User login
Abatacept, adalimumab equivalent for RA in 2-year head-to-head trial
MADRID – A 2-year head-to-head comparison of abatacept and adalimumab in rheumatoid arthritis patients who were on background methotrexate has found equal improvement with both biologics, according to results from a study presented at the annual European Congress of Rheumatology.
The randomized, investigator-blinded AMPLE trial is the first 2-year comparator study of biologics done in biologic-naive rheumatoid arthritis patients.
"Through 2 years of treatment, in this first active comparator study between biologic agents in rheumatoid arthritis patients with an inadequate response to methotrexate, this robust data set demonstrates that subcutaneous abatacept and adalimumab were equally efficacious in clinical, functional, and radiographic outcomes," said Dr. Michael H. Schiff, a professor of medicine at the University of Colorado, Denver.
Researchers recruited 646 patients with active RA and an inadequate response to methotrexate, and randomized them to either 125 mg of abatacept weekly (without an IV load) or 40 mg of adalimumab biweekly, with a stable dose of methotrexate.
The data show that both agents have an excellent retention rate, with 79% of the abatacept and 65% of the adalimumab groups completing the 2-year follow-up.
The two medications showed similar efficacy for American College of Rheumatology (ACR) 20, 50, 70, and 90 responses and rates of remission on the Disease Activity Score-28 (DAS28), Dr. Schiff said. For ACR 20, the 2-year response rate was 60% in each group. The ACR 50 response rate was 47% for the adalimumab group and 45% for the abatacept group. For the ACR 70, the rates were 29% for adalimumab and 31% for abatacept, and for the ACR 90, the rates were 8% for adalimumab and 15% for abatacept.
The 2-year DAS28 rate was virtually identical in each group, with a mean decrease of about 2.2 from baseline. X-ray non-progression was seen in 84% of each group at 2 years, Dr. Schiff said.
The study found similar numbers of serious adverse events in both arms (14% of the abatacept group and 17% of the adalimumab group). However, serious adverse events leading to discontinuation of the study medication occurred in 5% of patients taking adalimumab and 2% of those taking abatacept.
There was one death in each group – neither of which was related to the study drugs. There were seven malignancies in each group; four patients in each group discontinued their study medication due to neoplasm.
Infections were the most common serious side effects (31 total), occurring in 4% of the abatacept and 6% of the adalimumab groups). There were eight opportunistic infections – four in each group. The adalimumab group had two cases of pulmonary tuberculosis, one case of disseminated tuberculosis, and one case of disseminated histoplasmosis. There were three cases of pneumonia in the abatacept group and four in the adalimumab group.
Autoimmune events were also relatively common – 18 in all, with 12 in the abatacept group (4%) and 6 in the adalimumab group (2%). Dr. Schiff said none of these were serious or clinically important.
During the question-and-answer period, Dr. Schiff said it’s not currently possible to predict which patients would respond to the drugs. "We looked at responders in both groups and were not able to differentiate them based on clinical characteristics," he said. "We are now analyzing the biomarkers and hope to have that information for EULAR 2014."
"EULAR/ACR guidelines recommend starting a patient on methotrexate and then optimizing the dose over 3-6 months, and if a patient has an incomplete response to methotrexate, then to add a biological agent," said Dr. Schiff in an interview.
He noted that anti-tumor necrosis factor (anti-TNF) agents have been the first choice of most rheumatologists, and adalimumab is the most widely chosen anti-TNF agent worldwide, which is why it was selected as one of the agents for the head-to-head trial. Abatacept employs another method of action: T-cell inhibition.
"This paper has important clinical significance because a patient and his or her rheumatologist want to have data to make an informed choice of a biologic agent to add when an incomplete response to methotrexate occurs," Dr. Schiff said.
Senior writer Michele Sullivan contributed to this report.
MADRID – A 2-year head-to-head comparison of abatacept and adalimumab in rheumatoid arthritis patients who were on background methotrexate has found equal improvement with both biologics, according to results from a study presented at the annual European Congress of Rheumatology.
The randomized, investigator-blinded AMPLE trial is the first 2-year comparator study of biologics done in biologic-naive rheumatoid arthritis patients.
"Through 2 years of treatment, in this first active comparator study between biologic agents in rheumatoid arthritis patients with an inadequate response to methotrexate, this robust data set demonstrates that subcutaneous abatacept and adalimumab were equally efficacious in clinical, functional, and radiographic outcomes," said Dr. Michael H. Schiff, a professor of medicine at the University of Colorado, Denver.
Researchers recruited 646 patients with active RA and an inadequate response to methotrexate, and randomized them to either 125 mg of abatacept weekly (without an IV load) or 40 mg of adalimumab biweekly, with a stable dose of methotrexate.
The data show that both agents have an excellent retention rate, with 79% of the abatacept and 65% of the adalimumab groups completing the 2-year follow-up.
The two medications showed similar efficacy for American College of Rheumatology (ACR) 20, 50, 70, and 90 responses and rates of remission on the Disease Activity Score-28 (DAS28), Dr. Schiff said. For ACR 20, the 2-year response rate was 60% in each group. The ACR 50 response rate was 47% for the adalimumab group and 45% for the abatacept group. For the ACR 70, the rates were 29% for adalimumab and 31% for abatacept, and for the ACR 90, the rates were 8% for adalimumab and 15% for abatacept.
The 2-year DAS28 rate was virtually identical in each group, with a mean decrease of about 2.2 from baseline. X-ray non-progression was seen in 84% of each group at 2 years, Dr. Schiff said.
The study found similar numbers of serious adverse events in both arms (14% of the abatacept group and 17% of the adalimumab group). However, serious adverse events leading to discontinuation of the study medication occurred in 5% of patients taking adalimumab and 2% of those taking abatacept.
There was one death in each group – neither of which was related to the study drugs. There were seven malignancies in each group; four patients in each group discontinued their study medication due to neoplasm.
Infections were the most common serious side effects (31 total), occurring in 4% of the abatacept and 6% of the adalimumab groups). There were eight opportunistic infections – four in each group. The adalimumab group had two cases of pulmonary tuberculosis, one case of disseminated tuberculosis, and one case of disseminated histoplasmosis. There were three cases of pneumonia in the abatacept group and four in the adalimumab group.
Autoimmune events were also relatively common – 18 in all, with 12 in the abatacept group (4%) and 6 in the adalimumab group (2%). Dr. Schiff said none of these were serious or clinically important.
During the question-and-answer period, Dr. Schiff said it’s not currently possible to predict which patients would respond to the drugs. "We looked at responders in both groups and were not able to differentiate them based on clinical characteristics," he said. "We are now analyzing the biomarkers and hope to have that information for EULAR 2014."
"EULAR/ACR guidelines recommend starting a patient on methotrexate and then optimizing the dose over 3-6 months, and if a patient has an incomplete response to methotrexate, then to add a biological agent," said Dr. Schiff in an interview.
He noted that anti-tumor necrosis factor (anti-TNF) agents have been the first choice of most rheumatologists, and adalimumab is the most widely chosen anti-TNF agent worldwide, which is why it was selected as one of the agents for the head-to-head trial. Abatacept employs another method of action: T-cell inhibition.
"This paper has important clinical significance because a patient and his or her rheumatologist want to have data to make an informed choice of a biologic agent to add when an incomplete response to methotrexate occurs," Dr. Schiff said.
Senior writer Michele Sullivan contributed to this report.
MADRID – A 2-year head-to-head comparison of abatacept and adalimumab in rheumatoid arthritis patients who were on background methotrexate has found equal improvement with both biologics, according to results from a study presented at the annual European Congress of Rheumatology.
The randomized, investigator-blinded AMPLE trial is the first 2-year comparator study of biologics done in biologic-naive rheumatoid arthritis patients.
"Through 2 years of treatment, in this first active comparator study between biologic agents in rheumatoid arthritis patients with an inadequate response to methotrexate, this robust data set demonstrates that subcutaneous abatacept and adalimumab were equally efficacious in clinical, functional, and radiographic outcomes," said Dr. Michael H. Schiff, a professor of medicine at the University of Colorado, Denver.
Researchers recruited 646 patients with active RA and an inadequate response to methotrexate, and randomized them to either 125 mg of abatacept weekly (without an IV load) or 40 mg of adalimumab biweekly, with a stable dose of methotrexate.
The data show that both agents have an excellent retention rate, with 79% of the abatacept and 65% of the adalimumab groups completing the 2-year follow-up.
