User login
American College of Cardiology (ACC): Cardiovascular Conference at Snowmass
Aspirin for AF Fading Away
SNOWMASS, COLO.– The sun may be setting on the use of aspirin for thromboprophylaxis in patients with nonvalvular atrial fibrillation. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines are unique among the major international guidelines in giving a modest IIb, Level of Evidence C endorsement to the option of aspirin or no treatment in patients with atrial fibrillation (AF) and a CHA2DS2-VASc score of 1. In contrast, the 2014 U.K. NICE (National Institute for Health and Care Excellence) guidelines, the 2013 Asian Pacific Heart Rhythm Society guidelines, and the 2012 European Society of Cardiology guidelines all recommend consideration of oral anticoagulation – eschewing aspirin – in patients with a CHA2DS2-VASc score of 1 or more, Dr. Bernard J. Gersh noted at the Annual Cardiovascular Conference at Snowmass.
The NICE guidelines put the matter succinctly, stating, “The main departure from prior guidelines is that aspirin should not be used in AF simply to reduce the risk of stroke, as it is not as effective as NOACs [novel oral anticoagulants] ... and can cause more bleeding side effects.”
Dr. Gersh was coauthor of a recently published think piece that argued that exaggerated misperceptions of aspirin’s efficacy and safety have led to its inappropriate status as “the easy option” for stroke prevention in AF (Eur. Heart J. 2015;36:653-6).
“By giving physicians a soft option of aspirin in CHA2DS2-VASc 1 patients, we’re allowing them an excuse not to use an oral anticoagulant, which is what they should be using,” Dr. Gersh explained at the conference.
He noted that an analysis of more than 41,000 Medicare beneficiaries with AF in 2007-2008 showed that only 66.8% were on warfarin.
“There’s no doubt that warfarin, and for that matter the NOACs, are underutilized, and it’s possible that the misperception that aspirin is effective may contribute to that underutilization,” asserted Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
The cardiologist added that the widely held belief that aspirin reduces stroke risk by roughly 20%, compared with placebo, in patients with AF is based upon seriously flawed data. True, a meta-analysis of six placebo-controlled randomized trials done in an earlier era concluded that aspirin reduced the relative risk of stroke by 19%, but what’s often overlooked is that aspirin significantly outperformed placebo in only one of those six studies – the SPAF 1 trial – where in one arm aspirin achieved an “almost implausible” 94% relative risk reduction.
“Nothing reduces risk by 94%,” Dr. Gersh observed, adding that the SPAF 1 methodology was, upon careful examination, “completely unacceptable” by contemporary standards.
He was a coinvestigator in a study of 7,347 AF patients on oral anticoagulation therapy in 176 U.S. practices participating in the ORBIT-AF registry (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). The analysis showed that concomitant use of aspirin and an oral anticoagulant in patients with AF is common in everyday clinical practice, being employed in 35% of the study population. Of note, 39% of these patients had no history of CAD and therefore weren’t on aspirin for secondary cardiovascular prevention, and 17% of them were at elevated bleeding risk because of an ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding risk score of 5 or more.
The key, disturbing finding was this: The adjusted risks of major bleeding and hospitalization for bleeding were 53% and 52% greater, respectively, in patients on an oral anticoagulant plus aspirin than with an oral anticoagulant alone (Circulation 2013;128:721-8).
On the basis of this study and other evidence, Dr. Gersh believes the use of aspirin in patients with AF should be considered only in those with CAD, where a case can be made for its use in secondary prevention alongside an oral anticoagulant to reduce stroke risk. But even then, aspirin’s use is reasonable only in those at low risk of bleeding, and extra vigilance and prophylaxis with a proton pump inhibitor are called for.
“If a patient is at high risk of bleeding, I personally would not give aspirin,” the cardiologist added.
The big remaining question – and an area of current controversy – concerns the safety of halting aspirin in AF patients on an oral anticoagulant who’ve undergone coronary stenting. Ongoing studies are looking at this issue, and answers are expected within a year or 2.
Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for Baxter, Medtronic, and Teva.
SNOWMASS, COLO.– The sun may be setting on the use of aspirin for thromboprophylaxis in patients with nonvalvular atrial fibrillation. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines are unique among the major international guidelines in giving a modest IIb, Level of Evidence C endorsement to the option of aspirin or no treatment in patients with atrial fibrillation (AF) and a CHA2DS2-VASc score of 1. In contrast, the 2014 U.K. NICE (National Institute for Health and Care Excellence) guidelines, the 2013 Asian Pacific Heart Rhythm Society guidelines, and the 2012 European Society of Cardiology guidelines all recommend consideration of oral anticoagulation – eschewing aspirin – in patients with a CHA2DS2-VASc score of 1 or more, Dr. Bernard J. Gersh noted at the Annual Cardiovascular Conference at Snowmass.
The NICE guidelines put the matter succinctly, stating, “The main departure from prior guidelines is that aspirin should not be used in AF simply to reduce the risk of stroke, as it is not as effective as NOACs [novel oral anticoagulants] ... and can cause more bleeding side effects.”
Dr. Gersh was coauthor of a recently published think piece that argued that exaggerated misperceptions of aspirin’s efficacy and safety have led to its inappropriate status as “the easy option” for stroke prevention in AF (Eur. Heart J. 2015;36:653-6).
“By giving physicians a soft option of aspirin in CHA2DS2-VASc 1 patients, we’re allowing them an excuse not to use an oral anticoagulant, which is what they should be using,” Dr. Gersh explained at the conference.
He noted that an analysis of more than 41,000 Medicare beneficiaries with AF in 2007-2008 showed that only 66.8% were on warfarin.
“There’s no doubt that warfarin, and for that matter the NOACs, are underutilized, and it’s possible that the misperception that aspirin is effective may contribute to that underutilization,” asserted Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
The cardiologist added that the widely held belief that aspirin reduces stroke risk by roughly 20%, compared with placebo, in patients with AF is based upon seriously flawed data. True, a meta-analysis of six placebo-controlled randomized trials done in an earlier era concluded that aspirin reduced the relative risk of stroke by 19%, but what’s often overlooked is that aspirin significantly outperformed placebo in only one of those six studies – the SPAF 1 trial – where in one arm aspirin achieved an “almost implausible” 94% relative risk reduction.
“Nothing reduces risk by 94%,” Dr. Gersh observed, adding that the SPAF 1 methodology was, upon careful examination, “completely unacceptable” by contemporary standards.
He was a coinvestigator in a study of 7,347 AF patients on oral anticoagulation therapy in 176 U.S. practices participating in the ORBIT-AF registry (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). The analysis showed that concomitant use of aspirin and an oral anticoagulant in patients with AF is common in everyday clinical practice, being employed in 35% of the study population. Of note, 39% of these patients had no history of CAD and therefore weren’t on aspirin for secondary cardiovascular prevention, and 17% of them were at elevated bleeding risk because of an ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding risk score of 5 or more.
The key, disturbing finding was this: The adjusted risks of major bleeding and hospitalization for bleeding were 53% and 52% greater, respectively, in patients on an oral anticoagulant plus aspirin than with an oral anticoagulant alone (Circulation 2013;128:721-8).
On the basis of this study and other evidence, Dr. Gersh believes the use of aspirin in patients with AF should be considered only in those with CAD, where a case can be made for its use in secondary prevention alongside an oral anticoagulant to reduce stroke risk. But even then, aspirin’s use is reasonable only in those at low risk of bleeding, and extra vigilance and prophylaxis with a proton pump inhibitor are called for.
“If a patient is at high risk of bleeding, I personally would not give aspirin,” the cardiologist added.
The big remaining question – and an area of current controversy – concerns the safety of halting aspirin in AF patients on an oral anticoagulant who’ve undergone coronary stenting. Ongoing studies are looking at this issue, and answers are expected within a year or 2.
Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for Baxter, Medtronic, and Teva.
SNOWMASS, COLO.– The sun may be setting on the use of aspirin for thromboprophylaxis in patients with nonvalvular atrial fibrillation. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines are unique among the major international guidelines in giving a modest IIb, Level of Evidence C endorsement to the option of aspirin or no treatment in patients with atrial fibrillation (AF) and a CHA2DS2-VASc score of 1. In contrast, the 2014 U.K. NICE (National Institute for Health and Care Excellence) guidelines, the 2013 Asian Pacific Heart Rhythm Society guidelines, and the 2012 European Society of Cardiology guidelines all recommend consideration of oral anticoagulation – eschewing aspirin – in patients with a CHA2DS2-VASc score of 1 or more, Dr. Bernard J. Gersh noted at the Annual Cardiovascular Conference at Snowmass.
The NICE guidelines put the matter succinctly, stating, “The main departure from prior guidelines is that aspirin should not be used in AF simply to reduce the risk of stroke, as it is not as effective as NOACs [novel oral anticoagulants] ... and can cause more bleeding side effects.”
Dr. Gersh was coauthor of a recently published think piece that argued that exaggerated misperceptions of aspirin’s efficacy and safety have led to its inappropriate status as “the easy option” for stroke prevention in AF (Eur. Heart J. 2015;36:653-6).
“By giving physicians a soft option of aspirin in CHA2DS2-VASc 1 patients, we’re allowing them an excuse not to use an oral anticoagulant, which is what they should be using,” Dr. Gersh explained at the conference.
He noted that an analysis of more than 41,000 Medicare beneficiaries with AF in 2007-2008 showed that only 66.8% were on warfarin.
“There’s no doubt that warfarin, and for that matter the NOACs, are underutilized, and it’s possible that the misperception that aspirin is effective may contribute to that underutilization,” asserted Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
The cardiologist added that the widely held belief that aspirin reduces stroke risk by roughly 20%, compared with placebo, in patients with AF is based upon seriously flawed data. True, a meta-analysis of six placebo-controlled randomized trials done in an earlier era concluded that aspirin reduced the relative risk of stroke by 19%, but what’s often overlooked is that aspirin significantly outperformed placebo in only one of those six studies – the SPAF 1 trial – where in one arm aspirin achieved an “almost implausible” 94% relative risk reduction.
