User login
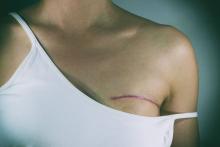
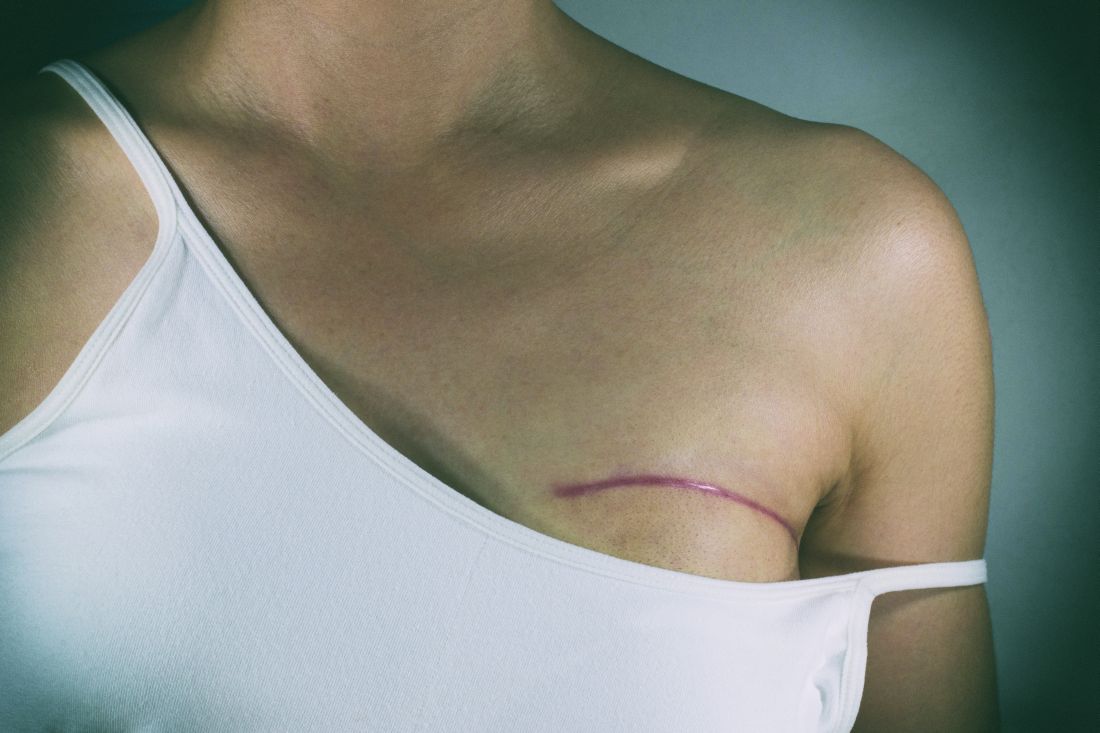
Sutureless, guided lumpectomy produced clean margins in majority
LAS VEGAS – An automated, minimally invasive, stereotactic-guided lumpectomy performed well in an outpatient setting, with no sutures required, potentially decreasing patient morbidity, according to Pat Whitworth, MD.
Pain scores were low, and all high-risk lesions were successfully removed.
The new technology is part of the natural progression of cancer resection, said Dr. Whitworth, director of the Nashville (Tenn.) Breast Center, at the annual meeting of the American Society of Breast Surgeons.
“We went from radical mastectomy, to modified radical mastectomy, to lumpectomy. This is just part of that progression where we match what we do to what the patient really needs,” he said.
Dr. Whitworth said he believes that radiologists will soon be using such technology to treat breast cancer, which puts the onus on breast cancer surgeons to adopt it themselves. “I think it’s important for breast surgeons to acquire the necessary skill and techniques to use the same tools and work collaboratively with radiologists, because this is coming,” he said.
To see if it were possible to achieve results similar to those with lumpectomy, Dr. Whitworth analyzed data from 279 women who had a small ductal carcinoma in situ (DCIS), invasive carcinoma, or high-risk lesion removed using a 15- or 20-mm radiofrequency basket capture with imaging guidance (lumpectomy). Patients who received a cancer diagnosis underwent a second, 20-mm basket capture to obtain shaved margins.
The procedure was conducted under local anesthesia and sedation, and the incisions were closed using Steri-Strip skin closures. The average pain score was 1.55 out of 10 (range, 0-7).
Of 125 patients found to have DCIS (n = 52) and invasive lesions (n = 73), the first capture achieved clear margins in 69 cases, and the shaved margin capture achieved clean margins in another 33 cases.
The remaining 23 patients (18%) had a positive margin by histologic standards following lumpectomy and shaved margin. Of the 22 with reported results, 17 (77%) had no residual lesion following open surgery.
The results convinced Dr. Wentworth of the technique’s utility, particularly in patients who may have a heightened surgical risk, he said. “We think this can replace open lumpectomy in selected patients, with favorable margin clearance.”
The approach is fairly simple, but Dr. Wentworth recommended beginning with stereotactic-guided technology before attempting the ultrasound-guided version, which requires a little more skill. “The biggest challenge is learning that you have to use a lot more local anesthetic. When this technology first came out, people tried to use the same amount of local anesthetic that they used for standard vacuum-assisted core biopsy, and it’s very painful unless you put 30 or 40 cc of dilute anesthetic in there,” said Dr. Wentworth.
The study was funded by Medtronic, which markets the technology. Dr. Whitworth is a principal at Targeted Medical Education, which receives funding from Medtronic.
LAS VEGAS – An automated, minimally invasive, stereotactic-guided lumpectomy performed well in an outpatient setting, with no sutures required, potentially decreasing patient morbidity, according to Pat Whitworth, MD.
Pain scores were low, and all high-risk lesions were successfully removed.
The new technology is part of the natural progression of cancer resection, said Dr. Whitworth, director of the Nashville (Tenn.) Breast Center, at the annual meeting of the American Society of Breast Surgeons.
“We went from radical mastectomy, to modified radical mastectomy, to lumpectomy. This is just part of that progression where we match what we do to what the patient really needs,” he said.
