User login
ATS: Glycopyrronium improves lung function, health status in COPD
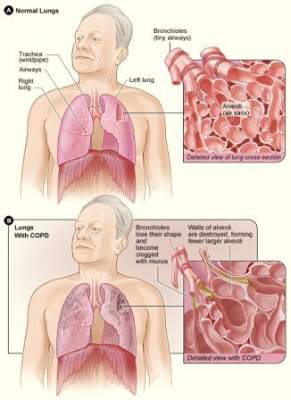
DENVER – Twice-daily treatment with the long-acting muscarinic antagonist glycopyrronium improved lung function and health status in patients with chronic obstructive pulmonary disorder (COPD) and moderate to severe airflow limitation in the randomized, double-blind, placebo-controlled GEM2 study.
In patients with stable symptomatic COPD with moderate to severe airflow limitation, twice-daily 12.5-mcg dosing of glycopyrronium provides clinically meaningful improvement in lung function over the 12-hour dosing interval, has early onset, is sustained over 12 weeks when compared with placebo, and is associated with significant improvements in COPD symptoms, health status, and rescue medication use, as well as numerical improvement in dyspnea scores, Dr. Edward Kerwin of the Clinical Research Institute of Southern Oregon, PC, Medford, and his colleagues reported in a poster at an international conference of the American Thoracic Society.
Lung function – as measured by forced expiratory volume in 1 second (FEV1) area under the curve from 0-12 hours (AUC 0-12h) – was significantly better both at day 1 and at week 12 of treatment in 216 patients who were randomized to receive a 12.5 mcg twice daily dose of the fast-onset, long-acting muscarinic antagonist (Novartis), compared with 216 patients who received placebo; there was a “significant and clinically meaningful between-treatment difference of 119 and 123 mL, respectively,” the researchers wrote.
“Glycopyrronium also showed consistently significant improvements in trough FEV1 vs. placebo at all assessed time points,” they said, adding that glycopyrronium showed an early onset of bronchodilation with significant improvements in FEV1 at 5 and 15 minutes post dose, compared with placebo at day 1 and week 12.
The least squares mean treatment differences for glycopyrronium vs. placebo for change from baseline in trough forced vital capacity (FVC) were 171 mL on day 2, and 130 mL at week 12. Peak FEV1 and peak FVC were significantly improved with glycopyrronium vs. placebo on day 1 (least squares mean treatment differences of 137 and 223 mL, respectively) and at week 12 (least squares mean treatment differences of 148 and 201 mL, respectively).
Health status was improved at week 12, with both significant and clinically meaningful improvements in St. George’s Respiratory Questionnaire total score in the treatment vs. placebo group (–6.4 vs. –1.2), and the percentage of patients achieving minimal clinically important differences (MCID), defined as at least 4 units, was significantly higher in the treatment vs. placebo group (54.9% vs. 42.3%), the investigators said.
Additionally, numerical improvements in transition dyspnea index total score, and percentage of patients achieving a MCID, defined as at least 1 unit, were observed at week 12 in the glycopyrronium vs. placebo group.
Patients in the glycopyrronium group showed improvement on all symptoms scores and endpoints, according to data recorded in patient e-diaries, and those in the treatment group also were able to perform usual daily activities significantly more often than those in the placebo group.
“A statistically significant decrease in daily, daytime, and nighttime number of puffs, and a significant increase in the percentage of days with no rescue medication use were observed,” the investigators noted.
Patients included in the multicenter GEM2 (Glycopyrronium Effect on Symptoms and Lung Function) study were adults aged 40 years and older with moderate to severe airflow limitation (GOLD 2011 strategy level 2 or 3), who were either current or former smokers with a smoking history of at least 10 pack-years. All had postbronchodilator FEV1 of at least 30% and less than 80% of the predicted value, and postbronchodilator FEV1/forced vital capacity ratio of less than 0.70 at a run-in visit. They also all had a modified Medical Research Council grade of 2 or greater at the run-in visit. Patients with a history of asthma or with a COPD exacerbation requiring treatment with antibiotics and/or systemic corticosteroids, and/or with hospitalization within 6 weeks of the screening and run-in periods were excluded, as were those with a history of long QT syndrome or whose corrected QT was greater than 450 ms at the run-in visit.
All underwent an initial 1- to 7-day washout period and a 2-week run-in period prior to randomization, as well as a safety follow-up period. Patients received either glycopyrronium 12. 5 mcg or placebo twice daily delivered via the Neohaler device for 12 weeks.
Treatment was generally well tolerated; of the 430 patients included in the safety set, 44 permanently discontinued treatment due to adverse events (4.6% and 4.2% in the treatment and placebo groups, respectively). The number who experienced at least one adverse event during the treatment period was similar in the two groups; COPD was the most common adverse event, occurring in 20.8% of those in the treatment group and 21.5% in the placebo group.
A nonfatal myocardial infraction occurred in one patient in the treatment group.
Laboratory parameters and vital sign findings were comparable in the two groups.
Based on the findings of the GEM studies, Novartis has submitted a New Drug Application to the Food and Drug Administration; glycopyrronium is already approved in more than 70 countries, including countries in Latin America and the European Union, as a once-daily treatment marketed as the Seebri Breezhaler.
The GEM2 study was sponsored by Novartis Pharmaceuticals. Two of the study researchers are Novartis employees.
DENVER – Twice-daily treatment with the long-acting muscarinic antagonist glycopyrronium improved lung function and health status in patients with chronic obstructive pulmonary disorder (COPD) and moderate to severe airflow limitation in the randomized, double-blind, placebo-controlled GEM2 study.
In patients with stable symptomatic COPD with moderate to severe airflow limitation, twice-daily 12.5-mcg dosing of glycopyrronium provides clinically meaningful improvement in lung function over the 12-hour dosing interval, has early onset, is sustained over 12 weeks when compared with placebo, and is associated with significant improvements in COPD symptoms, health status, and rescue medication use, as well as numerical improvement in dyspnea scores, Dr. Edward Kerwin of the Clinical Research Institute of Southern Oregon, PC, Medford, and his colleagues reported in a poster at an international conference of the American Thoracic Society.
Lung function – as measured by forced expiratory volume in 1 second (FEV1) area under the curve from 0-12 hours (AUC 0-12h) – was significantly better both at day 1 and at week 12 of treatment in 216 patients who were randomized to receive a 12.5 mcg twice daily dose of the fast-onset, long-acting muscarinic antagonist (Novartis), compared with 216 patients who received placebo; there was a “significant and clinically meaningful between-treatment difference of 119 and 123 mL, respectively,” the researchers wrote.
“Glycopyrronium also showed consistently significant improvements in trough FEV1 vs. placebo at all assessed time points,” they said, adding that glycopyrronium showed an early onset of bronchodilation with significant improvements in FEV1 at 5 and 15 minutes post dose, compared with placebo at day 1 and week 12.
The least squares mean treatment differences for glycopyrronium vs. placebo for change from baseline in trough forced vital capacity (FVC) were 171 mL on day 2, and 130 mL at week 12. Peak FEV1 and peak FVC were significantly improved with glycopyrronium vs. placebo on day 1 (least squares mean treatment differences of 137 and 223 mL, respectively) and at week 12 (least squares mean treatment differences of 148 and 201 mL, respectively).
Health status was improved at week 12, with both significant and clinically meaningful improvements in St. George’s Respiratory Questionnaire total score in the treatment vs. placebo group (–6.4 vs. –1.2), and the percentage of patients achieving minimal clinically important differences (MCID), defined as at least 4 units, was significantly higher in the treatment vs. placebo group (54.9% vs. 42.3%), the investigators said.
Additionally, numerical improvements in transition dyspnea index total score, and percentage of patients achieving a MCID, defined as at least 1 unit, were observed at week 12 in the glycopyrronium vs. placebo group.
Patients in the glycopyrronium group showed improvement on all symptoms scores and endpoints, according to data recorded in patient e-diaries, and those in the treatment group also were able to perform usual daily activities significantly more often than those in the placebo group.
“A statistically significant decrease in daily, daytime, and nighttime number of puffs, and a significant increase in the percentage of days with no rescue medication use were observed,” the investigators noted.
Patients included in the multicenter GEM2 (Glycopyrronium Effect on Symptoms and Lung Function) study were adults aged 40 years and older with moderate to severe airflow limitation (GOLD 2011 strategy level 2 or 3), who were either current or former smokers with a smoking history of at least 10 pack-years. All had postbronchodilator FEV1 of at least 30% and less than 80% of the predicted value, and postbronchodilator FEV1/forced vital capacity ratio of less than 0.70 at a run-in visit. They also all had a modified Medical Research Council grade of 2 or greater at the run-in visit. Patients with a history of asthma or with a COPD exacerbation requiring treatment with antibiotics and/or systemic corticosteroids, and/or with hospitalization within 6 weeks of the screening and run-in periods were excluded, as were those with a history of long QT syndrome or whose corrected QT was greater than 450 ms at the run-in visit.
All underwent an initial 1- to 7-day washout period and a 2-week run-in period prior to randomization, as well as a safety follow-up period. Patients received either glycopyrronium 12. 5 mcg or placebo twice daily delivered via the Neohaler device for 12 weeks.