“Nothing reduces risk by 94%,” Dr. Gersh observed, adding that the SPAF 1 methodology was, upon careful examination, “completely unacceptable” by contemporary standards.
He was a coinvestigator in a study of 7,347 AF patients on oral anticoagulation therapy in 176 U.S. practices participating in the ORBIT-AF registry (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). The analysis showed that concomitant use of aspirin and an oral anticoagulant in patients with AF is common in everyday clinical practice, being employed in 35% of the study population. Of note, 39% of these patients had no history of CAD and therefore weren’t on aspirin for secondary cardiovascular prevention, and 17% of them were at elevated bleeding risk because of an ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding risk score of 5 or more.
The key, disturbing finding was this: The adjusted risks of major bleeding and hospitalization for bleeding were 53% and 52% greater, respectively, in patients on an oral anticoagulant plus aspirin than with an oral anticoagulant alone (Circulation 2013;128:721-8).
On the basis of this study and other evidence, Dr. Gersh believes the use of aspirin in patients with AF should be considered only in those with CAD, where a case can be made for its use in secondary prevention alongside an oral anticoagulant to reduce stroke risk. But even then, aspirin’s use is reasonable only in those at low risk of bleeding, and extra vigilance and prophylaxis with a proton pump inhibitor are called for.
“If a patient is at high risk of bleeding, I personally would not give aspirin,” the cardiologist added.
The big remaining question – and an area of current controversy – concerns the safety of halting aspirin in AF patients on an oral anticoagulant who’ve undergone coronary stenting. Ongoing studies are looking at this issue, and answers are expected within a year or 2.
Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for Baxter, Medtronic, and Teva.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Aspirin for AF fading away
SNOWMASS, COLO.– The sun may be setting on the use of aspirin for thromboprophylaxis in patients with nonvalvular atrial fibrillation. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines are unique among the major international guidelines in giving a modest IIb, Level of Evidence C endorsement to the option of aspirin or no treatment in patients with atrial fibrillation (AF) and a CHA2DS2-VASc score of 1. In contrast, the 2014 U.K. NICE (National Institute for Health and Care Excellence) guidelines, the 2013 Asian Pacific Heart Rhythm Society guidelines, and the 2012 European Society of Cardiology guidelines all recommend consideration of oral anticoagulation – eschewing aspirin – in patients with a CHA2DS2-VASc score of 1 or more, Dr. Bernard J. Gersh noted at the Annual Cardiovascular Conference at Snowmass.
The NICE guidelines put the matter succinctly, stating, “The main departure from prior guidelines is that aspirin should not be used in AF simply to reduce the risk of stroke, as it is not as effective as NOACs [novel oral anticoagulants] ... and can cause more bleeding side effects.”
Dr. Gersh was coauthor of a recently published think piece that argued that exaggerated misperceptions of aspirin’s efficacy and safety have led to its inappropriate status as “the easy option” for stroke prevention in AF (Eur. Heart J. 2015;36:653-6).
“By giving physicians a soft option of aspirin in CHA2DS2-VASc 1 patients, we’re allowing them an excuse not to use an oral anticoagulant, which is what they should be using,” Dr. Gersh explained at the conference.
He noted that an analysis of more than 41,000 Medicare beneficiaries with AF in 2007-2008 showed that only 66.8% were on warfarin.
“There’s no doubt that warfarin, and for that matter the NOACs, are underutilized, and it’s possible that the misperception that aspirin is effective may contribute to that underutilization,” asserted Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
The cardiologist added that the widely held belief that aspirin reduces stroke risk by roughly 20%, compared with placebo, in patients with AF is based upon seriously flawed data. True, a meta-analysis of six placebo-controlled randomized trials done in an earlier era concluded that aspirin reduced the relative risk of stroke by 19%, but what’s often overlooked is that aspirin significantly outperformed placebo in only one of those six studies – the SPAF 1 trial – where in one arm aspirin achieved an “almost implausible” 94% relative risk reduction.
“Nothing reduces risk by 94%,” Dr. Gersh observed, adding that the SPAF 1 methodology was, upon careful examination, “completely unacceptable” by contemporary standards.
He was a coinvestigator in a study of 7,347 AF patients on oral anticoagulation therapy in 176 U.S. practices participating in the ORBIT-AF registry (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). The analysis showed that concomitant use of aspirin and an oral anticoagulant in patients with AF is common in everyday clinical practice, being employed in 35% of the study population. Of note, 39% of these patients had no history of CAD and therefore weren’t on aspirin for secondary cardiovascular prevention, and 17% of them were at elevated bleeding risk because of an ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding risk score of 5 or more.
The key, disturbing finding was this: The adjusted risks of major bleeding and hospitalization for bleeding were 53% and 52% greater, respectively, in patients on an oral anticoagulant plus aspirin than with an oral anticoagulant alone (Circulation 2013;128:721-8).
On the basis of this study and other evidence, Dr. Gersh believes the use of aspirin in patients with AF should be considered only in those with CAD, where a case can be made for its use in secondary prevention alongside an oral anticoagulant to reduce stroke risk. But even then, aspirin’s use is reasonable only in those at low risk of bleeding, and extra vigilance and prophylaxis with a proton pump inhibitor are called for.
“If a patient is at high risk of bleeding, I personally would not give aspirin,” the cardiologist added.
The big remaining question – and an area of current controversy – concerns the safety of halting aspirin in AF patients on an oral anticoagulant who’ve undergone coronary stenting. Ongoing studies are looking at this issue, and answers are expected within a year or 2.
Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for Baxter, Medtronic, and Teva.
SNOWMASS, COLO.– The sun may be setting on the use of aspirin for thromboprophylaxis in patients with nonvalvular atrial fibrillation. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines are unique among the major international guidelines in giving a modest IIb, Level of Evidence C endorsement to the option of aspirin or no treatment in patients with atrial fibrillation (AF) and a CHA2DS2-VASc score of 1. In contrast, the 2014 U.K. NICE (National Institute for Health and Care Excellence) guidelines, the 2013 Asian Pacific Heart Rhythm Society guidelines, and the 2012 European Society of Cardiology guidelines all recommend consideration of oral anticoagulation – eschewing aspirin – in patients with a CHA2DS2-VASc score of 1 or more, Dr. Bernard J. Gersh noted at the Annual Cardiovascular Conference at Snowmass.
The NICE guidelines put the matter succinctly, stating, “The main departure from prior guidelines is that aspirin should not be used in AF simply to reduce the risk of stroke, as it is not as effective as NOACs [novel oral anticoagulants] ... and can cause more bleeding side effects.”
Dr. Gersh was coauthor of a recently published think piece that argued that exaggerated misperceptions of aspirin’s efficacy and safety have led to its inappropriate status as “the easy option” for stroke prevention in AF (Eur. Heart J. 2015;36:653-6).
“By giving physicians a soft option of aspirin in CHA2DS2-VASc 1 patients, we’re allowing them an excuse not to use an oral anticoagulant, which is what they should be using,” Dr. Gersh explained at the conference.
He noted that an analysis of more than 41,000 Medicare beneficiaries with AF in 2007-2008 showed that only 66.8% were on warfarin.
“There’s no doubt that warfarin, and for that matter the NOACs, are underutilized, and it’s possible that the misperception that aspirin is effective may contribute to that underutilization,” asserted Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
The cardiologist added that the widely held belief that aspirin reduces stroke risk by roughly 20%, compared with placebo, in patients with AF is based upon seriously flawed data. True, a meta-analysis of six placebo-controlled randomized trials done in an earlier era concluded that aspirin reduced the relative risk of stroke by 19%, but what’s often overlooked is that aspirin significantly outperformed placebo in only one of those six studies – the SPAF 1 trial – where in one arm aspirin achieved an “almost implausible” 94% relative risk reduction.
“Nothing reduces risk by 94%,” Dr. Gersh observed, adding that the SPAF 1 methodology was, upon careful examination, “completely unacceptable” by contemporary standards.
He was a coinvestigator in a study of 7,347 AF patients on oral anticoagulation therapy in 176 U.S. practices participating in the ORBIT-AF registry (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). The analysis showed that concomitant use of aspirin and an oral anticoagulant in patients with AF is common in everyday clinical practice, being employed in 35% of the study population. Of note, 39% of these patients had no history of CAD and therefore weren’t on aspirin for secondary cardiovascular prevention, and 17% of them were at elevated bleeding risk because of an ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding risk score of 5 or more.
The key, disturbing finding was this: The adjusted risks of major bleeding and hospitalization for bleeding were 53% and 52% greater, respectively, in patients on an oral anticoagulant plus aspirin than with an oral anticoagulant alone (Circulation 2013;128:721-8).
On the basis of this study and other evidence, Dr. Gersh believes the use of aspirin in patients with AF should be considered only in those with CAD, where a case can be made for its use in secondary prevention alongside an oral anticoagulant to reduce stroke risk. But even then, aspirin’s use is reasonable only in those at low risk of bleeding, and extra vigilance and prophylaxis with a proton pump inhibitor are called for.
“If a patient is at high risk of bleeding, I personally would not give aspirin,” the cardiologist added.
The big remaining question – and an area of current controversy – concerns the safety of halting aspirin in AF patients on an oral anticoagulant who’ve undergone coronary stenting. Ongoing studies are looking at this issue, and answers are expected within a year or 2.
Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for Baxter, Medtronic, and Teva.
SNOWMASS, COLO.– The sun may be setting on the use of aspirin for thromboprophylaxis in patients with nonvalvular atrial fibrillation. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines are unique among the major international guidelines in giving a modest IIb, Level of Evidence C endorsement to the option of aspirin or no treatment in patients with atrial fibrillation (AF) and a CHA2DS2-VASc score of 1. In contrast, the 2014 U.K. NICE (National Institute for Health and Care Excellence) guidelines, the 2013 Asian Pacific Heart Rhythm Society guidelines, and the 2012 European Society of Cardiology guidelines all recommend consideration of oral anticoagulation – eschewing aspirin – in patients with a CHA2DS2-VASc score of 1 or more, Dr. Bernard J. Gersh noted at the Annual Cardiovascular Conference at Snowmass.
