User login
More weight loss with surgery than new obesity meds: meta-analysis
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.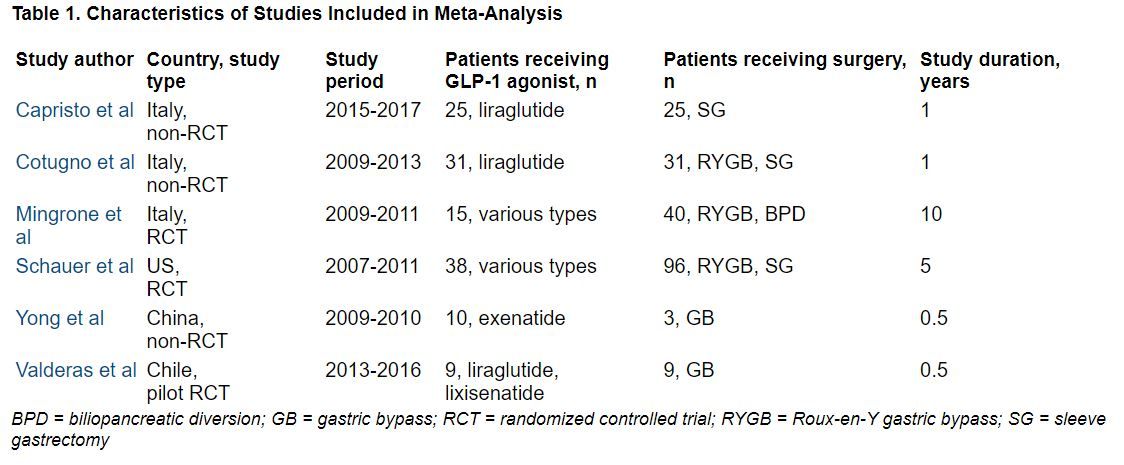
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK®
New dual-agonist weight-loss injection impressive, but early days
SAN DIEGO – A novel glucagonlike peptide-1 (GLP-1)/glucagon dual-receptor agonist, BI 456906, being developed by Boehringer Ingelheim and Zealand Pharma, led to “impressive” weight loss in a phase 2 dosing study of patients with overweight/obesity and type 2 diabetes – but this is early research.
Julio Rosenstock, MD, presented the study results, including weight loss and adverse events, at the annual meeting of the Obesity Society.
At the highest tested dose (1.8 mg twice weekly subcutaneous injections), 57% of patients lost at least 5% of their initial body weight and 35% lost at least 10% of their initial body weight at 16 weeks.
In contrast, among the patients who received a 1-mg semaglutide dose as a comparator, 38% lost at least 5% of their initial body weight and 16% lost at least 10% of their initial body weight at study end.
This is “very promising data as an anti-obesity compound,” said Dr. Rosenstock, professor of medicine, University of Texas Southwestern Medical Center in Dallas.
The researchers enrolled 411 adults and randomized them into eight groups of roughly 50 patients each.
They compared six doses of BI 456906 (from 0.3 mg/week to 1.8 mg twice weekly) versus 1 mg/week of the GLP-1 agonist semaglutide (Wegovy, Novo Nordisk) versus placebo.
Patients had a mean initial weight of 97 kg (214 pounds).
After 4 months, on average, patients who received the highest tested dose of BI 456906 lost 9% of their initial weight or roughly 8.7 kg (19 pounds).
Patients who received semaglutide lost 5.4% of their initial weight or roughly 5.2 kg (11.5 pounds), and patients who received placebo lost only 1.2% of their initial weight
The main adverse events were gastrointestinal.
‘Exciting data,’ but still early days
“This is very exciting data. It comes from another experienced company with a track record of successful products with a new compound in a class where other related compounds have shown efficacy and safety,” Dan Bessesen, MD, president of The Obesity Society, who was not involved with this research, told this news organization in an email.
“The degree of weight loss is impressive for a 16-week study,” Dr. Bessesen, professor of medicine in the division of endocrinology, metabolism and diabetes at the University of Colorado at Denver, Aurora, added. “The longer-term weight loss will likely be more.”