Treatment was generally well tolerated; of the 430 patients included in the safety set, 44 permanently discontinued treatment due to adverse events (4.6% and 4.2% in the treatment and placebo groups, respectively). The number who experienced at least one adverse event during the treatment period was similar in the two groups; COPD was the most common adverse event, occurring in 20.8% of those in the treatment group and 21.5% in the placebo group.
A nonfatal myocardial infraction occurred in one patient in the treatment group.
Laboratory parameters and vital sign findings were comparable in the two groups.
Based on the findings of the GEM studies, Novartis has submitted a New Drug Application to the Food and Drug Administration; glycopyrronium is already approved in more than 70 countries, including countries in Latin America and the European Union, as a once-daily treatment marketed as the Seebri Breezhaler.
The GEM2 study was sponsored by Novartis Pharmaceuticals. Two of the study researchers are Novartis employees.
DENVER – Twice-daily treatment with the long-acting muscarinic antagonist glycopyrronium improved lung function and health status in patients with chronic obstructive pulmonary disorder (COPD) and moderate to severe airflow limitation in the randomized, double-blind, placebo-controlled GEM2 study.
In patients with stable symptomatic COPD with moderate to severe airflow limitation, twice-daily 12.5-mcg dosing of glycopyrronium provides clinically meaningful improvement in lung function over the 12-hour dosing interval, has early onset, is sustained over 12 weeks when compared with placebo, and is associated with significant improvements in COPD symptoms, health status, and rescue medication use, as well as numerical improvement in dyspnea scores, Dr. Edward Kerwin of the Clinical Research Institute of Southern Oregon, PC, Medford, and his colleagues reported in a poster at an international conference of the American Thoracic Society.
Lung function – as measured by forced expiratory volume in 1 second (FEV1) area under the curve from 0-12 hours (AUC 0-12h) – was significantly better both at day 1 and at week 12 of treatment in 216 patients who were randomized to receive a 12.5 mcg twice daily dose of the fast-onset, long-acting muscarinic antagonist (Novartis), compared with 216 patients who received placebo; there was a “significant and clinically meaningful between-treatment difference of 119 and 123 mL, respectively,” the researchers wrote.
“Glycopyrronium also showed consistently significant improvements in trough FEV1 vs. placebo at all assessed time points,” they said, adding that glycopyrronium showed an early onset of bronchodilation with significant improvements in FEV1 at 5 and 15 minutes post dose, compared with placebo at day 1 and week 12.
The least squares mean treatment differences for glycopyrronium vs. placebo for change from baseline in trough forced vital capacity (FVC) were 171 mL on day 2, and 130 mL at week 12. Peak FEV1 and peak FVC were significantly improved with glycopyrronium vs. placebo on day 1 (least squares mean treatment differences of 137 and 223 mL, respectively) and at week 12 (least squares mean treatment differences of 148 and 201 mL, respectively).
Health status was improved at week 12, with both significant and clinically meaningful improvements in St. George’s Respiratory Questionnaire total score in the treatment vs. placebo group (–6.4 vs. –1.2), and the percentage of patients achieving minimal clinically important differences (MCID), defined as at least 4 units, was significantly higher in the treatment vs. placebo group (54.9% vs. 42.3%), the investigators said.
Additionally, numerical improvements in transition dyspnea index total score, and percentage of patients achieving a MCID, defined as at least 1 unit, were observed at week 12 in the glycopyrronium vs. placebo group.
Patients in the glycopyrronium group showed improvement on all symptoms scores and endpoints, according to data recorded in patient e-diaries, and those in the treatment group also were able to perform usual daily activities significantly more often than those in the placebo group.
“A statistically significant decrease in daily, daytime, and nighttime number of puffs, and a significant increase in the percentage of days with no rescue medication use were observed,” the investigators noted.
Patients included in the multicenter GEM2 (Glycopyrronium Effect on Symptoms and Lung Function) study were adults aged 40 years and older with moderate to severe airflow limitation (GOLD 2011 strategy level 2 or 3), who were either current or former smokers with a smoking history of at least 10 pack-years. All had postbronchodilator FEV1 of at least 30% and less than 80% of the predicted value, and postbronchodilator FEV1/forced vital capacity ratio of less than 0.70 at a run-in visit. They also all had a modified Medical Research Council grade of 2 or greater at the run-in visit. Patients with a history of asthma or with a COPD exacerbation requiring treatment with antibiotics and/or systemic corticosteroids, and/or with hospitalization within 6 weeks of the screening and run-in periods were excluded, as were those with a history of long QT syndrome or whose corrected QT was greater than 450 ms at the run-in visit.
All underwent an initial 1- to 7-day washout period and a 2-week run-in period prior to randomization, as well as a safety follow-up period. Patients received either glycopyrronium 12. 5 mcg or placebo twice daily delivered via the Neohaler device for 12 weeks.
Treatment was generally well tolerated; of the 430 patients included in the safety set, 44 permanently discontinued treatment due to adverse events (4.6% and 4.2% in the treatment and placebo groups, respectively). The number who experienced at least one adverse event during the treatment period was similar in the two groups; COPD was the most common adverse event, occurring in 20.8% of those in the treatment group and 21.5% in the placebo group.
A nonfatal myocardial infraction occurred in one patient in the treatment group.
Laboratory parameters and vital sign findings were comparable in the two groups.
Based on the findings of the GEM studies, Novartis has submitted a New Drug Application to the Food and Drug Administration; glycopyrronium is already approved in more than 70 countries, including countries in Latin America and the European Union, as a once-daily treatment marketed as the Seebri Breezhaler.
The GEM2 study was sponsored by Novartis Pharmaceuticals. Two of the study researchers are Novartis employees.
AT ATS 2015
Key clinical point: Glycopyrronium provides fast-onset, clinically meaningful improvement in lung function vs. placebo in COPD patients with moderate to severe airflow limitation.
Major finding: The between-treatment differences in FEV1 AUC (0-12 h) at day 1 and week 12 of treatment were 119 and 123 mL, respectively.
Data source: A randomized, double-blind, placebo-controlled trial of 432 patients.
Disclosures: The GEM2 study was sponsored by Novartis Pharmaceuticals. Two of the study researchers are Novartis employees.
Umeclidinium triple therapy improves lung function in COPD
DENVER – Lung function and health-related quality of life improved for COPD patients who received the long-acting muscarinic agent (LAMA) umeclidinium with fixed-dose inhaled corticosteroid/long-acting beta antagonist (LABA) therapy, based on a post hoc analysis of pooled data from four phase III trials.
Compared with inhaled corticosteroid (ICS)/LABA therapy alone, the triple therapy increased the number of rescue-free days, Dr. Thomas Siler, a pulmonologist with Midwest Chest Consultants, St. Charles, Mo., reported at an international conference of the American Thoracic Society.
The analysis involved 819 patients treated with 62.5 mcg of umeclidinium (Ellipta) – an approved maintenance treatment for COPD – plus ICS/LABA, 821 patients treated with 125 mg umeclidinium plus ICS/LABA, and 818 who received placebo and ICS/LABA. Statistically significant improvements were seen with active triple therapy vs. dual therapy plus placebo in forced expiratory volume in 1 second (FEV1) at day 85 (0.130 L) and at all other time points, as well as in 0-6 h weighted mean FEV1 at day 84 (0.152 L), Dr. Siler said.
With active triple therapy vs. dual therapy plus placebo, overall rescue use was reduced by 0.3 puffs/day and the number of rescue-free days increased by 7.1%. Also, St. George’s Respiratory Questionnaire (SGRQ) score at day 84 decreased by 1.55 vs. placebo, and the proportion of SGRQ responders was 41% vs. 31% for umeclidinium vs. placebo (odds ratio, 1.6).
Moderate/severe COPD exacerbations were experienced by 88 patients: 31 (4%) of the umeclidinium group patients and 57 (7%) of the placebo group patients (hazard ratio, 0.53).
The findings were similar in the patients who received off-label 125-mg dosing of umeclidinium, and the incidence of adverse events and serious adverse events was similar across treatment groups. Dr. Siler noted.
Data on the benefits of LAMAs in triple therapy in patients with moderate to very severe COPD are limited. This pooled analysis of data from four randomized, double-blind, parallel-group 12-week trials of once-daily add-on umeclidinium included COPD patients who entered a 4-week run-in on open-label ICS/LABA (either fluticasone furoate/vilanterol 100/25 mcg or fluticasone propionate/salmeterol 250/50 mcg), and who were then randomized to receive 62.5 or 125 mcg of umeclidinium or placebo.
GlaxoSmithKline funded the study.
DENVER – Lung function and health-related quality of life improved for COPD patients who received the long-acting muscarinic agent (LAMA) umeclidinium with fixed-dose inhaled corticosteroid/long-acting beta antagonist (LABA) therapy, based on a post hoc analysis of pooled data from four phase III trials.
Compared with inhaled corticosteroid (ICS)/LABA therapy alone, the triple therapy increased the number of rescue-free days, Dr. Thomas Siler, a pulmonologist with Midwest Chest Consultants, St. Charles, Mo., reported at an international conference of the American Thoracic Society.
The analysis involved 819 patients treated with 62.5 mcg of umeclidinium (Ellipta) – an approved maintenance treatment for COPD – plus ICS/LABA, 821 patients treated with 125 mg umeclidinium plus ICS/LABA, and 818 who received placebo and ICS/LABA. Statistically significant improvements were seen with active triple therapy vs. dual therapy plus placebo in forced expiratory volume in 1 second (FEV1) at day 85 (0.130 L) and at all other time points, as well as in 0-6 h weighted mean FEV1 at day 84 (0.152 L), Dr. Siler said.