The NICE guidelines put the matter succinctly, stating, “The main departure from prior guidelines is that aspirin should not be used in AF simply to reduce the risk of stroke, as it is not as effective as NOACs [novel oral anticoagulants] ... and can cause more bleeding side effects.”
Dr. Gersh was coauthor of a recently published think piece that argued that exaggerated misperceptions of aspirin’s efficacy and safety have led to its inappropriate status as “the easy option” for stroke prevention in AF (Eur. Heart J. 2015;36:653-6).
“By giving physicians a soft option of aspirin in CHA2DS2-VASc 1 patients, we’re allowing them an excuse not to use an oral anticoagulant, which is what they should be using,” Dr. Gersh explained at the conference.
He noted that an analysis of more than 41,000 Medicare beneficiaries with AF in 2007-2008 showed that only 66.8% were on warfarin.
“There’s no doubt that warfarin, and for that matter the NOACs, are underutilized, and it’s possible that the misperception that aspirin is effective may contribute to that underutilization,” asserted Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
The cardiologist added that the widely held belief that aspirin reduces stroke risk by roughly 20%, compared with placebo, in patients with AF is based upon seriously flawed data. True, a meta-analysis of six placebo-controlled randomized trials done in an earlier era concluded that aspirin reduced the relative risk of stroke by 19%, but what’s often overlooked is that aspirin significantly outperformed placebo in only one of those six studies – the SPAF 1 trial – where in one arm aspirin achieved an “almost implausible” 94% relative risk reduction.
“Nothing reduces risk by 94%,” Dr. Gersh observed, adding that the SPAF 1 methodology was, upon careful examination, “completely unacceptable” by contemporary standards.
He was a coinvestigator in a study of 7,347 AF patients on oral anticoagulation therapy in 176 U.S. practices participating in the ORBIT-AF registry (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). The analysis showed that concomitant use of aspirin and an oral anticoagulant in patients with AF is common in everyday clinical practice, being employed in 35% of the study population. Of note, 39% of these patients had no history of CAD and therefore weren’t on aspirin for secondary cardiovascular prevention, and 17% of them were at elevated bleeding risk because of an ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding risk score of 5 or more.
The key, disturbing finding was this: The adjusted risks of major bleeding and hospitalization for bleeding were 53% and 52% greater, respectively, in patients on an oral anticoagulant plus aspirin than with an oral anticoagulant alone (Circulation 2013;128:721-8).
On the basis of this study and other evidence, Dr. Gersh believes the use of aspirin in patients with AF should be considered only in those with CAD, where a case can be made for its use in secondary prevention alongside an oral anticoagulant to reduce stroke risk. But even then, aspirin’s use is reasonable only in those at low risk of bleeding, and extra vigilance and prophylaxis with a proton pump inhibitor are called for.
“If a patient is at high risk of bleeding, I personally would not give aspirin,” the cardiologist added.
The big remaining question – and an area of current controversy – concerns the safety of halting aspirin in AF patients on an oral anticoagulant who’ve undergone coronary stenting. Ongoing studies are looking at this issue, and answers are expected within a year or 2.
Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for Baxter, Medtronic, and Teva.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Post-TAVR brain health: An emerging concern
SNOWMASS, COLO. – New-onset brain lesions arising after transcatheter aortic valve replacement are the largely unacknowledged elephant in the room with regard to the boomingly popular procedure.
Multiple studies utilizing diffusion-weighted MRI have shown roughly a 70% incidence of new brain lesions following transcatheter aortic valve replacement (TAVR). And studies employing full neurocognitive test batteries have consistently shown a relationship between small brain infarcts much like these, cognitive decline, and dementia, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
“This is an incredibly alarming piece of information. People should be aware of this. There is interest in doing TAVR in younger and younger patients. But there is indeed an issue with unintended consequences. If we take younger and less and less symptomatic patients, their chance of dementia in 20 years is probably going to be increased. We’re going to have to follow these patients for a long period of time to look at that specific endpoint,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn.
Speaking of unintended consequences, there is also the issue of TAVR-related stroke. Among the more than 27,000 patient records submitted to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry through December 2014, the periprocedural stroke rate was 2.4%. One-year outcomes included 26.2% mortality and a 3.6% stroke rate.
Given that two-thirds of TAVR cases submitted to the TVT registry in 2014 involved patients age 80 or older, with New York Heart Association class III/IV symptoms present in 82%, and 50% of patients rated as being at extreme risk with a predicted 1-year mortality of 50% without intervention, a 3.6% stroke rate can be considered tolerable. But not so in the sort of younger asymptomatic patients with significant aortic stenosis increasingly under discussion as potential candidates for the procedure.
“Stroke rates are the real deal in patients undergoing TAVR. Maybe you’re going to take an asymptomatic person and give them a stroke rather than wait or give a surgical valve replacement,” the cardiologist said.
He predicted that within 10 years, the use of cerebral protection devices will be considered mandatory, not just during TAVR, but during percutaneous coronary intervention, CABG surgery, and probably during atrial fibrillation ablation as well. All of these procedures have been linked to new-onset brain lesions on diffusion-weighted MRI.
Promising new neuroprotection devices include Keystone Heart’s TriGuard, a filter-deflector that covers all three cerebral arteries, has no impact on cerebral blood flow, doesn’t require an additional access site, is supported by excellent safety data, and is approved in Europe but investigational in the United States, Dr. Holmes observed. Efficacy data are coming soon, when the results of the DETECT III (A Prospective Randomized Evaluation of The TriGuard HDH Embolic Deflection Device During Transcatheter Aortic Valve Replacement) will be presented at the ACC scientific sessions on March 15. In that study, 70 patients underwent neurocognitive testing before and 30 days after their TAVR procedure.
“We’re going to be using something – a filter or filter-deflector – in every single patient to prevent the abnormal brain hits that are seen with all of these procedures. The need for brain protection is not going away,” he forecast.
Dr. Holmes, who played a pivotal role in creating the TVT registry during his term as ACC president, pointed out an intriguing registry finding: Through 2014, only 36% of the procedures have been done percutaneously.
“When you go to meetings everybody says, ‘We do them all percutaneously with a dual Perclose.’ But when you look at the data, out of those 27,000 patients only about one-third were done percutaneously. Is that going to be different in the future? Probably. But it’s important to remember that we’re still not doing all that many percutaneous procedures,” the cardiologist said.
He reported serving as a consultant to Boston Scientific.
SNOWMASS, COLO. – New-onset brain lesions arising after transcatheter aortic valve replacement are the largely unacknowledged elephant in the room with regard to the boomingly popular procedure.
Multiple studies utilizing diffusion-weighted MRI have shown roughly a 70% incidence of new brain lesions following transcatheter aortic valve replacement (TAVR). And studies employing full neurocognitive test batteries have consistently shown a relationship between small brain infarcts much like these, cognitive decline, and dementia, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
“This is an incredibly alarming piece of information. People should be aware of this. There is interest in doing TAVR in younger and younger patients. But there is indeed an issue with unintended consequences. If we take younger and less and less symptomatic patients, their chance of dementia in 20 years is probably going to be increased. We’re going to have to follow these patients for a long period of time to look at that specific endpoint,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn.
Speaking of unintended consequences, there is also the issue of TAVR-related stroke. Among the more than 27,000 patient records submitted to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry through December 2014, the periprocedural stroke rate was 2.4%. One-year outcomes included 26.2% mortality and a 3.6% stroke rate.
Given that two-thirds of TAVR cases submitted to the TVT registry in 2014 involved patients age 80 or older, with New York Heart Association class III/IV symptoms present in 82%, and 50% of patients rated as being at extreme risk with a predicted 1-year mortality of 50% without intervention, a 3.6% stroke rate can be considered tolerable. But not so in the sort of younger asymptomatic patients with significant aortic stenosis increasingly under discussion as potential candidates for the procedure.
“Stroke rates are the real deal in patients undergoing TAVR. Maybe you’re going to take an asymptomatic person and give them a stroke rather than wait or give a surgical valve replacement,” the cardiologist said.
He predicted that within 10 years, the use of cerebral protection devices will be considered mandatory, not just during TAVR, but during percutaneous coronary intervention, CABG surgery, and probably during atrial fibrillation ablation as well. All of these procedures have been linked to new-onset brain lesions on diffusion-weighted MRI.
Promising new neuroprotection devices include Keystone Heart’s TriGuard, a filter-deflector that covers all three cerebral arteries, has no impact on cerebral blood flow, doesn’t require an additional access site, is supported by excellent safety data, and is approved in Europe but investigational in the United States, Dr. Holmes observed. Efficacy data are coming soon, when the results of the DETECT III (A Prospective Randomized Evaluation of The TriGuard HDH Embolic Deflection Device During Transcatheter Aortic Valve Replacement) will be presented at the ACC scientific sessions on March 15. In that study, 70 patients underwent neurocognitive testing before and 30 days after their TAVR procedure.
“We’re going to be using something – a filter or filter-deflector – in every single patient to prevent the abnormal brain hits that are seen with all of these procedures. The need for brain protection is not going away,” he forecast.
Dr. Holmes, who played a pivotal role in creating the TVT registry during his term as ACC president, pointed out an intriguing registry finding: Through 2014, only 36% of the procedures have been done percutaneously.
“When you go to meetings everybody says, ‘We do them all percutaneously with a dual Perclose.’ But when you look at the data, out of those 27,000 patients only about one-third were done percutaneously. Is that going to be different in the future? Probably. But it’s important to remember that we’re still not doing all that many percutaneous procedures,” the cardiologist said.
He reported serving as a consultant to Boston Scientific.
SNOWMASS, COLO. – New-onset brain lesions arising after transcatheter aortic valve replacement are the largely unacknowledged elephant in the room with regard to the boomingly popular procedure.
Multiple studies utilizing diffusion-weighted MRI have shown roughly a 70% incidence of new brain lesions following transcatheter aortic valve replacement (TAVR). And studies employing full neurocognitive test batteries have consistently shown a relationship between small brain infarcts much like these, cognitive decline, and dementia, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
“This is an incredibly alarming piece of information. People should be aware of this. There is interest in doing TAVR in younger and younger patients. But there is indeed an issue with unintended consequences. If we take younger and less and less symptomatic patients, their chance of dementia in 20 years is probably going to be increased. We’re going to have to follow these patients for a long period of time to look at that specific endpoint,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn.