Dr. Whitworth said he believes that radiologists will soon be using such technology to treat breast cancer, which puts the onus on breast cancer surgeons to adopt it themselves. “I think it’s important for breast surgeons to acquire the necessary skill and techniques to use the same tools and work collaboratively with radiologists, because this is coming,” he said.
To see if it were possible to achieve results similar to those with lumpectomy, Dr. Whitworth analyzed data from 279 women who had a small ductal carcinoma in situ (DCIS), invasive carcinoma, or high-risk lesion removed using a 15- or 20-mm radiofrequency basket capture with imaging guidance (lumpectomy). Patients who received a cancer diagnosis underwent a second, 20-mm basket capture to obtain shaved margins.
The procedure was conducted under local anesthesia and sedation, and the incisions were closed using Steri-Strip skin closures. The average pain score was 1.55 out of 10 (range, 0-7).
Of 125 patients found to have DCIS (n = 52) and invasive lesions (n = 73), the first capture achieved clear margins in 69 cases, and the shaved margin capture achieved clean margins in another 33 cases.
The remaining 23 patients (18%) had a positive margin by histologic standards following lumpectomy and shaved margin. Of the 22 with reported results, 17 (77%) had no residual lesion following open surgery.
The results convinced Dr. Wentworth of the technique’s utility, particularly in patients who may have a heightened surgical risk, he said. “We think this can replace open lumpectomy in selected patients, with favorable margin clearance.”
The approach is fairly simple, but Dr. Wentworth recommended beginning with stereotactic-guided technology before attempting the ultrasound-guided version, which requires a little more skill. “The biggest challenge is learning that you have to use a lot more local anesthetic. When this technology first came out, people tried to use the same amount of local anesthetic that they used for standard vacuum-assisted core biopsy, and it’s very painful unless you put 30 or 40 cc of dilute anesthetic in there,” said Dr. Wentworth.
The study was funded by Medtronic, which markets the technology. Dr. Whitworth is a principal at Targeted Medical Education, which receives funding from Medtronic.
LAS VEGAS – An automated, minimally invasive, stereotactic-guided lumpectomy performed well in an outpatient setting, with no sutures required, potentially decreasing patient morbidity, according to Pat Whitworth, MD.
Pain scores were low, and all high-risk lesions were successfully removed.
The new technology is part of the natural progression of cancer resection, said Dr. Whitworth, director of the Nashville (Tenn.) Breast Center, at the annual meeting of the American Society of Breast Surgeons.
“We went from radical mastectomy, to modified radical mastectomy, to lumpectomy. This is just part of that progression where we match what we do to what the patient really needs,” he said.
Dr. Whitworth said he believes that radiologists will soon be using such technology to treat breast cancer, which puts the onus on breast cancer surgeons to adopt it themselves. “I think it’s important for breast surgeons to acquire the necessary skill and techniques to use the same tools and work collaboratively with radiologists, because this is coming,” he said.
To see if it were possible to achieve results similar to those with lumpectomy, Dr. Whitworth analyzed data from 279 women who had a small ductal carcinoma in situ (DCIS), invasive carcinoma, or high-risk lesion removed using a 15- or 20-mm radiofrequency basket capture with imaging guidance (lumpectomy). Patients who received a cancer diagnosis underwent a second, 20-mm basket capture to obtain shaved margins.
The procedure was conducted under local anesthesia and sedation, and the incisions were closed using Steri-Strip skin closures. The average pain score was 1.55 out of 10 (range, 0-7).
Of 125 patients found to have DCIS (n = 52) and invasive lesions (n = 73), the first capture achieved clear margins in 69 cases, and the shaved margin capture achieved clean margins in another 33 cases.
The remaining 23 patients (18%) had a positive margin by histologic standards following lumpectomy and shaved margin. Of the 22 with reported results, 17 (77%) had no residual lesion following open surgery.
The results convinced Dr. Wentworth of the technique’s utility, particularly in patients who may have a heightened surgical risk, he said. “We think this can replace open lumpectomy in selected patients, with favorable margin clearance.”
The approach is fairly simple, but Dr. Wentworth recommended beginning with stereotactic-guided technology before attempting the ultrasound-guided version, which requires a little more skill. “The biggest challenge is learning that you have to use a lot more local anesthetic. When this technology first came out, people tried to use the same amount of local anesthetic that they used for standard vacuum-assisted core biopsy, and it’s very painful unless you put 30 or 40 cc of dilute anesthetic in there,” said Dr. Wentworth.
The study was funded by Medtronic, which markets the technology. Dr. Whitworth is a principal at Targeted Medical Education, which receives funding from Medtronic.
AT ASBS 2017
Key clinical point: The technique could replace standard lumpectomy in patients at high surgical risk.
Major finding: Clean margins were obtained in 102 of 125 women with diagnosed cancer.
Data source: A retrospective analysis of 279 patients.
Disclosures: The study was funded by Medtronic, which markets the technology. Dr. Whitworth is a principal at Targeted Medical Education, which receives funding from Medtronic.
VIDEO: Dr. Lisa Newman on triple negative breast cancer in African American women
LAS VEGAS – The heavy burden of triple negative and other aggressive breast cancers among African American women cannot be simplified to socioeconomic factors alone.
International investigations by Lisa Newman, MD, director of the Breast Oncology Program at the Henry Ford Health System, Detroit, and other researchers are making it clear that genetic factors play a significant role.
She explained the latest findings and what they mean for screening, genetic referral, and treatment in an interview at the American Society of Breast Surgeons annual meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – The heavy burden of triple negative and other aggressive breast cancers among African American women cannot be simplified to socioeconomic factors alone.
International investigations by Lisa Newman, MD, director of the Breast Oncology Program at the Henry Ford Health System, Detroit, and other researchers are making it clear that genetic factors play a significant role.
She explained the latest findings and what they mean for screening, genetic referral, and treatment in an interview at the American Society of Breast Surgeons annual meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – The heavy burden of triple negative and other aggressive breast cancers among African American women cannot be simplified to socioeconomic factors alone.
International investigations by Lisa Newman, MD, director of the Breast Oncology Program at the Henry Ford Health System, Detroit, and other researchers are making it clear that genetic factors play a significant role.