The two medications showed similar efficacy for American College of Rheumatology (ACR) 20, 50, 70, and 90 responses and rates of remission on the Disease Activity Score-28 (DAS28), Dr. Schiff said. For ACR 20, the 2-year response rate was 60% in each group. The ACR 50 response rate was 47% for the adalimumab group and 45% for the abatacept group. For the ACR 70, the rates were 29% for adalimumab and 31% for abatacept, and for the ACR 90, the rates were 8% for adalimumab and 15% for abatacept.
The 2-year DAS28 rate was virtually identical in each group, with a mean decrease of about 2.2 from baseline. X-ray non-progression was seen in 84% of each group at 2 years, Dr. Schiff said.
The study found similar numbers of serious adverse events in both arms (14% of the abatacept group and 17% of the adalimumab group). However, serious adverse events leading to discontinuation of the study medication occurred in 5% of patients taking adalimumab and 2% of those taking abatacept.
There was one death in each group – neither of which was related to the study drugs. There were seven malignancies in each group; four patients in each group discontinued their study medication due to neoplasm.
Infections were the most common serious side effects (31 total), occurring in 4% of the abatacept and 6% of the adalimumab groups). There were eight opportunistic infections – four in each group. The adalimumab group had two cases of pulmonary tuberculosis, one case of disseminated tuberculosis, and one case of disseminated histoplasmosis. There were three cases of pneumonia in the abatacept group and four in the adalimumab group.
Autoimmune events were also relatively common – 18 in all, with 12 in the abatacept group (4%) and 6 in the adalimumab group (2%). Dr. Schiff said none of these were serious or clinically important.
During the question-and-answer period, Dr. Schiff said it’s not currently possible to predict which patients would respond to the drugs. "We looked at responders in both groups and were not able to differentiate them based on clinical characteristics," he said. "We are now analyzing the biomarkers and hope to have that information for EULAR 2014."
"EULAR/ACR guidelines recommend starting a patient on methotrexate and then optimizing the dose over 3-6 months, and if a patient has an incomplete response to methotrexate, then to add a biological agent," said Dr. Schiff in an interview.
He noted that anti-tumor necrosis factor (anti-TNF) agents have been the first choice of most rheumatologists, and adalimumab is the most widely chosen anti-TNF agent worldwide, which is why it was selected as one of the agents for the head-to-head trial. Abatacept employs another method of action: T-cell inhibition.
"This paper has important clinical significance because a patient and his or her rheumatologist want to have data to make an informed choice of a biologic agent to add when an incomplete response to methotrexate occurs," Dr. Schiff said.
Senior writer Michele Sullivan contributed to this report.
AT THE EULAR CONGRESS 2013
Proposed ACR-EULAR scleroderma classification criteria 'more inclusive'
MADRID – New classification criteria for scleroderma presented at the annual European Congress of Rheumatology correctly identify more patients who could potentially be included in epidemiological studies and clinical trials than is possible with existing classification systems.
The new system is still a proposal and is under review by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), according to Dr. Frank van den Hoogen, who is the director of the rheumatology center at Sint Maartenskliniek in Nijmegen and head of the department of rheumatology at Radboud University in Nijmegen, both in the Netherlands.
In a validation cohort, the ACR-EULAR criteria had a sensitivity of 91% and a specificity of 92% to correctly identify patients with systemic sclerosis. By comparison, the 1980 Preliminary ARA Criteria had a sensitivity of 75% and a specificity of 72%.
The whole process of developing the ACR-EULAR criteria has taken about 5 years, Dr. van den Hoogen explained in an interview. "The ARA criteria were not as sensitive as we wanted because they excluded some patients with limited disease and also patients with newly diagnosed disease," he added.
"The purpose of classification criteria is to include similar patients in research," Dr. van den Hoogen said. "Classification criteria are not synonymous with diagnostic criteria," he explained, "[they] are generally more standardized and less inclusive." This is because physicians will see patients with multiple symptoms and it would not be possible to include every symptom seen in routine practice in a set of classification criteria. Nevertheless, diagnostic criteria do tend to mirror classification criteria.
The process of determining which items to include was driven by both data and consensus. Delphi exercises and a nominal group technique were used to create a set of potential items for the classification of systemic sclerosis.
Several patient cases were then reviewed by leading scleroderma experts based in Europe and North America. The cases represented the full spectrum of systemic sclerosis, including those with a low and those with a high probability of having the disease. Experts ranked the importance of the symptoms exhibited by each of these cases, and a whittled down list with a scoring system was obtained. Systemic sclerosis was present if a score of 9 or more was achieved.
Skin thickening of the fingers of both hands extending past the metacarpophalangeal (MCP) joints was considered to be indicative of scleroderma, and was given a score of 9. Conversely, patients with skin involvement likely to be due to another scleroderma-like disorder or skin thickening sparing the fingers were not likely to have systemic sclerosis.
Other items included skin thickening of the fingers, with subitems of puffy fingers (score = 2) and whole finger skin thickening, distal to the MCP (4); fingertip lesions, with subitems of digital tip ulcers (2) and pitting scars (3), telangiectasia (2), abnormal nailfold capillaries (2); pulmonary arterial hypertension, interstitial lung disease, or both (2); Raynaud’s phenomenon (3); and the presence of any scleroderma-related autoantibodies (3).
The ability of these criteria to correctly identify patients with and without systemic sclerosis was then prospectively tested in a random sample of 200 individuals and further validated in a cohort of 405 individuals that included both early and prevalent cases of scleroderma and its mimics who had been collected from several European and North American scleroderma centers.
"The proposed ACR-EULAR criteria for the classification of systemic sclerosis should allow more patients to be classified correctly as systemic sclerosis," Dr. van den Hoogen said. This includes those with early (less than 3 years) scleroderma and the 20% of patients who have limited disease but who do not meet current classification criteria.
"New ACR [EULAR] criteria show increased sensitivity in comparison to the old [ARA] criteria," concurred Dr. Suzana Jordan of University Hospital Zurich. She presented findings on the use of the proposed system in 317 patients from the Zurich scleroderma cohort. This cohort mainly consists of patients with early or mild disease.
Applying the criteria to this Swiss patient population, Dr. Jordan noted that "75% of systemic sclerosis patients were correctly identified compared to just over half of all patients (51%) using the ARA criteria." Furthermore, "50% of early scleroderma patients who did not fulfill the old criteria met the new," she concluded.
Dr. van den Hoogen said that he had no disclosures, except that this work was funded jointly by EULAR and the ACR.
MADRID – New classification criteria for scleroderma presented at the annual European Congress of Rheumatology correctly identify more patients who could potentially be included in epidemiological studies and clinical trials than is possible with existing classification systems.
The new system is still a proposal and is under review by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), according to Dr. Frank van den Hoogen, who is the director of the rheumatology center at Sint Maartenskliniek in Nijmegen and head of the department of rheumatology at Radboud University in Nijmegen, both in the Netherlands.
In a validation cohort, the ACR-EULAR criteria had a sensitivity of 91% and a specificity of 92% to correctly identify patients with systemic sclerosis. By comparison, the 1980 Preliminary ARA Criteria had a sensitivity of 75% and a specificity of 72%.
The whole process of developing the ACR-EULAR criteria has taken about 5 years, Dr. van den Hoogen explained in an interview. "The ARA criteria were not as sensitive as we wanted because they excluded some patients with limited disease and also patients with newly diagnosed disease," he added.
"The purpose of classification criteria is to include similar patients in research," Dr. van den Hoogen said. "Classification criteria are not synonymous with diagnostic criteria," he explained, "[they] are generally more standardized and less inclusive." This is because physicians will see patients with multiple symptoms and it would not be possible to include every symptom seen in routine practice in a set of classification criteria. Nevertheless, diagnostic criteria do tend to mirror classification criteria.
The process of determining which items to include was driven by both data and consensus. Delphi exercises and a nominal group technique were used to create a set of potential items for the classification of systemic sclerosis.
Several patient cases were then reviewed by leading scleroderma experts based in Europe and North America. The cases represented the full spectrum of systemic sclerosis, including those with a low and those with a high probability of having the disease. Experts ranked the importance of the symptoms exhibited by each of these cases, and a whittled down list with a scoring system was obtained. Systemic sclerosis was present if a score of 9 or more was achieved.
Skin thickening of the fingers of both hands extending past the metacarpophalangeal (MCP) joints was considered to be indicative of scleroderma, and was given a score of 9. Conversely, patients with skin involvement likely to be due to another scleroderma-like disorder or skin thickening sparing the fingers were not likely to have systemic sclerosis.
Other items included skin thickening of the fingers, with subitems of puffy fingers (score = 2) and whole finger skin thickening, distal to the MCP (4); fingertip lesions, with subitems of digital tip ulcers (2) and pitting scars (3), telangiectasia (2), abnormal nailfold capillaries (2); pulmonary arterial hypertension, interstitial lung disease, or both (2); Raynaud’s phenomenon (3); and the presence of any scleroderma-related autoantibodies (3).