Speaking of unintended consequences, there is also the issue of TAVR-related stroke. Among the more than 27,000 patient records submitted to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry through December 2014, the periprocedural stroke rate was 2.4%. One-year outcomes included 26.2% mortality and a 3.6% stroke rate.
Given that two-thirds of TAVR cases submitted to the TVT registry in 2014 involved patients age 80 or older, with New York Heart Association class III/IV symptoms present in 82%, and 50% of patients rated as being at extreme risk with a predicted 1-year mortality of 50% without intervention, a 3.6% stroke rate can be considered tolerable. But not so in the sort of younger asymptomatic patients with significant aortic stenosis increasingly under discussion as potential candidates for the procedure.
“Stroke rates are the real deal in patients undergoing TAVR. Maybe you’re going to take an asymptomatic person and give them a stroke rather than wait or give a surgical valve replacement,” the cardiologist said.
He predicted that within 10 years, the use of cerebral protection devices will be considered mandatory, not just during TAVR, but during percutaneous coronary intervention, CABG surgery, and probably during atrial fibrillation ablation as well. All of these procedures have been linked to new-onset brain lesions on diffusion-weighted MRI.
Promising new neuroprotection devices include Keystone Heart’s TriGuard, a filter-deflector that covers all three cerebral arteries, has no impact on cerebral blood flow, doesn’t require an additional access site, is supported by excellent safety data, and is approved in Europe but investigational in the United States, Dr. Holmes observed. Efficacy data are coming soon, when the results of the DETECT III (A Prospective Randomized Evaluation of The TriGuard HDH Embolic Deflection Device During Transcatheter Aortic Valve Replacement) will be presented at the ACC scientific sessions on March 15. In that study, 70 patients underwent neurocognitive testing before and 30 days after their TAVR procedure.
“We’re going to be using something – a filter or filter-deflector – in every single patient to prevent the abnormal brain hits that are seen with all of these procedures. The need for brain protection is not going away,” he forecast.
Dr. Holmes, who played a pivotal role in creating the TVT registry during his term as ACC president, pointed out an intriguing registry finding: Through 2014, only 36% of the procedures have been done percutaneously.
“When you go to meetings everybody says, ‘We do them all percutaneously with a dual Perclose.’ But when you look at the data, out of those 27,000 patients only about one-third were done percutaneously. Is that going to be different in the future? Probably. But it’s important to remember that we’re still not doing all that many percutaneous procedures,” the cardiologist said.
He reported serving as a consultant to Boston Scientific.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Nailing VT as the cause of wide complex tachycardia
SNOWMASS, COLO. – An ECG can offer a handful of easily interpreted clues that raise to 99% the certainty of diagnosing ventricular tachycardia as the cause of a patient’s wide complex tachycardia, according to Dr. Samuel J. Asirvatham.
The first thing to realize about wide complex tachycardias (WCTs), as defined by a heart rate in excess of 100 bpm and a QRS duration greater than 120 milliseconds, is that the cause is ventricular tachycardia (VT) rather than supraventricular tachycardia in 80% of cases, he said at the Annual Cardiovascular Conference at Snowmass.
“When you diagnose wide complex tachycardia as not VT, you’re much more likely to make a mistake than if you just closed your eyes and said VT,” said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
The history also provides a valuable clue: “If there’s anything wrong with the heart – a prior MI, structural disease, a scar on the heart from previous surgery, sarcoid – there’s a 95% chance that the WCT is due to VT,” he said.
Key ECG features will boost the diagnostic certainty of VT from 95% to 99%. Here are Dr. Asirvatham’s favorites:
• Atrioventricular dissociation. When the ventricular rate is faster than the atrial rate, the result is atrioventricular dissocation. In the setting of WCT, it’s highly specific for VT, and it’s present on the ECGs of up to 50% of affected patients.
“This is a very useful clue. I like looking for this. If you see it, you can be convinced the patient has VT,” he said.
• The wide QRS. The wider the wide QRS, the more likely it’s VT.If the QRS duration is greater than 150 milliseconds, there’s nearly a 98% likelihood of VT.
• Northwest axis. If both lead I and the inferior leads show negative deflection – that is, the electrical wave is moving away from the positive electrodes located over the left shoulder and closest to the feet – that means the wave is moving toward the northwest on the frontal plane axis. The odds are extremely high that this is VT.
• Chest lead concordance. If all of the chest leads V1-6 are positive or they’re all negative, that’s “powerful information” indicating VT, according to Dr. Asirvatham.
• Time from onset of R wave to S wave nadir. Even if chest concordance isn’t present, VT can be diagnosed with near-absolute certainty simply by measuring the time from onset of the R wave to the lowest point on the S wave. If it’s longer than 100 milliseconds, that finding strongly favors VT.
“Remember these ECG features and 99% of the time you will correctly diagnose VT,” the cardiologist said.
Dr. Asirvatham reported serving as a consultant to a dozen medical device companies.
SNOWMASS, COLO. – An ECG can offer a handful of easily interpreted clues that raise to 99% the certainty of diagnosing ventricular tachycardia as the cause of a patient’s wide complex tachycardia, according to Dr. Samuel J. Asirvatham.
The first thing to realize about wide complex tachycardias (WCTs), as defined by a heart rate in excess of 100 bpm and a QRS duration greater than 120 milliseconds, is that the cause is ventricular tachycardia (VT) rather than supraventricular tachycardia in 80% of cases, he said at the Annual Cardiovascular Conference at Snowmass.
“When you diagnose wide complex tachycardia as not VT, you’re much more likely to make a mistake than if you just closed your eyes and said VT,” said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
The history also provides a valuable clue: “If there’s anything wrong with the heart – a prior MI, structural disease, a scar on the heart from previous surgery, sarcoid – there’s a 95% chance that the WCT is due to VT,” he said.
Key ECG features will boost the diagnostic certainty of VT from 95% to 99%. Here are Dr. Asirvatham’s favorites:
• Atrioventricular dissociation. When the ventricular rate is faster than the atrial rate, the result is atrioventricular dissocation. In the setting of WCT, it’s highly specific for VT, and it’s present on the ECGs of up to 50% of affected patients.
“This is a very useful clue. I like looking for this. If you see it, you can be convinced the patient has VT,” he said.
• The wide QRS. The wider the wide QRS, the more likely it’s VT.If the QRS duration is greater than 150 milliseconds, there’s nearly a 98% likelihood of VT.
• Northwest axis. If both lead I and the inferior leads show negative deflection – that is, the electrical wave is moving away from the positive electrodes located over the left shoulder and closest to the feet – that means the wave is moving toward the northwest on the frontal plane axis. The odds are extremely high that this is VT.
• Chest lead concordance. If all of the chest leads V1-6 are positive or they’re all negative, that’s “powerful information” indicating VT, according to Dr. Asirvatham.
• Time from onset of R wave to S wave nadir. Even if chest concordance isn’t present, VT can be diagnosed with near-absolute certainty simply by measuring the time from onset of the R wave to the lowest point on the S wave. If it’s longer than 100 milliseconds, that finding strongly favors VT.
“Remember these ECG features and 99% of the time you will correctly diagnose VT,” the cardiologist said.
Dr. Asirvatham reported serving as a consultant to a dozen medical device companies.
SNOWMASS, COLO. – An ECG can offer a handful of easily interpreted clues that raise to 99% the certainty of diagnosing ventricular tachycardia as the cause of a patient’s wide complex tachycardia, according to Dr. Samuel J. Asirvatham.
The first thing to realize about wide complex tachycardias (WCTs), as defined by a heart rate in excess of 100 bpm and a QRS duration greater than 120 milliseconds, is that the cause is ventricular tachycardia (VT) rather than supraventricular tachycardia in 80% of cases, he said at the Annual Cardiovascular Conference at Snowmass.
“When you diagnose wide complex tachycardia as not VT, you’re much more likely to make a mistake than if you just closed your eyes and said VT,” said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
The history also provides a valuable clue: “If there’s anything wrong with the heart – a prior MI, structural disease, a scar on the heart from previous surgery, sarcoid – there’s a 95% chance that the WCT is due to VT,” he said.
Key ECG features will boost the diagnostic certainty of VT from 95% to 99%. Here are Dr. Asirvatham’s favorites:
• Atrioventricular dissociation. When the ventricular rate is faster than the atrial rate, the result is atrioventricular dissocation. In the setting of WCT, it’s highly specific for VT, and it’s present on the ECGs of up to 50% of affected patients.
“This is a very useful clue. I like looking for this. If you see it, you can be convinced the patient has VT,” he said.
• The wide QRS. The wider the wide QRS, the more likely it’s VT.If the QRS duration is greater than 150 milliseconds, there’s nearly a 98% likelihood of VT.
• Northwest axis. If both lead I and the inferior leads show negative deflection – that is, the electrical wave is moving away from the positive electrodes located over the left shoulder and closest to the feet – that means the wave is moving toward the northwest on the frontal plane axis. The odds are extremely high that this is VT.
• Chest lead concordance. If all of the chest leads V1-6 are positive or they’re all negative, that’s “powerful information” indicating VT, according to Dr. Asirvatham.
• Time from onset of R wave to S wave nadir. Even if chest concordance isn’t present, VT can be diagnosed with near-absolute certainty simply by measuring the time from onset of the R wave to the lowest point on the S wave. If it’s longer than 100 milliseconds, that finding strongly favors VT.
“Remember these ECG features and 99% of the time you will correctly diagnose VT,” the cardiologist said.
Dr. Asirvatham reported serving as a consultant to a dozen medical device companies.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Latest valvular disease guidelines bring big changes
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Tide turns in favor of multivessel PCI in STEMI
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Hybrid revascularization remains a rare bird
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.
