User login
HS cardiac troponin T shows MI rule-out potential
WASHINGTON – Patients presenting with chest pain with a negative ECG and an undetectable blood level of cardiac troponin T on a high-sensitivity assay had virtually no risk of a myocardial infarction during the following 30 days, in a retrospective review of more than 8,900 patients at one Swedish medical center.
Applying a high-sensitivity cardiac troponin T screen to rule out a possible MI in patients who present to emergency departments with chest pain could substantially reduce the rate of unnecessary hospitalizations such patients, Dr. Martin Holzmann said at the annual meeting of the American College of Cardiology.
During December 2010–December 2012, a total of 8,907 patients older than 25 years who presented to the emergency department at one large European medical center with chest pain, no identifiable ST-segment elevation, or depression on their ECG, and a blood level of cardiac troponin T that was undetectable at less than 5 ng/L on a high-sensitivity assay. From this group of patients, 15 subsequently developed an MI during the next 30 days of follow-up.
This finding showed that an undetectable level of high-sensitivity cardiac troponin T had a negative predictive accuracy of 99.8%, said Dr. Holzmann, an emergency medicine physician at Karolinska University Hospital in Stockholm. The researchers used an Elecsys 2010 device marketed by Roche for high-sensitivity measurement of cardiac troponin T.
But this finding, while consistent with prior reports suggesting that high-sensitivity measurement of cardiac troponin T could detect or rule out MI, came from a study that was not rigorously enough designed to justify routine high-sensitivity screening for cardiac troponin T in chest-pain patients in emergency departments, commented Dr. Allan S. Jaffe, a cardiologist and professor of medicine and laboratory medicine at the Mayo Clinic in Rochester, Minn. He also noted that the device used in the study is not approved for U.S. marketing.
"This is a strategy that has been hinted at since a paper in 2009 by Dr. Tobias Reichlin (N. Engl. J. Med. 2009;361:858-67)," but Dr. Jaffe questioned whether all patients in the new, Swedish study underwent adequate assessment for new-onset MIs during follow-up.
"I agree that in the long-term this strategy will likely work for low-risk people, but I have concerns" whether adequate proof is now in hand. "I want to see it tested in a study with all the ‘I’s dotted and ‘T’s crossed," including two or three serial measures of high-sensitivity cardiac troponin T, Dr. Jaffe said in an interview.
The study had no commercial funding, and Dr. Holzmann and his associates had no disclosures. Dr. Jaffe said that he has received research support and had consulted for several companies developing devices to measure cardiac troponin T.
On Twitter @mitchelzoler
WASHINGTON – Patients presenting with chest pain with a negative ECG and an undetectable blood level of cardiac troponin T on a high-sensitivity assay had virtually no risk of a myocardial infarction during the following 30 days, in a retrospective review of more than 8,900 patients at one Swedish medical center.
Applying a high-sensitivity cardiac troponin T screen to rule out a possible MI in patients who present to emergency departments with chest pain could substantially reduce the rate of unnecessary hospitalizations such patients, Dr. Martin Holzmann said at the annual meeting of the American College of Cardiology.
During December 2010–December 2012, a total of 8,907 patients older than 25 years who presented to the emergency department at one large European medical center with chest pain, no identifiable ST-segment elevation, or depression on their ECG, and a blood level of cardiac troponin T that was undetectable at less than 5 ng/L on a high-sensitivity assay. From this group of patients, 15 subsequently developed an MI during the next 30 days of follow-up.
This finding showed that an undetectable level of high-sensitivity cardiac troponin T had a negative predictive accuracy of 99.8%, said Dr. Holzmann, an emergency medicine physician at Karolinska University Hospital in Stockholm. The researchers used an Elecsys 2010 device marketed by Roche for high-sensitivity measurement of cardiac troponin T.
But this finding, while consistent with prior reports suggesting that high-sensitivity measurement of cardiac troponin T could detect or rule out MI, came from a study that was not rigorously enough designed to justify routine high-sensitivity screening for cardiac troponin T in chest-pain patients in emergency departments, commented Dr. Allan S. Jaffe, a cardiologist and professor of medicine and laboratory medicine at the Mayo Clinic in Rochester, Minn. He also noted that the device used in the study is not approved for U.S. marketing.
"This is a strategy that has been hinted at since a paper in 2009 by Dr. Tobias Reichlin (N. Engl. J. Med. 2009;361:858-67)," but Dr. Jaffe questioned whether all patients in the new, Swedish study underwent adequate assessment for new-onset MIs during follow-up.
"I agree that in the long-term this strategy will likely work for low-risk people, but I have concerns" whether adequate proof is now in hand. "I want to see it tested in a study with all the ‘I’s dotted and ‘T’s crossed," including two or three serial measures of high-sensitivity cardiac troponin T, Dr. Jaffe said in an interview.
The study had no commercial funding, and Dr. Holzmann and his associates had no disclosures. Dr. Jaffe said that he has received research support and had consulted for several companies developing devices to measure cardiac troponin T.
On Twitter @mitchelzoler
WASHINGTON – Patients presenting with chest pain with a negative ECG and an undetectable blood level of cardiac troponin T on a high-sensitivity assay had virtually no risk of a myocardial infarction during the following 30 days, in a retrospective review of more than 8,900 patients at one Swedish medical center.
Applying a high-sensitivity cardiac troponin T screen to rule out a possible MI in patients who present to emergency departments with chest pain could substantially reduce the rate of unnecessary hospitalizations such patients, Dr. Martin Holzmann said at the annual meeting of the American College of Cardiology.
During December 2010–December 2012, a total of 8,907 patients older than 25 years who presented to the emergency department at one large European medical center with chest pain, no identifiable ST-segment elevation, or depression on their ECG, and a blood level of cardiac troponin T that was undetectable at less than 5 ng/L on a high-sensitivity assay. From this group of patients, 15 subsequently developed an MI during the next 30 days of follow-up.
This finding showed that an undetectable level of high-sensitivity cardiac troponin T had a negative predictive accuracy of 99.8%, said Dr. Holzmann, an emergency medicine physician at Karolinska University Hospital in Stockholm. The researchers used an Elecsys 2010 device marketed by Roche for high-sensitivity measurement of cardiac troponin T.
But this finding, while consistent with prior reports suggesting that high-sensitivity measurement of cardiac troponin T could detect or rule out MI, came from a study that was not rigorously enough designed to justify routine high-sensitivity screening for cardiac troponin T in chest-pain patients in emergency departments, commented Dr. Allan S. Jaffe, a cardiologist and professor of medicine and laboratory medicine at the Mayo Clinic in Rochester, Minn. He also noted that the device used in the study is not approved for U.S. marketing.
"This is a strategy that has been hinted at since a paper in 2009 by Dr. Tobias Reichlin (N. Engl. J. Med. 2009;361:858-67)," but Dr. Jaffe questioned whether all patients in the new, Swedish study underwent adequate assessment for new-onset MIs during follow-up.
"I agree that in the long-term this strategy will likely work for low-risk people, but I have concerns" whether adequate proof is now in hand. "I want to see it tested in a study with all the ‘I’s dotted and ‘T’s crossed," including two or three serial measures of high-sensitivity cardiac troponin T, Dr. Jaffe said in an interview.
The study had no commercial funding, and Dr. Holzmann and his associates had no disclosures. Dr. Jaffe said that he has received research support and had consulted for several companies developing devices to measure cardiac troponin T.
On Twitter @mitchelzoler
AT ACC 2014
Major finding: Undetectable cardiac troponin T and no ECG change ruled out MI in emergency chest-pain patients with 99.8% accuracy.
Data source: Retrospective, single-center review of 8,907 patients with undetectable cardiac troponin T during emergency department visit.
Disclosures: The study had no commercial funding. Dr. Holzmann and his associates had no disclosures.
Obstructive sleep apnea complicates atrial fibrillation
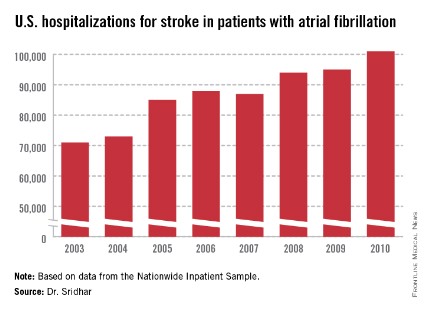
WASHINGTON – About 18% of U.S. patients with atrial fibrillation also have a diagnosis of obstructive sleep apnea, and the confluence of the two appeared linked to increased hospitalizations and further progression of atrial fibrillation, based on a registry of more than 10,000 U.S. atrial fibrillation patients.
In addition, patients who have AF and OSA and who are treated with continuous positive airway pressure (CPAP) have a reduced rate of AF progression, Dr. Jonathan P. Piccini Sr. said at the annual meeting of the American College of Cardiology.
"We probably are not screening for obstructive sleep apnea as aggressively as we should" in AF patients, said Dr. Piccini, an electrophysiologist at Duke University in Durham, N.C. "We know that if obstructive sleep apnea is treated [with CPAP,] the AF burden can be dramatically reduced."
The data Dr. Piccini and his associates analyzed came from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation), which starting in 2010 enrolled more than 10,000 AF patients from 172 U.S. locations with a variety of practice settings and followed them for more than 2 years. Their medical records showed that at enrollment, 1,841 of the 10,132 enrolled AF patients (18%) had also been diagnosed with OSA. Patients with OSA averaged 69 years old, while those without the disorder averaged 76 years old. Those with OSA had an average body mass index of 34 kg/m2, compared with 28 kg/m2 among those without OSA. Patients with OSA also had higher prevalence rates of dyspnea and fatigue than the enrolled AF patients without OSA.
During 2 years of follow-up, the two subgroups had similar rates of all-cause death, cardiovascular events, major bleeding events, and AF progression, but after multivariate adjustment, patients with OSA had a 12% higher rate of hospitalizations than did patients without OSA, a statistically significant difference.
Further analysis of the 1,624 OSA patients showed that 937 (58%) used CPAP during follow-up as a treatment for OSA. Comparison of the CPAP users and nonusers showed no significant difference in outcomes during follow-up for all-cause death, hospitalizations, cardiovascular events, or major bleeding events, but there was a statistically significant, 34% relative drop in the rate of AF progression among CPAP users compared with nonusers.
The ORBIT-AF registry is sponsored by Johnson & Johnson. Dr. Piccini has received remuneration from Johnson & Johnson, Forest Laboratories, and other companies.
On Twitter @mitchelzoler
WASHINGTON – About 18% of U.S. patients with atrial fibrillation also have a diagnosis of obstructive sleep apnea, and the confluence of the two appeared linked to increased hospitalizations and further progression of atrial fibrillation, based on a registry of more than 10,000 U.S. atrial fibrillation patients.
In addition, patients who have AF and OSA and who are treated with continuous positive airway pressure (CPAP) have a reduced rate of AF progression, Dr. Jonathan P. Piccini Sr. said at the annual meeting of the American College of Cardiology.
"We probably are not screening for obstructive sleep apnea as aggressively as we should" in AF patients, said Dr. Piccini, an electrophysiologist at Duke University in Durham, N.C. "We know that if obstructive sleep apnea is treated [with CPAP,] the AF burden can be dramatically reduced."
The data Dr. Piccini and his associates analyzed came from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation), which starting in 2010 enrolled more than 10,000 AF patients from 172 U.S. locations with a variety of practice settings and followed them for more than 2 years. Their medical records showed that at enrollment, 1,841 of the 10,132 enrolled AF patients (18%) had also been diagnosed with OSA. Patients with OSA averaged 69 years old, while those without the disorder averaged 76 years old. Those with OSA had an average body mass index of 34 kg/m2, compared with 28 kg/m2 among those without OSA. Patients with OSA also had higher prevalence rates of dyspnea and fatigue than the enrolled AF patients without OSA.
During 2 years of follow-up, the two subgroups had similar rates of all-cause death, cardiovascular events, major bleeding events, and AF progression, but after multivariate adjustment, patients with OSA had a 12% higher rate of hospitalizations than did patients without OSA, a statistically significant difference.
Further analysis of the 1,624 OSA patients showed that 937 (58%) used CPAP during follow-up as a treatment for OSA. Comparison of the CPAP users and nonusers showed no significant difference in outcomes during follow-up for all-cause death, hospitalizations, cardiovascular events, or major bleeding events, but there was a statistically significant, 34% relative drop in the rate of AF progression among CPAP users compared with nonusers.
The ORBIT-AF registry is sponsored by Johnson & Johnson. Dr. Piccini has received remuneration from Johnson & Johnson, Forest Laboratories, and other companies.
On Twitter @mitchelzoler
WASHINGTON – About 18% of U.S. patients with atrial fibrillation also have a diagnosis of obstructive sleep apnea, and the confluence of the two appeared linked to increased hospitalizations and further progression of atrial fibrillation, based on a registry of more than 10,000 U.S. atrial fibrillation patients.
In addition, patients who have AF and OSA and who are treated with continuous positive airway pressure (CPAP) have a reduced rate of AF progression, Dr. Jonathan P. Piccini Sr. said at the annual meeting of the American College of Cardiology.