She explained the latest findings and what they mean for screening, genetic referral, and treatment in an interview at the American Society of Breast Surgeons annual meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASBS 2017
DCIS tool IDs axillary node biopsy candidates
LAS VEGAS – A new tool identifies patients with ductal carcinoma in situ (DCIS) who are at high risk for being upstaged as a result of the pathology report. The screen could encourage patients to undergo axillary nodal staging during the core needle biopsy (CNB), thus avoiding a second procedure.
“The risk factors have been well described, but they haven’t helped give individual risk or individual percentages. Our goal was to try to individualize that risk so that we could counsel patients,” said lead researcher James Jakub, MD, chair of the division of breast endocrine and metabolic surgery at Mayo Clinic Rochester, at the annual meeting of the American Society of Breast Surgeons.
The researchers found that grade on CNB, mass lesion on imaging, multifocal/centric disease, and linear dimension combined to predict the likelihood of being upstaged to invasive disease (C statistic, 0.71; 95% confidence interval, 0.66-0.77).
They then combined those characteristics to create a nomogram and tested it against the validation set. In that group, 11% of patients were upstaged to invasive disease. The nomogram performed almost identically in the external validation set (C statistic, 0.71; 95% CI, 0.63-0.79). The model predicted 56 upstages, and 46 occurred.
This wasn’t the first attempt to create a nomogram to predict upstaging in DCIS patients, but others were not tested against external datasets. “That’s a weakness with other studies. Unless it’s validated externally, you don’t really know if you can apply it to your population. That was a very large plus: having colleagues who were willing to collaborate to validate it,” said Dr. Jakub.
The team is working on posting the nomogram online for widespread use.
It remains to be seen whether the availability of the nomogram will, in fact, change patients’ decision-making and result in more axillary nodal biopsies during CNB procedures in high risk patients.
“We haven’t looked into that. It will be interesting to see how it influences [sentinel lymph node] biopsy rate at our institution, and we’d love to hear from institutions who apply it. My sense is that patients who are at high risk are going to be interested in doing that. I think it will definitely change their management,” said Dr. Jakub.
LAS VEGAS – A new tool identifies patients with ductal carcinoma in situ (DCIS) who are at high risk for being upstaged as a result of the pathology report. The screen could encourage patients to undergo axillary nodal staging during the core needle biopsy (CNB), thus avoiding a second procedure.
“The risk factors have been well described, but they haven’t helped give individual risk or individual percentages. Our goal was to try to individualize that risk so that we could counsel patients,” said lead researcher James Jakub, MD, chair of the division of breast endocrine and metabolic surgery at Mayo Clinic Rochester, at the annual meeting of the American Society of Breast Surgeons.
The researchers found that grade on CNB, mass lesion on imaging, multifocal/centric disease, and linear dimension combined to predict the likelihood of being upstaged to invasive disease (C statistic, 0.71; 95% confidence interval, 0.66-0.77).
They then combined those characteristics to create a nomogram and tested it against the validation set. In that group, 11% of patients were upstaged to invasive disease. The nomogram performed almost identically in the external validation set (C statistic, 0.71; 95% CI, 0.63-0.79). The model predicted 56 upstages, and 46 occurred.
This wasn’t the first attempt to create a nomogram to predict upstaging in DCIS patients, but others were not tested against external datasets. “That’s a weakness with other studies. Unless it’s validated externally, you don’t really know if you can apply it to your population. That was a very large plus: having colleagues who were willing to collaborate to validate it,” said Dr. Jakub.
The team is working on posting the nomogram online for widespread use.
It remains to be seen whether the availability of the nomogram will, in fact, change patients’ decision-making and result in more axillary nodal biopsies during CNB procedures in high risk patients.
“We haven’t looked into that. It will be interesting to see how it influences [sentinel lymph node] biopsy rate at our institution, and we’d love to hear from institutions who apply it. My sense is that patients who are at high risk are going to be interested in doing that. I think it will definitely change their management,” said Dr. Jakub.
LAS VEGAS – A new tool identifies patients with ductal carcinoma in situ (DCIS) who are at high risk for being upstaged as a result of the pathology report. The screen could encourage patients to undergo axillary nodal staging during the core needle biopsy (CNB), thus avoiding a second procedure.
“The risk factors have been well described, but they haven’t helped give individual risk or individual percentages. Our goal was to try to individualize that risk so that we could counsel patients,” said lead researcher James Jakub, MD, chair of the division of breast endocrine and metabolic surgery at Mayo Clinic Rochester, at the annual meeting of the American Society of Breast Surgeons.
The researchers found that grade on CNB, mass lesion on imaging, multifocal/centric disease, and linear dimension combined to predict the likelihood of being upstaged to invasive disease (C statistic, 0.71; 95% confidence interval, 0.66-0.77).
They then combined those characteristics to create a nomogram and tested it against the validation set. In that group, 11% of patients were upstaged to invasive disease. The nomogram performed almost identically in the external validation set (C statistic, 0.71; 95% CI, 0.63-0.79). The model predicted 56 upstages, and 46 occurred.
This wasn’t the first attempt to create a nomogram to predict upstaging in DCIS patients, but others were not tested against external datasets. “That’s a weakness with other studies. Unless it’s validated externally, you don’t really know if you can apply it to your population. That was a very large plus: having colleagues who were willing to collaborate to validate it,” said Dr. Jakub.
The team is working on posting the nomogram online for widespread use.
It remains to be seen whether the availability of the nomogram will, in fact, change patients’ decision-making and result in more axillary nodal biopsies during CNB procedures in high risk patients.
“We haven’t looked into that. It will be interesting to see how it influences [sentinel lymph node] biopsy rate at our institution, and we’d love to hear from institutions who apply it. My sense is that patients who are at high risk are going to be interested in doing that. I think it will definitely change their management,” said Dr. Jakub.
AT ASBS 2017
Key clinical point: High risk patients who choose axillary biopsy could avoid a second procedure.
Major finding: The 4-item nomogram predicted upstaging with a C statistic of 0.71.
Data source: A retrospective sample (n = 827) and validation study (n = 579).
Disclosures: The source of funding was not disclosed. Dr. Jakub reported having no financial disclosures.