The ability of these criteria to correctly identify patients with and without systemic sclerosis was then prospectively tested in a random sample of 200 individuals and further validated in a cohort of 405 individuals that included both early and prevalent cases of scleroderma and its mimics who had been collected from several European and North American scleroderma centers.
"The proposed ACR-EULAR criteria for the classification of systemic sclerosis should allow more patients to be classified correctly as systemic sclerosis," Dr. van den Hoogen said. This includes those with early (less than 3 years) scleroderma and the 20% of patients who have limited disease but who do not meet current classification criteria.
"New ACR [EULAR] criteria show increased sensitivity in comparison to the old [ARA] criteria," concurred Dr. Suzana Jordan of University Hospital Zurich. She presented findings on the use of the proposed system in 317 patients from the Zurich scleroderma cohort. This cohort mainly consists of patients with early or mild disease.
Applying the criteria to this Swiss patient population, Dr. Jordan noted that "75% of systemic sclerosis patients were correctly identified compared to just over half of all patients (51%) using the ARA criteria." Furthermore, "50% of early scleroderma patients who did not fulfill the old criteria met the new," she concluded.
Dr. van den Hoogen said that he had no disclosures, except that this work was funded jointly by EULAR and the ACR.
MADRID – New classification criteria for scleroderma presented at the annual European Congress of Rheumatology correctly identify more patients who could potentially be included in epidemiological studies and clinical trials than is possible with existing classification systems.
The new system is still a proposal and is under review by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), according to Dr. Frank van den Hoogen, who is the director of the rheumatology center at Sint Maartenskliniek in Nijmegen and head of the department of rheumatology at Radboud University in Nijmegen, both in the Netherlands.
In a validation cohort, the ACR-EULAR criteria had a sensitivity of 91% and a specificity of 92% to correctly identify patients with systemic sclerosis. By comparison, the 1980 Preliminary ARA Criteria had a sensitivity of 75% and a specificity of 72%.
The whole process of developing the ACR-EULAR criteria has taken about 5 years, Dr. van den Hoogen explained in an interview. "The ARA criteria were not as sensitive as we wanted because they excluded some patients with limited disease and also patients with newly diagnosed disease," he added.
"The purpose of classification criteria is to include similar patients in research," Dr. van den Hoogen said. "Classification criteria are not synonymous with diagnostic criteria," he explained, "[they] are generally more standardized and less inclusive." This is because physicians will see patients with multiple symptoms and it would not be possible to include every symptom seen in routine practice in a set of classification criteria. Nevertheless, diagnostic criteria do tend to mirror classification criteria.
The process of determining which items to include was driven by both data and consensus. Delphi exercises and a nominal group technique were used to create a set of potential items for the classification of systemic sclerosis.
Several patient cases were then reviewed by leading scleroderma experts based in Europe and North America. The cases represented the full spectrum of systemic sclerosis, including those with a low and those with a high probability of having the disease. Experts ranked the importance of the symptoms exhibited by each of these cases, and a whittled down list with a scoring system was obtained. Systemic sclerosis was present if a score of 9 or more was achieved.
Skin thickening of the fingers of both hands extending past the metacarpophalangeal (MCP) joints was considered to be indicative of scleroderma, and was given a score of 9. Conversely, patients with skin involvement likely to be due to another scleroderma-like disorder or skin thickening sparing the fingers were not likely to have systemic sclerosis.
Other items included skin thickening of the fingers, with subitems of puffy fingers (score = 2) and whole finger skin thickening, distal to the MCP (4); fingertip lesions, with subitems of digital tip ulcers (2) and pitting scars (3), telangiectasia (2), abnormal nailfold capillaries (2); pulmonary arterial hypertension, interstitial lung disease, or both (2); Raynaud’s phenomenon (3); and the presence of any scleroderma-related autoantibodies (3).
The ability of these criteria to correctly identify patients with and without systemic sclerosis was then prospectively tested in a random sample of 200 individuals and further validated in a cohort of 405 individuals that included both early and prevalent cases of scleroderma and its mimics who had been collected from several European and North American scleroderma centers.
"The proposed ACR-EULAR criteria for the classification of systemic sclerosis should allow more patients to be classified correctly as systemic sclerosis," Dr. van den Hoogen said. This includes those with early (less than 3 years) scleroderma and the 20% of patients who have limited disease but who do not meet current classification criteria.
"New ACR [EULAR] criteria show increased sensitivity in comparison to the old [ARA] criteria," concurred Dr. Suzana Jordan of University Hospital Zurich. She presented findings on the use of the proposed system in 317 patients from the Zurich scleroderma cohort. This cohort mainly consists of patients with early or mild disease.
Applying the criteria to this Swiss patient population, Dr. Jordan noted that "75% of systemic sclerosis patients were correctly identified compared to just over half of all patients (51%) using the ARA criteria." Furthermore, "50% of early scleroderma patients who did not fulfill the old criteria met the new," she concluded.
Dr. van den Hoogen said that he had no disclosures, except that this work was funded jointly by EULAR and the ACR.
AT THE EULAR CONGRESS 2013
Exercise program improved strength, walking in AS
Patients with ankylosing spondylitis who participated in a progressive muscle strengthening program gained significant improvements in muscle strength and walking performance after 4 months, compared with those who did not participate, Dr. Fabio Jennings reported at the annual European Congress of Rheumatology.
The exercise program, which involved resistance training with the Swiss ball, was also well tolerated and was not associated with negative effects on disease activity, said Dr. Jennings of the rheumatology division at the Federal University of Sao Paulo, Brazil.
Dr. Jennings and his colleagues performed a randomized, controlled, single-blind, prospective trial of 60 patients with ankylosing spondylitis (AS).
Exercise is recommended for people with AS, but the benefits of a specific exercise program have not been well defined, Dr. Jennings noted.
In the study, 30 patients were randomized to the supervised exercise program, which entailed eight resistance exercises using free weights on a Swiss ball twice a week for 16 weeks, with increases in load every 4 weeks. The 30 patients in the control group continued regular treatment with medications, with no exercise. Demographics, clinical features, and medications were similar in the two groups at baseline.
The impact of the exercise on functional capacity, quality of life, muscle strength, and mobility was evaluated using the BASFI (Bath Ankylosing Spondylitis Functional Index), HAQ-S (Health Assessment Questionnaire for Spondyloarthropathies), the 6-minute walk test, and other assessment tools, every 4 weeks. Disease activity was measured with the BASDAI (Bath Ankylosing Spondylitis Disease Activity Index), erythrocyte sedimentation rate (ESR), and C-reactive protein levels.
After 4 months, there were statistically significant differences between the two groups in the strengths of the muscles used in performing the exercises (abdominal, squat, triceps, reverse fly, and rowing exercises), favoring the intervention group. These patients also had significant improvements in the 6-minute walk test; and they were satisfied with the treatment, as reflected by significant differences between the two groups in the Likert scale used to assess patient satisfaction at all time points measured.
In addition, disease activity – as measured by BASDAI, ESR, and C-reactive protein – did not worsen among those who participated in the exercise program.
The study showed that this type of exercise program, using a Swiss ball to improve muscle strength, "is a beneficial and safe intervention" in people with AS, lead author Mr. Marcelo Cardoso de Souza, a physiotherapist and member of a multidisciplinary rehabilitation team at the university, said in an interview. For these patients, improvement in muscular performance and functional capacity is important, he said, noting that before clinicians refer patients to such a program, patients should undergo a medical evaluation, and the program should be provided by an experienced professional.
None of the investigators had relevant financial conflicts of interest.
Patients with ankylosing spondylitis who participated in a progressive muscle strengthening program gained significant improvements in muscle strength and walking performance after 4 months, compared with those who did not participate, Dr. Fabio Jennings reported at the annual European Congress of Rheumatology.
The exercise program, which involved resistance training with the Swiss ball, was also well tolerated and was not associated with negative effects on disease activity, said Dr. Jennings of the rheumatology division at the Federal University of Sao Paulo, Brazil.
Dr. Jennings and his colleagues performed a randomized, controlled, single-blind, prospective trial of 60 patients with ankylosing spondylitis (AS).
Exercise is recommended for people with AS, but the benefits of a specific exercise program have not been well defined, Dr. Jennings noted.
In the study, 30 patients were randomized to the supervised exercise program, which entailed eight resistance exercises using free weights on a Swiss ball twice a week for 16 weeks, with increases in load every 4 weeks. The 30 patients in the control group continued regular treatment with medications, with no exercise. Demographics, clinical features, and medications were similar in the two groups at baseline.