"We probably are not screening for obstructive sleep apnea as aggressively as we should" in AF patients, said Dr. Piccini, an electrophysiologist at Duke University in Durham, N.C. "We know that if obstructive sleep apnea is treated [with CPAP,] the AF burden can be dramatically reduced."
The data Dr. Piccini and his associates analyzed came from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation), which starting in 2010 enrolled more than 10,000 AF patients from 172 U.S. locations with a variety of practice settings and followed them for more than 2 years. Their medical records showed that at enrollment, 1,841 of the 10,132 enrolled AF patients (18%) had also been diagnosed with OSA. Patients with OSA averaged 69 years old, while those without the disorder averaged 76 years old. Those with OSA had an average body mass index of 34 kg/m2, compared with 28 kg/m2 among those without OSA. Patients with OSA also had higher prevalence rates of dyspnea and fatigue than the enrolled AF patients without OSA.
During 2 years of follow-up, the two subgroups had similar rates of all-cause death, cardiovascular events, major bleeding events, and AF progression, but after multivariate adjustment, patients with OSA had a 12% higher rate of hospitalizations than did patients without OSA, a statistically significant difference.
Further analysis of the 1,624 OSA patients showed that 937 (58%) used CPAP during follow-up as a treatment for OSA. Comparison of the CPAP users and nonusers showed no significant difference in outcomes during follow-up for all-cause death, hospitalizations, cardiovascular events, or major bleeding events, but there was a statistically significant, 34% relative drop in the rate of AF progression among CPAP users compared with nonusers.
The ORBIT-AF registry is sponsored by Johnson & Johnson. Dr. Piccini has received remuneration from Johnson & Johnson, Forest Laboratories, and other companies.
On Twitter @mitchelzoler
AT ACC 2014
Major finding: Atrial fibrillation patients with sleep apnea had a significant, 12% increased rate of hospitalizations, compared with atrial fibrillation patients without sleep apnea.
Data source: The ORBIT-AF registry, which enrolled 10,132 U.S. patients with atrial fibrillation starting in 2010.
Disclosures: The ORBIT-AF registry is sponsored by Johnson & Johnson. Dr. Piccini has received remuneration from Johnson & Johnson, Forest Laboratories, and other companies.
Rivaroxaban replacing warfarin for AF stroke protection
WASHINGTON – Use of the new oral anticoagulant rivaroxaban to prevent stroke in atrial fibrillation patients doubled and then redoubled during 2010-2013, based on a review of nearly 10,000 atrial fibrillation patients who received their care through a large health network in Wisconsin and Illinois.
Data from 9,652 atrial fibrillation (AF) patients who received care through Aurora Health Care during the 4-year period also showed that two new oral anticoagulants, rivaroxaban (Xarelto) and dabigatran (Pradaxa), seemed roughly comparable to warfarin for preventing strokes and not causing bleeding, including in patients aged 80 years or older, a patient group poorly represented in the pivotal trials of the new drugs, Dr. Anton V. Strunets said at the annual meeting of the American College of Cardiology.
"In community-based, real-world practice, use of novel anticoagulants in the older population was substantial and increasing," and the results were also "reassuring" about the efficacy and safety of these drugs during routine use, particularly in the elderly, said Dr. Strunets, a physician on the staff of Aurora Health Care in Milwaukee.
Since 2010, "the type of anticoagulant used has changed dramatically," he said, with the use of warfarin and dabigatran as a percentage of AF patients falling and the use of rivaroxaban rising sharply. After rivaroxaban received Food and Drug Administration approval in 2011 for use in preventing stroke in AF patients, it entered the Aurora practice with roughly 8% of patients on the drug by the end of the year, rising to about 16% in 2012, and up to about 30% by the end of last year. In contrast, about 8% of patients were on dabigatran in 2010, and the level then dropped steadily during the next 3 years, so that last year about 1% of AF patients were on dabigatran. While warfarin remained the most widely used anticoagulant throughout, its share of the treated AF population fell from more than 90% in 2010 to less than 70% in 2013.
These numbers from Aurora matched numbers reported last November from a U.S. registry maintained by the American College of Cardiology, which showed that during June-September 2013, 72% of U.S. AF patients on an anticoagulant received warfarin.
Dr. Strunets cited several factors likely driving shifting anticoagulant use in AF patients, though none were documented by the data he reported: the attraction of once-daily dosing with rivaroxaban to boost compliance, especially in older patients; the coverage allowed by insurers and health plans; and recent concern about dabigatran tied to a new study of the drug’s safety launched by the Food and Drug Administration.
Another facet of oral anticoagulant use at Aurora is that during the 4-year period, 38% of AF patients were 80 years or older, including about 30% of those who received rivaroxaban and 25% of those on dabigatran. The efficacy and safety of treatment, based on the incidence of strokes and of intracranial and gastrointestinal bleeds, were roughly similar in the dabigatran, rivaroxaban, and warfarin subgroups, and the rates for the new anticoagulants also roughly matched the rates seen in the pivotal trials with those drugs, suggesting that the new anticoagulants were performing well when used in octo- and nonagenarians, Dr. Strunets said. But he cautioned that the patients who received rivaroxaban or dabigatran were significantly younger and had significantly lower stroke and bleeding risk scores than patients who received warfarin, a selection bias that may have affected outcomes.
Dr. Strunets and his associates had no disclosures.
On Twitter @mitchelzoler
WASHINGTON – Use of the new oral anticoagulant rivaroxaban to prevent stroke in atrial fibrillation patients doubled and then redoubled during 2010-2013, based on a review of nearly 10,000 atrial fibrillation patients who received their care through a large health network in Wisconsin and Illinois.
Data from 9,652 atrial fibrillation (AF) patients who received care through Aurora Health Care during the 4-year period also showed that two new oral anticoagulants, rivaroxaban (Xarelto) and dabigatran (Pradaxa), seemed roughly comparable to warfarin for preventing strokes and not causing bleeding, including in patients aged 80 years or older, a patient group poorly represented in the pivotal trials of the new drugs, Dr. Anton V. Strunets said at the annual meeting of the American College of Cardiology.
"In community-based, real-world practice, use of novel anticoagulants in the older population was substantial and increasing," and the results were also "reassuring" about the efficacy and safety of these drugs during routine use, particularly in the elderly, said Dr. Strunets, a physician on the staff of Aurora Health Care in Milwaukee.
Since 2010, "the type of anticoagulant used has changed dramatically," he said, with the use of warfarin and dabigatran as a percentage of AF patients falling and the use of rivaroxaban rising sharply. After rivaroxaban received Food and Drug Administration approval in 2011 for use in preventing stroke in AF patients, it entered the Aurora practice with roughly 8% of patients on the drug by the end of the year, rising to about 16% in 2012, and up to about 30% by the end of last year. In contrast, about 8% of patients were on dabigatran in 2010, and the level then dropped steadily during the next 3 years, so that last year about 1% of AF patients were on dabigatran. While warfarin remained the most widely used anticoagulant throughout, its share of the treated AF population fell from more than 90% in 2010 to less than 70% in 2013.
These numbers from Aurora matched numbers reported last November from a U.S. registry maintained by the American College of Cardiology, which showed that during June-September 2013, 72% of U.S. AF patients on an anticoagulant received warfarin.
Dr. Strunets cited several factors likely driving shifting anticoagulant use in AF patients, though none were documented by the data he reported: the attraction of once-daily dosing with rivaroxaban to boost compliance, especially in older patients; the coverage allowed by insurers and health plans; and recent concern about dabigatran tied to a new study of the drug’s safety launched by the Food and Drug Administration.
Another facet of oral anticoagulant use at Aurora is that during the 4-year period, 38% of AF patients were 80 years or older, including about 30% of those who received rivaroxaban and 25% of those on dabigatran. The efficacy and safety of treatment, based on the incidence of strokes and of intracranial and gastrointestinal bleeds, were roughly similar in the dabigatran, rivaroxaban, and warfarin subgroups, and the rates for the new anticoagulants also roughly matched the rates seen in the pivotal trials with those drugs, suggesting that the new anticoagulants were performing well when used in octo- and nonagenarians, Dr. Strunets said. But he cautioned that the patients who received rivaroxaban or dabigatran were significantly younger and had significantly lower stroke and bleeding risk scores than patients who received warfarin, a selection bias that may have affected outcomes.
Dr. Strunets and his associates had no disclosures.
On Twitter @mitchelzoler
WASHINGTON – Use of the new oral anticoagulant rivaroxaban to prevent stroke in atrial fibrillation patients doubled and then redoubled during 2010-2013, based on a review of nearly 10,000 atrial fibrillation patients who received their care through a large health network in Wisconsin and Illinois.
Data from 9,652 atrial fibrillation (AF) patients who received care through Aurora Health Care during the 4-year period also showed that two new oral anticoagulants, rivaroxaban (Xarelto) and dabigatran (Pradaxa), seemed roughly comparable to warfarin for preventing strokes and not causing bleeding, including in patients aged 80 years or older, a patient group poorly represented in the pivotal trials of the new drugs, Dr. Anton V. Strunets said at the annual meeting of the American College of Cardiology.
"In community-based, real-world practice, use of novel anticoagulants in the older population was substantial and increasing," and the results were also "reassuring" about the efficacy and safety of these drugs during routine use, particularly in the elderly, said Dr. Strunets, a physician on the staff of Aurora Health Care in Milwaukee.
Since 2010, "the type of anticoagulant used has changed dramatically," he said, with the use of warfarin and dabigatran as a percentage of AF patients falling and the use of rivaroxaban rising sharply. After rivaroxaban received Food and Drug Administration approval in 2011 for use in preventing stroke in AF patients, it entered the Aurora practice with roughly 8% of patients on the drug by the end of the year, rising to about 16% in 2012, and up to about 30% by the end of last year. In contrast, about 8% of patients were on dabigatran in 2010, and the level then dropped steadily during the next 3 years, so that last year about 1% of AF patients were on dabigatran. While warfarin remained the most widely used anticoagulant throughout, its share of the treated AF population fell from more than 90% in 2010 to less than 70% in 2013.
These numbers from Aurora matched numbers reported last November from a U.S. registry maintained by the American College of Cardiology, which showed that during June-September 2013, 72% of U.S. AF patients on an anticoagulant received warfarin.
Dr. Strunets cited several factors likely driving shifting anticoagulant use in AF patients, though none were documented by the data he reported: the attraction of once-daily dosing with rivaroxaban to boost compliance, especially in older patients; the coverage allowed by insurers and health plans; and recent concern about dabigatran tied to a new study of the drug’s safety launched by the Food and Drug Administration.
Another facet of oral anticoagulant use at Aurora is that during the 4-year period, 38% of AF patients were 80 years or older, including about 30% of those who received rivaroxaban and 25% of those on dabigatran. The efficacy and safety of treatment, based on the incidence of strokes and of intracranial and gastrointestinal bleeds, were roughly similar in the dabigatran, rivaroxaban, and warfarin subgroups, and the rates for the new anticoagulants also roughly matched the rates seen in the pivotal trials with those drugs, suggesting that the new anticoagulants were performing well when used in octo- and nonagenarians, Dr. Strunets said. But he cautioned that the patients who received rivaroxaban or dabigatran were significantly younger and had significantly lower stroke and bleeding risk scores than patients who received warfarin, a selection bias that may have affected outcomes.
Dr. Strunets and his associates had no disclosures.
On Twitter @mitchelzoler
AT ACC 2014
Major finding: Rivaroxaban use grew from approximately 8% of AF patients in 2011 to 16% in 2012 and 30% in 2013.
Data source: Review of anticoagulant care received by 9,652 AF patients treated through Aurora Health Care during 2010-2013.
Disclosures: Dr. Strunets and his associates had no disclosures.
EXACT-HF: Allopurinol flops for heart failure
WASHINGTON – Allopurinol has no effect on heart failure, according to results from the multicenter, double-blind EXACT-HF study.
"In heart failure patients with reduced ejection fraction and hyperuricemia, xanthine oxidase inhibition with allopurinol safely lowers uric acid levels, but has no beneficial effect on clinical status, exercise capacity, quality of life, or left ventricular structure and function. Other adjunctive therapies for high-risk heart failure patients are clearly needed," Dr. Michael M. Givertz said in presenting the EXACT-HF results at the annual meeting of the American College of Cardiology.
Hopes were high going into EXACT-HF that xanthine oxidase inhibition would provide a much needed novel therapeutic approach to heart failure. A growing body of evidence suggests that oxidative stress plays a major role in ventricular remodeling and disease progression, and xanthine oxidase – a potential source of oxidative stress in heart failure – was a logical therapeutic target.
Moreover, in animal models of heart failure, as well as earlier short-term patient studies, allopurinol caused regression of left ventricular hypertrophy, improved endothelial function, and boosted myocardial efficiency while reducing myocardial oxygen demand, noted Dr. Givertz, medical director of heart transplant and circulatory assist at Brigham and Women’s Hospital, Boston.