The side-effect profile is not particularly concerning and is like other drugs in this general class, he said.
However, he also noted a few caveats. This was only a phase 2 study, “so we should not make firm conclusions about efficacy from a study like this, as the number of subjects studied at each dose is relatively small and the follow-up not long.”
In addition, “the dose of semaglutide is the old ‘diabetes’ dose (1 mg) not the weight-loss dose of 2.4 mg or the new diabetes dose of 2 mg. It is not a real comparison with the maximal approved dose of semaglutide. So, we cannot say that it will be better than semaglutide.”
The next hurdle is the “need to see phase 3 studies in a larger group of patients studied for a longer time. Then [the company] will need FDA approval, so it may be a bit of time” before this drug potentially enters the marketplace.
The “bottom line” is that this potential new antiobesity/diabetes drug is “very promising, but [it is] still a little early to say where it ultimately will go.”
A1c results presented at EASD
To be included in this study, patients had to be 18-75 years old, have type 2 diabetes, a body mass index of 25-50 kg/m2, and hemoglobin A1c of 7%-10%, and be stable on metformin therapy.
The patients had a mean age of 57 years, and 57% were men. They had a mean A1c of 8.1%, a mean BMI of 34 kg/m2, and a mean waist circumference of 110 cm (43 inches).
“We just recently reported at the EASD conference last month, the effect of BI 456906 on A1c lowering,” Dr. Rosenstock said.
“It looks like the [drop in] A1c plateaus at 1.9%, which is pretty good when you consider the baseline A1c is around 8%. You get down to around 6%, which is what we regard as a very robust reduction in people with type 2 diabetes on metformin.”
The current analysis showed that patients who received doses of 0.3, 0.9, 1.8, and 2.7 mg/week of the novel drug lost 1.9%, 4.4%, 6.6%, and 6.7% of their initial body weight, respectively, after 16 weeks.
The patients who received 1.2 mg and 1.8 mg twice weekly lost even more weight, 7.2% and 9% of their initial weight, respectively.
At the highest dose, on average, patients lost 13 cm (5 inches) around their waist.
Adverse events were reported by 78% of the patients, most commonly nausea (34% of patients), vomiting (18%), and diarrhea (16%).
Only 1.3% of patients had a drug-related serious adverse event. A total of 16% of patients discontinued the therapy.
Most of the “gastrointestinal adverse events leading the treatment discontinuation were possibly dose and titration related,” Dr. Rosenstock said, “and it’s highly conceivable that for future studies a slower dose escalation may mitigate the occurrence of the gastrointestinal adverse events.”
BI 456906 was coinvented with Zealand Pharma. Under the licensing agreement, Boehringer Ingelheim funds all research, development, and commercialization.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A novel glucagonlike peptide-1 (GLP-1)/glucagon dual-receptor agonist, BI 456906, being developed by Boehringer Ingelheim and Zealand Pharma, led to “impressive” weight loss in a phase 2 dosing study of patients with overweight/obesity and type 2 diabetes – but this is early research.
Julio Rosenstock, MD, presented the study results, including weight loss and adverse events, at the annual meeting of the Obesity Society.
At the highest tested dose (1.8 mg twice weekly subcutaneous injections), 57% of patients lost at least 5% of their initial body weight and 35% lost at least 10% of their initial body weight at 16 weeks.
In contrast, among the patients who received a 1-mg semaglutide dose as a comparator, 38% lost at least 5% of their initial body weight and 16% lost at least 10% of their initial body weight at study end.
This is “very promising data as an anti-obesity compound,” said Dr. Rosenstock, professor of medicine, University of Texas Southwestern Medical Center in Dallas.
The researchers enrolled 411 adults and randomized them into eight groups of roughly 50 patients each.
They compared six doses of BI 456906 (from 0.3 mg/week to 1.8 mg twice weekly) versus 1 mg/week of the GLP-1 agonist semaglutide (Wegovy, Novo Nordisk) versus placebo.
Patients had a mean initial weight of 97 kg (214 pounds).
After 4 months, on average, patients who received the highest tested dose of BI 456906 lost 9% of their initial weight or roughly 8.7 kg (19 pounds).