With active triple therapy vs. dual therapy plus placebo, overall rescue use was reduced by 0.3 puffs/day and the number of rescue-free days increased by 7.1%. Also, St. George’s Respiratory Questionnaire (SGRQ) score at day 84 decreased by 1.55 vs. placebo, and the proportion of SGRQ responders was 41% vs. 31% for umeclidinium vs. placebo (odds ratio, 1.6).
Moderate/severe COPD exacerbations were experienced by 88 patients: 31 (4%) of the umeclidinium group patients and 57 (7%) of the placebo group patients (hazard ratio, 0.53).
The findings were similar in the patients who received off-label 125-mg dosing of umeclidinium, and the incidence of adverse events and serious adverse events was similar across treatment groups. Dr. Siler noted.
Data on the benefits of LAMAs in triple therapy in patients with moderate to very severe COPD are limited. This pooled analysis of data from four randomized, double-blind, parallel-group 12-week trials of once-daily add-on umeclidinium included COPD patients who entered a 4-week run-in on open-label ICS/LABA (either fluticasone furoate/vilanterol 100/25 mcg or fluticasone propionate/salmeterol 250/50 mcg), and who were then randomized to receive 62.5 or 125 mcg of umeclidinium or placebo.
GlaxoSmithKline funded the study.
DENVER – Lung function and health-related quality of life improved for COPD patients who received the long-acting muscarinic agent (LAMA) umeclidinium with fixed-dose inhaled corticosteroid/long-acting beta antagonist (LABA) therapy, based on a post hoc analysis of pooled data from four phase III trials.
Compared with inhaled corticosteroid (ICS)/LABA therapy alone, the triple therapy increased the number of rescue-free days, Dr. Thomas Siler, a pulmonologist with Midwest Chest Consultants, St. Charles, Mo., reported at an international conference of the American Thoracic Society.
The analysis involved 819 patients treated with 62.5 mcg of umeclidinium (Ellipta) – an approved maintenance treatment for COPD – plus ICS/LABA, 821 patients treated with 125 mg umeclidinium plus ICS/LABA, and 818 who received placebo and ICS/LABA. Statistically significant improvements were seen with active triple therapy vs. dual therapy plus placebo in forced expiratory volume in 1 second (FEV1) at day 85 (0.130 L) and at all other time points, as well as in 0-6 h weighted mean FEV1 at day 84 (0.152 L), Dr. Siler said.
With active triple therapy vs. dual therapy plus placebo, overall rescue use was reduced by 0.3 puffs/day and the number of rescue-free days increased by 7.1%. Also, St. George’s Respiratory Questionnaire (SGRQ) score at day 84 decreased by 1.55 vs. placebo, and the proportion of SGRQ responders was 41% vs. 31% for umeclidinium vs. placebo (odds ratio, 1.6).
Moderate/severe COPD exacerbations were experienced by 88 patients: 31 (4%) of the umeclidinium group patients and 57 (7%) of the placebo group patients (hazard ratio, 0.53).
The findings were similar in the patients who received off-label 125-mg dosing of umeclidinium, and the incidence of adverse events and serious adverse events was similar across treatment groups. Dr. Siler noted.
Data on the benefits of LAMAs in triple therapy in patients with moderate to very severe COPD are limited. This pooled analysis of data from four randomized, double-blind, parallel-group 12-week trials of once-daily add-on umeclidinium included COPD patients who entered a 4-week run-in on open-label ICS/LABA (either fluticasone furoate/vilanterol 100/25 mcg or fluticasone propionate/salmeterol 250/50 mcg), and who were then randomized to receive 62.5 or 125 mcg of umeclidinium or placebo.
GlaxoSmithKline funded the study.
AT ATS 2015
Key clinical point: Adding umeclidinium to fixed-dose inhaled corticosteroid/long-acting beta antagonist therapy might improve lung function and health-related quality of life in patients with chronic obstructive pulmonary disorder.
Major finding: Statistically significant improvements in FEV1 were seen vs. placebo, at day 85 (0.130 L) and at all other time points.
Data source: A post hoc analysis of data from four phase III trials comprising 2,458 patients.
Disclosures: GlaxoSmithKline funded the study.
ATS: Pulmonary rehab plus CPAP boosts sleep apnea outcomes
DENVER – Pulmonary rehabilitation plus continuous positive airway pressure therapy improved pulmonary function, compared with CPAP alone, in a randomized study of 40 patients with obstructive sleep apnea.
While CPAP remains the main treatment option, it doesn’t address the obesity and inactivity levels that can contribute to comorbidities associated with OSA, Katerina Neumonnova, Ph.D., reported during a press briefing at an international conference of the American Thoracic Society. Patients with OSA often describe respiratory symptoms associated with diminished functional capacity and decreased health-related quality of life. Adjunctive pulmonary rehabilitation might improve pulmonary function, allowing for increased activity levels that can lead to improved outcomes.
Obstructive sleep apnea (OSA) improved with CPAP in all patients in the study. However, percent predicted vital capacity increased from 96.4 to 103.2 in the 20 patients randomized to receive pulmonary rehabilitation and CPAP, and decreased from 99.5 to 94.8 in the 20 patients who received CPAP alone. Percent predicted forced expiratory volume in 1 second (FEV1) improved from 91.5 to 96.1 in the pulmonary rehabilitation plus CPAP group, and decreased from 92.1 to 86.3 in the CPAP-only group.
Those who received pulmonary rehabilitation and CPAP also had significantly greater reductions in body mass index and in neck, waist, and hip circumference, said Dr. Neumonnova of Palacky University, Olomouc, Czech Republic. Percentage of weight as body fat significantly decreased in both groups, but the reduction was greater in those who received pulmonary rehabilitation (from 39.4 to 36.9) than in those who got CPAP alone (from 40.1 to 39.1), she said.
The patients, 20 men and 20 women, mean age 54 years, had similar baseline apnea/hypopnea index (greater than 15), pulmonary function, BMI, percentage of body fat, and neck, waist, and hip circumferences.
The 6-week pulmonary rehabilitation program consisted of twice-weekly 60-minute individualized exercise training sessions, combined with education, breathing retraining, respiratory muscle training, and oropharyngeal exercises. Patients were encouraged to steadily increase their levels of activity, measured by steps per day.
Dr. Neumonnova reported having no disclosures.
DENVER – Pulmonary rehabilitation plus continuous positive airway pressure therapy improved pulmonary function, compared with CPAP alone, in a randomized study of 40 patients with obstructive sleep apnea.
While CPAP remains the main treatment option, it doesn’t address the obesity and inactivity levels that can contribute to comorbidities associated with OSA, Katerina Neumonnova, Ph.D., reported during a press briefing at an international conference of the American Thoracic Society. Patients with OSA often describe respiratory symptoms associated with diminished functional capacity and decreased health-related quality of life. Adjunctive pulmonary rehabilitation might improve pulmonary function, allowing for increased activity levels that can lead to improved outcomes.
Obstructive sleep apnea (OSA) improved with CPAP in all patients in the study. However, percent predicted vital capacity increased from 96.4 to 103.2 in the 20 patients randomized to receive pulmonary rehabilitation and CPAP, and decreased from 99.5 to 94.8 in the 20 patients who received CPAP alone. Percent predicted forced expiratory volume in 1 second (FEV1) improved from 91.5 to 96.1 in the pulmonary rehabilitation plus CPAP group, and decreased from 92.1 to 86.3 in the CPAP-only group.
Those who received pulmonary rehabilitation and CPAP also had significantly greater reductions in body mass index and in neck, waist, and hip circumference, said Dr. Neumonnova of Palacky University, Olomouc, Czech Republic. Percentage of weight as body fat significantly decreased in both groups, but the reduction was greater in those who received pulmonary rehabilitation (from 39.4 to 36.9) than in those who got CPAP alone (from 40.1 to 39.1), she said.
The patients, 20 men and 20 women, mean age 54 years, had similar baseline apnea/hypopnea index (greater than 15), pulmonary function, BMI, percentage of body fat, and neck, waist, and hip circumferences.
The 6-week pulmonary rehabilitation program consisted of twice-weekly 60-minute individualized exercise training sessions, combined with education, breathing retraining, respiratory muscle training, and oropharyngeal exercises. Patients were encouraged to steadily increase their levels of activity, measured by steps per day.
Dr. Neumonnova reported having no disclosures.
DENVER – Pulmonary rehabilitation plus continuous positive airway pressure therapy improved pulmonary function, compared with CPAP alone, in a randomized study of 40 patients with obstructive sleep apnea.
While CPAP remains the main treatment option, it doesn’t address the obesity and inactivity levels that can contribute to comorbidities associated with OSA, Katerina Neumonnova, Ph.D., reported during a press briefing at an international conference of the American Thoracic Society. Patients with OSA often describe respiratory symptoms associated with diminished functional capacity and decreased health-related quality of life. Adjunctive pulmonary rehabilitation might improve pulmonary function, allowing for increased activity levels that can lead to improved outcomes.
