User login
Propanolol Guidelines for Infant Hemangiomas Focus on Hospitalization
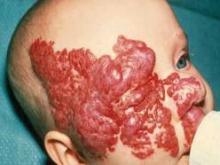
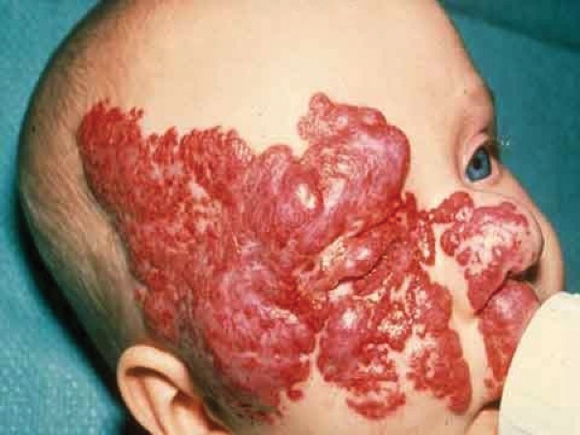
Inpatient hospitalization is recommended for infants with hemangiomas who are undergoing treatment with propanolol if they are younger than 8 weeks of gestational age, have inadequate social support, or have a comorbid condition affecting the cardiovascular or respiratory system, according to new consensus guidelines on the use of propranolol.
Outpatient initiation with monitoring can be considered for infants older than 8 weeks of age who have adequate social support and who do not have significant comorbidities, noted Dr. Ilona J. Frieden, who presented an overview of the soon-to-be published guidelines at the Women’s and Pediatric Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF).
Dr. Frieden was part of a multispecialty consensus group of experts who met in December 2011 to draft the recommendations on when to admit patients for hospitalization and which patients should be considered for outpatient therapy.
The guidelines also emphasize that all patients who receive propranolol should be monitored with heart rate and blood pressure measurements at baseline and at 1 and 2 hours after receiving the initial dose.
"Clinicians should repeat these measurements with dose increases greater than 0.5 mg/kg per day, including at least one set of measurements after the target dose has been achieved," said Dr. Frieden, professor of dermatology and pediatrics at the University of California, San Francisco. "If heart rate and blood pressure are abnormal, the child should be monitored until the vital signs normalize."
According to Dr. Frieden, the guidelines do not recommend routine glucose testing. "The risk of hypoglycemia is age dependent and begins after 8 hours of fasting in children 0-2 years of age," she said. The guidelines recommend that infants younger than 6 weeks of age be fed every 4 hours, those 6 weeks to 4 months of age every 5 hours, and those older than 4 months every 6-8 hours. They also state that propranolol should be discontinued during intercurrent illness, especially in the setting of restricted oral intake.
"These recommendations are based on current knowledge and should be considered subject to revision," Dr. Frieden said.
The efficacy of propranolol as a treatment for infantile hemangiomas is well documented, but its mechanism of action is not yet fully understood. "We don’t know why it works," she said.
Current hypotheses suggest that propranolol may decrease renin production, inhibit angiogenesis via vascular endothelial growth factor, or induce of apoptosis in microvascular endothelial cells.
A recent systematic review of 41 studies involving 1,264 patients who received beta blockers for infantile hemangiomas found that 28% of patients received prior medical therapy, most commonly oral prednisone. The review, which was reported during a poster session at the 2012 meeting of the Society for Pediatric Dermatology, found that propranolol was initiated at a mean age of 6.6 months with a mean dose of 2.1 mg/kg per day for a mean duration of 6.4 months.
The mean response rate was 98%, with a range between 82% and 100%. "Response measurements varied, but response rates were comparable irrespective of the anatomic site where the hemangioma was located," Dr. Frieden said.
Rebound growth occurred in 17% of cases and the adverse event rate ranged from 3% to 5%, most commonly sleep changes and acrocyanosis. Serious adverse events were rare.
Dr. Frieden disclosed that she has consulted for Pierre Fabre, which is developing propranolol as a treatment for infantile hemangiomas. SDEF and this news organization are owned by Frontline Medical Communications.
Inpatient hospitalization is recommended for infants with hemangiomas who are undergoing treatment with propanolol if they are younger than 8 weeks of gestational age, have inadequate social support, or have a comorbid condition affecting the cardiovascular or respiratory system, according to new consensus guidelines on the use of propranolol.
Outpatient initiation with monitoring can be considered for infants older than 8 weeks of age who have adequate social support and who do not have significant comorbidities, noted Dr. Ilona J. Frieden, who presented an overview of the soon-to-be published guidelines at the Women’s and Pediatric Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF).
Dr. Frieden was part of a multispecialty consensus group of experts who met in December 2011 to draft the recommendations on when to admit patients for hospitalization and which patients should be considered for outpatient therapy.
The guidelines also emphasize that all patients who receive propranolol should be monitored with heart rate and blood pressure measurements at baseline and at 1 and 2 hours after receiving the initial dose.
"Clinicians should repeat these measurements with dose increases greater than 0.5 mg/kg per day, including at least one set of measurements after the target dose has been achieved," said Dr. Frieden, professor of dermatology and pediatrics at the University of California, San Francisco. "If heart rate and blood pressure are abnormal, the child should be monitored until the vital signs normalize."