VIDEO: How to pick surgical margins with mixed breast lesions
LAS VEGAS – According to recent guidelines, no ink on tumor is the right surgical margin for early stage invasive breast cancer and 2 mm is the right lumpectomy margin for ductal carcinoma in situ (DCIS) treated with whole breast radiation. But, what do you do when invasive carcinoma is associated with DCIS?
It’s a common question for breast surgeons. Monica Morrow, MD, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York City, explained how to handle the situation in a video interview at the annual meeting of the American Society of Breast Surgeons.
She was the senior author on the 2014 invasive breast cancer guidelines and the lead author on the 2016 DCIS guidelines, both of which were consensus statements on surgical margins from the Society of Surgical Oncology and other groups (Ann Surg Oncol. 2014 Mar;21[3]:704-16; Ann Surg Oncol. 2016 Nov;23[12]:3801-10).
Dr. Morrow explained the thinking behind the guidelines and how to apply them to mixed lesions and other clinical scenarios, as well as their limitations and what remains to be determined. A key point is that a margin less than 2 mm is not by itself an indication for mastectomy in DCIS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – According to recent guidelines, no ink on tumor is the right surgical margin for early stage invasive breast cancer and 2 mm is the right lumpectomy margin for ductal carcinoma in situ (DCIS) treated with whole breast radiation. But, what do you do when invasive carcinoma is associated with DCIS?
It’s a common question for breast surgeons. Monica Morrow, MD, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York City, explained how to handle the situation in a video interview at the annual meeting of the American Society of Breast Surgeons.
She was the senior author on the 2014 invasive breast cancer guidelines and the lead author on the 2016 DCIS guidelines, both of which were consensus statements on surgical margins from the Society of Surgical Oncology and other groups (Ann Surg Oncol. 2014 Mar;21[3]:704-16; Ann Surg Oncol. 2016 Nov;23[12]:3801-10).
Dr. Morrow explained the thinking behind the guidelines and how to apply them to mixed lesions and other clinical scenarios, as well as their limitations and what remains to be determined. A key point is that a margin less than 2 mm is not by itself an indication for mastectomy in DCIS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – According to recent guidelines, no ink on tumor is the right surgical margin for early stage invasive breast cancer and 2 mm is the right lumpectomy margin for ductal carcinoma in situ (DCIS) treated with whole breast radiation. But, what do you do when invasive carcinoma is associated with DCIS?
It’s a common question for breast surgeons. Monica Morrow, MD, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York City, explained how to handle the situation in a video interview at the annual meeting of the American Society of Breast Surgeons.
She was the senior author on the 2014 invasive breast cancer guidelines and the lead author on the 2016 DCIS guidelines, both of which were consensus statements on surgical margins from the Society of Surgical Oncology and other groups (Ann Surg Oncol. 2014 Mar;21[3]:704-16; Ann Surg Oncol. 2016 Nov;23[12]:3801-10).
Dr. Morrow explained the thinking behind the guidelines and how to apply them to mixed lesions and other clinical scenarios, as well as their limitations and what remains to be determined. A key point is that a margin less than 2 mm is not by itself an indication for mastectomy in DCIS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
From ASBS 2017
Resection reduces inflammatory breast cancer lesion recurrence
LAS VEGAS – In patients with nonmetastatic inflammatory breast cancer (IBC), aggressive surgical resection can reduce local/regional recurrence rates to levels comparable to those seen in patients with noninflammatory breast cancer.
The key to the improved outcome is the presence of a plastic surgeon who can help close a tricky incision. “We have a multidisciplinary team that allows us to call upon the plastic surgeons if we can’t control the incision, and they help us with a flap closure,” said Kelly Rosso, MD, a breast surgical oncology fellow at the MD Anderson Cancer Center, Houston, who presented the study results at the annual meeting of the American Society of Breast Surgeons.
To see if their surgical interventions were producing better outcomes, the team analyzed data from 114 patients with nonmetastatic inflammatory breast cancer who had received trimodality therapy (neoadjuvant chemotherapy, surgery, and radiation therapy) from 2007 to 2015.
The median patient age was 52 years, and the median follow-up period was 3.6 years. In total, 55% of patients had N2 IBC, and 45% had N3 disease.
Nearly all (113 of 114) patients had negative surgical margins. A total of 29 patients died during the follow-up period, and the 5-year survival rate was 69%. The odds of a local/regional recurrence over 2 years was 3% (95% confidence interval, 1%-10%). The 2-year odds of recurrence or distant metastasis was 23% (95% CI, 16%-32%).
Dr. Lucci said that he suspects that many surgeons avoid resection of inflammatory breast cancer lesions under the assumption that the disease is metastatic in nature and fear that an attempt at local control would put the patient through unnecessary surgery with little benefit.
The new study challenges that belief. “I think this is a changing paradigm. We have to consider that those patients need to be thought of as still resectable and think about getting rid of that local disease, at the very least to improve their quality of life and hopefully to improve their overall outcome,” Dr. Lucci said.
The results should be readily achievable in other centers, he added. “If they have a plastic surgeon who’s willing to help them, they can resect to negative margins just as well [as in noninflammatory patients]. And, if they have a radiation therapist who’s dedicated to doing a more aggressive course, they can achieve the same results.”
Dr. Rosso and Dr. Lucci reported having no financial disclosures.
LAS VEGAS – In patients with nonmetastatic inflammatory breast cancer (IBC), aggressive surgical resection can reduce local/regional recurrence rates to levels comparable to those seen in patients with noninflammatory breast cancer.
The key to the improved outcome is the presence of a plastic surgeon who can help close a tricky incision. “We have a multidisciplinary team that allows us to call upon the plastic surgeons if we can’t control the incision, and they help us with a flap closure,” said Kelly Rosso, MD, a breast surgical oncology fellow at the MD Anderson Cancer Center, Houston, who presented the study results at the annual meeting of the American Society of Breast Surgeons.
To see if their surgical interventions were producing better outcomes, the team analyzed data from 114 patients with nonmetastatic inflammatory breast cancer who had received trimodality therapy (neoadjuvant chemotherapy, surgery, and radiation therapy) from 2007 to 2015.