Obstructive sleep apnea (OSA) improved with CPAP in all patients in the study. However, percent predicted vital capacity increased from 96.4 to 103.2 in the 20 patients randomized to receive pulmonary rehabilitation and CPAP, and decreased from 99.5 to 94.8 in the 20 patients who received CPAP alone. Percent predicted forced expiratory volume in 1 second (FEV1) improved from 91.5 to 96.1 in the pulmonary rehabilitation plus CPAP group, and decreased from 92.1 to 86.3 in the CPAP-only group.
Those who received pulmonary rehabilitation and CPAP also had significantly greater reductions in body mass index and in neck, waist, and hip circumference, said Dr. Neumonnova of Palacky University, Olomouc, Czech Republic. Percentage of weight as body fat significantly decreased in both groups, but the reduction was greater in those who received pulmonary rehabilitation (from 39.4 to 36.9) than in those who got CPAP alone (from 40.1 to 39.1), she said.
The patients, 20 men and 20 women, mean age 54 years, had similar baseline apnea/hypopnea index (greater than 15), pulmonary function, BMI, percentage of body fat, and neck, waist, and hip circumferences.
The 6-week pulmonary rehabilitation program consisted of twice-weekly 60-minute individualized exercise training sessions, combined with education, breathing retraining, respiratory muscle training, and oropharyngeal exercises. Patients were encouraged to steadily increase their levels of activity, measured by steps per day.
Dr. Neumonnova reported having no disclosures.
AT ATS 2015
Key clinical point: Pulmonary rehabilitation could boost outcomes in patients undergoing CPAP treatment for OSA.
Major finding: Percent predicted vital capacity increased from 96.4 to 103.2 in the pulmonary rehabilitation and CPAP group, and decreased from 99.5 to 94.8 in the CPAP-only group.
Data source: A randomized study of 40 patients.
Disclosures: Dr. Neumonnova reported having no disclosures.
ATS: Inhaled corticosteroid regimens being used in mild COPD
DENVER – Inhaled corticosteroid plus long-acting beta2-agonist therapy is overused in patients with mild COPD, based on a post hoc analysis of two pivotal phase III studies.
At entry in the phase III TONADO studies, nearly 40% of patients who were classified as having GOLD A or B disease were receiving ICS maintenance therapy either alone, in free combination, or as fixed-dose combination therapy, Dr. Henrik Watz of the Pulmonary Research Institute at Lung Clinic Grosshansdorf, Airway Research Center North, Grosshansdorf, Germany, and his colleagues reported at an international conference of the American Thoracic Society.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend that use of inhaled corticosteroids (ICS) and long-acting beta2-agonist (LABA) therapy be restricted to patients with severe or very severe COPD (category C or D disease) with frequent exacerbations.
The post-hoc analysis “confirms previous reports highlighting that treatment regimens containing ICS therapy are being used early in the management of patients with COPD, which may not be appropriate based on current GOLD recommendations. Furthermore, consistent improvements in lung function with tiotropium plus olodaterol versus the monocomponents were demonstrated in GOLD A, B, C, and D, regardless of previous ICS use,” Dr. Watz and his associates concluded.
The replicate TONADO studies (TONADO 1 and 2) were multicenter, randomized, double-blind, active-controlled studies evaluating the once-daily long-acting muscarinic agent (LAMA) tiotropium and the LABA olodaterol. The 5,162 patients were randomized to once-daily treatment with inhaled tiotropium plus olodaterol (Respimat FDC), to tiotropium, or to olodaterol for 52 weeks.
Of the study participants, 2,132 had GOLD A/B disease, and 3,030 had GOLD C/D disease, based on exacerbation history and lung function. All had postbronchodilator forced expiratory volume in 1 second (FEV1) that was less than 80% of predicted normal, and FEV1/forced vital capacity of less than 70%. All were current or exsmokers with a history of more than 10 pack-years.
At study entry, 7.2% of the GOLD A/B patients were treated with ICS without a LABA, and 31.1% were receiving ICS and a LABA. Of the GOLD C/D patients, 8.8% were receiving ICS without a LABA and 45% were receiving ICS with a LABA.
During the study, those who received both tiotropium and olodaterol had significant improvements in lung function, compared with those receiving only tiotropium. Among patients who had previously used ICS and received both drugs, the FEV1 area under the curve at 0-3 hours was 0.310 L for GOLD A/B patients and 0.236 L for GOLD C/D patients. For those with no prior ICS use, the FEV1 area under the curve at 0-3 hours was 0.277 L for GOLD A/B patients and 0.251 L for GOLD C/D patients.
For those with prior ICS use, trough FEV1 was 0.160 L for GOLD A/B patients and 0.122 L for GOLD C/D patients receiving both tiotropium and olodaterol. For those with no prior ICS use, trough FEV1 was 0.142 L for GOLD A/B patients and 0.149 L for GOLD C/D patients.
The TONADO studies included patients with moderate to very severe disease, but were conducted when the GOLD guidelines recommended that ICS plus LABA therapy be restricted to those with severe or very severe COPD and repeated exacerbations – before the guidelines were updated to take into account COPD symptoms. The updated guidelines call for ICS plus LABA maintenance therapy for patients in categories C and D disease with frequent exacerbations.
This study was supported by Boehringer Ingelheim, the maker of Respimat FDC.
DENVER – Inhaled corticosteroid plus long-acting beta2-agonist therapy is overused in patients with mild COPD, based on a post hoc analysis of two pivotal phase III studies.
At entry in the phase III TONADO studies, nearly 40% of patients who were classified as having GOLD A or B disease were receiving ICS maintenance therapy either alone, in free combination, or as fixed-dose combination therapy, Dr. Henrik Watz of the Pulmonary Research Institute at Lung Clinic Grosshansdorf, Airway Research Center North, Grosshansdorf, Germany, and his colleagues reported at an international conference of the American Thoracic Society.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend that use of inhaled corticosteroids (ICS) and long-acting beta2-agonist (LABA) therapy be restricted to patients with severe or very severe COPD (category C or D disease) with frequent exacerbations.
The post-hoc analysis “confirms previous reports highlighting that treatment regimens containing ICS therapy are being used early in the management of patients with COPD, which may not be appropriate based on current GOLD recommendations. Furthermore, consistent improvements in lung function with tiotropium plus olodaterol versus the monocomponents were demonstrated in GOLD A, B, C, and D, regardless of previous ICS use,” Dr. Watz and his associates concluded.
The replicate TONADO studies (TONADO 1 and 2) were multicenter, randomized, double-blind, active-controlled studies evaluating the once-daily long-acting muscarinic agent (LAMA) tiotropium and the LABA olodaterol. The 5,162 patients were randomized to once-daily treatment with inhaled tiotropium plus olodaterol (Respimat FDC), to tiotropium, or to olodaterol for 52 weeks.
Of the study participants, 2,132 had GOLD A/B disease, and 3,030 had GOLD C/D disease, based on exacerbation history and lung function. All had postbronchodilator forced expiratory volume in 1 second (FEV1) that was less than 80% of predicted normal, and FEV1/forced vital capacity of less than 70%. All were current or exsmokers with a history of more than 10 pack-years.
At study entry, 7.2% of the GOLD A/B patients were treated with ICS without a LABA, and 31.1% were receiving ICS and a LABA. Of the GOLD C/D patients, 8.8% were receiving ICS without a LABA and 45% were receiving ICS with a LABA.
During the study, those who received both tiotropium and olodaterol had significant improvements in lung function, compared with those receiving only tiotropium. Among patients who had previously used ICS and received both drugs, the FEV1 area under the curve at 0-3 hours was 0.310 L for GOLD A/B patients and 0.236 L for GOLD C/D patients. For those with no prior ICS use, the FEV1 area under the curve at 0-3 hours was 0.277 L for GOLD A/B patients and 0.251 L for GOLD C/D patients.
For those with prior ICS use, trough FEV1 was 0.160 L for GOLD A/B patients and 0.122 L for GOLD C/D patients receiving both tiotropium and olodaterol. For those with no prior ICS use, trough FEV1 was 0.142 L for GOLD A/B patients and 0.149 L for GOLD C/D patients.
The TONADO studies included patients with moderate to very severe disease, but were conducted when the GOLD guidelines recommended that ICS plus LABA therapy be restricted to those with severe or very severe COPD and repeated exacerbations – before the guidelines were updated to take into account COPD symptoms. The updated guidelines call for ICS plus LABA maintenance therapy for patients in categories C and D disease with frequent exacerbations.
This study was supported by Boehringer Ingelheim, the maker of Respimat FDC.
DENVER – Inhaled corticosteroid plus long-acting beta2-agonist therapy is overused in patients with mild COPD, based on a post hoc analysis of two pivotal phase III studies.
At entry in the phase III TONADO studies, nearly 40% of patients who were classified as having GOLD A or B disease were receiving ICS maintenance therapy either alone, in free combination, or as fixed-dose combination therapy, Dr. Henrik Watz of the Pulmonary Research Institute at Lung Clinic Grosshansdorf, Airway Research Center North, Grosshansdorf, Germany, and his colleagues reported at an international conference of the American Thoracic Society.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend that use of inhaled corticosteroids (ICS) and long-acting beta2-agonist (LABA) therapy be restricted to patients with severe or very severe COPD (category C or D disease) with frequent exacerbations.