According to Dr. Frieden, the guidelines do not recommend routine glucose testing. "The risk of hypoglycemia is age dependent and begins after 8 hours of fasting in children 0-2 years of age," she said. The guidelines recommend that infants younger than 6 weeks of age be fed every 4 hours, those 6 weeks to 4 months of age every 5 hours, and those older than 4 months every 6-8 hours. They also state that propranolol should be discontinued during intercurrent illness, especially in the setting of restricted oral intake.
"These recommendations are based on current knowledge and should be considered subject to revision," Dr. Frieden said.
The efficacy of propranolol as a treatment for infantile hemangiomas is well documented, but its mechanism of action is not yet fully understood. "We don’t know why it works," she said.
Current hypotheses suggest that propranolol may decrease renin production, inhibit angiogenesis via vascular endothelial growth factor, or induce of apoptosis in microvascular endothelial cells.
A recent systematic review of 41 studies involving 1,264 patients who received beta blockers for infantile hemangiomas found that 28% of patients received prior medical therapy, most commonly oral prednisone. The review, which was reported during a poster session at the 2012 meeting of the Society for Pediatric Dermatology, found that propranolol was initiated at a mean age of 6.6 months with a mean dose of 2.1 mg/kg per day for a mean duration of 6.4 months.
The mean response rate was 98%, with a range between 82% and 100%. "Response measurements varied, but response rates were comparable irrespective of the anatomic site where the hemangioma was located," Dr. Frieden said.
Rebound growth occurred in 17% of cases and the adverse event rate ranged from 3% to 5%, most commonly sleep changes and acrocyanosis. Serious adverse events were rare.
Dr. Frieden disclosed that she has consulted for Pierre Fabre, which is developing propranolol as a treatment for infantile hemangiomas. SDEF and this news organization are owned by Frontline Medical Communications.
Inpatient hospitalization is recommended for infants with hemangiomas who are undergoing treatment with propanolol if they are younger than 8 weeks of gestational age, have inadequate social support, or have a comorbid condition affecting the cardiovascular or respiratory system, according to new consensus guidelines on the use of propranolol.
Outpatient initiation with monitoring can be considered for infants older than 8 weeks of age who have adequate social support and who do not have significant comorbidities, noted Dr. Ilona J. Frieden, who presented an overview of the soon-to-be published guidelines at the Women’s and Pediatric Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF).
Dr. Frieden was part of a multispecialty consensus group of experts who met in December 2011 to draft the recommendations on when to admit patients for hospitalization and which patients should be considered for outpatient therapy.
The guidelines also emphasize that all patients who receive propranolol should be monitored with heart rate and blood pressure measurements at baseline and at 1 and 2 hours after receiving the initial dose.
"Clinicians should repeat these measurements with dose increases greater than 0.5 mg/kg per day, including at least one set of measurements after the target dose has been achieved," said Dr. Frieden, professor of dermatology and pediatrics at the University of California, San Francisco. "If heart rate and blood pressure are abnormal, the child should be monitored until the vital signs normalize."
According to Dr. Frieden, the guidelines do not recommend routine glucose testing. "The risk of hypoglycemia is age dependent and begins after 8 hours of fasting in children 0-2 years of age," she said. The guidelines recommend that infants younger than 6 weeks of age be fed every 4 hours, those 6 weeks to 4 months of age every 5 hours, and those older than 4 months every 6-8 hours. They also state that propranolol should be discontinued during intercurrent illness, especially in the setting of restricted oral intake.
"These recommendations are based on current knowledge and should be considered subject to revision," Dr. Frieden said.
The efficacy of propranolol as a treatment for infantile hemangiomas is well documented, but its mechanism of action is not yet fully understood. "We don’t know why it works," she said.
Current hypotheses suggest that propranolol may decrease renin production, inhibit angiogenesis via vascular endothelial growth factor, or induce of apoptosis in microvascular endothelial cells.
A recent systematic review of 41 studies involving 1,264 patients who received beta blockers for infantile hemangiomas found that 28% of patients received prior medical therapy, most commonly oral prednisone. The review, which was reported during a poster session at the 2012 meeting of the Society for Pediatric Dermatology, found that propranolol was initiated at a mean age of 6.6 months with a mean dose of 2.1 mg/kg per day for a mean duration of 6.4 months.
The mean response rate was 98%, with a range between 82% and 100%. "Response measurements varied, but response rates were comparable irrespective of the anatomic site where the hemangioma was located," Dr. Frieden said.
Rebound growth occurred in 17% of cases and the adverse event rate ranged from 3% to 5%, most commonly sleep changes and acrocyanosis. Serious adverse events were rare.
Dr. Frieden disclosed that she has consulted for Pierre Fabre, which is developing propranolol as a treatment for infantile hemangiomas. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE SDEF WOMEN'S & PEDIATRIC DERMATOLOGY SEMINAR
Even Simple Exanthems Warrant Thorough Evaluation
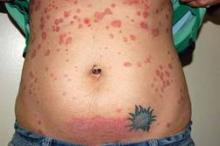
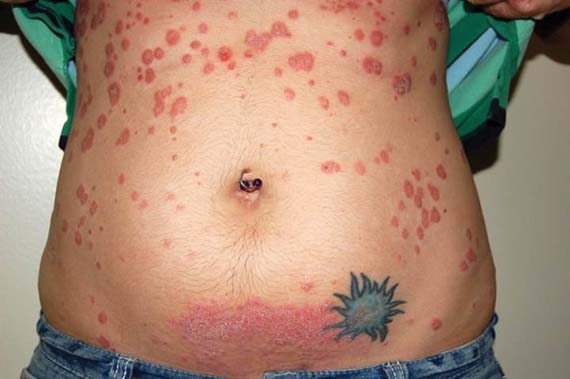
Exanthems in children are often benign and self-limited, but not always.