The median patient age was 52 years, and the median follow-up period was 3.6 years. In total, 55% of patients had N2 IBC, and 45% had N3 disease.
Nearly all (113 of 114) patients had negative surgical margins. A total of 29 patients died during the follow-up period, and the 5-year survival rate was 69%. The odds of a local/regional recurrence over 2 years was 3% (95% confidence interval, 1%-10%). The 2-year odds of recurrence or distant metastasis was 23% (95% CI, 16%-32%).
Dr. Lucci said that he suspects that many surgeons avoid resection of inflammatory breast cancer lesions under the assumption that the disease is metastatic in nature and fear that an attempt at local control would put the patient through unnecessary surgery with little benefit.
The new study challenges that belief. “I think this is a changing paradigm. We have to consider that those patients need to be thought of as still resectable and think about getting rid of that local disease, at the very least to improve their quality of life and hopefully to improve their overall outcome,” Dr. Lucci said.
The results should be readily achievable in other centers, he added. “If they have a plastic surgeon who’s willing to help them, they can resect to negative margins just as well [as in noninflammatory patients]. And, if they have a radiation therapist who’s dedicated to doing a more aggressive course, they can achieve the same results.”
Dr. Rosso and Dr. Lucci reported having no financial disclosures.
LAS VEGAS – In patients with nonmetastatic inflammatory breast cancer (IBC), aggressive surgical resection can reduce local/regional recurrence rates to levels comparable to those seen in patients with noninflammatory breast cancer.
The key to the improved outcome is the presence of a plastic surgeon who can help close a tricky incision. “We have a multidisciplinary team that allows us to call upon the plastic surgeons if we can’t control the incision, and they help us with a flap closure,” said Kelly Rosso, MD, a breast surgical oncology fellow at the MD Anderson Cancer Center, Houston, who presented the study results at the annual meeting of the American Society of Breast Surgeons.
To see if their surgical interventions were producing better outcomes, the team analyzed data from 114 patients with nonmetastatic inflammatory breast cancer who had received trimodality therapy (neoadjuvant chemotherapy, surgery, and radiation therapy) from 2007 to 2015.
The median patient age was 52 years, and the median follow-up period was 3.6 years. In total, 55% of patients had N2 IBC, and 45% had N3 disease.
Nearly all (113 of 114) patients had negative surgical margins. A total of 29 patients died during the follow-up period, and the 5-year survival rate was 69%. The odds of a local/regional recurrence over 2 years was 3% (95% confidence interval, 1%-10%). The 2-year odds of recurrence or distant metastasis was 23% (95% CI, 16%-32%).
Dr. Lucci said that he suspects that many surgeons avoid resection of inflammatory breast cancer lesions under the assumption that the disease is metastatic in nature and fear that an attempt at local control would put the patient through unnecessary surgery with little benefit.
The new study challenges that belief. “I think this is a changing paradigm. We have to consider that those patients need to be thought of as still resectable and think about getting rid of that local disease, at the very least to improve their quality of life and hopefully to improve their overall outcome,” Dr. Lucci said.
The results should be readily achievable in other centers, he added. “If they have a plastic surgeon who’s willing to help them, they can resect to negative margins just as well [as in noninflammatory patients]. And, if they have a radiation therapist who’s dedicated to doing a more aggressive course, they can achieve the same results.”
Dr. Rosso and Dr. Lucci reported having no financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: The odds of a local/regional recurrence over 2 years were 3%.
Data source: A retrospective analysis of 114 patients.
Disclosures: Dr. Rosso and Dr. Lucci reported having no financial disclosures.
Don’t rely on MRI findings after DCIS needle biopsy
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: Of 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology.
Data source: Single-center review of 54 DCIS cases.
Disclosures: There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
Lumpectomy plus reconstruction outperforms mastectomy plus reconstruction
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: Mastectomy with reconstruction had a 49% higher complication rate and $20,000 higher cost, compared with lumpectomy plus reconstruction.
Data source: Nationwide retrospective analysis of 39,518 women.
Disclosures: Dr. Hwang and Dr. Boughey reported having no financial disclosures.
Study underscores aggressive approach to inflammatory breast cancer
Aggressive resection to negative margins, combined with neoadjuvant chemotherapy and postsurgical radiation, resulted in a 96% 5-year locoregional recurrence-free survival in nonmetastatic inflammatory breast cancer, Kelly Rosso, MD, reported.
Dr. Rosso of MD Anderson Cancer Center, Houston, and her colleagues identified 277 women diagnosed with inflammatory breast cancer between 2007 and 2015 from a prospective database; 114 of those had nonmetastatic disease and received aggressive trimodality therapy with curative intent.
Median age at diagnosis was 52 years and all patients were diagnosed at Stage III; 55% presented with N2 disease while 45% presented with N3. Patients were followed for a median 3.6 years.
“Historically, prognosis for patients with inflammatory breast cancer has been very poor,” Dr. Rosso said at a press conference in advance of the annual meeting of the American Society of Breast Surgeons. “Data from our institution has failed to identify any significant improvement in survival from the 1970s to the 2000s.”
In this study, 29 patients died and 4 experienced a locoregional recurrence (3.5%) during follow-up. The 2-year probability of locoregional recurrence was low, at 3.19%, while the 2-year probability of recurrence or distant metastasis was 23.1%. The 5-year disease-free survival was 72.5%, significantly lower than local/regional recurrence-free survival because some patients developed metastatic cancer in other organs, Dr. Rosso reported.
Diminished overall survival and increased risk for recurrence or metastasis were more likely in women over the age of 65 years and those with HER2-negative status, limited clinical response to chemotherapy, and absence of a pathologically complete response. Recurrence or metastasis also were more likely in women with Stage IIIC disease and more lymphovascular involvement.
“It is encouraging to see the high 5-year breast cancer specific survival rates reported in this cohort,” Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., said in a statement. “This study supports that the current management of these patients with neoadjuvant chemotherapy, mastectomy and post-mastectomy radiation is the optimal multimodal approach for inflammatory breast cancer. The improvements in systemic therapy, with increased use of directed therapy, being used in breast cancer, together with appropriate local-regional therapies, is likely responsible for the improvement in survival over historical cohorts.”