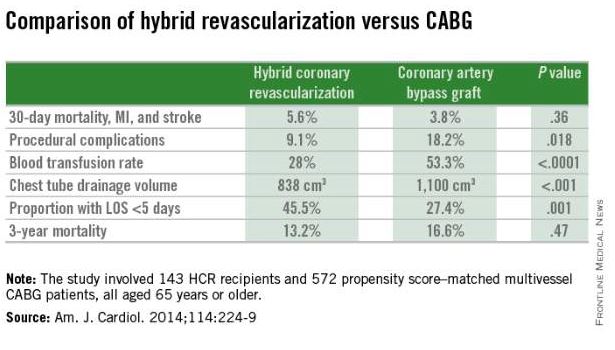
He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.

He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.

He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Emergency department holds key to early readmissions for heart failure
SNOWMASS, COLO. – Under intense fiscal pressure to curb early hospital readmissions for heart failure, cardiologists and hospital administrators are taking a hard look at the traditional role of the emergency department as the point of triage for patients with decompensated heart failure.
“Alternatives to the emergency department for ambulatory triage and intervention are essential,” Dr. Akshay Desai said at the Annual Cardiovascular Conference at Snowmass.
“Traditionally, when our patients become decompensated, we send them from our office or clinic to the ED. And 80%-90% of those who present to the ED with the diagnosis of heart failure are admitted to the hospital. So this means that the ED is a pretty ineffective triage point for heart failure patients. Most ED staff are concerned about ambulatory follow-up and feel it’s safer to follow patients in the hospital. The message here is we need a more robust ambulatory framework to manage patients with milder decompensation so they don’t all need to come into the hospital,” said Dr. Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital admission is of questionable value for a large fraction of decompensating heart failure patients. After all, not that much happens to them in the hospital that couldn’t take place in a less costly setting.
“For the most part, our patients in the hospital for decompensated heart failure get IV diuretics, with an average weight loss of about 4 kg. Surveillance is typically once- or twice-daily laboratory tests and a bedside clinical visit by a physician at 7:30 in the morning. Few patients get much else. There’s little diagnostic testing, few other therapies that require intensive monitoring, and most patients feel a little better by their first day in the hospital,” he said.
This suggests the need for what he called “an evolved model of heart failure care” in which the ED is replaced as the point of service by some form of ambulatory center that can serve as a buffer limiting the number of patients who need to come into the hospital.
“It could be a home-based strategy of IV diuretic administration, a clinic-based strategy of outpatient diuretic administration, or an observation unit based in the ED. All of these are now being tested in various models across the country as alternatives to help manage the readmission problem, and also to make life better for our patients, who’d prefer not to be in the hospital if they could be managed in other ways,” Dr. Desai continued.
Reducing 30-day readmission rates after a hospitalization for heart failure is seen by health policy makers as an opportunity to simultaneously improve care and reduce costs. But studies show only about half of readmissions in patients with heart failure are cardiovascular related, just half of those cardiovascular readmissions are heart failure related, and only about 30% of heart failure readmissions are truly preventable. However, wide variation exists across the country in risk-adjusted 30-day readmission rates, suggesting there is an opportunity for improvement in outlier hospitals.
Numerous factors have been linked to high heart failure readmission rates, including patient sociodemographic characteristics, comorbid conditions, and serum markers of heart failure severity. One underappreciated factor, in Dr. Desai’s view, is that readmission rates are significantly higher in hospitals that are financially and clinically resource poor, as shown in a Harvard School of Public Health analysis of Medicare claims data for more than 900,000 heart failure discharges. These resource-poor hospitals provide care for underserved populations, and they are experiencing a disproportionate burden of the financial penalties imposed for early readmission.
“As we seek to improve care for patients with heart failure, we should ensure that penalties for poor performance do not worsen disparities in quality of care,” according to the Harvard investigators (Circ. Cardiovasc. Qual. Outcomes 2011;4:53-9).
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – Under intense fiscal pressure to curb early hospital readmissions for heart failure, cardiologists and hospital administrators are taking a hard look at the traditional role of the emergency department as the point of triage for patients with decompensated heart failure.
“Alternatives to the emergency department for ambulatory triage and intervention are essential,” Dr. Akshay Desai said at the Annual Cardiovascular Conference at Snowmass.
“Traditionally, when our patients become decompensated, we send them from our office or clinic to the ED. And 80%-90% of those who present to the ED with the diagnosis of heart failure are admitted to the hospital. So this means that the ED is a pretty ineffective triage point for heart failure patients. Most ED staff are concerned about ambulatory follow-up and feel it’s safer to follow patients in the hospital. The message here is we need a more robust ambulatory framework to manage patients with milder decompensation so they don’t all need to come into the hospital,” said Dr. Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital admission is of questionable value for a large fraction of decompensating heart failure patients. After all, not that much happens to them in the hospital that couldn’t take place in a less costly setting.
“For the most part, our patients in the hospital for decompensated heart failure get IV diuretics, with an average weight loss of about 4 kg. Surveillance is typically once- or twice-daily laboratory tests and a bedside clinical visit by a physician at 7:30 in the morning. Few patients get much else. There’s little diagnostic testing, few other therapies that require intensive monitoring, and most patients feel a little better by their first day in the hospital,” he said.
This suggests the need for what he called “an evolved model of heart failure care” in which the ED is replaced as the point of service by some form of ambulatory center that can serve as a buffer limiting the number of patients who need to come into the hospital.
“It could be a home-based strategy of IV diuretic administration, a clinic-based strategy of outpatient diuretic administration, or an observation unit based in the ED. All of these are now being tested in various models across the country as alternatives to help manage the readmission problem, and also to make life better for our patients, who’d prefer not to be in the hospital if they could be managed in other ways,” Dr. Desai continued.
Reducing 30-day readmission rates after a hospitalization for heart failure is seen by health policy makers as an opportunity to simultaneously improve care and reduce costs. But studies show only about half of readmissions in patients with heart failure are cardiovascular related, just half of those cardiovascular readmissions are heart failure related, and only about 30% of heart failure readmissions are truly preventable. However, wide variation exists across the country in risk-adjusted 30-day readmission rates, suggesting there is an opportunity for improvement in outlier hospitals.
Numerous factors have been linked to high heart failure readmission rates, including patient sociodemographic characteristics, comorbid conditions, and serum markers of heart failure severity. One underappreciated factor, in Dr. Desai’s view, is that readmission rates are significantly higher in hospitals that are financially and clinically resource poor, as shown in a Harvard School of Public Health analysis of Medicare claims data for more than 900,000 heart failure discharges. These resource-poor hospitals provide care for underserved populations, and they are experiencing a disproportionate burden of the financial penalties imposed for early readmission.
“As we seek to improve care for patients with heart failure, we should ensure that penalties for poor performance do not worsen disparities in quality of care,” according to the Harvard investigators (Circ. Cardiovasc. Qual. Outcomes 2011;4:53-9).
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – Under intense fiscal pressure to curb early hospital readmissions for heart failure, cardiologists and hospital administrators are taking a hard look at the traditional role of the emergency department as the point of triage for patients with decompensated heart failure.
“Alternatives to the emergency department for ambulatory triage and intervention are essential,” Dr. Akshay Desai said at the Annual Cardiovascular Conference at Snowmass.
“Traditionally, when our patients become decompensated, we send them from our office or clinic to the ED. And 80%-90% of those who present to the ED with the diagnosis of heart failure are admitted to the hospital. So this means that the ED is a pretty ineffective triage point for heart failure patients. Most ED staff are concerned about ambulatory follow-up and feel it’s safer to follow patients in the hospital. The message here is we need a more robust ambulatory framework to manage patients with milder decompensation so they don’t all need to come into the hospital,” said Dr. Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital admission is of questionable value for a large fraction of decompensating heart failure patients. After all, not that much happens to them in the hospital that couldn’t take place in a less costly setting.
“For the most part, our patients in the hospital for decompensated heart failure get IV diuretics, with an average weight loss of about 4 kg. Surveillance is typically once- or twice-daily laboratory tests and a bedside clinical visit by a physician at 7:30 in the morning. Few patients get much else. There’s little diagnostic testing, few other therapies that require intensive monitoring, and most patients feel a little better by their first day in the hospital,” he said.
This suggests the need for what he called “an evolved model of heart failure care” in which the ED is replaced as the point of service by some form of ambulatory center that can serve as a buffer limiting the number of patients who need to come into the hospital.
“It could be a home-based strategy of IV diuretic administration, a clinic-based strategy of outpatient diuretic administration, or an observation unit based in the ED. All of these are now being tested in various models across the country as alternatives to help manage the readmission problem, and also to make life better for our patients, who’d prefer not to be in the hospital if they could be managed in other ways,” Dr. Desai continued.
Reducing 30-day readmission rates after a hospitalization for heart failure is seen by health policy makers as an opportunity to simultaneously improve care and reduce costs. But studies show only about half of readmissions in patients with heart failure are cardiovascular related, just half of those cardiovascular readmissions are heart failure related, and only about 30% of heart failure readmissions are truly preventable. However, wide variation exists across the country in risk-adjusted 30-day readmission rates, suggesting there is an opportunity for improvement in outlier hospitals.
Numerous factors have been linked to high heart failure readmission rates, including patient sociodemographic characteristics, comorbid conditions, and serum markers of heart failure severity. One underappreciated factor, in Dr. Desai’s view, is that readmission rates are significantly higher in hospitals that are financially and clinically resource poor, as shown in a Harvard School of Public Health analysis of Medicare claims data for more than 900,000 heart failure discharges. These resource-poor hospitals provide care for underserved populations, and they are experiencing a disproportionate burden of the financial penalties imposed for early readmission.
“As we seek to improve care for patients with heart failure, we should ensure that penalties for poor performance do not worsen disparities in quality of care,” according to the Harvard investigators (Circ. Cardiovasc. Qual. Outcomes 2011;4:53-9).
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Smart diet remains potent cardiovascular medicine
SNOWMASS, COLO. – Cutting dietary fat intake remains a highly effective strategy for reducing coronary heart disease risk – but only so long as the replacement nutrients aren’t even bigger offenders, Dr. Robert A. Vogel said at the Annual Cardiovascular Conference at Snowmass.