Patients who received semaglutide lost 5.4% of their initial weight or roughly 5.2 kg (11.5 pounds), and patients who received placebo lost only 1.2% of their initial weight
The main adverse events were gastrointestinal.
‘Exciting data,’ but still early days
“This is very exciting data. It comes from another experienced company with a track record of successful products with a new compound in a class where other related compounds have shown efficacy and safety,” Dan Bessesen, MD, president of The Obesity Society, who was not involved with this research, told this news organization in an email.
“The degree of weight loss is impressive for a 16-week study,” Dr. Bessesen, professor of medicine in the division of endocrinology, metabolism and diabetes at the University of Colorado at Denver, Aurora, added. “The longer-term weight loss will likely be more.”
The side-effect profile is not particularly concerning and is like other drugs in this general class, he said.
However, he also noted a few caveats. This was only a phase 2 study, “so we should not make firm conclusions about efficacy from a study like this, as the number of subjects studied at each dose is relatively small and the follow-up not long.”
In addition, “the dose of semaglutide is the old ‘diabetes’ dose (1 mg) not the weight-loss dose of 2.4 mg or the new diabetes dose of 2 mg. It is not a real comparison with the maximal approved dose of semaglutide. So, we cannot say that it will be better than semaglutide.”
The next hurdle is the “need to see phase 3 studies in a larger group of patients studied for a longer time. Then [the company] will need FDA approval, so it may be a bit of time” before this drug potentially enters the marketplace.
The “bottom line” is that this potential new antiobesity/diabetes drug is “very promising, but [it is] still a little early to say where it ultimately will go.”
A1c results presented at EASD
To be included in this study, patients had to be 18-75 years old, have type 2 diabetes, a body mass index of 25-50 kg/m2, and hemoglobin A1c of 7%-10%, and be stable on metformin therapy.
The patients had a mean age of 57 years, and 57% were men. They had a mean A1c of 8.1%, a mean BMI of 34 kg/m2, and a mean waist circumference of 110 cm (43 inches).
“We just recently reported at the EASD conference last month, the effect of BI 456906 on A1c lowering,” Dr. Rosenstock said.
“It looks like the [drop in] A1c plateaus at 1.9%, which is pretty good when you consider the baseline A1c is around 8%. You get down to around 6%, which is what we regard as a very robust reduction in people with type 2 diabetes on metformin.”
The current analysis showed that patients who received doses of 0.3, 0.9, 1.8, and 2.7 mg/week of the novel drug lost 1.9%, 4.4%, 6.6%, and 6.7% of their initial body weight, respectively, after 16 weeks.
The patients who received 1.2 mg and 1.8 mg twice weekly lost even more weight, 7.2% and 9% of their initial weight, respectively.
At the highest dose, on average, patients lost 13 cm (5 inches) around their waist.
Adverse events were reported by 78% of the patients, most commonly nausea (34% of patients), vomiting (18%), and diarrhea (16%).
Only 1.3% of patients had a drug-related serious adverse event. A total of 16% of patients discontinued the therapy.
Most of the “gastrointestinal adverse events leading the treatment discontinuation were possibly dose and titration related,” Dr. Rosenstock said, “and it’s highly conceivable that for future studies a slower dose escalation may mitigate the occurrence of the gastrointestinal adverse events.”
BI 456906 was coinvented with Zealand Pharma. Under the licensing agreement, Boehringer Ingelheim funds all research, development, and commercialization.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A novel glucagonlike peptide-1 (GLP-1)/glucagon dual-receptor agonist, BI 456906, being developed by Boehringer Ingelheim and Zealand Pharma, led to “impressive” weight loss in a phase 2 dosing study of patients with overweight/obesity and type 2 diabetes – but this is early research.
Julio Rosenstock, MD, presented the study results, including weight loss and adverse events, at the annual meeting of the Obesity Society.
At the highest tested dose (1.8 mg twice weekly subcutaneous injections), 57% of patients lost at least 5% of their initial body weight and 35% lost at least 10% of their initial body weight at 16 weeks.
In contrast, among the patients who received a 1-mg semaglutide dose as a comparator, 38% lost at least 5% of their initial body weight and 16% lost at least 10% of their initial body weight at study end.
This is “very promising data as an anti-obesity compound,” said Dr. Rosenstock, professor of medicine, University of Texas Southwestern Medical Center in Dallas.
The researchers enrolled 411 adults and randomized them into eight groups of roughly 50 patients each.
They compared six doses of BI 456906 (from 0.3 mg/week to 1.8 mg twice weekly) versus 1 mg/week of the GLP-1 agonist semaglutide (Wegovy, Novo Nordisk) versus placebo.
Patients had a mean initial weight of 97 kg (214 pounds).
After 4 months, on average, patients who received the highest tested dose of BI 456906 lost 9% of their initial weight or roughly 8.7 kg (19 pounds).
Patients who received semaglutide lost 5.4% of their initial weight or roughly 5.2 kg (11.5 pounds), and patients who received placebo lost only 1.2% of their initial weight
The main adverse events were gastrointestinal.
‘Exciting data,’ but still early days
“This is very exciting data. It comes from another experienced company with a track record of successful products with a new compound in a class where other related compounds have shown efficacy and safety,” Dan Bessesen, MD, president of The Obesity Society, who was not involved with this research, told this news organization in an email.
“The degree of weight loss is impressive for a 16-week study,” Dr. Bessesen, professor of medicine in the division of endocrinology, metabolism and diabetes at the University of Colorado at Denver, Aurora, added. “The longer-term weight loss will likely be more.”
The side-effect profile is not particularly concerning and is like other drugs in this general class, he said.
However, he also noted a few caveats. This was only a phase 2 study, “so we should not make firm conclusions about efficacy from a study like this, as the number of subjects studied at each dose is relatively small and the follow-up not long.”
In addition, “the dose of semaglutide is the old ‘diabetes’ dose (1 mg) not the weight-loss dose of 2.4 mg or the new diabetes dose of 2 mg. It is not a real comparison with the maximal approved dose of semaglutide. So, we cannot say that it will be better than semaglutide.”
The next hurdle is the “need to see phase 3 studies in a larger group of patients studied for a longer time. Then [the company] will need FDA approval, so it may be a bit of time” before this drug potentially enters the marketplace.
The “bottom line” is that this potential new antiobesity/diabetes drug is “very promising, but [it is] still a little early to say where it ultimately will go.”
A1c results presented at EASD
To be included in this study, patients had to be 18-75 years old, have type 2 diabetes, a body mass index of 25-50 kg/m2, and hemoglobin A1c of 7%-10%, and be stable on metformin therapy.
The patients had a mean age of 57 years, and 57% were men. They had a mean A1c of 8.1%, a mean BMI of 34 kg/m2, and a mean waist circumference of 110 cm (43 inches).
“We just recently reported at the EASD conference last month, the effect of BI 456906 on A1c lowering,” Dr. Rosenstock said.
“It looks like the [drop in] A1c plateaus at 1.9%, which is pretty good when you consider the baseline A1c is around 8%. You get down to around 6%, which is what we regard as a very robust reduction in people with type 2 diabetes on metformin.”
The current analysis showed that patients who received doses of 0.3, 0.9, 1.8, and 2.7 mg/week of the novel drug lost 1.9%, 4.4%, 6.6%, and 6.7% of their initial body weight, respectively, after 16 weeks.
The patients who received 1.2 mg and 1.8 mg twice weekly lost even more weight, 7.2% and 9% of their initial weight, respectively.
At the highest dose, on average, patients lost 13 cm (5 inches) around their waist.
Adverse events were reported by 78% of the patients, most commonly nausea (34% of patients), vomiting (18%), and diarrhea (16%).
Only 1.3% of patients had a drug-related serious adverse event. A total of 16% of patients discontinued the therapy.
Most of the “gastrointestinal adverse events leading the treatment discontinuation were possibly dose and titration related,” Dr. Rosenstock said, “and it’s highly conceivable that for future studies a slower dose escalation may mitigate the occurrence of the gastrointestinal adverse events.”
BI 456906 was coinvented with Zealand Pharma. Under the licensing agreement, Boehringer Ingelheim funds all research, development, and commercialization.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK® 2022
Tirzepatide lowers weight across all groups with obesity
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK® 2022
STEP TEENS: Semaglutide ‘gives hope’ to adolescents with obesity
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
FROM OBESITYWEEK® 2022
ObesityWeek 2022: What’s stopping effective treatment of obesity?
ObesityWeek 2022 is the largest international conference on obesity, with over 100 sessions, and coincides with the 40th anniversary of the Obesity Society. Being held Nov. 1-4, it is a hybrid meeting that participants can attend onsite in sunny San Diego or virtually.
“The meeting offers a wide perspective, from basic science, all the way to public policy on studies of treatment and prevention of obesity,” program planning chair for ObesityWeek, Kelly C. Allison, PhD, said in an interview.
The Presidential Plenary session on Nov. 1 will kick off the meeting with “a series of 10-minute rapid talks on cutting-edge topics in the field,” noted Dr. Allison, who is also director, Center for Weight and Eating Disorders, Hospital of the University of Pennsylvania, and professor of psychiatry, University of Pennsylvania, both in Philadelphia.
Among others, Ania M. Jastreboff, MD, PhD, will speak about “New developments in anti-obesity pharmacotherapy,” and Theodore K. Kyle, RPh, MBA, will discuss “Reducing barriers to treatment: Insurance coverage.”
“We’re seeing some pretty effective antiobesity medication, but still they are not being covered by many insurances,” said Dr. Allison. Some clinicians might be hesitant to prescribe antiobesity medications, remembering older drugs that were pulled from the market for health concerns, and some patients may also have concerns, she speculated. There is a need for greater education about the current antiobesity drugs.
In his presidential address, Dan Bessesen, MD, professor of medicine at the University of Colorado at Denver, Aurora, will discuss “Regulation of body weight and adaptive responses to weight loss.”
Pediatric obesity is a major focus of this year›s conference too, Allison noted.
At 8 a.m on Nov. 3, The Obesity Society, the World Obesity Federation, the European Association for the Study of Obesity, and Obesity Canada will present a joint symposium, “International innovations in pediatric obesity,” with speakers from Canada, Australia, and Ireland discussing ongoing paradigm shifts in the prevention and treatment of pediatric obesity.
Two hours later, at a joint symposium by the American Academy of Pediatrics/The Obesity Society, attendees will get a behind-the-scenes look at the making of the new AAP Obesity Clinical Practice Guideline for children and adolescents with obesity.
The conference tracks reflect the broad scope of this event: Track 1: Metabolism and Integrative Physiology; Track 2: Neuroscience; Track 3: Interventional and Clinical Studies; Track 4: Population Health; Track 5: Clinical/Professional Practice; Track 6: Policy/Public Health, and a subtrack: Eradicating Treatment Barriers.
Dr. Allison highlighted the following oral presentations and posters about antiobesity drugs:
- “Once-weekly subcutaneous semaglutide 2.4 mg in adolescents with overweight or obesity,” with an extended Q&A session, Nov. 2.
- “Clinical outcomes with medication use in tertiary pediatric weight management program,” by Enayet and colleagues. Poster 030.
- “The metabolically healthy obese paradigm and liver fat content in the Fels longitudinal study,” by Garza and colleagues Oral 055, Nov. 2.
- “Phase 3 clinical trial of metformin for treatment of COVID-19 in adults with overweight and obesity,” by Bramante and colleagues. Oral 067, Nov. 3. This trial was published in the (N Engl J Med. 2022;387:599-610).
- “Glucagon/GLP-1 receptor dual agonist BI 456906 reduces bodyweight in patients with type 2 diabetes,” by Rosenstock and colleagues. Oral-063, Nov. 3.
- “A randomized controlled trial of naltrexone and bupropion and behavior therapy for binge-eating disorder,” by Grilo and colleagues. Oral 066, Nov. 3.
And on Nov. 4, researchers will present four oral abstracts about the dual glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1) receptor agonist tirzepatide (Mounjaro), which is approved for type 2 diabetes and now has fast track designation for weight loss from the Food and Drug Administration. Oral abstracts 109, 110, 111, and 112 cover weight loss with tirzepatide across different age groups, body mass indexes, and comorbidities, as well as quality of life.
Dr. Allison also highlighted the following presentations that cover other diverse topics:
- Family-based treatment: “Pilot study to inform a randomized controlled trial of HeLP: Obesity prevention & treatment for the entire Hispanic family,” by Haemer and colleagues. Oral 029. November 2.
- Bariatric surgery: “Long-term outcomes of laparoscopic sleeve gastrectomy from 2010-2016: A nationwide cohort study,” Oral 014. Nov. 2.
- Prevention/public health: “Impact of positive and negative front-of-package food labels in a randomized experiment,” by Grummon and colleagues. Oral 068. Nov. 3.
- Time-restricted eating: “Effects of 8-hour time restricted eating for weight loss over 12 months,” by Gabel and colleagues. Oral 102. Nov. 4.
- Patient management: “Identifying interprofessional drivers of practice gaps in the management of patients with obesity,” by Robinson and colleagues. Poster 055.
On Nov. 4, researchers will present five winning papers that will be published in the December issue of the Obesity journal about GLP-1 agonists versus bariatric surgery; monoacylglycerol O-acyltransferase 1 in mice; a behavioral weight-loss intervention; the Canberra Obesity Management Service; and macronutrient (im)balance in an obesogenic environment.
“I’m always excited to hear some talks that are outside of my comfort area to understand the mechanisms of obesity better,” concluded Dr. Allison.
A version of this article first appeared on Medscape.com.
ObesityWeek 2022 is the largest international conference on obesity, with over 100 sessions, and coincides with the 40th anniversary of the Obesity Society. Being held Nov. 1-4, it is a hybrid meeting that participants can attend onsite in sunny San Diego or virtually.
“The meeting offers a wide perspective, from basic science, all the way to public policy on studies of treatment and prevention of obesity,” program planning chair for ObesityWeek, Kelly C. Allison, PhD, said in an interview.
The Presidential Plenary session on Nov. 1 will kick off the meeting with “a series of 10-minute rapid talks on cutting-edge topics in the field,” noted Dr. Allison, who is also director, Center for Weight and Eating Disorders, Hospital of the University of Pennsylvania, and professor of psychiatry, University of Pennsylvania, both in Philadelphia.
Among others, Ania M. Jastreboff, MD, PhD, will speak about “New developments in anti-obesity pharmacotherapy,” and Theodore K. Kyle, RPh, MBA, will discuss “Reducing barriers to treatment: Insurance coverage.”
“We’re seeing some pretty effective antiobesity medication, but still they are not being covered by many insurances,” said Dr. Allison. Some clinicians might be hesitant to prescribe antiobesity medications, remembering older drugs that were pulled from the market for health concerns, and some patients may also have concerns, she speculated. There is a need for greater education about the current antiobesity drugs.
In his presidential address, Dan Bessesen, MD, professor of medicine at the University of Colorado at Denver, Aurora, will discuss “Regulation of body weight and adaptive responses to weight loss.”
Pediatric obesity is a major focus of this year›s conference too, Allison noted.
At 8 a.m on Nov. 3, The Obesity Society, the World Obesity Federation, the European Association for the Study of Obesity, and Obesity Canada will present a joint symposium, “International innovations in pediatric obesity,” with speakers from Canada, Australia, and Ireland discussing ongoing paradigm shifts in the prevention and treatment of pediatric obesity.
Two hours later, at a joint symposium by the American Academy of Pediatrics/The Obesity Society, attendees will get a behind-the-scenes look at the making of the new AAP Obesity Clinical Practice Guideline for children and adolescents with obesity.
The conference tracks reflect the broad scope of this event: Track 1: Metabolism and Integrative Physiology; Track 2: Neuroscience; Track 3: Interventional and Clinical Studies; Track 4: Population Health; Track 5: Clinical/Professional Practice; Track 6: Policy/Public Health, and a subtrack: Eradicating Treatment Barriers.
Dr. Allison highlighted the following oral presentations and posters about antiobesity drugs:
- “Once-weekly subcutaneous semaglutide 2.4 mg in adolescents with overweight or obesity,” with an extended Q&A session, Nov. 2.
- “Clinical outcomes with medication use in tertiary pediatric weight management program,” by Enayet and colleagues. Poster 030.
- “The metabolically healthy obese paradigm and liver fat content in the Fels longitudinal study,” by Garza and colleagues Oral 055, Nov. 2.
- “Phase 3 clinical trial of metformin for treatment of COVID-19 in adults with overweight and obesity,” by Bramante and colleagues. Oral 067, Nov. 3. This trial was published in the (N Engl J Med. 2022;387:599-610).
- “Glucagon/GLP-1 receptor dual agonist BI 456906 reduces bodyweight in patients with type 2 diabetes,” by Rosenstock and colleagues. Oral-063, Nov. 3.
- “A randomized controlled trial of naltrexone and bupropion and behavior therapy for binge-eating disorder,” by Grilo and colleagues. Oral 066, Nov. 3.
And on Nov. 4, researchers will present four oral abstracts about the dual glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1) receptor agonist tirzepatide (Mounjaro), which is approved for type 2 diabetes and now has fast track designation for weight loss from the Food and Drug Administration. Oral abstracts 109, 110, 111, and 112 cover weight loss with tirzepatide across different age groups, body mass indexes, and comorbidities, as well as quality of life.
Dr. Allison also highlighted the following presentations that cover other diverse topics:
- Family-based treatment: “Pilot study to inform a randomized controlled trial of HeLP: Obesity prevention & treatment for the entire Hispanic family,” by Haemer and colleagues. Oral 029. November 2.
- Bariatric surgery: “Long-term outcomes of laparoscopic sleeve gastrectomy from 2010-2016: A nationwide cohort study,” Oral 014. Nov. 2.
- Prevention/public health: “Impact of positive and negative front-of-package food labels in a randomized experiment,” by Grummon and colleagues. Oral 068. Nov. 3.
- Time-restricted eating: “Effects of 8-hour time restricted eating for weight loss over 12 months,” by Gabel and colleagues. Oral 102. Nov. 4.
- Patient management: “Identifying interprofessional drivers of practice gaps in the management of patients with obesity,” by Robinson and colleagues. Poster 055.
On Nov. 4, researchers will present five winning papers that will be published in the December issue of the Obesity journal about GLP-1 agonists versus bariatric surgery; monoacylglycerol O-acyltransferase 1 in mice; a behavioral weight-loss intervention; the Canberra Obesity Management Service; and macronutrient (im)balance in an obesogenic environment.
“I’m always excited to hear some talks that are outside of my comfort area to understand the mechanisms of obesity better,” concluded Dr. Allison.
A version of this article first appeared on Medscape.com.
ObesityWeek 2022 is the largest international conference on obesity, with over 100 sessions, and coincides with the 40th anniversary of the Obesity Society. Being held Nov. 1-4, it is a hybrid meeting that participants can attend onsite in sunny San Diego or virtually.
“The meeting offers a wide perspective, from basic science, all the way to public policy on studies of treatment and prevention of obesity,” program planning chair for ObesityWeek, Kelly C. Allison, PhD, said in an interview.
The Presidential Plenary session on Nov. 1 will kick off the meeting with “a series of 10-minute rapid talks on cutting-edge topics in the field,” noted Dr. Allison, who is also director, Center for Weight and Eating Disorders, Hospital of the University of Pennsylvania, and professor of psychiatry, University of Pennsylvania, both in Philadelphia.
Among others, Ania M. Jastreboff, MD, PhD, will speak about “New developments in anti-obesity pharmacotherapy,” and Theodore K. Kyle, RPh, MBA, will discuss “Reducing barriers to treatment: Insurance coverage.”
“We’re seeing some pretty effective antiobesity medication, but still they are not being covered by many insurances,” said Dr. Allison. Some clinicians might be hesitant to prescribe antiobesity medications, remembering older drugs that were pulled from the market for health concerns, and some patients may also have concerns, she speculated. There is a need for greater education about the current antiobesity drugs.
In his presidential address, Dan Bessesen, MD, professor of medicine at the University of Colorado at Denver, Aurora, will discuss “Regulation of body weight and adaptive responses to weight loss.”
Pediatric obesity is a major focus of this year›s conference too, Allison noted.
At 8 a.m on Nov. 3, The Obesity Society, the World Obesity Federation, the European Association for the Study of Obesity, and Obesity Canada will present a joint symposium, “International innovations in pediatric obesity,” with speakers from Canada, Australia, and Ireland discussing ongoing paradigm shifts in the prevention and treatment of pediatric obesity.
Two hours later, at a joint symposium by the American Academy of Pediatrics/The Obesity Society, attendees will get a behind-the-scenes look at the making of the new AAP Obesity Clinical Practice Guideline for children and adolescents with obesity.
The conference tracks reflect the broad scope of this event: Track 1: Metabolism and Integrative Physiology; Track 2: Neuroscience; Track 3: Interventional and Clinical Studies; Track 4: Population Health; Track 5: Clinical/Professional Practice; Track 6: Policy/Public Health, and a subtrack: Eradicating Treatment Barriers.
Dr. Allison highlighted the following oral presentations and posters about antiobesity drugs:
- “Once-weekly subcutaneous semaglutide 2.4 mg in adolescents with overweight or obesity,” with an extended Q&A session, Nov. 2.
- “Clinical outcomes with medication use in tertiary pediatric weight management program,” by Enayet and colleagues. Poster 030.
- “The metabolically healthy obese paradigm and liver fat content in the Fels longitudinal study,” by Garza and colleagues Oral 055, Nov. 2.
- “Phase 3 clinical trial of metformin for treatment of COVID-19 in adults with overweight and obesity,” by Bramante and colleagues. Oral 067, Nov. 3. This trial was published in the (N Engl J Med. 2022;387:599-610).
- “Glucagon/GLP-1 receptor dual agonist BI 456906 reduces bodyweight in patients with type 2 diabetes,” by Rosenstock and colleagues. Oral-063, Nov. 3.
- “A randomized controlled trial of naltrexone and bupropion and behavior therapy for binge-eating disorder,” by Grilo and colleagues. Oral 066, Nov. 3.
And on Nov. 4, researchers will present four oral abstracts about the dual glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1) receptor agonist tirzepatide (Mounjaro), which is approved for type 2 diabetes and now has fast track designation for weight loss from the Food and Drug Administration. Oral abstracts 109, 110, 111, and 112 cover weight loss with tirzepatide across different age groups, body mass indexes, and comorbidities, as well as quality of life.
Dr. Allison also highlighted the following presentations that cover other diverse topics:
- Family-based treatment: “Pilot study to inform a randomized controlled trial of HeLP: Obesity prevention & treatment for the entire Hispanic family,” by Haemer and colleagues. Oral 029. November 2.
- Bariatric surgery: “Long-term outcomes of laparoscopic sleeve gastrectomy from 2010-2016: A nationwide cohort study,” Oral 014. Nov. 2.
- Prevention/public health: “Impact of positive and negative front-of-package food labels in a randomized experiment,” by Grummon and colleagues. Oral 068. Nov. 3.
- Time-restricted eating: “Effects of 8-hour time restricted eating for weight loss over 12 months,” by Gabel and colleagues. Oral 102. Nov. 4.
- Patient management: “Identifying interprofessional drivers of practice gaps in the management of patients with obesity,” by Robinson and colleagues. Poster 055.
On Nov. 4, researchers will present five winning papers that will be published in the December issue of the Obesity journal about GLP-1 agonists versus bariatric surgery; monoacylglycerol O-acyltransferase 1 in mice; a behavioral weight-loss intervention; the Canberra Obesity Management Service; and macronutrient (im)balance in an obesogenic environment.
“I’m always excited to hear some talks that are outside of my comfort area to understand the mechanisms of obesity better,” concluded Dr. Allison.
A version of this article first appeared on Medscape.com.