[email protected]
On Twitter @denisefulton
Aggressive resection to negative margins, combined with neoadjuvant chemotherapy and postsurgical radiation, resulted in a 96% 5-year locoregional recurrence-free survival in nonmetastatic inflammatory breast cancer, Kelly Rosso, MD, reported.
Dr. Rosso of MD Anderson Cancer Center, Houston, and her colleagues identified 277 women diagnosed with inflammatory breast cancer between 2007 and 2015 from a prospective database; 114 of those had nonmetastatic disease and received aggressive trimodality therapy with curative intent.
Median age at diagnosis was 52 years and all patients were diagnosed at Stage III; 55% presented with N2 disease while 45% presented with N3. Patients were followed for a median 3.6 years.
“Historically, prognosis for patients with inflammatory breast cancer has been very poor,” Dr. Rosso said at a press conference in advance of the annual meeting of the American Society of Breast Surgeons. “Data from our institution has failed to identify any significant improvement in survival from the 1970s to the 2000s.”
In this study, 29 patients died and 4 experienced a locoregional recurrence (3.5%) during follow-up. The 2-year probability of locoregional recurrence was low, at 3.19%, while the 2-year probability of recurrence or distant metastasis was 23.1%. The 5-year disease-free survival was 72.5%, significantly lower than local/regional recurrence-free survival because some patients developed metastatic cancer in other organs, Dr. Rosso reported.
Diminished overall survival and increased risk for recurrence or metastasis were more likely in women over the age of 65 years and those with HER2-negative status, limited clinical response to chemotherapy, and absence of a pathologically complete response. Recurrence or metastasis also were more likely in women with Stage IIIC disease and more lymphovascular involvement.
“It is encouraging to see the high 5-year breast cancer specific survival rates reported in this cohort,” Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., said in a statement. “This study supports that the current management of these patients with neoadjuvant chemotherapy, mastectomy and post-mastectomy radiation is the optimal multimodal approach for inflammatory breast cancer. The improvements in systemic therapy, with increased use of directed therapy, being used in breast cancer, together with appropriate local-regional therapies, is likely responsible for the improvement in survival over historical cohorts.”
[email protected]
On Twitter @denisefulton
Aggressive resection to negative margins, combined with neoadjuvant chemotherapy and postsurgical radiation, resulted in a 96% 5-year locoregional recurrence-free survival in nonmetastatic inflammatory breast cancer, Kelly Rosso, MD, reported.
Dr. Rosso of MD Anderson Cancer Center, Houston, and her colleagues identified 277 women diagnosed with inflammatory breast cancer between 2007 and 2015 from a prospective database; 114 of those had nonmetastatic disease and received aggressive trimodality therapy with curative intent.
Median age at diagnosis was 52 years and all patients were diagnosed at Stage III; 55% presented with N2 disease while 45% presented with N3. Patients were followed for a median 3.6 years.
“Historically, prognosis for patients with inflammatory breast cancer has been very poor,” Dr. Rosso said at a press conference in advance of the annual meeting of the American Society of Breast Surgeons. “Data from our institution has failed to identify any significant improvement in survival from the 1970s to the 2000s.”
In this study, 29 patients died and 4 experienced a locoregional recurrence (3.5%) during follow-up. The 2-year probability of locoregional recurrence was low, at 3.19%, while the 2-year probability of recurrence or distant metastasis was 23.1%. The 5-year disease-free survival was 72.5%, significantly lower than local/regional recurrence-free survival because some patients developed metastatic cancer in other organs, Dr. Rosso reported.
Diminished overall survival and increased risk for recurrence or metastasis were more likely in women over the age of 65 years and those with HER2-negative status, limited clinical response to chemotherapy, and absence of a pathologically complete response. Recurrence or metastasis also were more likely in women with Stage IIIC disease and more lymphovascular involvement.
“It is encouraging to see the high 5-year breast cancer specific survival rates reported in this cohort,” Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., said in a statement. “This study supports that the current management of these patients with neoadjuvant chemotherapy, mastectomy and post-mastectomy radiation is the optimal multimodal approach for inflammatory breast cancer. The improvements in systemic therapy, with increased use of directed therapy, being used in breast cancer, together with appropriate local-regional therapies, is likely responsible for the improvement in survival over historical cohorts.”
[email protected]
On Twitter @denisefulton
FROM ASBS 2017
Key clinical point:
Major finding: Locoregional recurrence occurred in 4 out of 114 women with inflammatory breast cancer treated with trimodality therapy.
Data source: A prospective database of 277 women diagnosed with inflammatory breast cancer between 2007 and 2015.
Disclosures: The study was unsponsored. Dr. Rosso disclosed no relevant conflicts of interest.
Multimodal breast cancer treatment linked with greater risk of lymphedema
Chemotherapy, radiation, and higher body mass index are strongly associated with increased risk of lymphedema in breast cancer patients who have undergone sentinel node biopsy or axillary lymph node dissection, according to analysis of data from the Rochester Epidemiology Project.
Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., and her associates performed a chart review of 1,794 patients diagnosed with a first breast cancer in Olmsted County, Minn., between 1990 and 2010. Patients’ median age at diagnosis was 60 years. About half (48%) were diagnosed at stage I, while 17% were diagnosed at Stage 0, 29% at Stage II, and 6% at Stage III.
At a median of 10 years of follow-up, 209 patients had been diagnosed with breast cancer–related lymphedema, with most diagnoses occurring within 5 years of surgery. No cases of lymphedema were found in patients who did not have axillary surgery.
There was no significant difference in the rate of lymphedema based on type of breast cancer surgery (mastectomy vs. lumpectomy with breast-conserving surgery); however, lymphedema occurred significantly more frequently in patients who received ALND as compared to those who received SLN (15.9% vs. 5.3%). Lymphedema rates did not differ based on type of axillary surgery (3.5% for ALND and 4.1% for SLN) in a subset of 453 patients who did not receive radiation or chemotherapy.