The impact of the exercise on functional capacity, quality of life, muscle strength, and mobility was evaluated using the BASFI (Bath Ankylosing Spondylitis Functional Index), HAQ-S (Health Assessment Questionnaire for Spondyloarthropathies), the 6-minute walk test, and other assessment tools, every 4 weeks. Disease activity was measured with the BASDAI (Bath Ankylosing Spondylitis Disease Activity Index), erythrocyte sedimentation rate (ESR), and C-reactive protein levels.
After 4 months, there were statistically significant differences between the two groups in the strengths of the muscles used in performing the exercises (abdominal, squat, triceps, reverse fly, and rowing exercises), favoring the intervention group. These patients also had significant improvements in the 6-minute walk test; and they were satisfied with the treatment, as reflected by significant differences between the two groups in the Likert scale used to assess patient satisfaction at all time points measured.
In addition, disease activity – as measured by BASDAI, ESR, and C-reactive protein – did not worsen among those who participated in the exercise program.
The study showed that this type of exercise program, using a Swiss ball to improve muscle strength, "is a beneficial and safe intervention" in people with AS, lead author Mr. Marcelo Cardoso de Souza, a physiotherapist and member of a multidisciplinary rehabilitation team at the university, said in an interview. For these patients, improvement in muscular performance and functional capacity is important, he said, noting that before clinicians refer patients to such a program, patients should undergo a medical evaluation, and the program should be provided by an experienced professional.
None of the investigators had relevant financial conflicts of interest.
Patients with ankylosing spondylitis who participated in a progressive muscle strengthening program gained significant improvements in muscle strength and walking performance after 4 months, compared with those who did not participate, Dr. Fabio Jennings reported at the annual European Congress of Rheumatology.
The exercise program, which involved resistance training with the Swiss ball, was also well tolerated and was not associated with negative effects on disease activity, said Dr. Jennings of the rheumatology division at the Federal University of Sao Paulo, Brazil.
Dr. Jennings and his colleagues performed a randomized, controlled, single-blind, prospective trial of 60 patients with ankylosing spondylitis (AS).
Exercise is recommended for people with AS, but the benefits of a specific exercise program have not been well defined, Dr. Jennings noted.
In the study, 30 patients were randomized to the supervised exercise program, which entailed eight resistance exercises using free weights on a Swiss ball twice a week for 16 weeks, with increases in load every 4 weeks. The 30 patients in the control group continued regular treatment with medications, with no exercise. Demographics, clinical features, and medications were similar in the two groups at baseline.
The impact of the exercise on functional capacity, quality of life, muscle strength, and mobility was evaluated using the BASFI (Bath Ankylosing Spondylitis Functional Index), HAQ-S (Health Assessment Questionnaire for Spondyloarthropathies), the 6-minute walk test, and other assessment tools, every 4 weeks. Disease activity was measured with the BASDAI (Bath Ankylosing Spondylitis Disease Activity Index), erythrocyte sedimentation rate (ESR), and C-reactive protein levels.
After 4 months, there were statistically significant differences between the two groups in the strengths of the muscles used in performing the exercises (abdominal, squat, triceps, reverse fly, and rowing exercises), favoring the intervention group. These patients also had significant improvements in the 6-minute walk test; and they were satisfied with the treatment, as reflected by significant differences between the two groups in the Likert scale used to assess patient satisfaction at all time points measured.
In addition, disease activity – as measured by BASDAI, ESR, and C-reactive protein – did not worsen among those who participated in the exercise program.
The study showed that this type of exercise program, using a Swiss ball to improve muscle strength, "is a beneficial and safe intervention" in people with AS, lead author Mr. Marcelo Cardoso de Souza, a physiotherapist and member of a multidisciplinary rehabilitation team at the university, said in an interview. For these patients, improvement in muscular performance and functional capacity is important, he said, noting that before clinicians refer patients to such a program, patients should undergo a medical evaluation, and the program should be provided by an experienced professional.
None of the investigators had relevant financial conflicts of interest.
FROM THE EULAR CONGRESS 2013
Adalimumab can serve as first- and second-line biologic in JIA
Adalimumab is safe and effective as both a first- and second-line biologic agent in patients with juvenile idiopathic arthritis, according to data presented by Dr. Gerd Horneff.
Dr. Horneff from the Asklepios Klinik, St. Augustin, Germany, and Dr. Heinrike Schmeling from the Alberta Children’s Hospital, Calgary, analyzed data from 329 children aged 4-18 years with juvenile idiopathic arthritis (JIA) in the German Biologics Register, finding that a high proportion of patients showed a significant response to treatment with adalimumab.
"The results of this study indicate that adalimumab is highly effective as a first and also as a second introduced biological agent resulting in substantial improvements in clinical signs and symptoms in children with juvenile idiopathic arthritis," Dr. Schmeling said in an interview prior to the annual European Congress of Rheumatology.
Even patients who were using adalimumab as a second or third biologic gained an additional response to adalimumab on top of their responses to previous biologics. The percentage of patients who met the criteria of 30%/50%/70% improvement at last documentation when adalimumab was the first introduced biologic agent was 65%/61%/46%, compared with 73%/65%/48% when adalimumab was initiated after other biologics, the investigators reported.
Around one-third of patients had rheumatoid factor–negative polyarticular JIA, 22.9% had extended oligoarticular JIA, and 25.2% of patients had a history of uveitis.
The median age of onset of disease was 6.7 years, and median age at treatment initiation was 13.8 years, with median disease duration of 5.1 years. ANA positivity was present in 51% of patients and HLB27 positivity in 19.8%. Most patients had been previously treated with methotrexate (92.4%), approximately one-third had received other disease-modifying antirheumatic drugs (DMARDs), and two-thirds had been treated with biologics, mostly etanercept.
Many were also receiving concomitant treatment with NSAIDs (55.3%), steroids (38%), methotrexate (57.8%), and other DMARDs (13%).
However, Dr. Schmeling emphasized that this study was not an attempt to compare adalimumab to other biologic treatments.
While no malignancies were observed during adalimumab treatment, 96 patients reported 220 adverse events, 14 of which were serious. There were also more autoimmune adverse events among patients treated with adalimumab, but Dr. Schmeling said the numbers were too small to draw any conclusions.
Uveitis developed in 18 children while on treatment, 13 of them had a history of uveitis prior to adalimumab initiation. Treatment was discontinued in 61 patients because of inefficacy (9%), adverse events (5%), remission (3%), patient request (9%), and other unspecified reasons (2%).
Disease register is supported by Abbott, Pfizer (Wyeth Pharma), and Roche, Germany. Dr. Schmeling had no financial disclosures. Dr Horneff declared consultancies, speakers bureau engagement, and research support from Abbott, Pfizer, and Roche.
Adalimumab is safe and effective as both a first- and second-line biologic agent in patients with juvenile idiopathic arthritis, according to data presented by Dr. Gerd Horneff.
Dr. Horneff from the Asklepios Klinik, St. Augustin, Germany, and Dr. Heinrike Schmeling from the Alberta Children’s Hospital, Calgary, analyzed data from 329 children aged 4-18 years with juvenile idiopathic arthritis (JIA) in the German Biologics Register, finding that a high proportion of patients showed a significant response to treatment with adalimumab.
"The results of this study indicate that adalimumab is highly effective as a first and also as a second introduced biological agent resulting in substantial improvements in clinical signs and symptoms in children with juvenile idiopathic arthritis," Dr. Schmeling said in an interview prior to the annual European Congress of Rheumatology.
Even patients who were using adalimumab as a second or third biologic gained an additional response to adalimumab on top of their responses to previous biologics. The percentage of patients who met the criteria of 30%/50%/70% improvement at last documentation when adalimumab was the first introduced biologic agent was 65%/61%/46%, compared with 73%/65%/48% when adalimumab was initiated after other biologics, the investigators reported.
Around one-third of patients had rheumatoid factor–negative polyarticular JIA, 22.9% had extended oligoarticular JIA, and 25.2% of patients had a history of uveitis.
The median age of onset of disease was 6.7 years, and median age at treatment initiation was 13.8 years, with median disease duration of 5.1 years. ANA positivity was present in 51% of patients and HLB27 positivity in 19.8%. Most patients had been previously treated with methotrexate (92.4%), approximately one-third had received other disease-modifying antirheumatic drugs (DMARDs), and two-thirds had been treated with biologics, mostly etanercept.
Many were also receiving concomitant treatment with NSAIDs (55.3%), steroids (38%), methotrexate (57.8%), and other DMARDs (13%).
However, Dr. Schmeling emphasized that this study was not an attempt to compare adalimumab to other biologic treatments.