In some cases – particularly those involving purpura, blisters, mucosal involvement, a high fever, or extracutaneous organ involvement – it is important to thoroughly evaluate the child for more serious disease, Dr. Anthony J. Mancini said at the seminar, sponsored by the Skin Disease Education Foundation (SDEF).
Dr. Mancini, head of the division of dermatology at the Ann and Robert H. Lurie Children’s Hospital of Chicago and professor of pediatrics and dermatology at Northwestern University, Chicago, provided tips for assessing the child presenting with an exanthem:
• If purpura is present, consider parvovirus, enterovirus, rickettsia, Neisseria meningitidis, Henoch-Schönlein purpura, and even group A strep.
• If edema is present, consider Kawasaki disease, serum sickness-like reactions (most often distal edema), or drug hypersensitivity syndrome (notable for facial edema, especially periorbitally).
• If an associated enanthem is present, consider Kawasaki disease, drug hypersensitivity, measles, rubella, enterovirus, parvovirus, adenovirus, group A strep, and Epstein-Barr virus (EBV).
• If conjunctivitis is present, consider Kawasaki disease, drug hypersensitivity, measles, and adenovirus (the most common mimicker of Kawasaki disease in children).
Parvovirus B19 Infection
Among the exanthems that Dr. Mancini discussed were those related to parvovirus B19 infection, including erythema infectiosum (fifth disease) and papular-purpuric gloves and socks syndrome. The latter entity is notable for symmetric swelling of the hands and feet, with palmoplantar purpuric erythema and a sharp demarcation at the wrists and ankles.
Children with gloves and socks syndrome may also present with enanthem of the soft and hard palate, and importantly, children with this exanthem may still be viremic (and hence contagious), compared with those who have classic fifth disease, he said.
Parvovirus B19 can also be associated with a bathing trunk eruption with petechiae, notable for accentuation in the flexures and a prominent petechial component. The infection was found to be the culprit in 13 of 17 children who presented with generalized petechial exanthems in a study based in Wisconsin, Dr. Mancini said. These exanthems also revealed accentuation in the fold areas, as well as the acral extremities. Fever was present in 85% of patients, and many had a history of mild upper respiratory symptoms. Additionally, most of the children (83%) had leukopenia (Pediatrics 2010;125:e787).
Breakthrough Varicella
Breakthrough varicella still occurs in a significant proportion of varicella vaccinees, especially in children from countries with a one-dose vaccination schedule, such as Taiwan.
This association is important to recognize, given that many practicing clinicians may have never seen acute varicella. The "dewdrop on a rose petal" presentation is characteristic, but lesions may also develop severe crusting or ulceration when secondary bacterial infection is present. Acute varicella may also accentuate in sites of trauma or sunburn, and has been termed "occult varicella," he noted.
Herpes zoster, caused by reactivation of latent varicella zoster virus (VZV) in the dorsal sensory or cranial nerve ganglia, is not unusual in healthy children and may be caused by either the wild type or vaccine strain of VZV, he said.
The greatest risk factor for having herpes zoster during childhood is a history of acute varicella under 1 year of age. Fortunately, postherpetic neuralgia following herpes zoster is rare in children.
Hand-Foot-and-Mouth Disease
In a recent nationwide epidemic of severe hand-foot-and-mouth disease (HFMD), patients presented in clusters with fevers and a more widespread HFMD eruption than is characteristic of the self-limited disease, noted Dr. Mancini. Hospitalization was common, and blistering was prominent, with frequent perioral involvement and lesions on the arms and legs in nearly half of patients.
Of note, post-HFMD nail matrix arrest, first recognized by Dr. Mancini and his colleague (Pediatr. Dermatol. 2000;17:7-11), presents on average 40 days after exanthematous illness. Deep transverse ridges with nail shedding occur, more often affecting fingers than toes, and the process is self-limited with eventual spontaneous nail regrowth.
Gianotti-Crosti Syndrome
This syndrome was classically described in association with acute hepatitis B infection, although it is becoming exceedingly uncommon in the United States, where EBV appears to be the most common cause. Patients present with upper respiratory infection prodrome and monomorphous edematous papules on the cheeks, extensor extremities, and buttocks.
While the patient should be assessed for gastrointestinal symptoms, hepatosplenomegaly, lymphadenopathy, and hepatitis risk factors, blind hepatitis blood evaluations are not warranted in this country, Dr. Mancini said. The condition typically resolves in 3-12 weeks with supportive therapy.
Unilateral Laterothoracic Exanthem
Another atypical, parainfectious exanthem, unilateral laterothoracic exanthem, also known as "asymmetric periflexural exanthem," usually presents in children 1-5 years of age. It is initially unilateral in distribution, most often starting in the axilla or on the trunk or flank. At this stage, it may be misdiagnosed as contact dermatitis. The process eventually becomes more generalized, although it tends to maintain a unilateral predominance. Sixty percent of children have associated pruritus, Dr. Mancini said.