Almost a third (31.3%) of patients who had nodal radiation, with or without breast or chest wall radiation, developed lymphedema by 5 years, as compared with 5.9% of patients who did not receive radiation (P less than .001). Similarly, patients who received chemotherapy were significantly more likely to develop lymphedema. At 5 years, lymphedema was present in 27.2% of patients who received anthracylcline/cyclophosphamide (AC) with a taxane agent, 29.7% of those who received a taxane agent without AC , and 6.0% of those who did not get chemotherapy (P less than .001).
Five-year incidence of lymphedema also increased significantly with body mass index, occurring in 17.1% of patients with a BMI greater than 35 kg/m2, 13% of those with a BMI between 30-34.99, and 14.4% of those with a BMI between 25-29.99, as compared with 8% of those with a BMI below 25.
On univariate analysis, increased stage was associated with risk of lymphedema, Dr. Boughey said. However, on multivariate analysis, stage was no longer associated with risk of lymphedema and “the dominate factors were BMI, type of axillary surgery, use of radiation therapy and particularly nodal radiation, and the use of chemotherapy.”
The highest rate of lymphedema was seen in a patients with the most advanced disease who had ALND with nodal radiation and AC with a taxane agent, she said.
“We think this is primarily due to the modalities of treatment rather that the pure phenomenon of stage,” Dr. Boughey added. “This study can help identify patients at a higher risk of lymphedema so that we can individualize the surveillance of these patients to allow them to have earlier identification and earlier treatment of lymphedema.”
[email protected]
On Twitter @denisefulton
Chemotherapy, radiation, and higher body mass index are strongly associated with increased risk of lymphedema in breast cancer patients who have undergone sentinel node biopsy or axillary lymph node dissection, according to analysis of data from the Rochester Epidemiology Project.
Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., and her associates performed a chart review of 1,794 patients diagnosed with a first breast cancer in Olmsted County, Minn., between 1990 and 2010. Patients’ median age at diagnosis was 60 years. About half (48%) were diagnosed at stage I, while 17% were diagnosed at Stage 0, 29% at Stage II, and 6% at Stage III.
At a median of 10 years of follow-up, 209 patients had been diagnosed with breast cancer–related lymphedema, with most diagnoses occurring within 5 years of surgery. No cases of lymphedema were found in patients who did not have axillary surgery.
There was no significant difference in the rate of lymphedema based on type of breast cancer surgery (mastectomy vs. lumpectomy with breast-conserving surgery); however, lymphedema occurred significantly more frequently in patients who received ALND as compared to those who received SLN (15.9% vs. 5.3%). Lymphedema rates did not differ based on type of axillary surgery (3.5% for ALND and 4.1% for SLN) in a subset of 453 patients who did not receive radiation or chemotherapy.
Almost a third (31.3%) of patients who had nodal radiation, with or without breast or chest wall radiation, developed lymphedema by 5 years, as compared with 5.9% of patients who did not receive radiation (P less than .001). Similarly, patients who received chemotherapy were significantly more likely to develop lymphedema. At 5 years, lymphedema was present in 27.2% of patients who received anthracylcline/cyclophosphamide (AC) with a taxane agent, 29.7% of those who received a taxane agent without AC , and 6.0% of those who did not get chemotherapy (P less than .001).
Five-year incidence of lymphedema also increased significantly with body mass index, occurring in 17.1% of patients with a BMI greater than 35 kg/m2, 13% of those with a BMI between 30-34.99, and 14.4% of those with a BMI between 25-29.99, as compared with 8% of those with a BMI below 25.
On univariate analysis, increased stage was associated with risk of lymphedema, Dr. Boughey said. However, on multivariate analysis, stage was no longer associated with risk of lymphedema and “the dominate factors were BMI, type of axillary surgery, use of radiation therapy and particularly nodal radiation, and the use of chemotherapy.”
The highest rate of lymphedema was seen in a patients with the most advanced disease who had ALND with nodal radiation and AC with a taxane agent, she said.
“We think this is primarily due to the modalities of treatment rather that the pure phenomenon of stage,” Dr. Boughey added. “This study can help identify patients at a higher risk of lymphedema so that we can individualize the surveillance of these patients to allow them to have earlier identification and earlier treatment of lymphedema.”
[email protected]
On Twitter @denisefulton
Chemotherapy, radiation, and higher body mass index are strongly associated with increased risk of lymphedema in breast cancer patients who have undergone sentinel node biopsy or axillary lymph node dissection, according to analysis of data from the Rochester Epidemiology Project.
Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., and her associates performed a chart review of 1,794 patients diagnosed with a first breast cancer in Olmsted County, Minn., between 1990 and 2010. Patients’ median age at diagnosis was 60 years. About half (48%) were diagnosed at stage I, while 17% were diagnosed at Stage 0, 29% at Stage II, and 6% at Stage III.
At a median of 10 years of follow-up, 209 patients had been diagnosed with breast cancer–related lymphedema, with most diagnoses occurring within 5 years of surgery. No cases of lymphedema were found in patients who did not have axillary surgery.
There was no significant difference in the rate of lymphedema based on type of breast cancer surgery (mastectomy vs. lumpectomy with breast-conserving surgery); however, lymphedema occurred significantly more frequently in patients who received ALND as compared to those who received SLN (15.9% vs. 5.3%). Lymphedema rates did not differ based on type of axillary surgery (3.5% for ALND and 4.1% for SLN) in a subset of 453 patients who did not receive radiation or chemotherapy.
Almost a third (31.3%) of patients who had nodal radiation, with or without breast or chest wall radiation, developed lymphedema by 5 years, as compared with 5.9% of patients who did not receive radiation (P less than .001). Similarly, patients who received chemotherapy were significantly more likely to develop lymphedema. At 5 years, lymphedema was present in 27.2% of patients who received anthracylcline/cyclophosphamide (AC) with a taxane agent, 29.7% of those who received a taxane agent without AC , and 6.0% of those who did not get chemotherapy (P less than .001).
Five-year incidence of lymphedema also increased significantly with body mass index, occurring in 17.1% of patients with a BMI greater than 35 kg/m2, 13% of those with a BMI between 30-34.99, and 14.4% of those with a BMI between 25-29.99, as compared with 8% of those with a BMI below 25.