While no malignancies were observed during adalimumab treatment, 96 patients reported 220 adverse events, 14 of which were serious. There were also more autoimmune adverse events among patients treated with adalimumab, but Dr. Schmeling said the numbers were too small to draw any conclusions.
Uveitis developed in 18 children while on treatment, 13 of them had a history of uveitis prior to adalimumab initiation. Treatment was discontinued in 61 patients because of inefficacy (9%), adverse events (5%), remission (3%), patient request (9%), and other unspecified reasons (2%).
Disease register is supported by Abbott, Pfizer (Wyeth Pharma), and Roche, Germany. Dr. Schmeling had no financial disclosures. Dr Horneff declared consultancies, speakers bureau engagement, and research support from Abbott, Pfizer, and Roche.
Adalimumab is safe and effective as both a first- and second-line biologic agent in patients with juvenile idiopathic arthritis, according to data presented by Dr. Gerd Horneff.
Dr. Horneff from the Asklepios Klinik, St. Augustin, Germany, and Dr. Heinrike Schmeling from the Alberta Children’s Hospital, Calgary, analyzed data from 329 children aged 4-18 years with juvenile idiopathic arthritis (JIA) in the German Biologics Register, finding that a high proportion of patients showed a significant response to treatment with adalimumab.
"The results of this study indicate that adalimumab is highly effective as a first and also as a second introduced biological agent resulting in substantial improvements in clinical signs and symptoms in children with juvenile idiopathic arthritis," Dr. Schmeling said in an interview prior to the annual European Congress of Rheumatology.
Even patients who were using adalimumab as a second or third biologic gained an additional response to adalimumab on top of their responses to previous biologics. The percentage of patients who met the criteria of 30%/50%/70% improvement at last documentation when adalimumab was the first introduced biologic agent was 65%/61%/46%, compared with 73%/65%/48% when adalimumab was initiated after other biologics, the investigators reported.
Around one-third of patients had rheumatoid factor–negative polyarticular JIA, 22.9% had extended oligoarticular JIA, and 25.2% of patients had a history of uveitis.
The median age of onset of disease was 6.7 years, and median age at treatment initiation was 13.8 years, with median disease duration of 5.1 years. ANA positivity was present in 51% of patients and HLB27 positivity in 19.8%. Most patients had been previously treated with methotrexate (92.4%), approximately one-third had received other disease-modifying antirheumatic drugs (DMARDs), and two-thirds had been treated with biologics, mostly etanercept.
Many were also receiving concomitant treatment with NSAIDs (55.3%), steroids (38%), methotrexate (57.8%), and other DMARDs (13%).
However, Dr. Schmeling emphasized that this study was not an attempt to compare adalimumab to other biologic treatments.
While no malignancies were observed during adalimumab treatment, 96 patients reported 220 adverse events, 14 of which were serious. There were also more autoimmune adverse events among patients treated with adalimumab, but Dr. Schmeling said the numbers were too small to draw any conclusions.
Uveitis developed in 18 children while on treatment, 13 of them had a history of uveitis prior to adalimumab initiation. Treatment was discontinued in 61 patients because of inefficacy (9%), adverse events (5%), remission (3%), patient request (9%), and other unspecified reasons (2%).
Disease register is supported by Abbott, Pfizer (Wyeth Pharma), and Roche, Germany. Dr. Schmeling had no financial disclosures. Dr Horneff declared consultancies, speakers bureau engagement, and research support from Abbott, Pfizer, and Roche.
FROM THE EULAR CONGRESS 2013
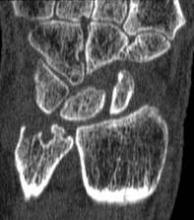
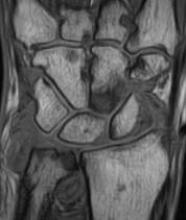
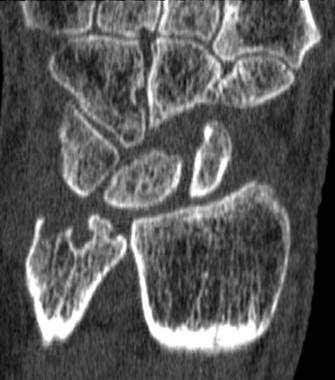
MRI score of joint narrowing has research promise
A magnetic resonance imaging scoring system of joint-space narrowing in rheumatoid arthritis showed "a very high" agreement with computed tomography scores and may become a useful tool in rheumatoid arthritis clinical trials after further validation, judging from data presented by Dr. Uffe Møller Døhn.
In a small study, which was conducted to validate the OMERACT-RAMRIS MRI JSN scoring system in the wrists and metacarpophalangeal (MCP) joints, there was a very high agreement between the joint-space narrowing scores on MRI and CT and moderate agreement between scores on MRI and x-ray, said Dr. Møller Døhn of Copenhagen University Hospital at Glostrup at the annual European Congress of Rheumatology. In addition, there was "high to very high" inter- and intrareader reliability, particularly for the wrist joints.
An OMERACT (Outcome Measures in Rheumatology) initiative, this scoring system is being developed to provide a more precise and sensitive method of measuring joint space damage in patients with rheumatoid arthritis (RA), but it needs to be validated through comparisons to other imaging methods.
To evaluate the degree of agreement with CT and x-ray scores, this study assessed MRI and CT images of the wrist and the second to fifth metacarpophalangeal (MCP 2-5) joints of 14 people with RA and one healthy control, who were from a clinical trial. Three readers assessed the images twice, and a single reader scored x-rays using the Sharp-Van der Heidje method, said Dr. Møller Døhn, who is in the center for rheumatology and spine diseases at the hospital.
The MRI scores of joint space narrowing "were very highly correlated" with CT scores, when comparing the wrist and MCP scores both separately and combined: Using intraclass correlation coefficients (ICCs) as a measure of agreement between scores and scorers, the MRI and CT scores for joint space narrowing were 0.94 for the MCP joints, 0.92 for the wrist, and 0.92 for the wrist and MCP joints combined. But the ICCs for the x-ray joint space narrowing scores were lower: With MRI scores, the ICCs were 0.49 for the MCP 2-5 joints and 0.55 for the wrist. With CT scores, the ICCs were 0.56 for the MCP 2-5 joints and 0.43 for the wrist.
"The most important next step is to test the scoring system in a longitudinal setting, in order to investigate the sensitivity to change," Dr. Møller Døhn said in an interview before the congress. "Before the system can be implemented as an outcome measure in clinical trials, we need to know if it is more sensitive than other methods that are already available. If it turns out that [joint space narrowing] assessment of several joints on x-ray is just as good as - or better than - MRI, then it does not add information to what we already use today."
Dr. Møller Døhn reported that he had no relevant financial disclosures.
A magnetic resonance imaging scoring system of joint-space narrowing in rheumatoid arthritis showed "a very high" agreement with computed tomography scores and may become a useful tool in rheumatoid arthritis clinical trials after further validation, judging from data presented by Dr. Uffe Møller Døhn.
In a small study, which was conducted to validate the OMERACT-RAMRIS MRI JSN scoring system in the wrists and metacarpophalangeal (MCP) joints, there was a very high agreement between the joint-space narrowing scores on MRI and CT and moderate agreement between scores on MRI and x-ray, said Dr. Møller Døhn of Copenhagen University Hospital at Glostrup at the annual European Congress of Rheumatology. In addition, there was "high to very high" inter- and intrareader reliability, particularly for the wrist joints.
An OMERACT (Outcome Measures in Rheumatology) initiative, this scoring system is being developed to provide a more precise and sensitive method of measuring joint space damage in patients with rheumatoid arthritis (RA), but it needs to be validated through comparisons to other imaging methods.
To evaluate the degree of agreement with CT and x-ray scores, this study assessed MRI and CT images of the wrist and the second to fifth metacarpophalangeal (MCP 2-5) joints of 14 people with RA and one healthy control, who were from a clinical trial. Three readers assessed the images twice, and a single reader scored x-rays using the Sharp-Van der Heidje method, said Dr. Møller Døhn, who is in the center for rheumatology and spine diseases at the hospital.
The MRI scores of joint space narrowing "were very highly correlated" with CT scores, when comparing the wrist and MCP scores both separately and combined: Using intraclass correlation coefficients (ICCs) as a measure of agreement between scores and scorers, the MRI and CT scores for joint space narrowing were 0.94 for the MCP joints, 0.92 for the wrist, and 0.92 for the wrist and MCP joints combined. But the ICCs for the x-ray joint space narrowing scores were lower: With MRI scores, the ICCs were 0.49 for the MCP 2-5 joints and 0.55 for the wrist. With CT scores, the ICCs were 0.56 for the MCP 2-5 joints and 0.43 for the wrist.