A prodrome occurs in 60%-75% of patients, and may include rhinitis, pharyngitis, and gastrointestinal complaints. Fever occurs in roughly 50% of patients, and while the etiology is unknown, it is believed to be a viral-associated exanthem. unilateral laterothoracic exanthem resolves over a 4- to 8-week period with supportive therapy.
Dr. Mancini reported having no relevant financial disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
Exanthems in children are often benign and self-limited, but not always.
In some cases – particularly those involving purpura, blisters, mucosal involvement, a high fever, or extracutaneous organ involvement – it is important to thoroughly evaluate the child for more serious disease, Dr. Anthony J. Mancini said at the seminar, sponsored by the Skin Disease Education Foundation (SDEF).
Dr. Mancini, head of the division of dermatology at the Ann and Robert H. Lurie Children’s Hospital of Chicago and professor of pediatrics and dermatology at Northwestern University, Chicago, provided tips for assessing the child presenting with an exanthem:
• If purpura is present, consider parvovirus, enterovirus, rickettsia, Neisseria meningitidis, Henoch-Schönlein purpura, and even group A strep.
• If edema is present, consider Kawasaki disease, serum sickness-like reactions (most often distal edema), or drug hypersensitivity syndrome (notable for facial edema, especially periorbitally).
• If an associated enanthem is present, consider Kawasaki disease, drug hypersensitivity, measles, rubella, enterovirus, parvovirus, adenovirus, group A strep, and Epstein-Barr virus (EBV).
• If conjunctivitis is present, consider Kawasaki disease, drug hypersensitivity, measles, and adenovirus (the most common mimicker of Kawasaki disease in children).
Parvovirus B19 Infection
Among the exanthems that Dr. Mancini discussed were those related to parvovirus B19 infection, including erythema infectiosum (fifth disease) and papular-purpuric gloves and socks syndrome. The latter entity is notable for symmetric swelling of the hands and feet, with palmoplantar purpuric erythema and a sharp demarcation at the wrists and ankles.
Children with gloves and socks syndrome may also present with enanthem of the soft and hard palate, and importantly, children with this exanthem may still be viremic (and hence contagious), compared with those who have classic fifth disease, he said.
Parvovirus B19 can also be associated with a bathing trunk eruption with petechiae, notable for accentuation in the flexures and a prominent petechial component. The infection was found to be the culprit in 13 of 17 children who presented with generalized petechial exanthems in a study based in Wisconsin, Dr. Mancini said. These exanthems also revealed accentuation in the fold areas, as well as the acral extremities. Fever was present in 85% of patients, and many had a history of mild upper respiratory symptoms. Additionally, most of the children (83%) had leukopenia (Pediatrics 2010;125:e787).
Breakthrough Varicella
Breakthrough varicella still occurs in a significant proportion of varicella vaccinees, especially in children from countries with a one-dose vaccination schedule, such as Taiwan.
This association is important to recognize, given that many practicing clinicians may have never seen acute varicella. The "dewdrop on a rose petal" presentation is characteristic, but lesions may also develop severe crusting or ulceration when secondary bacterial infection is present. Acute varicella may also accentuate in sites of trauma or sunburn, and has been termed "occult varicella," he noted.
Herpes zoster, caused by reactivation of latent varicella zoster virus (VZV) in the dorsal sensory or cranial nerve ganglia, is not unusual in healthy children and may be caused by either the wild type or vaccine strain of VZV, he said.
The greatest risk factor for having herpes zoster during childhood is a history of acute varicella under 1 year of age. Fortunately, postherpetic neuralgia following herpes zoster is rare in children.
Hand-Foot-and-Mouth Disease
In a recent nationwide epidemic of severe hand-foot-and-mouth disease (HFMD), patients presented in clusters with fevers and a more widespread HFMD eruption than is characteristic of the self-limited disease, noted Dr. Mancini. Hospitalization was common, and blistering was prominent, with frequent perioral involvement and lesions on the arms and legs in nearly half of patients.
Of note, post-HFMD nail matrix arrest, first recognized by Dr. Mancini and his colleague (Pediatr. Dermatol. 2000;17:7-11), presents on average 40 days after exanthematous illness. Deep transverse ridges with nail shedding occur, more often affecting fingers than toes, and the process is self-limited with eventual spontaneous nail regrowth.
Gianotti-Crosti Syndrome
This syndrome was classically described in association with acute hepatitis B infection, although it is becoming exceedingly uncommon in the United States, where EBV appears to be the most common cause. Patients present with upper respiratory infection prodrome and monomorphous edematous papules on the cheeks, extensor extremities, and buttocks.
While the patient should be assessed for gastrointestinal symptoms, hepatosplenomegaly, lymphadenopathy, and hepatitis risk factors, blind hepatitis blood evaluations are not warranted in this country, Dr. Mancini said. The condition typically resolves in 3-12 weeks with supportive therapy.
Unilateral Laterothoracic Exanthem
Another atypical, parainfectious exanthem, unilateral laterothoracic exanthem, also known as "asymmetric periflexural exanthem," usually presents in children 1-5 years of age. It is initially unilateral in distribution, most often starting in the axilla or on the trunk or flank. At this stage, it may be misdiagnosed as contact dermatitis. The process eventually becomes more generalized, although it tends to maintain a unilateral predominance. Sixty percent of children have associated pruritus, Dr. Mancini said.