EXACT-HF was a double-blind study in which 253 hyperuricemic heart failure patients with a median left ventricular ejection fraction (LVEF) of 24% and a median uric acid level of 11 mg/dL were placed on allopurinol or placebo and followed prospectively for 6 months. Allopurinol was started at 300 mg/day and, after 1 week, boosted to a target dose of 600 mg/day if tolerated.
The primary endpoint was a composite comprising death, hospitalization, or an emergency department or urgent care clinic visit for worsening heart failure; a medication change due to worsening heart failure; and patient global assessment. By this standard, roughly 45% of patients in both study arms were worse after 6 months, 16% were improved, and the rest were unchanged. Nor did the two groups differ significantly in terms of the various secondary and tertiary endpoints, including quality of life as assessed by the Kansas City Cardiomyopathy Score; the 6-minute walk test; levels of cystatin C, myeloperoxidase, and NT-proBNP (N-terminal of the prohormone brain natriuretic peptide); and left ventricular volume, mass, and ejection fraction.
Uric acid levels were reduced by about 45% in the allopurinol group. However, investigators never viewed hyperuricemia as a mediator of heart failure, but rather as a marker of more severe disease. And this was a population with fairly severe disease, as reflected in the 6% mortality rate at 6 months, along with a 30% rate of unscheduled outpatient visits and 38% rate of all-cause hospitalization, Dr. Givertz said.
Asked if he thought a longer treatment period might have shown therapeutic benefit, the cardiologist replied that there was one positive signal: The risk of heart failure hospitalization over the 6-month study was reduced by 33% in the allopurinol group, with the curves separating after 8-10 weeks, although the difference wasn’t statistically significant. But he was reluctant to make too much of this.
"It’s conceivable that if one treated for a year or longer with high doses of allopurinol, perhaps one might see a benefit. The argument against that is the consistency in the neutrality of the other endpoints at 6 months," according to Dr. Givertz.
Intriguingly, several recent studies suggest that colchicine – an even more venerable gout drug than allopurinol – may protect gout patients against cardiovascular events.
The EXACT-HF trial was sponsored by the National Heart, Lung, and Blood Institute and carried out by the NHLBI Heart Failure Clinical Research Network. Dr. Givertz reported serving as a consultant to Merck, Cardioxyl, and Janssen.
WASHINGTON – Allopurinol has no effect on heart failure, according to results from the multicenter, double-blind EXACT-HF study.
"In heart failure patients with reduced ejection fraction and hyperuricemia, xanthine oxidase inhibition with allopurinol safely lowers uric acid levels, but has no beneficial effect on clinical status, exercise capacity, quality of life, or left ventricular structure and function. Other adjunctive therapies for high-risk heart failure patients are clearly needed," Dr. Michael M. Givertz said in presenting the EXACT-HF results at the annual meeting of the American College of Cardiology.
Hopes were high going into EXACT-HF that xanthine oxidase inhibition would provide a much needed novel therapeutic approach to heart failure. A growing body of evidence suggests that oxidative stress plays a major role in ventricular remodeling and disease progression, and xanthine oxidase – a potential source of oxidative stress in heart failure – was a logical therapeutic target.
Moreover, in animal models of heart failure, as well as earlier short-term patient studies, allopurinol caused regression of left ventricular hypertrophy, improved endothelial function, and boosted myocardial efficiency while reducing myocardial oxygen demand, noted Dr. Givertz, medical director of heart transplant and circulatory assist at Brigham and Women’s Hospital, Boston.
EXACT-HF was a double-blind study in which 253 hyperuricemic heart failure patients with a median left ventricular ejection fraction (LVEF) of 24% and a median uric acid level of 11 mg/dL were placed on allopurinol or placebo and followed prospectively for 6 months. Allopurinol was started at 300 mg/day and, after 1 week, boosted to a target dose of 600 mg/day if tolerated.
The primary endpoint was a composite comprising death, hospitalization, or an emergency department or urgent care clinic visit for worsening heart failure; a medication change due to worsening heart failure; and patient global assessment. By this standard, roughly 45% of patients in both study arms were worse after 6 months, 16% were improved, and the rest were unchanged. Nor did the two groups differ significantly in terms of the various secondary and tertiary endpoints, including quality of life as assessed by the Kansas City Cardiomyopathy Score; the 6-minute walk test; levels of cystatin C, myeloperoxidase, and NT-proBNP (N-terminal of the prohormone brain natriuretic peptide); and left ventricular volume, mass, and ejection fraction.
Uric acid levels were reduced by about 45% in the allopurinol group. However, investigators never viewed hyperuricemia as a mediator of heart failure, but rather as a marker of more severe disease. And this was a population with fairly severe disease, as reflected in the 6% mortality rate at 6 months, along with a 30% rate of unscheduled outpatient visits and 38% rate of all-cause hospitalization, Dr. Givertz said.
Asked if he thought a longer treatment period might have shown therapeutic benefit, the cardiologist replied that there was one positive signal: The risk of heart failure hospitalization over the 6-month study was reduced by 33% in the allopurinol group, with the curves separating after 8-10 weeks, although the difference wasn’t statistically significant. But he was reluctant to make too much of this.
"It’s conceivable that if one treated for a year or longer with high doses of allopurinol, perhaps one might see a benefit. The argument against that is the consistency in the neutrality of the other endpoints at 6 months," according to Dr. Givertz.
Intriguingly, several recent studies suggest that colchicine – an even more venerable gout drug than allopurinol – may protect gout patients against cardiovascular events.
The EXACT-HF trial was sponsored by the National Heart, Lung, and Blood Institute and carried out by the NHLBI Heart Failure Clinical Research Network. Dr. Givertz reported serving as a consultant to Merck, Cardioxyl, and Janssen.
WASHINGTON – Allopurinol has no effect on heart failure, according to results from the multicenter, double-blind EXACT-HF study.
"In heart failure patients with reduced ejection fraction and hyperuricemia, xanthine oxidase inhibition with allopurinol safely lowers uric acid levels, but has no beneficial effect on clinical status, exercise capacity, quality of life, or left ventricular structure and function. Other adjunctive therapies for high-risk heart failure patients are clearly needed," Dr. Michael M. Givertz said in presenting the EXACT-HF results at the annual meeting of the American College of Cardiology.
Hopes were high going into EXACT-HF that xanthine oxidase inhibition would provide a much needed novel therapeutic approach to heart failure. A growing body of evidence suggests that oxidative stress plays a major role in ventricular remodeling and disease progression, and xanthine oxidase – a potential source of oxidative stress in heart failure – was a logical therapeutic target.
Moreover, in animal models of heart failure, as well as earlier short-term patient studies, allopurinol caused regression of left ventricular hypertrophy, improved endothelial function, and boosted myocardial efficiency while reducing myocardial oxygen demand, noted Dr. Givertz, medical director of heart transplant and circulatory assist at Brigham and Women’s Hospital, Boston.
EXACT-HF was a double-blind study in which 253 hyperuricemic heart failure patients with a median left ventricular ejection fraction (LVEF) of 24% and a median uric acid level of 11 mg/dL were placed on allopurinol or placebo and followed prospectively for 6 months. Allopurinol was started at 300 mg/day and, after 1 week, boosted to a target dose of 600 mg/day if tolerated.
The primary endpoint was a composite comprising death, hospitalization, or an emergency department or urgent care clinic visit for worsening heart failure; a medication change due to worsening heart failure; and patient global assessment. By this standard, roughly 45% of patients in both study arms were worse after 6 months, 16% were improved, and the rest were unchanged. Nor did the two groups differ significantly in terms of the various secondary and tertiary endpoints, including quality of life as assessed by the Kansas City Cardiomyopathy Score; the 6-minute walk test; levels of cystatin C, myeloperoxidase, and NT-proBNP (N-terminal of the prohormone brain natriuretic peptide); and left ventricular volume, mass, and ejection fraction.
Uric acid levels were reduced by about 45% in the allopurinol group. However, investigators never viewed hyperuricemia as a mediator of heart failure, but rather as a marker of more severe disease. And this was a population with fairly severe disease, as reflected in the 6% mortality rate at 6 months, along with a 30% rate of unscheduled outpatient visits and 38% rate of all-cause hospitalization, Dr. Givertz said.
Asked if he thought a longer treatment period might have shown therapeutic benefit, the cardiologist replied that there was one positive signal: The risk of heart failure hospitalization over the 6-month study was reduced by 33% in the allopurinol group, with the curves separating after 8-10 weeks, although the difference wasn’t statistically significant. But he was reluctant to make too much of this.
"It’s conceivable that if one treated for a year or longer with high doses of allopurinol, perhaps one might see a benefit. The argument against that is the consistency in the neutrality of the other endpoints at 6 months," according to Dr. Givertz.
Intriguingly, several recent studies suggest that colchicine – an even more venerable gout drug than allopurinol – may protect gout patients against cardiovascular events.
The EXACT-HF trial was sponsored by the National Heart, Lung, and Blood Institute and carried out by the NHLBI Heart Failure Clinical Research Network. Dr. Givertz reported serving as a consultant to Merck, Cardioxyl, and Janssen.
AT ACC 14
Major finding: Six months of therapy with allopurinol in patients with hyperuricemia and heart failure with reduced ejection fraction lowered their serum uric acid levels but had no effect on their heart failure.
Data source: EXACT-HF, a 6-month, double-blind, placebo-controlled trial conducted in 253 patients.
Disclosures: The EXACT-HF trial was sponsored by the National Heart, Lung, and Blood Institute and carried out by the NHLBI Heart Failure Clinical Research Network. Dr. Givertz reported serving as a consultant to Merck, Cardioxyl, and Janssen.
Delayed consent in HEAT-PPCI draws criticism
WASHINGTON – The delayed consent approach that investigators used to run the HEAT-PPCI trial generated almost as much controversy as the surprising finding that unfractionated heparin was safer than bivalirudin for anticoagulating ST-segment elevation myocardial infarction patients undergoing primary coronary stenting.
"I’m extremely bothered by the fact that you entered patients into the study without getting their permission," said Dr. William W. O’Neill, who spoke from the stage following the HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention) report as a member of the discussion panel for the session at the annual meeting of the American College of Cardiology.
"There have been people who say that no one [experiencing an acute myocardial infarction] remembers their informed consent, but most of us have a very strong ethical concern. There is a social contract we make with our patients to make sure they are not subject to research without their permission. That’s in the Declaration of Helsinki," said Dr. O’Neill, medical director of the Center for Structural Heart Disease at Henry Ford Hospital in Detroit.
But Dr. Adeel Shahzad, who presented the study’s findings, and Dr. Rod Stables, the study’s principal investigator, unequivocally defended the ethics of their approach, and stressed that the design had been approved by three separate national bodies charged with ethical oversight of U.K. medical studies.
"We’re not advocating the use of delayed consent for all trials. Every trial has different circumstances. We compared two drugs that were in routine use throughout the world and that were used for licensed indications," Dr. Shahzad, an interventional cardiologist at Liverpool (England) Heart and Chest Hospital, said in reply to Dr. O’Neill’s criticism. "In the primary PCI [percutaneous coronary intervention] setting the patient is in pain and possibly under the influence of drugs. In this setting and with an average door-to-balloon time of 29 minutes we don’t believe that informed consent is possible."
"In HEAT-PPCI, 75% of patients were randomized within 9 minutes or arrival. It’s a debatable point whether it is possible to obtain any meaningful consent on that time schedule," said Dr. Stables, director of the cardiac catheterization laboratory at Liverpool Heart and Chest Hospital.
The study’s design called for the informed consent process to occur following intervention, once patients had stabilized. Of the 1,829 patients treated in Liverpool who met the study’s entry criteria, 1,812 (99%) subsequently gave their consent to be included in the trial. Dr. Stables also noted that in addition to the formal approval obtained from three U.K. medical ethics groups, the study’s design also won approval from a panel composed of patients, and the trial also employed a full-time patient advocate.
The ethics of the approach used in HEAT-PPCI were endorsed by both Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Health System in Atlanta, and by Dr. David R. Holmes Jr., an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Holmes recalled his own experience when hospitalized on an emergency basis for "massive" gastrointestinal bleeding. A few weeks later he was contacted about a study that he had agreed to participate in while in a very debilitated state before he received treatment; he had absolutely no recollection of ever agreeing to be in the study.
Dr. O’Neill, Dr. Shahzad, Dr. Stables, Dr. King, and Dr. Holmes had no relevant disclosures.
On Twitter @mitchelzoler
WASHINGTON – The delayed consent approach that investigators used to run the HEAT-PPCI trial generated almost as much controversy as the surprising finding that unfractionated heparin was safer than bivalirudin for anticoagulating ST-segment elevation myocardial infarction patients undergoing primary coronary stenting.
"I’m extremely bothered by the fact that you entered patients into the study without getting their permission," said Dr. William W. O’Neill, who spoke from the stage following the HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention) report as a member of the discussion panel for the session at the annual meeting of the American College of Cardiology.