"The most important next step is to test the scoring system in a longitudinal setting, in order to investigate the sensitivity to change," Dr. Møller Døhn said in an interview before the congress. "Before the system can be implemented as an outcome measure in clinical trials, we need to know if it is more sensitive than other methods that are already available. If it turns out that [joint space narrowing] assessment of several joints on x-ray is just as good as - or better than - MRI, then it does not add information to what we already use today."
Dr. Møller Døhn reported that he had no relevant financial disclosures.
A magnetic resonance imaging scoring system of joint-space narrowing in rheumatoid arthritis showed "a very high" agreement with computed tomography scores and may become a useful tool in rheumatoid arthritis clinical trials after further validation, judging from data presented by Dr. Uffe Møller Døhn.
In a small study, which was conducted to validate the OMERACT-RAMRIS MRI JSN scoring system in the wrists and metacarpophalangeal (MCP) joints, there was a very high agreement between the joint-space narrowing scores on MRI and CT and moderate agreement between scores on MRI and x-ray, said Dr. Møller Døhn of Copenhagen University Hospital at Glostrup at the annual European Congress of Rheumatology. In addition, there was "high to very high" inter- and intrareader reliability, particularly for the wrist joints.
An OMERACT (Outcome Measures in Rheumatology) initiative, this scoring system is being developed to provide a more precise and sensitive method of measuring joint space damage in patients with rheumatoid arthritis (RA), but it needs to be validated through comparisons to other imaging methods.
To evaluate the degree of agreement with CT and x-ray scores, this study assessed MRI and CT images of the wrist and the second to fifth metacarpophalangeal (MCP 2-5) joints of 14 people with RA and one healthy control, who were from a clinical trial. Three readers assessed the images twice, and a single reader scored x-rays using the Sharp-Van der Heidje method, said Dr. Møller Døhn, who is in the center for rheumatology and spine diseases at the hospital.
The MRI scores of joint space narrowing "were very highly correlated" with CT scores, when comparing the wrist and MCP scores both separately and combined: Using intraclass correlation coefficients (ICCs) as a measure of agreement between scores and scorers, the MRI and CT scores for joint space narrowing were 0.94 for the MCP joints, 0.92 for the wrist, and 0.92 for the wrist and MCP joints combined. But the ICCs for the x-ray joint space narrowing scores were lower: With MRI scores, the ICCs were 0.49 for the MCP 2-5 joints and 0.55 for the wrist. With CT scores, the ICCs were 0.56 for the MCP 2-5 joints and 0.43 for the wrist.
"The most important next step is to test the scoring system in a longitudinal setting, in order to investigate the sensitivity to change," Dr. Møller Døhn said in an interview before the congress. "Before the system can be implemented as an outcome measure in clinical trials, we need to know if it is more sensitive than other methods that are already available. If it turns out that [joint space narrowing] assessment of several joints on x-ray is just as good as - or better than - MRI, then it does not add information to what we already use today."
Dr. Møller Døhn reported that he had no relevant financial disclosures.
FROM THE EULAR CONGRESS 2013
Biologics reduced sick leave in RA patients
Biologics and improved strategies for their use have significantly reduced the relatively high rate of sick leave among patients with rheumatoid arthritis, but more efficient, multiprofessional intervention strategies are still needed to reduce its incidence, Mathilda Bjork, Ph.D., reported at the annual European Congress of Rheumatism.
Dr. Bjork, of Jonkoping University, Sweden, conducted a subanalysis of the Swedish Early Rheumatoid Arthritis cohort study (Swedish acronym – TIRA). The study included patients with early rheumatoid arthritis, and was designed to calculate direct and indirect costs of the disease over a 3-year period. There have been two TIRA cohorts – one in 1996-1998 and one in 2005-2008. All had early disease; they were a mean of 62 years at baseline.
At all follow-up visits, the patients met with a multidisciplinary team including a physician, an occupational therapist and a physiotherapist, and were given individual treatment based on their needs.
Those in the more recent cohort were treated more aggressively with disease-modifying antirheumatic drugs (DMARDs), mainly methotrexate, starting at their first visit. They also received biologics when required.
Dr. Bjork’s study examined sick leave rates between the two TIRA cohorts: 1996-1998 and 2005-2008. The comparison found that sick leave rates in the newer cohort declined about 50% compared to those in the older cohort.
In the early cohort, sick leave rates were stable over the 3-year study period. At baseline, 60% of the patients were taking sick leave due to their RA; that number was unchanged at 3 years.
In the newer cohort, the baseline sick leave rate was similar, with 55% taking leave due to their disease. But at the 3-year follow-up, only 30% were on sick leave.
"I think it’s good news," Dr. Bjork said in an interview. Since both groups were making use of the multidisciplinary team treatment, DMARD treatment appeared to be the main driver behind the difference. "They are being used more frequently and in higher doses, and it’s working."
Dr. Bjork said the study came about not only because RA is associated with such high indirect costs for sick leave, but also because of the direct treatment costs of new medications such as biological agents.
"The rationale behind the study was to explore whether more effective disease control reduces sick leave in a postbiologic cohort compared to a prebiologic cohort, with the potential for compensating some of the increased treatment cost."
The researchers suggested that changes in political policies and the sickness insurance system may also have had some impact on the differences in sick leave between the two cohorts.
Despite the significant reductions in sick leave, she suggested that more could be done to address the persistently high rate of sick leave among individuals with RA.
"The impact of rheumatoid arthritis on an individual’s ability to work is a complex interaction of biological, psychological, social, and occupational factors," she said. "The interventions need to have a wider perspective than the rheumatoid arthritis per se and [should be] done in a close interaction between the patient, clinicians, employers, and policy makers early in the disease process."
Dr. Bjork had no conflicts of interest relevant to the study.
Biologics and improved strategies for their use have significantly reduced the relatively high rate of sick leave among patients with rheumatoid arthritis, but more efficient, multiprofessional intervention strategies are still needed to reduce its incidence, Mathilda Bjork, Ph.D., reported at the annual European Congress of Rheumatism.
Dr. Bjork, of Jonkoping University, Sweden, conducted a subanalysis of the Swedish Early Rheumatoid Arthritis cohort study (Swedish acronym – TIRA). The study included patients with early rheumatoid arthritis, and was designed to calculate direct and indirect costs of the disease over a 3-year period. There have been two TIRA cohorts – one in 1996-1998 and one in 2005-2008. All had early disease; they were a mean of 62 years at baseline.
At all follow-up visits, the patients met with a multidisciplinary team including a physician, an occupational therapist and a physiotherapist, and were given individual treatment based on their needs.
Those in the more recent cohort were treated more aggressively with disease-modifying antirheumatic drugs (DMARDs), mainly methotrexate, starting at their first visit. They also received biologics when required.
Dr. Bjork’s study examined sick leave rates between the two TIRA cohorts: 1996-1998 and 2005-2008. The comparison found that sick leave rates in the newer cohort declined about 50% compared to those in the older cohort.
In the early cohort, sick leave rates were stable over the 3-year study period. At baseline, 60% of the patients were taking sick leave due to their RA; that number was unchanged at 3 years.
In the newer cohort, the baseline sick leave rate was similar, with 55% taking leave due to their disease. But at the 3-year follow-up, only 30% were on sick leave.
"I think it’s good news," Dr. Bjork said in an interview. Since both groups were making use of the multidisciplinary team treatment, DMARD treatment appeared to be the main driver behind the difference. "They are being used more frequently and in higher doses, and it’s working."
Dr. Bjork said the study came about not only because RA is associated with such high indirect costs for sick leave, but also because of the direct treatment costs of new medications such as biological agents.
"The rationale behind the study was to explore whether more effective disease control reduces sick leave in a postbiologic cohort compared to a prebiologic cohort, with the potential for compensating some of the increased treatment cost."
The researchers suggested that changes in political policies and the sickness insurance system may also have had some impact on the differences in sick leave between the two cohorts.
Despite the significant reductions in sick leave, she suggested that more could be done to address the persistently high rate of sick leave among individuals with RA.
"The impact of rheumatoid arthritis on an individual’s ability to work is a complex interaction of biological, psychological, social, and occupational factors," she said. "The interventions need to have a wider perspective than the rheumatoid arthritis per se and [should be] done in a close interaction between the patient, clinicians, employers, and policy makers early in the disease process."
Dr. Bjork had no conflicts of interest relevant to the study.
Biologics and improved strategies for their use have significantly reduced the relatively high rate of sick leave among patients with rheumatoid arthritis, but more efficient, multiprofessional intervention strategies are still needed to reduce its incidence, Mathilda Bjork, Ph.D., reported at the annual European Congress of Rheumatism.