A prodrome occurs in 60%-75% of patients, and may include rhinitis, pharyngitis, and gastrointestinal complaints. Fever occurs in roughly 50% of patients, and while the etiology is unknown, it is believed to be a viral-associated exanthem. unilateral laterothoracic exanthem resolves over a 4- to 8-week period with supportive therapy.
Dr. Mancini reported having no relevant financial disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
Exanthems in children are often benign and self-limited, but not always.
In some cases – particularly those involving purpura, blisters, mucosal involvement, a high fever, or extracutaneous organ involvement – it is important to thoroughly evaluate the child for more serious disease, Dr. Anthony J. Mancini said at the seminar, sponsored by the Skin Disease Education Foundation (SDEF).
Dr. Mancini, head of the division of dermatology at the Ann and Robert H. Lurie Children’s Hospital of Chicago and professor of pediatrics and dermatology at Northwestern University, Chicago, provided tips for assessing the child presenting with an exanthem:
• If purpura is present, consider parvovirus, enterovirus, rickettsia, Neisseria meningitidis, Henoch-Schönlein purpura, and even group A strep.
• If edema is present, consider Kawasaki disease, serum sickness-like reactions (most often distal edema), or drug hypersensitivity syndrome (notable for facial edema, especially periorbitally).
• If an associated enanthem is present, consider Kawasaki disease, drug hypersensitivity, measles, rubella, enterovirus, parvovirus, adenovirus, group A strep, and Epstein-Barr virus (EBV).
• If conjunctivitis is present, consider Kawasaki disease, drug hypersensitivity, measles, and adenovirus (the most common mimicker of Kawasaki disease in children).
Parvovirus B19 Infection
Among the exanthems that Dr. Mancini discussed were those related to parvovirus B19 infection, including erythema infectiosum (fifth disease) and papular-purpuric gloves and socks syndrome. The latter entity is notable for symmetric swelling of the hands and feet, with palmoplantar purpuric erythema and a sharp demarcation at the wrists and ankles.
Children with gloves and socks syndrome may also present with enanthem of the soft and hard palate, and importantly, children with this exanthem may still be viremic (and hence contagious), compared with those who have classic fifth disease, he said.
Parvovirus B19 can also be associated with a bathing trunk eruption with petechiae, notable for accentuation in the flexures and a prominent petechial component. The infection was found to be the culprit in 13 of 17 children who presented with generalized petechial exanthems in a study based in Wisconsin, Dr. Mancini said. These exanthems also revealed accentuation in the fold areas, as well as the acral extremities. Fever was present in 85% of patients, and many had a history of mild upper respiratory symptoms. Additionally, most of the children (83%) had leukopenia (Pediatrics 2010;125:e787).
Breakthrough Varicella
Breakthrough varicella still occurs in a significant proportion of varicella vaccinees, especially in children from countries with a one-dose vaccination schedule, such as Taiwan.
This association is important to recognize, given that many practicing clinicians may have never seen acute varicella. The "dewdrop on a rose petal" presentation is characteristic, but lesions may also develop severe crusting or ulceration when secondary bacterial infection is present. Acute varicella may also accentuate in sites of trauma or sunburn, and has been termed "occult varicella," he noted.
Herpes zoster, caused by reactivation of latent varicella zoster virus (VZV) in the dorsal sensory or cranial nerve ganglia, is not unusual in healthy children and may be caused by either the wild type or vaccine strain of VZV, he said.
The greatest risk factor for having herpes zoster during childhood is a history of acute varicella under 1 year of age. Fortunately, postherpetic neuralgia following herpes zoster is rare in children.
Hand-Foot-and-Mouth Disease
In a recent nationwide epidemic of severe hand-foot-and-mouth disease (HFMD), patients presented in clusters with fevers and a more widespread HFMD eruption than is characteristic of the self-limited disease, noted Dr. Mancini. Hospitalization was common, and blistering was prominent, with frequent perioral involvement and lesions on the arms and legs in nearly half of patients.
Of note, post-HFMD nail matrix arrest, first recognized by Dr. Mancini and his colleague (Pediatr. Dermatol. 2000;17:7-11), presents on average 40 days after exanthematous illness. Deep transverse ridges with nail shedding occur, more often affecting fingers than toes, and the process is self-limited with eventual spontaneous nail regrowth.
Gianotti-Crosti Syndrome
This syndrome was classically described in association with acute hepatitis B infection, although it is becoming exceedingly uncommon in the United States, where EBV appears to be the most common cause. Patients present with upper respiratory infection prodrome and monomorphous edematous papules on the cheeks, extensor extremities, and buttocks.
While the patient should be assessed for gastrointestinal symptoms, hepatosplenomegaly, lymphadenopathy, and hepatitis risk factors, blind hepatitis blood evaluations are not warranted in this country, Dr. Mancini said. The condition typically resolves in 3-12 weeks with supportive therapy.
Unilateral Laterothoracic Exanthem
Another atypical, parainfectious exanthem, unilateral laterothoracic exanthem, also known as "asymmetric periflexural exanthem," usually presents in children 1-5 years of age. It is initially unilateral in distribution, most often starting in the axilla or on the trunk or flank. At this stage, it may be misdiagnosed as contact dermatitis. The process eventually becomes more generalized, although it tends to maintain a unilateral predominance. Sixty percent of children have associated pruritus, Dr. Mancini said.