The post-hoc analysis “confirms previous reports highlighting that treatment regimens containing ICS therapy are being used early in the management of patients with COPD, which may not be appropriate based on current GOLD recommendations. Furthermore, consistent improvements in lung function with tiotropium plus olodaterol versus the monocomponents were demonstrated in GOLD A, B, C, and D, regardless of previous ICS use,” Dr. Watz and his associates concluded.
The replicate TONADO studies (TONADO 1 and 2) were multicenter, randomized, double-blind, active-controlled studies evaluating the once-daily long-acting muscarinic agent (LAMA) tiotropium and the LABA olodaterol. The 5,162 patients were randomized to once-daily treatment with inhaled tiotropium plus olodaterol (Respimat FDC), to tiotropium, or to olodaterol for 52 weeks.
Of the study participants, 2,132 had GOLD A/B disease, and 3,030 had GOLD C/D disease, based on exacerbation history and lung function. All had postbronchodilator forced expiratory volume in 1 second (FEV1) that was less than 80% of predicted normal, and FEV1/forced vital capacity of less than 70%. All were current or exsmokers with a history of more than 10 pack-years.
At study entry, 7.2% of the GOLD A/B patients were treated with ICS without a LABA, and 31.1% were receiving ICS and a LABA. Of the GOLD C/D patients, 8.8% were receiving ICS without a LABA and 45% were receiving ICS with a LABA.
During the study, those who received both tiotropium and olodaterol had significant improvements in lung function, compared with those receiving only tiotropium. Among patients who had previously used ICS and received both drugs, the FEV1 area under the curve at 0-3 hours was 0.310 L for GOLD A/B patients and 0.236 L for GOLD C/D patients. For those with no prior ICS use, the FEV1 area under the curve at 0-3 hours was 0.277 L for GOLD A/B patients and 0.251 L for GOLD C/D patients.
For those with prior ICS use, trough FEV1 was 0.160 L for GOLD A/B patients and 0.122 L for GOLD C/D patients receiving both tiotropium and olodaterol. For those with no prior ICS use, trough FEV1 was 0.142 L for GOLD A/B patients and 0.149 L for GOLD C/D patients.
The TONADO studies included patients with moderate to very severe disease, but were conducted when the GOLD guidelines recommended that ICS plus LABA therapy be restricted to those with severe or very severe COPD and repeated exacerbations – before the guidelines were updated to take into account COPD symptoms. The updated guidelines call for ICS plus LABA maintenance therapy for patients in categories C and D disease with frequent exacerbations.
This study was supported by Boehringer Ingelheim, the maker of Respimat FDC.
AT ATS 2015
Key clinical point: Inhaled corticosteroid plus long-acting beta2-agonist therapy is overused in patients with mild COPD.
Major finding: Before study entry, 7.2% of GOLD A/B patients were receiving ICS and 31.1% were receiving ICS plus LABA; 8.8% of GOLD C/D patients were receiving ICS and and 45% were receiving ICS plus LABA.
Data source: A post hoc analysis of data for 5,162 patients from the phase III TONADO studies.
Disclosures: The study was supported by Boehringer Ingelheim, the maker of Respimat FDC.
ATS: QAW039 lowered sputum eosinophilia in eosinophilic asthma
DENVER – After 12 weeks of treatment, a novel oral prostaglandin D2–receptor antagonist significantly suppressed sputum and submucosal eosinophilia without affecting blood eosinophilia levels in patients with eosinophilic asthma, based on results of a single-center randomized trial.
QAW039 given at 225 mg b.i.d. for 12 weeks improved symptom control and asthma-related quality of life, and had a favorable safety profile, lead study author Dr. Rachid Berair said at an international conference of the American Thoracic Society.
QAW039 is under clinical development by Novartis Pharmaceuticals, the sponsor of the trial.
For the study 61 asthmatics with a sputum eosinophil count of 2% or greater were randomized to either QAW039 (30 patients) or placebo (31 patients) for 12 weeks after a 2-week placebo run-in. The study’s primary endpoint was the reduction of sputum eosinophils, while secondary objectives were improvement of asthma control and safety and tolerability. Other endpoints were improvement on the Asthma-related Quality of Life Questionnaire (AQLQ), FEV1, and histologic features of eosinophilic inflammation and airway remodeling, Dr. Berair of the University of Leicester, England, reported.
Mean patient age was 50 years; 90% of patients had asthma at Global Initiative for Asthma (GINA) level IV or greater, while 10% had asthma at GINA V and required up to 10 mg prednisolone daily.
In the trial, patients in the treatment group experienced a 3.5-fold reduction in sputum eosinophils, compared with those in the placebo group (P = .001); as well as a 2.3-fold reduction in submucosal eosinophils, compared with those in the placebo group (P = .041). The numbers of bronchial epithelial eosinophils did not differ between the two groups and there were no significant reductions in blood eosinophil levels between the groups.
Prebronchodilator FEV1 improved in the treatment group compared with the placebo group, but the differences did not reach statistical significance. However postbronchodilator FEV1 improved significantly in the treatment group, compared with the placebo group (P = .020).
The overall AQLQ scores improved significantly in the treatment group, compared with the placebo group (a mean of .59 points; P = .008), as did the AQLQ subdomains of symptoms, activities, emotions, and environmental exposure.
Adverse events were mild and moderate and balanced between both groups. The most common adverse events were respiratory tract infections and asthma exacerbations.
The clinical benefits of QAW039 on the AQLQ, postbronchodilator FEV1, and ACQ7 were no longer evident at 6 weeks after patients stopped taking the drug. “Given the effect of this drug on eosinophils, we can postulate that it would have an effect on asthma exacerbation rates,” Dr. Berair said. “However, our study was not designed nor was of the required duration to show that effect. We need longer studies to look at the effect of this drug on asthma exacerbations.”
The study was sponsored by Novartis. Dr. Berair reported having no relevant financial conflicts.
On Twitter @dougbrunk
DENVER – After 12 weeks of treatment, a novel oral prostaglandin D2–receptor antagonist significantly suppressed sputum and submucosal eosinophilia without affecting blood eosinophilia levels in patients with eosinophilic asthma, based on results of a single-center randomized trial.
QAW039 given at 225 mg b.i.d. for 12 weeks improved symptom control and asthma-related quality of life, and had a favorable safety profile, lead study author Dr. Rachid Berair said at an international conference of the American Thoracic Society.
QAW039 is under clinical development by Novartis Pharmaceuticals, the sponsor of the trial.
For the study 61 asthmatics with a sputum eosinophil count of 2% or greater were randomized to either QAW039 (30 patients) or placebo (31 patients) for 12 weeks after a 2-week placebo run-in. The study’s primary endpoint was the reduction of sputum eosinophils, while secondary objectives were improvement of asthma control and safety and tolerability. Other endpoints were improvement on the Asthma-related Quality of Life Questionnaire (AQLQ), FEV1, and histologic features of eosinophilic inflammation and airway remodeling, Dr. Berair of the University of Leicester, England, reported.
Mean patient age was 50 years; 90% of patients had asthma at Global Initiative for Asthma (GINA) level IV or greater, while 10% had asthma at GINA V and required up to 10 mg prednisolone daily.
In the trial, patients in the treatment group experienced a 3.5-fold reduction in sputum eosinophils, compared with those in the placebo group (P = .001); as well as a 2.3-fold reduction in submucosal eosinophils, compared with those in the placebo group (P = .041). The numbers of bronchial epithelial eosinophils did not differ between the two groups and there were no significant reductions in blood eosinophil levels between the groups.
Prebronchodilator FEV1 improved in the treatment group compared with the placebo group, but the differences did not reach statistical significance. However postbronchodilator FEV1 improved significantly in the treatment group, compared with the placebo group (P = .020).
The overall AQLQ scores improved significantly in the treatment group, compared with the placebo group (a mean of .59 points; P = .008), as did the AQLQ subdomains of symptoms, activities, emotions, and environmental exposure.
Adverse events were mild and moderate and balanced between both groups. The most common adverse events were respiratory tract infections and asthma exacerbations.
The clinical benefits of QAW039 on the AQLQ, postbronchodilator FEV1, and ACQ7 were no longer evident at 6 weeks after patients stopped taking the drug. “Given the effect of this drug on eosinophils, we can postulate that it would have an effect on asthma exacerbation rates,” Dr. Berair said. “However, our study was not designed nor was of the required duration to show that effect. We need longer studies to look at the effect of this drug on asthma exacerbations.”
The study was sponsored by Novartis. Dr. Berair reported having no relevant financial conflicts.
On Twitter @dougbrunk
DENVER – After 12 weeks of treatment, a novel oral prostaglandin D2–receptor antagonist significantly suppressed sputum and submucosal eosinophilia without affecting blood eosinophilia levels in patients with eosinophilic asthma, based on results of a single-center randomized trial.
QAW039 given at 225 mg b.i.d. for 12 weeks improved symptom control and asthma-related quality of life, and had a favorable safety profile, lead study author Dr. Rachid Berair said at an international conference of the American Thoracic Society.
QAW039 is under clinical development by Novartis Pharmaceuticals, the sponsor of the trial.
For the study 61 asthmatics with a sputum eosinophil count of 2% or greater were randomized to either QAW039 (30 patients) or placebo (31 patients) for 12 weeks after a 2-week placebo run-in. The study’s primary endpoint was the reduction of sputum eosinophils, while secondary objectives were improvement of asthma control and safety and tolerability. Other endpoints were improvement on the Asthma-related Quality of Life Questionnaire (AQLQ), FEV1, and histologic features of eosinophilic inflammation and airway remodeling, Dr. Berair of the University of Leicester, England, reported.