Dr. Bjork, of Jonkoping University, Sweden, conducted a subanalysis of the Swedish Early Rheumatoid Arthritis cohort study (Swedish acronym – TIRA). The study included patients with early rheumatoid arthritis, and was designed to calculate direct and indirect costs of the disease over a 3-year period. There have been two TIRA cohorts – one in 1996-1998 and one in 2005-2008. All had early disease; they were a mean of 62 years at baseline.
At all follow-up visits, the patients met with a multidisciplinary team including a physician, an occupational therapist and a physiotherapist, and were given individual treatment based on their needs.
Those in the more recent cohort were treated more aggressively with disease-modifying antirheumatic drugs (DMARDs), mainly methotrexate, starting at their first visit. They also received biologics when required.
Dr. Bjork’s study examined sick leave rates between the two TIRA cohorts: 1996-1998 and 2005-2008. The comparison found that sick leave rates in the newer cohort declined about 50% compared to those in the older cohort.
In the early cohort, sick leave rates were stable over the 3-year study period. At baseline, 60% of the patients were taking sick leave due to their RA; that number was unchanged at 3 years.
In the newer cohort, the baseline sick leave rate was similar, with 55% taking leave due to their disease. But at the 3-year follow-up, only 30% were on sick leave.
"I think it’s good news," Dr. Bjork said in an interview. Since both groups were making use of the multidisciplinary team treatment, DMARD treatment appeared to be the main driver behind the difference. "They are being used more frequently and in higher doses, and it’s working."
Dr. Bjork said the study came about not only because RA is associated with such high indirect costs for sick leave, but also because of the direct treatment costs of new medications such as biological agents.
"The rationale behind the study was to explore whether more effective disease control reduces sick leave in a postbiologic cohort compared to a prebiologic cohort, with the potential for compensating some of the increased treatment cost."
The researchers suggested that changes in political policies and the sickness insurance system may also have had some impact on the differences in sick leave between the two cohorts.
Despite the significant reductions in sick leave, she suggested that more could be done to address the persistently high rate of sick leave among individuals with RA.
"The impact of rheumatoid arthritis on an individual’s ability to work is a complex interaction of biological, psychological, social, and occupational factors," she said. "The interventions need to have a wider perspective than the rheumatoid arthritis per se and [should be] done in a close interaction between the patient, clinicians, employers, and policy makers early in the disease process."
Dr. Bjork had no conflicts of interest relevant to the study.
AT THE EULAR CONGRESS 2013
Ustekinumab benefits in PsA sustained through 1 year
MADRID – The lessening of the signs and symptoms of psoriatic arthritis that occurs during the first 6 months of ustekinumab treatment persisted and improved further at the end of 1 year, with a favorable safety profile, according to 52-week data from the PSUMMIT II trial.
The sustained benefits in American College of Rheumatology (ACR) 20 responses and other efficacy endpoints were evident even in patients who had been treated previously with anti-tumor necrosis factor (anti-TNF) agents and among those who were anti-TNF naïve, although the benefits were greater in the latter group patients, according to Dr. Christopher T. Ritchlin, a professor in the department of medicine, allergy/immunology, and rheumatology at the University of Rochester (N.Y.). This includes beneficial effects on skin and enthesitis, Dr. Ritchlin said at the annual European Congress of Rheumatology.
The PSUMMIT II study is a follow-up to the PSUMMIT I study, the findings of which showed that ustekinumab, a human interleukin (IL)-12 and IL-23 antagonist, showed significant effectiveness in patients with psoriatic arthritis (PsA) who had not been exposed to anti-TNF drugs.
Ustekinumab is currently approved for treating moderate to severe plaque psoriasis in adults who are candidates for phototherapy or systemic therapy in the United States, or those who have failed to respond to, have a contraindication to, or are intolerant of other systemic therapies in Europe. In December 2012, the manufacturer, Janssen, announced that it had filed for further approval for ustekinumab in both the United States and Europe for the treatment of active disease.
The PSUMMIT II study enrolled 312 patients with active PsA who had five or more tender and five or more swollen joints, and a C-reactive protein level of 0.3 mg/dL or higher. Patients who had been treated previously with anti-TNF therapy (n = 180) and those naive to anti-TNF therapy (n = 132) were included and randomized to one of two doses of ustekinumab (45 mg or 90 mg) or placebo administered at week 0, 4, and 12. At 16 weeks, patients with less than a 5% improvement in tender and swollen joint counts on placebo were switched to active treatment, those on 45 mg ustekinumab had their dose upped to 90 mg, and those on 90 mg remained on that dose.
At 6 months, significantly more patients treated with ustekinumab than placebo achieved the primary endpoint of an ACR 20, and more patients on active treatment had an ACR 50, and at least a 75% improvement in the Psoriasis Area and Severity Index (PASI 75).
These results were sustained at 1 year, with 47%-48% of those on 45 mg and 90 mg, and 56% of those who switched from placebo to the 45-mg dose, achieving an ACR 20. In addition, 26%-29% achieved an ACR 50 (29% for those switched from placebo), and 13%-18% achieved an ACR 70 (15% for placebo). There were also improvements associated with treatment in HAQ-DI (Health Assessment Questionnaire-Disability Index) scores at week 52, according to Dr. Ritchlin. The mean change in HAQ-DI scores from baseline to week 52 were -0.21 for placebo, -0.20 for the 45-mg dose of ustekinumab, and -0.28 for the 90-mg dose.
Among those who had not been treated before with an anti-TNF agent, 59%-60% of those on ustekinumab (73% for those switched from placebo) achieved an ACR 20 at week 52, compared with 37%-41% of those who had taken an anti-TNF agent previously before being treated with ustekinumab (30% for placebo). Although responses among anti-TNF naive patients were superior, the responses among those who had been treated with these agents previously were still significantly improved, an indication that ustekinumab "offers an alternative for patients who cannot take or fail anti-TNF agents," Dr. Ritchlin said in the interview.
Treatment was "very effective" for skin symptoms and for enthesitis, he noted. Compared with baseline, dactylitis was improved by a median of 95% among those on the 45-mg dose, 91% among those on the 90-mg dose, and 100% in those who switched from placebo to the 45-mg dose of ustekinumab. Similar improvements in enthesitis were seen, with the highest improvement (60%) seen with the highest dose of ustekinumab. PASI scores at baseline ranged from 11 to 13 and improved by 56%-64% by follow-up at week 52.
In general, ustekinumab was well tolerated, with no deaths or cases of tuberculosis reported and with similar rates of adverse events and serious adverse events between the two doses (just under 6%). There were two malignancies: one breast cancer and one squamous cell carcinoma in two patients taking the 90-mg dose of ustekinumab, who had both been treated with anti-TNFs previously. The rate of serious infections was less than 1% among those treated with ustekinumab. Through 60 weeks of treatment, there were three major adverse cardiovascular events, all myocardial infarctions, in patients treated with ustekinumab. These patients all had multiple cardiovascular risk factors, Dr. Ritchlin said. They had also been exposed previously to anti-TNF treatment.
Radiographic data from the trial are expected and likely to be available by the end of the year for presentation at the annual American College of Rheumatology meeting.
Dr. Ritchlin disclosed having received grant and research support from Janssen. Four of the nine remaining authors are Janssen employees and shareholders of Johnson & Johnson, Janssen’s parent company. Ustekinumab is marketed as Stelara in the United States.
Sara Freeman contributed to this report.
MADRID – The lessening of the signs and symptoms of psoriatic arthritis that occurs during the first 6 months of ustekinumab treatment persisted and improved further at the end of 1 year, with a favorable safety profile, according to 52-week data from the PSUMMIT II trial.
The sustained benefits in American College of Rheumatology (ACR) 20 responses and other efficacy endpoints were evident even in patients who had been treated previously with anti-tumor necrosis factor (anti-TNF) agents and among those who were anti-TNF naïve, although the benefits were greater in the latter group patients, according to Dr. Christopher T. Ritchlin, a professor in the department of medicine, allergy/immunology, and rheumatology at the University of Rochester (N.Y.). This includes beneficial effects on skin and enthesitis, Dr. Ritchlin said at the annual European Congress of Rheumatology.
The PSUMMIT II study is a follow-up to the PSUMMIT I study, the findings of which showed that ustekinumab, a human interleukin (IL)-12 and IL-23 antagonist, showed significant effectiveness in patients with psoriatic arthritis (PsA) who had not been exposed to anti-TNF drugs.