"There have been people who say that no one [experiencing an acute myocardial infarction] remembers their informed consent, but most of us have a very strong ethical concern. There is a social contract we make with our patients to make sure they are not subject to research without their permission. That’s in the Declaration of Helsinki," said Dr. O’Neill, medical director of the Center for Structural Heart Disease at Henry Ford Hospital in Detroit.
But Dr. Adeel Shahzad, who presented the study’s findings, and Dr. Rod Stables, the study’s principal investigator, unequivocally defended the ethics of their approach, and stressed that the design had been approved by three separate national bodies charged with ethical oversight of U.K. medical studies.
"We’re not advocating the use of delayed consent for all trials. Every trial has different circumstances. We compared two drugs that were in routine use throughout the world and that were used for licensed indications," Dr. Shahzad, an interventional cardiologist at Liverpool (England) Heart and Chest Hospital, said in reply to Dr. O’Neill’s criticism. "In the primary PCI [percutaneous coronary intervention] setting the patient is in pain and possibly under the influence of drugs. In this setting and with an average door-to-balloon time of 29 minutes we don’t believe that informed consent is possible."
"In HEAT-PPCI, 75% of patients were randomized within 9 minutes or arrival. It’s a debatable point whether it is possible to obtain any meaningful consent on that time schedule," said Dr. Stables, director of the cardiac catheterization laboratory at Liverpool Heart and Chest Hospital.
The study’s design called for the informed consent process to occur following intervention, once patients had stabilized. Of the 1,829 patients treated in Liverpool who met the study’s entry criteria, 1,812 (99%) subsequently gave their consent to be included in the trial. Dr. Stables also noted that in addition to the formal approval obtained from three U.K. medical ethics groups, the study’s design also won approval from a panel composed of patients, and the trial also employed a full-time patient advocate.
The ethics of the approach used in HEAT-PPCI were endorsed by both Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Health System in Atlanta, and by Dr. David R. Holmes Jr., an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Holmes recalled his own experience when hospitalized on an emergency basis for "massive" gastrointestinal bleeding. A few weeks later he was contacted about a study that he had agreed to participate in while in a very debilitated state before he received treatment; he had absolutely no recollection of ever agreeing to be in the study.
Dr. O’Neill, Dr. Shahzad, Dr. Stables, Dr. King, and Dr. Holmes had no relevant disclosures.
On Twitter @mitchelzoler
WASHINGTON – The delayed consent approach that investigators used to run the HEAT-PPCI trial generated almost as much controversy as the surprising finding that unfractionated heparin was safer than bivalirudin for anticoagulating ST-segment elevation myocardial infarction patients undergoing primary coronary stenting.
"I’m extremely bothered by the fact that you entered patients into the study without getting their permission," said Dr. William W. O’Neill, who spoke from the stage following the HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention) report as a member of the discussion panel for the session at the annual meeting of the American College of Cardiology.
"There have been people who say that no one [experiencing an acute myocardial infarction] remembers their informed consent, but most of us have a very strong ethical concern. There is a social contract we make with our patients to make sure they are not subject to research without their permission. That’s in the Declaration of Helsinki," said Dr. O’Neill, medical director of the Center for Structural Heart Disease at Henry Ford Hospital in Detroit.
But Dr. Adeel Shahzad, who presented the study’s findings, and Dr. Rod Stables, the study’s principal investigator, unequivocally defended the ethics of their approach, and stressed that the design had been approved by three separate national bodies charged with ethical oversight of U.K. medical studies.
"We’re not advocating the use of delayed consent for all trials. Every trial has different circumstances. We compared two drugs that were in routine use throughout the world and that were used for licensed indications," Dr. Shahzad, an interventional cardiologist at Liverpool (England) Heart and Chest Hospital, said in reply to Dr. O’Neill’s criticism. "In the primary PCI [percutaneous coronary intervention] setting the patient is in pain and possibly under the influence of drugs. In this setting and with an average door-to-balloon time of 29 minutes we don’t believe that informed consent is possible."
"In HEAT-PPCI, 75% of patients were randomized within 9 minutes or arrival. It’s a debatable point whether it is possible to obtain any meaningful consent on that time schedule," said Dr. Stables, director of the cardiac catheterization laboratory at Liverpool Heart and Chest Hospital.
The study’s design called for the informed consent process to occur following intervention, once patients had stabilized. Of the 1,829 patients treated in Liverpool who met the study’s entry criteria, 1,812 (99%) subsequently gave their consent to be included in the trial. Dr. Stables also noted that in addition to the formal approval obtained from three U.K. medical ethics groups, the study’s design also won approval from a panel composed of patients, and the trial also employed a full-time patient advocate.
The ethics of the approach used in HEAT-PPCI were endorsed by both Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Health System in Atlanta, and by Dr. David R. Holmes Jr., an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Holmes recalled his own experience when hospitalized on an emergency basis for "massive" gastrointestinal bleeding. A few weeks later he was contacted about a study that he had agreed to participate in while in a very debilitated state before he received treatment; he had absolutely no recollection of ever agreeing to be in the study.
Dr. O’Neill, Dr. Shahzad, Dr. Stables, Dr. King, and Dr. Holmes had no relevant disclosures.
On Twitter @mitchelzoler
EXPERT OPINION AT ACC 2014
Heparin surpasses bivalirudin in STEMI primary PCI
WASHINGTON – Unfractionated heparin outperformed bivalirudin for 28-day outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention in a single-center randomized trial of more than 1,800 patients when use of a glycoprotein IIb/IIIa inhibitor was kept to a minimum.
Use of unfractionated heparin (UFH) compared with bivalirudin (Angiomax) led to a significant reduction in the rate of combined major adverse coronary events after 28 days compared with bivalirudin driven primarily by a significant cut in the rate of acute stent thrombosis and repeat myocardial infarctions with no increased risk for major bleeding events, consistent results across all subgroups examined, and the potential for substantial savings in drug costs, Dr. Adeel Shahzad said at the annual meeting of the American College of Cardiology.
The combined rate of major adverse coronary events was 6% in patients treated with UFH and 9% in those treated with bivalirudin, a statistically significant difference for the study’s primary efficacy endpoint. The rate of major bleeding events, the study’s primary safety endpoint, occurred in 3.5% of patients treated with bivalirudin and in 3.1% of those who received UFH, a difference that was not statistically significant.
The superior outcome with UFH in this study, which contrasted sharply with the results of several prior head-to-head comparisons of the two drugs in patients with a ST-segment elevation myocardial infarction (STEMI) or other acute coronary syndrome events undergoing primary percutaneous coronary intervention (PCI) seemed closely tied to the study’s protocol that restricted coadministration of a glycoprotein IIb/IIIa inhibitor (such as abciximab [ReoPro]) to only those patients experiencing thrombotic complications during PCI ("bailout" use).
"For the first time, unfractionated heparin was used like bivalirudin," with low use of a glycoprotein IIb/IIIa inhibitor, noted the study’s principal investigator, Dr. Rod Stables, director of the cardiac catheterization laboratory at Liverpool (England) Heart and Chest Hospital.
Current STEMI management guidelines from the American College of Cardiology and American Heart Association (J. Am. Coll. Cardiol. 2013;61:e78-140) and from the European Society of Cardiology (Eur. Heart J. 2012;33:2569-619) endorse use of either bivalirudin or UFH as an anticoagulant when patients undergo primary PCI. The European guidelines say that bivalirudin is preferred over UFH when a glycoprotein IIb/IIIa antagonist is administered along with UFH.
"It’s perfectly legitimate with the guidelines to use unfractionated heparin, but most people [in the United States] use bivalirudin or a glycoprotein IIb/IIIa inhibitor with heparin," said Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Health System in Atlanta.
Based on the new results, "people will say it’s really interesting, and they may at least think about not using bivalirudin all the time," said Dr. David R. Holmes Jr., an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "Those who are believers in bivalirudin will continue to use it, but others will say that heparin did just fine [in this trial] and is less expensive.
Dr. Stables predicted that based on these results, interventionalists at his institution who can choose between bivalirudin and UFH will increasingly use heparin, a change that he estimated could save his hospital as much as 500,000 pounds per year (about $800,000).
The HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary PCI) trial randomized 1,829 STEMI patients at Liverpool Heart and Chest Hospital during February 2012–November 2013. The data analysis included the 1,812 patients from the series who agreed to participate in the study, which had a delayed-consent design (see sidebar). A total of 1,917 patients with STEMI presented at Liverpool during this 22-month period, with about 5% of patients excluded because of prespecified exclusion criteria. This produced a highly representative, "real-world" population. "It’s the closest you can get to an all-comers trial," Dr. Stables said.
About 62% of the patients received ticagrelor (Brilinta) as an antiplatelet drug, about 27% received prasugrel (Effient), and the remaining 11% received clopidogrel. About 14% of the bivalirudin-treated patients and about 16% of those randomized to receive UFH were treated with a glycoprotein IIb/IIIa inhibitor. The door-to-balloon time for patients in the study averaged 29 minutes, and the average age of the patients was 63 years. About 81% of patients underwent coronary catheterization by radial-artery access, with the remainder undergoing femoral-artery access; about 82% of all patients had a PCI procedure actually performed. And of those who underwent primary PCI, about 92% of all patients received at least one coronary stent. The 907 consenting patients randomized to UFH received a dose of 70 units/kg prior to catheterization. The 905 consenting patients randomized to bivalirudin received a 0.75-mg/kg bolus and a 1.75-mg/kg/hour infusion during their procedure.
The difference in rates of major adverse coronary events during the 28-day follow-up seemed driven primarily by a difference in the rate of myocardial infarctions, which occurred in 2.3% of the bivalirudin patients and in 0.8% of the UFH patients, Dr. Shahzad said. The rate of acute stent thrombosis was 2.9% in the bivalirudin arm and 0.9% in patients on UFH.
Several prior trials that compared UFH and bivalirudin in STEMI or acute coronary syndrome patients undergoing PCI or other invasive procedures used either mandatory coadministration of a glycoprotein IIb/IIIa inhibitor along with UHF, or a much higher rate of discretionary use, such as in the ACUITY (N. Engl. J. Med. 2006;355:2203-16), HORIZONS (N. Engl. J. Med. 2008;358:2218-30), and EUROMAX (N. Engl. J. Med. 2013;369:2207-17) studies. In all these trials, the rates of major bleeding events were significantly higher in the UFH arm, and this appeared to lead to an increased number of adverse coronary events during the first month following intervention.
"What we’ve seen in every trial before was a reduction in bleeding complications of about 40% to 50%. The National Cardiovascular Data Registry shows a tremendous decrease [in bleeding] when you change from heparin to bivalirudin," said Dr. Roxana Mehran, an interventional cardiologist and professor of medicine at Mount Sinai Hospital in New York.
"All the trials had differential use of glycoprotein IIb/IIIa inhibitors" in the UFH and bivalirudin arms, noted Dr. Shahzad, an interventional cardiologist at Liverpool Heart and Chest Hospital.
Dr. Stables said that the low bleeding rates with UFH in the new study seemed largely dependent on the relatively low rate at which patients received a glycoprotein IIb/IIIa inhibitor and did not correlate with radial-artery access. "We did a prespecified subgroup analysis of patients who underwent radial or femoral access and the outcomes were the same in both groups," he said.
Dr. Gregg W. Stone, who served as lead investigator for ACUITY and HORIZONS, contended that the trialists in HEAT-PPCI underdosed bivalirudin based on the average activated clotting time (ACT) of 270 seconds in the bivalirudin-treated patients in the study. Most patients properly treated with bivalirudin achieve ACTs of 350-450 seconds, said Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University in New York. Dr. Stone also noted that the "results of this single-center study were markedly different from three randomized, controlled trials done at 250 centers and involving more than 8,000 patients."
Dr. Shahzad countered that the ACT-measuring device used at his institution was consistently accurate but measures ACT levels that are typically 50 seconds below values obtained with most other devices.
HEAT-PPCI did not receive any commercial funding. Dr. Shahzad, Dr. Stables, Dr. King, and Dr. Holmes had no disclosures. Dr. Mehran has received consultant fees, honoraria, and/or research grants from 10 drug and device companies, but not from The Medicines Company, which markets Angiomax. Dr. Stone has grants or fees from, or has an ownership position in, several drug or device companies, but not The Medicines Company.
On Twitter @mitchelzoler

|
Mitchel L. Zoler/Frontline Medical News
|
This was an extremely well-conducted trial with a very large patient population and meticulous oversight and exceptionally complete follow-up.
There has been a signal of increased stent thrombosis early on with bivalirudin, as was seen in the EUROMAX trial (N. Engl. J. Med. 2013;369:2207-17), but over the longer term these differences in event rates have been mitigated. A surprise in the current study was the absence of a difference in major bleeding complications between the two drug arms. The trial used radial access in 80% of patients, and radial access has been associated with significant decreases in access-site bleeding compared with transfemoral access. Future studies should examine the role of transradial access in leading to reduced bleeding in this setting.
David Kandzari, M.D., is an interventional cardiologist with Piedmont Healthcare in Atlanta. He has been a consultant to, and has received honoraria from, Micell Technologies, Biotronik, Boston Scientific, Thoratec, and Medtronic. He made these comments as a discussant for the trial.