Mean patient age was 50 years; 90% of patients had asthma at Global Initiative for Asthma (GINA) level IV or greater, while 10% had asthma at GINA V and required up to 10 mg prednisolone daily.
In the trial, patients in the treatment group experienced a 3.5-fold reduction in sputum eosinophils, compared with those in the placebo group (P = .001); as well as a 2.3-fold reduction in submucosal eosinophils, compared with those in the placebo group (P = .041). The numbers of bronchial epithelial eosinophils did not differ between the two groups and there were no significant reductions in blood eosinophil levels between the groups.
Prebronchodilator FEV1 improved in the treatment group compared with the placebo group, but the differences did not reach statistical significance. However postbronchodilator FEV1 improved significantly in the treatment group, compared with the placebo group (P = .020).
The overall AQLQ scores improved significantly in the treatment group, compared with the placebo group (a mean of .59 points; P = .008), as did the AQLQ subdomains of symptoms, activities, emotions, and environmental exposure.
Adverse events were mild and moderate and balanced between both groups. The most common adverse events were respiratory tract infections and asthma exacerbations.
The clinical benefits of QAW039 on the AQLQ, postbronchodilator FEV1, and ACQ7 were no longer evident at 6 weeks after patients stopped taking the drug. “Given the effect of this drug on eosinophils, we can postulate that it would have an effect on asthma exacerbation rates,” Dr. Berair said. “However, our study was not designed nor was of the required duration to show that effect. We need longer studies to look at the effect of this drug on asthma exacerbations.”
The study was sponsored by Novartis. Dr. Berair reported having no relevant financial conflicts.
On Twitter @dougbrunk
AT ATS 2015
Key clinical point: QAW039 appears to reduce treatment-resistant eosinophilic inflammation in severe asthma.
Major finding: Patients in the QAW039 treatment group experienced a 3.5-fold reduction in sputum eosinophils, compared with those in the placebo group (P = .001), as well as a 2.3-fold reduction in submucosal eosinophils, compared with those in the placebo group (P = .041).
Data source: A randomized trial of 61 patients with eosinophilic asthma who received QAW039 225 b.i.d. or placebo for 12 weeks after a 2-week placebo run-in.
Disclosures: The study was sponsored by Novartis. Dr. Berair reported having no relevant financial conflicts.
ATS: Metformin failed to improve outcomes after COPD exacerbations
DENVER – Among patients hospitalized for chronic obstructive pulmonary disease (COPD) exacerbations, acute administration of metformin had no detectable impact on clinical recovery, based on results from a single-center study.
Patients hospitalized for COPD exacerbations are more likely to have longer hospital stays and to experience adverse outcomes if their blood glucose levels are elevated, Emma H. Baker, Ph.D., said in an interview at an international conference of the American Thoracic Society.
“The aim of the current study was to see if lowering blood glucose in COPD patients would reduce adverse outcomes,” said Dr. Baker, professor of clinical pharmacology at the Institute for Infection and Immunity at St. George’s University of London. A previous study suggested that tight glycemic control with insulin improved outcomes among COPD patients in the intensive care unit; however, a large, randomized, multicenter trial showed no difference in outcomes and a risk for hypoglycemia. The researchers wanted to see whether metformin would prove a safer option.
Dr. Baker and her associates randomized 51 patients in a 2:1 fashion to receive metformin or placebo. The metformin dose was escalated to 2 g/day over 4 days and continued for 1 month. Outcome measures included the Exacerbations of Chronic Pulmonary Disease Tool (EXACT), the COPD Assessment Test (CAT), and the rates of recurrent health care use events such as antibiotic or steroid prescription and readmission to the hospital. Of the 51 patients, 34 received metformin and 18 received placebo. The mean age of study participants was 67 years and 39% were women.
Compared with baseline, the EXACT scores improved by 4.2 points at day 5, 5-7 points at day 10, and 6.3 points at day 28 (P < .05 for all). However, the researchers observed no significance differences between the metformin and placebo groups in terms of improvements on EXACT scores. Similarly, CAT scores improved by a mean of 4.2 points over the course of the study, largely during the time between baseline and hospital discharge. There were no significant differences between the metformin and placebo groups at any time point. Meanwhile, the median time to a recurrent health care use event was similar between the groups (46 days in the metformin group, compared with 40 days in the placebo group; P = .682).
“Metformin works through reducing hepatic glucose output and increasing insulin sensitivity – and the effect may take several months,” Dr. Baker said. “What we did show is that metformin seems to be safe in COPD patients,” with no evidence of lactic acidosis.
Diabetes and hyperglycemia are very common in COPD, and were associated with more frequent COPD exacerbations and worse outcomes from exacerbations, according to Dr. Baker.
The study was sponsored by the Medical Research Council of the United Kingdom and the British Lung Foundation. Dr. Baker reported having no relevant financial conflicts.
On Twitter @dougbrunk
DENVER – Among patients hospitalized for chronic obstructive pulmonary disease (COPD) exacerbations, acute administration of metformin had no detectable impact on clinical recovery, based on results from a single-center study.
Patients hospitalized for COPD exacerbations are more likely to have longer hospital stays and to experience adverse outcomes if their blood glucose levels are elevated, Emma H. Baker, Ph.D., said in an interview at an international conference of the American Thoracic Society.
“The aim of the current study was to see if lowering blood glucose in COPD patients would reduce adverse outcomes,” said Dr. Baker, professor of clinical pharmacology at the Institute for Infection and Immunity at St. George’s University of London. A previous study suggested that tight glycemic control with insulin improved outcomes among COPD patients in the intensive care unit; however, a large, randomized, multicenter trial showed no difference in outcomes and a risk for hypoglycemia. The researchers wanted to see whether metformin would prove a safer option.
Dr. Baker and her associates randomized 51 patients in a 2:1 fashion to receive metformin or placebo. The metformin dose was escalated to 2 g/day over 4 days and continued for 1 month. Outcome measures included the Exacerbations of Chronic Pulmonary Disease Tool (EXACT), the COPD Assessment Test (CAT), and the rates of recurrent health care use events such as antibiotic or steroid prescription and readmission to the hospital. Of the 51 patients, 34 received metformin and 18 received placebo. The mean age of study participants was 67 years and 39% were women.
Compared with baseline, the EXACT scores improved by 4.2 points at day 5, 5-7 points at day 10, and 6.3 points at day 28 (P < .05 for all). However, the researchers observed no significance differences between the metformin and placebo groups in terms of improvements on EXACT scores. Similarly, CAT scores improved by a mean of 4.2 points over the course of the study, largely during the time between baseline and hospital discharge. There were no significant differences between the metformin and placebo groups at any time point. Meanwhile, the median time to a recurrent health care use event was similar between the groups (46 days in the metformin group, compared with 40 days in the placebo group; P = .682).
“Metformin works through reducing hepatic glucose output and increasing insulin sensitivity – and the effect may take several months,” Dr. Baker said. “What we did show is that metformin seems to be safe in COPD patients,” with no evidence of lactic acidosis.
Diabetes and hyperglycemia are very common in COPD, and were associated with more frequent COPD exacerbations and worse outcomes from exacerbations, according to Dr. Baker.
The study was sponsored by the Medical Research Council of the United Kingdom and the British Lung Foundation. Dr. Baker reported having no relevant financial conflicts.
On Twitter @dougbrunk
DENVER – Among patients hospitalized for chronic obstructive pulmonary disease (COPD) exacerbations, acute administration of metformin had no detectable impact on clinical recovery, based on results from a single-center study.
Patients hospitalized for COPD exacerbations are more likely to have longer hospital stays and to experience adverse outcomes if their blood glucose levels are elevated, Emma H. Baker, Ph.D., said in an interview at an international conference of the American Thoracic Society.
“The aim of the current study was to see if lowering blood glucose in COPD patients would reduce adverse outcomes,” said Dr. Baker, professor of clinical pharmacology at the Institute for Infection and Immunity at St. George’s University of London. A previous study suggested that tight glycemic control with insulin improved outcomes among COPD patients in the intensive care unit; however, a large, randomized, multicenter trial showed no difference in outcomes and a risk for hypoglycemia. The researchers wanted to see whether metformin would prove a safer option.
Dr. Baker and her associates randomized 51 patients in a 2:1 fashion to receive metformin or placebo. The metformin dose was escalated to 2 g/day over 4 days and continued for 1 month. Outcome measures included the Exacerbations of Chronic Pulmonary Disease Tool (EXACT), the COPD Assessment Test (CAT), and the rates of recurrent health care use events such as antibiotic or steroid prescription and readmission to the hospital. Of the 51 patients, 34 received metformin and 18 received placebo. The mean age of study participants was 67 years and 39% were women.
Compared with baseline, the EXACT scores improved by 4.2 points at day 5, 5-7 points at day 10, and 6.3 points at day 28 (P < .05 for all). However, the researchers observed no significance differences between the metformin and placebo groups in terms of improvements on EXACT scores. Similarly, CAT scores improved by a mean of 4.2 points over the course of the study, largely during the time between baseline and hospital discharge. There were no significant differences between the metformin and placebo groups at any time point. Meanwhile, the median time to a recurrent health care use event was similar between the groups (46 days in the metformin group, compared with 40 days in the placebo group; P = .682).
“Metformin works through reducing hepatic glucose output and increasing insulin sensitivity – and the effect may take several months,” Dr. Baker said. “What we did show is that metformin seems to be safe in COPD patients,” with no evidence of lactic acidosis.
Diabetes and hyperglycemia are very common in COPD, and were associated with more frequent COPD exacerbations and worse outcomes from exacerbations, according to Dr. Baker.
The study was sponsored by the Medical Research Council of the United Kingdom and the British Lung Foundation. Dr. Baker reported having no relevant financial conflicts.
On Twitter @dougbrunk
AT ATS 2015
Key clinical point:A 1-month course of metformin had no impact on clinical recovery from COPD exacerbations.
Major finding: From baseline to day 28, no significant differences between the metformin and placebo groups were observed in scores on the Exacerbations of Chronic Pulmonary Disease Tool (EXACT) and the COPD Assessment Test (CAT).
Data source: A randomized trial of 51 patients hospitalized for a COPD exacerbations.
Disclosures: The study was sponsored by the Medical Research Council of the United Kingdom and the British Lung Foundation. Dr. Baker reported having no relevant financial conflicts.
ATS: COPD patients on arformoterol tartrate reported better quality of life
DENVER – In a year-long multicenter trial, improvements in respiratory health status were more likely to be reported by COPD patients who received nebulized arformoterol tartrate, compared with those who received placebo as part of their treatment regimen.
“We can’t say it’s a blockbuster improvement in quality of life, but it’s still good,” lead study author Dr. James F. Donohue said in an interview at an international conference of the American Thoracic Society.
In a secondary analysis of data from a published clinical trial (Chest 2014;146:1531-42), Dr. Donohue and his associates evaluated 420 patients with moderate to severe COPD who received nebulized arformoterol 15 mcg b.i.d. and 421 who received matched placebo for 52 weeks. Eligibility criteria included being at least 40 years of age and having a documented primary diagnosis of nonasthmatic COPD. With the exception of other long-acting beta-2 agonists, treatment with other COPD medications was permitted.
At months 3, 6, and 12, patients completed the 10-item Clinical COPD Questionnaire(CCQ), a validated, self-administered instrument. Improvement of 0.4 points or more in the CCQ total score is considered clinically significant.
At baseline, the mean age of the study participants was 63 years, 43% were female, and 51% were current smokers. A total of 466 participants completed the 12-month trial, including 255 in the arformoterol group (61%) and 211 in the placebo group (51%). Compared with patients in the placebo group, those in the arformoterol group had greater improvements on CCQ total score (–0.18 vs. 0.02, respectively; P = 0.001), symptoms (–0.21 vs. 0.01; P = 0.002), functional state (–0.17 vs. 0.02; P = 0.018), and mental state (–0.20 vs. 0.02; P = 0.023) across the postbaseline visits. At the study endpoint, 38% of patients in the arformoterol group and 31% of patients in the placebo group had clinically significant improvements on the CCQ total score.
“These are good results in a 52-week study,” said Dr. Donohue, professor of pulmonary and critical care medicine at the University of North Carolina, Chapel Hill. “What we really want is to help the patient feel better, be more mentally alert, more functional, and have less symptoms.”
The study was funded by Sunovion Pharmaceuticals, the manufacturer of arformoterol. Dr. Donohue disclosed that he is a consultant for the company.
On Twitter @dougbrunk
DENVER – In a year-long multicenter trial, improvements in respiratory health status were more likely to be reported by COPD patients who received nebulized arformoterol tartrate, compared with those who received placebo as part of their treatment regimen.
“We can’t say it’s a blockbuster improvement in quality of life, but it’s still good,” lead study author Dr. James F. Donohue said in an interview at an international conference of the American Thoracic Society.
In a secondary analysis of data from a published clinical trial (Chest 2014;146:1531-42), Dr. Donohue and his associates evaluated 420 patients with moderate to severe COPD who received nebulized arformoterol 15 mcg b.i.d. and 421 who received matched placebo for 52 weeks. Eligibility criteria included being at least 40 years of age and having a documented primary diagnosis of nonasthmatic COPD. With the exception of other long-acting beta-2 agonists, treatment with other COPD medications was permitted.
At months 3, 6, and 12, patients completed the 10-item Clinical COPD Questionnaire(CCQ), a validated, self-administered instrument. Improvement of 0.4 points or more in the CCQ total score is considered clinically significant.
At baseline, the mean age of the study participants was 63 years, 43% were female, and 51% were current smokers. A total of 466 participants completed the 12-month trial, including 255 in the arformoterol group (61%) and 211 in the placebo group (51%). Compared with patients in the placebo group, those in the arformoterol group had greater improvements on CCQ total score (–0.18 vs. 0.02, respectively; P = 0.001), symptoms (–0.21 vs. 0.01; P = 0.002), functional state (–0.17 vs. 0.02; P = 0.018), and mental state (–0.20 vs. 0.02; P = 0.023) across the postbaseline visits. At the study endpoint, 38% of patients in the arformoterol group and 31% of patients in the placebo group had clinically significant improvements on the CCQ total score.
“These are good results in a 52-week study,” said Dr. Donohue, professor of pulmonary and critical care medicine at the University of North Carolina, Chapel Hill. “What we really want is to help the patient feel better, be more mentally alert, more functional, and have less symptoms.”
The study was funded by Sunovion Pharmaceuticals, the manufacturer of arformoterol. Dr. Donohue disclosed that he is a consultant for the company.
On Twitter @dougbrunk
DENVER – In a year-long multicenter trial, improvements in respiratory health status were more likely to be reported by COPD patients who received nebulized arformoterol tartrate, compared with those who received placebo as part of their treatment regimen.
“We can’t say it’s a blockbuster improvement in quality of life, but it’s still good,” lead study author Dr. James F. Donohue said in an interview at an international conference of the American Thoracic Society.
In a secondary analysis of data from a published clinical trial (Chest 2014;146:1531-42), Dr. Donohue and his associates evaluated 420 patients with moderate to severe COPD who received nebulized arformoterol 15 mcg b.i.d. and 421 who received matched placebo for 52 weeks. Eligibility criteria included being at least 40 years of age and having a documented primary diagnosis of nonasthmatic COPD. With the exception of other long-acting beta-2 agonists, treatment with other COPD medications was permitted.
At months 3, 6, and 12, patients completed the 10-item Clinical COPD Questionnaire(CCQ), a validated, self-administered instrument. Improvement of 0.4 points or more in the CCQ total score is considered clinically significant.
At baseline, the mean age of the study participants was 63 years, 43% were female, and 51% were current smokers. A total of 466 participants completed the 12-month trial, including 255 in the arformoterol group (61%) and 211 in the placebo group (51%). Compared with patients in the placebo group, those in the arformoterol group had greater improvements on CCQ total score (–0.18 vs. 0.02, respectively; P = 0.001), symptoms (–0.21 vs. 0.01; P = 0.002), functional state (–0.17 vs. 0.02; P = 0.018), and mental state (–0.20 vs. 0.02; P = 0.023) across the postbaseline visits. At the study endpoint, 38% of patients in the arformoterol group and 31% of patients in the placebo group had clinically significant improvements on the CCQ total score.
“These are good results in a 52-week study,” said Dr. Donohue, professor of pulmonary and critical care medicine at the University of North Carolina, Chapel Hill. “What we really want is to help the patient feel better, be more mentally alert, more functional, and have less symptoms.”
The study was funded by Sunovion Pharmaceuticals, the manufacturer of arformoterol. Dr. Donohue disclosed that he is a consultant for the company.
On Twitter @dougbrunk
AT ATS 2015
Key clinical point: Assessing health status along with lung function provides additional information about the effectiveness of COPD maintenance treatments.
Major finding: After a year, 38% of patients in the arformoterol group and 31% of patients in the placebo group reported clinically significant improvements on the Clinical COPD Questionnaire (CCQ).
Data source: A secondary analysis of data from 420 patients who had moderate to severe COPD and received nebulized arformoterol 15 mcg b.i.d. and 421 similar patients who received a matched placebo.
Disclosures: The study was funded by Sunovion Pharmaceuticals. Dr. Donohue disclosed that he is a consultant for the company.
Mepolizumab reduces asthma exacerbations more in the elderly
DENVER – The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo as part of the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) trial, according to a post hoc analysis of the trial data.
The analysis also demonstrated that mepolizumab improved quality of life, compared with standard care, in both older and younger patients, although little differentiation was seen with respect to asthma control, Dr. Hector Ortega reported in a poster at an international conference of the American Thoracic Society.
A 76% greater reduction in clinically significant exacerbations was seen in 54 mepolizumab-treated patients aged 65 years and older in the trial, compared with 26 in that age group who received placebo (mean exacerbation rate per year, 0.92 vs. 1.65); a 44% greater reduction was seen in 331 mepolizumab-treated patients under age 65 years, compared with 165 in that age group who received placebo (mean exacerbation rate per year, 0.42 vs. 1.78), said Dr. Ortega, medical director at GlaxoSmithKline, Research Triangle Park, N.C.
The adjusted mean difference vs. placebo in change in St. George’s Respiratory Questionnaire scores from baseline to 32 weeks was -4.5 in the older patients, and -7.3 in the younger patients, and the adjusted mean difference vs. placebo in change in Asthma Control Questionnaire scores from baseline to 32 weeks was -0.1 and -0.5 in the older and younger patients, respectively, he noted.
The older patients, as expected, suffered more frequently from comorbidities, and this may have been accentuated by the chronic use of inhaled and oral corticosteroids in the older patients.
However, comorbidities had no impact on the response to treatment, and the safety profile of mepolizumab was similar to placebo in both age groups, he said.
Patients in the multicenter, randomized, placebo-controlled MENSA trial received high dose inhaled corticosteroids plus at least one additional controller, had a history of frequent exacerbations and a predefined eosinophilic threshold, and were randomized to receive add-on therapy with either 75 mg of intravenous mepolizumab, 100 mg subcutaneous mepolizumab, or corresponding placebo every 4 weeks for 32 weeks. In the primary planned analysis, the responses to the two doses of mepolizumab were similar, but efficacy in older patients had not been previously reported.
The MENSA trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
DENVER – The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo as part of the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) trial, according to a post hoc analysis of the trial data.
The analysis also demonstrated that mepolizumab improved quality of life, compared with standard care, in both older and younger patients, although little differentiation was seen with respect to asthma control, Dr. Hector Ortega reported in a poster at an international conference of the American Thoracic Society.
A 76% greater reduction in clinically significant exacerbations was seen in 54 mepolizumab-treated patients aged 65 years and older in the trial, compared with 26 in that age group who received placebo (mean exacerbation rate per year, 0.92 vs. 1.65); a 44% greater reduction was seen in 331 mepolizumab-treated patients under age 65 years, compared with 165 in that age group who received placebo (mean exacerbation rate per year, 0.42 vs. 1.78), said Dr. Ortega, medical director at GlaxoSmithKline, Research Triangle Park, N.C.
The adjusted mean difference vs. placebo in change in St. George’s Respiratory Questionnaire scores from baseline to 32 weeks was -4.5 in the older patients, and -7.3 in the younger patients, and the adjusted mean difference vs. placebo in change in Asthma Control Questionnaire scores from baseline to 32 weeks was -0.1 and -0.5 in the older and younger patients, respectively, he noted.
The older patients, as expected, suffered more frequently from comorbidities, and this may have been accentuated by the chronic use of inhaled and oral corticosteroids in the older patients.
However, comorbidities had no impact on the response to treatment, and the safety profile of mepolizumab was similar to placebo in both age groups, he said.
Patients in the multicenter, randomized, placebo-controlled MENSA trial received high dose inhaled corticosteroids plus at least one additional controller, had a history of frequent exacerbations and a predefined eosinophilic threshold, and were randomized to receive add-on therapy with either 75 mg of intravenous mepolizumab, 100 mg subcutaneous mepolizumab, or corresponding placebo every 4 weeks for 32 weeks. In the primary planned analysis, the responses to the two doses of mepolizumab were similar, but efficacy in older patients had not been previously reported.
The MENSA trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
DENVER – The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo as part of the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) trial, according to a post hoc analysis of the trial data.
The analysis also demonstrated that mepolizumab improved quality of life, compared with standard care, in both older and younger patients, although little differentiation was seen with respect to asthma control, Dr. Hector Ortega reported in a poster at an international conference of the American Thoracic Society.
A 76% greater reduction in clinically significant exacerbations was seen in 54 mepolizumab-treated patients aged 65 years and older in the trial, compared with 26 in that age group who received placebo (mean exacerbation rate per year, 0.92 vs. 1.65); a 44% greater reduction was seen in 331 mepolizumab-treated patients under age 65 years, compared with 165 in that age group who received placebo (mean exacerbation rate per year, 0.42 vs. 1.78), said Dr. Ortega, medical director at GlaxoSmithKline, Research Triangle Park, N.C.
The adjusted mean difference vs. placebo in change in St. George’s Respiratory Questionnaire scores from baseline to 32 weeks was -4.5 in the older patients, and -7.3 in the younger patients, and the adjusted mean difference vs. placebo in change in Asthma Control Questionnaire scores from baseline to 32 weeks was -0.1 and -0.5 in the older and younger patients, respectively, he noted.
The older patients, as expected, suffered more frequently from comorbidities, and this may have been accentuated by the chronic use of inhaled and oral corticosteroids in the older patients.
However, comorbidities had no impact on the response to treatment, and the safety profile of mepolizumab was similar to placebo in both age groups, he said.
Patients in the multicenter, randomized, placebo-controlled MENSA trial received high dose inhaled corticosteroids plus at least one additional controller, had a history of frequent exacerbations and a predefined eosinophilic threshold, and were randomized to receive add-on therapy with either 75 mg of intravenous mepolizumab, 100 mg subcutaneous mepolizumab, or corresponding placebo every 4 weeks for 32 weeks. In the primary planned analysis, the responses to the two doses of mepolizumab were similar, but efficacy in older patients had not been previously reported.
The MENSA trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
AT ATS 2015
Key clinical point: The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo.
Major finding: The mean reduction in the exacerbation rate with mepolizumab vs. placebo was 76% for those aged 65 and older vs. 44% in those under age 65.
Data source: A post hoc analysis of data from 576 patients in the multicenter, randomized, placebo-controlled MENSA trial.
Disclosures: The trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
Traditional Diagnostic Criteria Miss OSA in Coronary Artery Disease Patients
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
AT ATS 2015
Traditional diagnostic criteria miss OSA in coronary artery disease patients
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
DENVER – Obstructive sleep apnea was common among patients in the multinational Sleep and Stent Study who underwent successful percutaneous coronary intervention, but most of those affected had no daytime sleepiness, and only about half had positive findings on the Berlin Questionnaire.
Further, findings at up to 4 years of follow-up show that coronary artery disease patients with vs. without obstructive sleep apnea (OSA) had nearly twice the risk of adverse events.
The findings suggest that OSA has important clinical implications for coronary artery disease (CAD), and that validated tools for identifying OSA risk in the general population may not be useful in those with cardiovascular disease, Sofia Furlan, Ph.D. said in a press briefing at an international conference of the American Thoracic Society.
Of 1,305 patients included in the ongoing observational study, 45% had OSA, including 21.8% who had severe OSA defined by an apnea-hypopnea index of 30 or more events/hour. Excessive daytime sleepiness, defined as an Epworth Sleepiness Scale score of greater than 10, was identified in 24.5% of the OSA patients, and a Berlin Questionnaire score indicative of high risk for OSA was found in 54.3% of patients with OSA, said Dr. Furlan of the University of Sao Paulo, Brazil.
The adverse event rate was 11% in CAD patients with OSA, compared with 6.5% in those without OSA – a significant difference thus far in the study, Dr. Furlan noted.
Patients in the Sleep and Stent Study, which was designed to look at the relationships between OSA and cardiovascular outcomes in adults treated with percutaneous coronary intervention, were aged 18-80 years and were enrolled from eight centers in five countries: Singapore, China and Hong Kong, India, Myanmar, and Brazil.
Indications for percutaneous coronary intervention included ST segment elevation myocardial infarction in 33% of subjects, non-ST segment elevation myocardial infarction in 20% of subjects, unstable angina in 16% of subjects, and stable angina in 31% of subjects. All patients underwent an overnight sleep study using a level-3 portable diagnostic device prior to hospital discharge, and the tracings were analyzed by a blinded sleep physician.
The prevalence of OSA was comparable across study sites, Dr. Furlan said.
The findings reinforce the known association between OSA and cardiovascular disease, lead researcher Dr. Luciano Drager, also of the University of Sao Paulo, said in a press statement.
“Earlier studies have shown strong relationships between sleep apnea and a number of cardiovascular conditions, including high blood pressure, arrhythmia, stroke, and heart failure. Our study supports this strong association between OSA and heart disease and also suggests that the methods used to screen for OSA in patients with cardiovascular disease need to be improved,” Dr. Drager said.
Given the apparent association between sleep apnea and adverse outcomes in patients with CAD, and the fact that sleep apnea is underdiagnosed, it may be time to consider monitoring for sleep apnea in CAD patients, Dr. Furlan added.
Non–sleep related predictors of OSA in patients with cardiovascular disease also should be explored, she said.
Press briefing moderator Dr. Mihaela Teodorescu of the University of Wisconsin, Madison, noted that other studies also have suggested that coexistent OSA manifests differently in different disease states.
“This is a very good example. Whereas in the general population sleepiness is one of the major presenting symptoms, in this particularly vulnerable population with heart attacks and severe coronary artery disease, these people are not sleepy, ” she said, adding that “current screening questionnaires, which are relatively widely used in clinical populations, may not do the job.”
In fact, if traditional criteria for diagnosis are used, a large proportion of patients with OSA will be missed, she said.
“To me as a clinician, this is the major take-home message: Don’t rely on symptoms, because we will miss a large proportion of patients simply based on that.”
The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.
AT ATS 2015
Key clinical point: OSA presents differently in CAD patients, so traditional diagnostic criteria will miss a large proportion of cases.
Major finding: Only 24.5% of the OSA patients reported excessive daytime sleepiness, and only 54.3% had a Berlin Questionnaire score indicative of high risk for OSA.
Data source: A multicenter observational study involving 1,305 patients.
Disclosures: The investigators reported having no disclosures. The Sleep and Stent Study is sponsored by Boston Scientific Corporation.