Ustekinumab is currently approved for treating moderate to severe plaque psoriasis in adults who are candidates for phototherapy or systemic therapy in the United States, or those who have failed to respond to, have a contraindication to, or are intolerant of other systemic therapies in Europe. In December 2012, the manufacturer, Janssen, announced that it had filed for further approval for ustekinumab in both the United States and Europe for the treatment of active disease.
The PSUMMIT II study enrolled 312 patients with active PsA who had five or more tender and five or more swollen joints, and a C-reactive protein level of 0.3 mg/dL or higher. Patients who had been treated previously with anti-TNF therapy (n = 180) and those naive to anti-TNF therapy (n = 132) were included and randomized to one of two doses of ustekinumab (45 mg or 90 mg) or placebo administered at week 0, 4, and 12. At 16 weeks, patients with less than a 5% improvement in tender and swollen joint counts on placebo were switched to active treatment, those on 45 mg ustekinumab had their dose upped to 90 mg, and those on 90 mg remained on that dose.
At 6 months, significantly more patients treated with ustekinumab than placebo achieved the primary endpoint of an ACR 20, and more patients on active treatment had an ACR 50, and at least a 75% improvement in the Psoriasis Area and Severity Index (PASI 75).
These results were sustained at 1 year, with 47%-48% of those on 45 mg and 90 mg, and 56% of those who switched from placebo to the 45-mg dose, achieving an ACR 20. In addition, 26%-29% achieved an ACR 50 (29% for those switched from placebo), and 13%-18% achieved an ACR 70 (15% for placebo). There were also improvements associated with treatment in HAQ-DI (Health Assessment Questionnaire-Disability Index) scores at week 52, according to Dr. Ritchlin. The mean change in HAQ-DI scores from baseline to week 52 were -0.21 for placebo, -0.20 for the 45-mg dose of ustekinumab, and -0.28 for the 90-mg dose.
Among those who had not been treated before with an anti-TNF agent, 59%-60% of those on ustekinumab (73% for those switched from placebo) achieved an ACR 20 at week 52, compared with 37%-41% of those who had taken an anti-TNF agent previously before being treated with ustekinumab (30% for placebo). Although responses among anti-TNF naive patients were superior, the responses among those who had been treated with these agents previously were still significantly improved, an indication that ustekinumab "offers an alternative for patients who cannot take or fail anti-TNF agents," Dr. Ritchlin said in the interview.
Treatment was "very effective" for skin symptoms and for enthesitis, he noted. Compared with baseline, dactylitis was improved by a median of 95% among those on the 45-mg dose, 91% among those on the 90-mg dose, and 100% in those who switched from placebo to the 45-mg dose of ustekinumab. Similar improvements in enthesitis were seen, with the highest improvement (60%) seen with the highest dose of ustekinumab. PASI scores at baseline ranged from 11 to 13 and improved by 56%-64% by follow-up at week 52.
In general, ustekinumab was well tolerated, with no deaths or cases of tuberculosis reported and with similar rates of adverse events and serious adverse events between the two doses (just under 6%). There were two malignancies: one breast cancer and one squamous cell carcinoma in two patients taking the 90-mg dose of ustekinumab, who had both been treated with anti-TNFs previously. The rate of serious infections was less than 1% among those treated with ustekinumab. Through 60 weeks of treatment, there were three major adverse cardiovascular events, all myocardial infarctions, in patients treated with ustekinumab. These patients all had multiple cardiovascular risk factors, Dr. Ritchlin said. They had also been exposed previously to anti-TNF treatment.
Radiographic data from the trial are expected and likely to be available by the end of the year for presentation at the annual American College of Rheumatology meeting.
Dr. Ritchlin disclosed having received grant and research support from Janssen. Four of the nine remaining authors are Janssen employees and shareholders of Johnson & Johnson, Janssen’s parent company. Ustekinumab is marketed as Stelara in the United States.
Sara Freeman contributed to this report.
MADRID – The lessening of the signs and symptoms of psoriatic arthritis that occurs during the first 6 months of ustekinumab treatment persisted and improved further at the end of 1 year, with a favorable safety profile, according to 52-week data from the PSUMMIT II trial.
The sustained benefits in American College of Rheumatology (ACR) 20 responses and other efficacy endpoints were evident even in patients who had been treated previously with anti-tumor necrosis factor (anti-TNF) agents and among those who were anti-TNF naïve, although the benefits were greater in the latter group patients, according to Dr. Christopher T. Ritchlin, a professor in the department of medicine, allergy/immunology, and rheumatology at the University of Rochester (N.Y.). This includes beneficial effects on skin and enthesitis, Dr. Ritchlin said at the annual European Congress of Rheumatology.
The PSUMMIT II study is a follow-up to the PSUMMIT I study, the findings of which showed that ustekinumab, a human interleukin (IL)-12 and IL-23 antagonist, showed significant effectiveness in patients with psoriatic arthritis (PsA) who had not been exposed to anti-TNF drugs.
Ustekinumab is currently approved for treating moderate to severe plaque psoriasis in adults who are candidates for phototherapy or systemic therapy in the United States, or those who have failed to respond to, have a contraindication to, or are intolerant of other systemic therapies in Europe. In December 2012, the manufacturer, Janssen, announced that it had filed for further approval for ustekinumab in both the United States and Europe for the treatment of active disease.
The PSUMMIT II study enrolled 312 patients with active PsA who had five or more tender and five or more swollen joints, and a C-reactive protein level of 0.3 mg/dL or higher. Patients who had been treated previously with anti-TNF therapy (n = 180) and those naive to anti-TNF therapy (n = 132) were included and randomized to one of two doses of ustekinumab (45 mg or 90 mg) or placebo administered at week 0, 4, and 12. At 16 weeks, patients with less than a 5% improvement in tender and swollen joint counts on placebo were switched to active treatment, those on 45 mg ustekinumab had their dose upped to 90 mg, and those on 90 mg remained on that dose.
At 6 months, significantly more patients treated with ustekinumab than placebo achieved the primary endpoint of an ACR 20, and more patients on active treatment had an ACR 50, and at least a 75% improvement in the Psoriasis Area and Severity Index (PASI 75).
These results were sustained at 1 year, with 47%-48% of those on 45 mg and 90 mg, and 56% of those who switched from placebo to the 45-mg dose, achieving an ACR 20. In addition, 26%-29% achieved an ACR 50 (29% for those switched from placebo), and 13%-18% achieved an ACR 70 (15% for placebo). There were also improvements associated with treatment in HAQ-DI (Health Assessment Questionnaire-Disability Index) scores at week 52, according to Dr. Ritchlin. The mean change in HAQ-DI scores from baseline to week 52 were -0.21 for placebo, -0.20 for the 45-mg dose of ustekinumab, and -0.28 for the 90-mg dose.
Among those who had not been treated before with an anti-TNF agent, 59%-60% of those on ustekinumab (73% for those switched from placebo) achieved an ACR 20 at week 52, compared with 37%-41% of those who had taken an anti-TNF agent previously before being treated with ustekinumab (30% for placebo). Although responses among anti-TNF naive patients were superior, the responses among those who had been treated with these agents previously were still significantly improved, an indication that ustekinumab "offers an alternative for patients who cannot take or fail anti-TNF agents," Dr. Ritchlin said in the interview.
Treatment was "very effective" for skin symptoms and for enthesitis, he noted. Compared with baseline, dactylitis was improved by a median of 95% among those on the 45-mg dose, 91% among those on the 90-mg dose, and 100% in those who switched from placebo to the 45-mg dose of ustekinumab. Similar improvements in enthesitis were seen, with the highest improvement (60%) seen with the highest dose of ustekinumab. PASI scores at baseline ranged from 11 to 13 and improved by 56%-64% by follow-up at week 52.
In general, ustekinumab was well tolerated, with no deaths or cases of tuberculosis reported and with similar rates of adverse events and serious adverse events between the two doses (just under 6%). There were two malignancies: one breast cancer and one squamous cell carcinoma in two patients taking the 90-mg dose of ustekinumab, who had both been treated with anti-TNFs previously. The rate of serious infections was less than 1% among those treated with ustekinumab. Through 60 weeks of treatment, there were three major adverse cardiovascular events, all myocardial infarctions, in patients treated with ustekinumab. These patients all had multiple cardiovascular risk factors, Dr. Ritchlin said. They had also been exposed previously to anti-TNF treatment.
Radiographic data from the trial are expected and likely to be available by the end of the year for presentation at the annual American College of Rheumatology meeting.
Dr. Ritchlin disclosed having received grant and research support from Janssen. Four of the nine remaining authors are Janssen employees and shareholders of Johnson & Johnson, Janssen’s parent company. Ustekinumab is marketed as Stelara in the United States.
Sara Freeman contributed to this report.
AT THE EULAR CONGRESS 2013