In the face of decades of public health admonitions to reduce saturated fat intake, most Americans have increased their consumption of trans fats and simple carbohydrates, especially sugar. And therein lies a problem. Trans fats are far more harmful than saturated fats in terms of cardiovascular risk. And excessive sugar consumption is a major contributor to abdominal obesity, metabolic syndrome, hypertension, and endothelial dysfunction.
“In the United States, sugar is a bigger source of hypertension than is salt,” asserted Dr. Vogel, a cardiologist at the University of Colorado, Denver.
The editors of Time magazine ignited a public controversy last year with a cover story arrestingly titled, “Eat Butter – Scientists labelled fat the enemy. Why they were wrong.” The editors were picking up on a British meta-analysis of 32 observational studies that concluded there is no clear evidence to support the notion that saturated fats are harmful to cardiovascular health and that swapping them out for consumption of polyunsaturated fatty acids (PUFAs) is beneficial (Ann. Intern. Med. 2014;160:398-406).
Dr. Vogel said those investigators are in fact correct: Many of the observational studies – going all the way back to the pioneering work by Dr. Ancel Keys in the 1950s – are flawed. They don’t convincingly prove the case for PUFAs as a healthier alternative. But there is persuasive evidence from well-conducted, randomized, controlled trials that this is indeed so, he added.
Several of these studies were done in an earlier era when it was possible to slip around the challenges and limitations of dietary studies in free-living populations. These trials wouldn’t be possible today for ethical reasons involving lack of informed consent.
For example, in the Finnish Mental Hospital Study conducted during 1959-1971, the food served at two mental institutions was altered. Patients at one hospital got 6 years of a diet high in PUFAs, then were crossed over to a typical Finnish diet. At the other mental hospital, patients were fed a normal Finnish diet for 6 years, then crossed over to the high-PUFA diet for 6 years. During the experimental-diet years, the coronary heart disease event rate was reduced by nearly 60% (Int. J. Epidemiol. 1979;8:99-118).
Similarly, in a prospective randomized trial conducted at a Los Angeles Veterans Affairs institution for older, cognitively impaired men, a no-choice shift to a diet high in PUFAs with reduced saturated fats resulted in roughly a 30% reduction in CHD events compared to the usual institutional diet (Lancet 1968;2:1060-2). A similar magnitude of CHD event reduction was seen with a high-PUFA dietary intervention in the Oslo Diet-Heart Study, a prospective secondary prevention trial (Circulation 1970;42:935-42).
In the contemporary era, the standout randomized dietary intervention trial is the Lyon Diet Heart Study, a 46-month prospective secondary prevention trial in which a Mediterranean diet low in saturated fat and high in alpha-linoleic acid, a PUFA, reduced the combined endpoint of cardiac death and nonfatal MI by 70%, compared with the usual post-MI prudent diet recommended at that time. Yet total cholesterol levels in the two study arms did not differ (Circulation 1999;99:779-85).
To put these results into context, Dr. Vogel noted that the Cholesterol Treatment Trialists Collaboration headquartered at the University of Oxford (England) has shown that for every 40 mg/dL of LDL-lowering achieved with statin therapy, the result is roughly a 20% reduction in CHD. In contrast, the classic nonpharmacologic diet studies resulted in 30%-70% relative risk reductions.
“Heart disease is a dietary disease,” the cardiologist emphasized. “When you compare diet intervention to LDL lowering with statins, you see that diet is very, very effective. But you have to know the details of the diet. You can’t take something out and put just anything in. It doesn’t work like that.”
For example, an analysis of data from the National Health and Nutrition Examination Survey concluded that individuals who consumed 25% of their calories from added sugar – that’s the equivalent of three 12-oz cans of a sugary cola per day – had a 175% increased risk of cardiovascular mortality during a median 14.6 years of follow-up, compared with those who got less than 10% of their calories from added sugar (JAMA Intern. Med. 2014;174:516-24).
And as for the impact of the trans fat that’s liberally present in many processed foods, the Nurses Health Study showed that for every 5% increase in energy intake from saturated fat – that’s equivalent to one 8-oz steak per day – the relative risk for CHD rose by a relatively modest 17%, while for a 5% increase in energy intake from trans fat – the equivalent of 4 oz of butter – CHD risk shot up by 382% (N. Engl. J. Med. 1997;337:1491-9).
Dr. Vogel reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center and receiving a research grant from Sanofi.
SNOWMASS, COLO. – Cutting dietary fat intake remains a highly effective strategy for reducing coronary heart disease risk – but only so long as the replacement nutrients aren’t even bigger offenders, Dr. Robert A. Vogel said at the Annual Cardiovascular Conference at Snowmass.
In the face of decades of public health admonitions to reduce saturated fat intake, most Americans have increased their consumption of trans fats and simple carbohydrates, especially sugar. And therein lies a problem. Trans fats are far more harmful than saturated fats in terms of cardiovascular risk. And excessive sugar consumption is a major contributor to abdominal obesity, metabolic syndrome, hypertension, and endothelial dysfunction.
“In the United States, sugar is a bigger source of hypertension than is salt,” asserted Dr. Vogel, a cardiologist at the University of Colorado, Denver.
The editors of Time magazine ignited a public controversy last year with a cover story arrestingly titled, “Eat Butter – Scientists labelled fat the enemy. Why they were wrong.” The editors were picking up on a British meta-analysis of 32 observational studies that concluded there is no clear evidence to support the notion that saturated fats are harmful to cardiovascular health and that swapping them out for consumption of polyunsaturated fatty acids (PUFAs) is beneficial (Ann. Intern. Med. 2014;160:398-406).
Dr. Vogel said those investigators are in fact correct: Many of the observational studies – going all the way back to the pioneering work by Dr. Ancel Keys in the 1950s – are flawed. They don’t convincingly prove the case for PUFAs as a healthier alternative. But there is persuasive evidence from well-conducted, randomized, controlled trials that this is indeed so, he added.
Several of these studies were done in an earlier era when it was possible to slip around the challenges and limitations of dietary studies in free-living populations. These trials wouldn’t be possible today for ethical reasons involving lack of informed consent.
For example, in the Finnish Mental Hospital Study conducted during 1959-1971, the food served at two mental institutions was altered. Patients at one hospital got 6 years of a diet high in PUFAs, then were crossed over to a typical Finnish diet. At the other mental hospital, patients were fed a normal Finnish diet for 6 years, then crossed over to the high-PUFA diet for 6 years. During the experimental-diet years, the coronary heart disease event rate was reduced by nearly 60% (Int. J. Epidemiol. 1979;8:99-118).
Similarly, in a prospective randomized trial conducted at a Los Angeles Veterans Affairs institution for older, cognitively impaired men, a no-choice shift to a diet high in PUFAs with reduced saturated fats resulted in roughly a 30% reduction in CHD events compared to the usual institutional diet (Lancet 1968;2:1060-2). A similar magnitude of CHD event reduction was seen with a high-PUFA dietary intervention in the Oslo Diet-Heart Study, a prospective secondary prevention trial (Circulation 1970;42:935-42).
In the contemporary era, the standout randomized dietary intervention trial is the Lyon Diet Heart Study, a 46-month prospective secondary prevention trial in which a Mediterranean diet low in saturated fat and high in alpha-linoleic acid, a PUFA, reduced the combined endpoint of cardiac death and nonfatal MI by 70%, compared with the usual post-MI prudent diet recommended at that time. Yet total cholesterol levels in the two study arms did not differ (Circulation 1999;99:779-85).
To put these results into context, Dr. Vogel noted that the Cholesterol Treatment Trialists Collaboration headquartered at the University of Oxford (England) has shown that for every 40 mg/dL of LDL-lowering achieved with statin therapy, the result is roughly a 20% reduction in CHD. In contrast, the classic nonpharmacologic diet studies resulted in 30%-70% relative risk reductions.
“Heart disease is a dietary disease,” the cardiologist emphasized. “When you compare diet intervention to LDL lowering with statins, you see that diet is very, very effective. But you have to know the details of the diet. You can’t take something out and put just anything in. It doesn’t work like that.”
For example, an analysis of data from the National Health and Nutrition Examination Survey concluded that individuals who consumed 25% of their calories from added sugar – that’s the equivalent of three 12-oz cans of a sugary cola per day – had a 175% increased risk of cardiovascular mortality during a median 14.6 years of follow-up, compared with those who got less than 10% of their calories from added sugar (JAMA Intern. Med. 2014;174:516-24).
And as for the impact of the trans fat that’s liberally present in many processed foods, the Nurses Health Study showed that for every 5% increase in energy intake from saturated fat – that’s equivalent to one 8-oz steak per day – the relative risk for CHD rose by a relatively modest 17%, while for a 5% increase in energy intake from trans fat – the equivalent of 4 oz of butter – CHD risk shot up by 382% (N. Engl. J. Med. 1997;337:1491-9).
Dr. Vogel reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center and receiving a research grant from Sanofi.
SNOWMASS, COLO. – Cutting dietary fat intake remains a highly effective strategy for reducing coronary heart disease risk – but only so long as the replacement nutrients aren’t even bigger offenders, Dr. Robert A. Vogel said at the Annual Cardiovascular Conference at Snowmass.
In the face of decades of public health admonitions to reduce saturated fat intake, most Americans have increased their consumption of trans fats and simple carbohydrates, especially sugar. And therein lies a problem. Trans fats are far more harmful than saturated fats in terms of cardiovascular risk. And excessive sugar consumption is a major contributor to abdominal obesity, metabolic syndrome, hypertension, and endothelial dysfunction.
“In the United States, sugar is a bigger source of hypertension than is salt,” asserted Dr. Vogel, a cardiologist at the University of Colorado, Denver.
The editors of Time magazine ignited a public controversy last year with a cover story arrestingly titled, “Eat Butter – Scientists labelled fat the enemy. Why they were wrong.” The editors were picking up on a British meta-analysis of 32 observational studies that concluded there is no clear evidence to support the notion that saturated fats are harmful to cardiovascular health and that swapping them out for consumption of polyunsaturated fatty acids (PUFAs) is beneficial (Ann. Intern. Med. 2014;160:398-406).