On univariate analysis, increased stage was associated with risk of lymphedema, Dr. Boughey said. However, on multivariate analysis, stage was no longer associated with risk of lymphedema and “the dominate factors were BMI, type of axillary surgery, use of radiation therapy and particularly nodal radiation, and the use of chemotherapy.”
The highest rate of lymphedema was seen in a patients with the most advanced disease who had ALND with nodal radiation and AC with a taxane agent, she said.
“We think this is primarily due to the modalities of treatment rather that the pure phenomenon of stage,” Dr. Boughey added. “This study can help identify patients at a higher risk of lymphedema so that we can individualize the surveillance of these patients to allow them to have earlier identification and earlier treatment of lymphedema.”
[email protected]
On Twitter @denisefulton
FROM ASBS 2017
Key clinical point:
Major finding: More than 30% of patients receiving nodal radiation developed lymphedema vs. 5.9% of those who did not. Similarly, lymphedema developed in just under 30% of patients who received a taxane vs. 6.9% of those who did not.
Data source: Analysis of all 1,794 cases of first breast cancers diagnosed in Olmsted County, Minn., 1990-2010.
Disclosures: The Rochester Epidemiology Project receives federal funding from agencies of the Health & Human Services Department. Dr. Boughey reported no relevant financial conflicts of interest.
Risk of recurrence outweighs risk of contralateral breast cancer for DCIS patients
LAS VEGAS – The risk of ipsilateral breast tumor recurrence was greater than the risk of contralateral breast cancer at both 5 and 10 years following diagnosis of ductal carcinoma in situ (DCIS), investigators report at a press conference in advance of the annual meeting of the American Society of Breast Surgeons.
“A rapidly growing number of women are choosing double mastectomies for DCIS, perhaps because they misperceive their risk of future cancer. Our research provides important data for treatment decision-making,” said Megan Miller, MD, of Memorial Sloan Kettering Cancer Center. “It suggests patients and their doctors should focus on risk factors and appropriate therapy for the diseased breast, not the opposite breast, and that ipsilateral DCIS should not prompt a bilateral mastectomy.”
The investigators also found that CBC did not correlate with age, family history, and initial DCIS characteristics, though these factors did correlate with the risk of IBTR.
Dr. Miller and her colleagues found radiation had no impact on risk of CBC (4.9% vs. 6.3%; P = .1), though it significantly reduced the risk for IBTR (10.3% vs. 19.3%; P less than .0001).
More research is needed on risk factors for patients with a preinvasive condition, Dr. Miller said.
“Most studies examining the benefits of bilateral mastectomy focus on invasive cancer,” she said. “This study is unique in providing hard data for women with preinvasive disease. For these patients, examining risk factors for recurrence and the benefits of radiation and endocrine therapy to treat the existing cancer are important.”
[email protected]
On Twitter @eaztweets
LAS VEGAS – The risk of ipsilateral breast tumor recurrence was greater than the risk of contralateral breast cancer at both 5 and 10 years following diagnosis of ductal carcinoma in situ (DCIS), investigators report at a press conference in advance of the annual meeting of the American Society of Breast Surgeons.
“A rapidly growing number of women are choosing double mastectomies for DCIS, perhaps because they misperceive their risk of future cancer. Our research provides important data for treatment decision-making,” said Megan Miller, MD, of Memorial Sloan Kettering Cancer Center. “It suggests patients and their doctors should focus on risk factors and appropriate therapy for the diseased breast, not the opposite breast, and that ipsilateral DCIS should not prompt a bilateral mastectomy.”
The investigators also found that CBC did not correlate with age, family history, and initial DCIS characteristics, though these factors did correlate with the risk of IBTR.
Dr. Miller and her colleagues found radiation had no impact on risk of CBC (4.9% vs. 6.3%; P = .1), though it significantly reduced the risk for IBTR (10.3% vs. 19.3%; P less than .0001).
More research is needed on risk factors for patients with a preinvasive condition, Dr. Miller said.
“Most studies examining the benefits of bilateral mastectomy focus on invasive cancer,” she said. “This study is unique in providing hard data for women with preinvasive disease. For these patients, examining risk factors for recurrence and the benefits of radiation and endocrine therapy to treat the existing cancer are important.”
[email protected]
On Twitter @eaztweets
LAS VEGAS – The risk of ipsilateral breast tumor recurrence was greater than the risk of contralateral breast cancer at both 5 and 10 years following diagnosis of ductal carcinoma in situ (DCIS), investigators report at a press conference in advance of the annual meeting of the American Society of Breast Surgeons.
“A rapidly growing number of women are choosing double mastectomies for DCIS, perhaps because they misperceive their risk of future cancer. Our research provides important data for treatment decision-making,” said Megan Miller, MD, of Memorial Sloan Kettering Cancer Center. “It suggests patients and their doctors should focus on risk factors and appropriate therapy for the diseased breast, not the opposite breast, and that ipsilateral DCIS should not prompt a bilateral mastectomy.”
The investigators also found that CBC did not correlate with age, family history, and initial DCIS characteristics, though these factors did correlate with the risk of IBTR.
Dr. Miller and her colleagues found radiation had no impact on risk of CBC (4.9% vs. 6.3%; P = .1), though it significantly reduced the risk for IBTR (10.3% vs. 19.3%; P less than .0001).
More research is needed on risk factors for patients with a preinvasive condition, Dr. Miller said.
“Most studies examining the benefits of bilateral mastectomy focus on invasive cancer,” she said. “This study is unique in providing hard data for women with preinvasive disease. For these patients, examining risk factors for recurrence and the benefits of radiation and endocrine therapy to treat the existing cancer are important.”
[email protected]
On Twitter @eaztweets
FROM ASBS 2017
Key clinical point:
Major finding: Among 2,759 patients, incidence rate of contralateral breast cancer was 2.8% and 5.6% over 5 and 10 years, respectively, while risk of ipsilateral breast tumor recurrence was 7.8% and 14.3% over 5 and 10 years, respectively.
Data source: Study of 2,759 DCIS patients who underwent breast conserving surgery between 1978-2011, who were followed for a median of 6.8 years.
Disclosures: The investigators reported no relevant disclosures.