|
Mitchel L. Zoler/Frontline Medical News
|
This was an extremely well-conducted trial with a very large patient population and meticulous oversight and exceptionally complete follow-up.
There has been a signal of increased stent thrombosis early on with bivalirudin, as was seen in the EUROMAX trial (N. Engl. J. Med. 2013;369:2207-17), but over the longer term these differences in event rates have been mitigated. A surprise in the current study was the absence of a difference in major bleeding complications between the two drug arms. The trial used radial access in 80% of patients, and radial access has been associated with significant decreases in access-site bleeding compared with transfemoral access. Future studies should examine the role of transradial access in leading to reduced bleeding in this setting.
David Kandzari, M.D., is an interventional cardiologist with Piedmont Healthcare in Atlanta. He has been a consultant to, and has received honoraria from, Micell Technologies, Biotronik, Boston Scientific, Thoratec, and Medtronic. He made these comments as a discussant for the trial.

|
Mitchel L. Zoler/Frontline Medical News
|
This was an extremely well-conducted trial with a very large patient population and meticulous oversight and exceptionally complete follow-up.
There has been a signal of increased stent thrombosis early on with bivalirudin, as was seen in the EUROMAX trial (N. Engl. J. Med. 2013;369:2207-17), but over the longer term these differences in event rates have been mitigated. A surprise in the current study was the absence of a difference in major bleeding complications between the two drug arms. The trial used radial access in 80% of patients, and radial access has been associated with significant decreases in access-site bleeding compared with transfemoral access. Future studies should examine the role of transradial access in leading to reduced bleeding in this setting.
David Kandzari, M.D., is an interventional cardiologist with Piedmont Healthcare in Atlanta. He has been a consultant to, and has received honoraria from, Micell Technologies, Biotronik, Boston Scientific, Thoratec, and Medtronic. He made these comments as a discussant for the trial.
WASHINGTON – Unfractionated heparin outperformed bivalirudin for 28-day outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention in a single-center randomized trial of more than 1,800 patients when use of a glycoprotein IIb/IIIa inhibitor was kept to a minimum.
Use of unfractionated heparin (UFH) compared with bivalirudin (Angiomax) led to a significant reduction in the rate of combined major adverse coronary events after 28 days compared with bivalirudin driven primarily by a significant cut in the rate of acute stent thrombosis and repeat myocardial infarctions with no increased risk for major bleeding events, consistent results across all subgroups examined, and the potential for substantial savings in drug costs, Dr. Adeel Shahzad said at the annual meeting of the American College of Cardiology.
The combined rate of major adverse coronary events was 6% in patients treated with UFH and 9% in those treated with bivalirudin, a statistically significant difference for the study’s primary efficacy endpoint. The rate of major bleeding events, the study’s primary safety endpoint, occurred in 3.5% of patients treated with bivalirudin and in 3.1% of those who received UFH, a difference that was not statistically significant.
The superior outcome with UFH in this study, which contrasted sharply with the results of several prior head-to-head comparisons of the two drugs in patients with a ST-segment elevation myocardial infarction (STEMI) or other acute coronary syndrome events undergoing primary percutaneous coronary intervention (PCI) seemed closely tied to the study’s protocol that restricted coadministration of a glycoprotein IIb/IIIa inhibitor (such as abciximab [ReoPro]) to only those patients experiencing thrombotic complications during PCI ("bailout" use).
"For the first time, unfractionated heparin was used like bivalirudin," with low use of a glycoprotein IIb/IIIa inhibitor, noted the study’s principal investigator, Dr. Rod Stables, director of the cardiac catheterization laboratory at Liverpool (England) Heart and Chest Hospital.
Current STEMI management guidelines from the American College of Cardiology and American Heart Association (J. Am. Coll. Cardiol. 2013;61:e78-140) and from the European Society of Cardiology (Eur. Heart J. 2012;33:2569-619) endorse use of either bivalirudin or UFH as an anticoagulant when patients undergo primary PCI. The European guidelines say that bivalirudin is preferred over UFH when a glycoprotein IIb/IIIa antagonist is administered along with UFH.
"It’s perfectly legitimate with the guidelines to use unfractionated heparin, but most people [in the United States] use bivalirudin or a glycoprotein IIb/IIIa inhibitor with heparin," said Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Health System in Atlanta.
Based on the new results, "people will say it’s really interesting, and they may at least think about not using bivalirudin all the time," said Dr. David R. Holmes Jr., an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "Those who are believers in bivalirudin will continue to use it, but others will say that heparin did just fine [in this trial] and is less expensive.
Dr. Stables predicted that based on these results, interventionalists at his institution who can choose between bivalirudin and UFH will increasingly use heparin, a change that he estimated could save his hospital as much as 500,000 pounds per year (about $800,000).
The HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary PCI) trial randomized 1,829 STEMI patients at Liverpool Heart and Chest Hospital during February 2012–November 2013. The data analysis included the 1,812 patients from the series who agreed to participate in the study, which had a delayed-consent design (see sidebar). A total of 1,917 patients with STEMI presented at Liverpool during this 22-month period, with about 5% of patients excluded because of prespecified exclusion criteria. This produced a highly representative, "real-world" population. "It’s the closest you can get to an all-comers trial," Dr. Stables said.
About 62% of the patients received ticagrelor (Brilinta) as an antiplatelet drug, about 27% received prasugrel (Effient), and the remaining 11% received clopidogrel. About 14% of the bivalirudin-treated patients and about 16% of those randomized to receive UFH were treated with a glycoprotein IIb/IIIa inhibitor. The door-to-balloon time for patients in the study averaged 29 minutes, and the average age of the patients was 63 years. About 81% of patients underwent coronary catheterization by radial-artery access, with the remainder undergoing femoral-artery access; about 82% of all patients had a PCI procedure actually performed. And of those who underwent primary PCI, about 92% of all patients received at least one coronary stent. The 907 consenting patients randomized to UFH received a dose of 70 units/kg prior to catheterization. The 905 consenting patients randomized to bivalirudin received a 0.75-mg/kg bolus and a 1.75-mg/kg/hour infusion during their procedure.
The difference in rates of major adverse coronary events during the 28-day follow-up seemed driven primarily by a difference in the rate of myocardial infarctions, which occurred in 2.3% of the bivalirudin patients and in 0.8% of the UFH patients, Dr. Shahzad said. The rate of acute stent thrombosis was 2.9% in the bivalirudin arm and 0.9% in patients on UFH.
Several prior trials that compared UFH and bivalirudin in STEMI or acute coronary syndrome patients undergoing PCI or other invasive procedures used either mandatory coadministration of a glycoprotein IIb/IIIa inhibitor along with UHF, or a much higher rate of discretionary use, such as in the ACUITY (N. Engl. J. Med. 2006;355:2203-16), HORIZONS (N. Engl. J. Med. 2008;358:2218-30), and EUROMAX (N. Engl. J. Med. 2013;369:2207-17) studies. In all these trials, the rates of major bleeding events were significantly higher in the UFH arm, and this appeared to lead to an increased number of adverse coronary events during the first month following intervention.
"What we’ve seen in every trial before was a reduction in bleeding complications of about 40% to 50%. The National Cardiovascular Data Registry shows a tremendous decrease [in bleeding] when you change from heparin to bivalirudin," said Dr. Roxana Mehran, an interventional cardiologist and professor of medicine at Mount Sinai Hospital in New York.
"All the trials had differential use of glycoprotein IIb/IIIa inhibitors" in the UFH and bivalirudin arms, noted Dr. Shahzad, an interventional cardiologist at Liverpool Heart and Chest Hospital.
Dr. Stables said that the low bleeding rates with UFH in the new study seemed largely dependent on the relatively low rate at which patients received a glycoprotein IIb/IIIa inhibitor and did not correlate with radial-artery access. "We did a prespecified subgroup analysis of patients who underwent radial or femoral access and the outcomes were the same in both groups," he said.
Dr. Gregg W. Stone, who served as lead investigator for ACUITY and HORIZONS, contended that the trialists in HEAT-PPCI underdosed bivalirudin based on the average activated clotting time (ACT) of 270 seconds in the bivalirudin-treated patients in the study. Most patients properly treated with bivalirudin achieve ACTs of 350-450 seconds, said Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University in New York. Dr. Stone also noted that the "results of this single-center study were markedly different from three randomized, controlled trials done at 250 centers and involving more than 8,000 patients."
Dr. Shahzad countered that the ACT-measuring device used at his institution was consistently accurate but measures ACT levels that are typically 50 seconds below values obtained with most other devices.
HEAT-PPCI did not receive any commercial funding. Dr. Shahzad, Dr. Stables, Dr. King, and Dr. Holmes had no disclosures. Dr. Mehran has received consultant fees, honoraria, and/or research grants from 10 drug and device companies, but not from The Medicines Company, which markets Angiomax. Dr. Stone has grants or fees from, or has an ownership position in, several drug or device companies, but not The Medicines Company.
On Twitter @mitchelzoler
WASHINGTON – Unfractionated heparin outperformed bivalirudin for 28-day outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention in a single-center randomized trial of more than 1,800 patients when use of a glycoprotein IIb/IIIa inhibitor was kept to a minimum.
Use of unfractionated heparin (UFH) compared with bivalirudin (Angiomax) led to a significant reduction in the rate of combined major adverse coronary events after 28 days compared with bivalirudin driven primarily by a significant cut in the rate of acute stent thrombosis and repeat myocardial infarctions with no increased risk for major bleeding events, consistent results across all subgroups examined, and the potential for substantial savings in drug costs, Dr. Adeel Shahzad said at the annual meeting of the American College of Cardiology.
The combined rate of major adverse coronary events was 6% in patients treated with UFH and 9% in those treated with bivalirudin, a statistically significant difference for the study’s primary efficacy endpoint. The rate of major bleeding events, the study’s primary safety endpoint, occurred in 3.5% of patients treated with bivalirudin and in 3.1% of those who received UFH, a difference that was not statistically significant.
The superior outcome with UFH in this study, which contrasted sharply with the results of several prior head-to-head comparisons of the two drugs in patients with a ST-segment elevation myocardial infarction (STEMI) or other acute coronary syndrome events undergoing primary percutaneous coronary intervention (PCI) seemed closely tied to the study’s protocol that restricted coadministration of a glycoprotein IIb/IIIa inhibitor (such as abciximab [ReoPro]) to only those patients experiencing thrombotic complications during PCI ("bailout" use).
"For the first time, unfractionated heparin was used like bivalirudin," with low use of a glycoprotein IIb/IIIa inhibitor, noted the study’s principal investigator, Dr. Rod Stables, director of the cardiac catheterization laboratory at Liverpool (England) Heart and Chest Hospital.
Current STEMI management guidelines from the American College of Cardiology and American Heart Association (J. Am. Coll. Cardiol. 2013;61:e78-140) and from the European Society of Cardiology (Eur. Heart J. 2012;33:2569-619) endorse use of either bivalirudin or UFH as an anticoagulant when patients undergo primary PCI. The European guidelines say that bivalirudin is preferred over UFH when a glycoprotein IIb/IIIa antagonist is administered along with UFH.
"It’s perfectly legitimate with the guidelines to use unfractionated heparin, but most people [in the United States] use bivalirudin or a glycoprotein IIb/IIIa inhibitor with heparin," said Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Health System in Atlanta.
Based on the new results, "people will say it’s really interesting, and they may at least think about not using bivalirudin all the time," said Dr. David R. Holmes Jr., an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "Those who are believers in bivalirudin will continue to use it, but others will say that heparin did just fine [in this trial] and is less expensive.
Dr. Stables predicted that based on these results, interventionalists at his institution who can choose between bivalirudin and UFH will increasingly use heparin, a change that he estimated could save his hospital as much as 500,000 pounds per year (about $800,000).
The HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary PCI) trial randomized 1,829 STEMI patients at Liverpool Heart and Chest Hospital during February 2012–November 2013. The data analysis included the 1,812 patients from the series who agreed to participate in the study, which had a delayed-consent design (see sidebar). A total of 1,917 patients with STEMI presented at Liverpool during this 22-month period, with about 5% of patients excluded because of prespecified exclusion criteria. This produced a highly representative, "real-world" population. "It’s the closest you can get to an all-comers trial," Dr. Stables said.
About 62% of the patients received ticagrelor (Brilinta) as an antiplatelet drug, about 27% received prasugrel (Effient), and the remaining 11% received clopidogrel. About 14% of the bivalirudin-treated patients and about 16% of those randomized to receive UFH were treated with a glycoprotein IIb/IIIa inhibitor. The door-to-balloon time for patients in the study averaged 29 minutes, and the average age of the patients was 63 years. About 81% of patients underwent coronary catheterization by radial-artery access, with the remainder undergoing femoral-artery access; about 82% of all patients had a PCI procedure actually performed. And of those who underwent primary PCI, about 92% of all patients received at least one coronary stent. The 907 consenting patients randomized to UFH received a dose of 70 units/kg prior to catheterization. The 905 consenting patients randomized to bivalirudin received a 0.75-mg/kg bolus and a 1.75-mg/kg/hour infusion during their procedure.