Dr. Vogel said those investigators are in fact correct: Many of the observational studies – going all the way back to the pioneering work by Dr. Ancel Keys in the 1950s – are flawed. They don’t convincingly prove the case for PUFAs as a healthier alternative. But there is persuasive evidence from well-conducted, randomized, controlled trials that this is indeed so, he added.
Several of these studies were done in an earlier era when it was possible to slip around the challenges and limitations of dietary studies in free-living populations. These trials wouldn’t be possible today for ethical reasons involving lack of informed consent.
For example, in the Finnish Mental Hospital Study conducted during 1959-1971, the food served at two mental institutions was altered. Patients at one hospital got 6 years of a diet high in PUFAs, then were crossed over to a typical Finnish diet. At the other mental hospital, patients were fed a normal Finnish diet for 6 years, then crossed over to the high-PUFA diet for 6 years. During the experimental-diet years, the coronary heart disease event rate was reduced by nearly 60% (Int. J. Epidemiol. 1979;8:99-118).
Similarly, in a prospective randomized trial conducted at a Los Angeles Veterans Affairs institution for older, cognitively impaired men, a no-choice shift to a diet high in PUFAs with reduced saturated fats resulted in roughly a 30% reduction in CHD events compared to the usual institutional diet (Lancet 1968;2:1060-2). A similar magnitude of CHD event reduction was seen with a high-PUFA dietary intervention in the Oslo Diet-Heart Study, a prospective secondary prevention trial (Circulation 1970;42:935-42).
In the contemporary era, the standout randomized dietary intervention trial is the Lyon Diet Heart Study, a 46-month prospective secondary prevention trial in which a Mediterranean diet low in saturated fat and high in alpha-linoleic acid, a PUFA, reduced the combined endpoint of cardiac death and nonfatal MI by 70%, compared with the usual post-MI prudent diet recommended at that time. Yet total cholesterol levels in the two study arms did not differ (Circulation 1999;99:779-85).
To put these results into context, Dr. Vogel noted that the Cholesterol Treatment Trialists Collaboration headquartered at the University of Oxford (England) has shown that for every 40 mg/dL of LDL-lowering achieved with statin therapy, the result is roughly a 20% reduction in CHD. In contrast, the classic nonpharmacologic diet studies resulted in 30%-70% relative risk reductions.
“Heart disease is a dietary disease,” the cardiologist emphasized. “When you compare diet intervention to LDL lowering with statins, you see that diet is very, very effective. But you have to know the details of the diet. You can’t take something out and put just anything in. It doesn’t work like that.”
For example, an analysis of data from the National Health and Nutrition Examination Survey concluded that individuals who consumed 25% of their calories from added sugar – that’s the equivalent of three 12-oz cans of a sugary cola per day – had a 175% increased risk of cardiovascular mortality during a median 14.6 years of follow-up, compared with those who got less than 10% of their calories from added sugar (JAMA Intern. Med. 2014;174:516-24).
And as for the impact of the trans fat that’s liberally present in many processed foods, the Nurses Health Study showed that for every 5% increase in energy intake from saturated fat – that’s equivalent to one 8-oz steak per day – the relative risk for CHD rose by a relatively modest 17%, while for a 5% increase in energy intake from trans fat – the equivalent of 4 oz of butter – CHD risk shot up by 382% (N. Engl. J. Med. 1997;337:1491-9).
Dr. Vogel reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center and receiving a research grant from Sanofi.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
How to cut the CRT nonresponder rate
SNOWMASS, COLO. – The 12-lead Holter monitor is an invaluable and underutilized tool for turning cardiac resynchronization therapy nonresponders into responders, according to Dr. N.A. Mark Estes III.
“The Achilles heel of CRT right now is the failure to respond that occurs in one-quarter to one-third of patients who receive the device. And I think it’s less and less due to a problem of poor patient selection or technical failure, but instead due to common mistakes made in patients who’ve been properly selected and in whom a CRT device has been placed appropriately. I think there’s a lot of room for improvement here,” said Dr. Estes, director of the cardiac arrhythmia center and professor of medicine at Tufts University in Boston.
One common mistake is to accept at face value the CRT device counter when it shows a high percentage of ventricular pacing that’s supposed to be indicative of adequate biventricular pacing in patients. That’s not reliable in patients with coexisting heart failure and atrial fibrillation. In fact, the device pacing counter can markedly overestimate the degree of effective biventricular pacing in patients with permanent atrial fibrillation undergoing CRT. That’s because it may incorrectly count fusion and pseudofusion beats as paced events, even though ventricular capture and atrioventricular resynchronization fail to occur during such beats. The 12-lead Holter monitor will tell the real story regarding ventricular rate control and the percentage of fully paced beats, Dr. Estes said at the Annual Cardiovascular Conference at Snowmass.
“In patients with atrial fibrillation, you can’t just do an EKG, and you can’t just do an interrogation of the device and see that they have 90% or 95% biventricular pacing, which is the sweet spot you need to be North of to make sure they’re having benefit. You really need to do the 24-hour Holter monitor,” the cardiologist emphasized.
“It happens all the time,” he continued. “It happens in my own institution, where we have people come in with heart failure who are being evaluated for an LVAD [left ventricular assist device] because when their CRT device is interrogated, it shows 95% pacing. Everyone’s getting ready to put in the LVAD, and we put them on a Holter monitor and guess what? They’re 60% paced. And sometimes you can have dramatic – really dramatic – improvements by just slowing the ventricular response.”
Increasingly, however, he and his fellow electrophysiologists are alternatively turning to atrioventricular junction ablation in suitable candidates for the catheter-based procedure in order to improve their clinical response to CRT.
Dr. Estes cited a study by Columbia University cardiologists as being highly influential in shaping the new understanding of how to use 12-lead Holter monitoring to identify patients with atrial fibrillation who aren’t responding to CRT. They studied 19 patients with permanent atrial fibrillation who were on CRT, all of whom were on beta-blockers, amiodarone, and digoxin for rate control. Although device interrogation showed all 19 patients had in excess of 90% biventricular pacing 12 months after CRT implantation, only 9 patients were actually clinical responders to CRT as defined by at least a 1-grade improvement in New York Heart Association functional class.
Upon wearing an ambulatory 12-lead Holter monitor for 24 hours, only the nine patients with clinical improvement in response to CRT actually had effective pacing; 94% of their heart beats over the course of 24 hours were fully paced, with a mere 2% fusion beats and less than 4% pseudofusion beats. The other 10 patients had an average of only 60% fully paced beats, along with 16% fusion and 24% pseudofusion beats.
The CRT clinical responders also showed evidence of reverse remodeling, with significantly greater improvements in left ventricular ejection fraction, end-systolic dimension, end-diastolic diameter, and end-systolic and end-diastolic volume, compared with nonresponders.
The lesson, according to the investigators, is that the percentage of biventricular pacing, as obtained from CRT device interrogation, is an unreliable indicator of effective pacing in patients with permanent atrial fibrillation. The goal of CRT programming in this population is to try to achieve 100% biventricular capture on the 12-lead Holter monitor (J. Am. Coll. Cardiol. 2009;53:1050-5).
A series of landmark randomized trials has established that in suitable candidates for CRT with NYHA class I through ambulatory class IV heart failure with reduced ejection fraction, the device therapy provides on average about a 17% reduction in all-cause mortality and a 29% decrease in hospitalizations for heart failure above and beyond the benefits obtained through guideline-directed medical therapy. However, the improvements seen in patients with NYHA class I symptoms are less impressive than in those with NYHA class II-IV disease. And it’s possible that the randomized controlled trials overestimate the true benefit from CRT.
“This is a group of extremely experienced investigators who know the literature well. They’re typically quite savvy with patient selection, and they’re vested in getting the right patients into these trials. Unfortunately, there’s not a registry of all eligible patients, so we can’t tell exactly how those patients were selected. It would have been helpful,” Dr. Estes said.
It’s clear from the randomized trials that the ideal patient for CRT is a woman under age 65 with nonischemic cardiomyopathy with a left bundle branch block, normal sinus rhythm, and a QRS duration of 150 milliseconds or more. That’s who’s most likely to obtain clinical benefit from the therapy. The flip side of that is that an older male patient with ischemia, atrial fibrillation, a QRS of 120-150 milliseconds, and non–left bundle branch block is less likely to respond.
“We’ve figured this out. I think we’ve gotten pretty good at giving patients an honest estimate of their probability of responding independent of our ability to get a lead into a good spot in the coronary sinus,” Dr. Estes said. “More than 95% of patients can be adequately resynchronized currently with the tools we have, so it’s not the barrier that it once represented. We have many different techniques available.”
Despite intensive efforts by investigators using sophisticated nuclear studies, echocardiography, and MRI, however, nothing to date has been able to predict who’s going to respond to CRT as well as a simple electrical parameter: the QRS duration.
“It remains really the gold standard. It’s an electrical marker for what is a mechanical event,” Dr. Estes said.
He reported serving as a consultant to Medtronic, St. Jude Medical, and Boston Scientific and receiving research support from Boston Scientific and St. Jude Medical.
SNOWMASS, COLO. – The 12-lead Holter monitor is an invaluable and underutilized tool for turning cardiac resynchronization therapy nonresponders into responders, according to Dr. N.A. Mark Estes III.
“The Achilles heel of CRT right now is the failure to respond that occurs in one-quarter to one-third of patients who receive the device. And I think it’s less and less due to a problem of poor patient selection or technical failure, but instead due to common mistakes made in patients who’ve been properly selected and in whom a CRT device has been placed appropriately. I think there’s a lot of room for improvement here,” said Dr. Estes, director of the cardiac arrhythmia center and professor of medicine at Tufts University in Boston.
One common mistake is to accept at face value the CRT device counter when it shows a high percentage of ventricular pacing that’s supposed to be indicative of adequate biventricular pacing in patients. That’s not reliable in patients with coexisting heart failure and atrial fibrillation. In fact, the device pacing counter can markedly overestimate the degree of effective biventricular pacing in patients with permanent atrial fibrillation undergoing CRT. That’s because it may incorrectly count fusion and pseudofusion beats as paced events, even though ventricular capture and atrioventricular resynchronization fail to occur during such beats. The 12-lead Holter monitor will tell the real story regarding ventricular rate control and the percentage of fully paced beats, Dr. Estes said at the Annual Cardiovascular Conference at Snowmass.