A prodrome occurs in 60%-75% of patients, and may include rhinitis, pharyngitis, and gastrointestinal complaints. Fever occurs in roughly 50% of patients, and while the etiology is unknown, it is believed to be a viral-associated exanthem. unilateral laterothoracic exanthem resolves over a 4- to 8-week period with supportive therapy.
Dr. Mancini reported having no relevant financial disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE SDEF WOMEN'S AND PEDIATRIC DERMATOLOGY SEMINAR
Psoriasis Flares Rapidly Postpartum
Managing psoriasis during pregnancy includes consideration for quality of life and medication safety issues, according to Dr. Alan Menter.
"Treatment of pregnant women with psoriasis should take into consideration the benefit of the therapy to her and the safety of her fetus. The full spectrum of safe and effective therapies during pregnancy needs to be reviewed," Dr. Menter said at the Women’s & Pediatric Dermatology Seminar, sponsored by the Skin Disease Education Foundation (SDEF).
"Most people present with psoriasis before age 40 years. The disease affects women and men equally, so many women with psoriasis are of reproductive age and are considering pregnancy," said Dr. Menter, chairman of the dermatology division at Baylor University Medical Center, Dallas.
"Maintaining quality of life is particularly important for females with psoriasis who are considering becoming pregnant, as well as during pregnancy and while breastfeeding."
Treatment Options
Patients with mild psoriasis can be treated safely with topical agents during pregnancy, most commonly with steroid and vitamin D preparations. For women with psoriasis unresponsive to topicals, a course of phototherapy with narrow-band UVB is safe and effective before conception, during pregnancy, and during the lactation period, Dr. Menter said.
Methotrexate, which should be stopped 3 months before conceiving, and the retinoid acitretin (Soriatane), which is contraindicated in females of childbearing potential, are pregnancy category X drugs and are not options during pregnancy.
As for cyclosporine, more than 20 years of pregnancy registry data on organ transplant recipients treated with the drug – who are on higher doses than used in psoriasis – indicate that cyclosporine is a "viable drug to be used during pregnancy if absolutely necessary," he said.
For moderate to severe psoriasis, "cyclosporine works very well at relatively low dosages, but has to be discontinued before the baby is born because it is excreted in breast milk," he added. A potential adverse effect of cyclosporine is that it may aggravate hypertension of pregnancy, and, in a small percentage of patients, it produces a pregnancy that is 3-4 weeks shorter than normal.
The biologic treatments etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), and ustekinumab (Stelara), which are all category B drugs, could interfere with normal embryonic development and affect fetal and newborn immunity. However, there are numerous pregnancy registries for both psoriasis and other inflammatory diseases, such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis, which show that biologics have had no long-term implications for the normal development of the infant immune system.
Psoriasis improves spontaneously in about 60% of women during pregnancy, although they are at an increased risk for postpartum flares. After delivery, "psoriasis can flare up very quickly and can spread from head to toe within days or weeks ... [and is] sometimes a lot worse than what they had previously," he said.
Patients should be advised to alert their physicians at the first sign of a flare after delivery. Phototherapy and topical treatments are options, but cyclosporine should not be used in breastfeeding patients. There is almost no excretion of the four injectable biologics into breast milk, which can thus be considered safe in lactating women.
Case Studies
Among the cases he presented at the conference was a 26-year-old female with a 5-year history of severe psoriasis who started treatment with cyclosporine. At 12 months, she was 90% clear and the dose was tapered. She became pregnant at 16 weeks. Cyclosporine was discontinued at 27 weeks gestation, and she remained relatively clear for the rest of her pregnancy and delivered a healthy baby at term.
She had a severe postpartum flare, with only a moderate response obtained with the reintroduction of cyclosporine after she stopped breastfeeding. She was then started on a 12-week course of the biologic agent alefacept and UVB treatment, which produced a moderate response. During the second month of alefacept treatment, she became pregnant again. The moderate response achieved with the alefacept was maintained throughout the pregnancy, even after the 12-week course of alefacept was stopped. She delivered a normal, healthy baby.
The same patient was started on infliximab for a significant flare of psoriasis a month after her second delivery. She was 95% clear after three induction infusions and was maintained on infliximab for 5 years. She became pregnant again during treatment with infliximab and had a healthy baby, with a normal delivery. She remains 90% clear to date on infliximab at a dose of 7.5 mg/kg every 8 weeks.
Another patient Dr. Menter described became pregnant while on methotrexate, and had a healthy baby despite the risk of miscarriage and fetal abnormalities with methotrexate. She stopped methotrexate treatment at week 13 of pregnancy, but developed a significant flare and started treatment with cyclosporine, which was tapered and discontinued at 8 months of pregnancy when the patient’s ustekinumab therapy was initiated. Three weeks later, she delivered a healthy baby who is "thriving" at age 3 years; her psoriasis remains 75% clear on a dose of 45 mg of ustekinumab every 12 weeks, Dr. Menter said.
Clinicians should be aware that women with psoriasis are at a slightly increased risk for spontaneous abortion and premature rupture of the membranes, he said, referring to the results of a case-control study that found an increased risk for these pregnancy complications, as well as macrosomia and induced abortions in women with psoriasis (J. Eur. Acad. Dermatol. Venereol. 2011:25;1041-7).
Dr. Menter has received research support from, been a consultant to, or served as a lecturer for Abbott, Amgen, and other companies.
SDEF and this news organization are owned by Frontline Medical Communications.
Managing psoriasis during pregnancy includes consideration for quality of life and medication safety issues, according to Dr. Alan Menter.
"Treatment of pregnant women with psoriasis should take into consideration the benefit of the therapy to her and the safety of her fetus. The full spectrum of safe and effective therapies during pregnancy needs to be reviewed," Dr. Menter said at the Women’s & Pediatric Dermatology Seminar, sponsored by the Skin Disease Education Foundation (SDEF).
"Most people present with psoriasis before age 40 years. The disease affects women and men equally, so many women with psoriasis are of reproductive age and are considering pregnancy," said Dr. Menter, chairman of the dermatology division at Baylor University Medical Center, Dallas.
"Maintaining quality of life is particularly important for females with psoriasis who are considering becoming pregnant, as well as during pregnancy and while breastfeeding."
Treatment Options
Patients with mild psoriasis can be treated safely with topical agents during pregnancy, most commonly with steroid and vitamin D preparations. For women with psoriasis unresponsive to topicals, a course of phototherapy with narrow-band UVB is safe and effective before conception, during pregnancy, and during the lactation period, Dr. Menter said.
Methotrexate, which should be stopped 3 months before conceiving, and the retinoid acitretin (Soriatane), which is contraindicated in females of childbearing potential, are pregnancy category X drugs and are not options during pregnancy.
As for cyclosporine, more than 20 years of pregnancy registry data on organ transplant recipients treated with the drug – who are on higher doses than used in psoriasis – indicate that cyclosporine is a "viable drug to be used during pregnancy if absolutely necessary," he said.
For moderate to severe psoriasis, "cyclosporine works very well at relatively low dosages, but has to be discontinued before the baby is born because it is excreted in breast milk," he added. A potential adverse effect of cyclosporine is that it may aggravate hypertension of pregnancy, and, in a small percentage of patients, it produces a pregnancy that is 3-4 weeks shorter than normal.
The biologic treatments etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), and ustekinumab (Stelara), which are all category B drugs, could interfere with normal embryonic development and affect fetal and newborn immunity. However, there are numerous pregnancy registries for both psoriasis and other inflammatory diseases, such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis, which show that biologics have had no long-term implications for the normal development of the infant immune system.
Psoriasis improves spontaneously in about 60% of women during pregnancy, although they are at an increased risk for postpartum flares. After delivery, "psoriasis can flare up very quickly and can spread from head to toe within days or weeks ... [and is] sometimes a lot worse than what they had previously," he said.
Patients should be advised to alert their physicians at the first sign of a flare after delivery. Phototherapy and topical treatments are options, but cyclosporine should not be used in breastfeeding patients. There is almost no excretion of the four injectable biologics into breast milk, which can thus be considered safe in lactating women.
Case Studies
Among the cases he presented at the conference was a 26-year-old female with a 5-year history of severe psoriasis who started treatment with cyclosporine. At 12 months, she was 90% clear and the dose was tapered. She became pregnant at 16 weeks. Cyclosporine was discontinued at 27 weeks gestation, and she remained relatively clear for the rest of her pregnancy and delivered a healthy baby at term.
She had a severe postpartum flare, with only a moderate response obtained with the reintroduction of cyclosporine after she stopped breastfeeding. She was then started on a 12-week course of the biologic agent alefacept and UVB treatment, which produced a moderate response. During the second month of alefacept treatment, she became pregnant again. The moderate response achieved with the alefacept was maintained throughout the pregnancy, even after the 12-week course of alefacept was stopped. She delivered a normal, healthy baby.
The same patient was started on infliximab for a significant flare of psoriasis a month after her second delivery. She was 95% clear after three induction infusions and was maintained on infliximab for 5 years. She became pregnant again during treatment with infliximab and had a healthy baby, with a normal delivery. She remains 90% clear to date on infliximab at a dose of 7.5 mg/kg every 8 weeks.
Another patient Dr. Menter described became pregnant while on methotrexate, and had a healthy baby despite the risk of miscarriage and fetal abnormalities with methotrexate. She stopped methotrexate treatment at week 13 of pregnancy, but developed a significant flare and started treatment with cyclosporine, which was tapered and discontinued at 8 months of pregnancy when the patient’s ustekinumab therapy was initiated. Three weeks later, she delivered a healthy baby who is "thriving" at age 3 years; her psoriasis remains 75% clear on a dose of 45 mg of ustekinumab every 12 weeks, Dr. Menter said.
Clinicians should be aware that women with psoriasis are at a slightly increased risk for spontaneous abortion and premature rupture of the membranes, he said, referring to the results of a case-control study that found an increased risk for these pregnancy complications, as well as macrosomia and induced abortions in women with psoriasis (J. Eur. Acad. Dermatol. Venereol. 2011:25;1041-7).
Dr. Menter has received research support from, been a consultant to, or served as a lecturer for Abbott, Amgen, and other companies.
SDEF and this news organization are owned by Frontline Medical Communications.
Managing psoriasis during pregnancy includes consideration for quality of life and medication safety issues, according to Dr. Alan Menter.
"Treatment of pregnant women with psoriasis should take into consideration the benefit of the therapy to her and the safety of her fetus. The full spectrum of safe and effective therapies during pregnancy needs to be reviewed," Dr. Menter said at the Women’s & Pediatric Dermatology Seminar, sponsored by the Skin Disease Education Foundation (SDEF).
"Most people present with psoriasis before age 40 years. The disease affects women and men equally, so many women with psoriasis are of reproductive age and are considering pregnancy," said Dr. Menter, chairman of the dermatology division at Baylor University Medical Center, Dallas.
"Maintaining quality of life is particularly important for females with psoriasis who are considering becoming pregnant, as well as during pregnancy and while breastfeeding."
Treatment Options
Patients with mild psoriasis can be treated safely with topical agents during pregnancy, most commonly with steroid and vitamin D preparations. For women with psoriasis unresponsive to topicals, a course of phototherapy with narrow-band UVB is safe and effective before conception, during pregnancy, and during the lactation period, Dr. Menter said.
Methotrexate, which should be stopped 3 months before conceiving, and the retinoid acitretin (Soriatane), which is contraindicated in females of childbearing potential, are pregnancy category X drugs and are not options during pregnancy.
As for cyclosporine, more than 20 years of pregnancy registry data on organ transplant recipients treated with the drug – who are on higher doses than used in psoriasis – indicate that cyclosporine is a "viable drug to be used during pregnancy if absolutely necessary," he said.
For moderate to severe psoriasis, "cyclosporine works very well at relatively low dosages, but has to be discontinued before the baby is born because it is excreted in breast milk," he added. A potential adverse effect of cyclosporine is that it may aggravate hypertension of pregnancy, and, in a small percentage of patients, it produces a pregnancy that is 3-4 weeks shorter than normal.
The biologic treatments etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), and ustekinumab (Stelara), which are all category B drugs, could interfere with normal embryonic development and affect fetal and newborn immunity. However, there are numerous pregnancy registries for both psoriasis and other inflammatory diseases, such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis, which show that biologics have had no long-term implications for the normal development of the infant immune system.
Psoriasis improves spontaneously in about 60% of women during pregnancy, although they are at an increased risk for postpartum flares. After delivery, "psoriasis can flare up very quickly and can spread from head to toe within days or weeks ... [and is] sometimes a lot worse than what they had previously," he said.
Patients should be advised to alert their physicians at the first sign of a flare after delivery. Phototherapy and topical treatments are options, but cyclosporine should not be used in breastfeeding patients. There is almost no excretion of the four injectable biologics into breast milk, which can thus be considered safe in lactating women.
Case Studies
Among the cases he presented at the conference was a 26-year-old female with a 5-year history of severe psoriasis who started treatment with cyclosporine. At 12 months, she was 90% clear and the dose was tapered. She became pregnant at 16 weeks. Cyclosporine was discontinued at 27 weeks gestation, and she remained relatively clear for the rest of her pregnancy and delivered a healthy baby at term.
She had a severe postpartum flare, with only a moderate response obtained with the reintroduction of cyclosporine after she stopped breastfeeding. She was then started on a 12-week course of the biologic agent alefacept and UVB treatment, which produced a moderate response. During the second month of alefacept treatment, she became pregnant again. The moderate response achieved with the alefacept was maintained throughout the pregnancy, even after the 12-week course of alefacept was stopped. She delivered a normal, healthy baby.
The same patient was started on infliximab for a significant flare of psoriasis a month after her second delivery. She was 95% clear after three induction infusions and was maintained on infliximab for 5 years. She became pregnant again during treatment with infliximab and had a healthy baby, with a normal delivery. She remains 90% clear to date on infliximab at a dose of 7.5 mg/kg every 8 weeks.
Another patient Dr. Menter described became pregnant while on methotrexate, and had a healthy baby despite the risk of miscarriage and fetal abnormalities with methotrexate. She stopped methotrexate treatment at week 13 of pregnancy, but developed a significant flare and started treatment with cyclosporine, which was tapered and discontinued at 8 months of pregnancy when the patient’s ustekinumab therapy was initiated. Three weeks later, she delivered a healthy baby who is "thriving" at age 3 years; her psoriasis remains 75% clear on a dose of 45 mg of ustekinumab every 12 weeks, Dr. Menter said.
Clinicians should be aware that women with psoriasis are at a slightly increased risk for spontaneous abortion and premature rupture of the membranes, he said, referring to the results of a case-control study that found an increased risk for these pregnancy complications, as well as macrosomia and induced abortions in women with psoriasis (J. Eur. Acad. Dermatol. Venereol. 2011:25;1041-7).
Dr. Menter has received research support from, been a consultant to, or served as a lecturer for Abbott, Amgen, and other companies.
SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE SDEF WOMEN'S AND PEDIATRIC DERMATOLOGY SEMINAR
SDEF Women's & Pediatric Dermatology Seminar - Conference Coverage
The SDEF Women’s & Pediatric Dermatology meeting is being held in San Francisco, Oct. 19-21. Our reporting team will provide coverage for this conference. Check back here for the latest from this meeting.
The SDEF Women’s & Pediatric Dermatology meeting is being held in San Francisco, Oct. 19-21. Our reporting team will provide coverage for this conference. Check back here for the latest from this meeting.
The SDEF Women’s & Pediatric Dermatology meeting is being held in San Francisco, Oct. 19-21. Our reporting team will provide coverage for this conference. Check back here for the latest from this meeting.