The difference in rates of major adverse coronary events during the 28-day follow-up seemed driven primarily by a difference in the rate of myocardial infarctions, which occurred in 2.3% of the bivalirudin patients and in 0.8% of the UFH patients, Dr. Shahzad said. The rate of acute stent thrombosis was 2.9% in the bivalirudin arm and 0.9% in patients on UFH.
Several prior trials that compared UFH and bivalirudin in STEMI or acute coronary syndrome patients undergoing PCI or other invasive procedures used either mandatory coadministration of a glycoprotein IIb/IIIa inhibitor along with UHF, or a much higher rate of discretionary use, such as in the ACUITY (N. Engl. J. Med. 2006;355:2203-16), HORIZONS (N. Engl. J. Med. 2008;358:2218-30), and EUROMAX (N. Engl. J. Med. 2013;369:2207-17) studies. In all these trials, the rates of major bleeding events were significantly higher in the UFH arm, and this appeared to lead to an increased number of adverse coronary events during the first month following intervention.
"What we’ve seen in every trial before was a reduction in bleeding complications of about 40% to 50%. The National Cardiovascular Data Registry shows a tremendous decrease [in bleeding] when you change from heparin to bivalirudin," said Dr. Roxana Mehran, an interventional cardiologist and professor of medicine at Mount Sinai Hospital in New York.
"All the trials had differential use of glycoprotein IIb/IIIa inhibitors" in the UFH and bivalirudin arms, noted Dr. Shahzad, an interventional cardiologist at Liverpool Heart and Chest Hospital.
Dr. Stables said that the low bleeding rates with UFH in the new study seemed largely dependent on the relatively low rate at which patients received a glycoprotein IIb/IIIa inhibitor and did not correlate with radial-artery access. "We did a prespecified subgroup analysis of patients who underwent radial or femoral access and the outcomes were the same in both groups," he said.
Dr. Gregg W. Stone, who served as lead investigator for ACUITY and HORIZONS, contended that the trialists in HEAT-PPCI underdosed bivalirudin based on the average activated clotting time (ACT) of 270 seconds in the bivalirudin-treated patients in the study. Most patients properly treated with bivalirudin achieve ACTs of 350-450 seconds, said Dr. Stone, professor of medicine and director of cardiovascular research and education at Columbia University in New York. Dr. Stone also noted that the "results of this single-center study were markedly different from three randomized, controlled trials done at 250 centers and involving more than 8,000 patients."
Dr. Shahzad countered that the ACT-measuring device used at his institution was consistently accurate but measures ACT levels that are typically 50 seconds below values obtained with most other devices.
HEAT-PPCI did not receive any commercial funding. Dr. Shahzad, Dr. Stables, Dr. King, and Dr. Holmes had no disclosures. Dr. Mehran has received consultant fees, honoraria, and/or research grants from 10 drug and device companies, but not from The Medicines Company, which markets Angiomax. Dr. Stone has grants or fees from, or has an ownership position in, several drug or device companies, but not The Medicines Company.
On Twitter @mitchelzoler
AT ACC 14
Mortality nearly halved with dual antiplatelet therapy in severe PAD
WASHINGTON – Long-term dual-antiplatelet therapy may provide a mortality benefit over aspirin alone in patients with symptomatic peripheral artery disease.
In an observational study of 629 patients with claudication or critical limb ischemia, the 348 who were on dual antiplatelet therapy (DAPT) with aspirin plus clopidogrel had a 3-year all-cause mortality rate of 11%, compared with 21% for those on aspirin monotherapy, Dr. Ehrin J. Armstrong said at the annual meeting of the American College of Cardiology.
The group on DAPT also had a significantly lower 3-year rate of major adverse cardiovascular events: 20%, compared with 28% in the monotherapy group. However, this was driven by the reduced risk of mortality. Rates of nonfatal MI and stroke were similar in the two groups. So were rates of lower extremity bypass surgery and major amputations, according to Dr. Armstrong, a cardiologist at the University of California, Davis.
The group on DAPT had a significantly higher baseline prevalence of diabetes at the time of angiography: 54%, compared with 45% for patients on aspirin alone. The DAPT group also had a higher baseline prevalence of known coronary artery disease – 56% vs. 45% – and greater use of beta-blockers, by a margin of 55%, compared with 48%. In a multivariate regression analysis adjusted for these and other potential confounders, DAPT was associated with a 45% reduction in the risk of mortality and a 35% decrease in major adverse cardiovascular events.
The rate of the combined endpoint of death or major amputation was 18% in the DAPT group and 27% with aspirin monotherapy, for a highly significant 47% relative risk reduction.
Although a study such as this can’t be considered definitive, these data suggest DAPT is worth considering in patients with symptomatic peripheral artery disease, the cardiologist said.
He reported having no financial conflicts of interest.
WASHINGTON – Long-term dual-antiplatelet therapy may provide a mortality benefit over aspirin alone in patients with symptomatic peripheral artery disease.
In an observational study of 629 patients with claudication or critical limb ischemia, the 348 who were on dual antiplatelet therapy (DAPT) with aspirin plus clopidogrel had a 3-year all-cause mortality rate of 11%, compared with 21% for those on aspirin monotherapy, Dr. Ehrin J. Armstrong said at the annual meeting of the American College of Cardiology.
The group on DAPT also had a significantly lower 3-year rate of major adverse cardiovascular events: 20%, compared with 28% in the monotherapy group. However, this was driven by the reduced risk of mortality. Rates of nonfatal MI and stroke were similar in the two groups. So were rates of lower extremity bypass surgery and major amputations, according to Dr. Armstrong, a cardiologist at the University of California, Davis.
The group on DAPT had a significantly higher baseline prevalence of diabetes at the time of angiography: 54%, compared with 45% for patients on aspirin alone. The DAPT group also had a higher baseline prevalence of known coronary artery disease – 56% vs. 45% – and greater use of beta-blockers, by a margin of 55%, compared with 48%. In a multivariate regression analysis adjusted for these and other potential confounders, DAPT was associated with a 45% reduction in the risk of mortality and a 35% decrease in major adverse cardiovascular events.
The rate of the combined endpoint of death or major amputation was 18% in the DAPT group and 27% with aspirin monotherapy, for a highly significant 47% relative risk reduction.
Although a study such as this can’t be considered definitive, these data suggest DAPT is worth considering in patients with symptomatic peripheral artery disease, the cardiologist said.
He reported having no financial conflicts of interest.
WASHINGTON – Long-term dual-antiplatelet therapy may provide a mortality benefit over aspirin alone in patients with symptomatic peripheral artery disease.
In an observational study of 629 patients with claudication or critical limb ischemia, the 348 who were on dual antiplatelet therapy (DAPT) with aspirin plus clopidogrel had a 3-year all-cause mortality rate of 11%, compared with 21% for those on aspirin monotherapy, Dr. Ehrin J. Armstrong said at the annual meeting of the American College of Cardiology.
The group on DAPT also had a significantly lower 3-year rate of major adverse cardiovascular events: 20%, compared with 28% in the monotherapy group. However, this was driven by the reduced risk of mortality. Rates of nonfatal MI and stroke were similar in the two groups. So were rates of lower extremity bypass surgery and major amputations, according to Dr. Armstrong, a cardiologist at the University of California, Davis.
The group on DAPT had a significantly higher baseline prevalence of diabetes at the time of angiography: 54%, compared with 45% for patients on aspirin alone. The DAPT group also had a higher baseline prevalence of known coronary artery disease – 56% vs. 45% – and greater use of beta-blockers, by a margin of 55%, compared with 48%. In a multivariate regression analysis adjusted for these and other potential confounders, DAPT was associated with a 45% reduction in the risk of mortality and a 35% decrease in major adverse cardiovascular events.
The rate of the combined endpoint of death or major amputation was 18% in the DAPT group and 27% with aspirin monotherapy, for a highly significant 47% relative risk reduction.
Although a study such as this can’t be considered definitive, these data suggest DAPT is worth considering in patients with symptomatic peripheral artery disease, the cardiologist said.
He reported having no financial conflicts of interest.
AT ACC 14
Major finding: Patients with severe peripheral artery disease who were on dual antiplatelet therapy had an adjusted 45% reduction in the 3-year risk of all-cause mortality compared with those on aspirin monotherapy.
Data source: A retrospective, single-center study of 348 patients on DAPT with aspirin and clopidogrel and 281 on aspirin alone, all with PAD marked by claudication or critical limb ischemia.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
LDL particle number advantageous in managing cardiovascular risk
WASHINGTON – High-risk patients managed to a target LDL particle number rather than to an LDL cholesterol goal had 25% fewer cardiovascular events over 3 years of follow-up.
"These new data add to the growing body of evidence suggesting that [nuclear magnetic resonance] measurement of LDL particle number, when used in conjunction with other lipid measurements, is a valuable cardiovascular risk management tool," Dr. Terry A. Jacobson declared at the annual meeting of the American College of Cardiology.
LDL cholesterol level and LDL particle number are often discordant, particularly in patients with diabetes, metabolic syndrome, or hypertriglyceridemia. Data from several major epidemiologic studies, including the Framingham Offspring Study (J. Clin. Lipidol. 2007;1:583-92), have shown that LDL particle number better predicts cardiovascular events than LDL cholesterol level.
Until now, however, what has been missing is real-world evidence that treating to a target LDL particle number leads to better clinical outcomes than treating to a target LDL cholesterol number, explained Dr. Jacobson, professor of medicine at Emory University and director of the Office of Health Promotion and Disease Prevention at Grady Health Systems, both in Atlanta.
He presented a retrospective, nonrandomized analysis of 705 patients with coronary heart disease or a CHD equivalent who were placed on lipid-lowering therapy aimed at lowering their LDL particle number to less than 1,000 nmol/L and were then followed for 3 years. They were matched based upon demographics and comorbidities to an equal number of high-risk patients whose lipid-lowering regimen brought them below a target LDL cholesterol of 100 mg/dL.
During 3 years of follow-up, one or more CHD events or strokes – the composite primary end point – occurred in 14.6% of the group targeted to an LDL particle number below 1,000 nmol/L, compared with 19% of those whose goal was an LDL cholesterol level below 100 mg/dL, for a 25% relative risk reduction.
In a separate cohort of 4,188 matched high-risk patients followed for 12 months, the composite end point occurred in 6.3% of the group with an LDL particle number target, compared with 8.1% with an LDL cholesterol target of less than 100 mg/dL, for a 24% relative risk reduction, he added.
All participants in this study were selected from the HealthCore Integrated Research Database, which is associated with WellPoint, a large commercial health plan. These were high-risk patients: 60% had cardiovascular disease at baseline, and 57% had diabetes.
Attaining an LDL particle number of less than 1,000 nmol/L entailed a more aggressive lipid-lowering strategy than the one employed to drive LDL cholesterol below 100 mg/dL. Patients managed to the LDL particle number target were more likely to receive higher-potency statins at baseline. As a result, their mean LDL cholesterol was lower: 73 mg/dL in the group followed for 3 years, compared with 79 mg/dL in patients with an LDL cholesterol target of less than 100 mg/dL.
LDL particle number was measured via nuclear magnetic resonance technology using the NMR LipoProfile test, the sole Food and Drug Administration-approved blood test for this purpose.
The proprietary test is marketed by LipoScience, which funded this study. Dr. Jacobson is a consultant to the company as well as to Amarin, AstraZeneca, HealthCore, Merck, and Regeneron/Sanofi.
WASHINGTON – High-risk patients managed to a target LDL particle number rather than to an LDL cholesterol goal had 25% fewer cardiovascular events over 3 years of follow-up.
"These new data add to the growing body of evidence suggesting that [nuclear magnetic resonance] measurement of LDL particle number, when used in conjunction with other lipid measurements, is a valuable cardiovascular risk management tool," Dr. Terry A. Jacobson declared at the annual meeting of the American College of Cardiology.
LDL cholesterol level and LDL particle number are often discordant, particularly in patients with diabetes, metabolic syndrome, or hypertriglyceridemia. Data from several major epidemiologic studies, including the Framingham Offspring Study (J. Clin. Lipidol. 2007;1:583-92), have shown that LDL particle number better predicts cardiovascular events than LDL cholesterol level.
Until now, however, what has been missing is real-world evidence that treating to a target LDL particle number leads to better clinical outcomes than treating to a target LDL cholesterol number, explained Dr. Jacobson, professor of medicine at Emory University and director of the Office of Health Promotion and Disease Prevention at Grady Health Systems, both in Atlanta.
He presented a retrospective, nonrandomized analysis of 705 patients with coronary heart disease or a CHD equivalent who were placed on lipid-lowering therapy aimed at lowering their LDL particle number to less than 1,000 nmol/L and were then followed for 3 years. They were matched based upon demographics and comorbidities to an equal number of high-risk patients whose lipid-lowering regimen brought them below a target LDL cholesterol of 100 mg/dL.
During 3 years of follow-up, one or more CHD events or strokes – the composite primary end point – occurred in 14.6% of the group targeted to an LDL particle number below 1,000 nmol/L, compared with 19% of those whose goal was an LDL cholesterol level below 100 mg/dL, for a 25% relative risk reduction.
In a separate cohort of 4,188 matched high-risk patients followed for 12 months, the composite end point occurred in 6.3% of the group with an LDL particle number target, compared with 8.1% with an LDL cholesterol target of less than 100 mg/dL, for a 24% relative risk reduction, he added.
All participants in this study were selected from the HealthCore Integrated Research Database, which is associated with WellPoint, a large commercial health plan. These were high-risk patients: 60% had cardiovascular disease at baseline, and 57% had diabetes.
Attaining an LDL particle number of less than 1,000 nmol/L entailed a more aggressive lipid-lowering strategy than the one employed to drive LDL cholesterol below 100 mg/dL. Patients managed to the LDL particle number target were more likely to receive higher-potency statins at baseline. As a result, their mean LDL cholesterol was lower: 73 mg/dL in the group followed for 3 years, compared with 79 mg/dL in patients with an LDL cholesterol target of less than 100 mg/dL.
LDL particle number was measured via nuclear magnetic resonance technology using the NMR LipoProfile test, the sole Food and Drug Administration-approved blood test for this purpose.
The proprietary test is marketed by LipoScience, which funded this study. Dr. Jacobson is a consultant to the company as well as to Amarin, AstraZeneca, HealthCore, Merck, and Regeneron/Sanofi.
WASHINGTON – High-risk patients managed to a target LDL particle number rather than to an LDL cholesterol goal had 25% fewer cardiovascular events over 3 years of follow-up.
"These new data add to the growing body of evidence suggesting that [nuclear magnetic resonance] measurement of LDL particle number, when used in conjunction with other lipid measurements, is a valuable cardiovascular risk management tool," Dr. Terry A. Jacobson declared at the annual meeting of the American College of Cardiology.
LDL cholesterol level and LDL particle number are often discordant, particularly in patients with diabetes, metabolic syndrome, or hypertriglyceridemia. Data from several major epidemiologic studies, including the Framingham Offspring Study (J. Clin. Lipidol. 2007;1:583-92), have shown that LDL particle number better predicts cardiovascular events than LDL cholesterol level.
Until now, however, what has been missing is real-world evidence that treating to a target LDL particle number leads to better clinical outcomes than treating to a target LDL cholesterol number, explained Dr. Jacobson, professor of medicine at Emory University and director of the Office of Health Promotion and Disease Prevention at Grady Health Systems, both in Atlanta.
He presented a retrospective, nonrandomized analysis of 705 patients with coronary heart disease or a CHD equivalent who were placed on lipid-lowering therapy aimed at lowering their LDL particle number to less than 1,000 nmol/L and were then followed for 3 years. They were matched based upon demographics and comorbidities to an equal number of high-risk patients whose lipid-lowering regimen brought them below a target LDL cholesterol of 100 mg/dL.
During 3 years of follow-up, one or more CHD events or strokes – the composite primary end point – occurred in 14.6% of the group targeted to an LDL particle number below 1,000 nmol/L, compared with 19% of those whose goal was an LDL cholesterol level below 100 mg/dL, for a 25% relative risk reduction.
In a separate cohort of 4,188 matched high-risk patients followed for 12 months, the composite end point occurred in 6.3% of the group with an LDL particle number target, compared with 8.1% with an LDL cholesterol target of less than 100 mg/dL, for a 24% relative risk reduction, he added.
All participants in this study were selected from the HealthCore Integrated Research Database, which is associated with WellPoint, a large commercial health plan. These were high-risk patients: 60% had cardiovascular disease at baseline, and 57% had diabetes.
Attaining an LDL particle number of less than 1,000 nmol/L entailed a more aggressive lipid-lowering strategy than the one employed to drive LDL cholesterol below 100 mg/dL. Patients managed to the LDL particle number target were more likely to receive higher-potency statins at baseline. As a result, their mean LDL cholesterol was lower: 73 mg/dL in the group followed for 3 years, compared with 79 mg/dL in patients with an LDL cholesterol target of less than 100 mg/dL.
LDL particle number was measured via nuclear magnetic resonance technology using the NMR LipoProfile test, the sole Food and Drug Administration-approved blood test for this purpose.
The proprietary test is marketed by LipoScience, which funded this study. Dr. Jacobson is a consultant to the company as well as to Amarin, AstraZeneca, HealthCore, Merck, and Regeneron/Sanofi.
AT ACC 14
Major finding: The 3-year incidence of coronary heart disease events or stroke was 14.6% in high-risk patients managed to an LDL particle number target of less than 1,000 nmol/L, compared with 19% in those managed to a target LDL cholesterol of less than 100 mg/dL.
Data source: This was a retrospective case-control study in which 1,410 patients with baseline coronary heart disease or a CHD equivalent were placed on lipid-lowering therapy with a target of either an LDL cholesterol below 100 mg/dL or an LDL particle number of less than 1,000 nmol/L.
Disclosures: The study was sponsored by LipoScience. The presenter is a consultant to the company.
Bivalirudin called safe and effective in HIT
WASHINGTON – Bivalirudin is an attractive off-label option for the treatment of heparin-induced thrombocytopenia, Dr. Lee Joseph said at the annual meeting of the American College of Cardiology.
She presented what is believed to be the largest-ever case series of patients treated for heparin-induced thrombocytopenia (HIT) using bivalirudin (Angiomax): 641 patients treated over a 9-year period at the Cleveland Clinic, where bivalirudin has been the treatment of choice for HIT since 2002.
Roughly half of the bivalirudin-treated patients with confirmed or suspected HIT were medical patients, the other half surgical. One-quarter of the patients were in the intensive care unit. Chronic renal failure was present in 27% of patients, and 8% had chronic liver disease. Their average duration of heparin exposure was 7 days. At the time when HIT was diagnosed, 55% of the patients had a venous thromboembolism, and 10% had an arterial thromboembolic event.
The treatment outcomes were impressive, particularly when compared with studies involving the other anticoagulants used in treating HIT, Dr. Joseph said. There were no HIT-related amputations in bivalirudin-treated patients. The rate of new thrombosis was 4.6%. All-cause 30-day mortality was 14.5%, with a 0.2% incidence of death due to HIT-related thrombosis. The major bleeding rate was 7.6%, with a 2.4% rate of nonmajor bleeding. Fatal bleeding occurred in 1.5% of patients treated with the direct thrombin inhibitor.
Patients received bivalirudin for a median of 9 days. A therapeutic activated partial thromboplastin time was attained within a median of 12 hours.
A search for predictors of treatment outcome turned up three risk factors for major bleeding events and/or 30-day mortality on bivalirudin: dialysis dependence, being in the intensive care unit, and having a platelet count nadir below 60,000/uL, according to Dr. Joseph, who is now with the department of cardiovascular medicine at the University of Iowa in Iowa City.
The sole available agent approved in the United States for treatment of HIT is argatroban. Its use is problematic because of its very long half-life and resulting difficulty in transitioning to long-term anticoagulation with warfarin. Bivalirudin and fondaparinux (Arixtra), an indirect factor Xa inhibitor, are used off-label in treating HIT. Physicians at the Cleveland Clinic have used bivalirudin almost exclusively for more than a decade because of its short half-life of about 25 minutes, its low immunogenicity, the fact that only 20% of the medication is eliminated renally, the potential for rapid dose titration with no need for an initial bolus, and bivalirudin’s minimal interference with INR measurement. Plus cardiologists were already comfortable using bivalirudin in the cardiac catheterization lab, she explained.
In published studies of argatroban, fondaparinux, and lepirudin – an agent no longer available in the United States – HIT-related amputation rates of 5%-14% were reported, along with 30-day all-cause mortality rates of 7%-18% and major bleeding rates of 6%-15%.
Dr. Joseph said a prospective study comparing bivalirudin with other agents for HIT is in the planning stages.
An audience member noted that bivalirudin is an expensive drug and wondered about the possibility of using dabigatran, a less costly oral direct thrombin inhibitor, in treating HIT. Dr. Joseph replied that nearly all patients with HIT are hospitalized, and many are seriously ill, so an intravenously administered agent with a short half-life or rapid reversibility is the preferred strategy.
She reported having no financial conflicts with regard to this study, which was free of commercial support.
WASHINGTON – Bivalirudin is an attractive off-label option for the treatment of heparin-induced thrombocytopenia, Dr. Lee Joseph said at the annual meeting of the American College of Cardiology.
She presented what is believed to be the largest-ever case series of patients treated for heparin-induced thrombocytopenia (HIT) using bivalirudin (Angiomax): 641 patients treated over a 9-year period at the Cleveland Clinic, where bivalirudin has been the treatment of choice for HIT since 2002.
Roughly half of the bivalirudin-treated patients with confirmed or suspected HIT were medical patients, the other half surgical. One-quarter of the patients were in the intensive care unit. Chronic renal failure was present in 27% of patients, and 8% had chronic liver disease. Their average duration of heparin exposure was 7 days. At the time when HIT was diagnosed, 55% of the patients had a venous thromboembolism, and 10% had an arterial thromboembolic event.
The treatment outcomes were impressive, particularly when compared with studies involving the other anticoagulants used in treating HIT, Dr. Joseph said. There were no HIT-related amputations in bivalirudin-treated patients. The rate of new thrombosis was 4.6%. All-cause 30-day mortality was 14.5%, with a 0.2% incidence of death due to HIT-related thrombosis. The major bleeding rate was 7.6%, with a 2.4% rate of nonmajor bleeding. Fatal bleeding occurred in 1.5% of patients treated with the direct thrombin inhibitor.
Patients received bivalirudin for a median of 9 days. A therapeutic activated partial thromboplastin time was attained within a median of 12 hours.
A search for predictors of treatment outcome turned up three risk factors for major bleeding events and/or 30-day mortality on bivalirudin: dialysis dependence, being in the intensive care unit, and having a platelet count nadir below 60,000/uL, according to Dr. Joseph, who is now with the department of cardiovascular medicine at the University of Iowa in Iowa City.
The sole available agent approved in the United States for treatment of HIT is argatroban. Its use is problematic because of its very long half-life and resulting difficulty in transitioning to long-term anticoagulation with warfarin. Bivalirudin and fondaparinux (Arixtra), an indirect factor Xa inhibitor, are used off-label in treating HIT. Physicians at the Cleveland Clinic have used bivalirudin almost exclusively for more than a decade because of its short half-life of about 25 minutes, its low immunogenicity, the fact that only 20% of the medication is eliminated renally, the potential for rapid dose titration with no need for an initial bolus, and bivalirudin’s minimal interference with INR measurement. Plus cardiologists were already comfortable using bivalirudin in the cardiac catheterization lab, she explained.
In published studies of argatroban, fondaparinux, and lepirudin – an agent no longer available in the United States – HIT-related amputation rates of 5%-14% were reported, along with 30-day all-cause mortality rates of 7%-18% and major bleeding rates of 6%-15%.
Dr. Joseph said a prospective study comparing bivalirudin with other agents for HIT is in the planning stages.
An audience member noted that bivalirudin is an expensive drug and wondered about the possibility of using dabigatran, a less costly oral direct thrombin inhibitor, in treating HIT. Dr. Joseph replied that nearly all patients with HIT are hospitalized, and many are seriously ill, so an intravenously administered agent with a short half-life or rapid reversibility is the preferred strategy.
She reported having no financial conflicts with regard to this study, which was free of commercial support.
WASHINGTON – Bivalirudin is an attractive off-label option for the treatment of heparin-induced thrombocytopenia, Dr. Lee Joseph said at the annual meeting of the American College of Cardiology.
She presented what is believed to be the largest-ever case series of patients treated for heparin-induced thrombocytopenia (HIT) using bivalirudin (Angiomax): 641 patients treated over a 9-year period at the Cleveland Clinic, where bivalirudin has been the treatment of choice for HIT since 2002.
Roughly half of the bivalirudin-treated patients with confirmed or suspected HIT were medical patients, the other half surgical. One-quarter of the patients were in the intensive care unit. Chronic renal failure was present in 27% of patients, and 8% had chronic liver disease. Their average duration of heparin exposure was 7 days. At the time when HIT was diagnosed, 55% of the patients had a venous thromboembolism, and 10% had an arterial thromboembolic event.
The treatment outcomes were impressive, particularly when compared with studies involving the other anticoagulants used in treating HIT, Dr. Joseph said. There were no HIT-related amputations in bivalirudin-treated patients. The rate of new thrombosis was 4.6%. All-cause 30-day mortality was 14.5%, with a 0.2% incidence of death due to HIT-related thrombosis. The major bleeding rate was 7.6%, with a 2.4% rate of nonmajor bleeding. Fatal bleeding occurred in 1.5% of patients treated with the direct thrombin inhibitor.
Patients received bivalirudin for a median of 9 days. A therapeutic activated partial thromboplastin time was attained within a median of 12 hours.
A search for predictors of treatment outcome turned up three risk factors for major bleeding events and/or 30-day mortality on bivalirudin: dialysis dependence, being in the intensive care unit, and having a platelet count nadir below 60,000/uL, according to Dr. Joseph, who is now with the department of cardiovascular medicine at the University of Iowa in Iowa City.
The sole available agent approved in the United States for treatment of HIT is argatroban. Its use is problematic because of its very long half-life and resulting difficulty in transitioning to long-term anticoagulation with warfarin. Bivalirudin and fondaparinux (Arixtra), an indirect factor Xa inhibitor, are used off-label in treating HIT. Physicians at the Cleveland Clinic have used bivalirudin almost exclusively for more than a decade because of its short half-life of about 25 minutes, its low immunogenicity, the fact that only 20% of the medication is eliminated renally, the potential for rapid dose titration with no need for an initial bolus, and bivalirudin’s minimal interference with INR measurement. Plus cardiologists were already comfortable using bivalirudin in the cardiac catheterization lab, she explained.
In published studies of argatroban, fondaparinux, and lepirudin – an agent no longer available in the United States – HIT-related amputation rates of 5%-14% were reported, along with 30-day all-cause mortality rates of 7%-18% and major bleeding rates of 6%-15%.
Dr. Joseph said a prospective study comparing bivalirudin with other agents for HIT is in the planning stages.
An audience member noted that bivalirudin is an expensive drug and wondered about the possibility of using dabigatran, a less costly oral direct thrombin inhibitor, in treating HIT. Dr. Joseph replied that nearly all patients with HIT are hospitalized, and many are seriously ill, so an intravenously administered agent with a short half-life or rapid reversibility is the preferred strategy.
She reported having no financial conflicts with regard to this study, which was free of commercial support.
AT ACC 14
Major finding: In a large series of patients who received bivalirudin for heparin-induced thrombocytopenia, there were no amputations, a 4.6% rate of new thrombosis, a 0.2% incidence of death due to thrombosis, and a 7.6% major bleeding rate.
Data source: This was a retrospective case series of 641 patients with heparin-induced thrombocytopenia treated with bivalirudin at the Cleveland Clinic over a 9-year period.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted without commercial support.
A fib–associated stroke jumped 42% in 2000s
WASHINGTON – A third more Americans were hospitalized for ischemic stroke in 2010 than 7 years before, and the incidence of stroke associated with atrial fibrillation grew even more sharply, up by 42% in 2010 relative to the 2003 rate, according to nationwide data collected in the Nationwide Inpatient Sample.
The period 2003-2010 also witnessed other notable changes in hospitalized U.S. patients with stroke or atrial fibrillation (AF) or both. The in-hospital mortality rate of hospitalized stroke patients with AF dropped from 12% in 2003 to 9% in 2010. During the same period the prevalence of hospitalized AF patients with a high stroke risk, identified by a CHADS (congestive heart failure, hypertension, age > 75 years, diabetes, and prior stroke or transient ischemic attack) score greater than two rose from 20% of hospitalized AF patients in 2003 to 29% in 2010, reflecting an increased prevalence of the comorbidities that drive the CHADS score. The prevalence of a prior stroke or transient ischemic attack (TIA) among hospitalized American AF patients jumped from 5% in 2003 to 12% in 2010, Dr. Arun R.M. Sridhar said at the annual meeting of the American College of Cardiology.
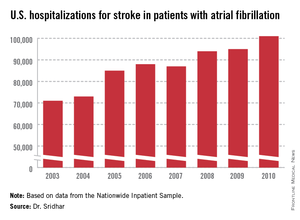
In a multivariate analysis, prior stroke or TIA was the strongest risk predictor for a subsequent stroke among the hospitalized AF cases in the data base, said Dr. Sridhar, a physician at the University of Kansas Medical Center in Kansas City.
But increases in the prevalence of prior stroke and TIA as well as in other measured comorbidities and the growing elderly population with AF and stroke were together unable to fully explain the rising rate of AF-associated strokes. "The rise in strokes persists despite accounting for the changes in demographics. The reasons are not very clear," Dr. Sridhar said.
The Nationwide Inpatient Sample data, collected annually by the Agency for Healthcare Research and Quality, showed that total U.S. hospitalizations for stroke rose from about 335,000 in 2003 to 448,000 in 2010, a 34% jump over 8 years. During the same time stroke hospitalizations for patients with AF increased from about 71,000 in 2003 to 101,000 in 2010, a 42% relative spike. This means the percent of hospitalized stroke patients who also had AF inched up from 21.2% in 2003 to 22.5% in 2010.
One of the clear drivers of the increase in AF-associated strokes was an aging population. In 2003, 37% of AF associated, hospitalized strokes were in patients aged 85 or older. By 2010, the 85 and older segment had swelled to 42%. The statistics also showed discernable but slower growth in patients aged 65-84 years, as well as in women and whites.
Mortality among hospitalized stroke patients fell across the board, not just among those with coexistent AF. For hospitalized stroke patients without AF in-hospital mortality fell from 5.1% in 2003 to 4.1% in 2010. During the 8 years, the average length of hospitalization dropped by about a day for all stroke patients, but despite that inflation-adjusted hospitalization charges jumped by 27% for patients with stroke and AF and by 39% for those with stroke and no AF.
Dr. Sridhar and his associates also analyzed data from more than 27 million Americans hospitalized with AF during the 8-year period. During this time the prevalence of several comorbidities in AF patients all rose and drove up their CHADS scores, starting with the increased prevalence of a history of stroke or TIA. The prevalence of hypertension in hospitalized AF patients rose from 45% to 48%, the prevalence of concomitant heart failure rose from 31% to 37%, and the prevalence of diabetes rose from 24% to 29%. But all these increases, as well as older patient ages, could not fully account for the 42% jump in the total of hospitalized stroke patients with AF from 2003 to 2010, Dr. Sridhar said.
Dr. Sridhar and his associates said that they had no disclosures.
On Twitter @mitchelzoler
WASHINGTON – A third more Americans were hospitalized for ischemic stroke in 2010 than 7 years before, and the incidence of stroke associated with atrial fibrillation grew even more sharply, up by 42% in 2010 relative to the 2003 rate, according to nationwide data collected in the Nationwide Inpatient Sample.
The period 2003-2010 also witnessed other notable changes in hospitalized U.S. patients with stroke or atrial fibrillation (AF) or both. The in-hospital mortality rate of hospitalized stroke patients with AF dropped from 12% in 2003 to 9% in 2010. During the same period the prevalence of hospitalized AF patients with a high stroke risk, identified by a CHADS (congestive heart failure, hypertension, age > 75 years, diabetes, and prior stroke or transient ischemic attack) score greater than two rose from 20% of hospitalized AF patients in 2003 to 29% in 2010, reflecting an increased prevalence of the comorbidities that drive the CHADS score. The prevalence of a prior stroke or transient ischemic attack (TIA) among hospitalized American AF patients jumped from 5% in 2003 to 12% in 2010, Dr. Arun R.M. Sridhar said at the annual meeting of the American College of Cardiology.

In a multivariate analysis, prior stroke or TIA was the strongest risk predictor for a subsequent stroke among the hospitalized AF cases in the data base, said Dr. Sridhar, a physician at the University of Kansas Medical Center in Kansas City.
But increases in the prevalence of prior stroke and TIA as well as in other measured comorbidities and the growing elderly population with AF and stroke were together unable to fully explain the rising rate of AF-associated strokes. "The rise in strokes persists despite accounting for the changes in demographics. The reasons are not very clear," Dr. Sridhar said.
The Nationwide Inpatient Sample data, collected annually by the Agency for Healthcare Research and Quality, showed that total U.S. hospitalizations for stroke rose from about 335,000 in 2003 to 448,000 in 2010, a 34% jump over 8 years. During the same time stroke hospitalizations for patients with AF increased from about 71,000 in 2003 to 101,000 in 2010, a 42% relative spike. This means the percent of hospitalized stroke patients who also had AF inched up from 21.2% in 2003 to 22.5% in 2010.
One of the clear drivers of the increase in AF-associated strokes was an aging population. In 2003, 37% of AF associated, hospitalized strokes were in patients aged 85 or older. By 2010, the 85 and older segment had swelled to 42%. The statistics also showed discernable but slower growth in patients aged 65-84 years, as well as in women and whites.
Mortality among hospitalized stroke patients fell across the board, not just among those with coexistent AF. For hospitalized stroke patients without AF in-hospital mortality fell from 5.1% in 2003 to 4.1% in 2010. During the 8 years, the average length of hospitalization dropped by about a day for all stroke patients, but despite that inflation-adjusted hospitalization charges jumped by 27% for patients with stroke and AF and by 39% for those with stroke and no AF.
Dr. Sridhar and his associates also analyzed data from more than 27 million Americans hospitalized with AF during the 8-year period. During this time the prevalence of several comorbidities in AF patients all rose and drove up their CHADS scores, starting with the increased prevalence of a history of stroke or TIA. The prevalence of hypertension in hospitalized AF patients rose from 45% to 48%, the prevalence of concomitant heart failure rose from 31% to 37%, and the prevalence of diabetes rose from 24% to 29%. But all these increases, as well as older patient ages, could not fully account for the 42% jump in the total of hospitalized stroke patients with AF from 2003 to 2010, Dr. Sridhar said.
Dr. Sridhar and his associates said that they had no disclosures.
On Twitter @mitchelzoler
WASHINGTON – A third more Americans were hospitalized for ischemic stroke in 2010 than 7 years before, and the incidence of stroke associated with atrial fibrillation grew even more sharply, up by 42% in 2010 relative to the 2003 rate, according to nationwide data collected in the Nationwide Inpatient Sample.
The period 2003-2010 also witnessed other notable changes in hospitalized U.S. patients with stroke or atrial fibrillation (AF) or both. The in-hospital mortality rate of hospitalized stroke patients with AF dropped from 12% in 2003 to 9% in 2010. During the same period the prevalence of hospitalized AF patients with a high stroke risk, identified by a CHADS (congestive heart failure, hypertension, age > 75 years, diabetes, and prior stroke or transient ischemic attack) score greater than two rose from 20% of hospitalized AF patients in 2003 to 29% in 2010, reflecting an increased prevalence of the comorbidities that drive the CHADS score. The prevalence of a prior stroke or transient ischemic attack (TIA) among hospitalized American AF patients jumped from 5% in 2003 to 12% in 2010, Dr. Arun R.M. Sridhar said at the annual meeting of the American College of Cardiology.

In a multivariate analysis, prior stroke or TIA was the strongest risk predictor for a subsequent stroke among the hospitalized AF cases in the data base, said Dr. Sridhar, a physician at the University of Kansas Medical Center in Kansas City.
But increases in the prevalence of prior stroke and TIA as well as in other measured comorbidities and the growing elderly population with AF and stroke were together unable to fully explain the rising rate of AF-associated strokes. "The rise in strokes persists despite accounting for the changes in demographics. The reasons are not very clear," Dr. Sridhar said.
The Nationwide Inpatient Sample data, collected annually by the Agency for Healthcare Research and Quality, showed that total U.S. hospitalizations for stroke rose from about 335,000 in 2003 to 448,000 in 2010, a 34% jump over 8 years. During the same time stroke hospitalizations for patients with AF increased from about 71,000 in 2003 to 101,000 in 2010, a 42% relative spike. This means the percent of hospitalized stroke patients who also had AF inched up from 21.2% in 2003 to 22.5% in 2010.
One of the clear drivers of the increase in AF-associated strokes was an aging population. In 2003, 37% of AF associated, hospitalized strokes were in patients aged 85 or older. By 2010, the 85 and older segment had swelled to 42%. The statistics also showed discernable but slower growth in patients aged 65-84 years, as well as in women and whites.
Mortality among hospitalized stroke patients fell across the board, not just among those with coexistent AF. For hospitalized stroke patients without AF in-hospital mortality fell from 5.1% in 2003 to 4.1% in 2010. During the 8 years, the average length of hospitalization dropped by about a day for all stroke patients, but despite that inflation-adjusted hospitalization charges jumped by 27% for patients with stroke and AF and by 39% for those with stroke and no AF.
Dr. Sridhar and his associates also analyzed data from more than 27 million Americans hospitalized with AF during the 8-year period. During this time the prevalence of several comorbidities in AF patients all rose and drove up their CHADS scores, starting with the increased prevalence of a history of stroke or TIA. The prevalence of hypertension in hospitalized AF patients rose from 45% to 48%, the prevalence of concomitant heart failure rose from 31% to 37%, and the prevalence of diabetes rose from 24% to 29%. But all these increases, as well as older patient ages, could not fully account for the 42% jump in the total of hospitalized stroke patients with AF from 2003 to 2010, Dr. Sridhar said.
Dr. Sridhar and his associates said that they had no disclosures.
On Twitter @mitchelzoler
AT ACC 2014
Major finding: During 2003-2010, annual stroke hospitalizations in U.S. atrial fibrillation patients rose from 71,000 to 101,000, a 42% increase.
Data source: Review of U.S. stroke hospitalizations during 2003-2010 recorded by the Nationwide Inpatient Sample.
Disclosures: Dr. Sridhar and his associates said that they had no disclosures.