“In patients with atrial fibrillation, you can’t just do an EKG, and you can’t just do an interrogation of the device and see that they have 90% or 95% biventricular pacing, which is the sweet spot you need to be North of to make sure they’re having benefit. You really need to do the 24-hour Holter monitor,” the cardiologist emphasized.
“It happens all the time,” he continued. “It happens in my own institution, where we have people come in with heart failure who are being evaluated for an LVAD [left ventricular assist device] because when their CRT device is interrogated, it shows 95% pacing. Everyone’s getting ready to put in the LVAD, and we put them on a Holter monitor and guess what? They’re 60% paced. And sometimes you can have dramatic – really dramatic – improvements by just slowing the ventricular response.”
Increasingly, however, he and his fellow electrophysiologists are alternatively turning to atrioventricular junction ablation in suitable candidates for the catheter-based procedure in order to improve their clinical response to CRT.
Dr. Estes cited a study by Columbia University cardiologists as being highly influential in shaping the new understanding of how to use 12-lead Holter monitoring to identify patients with atrial fibrillation who aren’t responding to CRT. They studied 19 patients with permanent atrial fibrillation who were on CRT, all of whom were on beta-blockers, amiodarone, and digoxin for rate control. Although device interrogation showed all 19 patients had in excess of 90% biventricular pacing 12 months after CRT implantation, only 9 patients were actually clinical responders to CRT as defined by at least a 1-grade improvement in New York Heart Association functional class.
Upon wearing an ambulatory 12-lead Holter monitor for 24 hours, only the nine patients with clinical improvement in response to CRT actually had effective pacing; 94% of their heart beats over the course of 24 hours were fully paced, with a mere 2% fusion beats and less than 4% pseudofusion beats. The other 10 patients had an average of only 60% fully paced beats, along with 16% fusion and 24% pseudofusion beats.
The CRT clinical responders also showed evidence of reverse remodeling, with significantly greater improvements in left ventricular ejection fraction, end-systolic dimension, end-diastolic diameter, and end-systolic and end-diastolic volume, compared with nonresponders.
The lesson, according to the investigators, is that the percentage of biventricular pacing, as obtained from CRT device interrogation, is an unreliable indicator of effective pacing in patients with permanent atrial fibrillation. The goal of CRT programming in this population is to try to achieve 100% biventricular capture on the 12-lead Holter monitor (J. Am. Coll. Cardiol. 2009;53:1050-5).
A series of landmark randomized trials has established that in suitable candidates for CRT with NYHA class I through ambulatory class IV heart failure with reduced ejection fraction, the device therapy provides on average about a 17% reduction in all-cause mortality and a 29% decrease in hospitalizations for heart failure above and beyond the benefits obtained through guideline-directed medical therapy. However, the improvements seen in patients with NYHA class I symptoms are less impressive than in those with NYHA class II-IV disease. And it’s possible that the randomized controlled trials overestimate the true benefit from CRT.
“This is a group of extremely experienced investigators who know the literature well. They’re typically quite savvy with patient selection, and they’re vested in getting the right patients into these trials. Unfortunately, there’s not a registry of all eligible patients, so we can’t tell exactly how those patients were selected. It would have been helpful,” Dr. Estes said.
It’s clear from the randomized trials that the ideal patient for CRT is a woman under age 65 with nonischemic cardiomyopathy with a left bundle branch block, normal sinus rhythm, and a QRS duration of 150 milliseconds or more. That’s who’s most likely to obtain clinical benefit from the therapy. The flip side of that is that an older male patient with ischemia, atrial fibrillation, a QRS of 120-150 milliseconds, and non–left bundle branch block is less likely to respond.
“We’ve figured this out. I think we’ve gotten pretty good at giving patients an honest estimate of their probability of responding independent of our ability to get a lead into a good spot in the coronary sinus,” Dr. Estes said. “More than 95% of patients can be adequately resynchronized currently with the tools we have, so it’s not the barrier that it once represented. We have many different techniques available.”
Despite intensive efforts by investigators using sophisticated nuclear studies, echocardiography, and MRI, however, nothing to date has been able to predict who’s going to respond to CRT as well as a simple electrical parameter: the QRS duration.
“It remains really the gold standard. It’s an electrical marker for what is a mechanical event,” Dr. Estes said.
He reported serving as a consultant to Medtronic, St. Jude Medical, and Boston Scientific and receiving research support from Boston Scientific and St. Jude Medical.
SNOWMASS, COLO. – The 12-lead Holter monitor is an invaluable and underutilized tool for turning cardiac resynchronization therapy nonresponders into responders, according to Dr. N.A. Mark Estes III.
“The Achilles heel of CRT right now is the failure to respond that occurs in one-quarter to one-third of patients who receive the device. And I think it’s less and less due to a problem of poor patient selection or technical failure, but instead due to common mistakes made in patients who’ve been properly selected and in whom a CRT device has been placed appropriately. I think there’s a lot of room for improvement here,” said Dr. Estes, director of the cardiac arrhythmia center and professor of medicine at Tufts University in Boston.
One common mistake is to accept at face value the CRT device counter when it shows a high percentage of ventricular pacing that’s supposed to be indicative of adequate biventricular pacing in patients. That’s not reliable in patients with coexisting heart failure and atrial fibrillation. In fact, the device pacing counter can markedly overestimate the degree of effective biventricular pacing in patients with permanent atrial fibrillation undergoing CRT. That’s because it may incorrectly count fusion and pseudofusion beats as paced events, even though ventricular capture and atrioventricular resynchronization fail to occur during such beats. The 12-lead Holter monitor will tell the real story regarding ventricular rate control and the percentage of fully paced beats, Dr. Estes said at the Annual Cardiovascular Conference at Snowmass.
“In patients with atrial fibrillation, you can’t just do an EKG, and you can’t just do an interrogation of the device and see that they have 90% or 95% biventricular pacing, which is the sweet spot you need to be North of to make sure they’re having benefit. You really need to do the 24-hour Holter monitor,” the cardiologist emphasized.
“It happens all the time,” he continued. “It happens in my own institution, where we have people come in with heart failure who are being evaluated for an LVAD [left ventricular assist device] because when their CRT device is interrogated, it shows 95% pacing. Everyone’s getting ready to put in the LVAD, and we put them on a Holter monitor and guess what? They’re 60% paced. And sometimes you can have dramatic – really dramatic – improvements by just slowing the ventricular response.”
Increasingly, however, he and his fellow electrophysiologists are alternatively turning to atrioventricular junction ablation in suitable candidates for the catheter-based procedure in order to improve their clinical response to CRT.
Dr. Estes cited a study by Columbia University cardiologists as being highly influential in shaping the new understanding of how to use 12-lead Holter monitoring to identify patients with atrial fibrillation who aren’t responding to CRT. They studied 19 patients with permanent atrial fibrillation who were on CRT, all of whom were on beta-blockers, amiodarone, and digoxin for rate control. Although device interrogation showed all 19 patients had in excess of 90% biventricular pacing 12 months after CRT implantation, only 9 patients were actually clinical responders to CRT as defined by at least a 1-grade improvement in New York Heart Association functional class.
Upon wearing an ambulatory 12-lead Holter monitor for 24 hours, only the nine patients with clinical improvement in response to CRT actually had effective pacing; 94% of their heart beats over the course of 24 hours were fully paced, with a mere 2% fusion beats and less than 4% pseudofusion beats. The other 10 patients had an average of only 60% fully paced beats, along with 16% fusion and 24% pseudofusion beats.
The CRT clinical responders also showed evidence of reverse remodeling, with significantly greater improvements in left ventricular ejection fraction, end-systolic dimension, end-diastolic diameter, and end-systolic and end-diastolic volume, compared with nonresponders.
The lesson, according to the investigators, is that the percentage of biventricular pacing, as obtained from CRT device interrogation, is an unreliable indicator of effective pacing in patients with permanent atrial fibrillation. The goal of CRT programming in this population is to try to achieve 100% biventricular capture on the 12-lead Holter monitor (J. Am. Coll. Cardiol. 2009;53:1050-5).
A series of landmark randomized trials has established that in suitable candidates for CRT with NYHA class I through ambulatory class IV heart failure with reduced ejection fraction, the device therapy provides on average about a 17% reduction in all-cause mortality and a 29% decrease in hospitalizations for heart failure above and beyond the benefits obtained through guideline-directed medical therapy. However, the improvements seen in patients with NYHA class I symptoms are less impressive than in those with NYHA class II-IV disease. And it’s possible that the randomized controlled trials overestimate the true benefit from CRT.
“This is a group of extremely experienced investigators who know the literature well. They’re typically quite savvy with patient selection, and they’re vested in getting the right patients into these trials. Unfortunately, there’s not a registry of all eligible patients, so we can’t tell exactly how those patients were selected. It would have been helpful,” Dr. Estes said.
It’s clear from the randomized trials that the ideal patient for CRT is a woman under age 65 with nonischemic cardiomyopathy with a left bundle branch block, normal sinus rhythm, and a QRS duration of 150 milliseconds or more. That’s who’s most likely to obtain clinical benefit from the therapy. The flip side of that is that an older male patient with ischemia, atrial fibrillation, a QRS of 120-150 milliseconds, and non–left bundle branch block is less likely to respond.
“We’ve figured this out. I think we’ve gotten pretty good at giving patients an honest estimate of their probability of responding independent of our ability to get a lead into a good spot in the coronary sinus,” Dr. Estes said. “More than 95% of patients can be adequately resynchronized currently with the tools we have, so it’s not the barrier that it once represented. We have many different techniques available.”
Despite intensive efforts by investigators using sophisticated nuclear studies, echocardiography, and MRI, however, nothing to date has been able to predict who’s going to respond to CRT as well as a simple electrical parameter: the QRS duration.
“It remains really the gold standard. It’s an electrical marker for what is a mechanical event,” Dr. Estes said.
He reported serving as a consultant to Medtronic, St. Jude Medical, and Boston Scientific and receiving research support from Boston Scientific and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS