developed by U.S. pharmaceutical company Axcella Therapeutics.
“Potential causes [of long COVID fatigue] include reduced mitochondrial function and cellular bioenergetics,” the researchers reported.
“AXA1125 was tested in long COVID fatigue as previous data from Axcella showed effects on cellular energetics and inflammation. Emerging data on long COVID suggests that the virus targets the mitochondrial, which are essential to normal energy generation and control of inflammation,” the university noted in its press release. “AXA1125 may improve energy generation and reduce the amount of inflammation in the body.”
The study’s authors wrote that AXA1125 was tied to a “significant reduction in 28-day Chalder Fatigue Questionnaire score relative to placebo.” They said participants who reported less fatigue also had better mitochondrial health and walked farther in a 6-minute test.
A version of this article first appeared on WebMD.com.
In a phase 2 clinical trial of a potential treatment for fatigue associated with long COVID-19, people who received the medicine reported positive results over those receiving a placebo.
The study was conducted by researchers at the University of Oxford, England, and published in eClinical Medicine.
It was one of the first randomized double-blind placebo controlled trial for a possible treatment for long COVID, according to a press release from the university.
the university reported.
Forty-one people participated. They had fatigue for 18 months beforehand. All completed the study, and none reported serious side effects.
AXA1125 was developed by U.S. pharmaceutical company Axcella Therapeutics.
“Potential causes [of long COVID fatigue] include reduced mitochondrial function and cellular bioenergetics,” the researchers reported.
“AXA1125 was tested in long COVID fatigue as previous data from Axcella showed effects on cellular energetics and inflammation. Emerging data on long COVID suggests that the virus targets the mitochondrial, which are essential to normal energy generation and control of inflammation,” the university noted in its press release. “AXA1125 may improve energy generation and reduce the amount of inflammation in the body.”
The study’s authors wrote that AXA1125 was tied to a “significant reduction in 28-day Chalder Fatigue Questionnaire score relative to placebo.” They said participants who reported less fatigue also had better mitochondrial health and walked farther in a 6-minute test.
A version of this article first appeared on WebMD.com.
Severe COVID-19 linked to new diabetes diagnoses
COVID can more than triple the chance of being diagnosed with type 2 diabetes within a year of being infected, according to a new Canadian study.
Men who had even a mild case of COVID were significantly more likely than were noninfected men to be diagnosed with type 2 diabetes. Women didn’t have an increased risk unless they were severely ill.
Both men and women who had severe cases were at the highest risk. , and those who were admitted to intensive care units had more than a tripled risk.
“This is definitely a concern in terms of long-term outcomes,” researcher and University of British Columbia professor Naveed Z. Janjua, PhD, told The New York Times. “With a respiratory infection, you usually think, ‘Seven or eight days and I’m done with it, that’s it.’ [But] here we’re seeing lingering effects that are lifelong.”
The study was published in JAMA Network Open. Researchers analyzed health data from 2020 and 2021 for 629,935 people, 20% of whom were diagnosed with COVID during that time. Most people in the study had not been vaccinated because vaccines were not widely available then. The health information came from a registry maintained by public health officials in British Columbia. The follow-up period was 257 days.
The authors cautioned that their findings could not say that COVID causes type 2 diabetes; rather, in a commentary published along with the study, Pamela B. Davis, MD, PhD, said the link makes sense because COVID is known to impact the pancreas.
“Such a stress may move a patient from a prediabetic state into diabetes,” wrote Dr. Davis, former dean of Case Western Reserve University, Cleveland, where she is now a professor.
The researchers estimated that the increased pattern of diagnoses of diabetes following COVID infection could increase the rate of the disease occurring in the general population by 3%-5% overall.
A version of this article first appeared on WebMD.com.
COVID can more than triple the chance of being diagnosed with type 2 diabetes within a year of being infected, according to a new Canadian study.
Men who had even a mild case of COVID were significantly more likely than were noninfected men to be diagnosed with type 2 diabetes. Women didn’t have an increased risk unless they were severely ill.
Both men and women who had severe cases were at the highest risk. , and those who were admitted to intensive care units had more than a tripled risk.
“This is definitely a concern in terms of long-term outcomes,” researcher and University of British Columbia professor Naveed Z. Janjua, PhD, told The New York Times. “With a respiratory infection, you usually think, ‘Seven or eight days and I’m done with it, that’s it.’ [But] here we’re seeing lingering effects that are lifelong.”
The study was published in JAMA Network Open. Researchers analyzed health data from 2020 and 2021 for 629,935 people, 20% of whom were diagnosed with COVID during that time. Most people in the study had not been vaccinated because vaccines were not widely available then. The health information came from a registry maintained by public health officials in British Columbia. The follow-up period was 257 days.
The authors cautioned that their findings could not say that COVID causes type 2 diabetes; rather, in a commentary published along with the study, Pamela B. Davis, MD, PhD, said the link makes sense because COVID is known to impact the pancreas.
“Such a stress may move a patient from a prediabetic state into diabetes,” wrote Dr. Davis, former dean of Case Western Reserve University, Cleveland, where she is now a professor.
The researchers estimated that the increased pattern of diagnoses of diabetes following COVID infection could increase the rate of the disease occurring in the general population by 3%-5% overall.
A version of this article first appeared on WebMD.com.
COVID can more than triple the chance of being diagnosed with type 2 diabetes within a year of being infected, according to a new Canadian study.
Men who had even a mild case of COVID were significantly more likely than were noninfected men to be diagnosed with type 2 diabetes. Women didn’t have an increased risk unless they were severely ill.
Both men and women who had severe cases were at the highest risk. , and those who were admitted to intensive care units had more than a tripled risk.
“This is definitely a concern in terms of long-term outcomes,” researcher and University of British Columbia professor Naveed Z. Janjua, PhD, told The New York Times. “With a respiratory infection, you usually think, ‘Seven or eight days and I’m done with it, that’s it.’ [But] here we’re seeing lingering effects that are lifelong.”
The study was published in JAMA Network Open. Researchers analyzed health data from 2020 and 2021 for 629,935 people, 20% of whom were diagnosed with COVID during that time. Most people in the study had not been vaccinated because vaccines were not widely available then. The health information came from a registry maintained by public health officials in British Columbia. The follow-up period was 257 days.
The authors cautioned that their findings could not say that COVID causes type 2 diabetes; rather, in a commentary published along with the study, Pamela B. Davis, MD, PhD, said the link makes sense because COVID is known to impact the pancreas.
“Such a stress may move a patient from a prediabetic state into diabetes,” wrote Dr. Davis, former dean of Case Western Reserve University, Cleveland, where she is now a professor.
The researchers estimated that the increased pattern of diagnoses of diabetes following COVID infection could increase the rate of the disease occurring in the general population by 3%-5% overall.
A version of this article first appeared on WebMD.com.
CDC backs FDA’s call for second COVID booster for those at high risk
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .