User login
Low-dose CT has a place in spondyloarthritis imaging toolbox
MADISON, WISC. – said Robert Lambert, MD, speaking at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
“Low-dose CT of the SI [sacroiliac] joints is probably underutilized,” said Dr. Lambert, chair of the department of radiology and diagnostic imaging at the University of Alberta, Edmonton. “Subtle bony changes are demonstrated very well, and it can be an excellent test for resolving equivocal findings on x-ray or MRI.”
An important first step in making imaging decisions is to put concerns about radiation exposure in context, said Dr. Lambert. “Today, almost all CT effective exposure doses are considered low risk.”
Older studies have shown that a conventional two-view chest radiograph delivers a dose of 0.1 mSv – the equivalent of 10 days of background radiation – whereas the highest radiation dose is delivered by a CT scan of the abdomen and pelvis with and without contrast. This examination delivers an effective dose of 20 mSv, the equivalent of 7 years of background radiation. Dr. Lambert pointed out what the “moderate” additional lifetime risk of malignancy – 1:500 – associated with this scan looks like in real-world numbers: “So the lifetime risk of cancer would increase from 20% to 20.2%.”
Recently, measurements of effective doses delivered in low-dose CT (ldCT) have shown that “most doses are significantly lower than previously quoted,” said Dr. Lambert. For example, ldCT of the SI joints delivers just 0.42 mSv, a radiation dose that’s in the same minimal risk category as a chest radiograph. In fact, for patients with high body mass, the radiation dose from ldCT of the SI joints can be less than that from a conventional radiograph.
“Could low-dose CT of the spine better detect new bone formation, compared to x-ray?” Dr. Lambert asked. A recent study attempted to answer the question, looking at 40 patients with ankylosing spondylitis who received ldCT at baseline and 2 years later (Ann Rheum Dis. 2018;77:371-7). In developing a CT syndesmophyte score (CTSS), two independent readers, blinded to the time order in which images were obtained, assessed vertebral syndesmophytes in the coronal and sagittal planes for each patient. The conclusion was that “new bone formation in the spine of patients with ankylosing spondylitis can be assessed reliably,” Dr. Lambert said.
A related study directly compared the new CTSS system with the modified Stoke Ankylosing Spondylitis Spine Score, used for conventional radiographs. Both studies used data from the Sensitive Imaging in Ankylosing Spondylitis cohort.
In this latter study, whole spine ldCT tracked progression better than conventional radiographs because it detected more new and growing syndesmophytes, Dr. Lambert said. One important reason for this was that conventional radiography only has utility in the cervical and lumbar spine and the pelvis, while most progression was seen in the thoracic spine with ldCT (Ann Rheum Dis. 2018;77:293-9).
The radiation dose for ldCT of the spine – approximately 4 mSv – is about 10 times that for ldCT of the SI joints, but still one-half to three-quarters of the dose for a whole-spine CT, Dr. Lambert said. Put another way, the ldCT whole-spine dose is nearly equivalent to the dose for the three radiographic studies required to image the cervical, thoracic, and lumbar spine.
Another imaging approach using CT zooms in on the thoracolumbar spine, imaging vertebrae T10-L4. Through sophisticated computational reconstruction techniques, the researchers were able to quantify syndesmophyte height circumferentially around each vertebra (J Rheumatol. 2015;42[3]:472-8).
The study, which imaged 33 patients at baseline and then at year 1 and year 2, found that the circumferential syndesmophyte height correlated well with spinal flexibility. Variation was low between two scans performed on the same day, at 0.893% per patient. Despite these advantages of high reliability and good sensitivity to change, one consideration for clinical consideration is the radiation dose, estimated about 8 mSv, Dr. Lambert noted.
Though MRI is a keystone for diagnosis and management of spondyloarthritis, Dr. Lambert pointed out that it’s more expensive than CT and still not routinely available everywhere. He also noted that reimbursement and prior authorizations may be easier to obtain for CT.
“Low-dose CT has tremendous research potential, especially in the thoracic spine,” said Dr. Lambert. “But it’s not ready for routine clinical use. First, the dose is not trivial, at about 4 mSv.” Also, it’s time consuming to interpret and not all CT scanners are compatible with ldCT techniques. “Lower dose can mean lower imaging quality,” and syndesmophytes can be harder to detect in larger individuals.
Dr. Lambert reported no relevant conflicts of interest.
MADISON, WISC. – said Robert Lambert, MD, speaking at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
“Low-dose CT of the SI [sacroiliac] joints is probably underutilized,” said Dr. Lambert, chair of the department of radiology and diagnostic imaging at the University of Alberta, Edmonton. “Subtle bony changes are demonstrated very well, and it can be an excellent test for resolving equivocal findings on x-ray or MRI.”
An important first step in making imaging decisions is to put concerns about radiation exposure in context, said Dr. Lambert. “Today, almost all CT effective exposure doses are considered low risk.”
Older studies have shown that a conventional two-view chest radiograph delivers a dose of 0.1 mSv – the equivalent of 10 days of background radiation – whereas the highest radiation dose is delivered by a CT scan of the abdomen and pelvis with and without contrast. This examination delivers an effective dose of 20 mSv, the equivalent of 7 years of background radiation. Dr. Lambert pointed out what the “moderate” additional lifetime risk of malignancy – 1:500 – associated with this scan looks like in real-world numbers: “So the lifetime risk of cancer would increase from 20% to 20.2%.”
Recently, measurements of effective doses delivered in low-dose CT (ldCT) have shown that “most doses are significantly lower than previously quoted,” said Dr. Lambert. For example, ldCT of the SI joints delivers just 0.42 mSv, a radiation dose that’s in the same minimal risk category as a chest radiograph. In fact, for patients with high body mass, the radiation dose from ldCT of the SI joints can be less than that from a conventional radiograph.
“Could low-dose CT of the spine better detect new bone formation, compared to x-ray?” Dr. Lambert asked. A recent study attempted to answer the question, looking at 40 patients with ankylosing spondylitis who received ldCT at baseline and 2 years later (Ann Rheum Dis. 2018;77:371-7). In developing a CT syndesmophyte score (CTSS), two independent readers, blinded to the time order in which images were obtained, assessed vertebral syndesmophytes in the coronal and sagittal planes for each patient. The conclusion was that “new bone formation in the spine of patients with ankylosing spondylitis can be assessed reliably,” Dr. Lambert said.
A related study directly compared the new CTSS system with the modified Stoke Ankylosing Spondylitis Spine Score, used for conventional radiographs. Both studies used data from the Sensitive Imaging in Ankylosing Spondylitis cohort.
In this latter study, whole spine ldCT tracked progression better than conventional radiographs because it detected more new and growing syndesmophytes, Dr. Lambert said. One important reason for this was that conventional radiography only has utility in the cervical and lumbar spine and the pelvis, while most progression was seen in the thoracic spine with ldCT (Ann Rheum Dis. 2018;77:293-9).
The radiation dose for ldCT of the spine – approximately 4 mSv – is about 10 times that for ldCT of the SI joints, but still one-half to three-quarters of the dose for a whole-spine CT, Dr. Lambert said. Put another way, the ldCT whole-spine dose is nearly equivalent to the dose for the three radiographic studies required to image the cervical, thoracic, and lumbar spine.
Another imaging approach using CT zooms in on the thoracolumbar spine, imaging vertebrae T10-L4. Through sophisticated computational reconstruction techniques, the researchers were able to quantify syndesmophyte height circumferentially around each vertebra (J Rheumatol. 2015;42[3]:472-8).
The study, which imaged 33 patients at baseline and then at year 1 and year 2, found that the circumferential syndesmophyte height correlated well with spinal flexibility. Variation was low between two scans performed on the same day, at 0.893% per patient. Despite these advantages of high reliability and good sensitivity to change, one consideration for clinical consideration is the radiation dose, estimated about 8 mSv, Dr. Lambert noted.
Though MRI is a keystone for diagnosis and management of spondyloarthritis, Dr. Lambert pointed out that it’s more expensive than CT and still not routinely available everywhere. He also noted that reimbursement and prior authorizations may be easier to obtain for CT.
“Low-dose CT has tremendous research potential, especially in the thoracic spine,” said Dr. Lambert. “But it’s not ready for routine clinical use. First, the dose is not trivial, at about 4 mSv.” Also, it’s time consuming to interpret and not all CT scanners are compatible with ldCT techniques. “Lower dose can mean lower imaging quality,” and syndesmophytes can be harder to detect in larger individuals.
Dr. Lambert reported no relevant conflicts of interest.
MADISON, WISC. – said Robert Lambert, MD, speaking at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
“Low-dose CT of the SI [sacroiliac] joints is probably underutilized,” said Dr. Lambert, chair of the department of radiology and diagnostic imaging at the University of Alberta, Edmonton. “Subtle bony changes are demonstrated very well, and it can be an excellent test for resolving equivocal findings on x-ray or MRI.”
An important first step in making imaging decisions is to put concerns about radiation exposure in context, said Dr. Lambert. “Today, almost all CT effective exposure doses are considered low risk.”
Older studies have shown that a conventional two-view chest radiograph delivers a dose of 0.1 mSv – the equivalent of 10 days of background radiation – whereas the highest radiation dose is delivered by a CT scan of the abdomen and pelvis with and without contrast. This examination delivers an effective dose of 20 mSv, the equivalent of 7 years of background radiation. Dr. Lambert pointed out what the “moderate” additional lifetime risk of malignancy – 1:500 – associated with this scan looks like in real-world numbers: “So the lifetime risk of cancer would increase from 20% to 20.2%.”
Recently, measurements of effective doses delivered in low-dose CT (ldCT) have shown that “most doses are significantly lower than previously quoted,” said Dr. Lambert. For example, ldCT of the SI joints delivers just 0.42 mSv, a radiation dose that’s in the same minimal risk category as a chest radiograph. In fact, for patients with high body mass, the radiation dose from ldCT of the SI joints can be less than that from a conventional radiograph.
“Could low-dose CT of the spine better detect new bone formation, compared to x-ray?” Dr. Lambert asked. A recent study attempted to answer the question, looking at 40 patients with ankylosing spondylitis who received ldCT at baseline and 2 years later (Ann Rheum Dis. 2018;77:371-7). In developing a CT syndesmophyte score (CTSS), two independent readers, blinded to the time order in which images were obtained, assessed vertebral syndesmophytes in the coronal and sagittal planes for each patient. The conclusion was that “new bone formation in the spine of patients with ankylosing spondylitis can be assessed reliably,” Dr. Lambert said.
A related study directly compared the new CTSS system with the modified Stoke Ankylosing Spondylitis Spine Score, used for conventional radiographs. Both studies used data from the Sensitive Imaging in Ankylosing Spondylitis cohort.
In this latter study, whole spine ldCT tracked progression better than conventional radiographs because it detected more new and growing syndesmophytes, Dr. Lambert said. One important reason for this was that conventional radiography only has utility in the cervical and lumbar spine and the pelvis, while most progression was seen in the thoracic spine with ldCT (Ann Rheum Dis. 2018;77:293-9).
The radiation dose for ldCT of the spine – approximately 4 mSv – is about 10 times that for ldCT of the SI joints, but still one-half to three-quarters of the dose for a whole-spine CT, Dr. Lambert said. Put another way, the ldCT whole-spine dose is nearly equivalent to the dose for the three radiographic studies required to image the cervical, thoracic, and lumbar spine.
Another imaging approach using CT zooms in on the thoracolumbar spine, imaging vertebrae T10-L4. Through sophisticated computational reconstruction techniques, the researchers were able to quantify syndesmophyte height circumferentially around each vertebra (J Rheumatol. 2015;42[3]:472-8).
The study, which imaged 33 patients at baseline and then at year 1 and year 2, found that the circumferential syndesmophyte height correlated well with spinal flexibility. Variation was low between two scans performed on the same day, at 0.893% per patient. Despite these advantages of high reliability and good sensitivity to change, one consideration for clinical consideration is the radiation dose, estimated about 8 mSv, Dr. Lambert noted.
Though MRI is a keystone for diagnosis and management of spondyloarthritis, Dr. Lambert pointed out that it’s more expensive than CT and still not routinely available everywhere. He also noted that reimbursement and prior authorizations may be easier to obtain for CT.
“Low-dose CT has tremendous research potential, especially in the thoracic spine,” said Dr. Lambert. “But it’s not ready for routine clinical use. First, the dose is not trivial, at about 4 mSv.” Also, it’s time consuming to interpret and not all CT scanners are compatible with ldCT techniques. “Lower dose can mean lower imaging quality,” and syndesmophytes can be harder to detect in larger individuals.
Dr. Lambert reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM SPARTAN 2019
VA system lags in getting DMARDs to veterans with inflammatory arthritis
MADISON, WISC. – Only half of United States veterans with inflammatory arthritis received disease-modifying medication within 90 days of diagnosis if they received care within the Veterans Health Administration, according to a study presented at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Over the study period, 58.2% of all inflammatory arthritis patients began a disease-modifying antirheumatic drug (DMARD) within 12 months of diagnosis. Rates of DMARD initiation were similar for patients with rheumatoid arthritis (RA, 57.7%) and psoriatic arthritis (PsA, 64.3%), said the first author of the poster presentation, Sogol S. Amjadi, DO, a resident physician at Bingham Memorial Hospital, Blackfoot, Idaho.
However, at 12 months after diagnosis, only 29.6% of ankylosing spondylitis (AS) patients had not been started on a DMARD. “The ankylosing spondylitis group really had the lowest DMARD initiation over time,” said Dr. Amjadi in an interview.
The study used diagnosis codes and natural language processing to look for incident cases of the three inflammatory arthritides (IAs) among patients receiving care within the Veterans Health Administration from 2007 through 2015.
In all, 12,118 patients with incident IA were identified. Of these, 9,711 had RA, 1,472 had PsA, and 935 had AS. Patients were mostly (91.3%) male, with a mean age of 63.7 years.
Over the study period, 41.2% of IA patients were dispensed a DMARD within 30 days of diagnosis, and 50% received a DMARD within 90 days of diagnosis. Patients with PsA or RA had similar rates of DMARD prescription within 30 days of diagnosis (about 42% and 43%, respectively).
The investigators discovered in their analysis that another factor in prompt treatment was access to specialty care.“Timely access to a rheumatology provider is likely important for early DMARD treatment,” wrote Dr. Amjadi and her coauthors in the poster accompanying the presentation. Of patients who did receive a DMARD, 82.7% had received rheumatology specialty care before nonbiologic DMARD dispensing, as had 90.0% of patients receiving biologic DMARDs. Over the entire study period, about 10% of all IA patients had biologic DMARD exposure.
There was a trend over time for increased DMARD dispensing, said the investigators. “The percentage of IA patients with DMARD exposure during the 12-month follow-up period increased from 48.8% in 2008 to 66.4% in 2015.”
For AS patients, early DMARD prescribing rates rose from about 20% in 2007 to nearly 30% in 2015. “DMARD treatment rates during the initial 12 months after diagnosis increased between 2007 and 2015, but nontreatment remained common, particularly in patients with AS,” wrote the investigators. “Delays in treatment for inflammatory arthritis are associated with unfavorable outcomes, including impaired quality of life, irreversible joint damage, and disability.”
The authors reported no conflicts of interest and no outside sources of funding.
MADISON, WISC. – Only half of United States veterans with inflammatory arthritis received disease-modifying medication within 90 days of diagnosis if they received care within the Veterans Health Administration, according to a study presented at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Over the study period, 58.2% of all inflammatory arthritis patients began a disease-modifying antirheumatic drug (DMARD) within 12 months of diagnosis. Rates of DMARD initiation were similar for patients with rheumatoid arthritis (RA, 57.7%) and psoriatic arthritis (PsA, 64.3%), said the first author of the poster presentation, Sogol S. Amjadi, DO, a resident physician at Bingham Memorial Hospital, Blackfoot, Idaho.
However, at 12 months after diagnosis, only 29.6% of ankylosing spondylitis (AS) patients had not been started on a DMARD. “The ankylosing spondylitis group really had the lowest DMARD initiation over time,” said Dr. Amjadi in an interview.
The study used diagnosis codes and natural language processing to look for incident cases of the three inflammatory arthritides (IAs) among patients receiving care within the Veterans Health Administration from 2007 through 2015.
In all, 12,118 patients with incident IA were identified. Of these, 9,711 had RA, 1,472 had PsA, and 935 had AS. Patients were mostly (91.3%) male, with a mean age of 63.7 years.
Over the study period, 41.2% of IA patients were dispensed a DMARD within 30 days of diagnosis, and 50% received a DMARD within 90 days of diagnosis. Patients with PsA or RA had similar rates of DMARD prescription within 30 days of diagnosis (about 42% and 43%, respectively).
The investigators discovered in their analysis that another factor in prompt treatment was access to specialty care.“Timely access to a rheumatology provider is likely important for early DMARD treatment,” wrote Dr. Amjadi and her coauthors in the poster accompanying the presentation. Of patients who did receive a DMARD, 82.7% had received rheumatology specialty care before nonbiologic DMARD dispensing, as had 90.0% of patients receiving biologic DMARDs. Over the entire study period, about 10% of all IA patients had biologic DMARD exposure.
There was a trend over time for increased DMARD dispensing, said the investigators. “The percentage of IA patients with DMARD exposure during the 12-month follow-up period increased from 48.8% in 2008 to 66.4% in 2015.”
For AS patients, early DMARD prescribing rates rose from about 20% in 2007 to nearly 30% in 2015. “DMARD treatment rates during the initial 12 months after diagnosis increased between 2007 and 2015, but nontreatment remained common, particularly in patients with AS,” wrote the investigators. “Delays in treatment for inflammatory arthritis are associated with unfavorable outcomes, including impaired quality of life, irreversible joint damage, and disability.”
The authors reported no conflicts of interest and no outside sources of funding.
MADISON, WISC. – Only half of United States veterans with inflammatory arthritis received disease-modifying medication within 90 days of diagnosis if they received care within the Veterans Health Administration, according to a study presented at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Over the study period, 58.2% of all inflammatory arthritis patients began a disease-modifying antirheumatic drug (DMARD) within 12 months of diagnosis. Rates of DMARD initiation were similar for patients with rheumatoid arthritis (RA, 57.7%) and psoriatic arthritis (PsA, 64.3%), said the first author of the poster presentation, Sogol S. Amjadi, DO, a resident physician at Bingham Memorial Hospital, Blackfoot, Idaho.
However, at 12 months after diagnosis, only 29.6% of ankylosing spondylitis (AS) patients had not been started on a DMARD. “The ankylosing spondylitis group really had the lowest DMARD initiation over time,” said Dr. Amjadi in an interview.
The study used diagnosis codes and natural language processing to look for incident cases of the three inflammatory arthritides (IAs) among patients receiving care within the Veterans Health Administration from 2007 through 2015.
In all, 12,118 patients with incident IA were identified. Of these, 9,711 had RA, 1,472 had PsA, and 935 had AS. Patients were mostly (91.3%) male, with a mean age of 63.7 years.
Over the study period, 41.2% of IA patients were dispensed a DMARD within 30 days of diagnosis, and 50% received a DMARD within 90 days of diagnosis. Patients with PsA or RA had similar rates of DMARD prescription within 30 days of diagnosis (about 42% and 43%, respectively).
The investigators discovered in their analysis that another factor in prompt treatment was access to specialty care.“Timely access to a rheumatology provider is likely important for early DMARD treatment,” wrote Dr. Amjadi and her coauthors in the poster accompanying the presentation. Of patients who did receive a DMARD, 82.7% had received rheumatology specialty care before nonbiologic DMARD dispensing, as had 90.0% of patients receiving biologic DMARDs. Over the entire study period, about 10% of all IA patients had biologic DMARD exposure.
There was a trend over time for increased DMARD dispensing, said the investigators. “The percentage of IA patients with DMARD exposure during the 12-month follow-up period increased from 48.8% in 2008 to 66.4% in 2015.”
For AS patients, early DMARD prescribing rates rose from about 20% in 2007 to nearly 30% in 2015. “DMARD treatment rates during the initial 12 months after diagnosis increased between 2007 and 2015, but nontreatment remained common, particularly in patients with AS,” wrote the investigators. “Delays in treatment for inflammatory arthritis are associated with unfavorable outcomes, including impaired quality of life, irreversible joint damage, and disability.”
The authors reported no conflicts of interest and no outside sources of funding.
REPORTING FROM SPARTAN 2019
Key clinical point:
Major finding: Overall, 58.2% of inflammatory arthritis patients received a DMARD within the first year of diagnosis.
Study details: Retrospective review of 12,118 incident cases of inflammatory arthritis in the Veterans Health Administration during the period from 2007 through 2015.
Disclosures: The authors reported no conflicts of interest and no outside sources of funding.
Source: Amjadi SS et al. SPARTAN 2019.
Ankylosing spondylitis patients taking COX-2 inhibitors may see fewer cardiovascular events
MADISON, WISC. – Patients with ankylosing spondylitis had a small but significant reduction in risk for cardiovascular events if they were taking cyclooxygenase-2 (COX-2) inhibitors, according to a new systematic review and meta-analysis.
The reduced risk observed in this analysis (risk ratio, 0.48; 95% confidence interval, 0.33-0.70) contrasts with the increased risk for cardiovascular events seen with COX-2 inhibitor use in the general population, said Paras Karmacharya, MBBS, speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN). The overall effect was highly statistically significant (P = .0001), and the finding provides “reassuring” data for a population that’s known to have an elevated risk for cardiovascular events, he said.
“[W]e found in the subgroup analysis that COX-2 inhibitors were associated with a reduced risk of cardiovascular events as a whole,” an association also seen when looking just at ischemic stroke, Dr. Karmacharya said. “So that was sort of surprising; in the general population, there are some concerns about using COX-2 inhibitors.”
Looking at data for the nine studies that met criteria for inclusion in the meta-analysis, Dr. Karmacharya, a rheumatology fellow at the Mayo Clinic, Rochester, Minn., and his collaborators calculated risk ratios for a composite outcome of all cardiovascular events (CVE) for all individuals taking NSAIDs, compared with individuals with ankylosing spondylitis (AS) who were not taking NSAIDs. Here, they found a relative risk of 0.94 (95% CI, 0.50-1.75; P = .84).
Next, the investigators calculated a relative risk of 0.78 for the composite CVE outcome just for those taking nonselective NSAIDs (95% CI, 0.44-1.38; P = .40).
Along with NSAIDs, Dr. Karmacharya and his coauthors also looked at the relationship between tumor necrosis factor inhibitors (TNFIs) and cardiovascular events. They found a significantly increased risk for the composite endpoint among AS patients taking TNFIs (RR, 1.60; 95% CI, 1.05-2.41; P = .03), but the comparison was limited to only one study.
In their analysis, the investigators also broke out risk of acute coronary syndrome (ACS)/ischemic heart disease. “The only place where we found some increased risk was ACS and ischemic heart disease, and that was with nonselective NSAIDS,” Dr. Karmacharya said (RR, 1.21; 95% CI, 1.06-1.39; P = .005). No significant changes in relative risk for ACS and ischemic heart disease were seen for the total group of NSAID users, for the subgroups taking COX-2 inhibitors, or for those taking TNFIs.
Finally, the investigators found a relative risk of 0.58 for stroke among the full group of NSAID users and a relative risk of 0.59 for those taking COX-2 inhibitors, but no reduced risk for the subgroup taking nonselective NSAIDs (P = .02, .04, and .37, respectively).
“While NSAIDs are known to be associated with an increased risk of CVE in the general population, whether the anti-inflammatory effects of NSAIDs reduce or modify the CVE risk in AS is controversial,” Dr. Karmacharya and his collaborators wrote. In this context, the meta-analysis provides a useful perspective for rheumatologists who care for AS patients, Dr. Karmacharya said: “I think it’s important, because most of these patients are on NSAIDs long-term.”
However, all of the studies included in the meta-analysis were observational, with no randomized, controlled trials meeting inclusion criteria. Also, some analyses presented in the poster involved as few as two studies, so findings should be interpreted with caution, he added. “We don’t have a lot of studies included in the analysis. ... so we need more data for sure, but I think what data we have so far look reassuring.”
Dr. Karmacharya reported that he had no conflicts of interest, and reported no outside sources of funding.
MADISON, WISC. – Patients with ankylosing spondylitis had a small but significant reduction in risk for cardiovascular events if they were taking cyclooxygenase-2 (COX-2) inhibitors, according to a new systematic review and meta-analysis.
The reduced risk observed in this analysis (risk ratio, 0.48; 95% confidence interval, 0.33-0.70) contrasts with the increased risk for cardiovascular events seen with COX-2 inhibitor use in the general population, said Paras Karmacharya, MBBS, speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN). The overall effect was highly statistically significant (P = .0001), and the finding provides “reassuring” data for a population that’s known to have an elevated risk for cardiovascular events, he said.
“[W]e found in the subgroup analysis that COX-2 inhibitors were associated with a reduced risk of cardiovascular events as a whole,” an association also seen when looking just at ischemic stroke, Dr. Karmacharya said. “So that was sort of surprising; in the general population, there are some concerns about using COX-2 inhibitors.”
Looking at data for the nine studies that met criteria for inclusion in the meta-analysis, Dr. Karmacharya, a rheumatology fellow at the Mayo Clinic, Rochester, Minn., and his collaborators calculated risk ratios for a composite outcome of all cardiovascular events (CVE) for all individuals taking NSAIDs, compared with individuals with ankylosing spondylitis (AS) who were not taking NSAIDs. Here, they found a relative risk of 0.94 (95% CI, 0.50-1.75; P = .84).
Next, the investigators calculated a relative risk of 0.78 for the composite CVE outcome just for those taking nonselective NSAIDs (95% CI, 0.44-1.38; P = .40).
Along with NSAIDs, Dr. Karmacharya and his coauthors also looked at the relationship between tumor necrosis factor inhibitors (TNFIs) and cardiovascular events. They found a significantly increased risk for the composite endpoint among AS patients taking TNFIs (RR, 1.60; 95% CI, 1.05-2.41; P = .03), but the comparison was limited to only one study.
In their analysis, the investigators also broke out risk of acute coronary syndrome (ACS)/ischemic heart disease. “The only place where we found some increased risk was ACS and ischemic heart disease, and that was with nonselective NSAIDS,” Dr. Karmacharya said (RR, 1.21; 95% CI, 1.06-1.39; P = .005). No significant changes in relative risk for ACS and ischemic heart disease were seen for the total group of NSAID users, for the subgroups taking COX-2 inhibitors, or for those taking TNFIs.
Finally, the investigators found a relative risk of 0.58 for stroke among the full group of NSAID users and a relative risk of 0.59 for those taking COX-2 inhibitors, but no reduced risk for the subgroup taking nonselective NSAIDs (P = .02, .04, and .37, respectively).
“While NSAIDs are known to be associated with an increased risk of CVE in the general population, whether the anti-inflammatory effects of NSAIDs reduce or modify the CVE risk in AS is controversial,” Dr. Karmacharya and his collaborators wrote. In this context, the meta-analysis provides a useful perspective for rheumatologists who care for AS patients, Dr. Karmacharya said: “I think it’s important, because most of these patients are on NSAIDs long-term.”
However, all of the studies included in the meta-analysis were observational, with no randomized, controlled trials meeting inclusion criteria. Also, some analyses presented in the poster involved as few as two studies, so findings should be interpreted with caution, he added. “We don’t have a lot of studies included in the analysis. ... so we need more data for sure, but I think what data we have so far look reassuring.”
Dr. Karmacharya reported that he had no conflicts of interest, and reported no outside sources of funding.
MADISON, WISC. – Patients with ankylosing spondylitis had a small but significant reduction in risk for cardiovascular events if they were taking cyclooxygenase-2 (COX-2) inhibitors, according to a new systematic review and meta-analysis.
The reduced risk observed in this analysis (risk ratio, 0.48; 95% confidence interval, 0.33-0.70) contrasts with the increased risk for cardiovascular events seen with COX-2 inhibitor use in the general population, said Paras Karmacharya, MBBS, speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN). The overall effect was highly statistically significant (P = .0001), and the finding provides “reassuring” data for a population that’s known to have an elevated risk for cardiovascular events, he said.
“[W]e found in the subgroup analysis that COX-2 inhibitors were associated with a reduced risk of cardiovascular events as a whole,” an association also seen when looking just at ischemic stroke, Dr. Karmacharya said. “So that was sort of surprising; in the general population, there are some concerns about using COX-2 inhibitors.”
Looking at data for the nine studies that met criteria for inclusion in the meta-analysis, Dr. Karmacharya, a rheumatology fellow at the Mayo Clinic, Rochester, Minn., and his collaborators calculated risk ratios for a composite outcome of all cardiovascular events (CVE) for all individuals taking NSAIDs, compared with individuals with ankylosing spondylitis (AS) who were not taking NSAIDs. Here, they found a relative risk of 0.94 (95% CI, 0.50-1.75; P = .84).
Next, the investigators calculated a relative risk of 0.78 for the composite CVE outcome just for those taking nonselective NSAIDs (95% CI, 0.44-1.38; P = .40).
Along with NSAIDs, Dr. Karmacharya and his coauthors also looked at the relationship between tumor necrosis factor inhibitors (TNFIs) and cardiovascular events. They found a significantly increased risk for the composite endpoint among AS patients taking TNFIs (RR, 1.60; 95% CI, 1.05-2.41; P = .03), but the comparison was limited to only one study.
In their analysis, the investigators also broke out risk of acute coronary syndrome (ACS)/ischemic heart disease. “The only place where we found some increased risk was ACS and ischemic heart disease, and that was with nonselective NSAIDS,” Dr. Karmacharya said (RR, 1.21; 95% CI, 1.06-1.39; P = .005). No significant changes in relative risk for ACS and ischemic heart disease were seen for the total group of NSAID users, for the subgroups taking COX-2 inhibitors, or for those taking TNFIs.
Finally, the investigators found a relative risk of 0.58 for stroke among the full group of NSAID users and a relative risk of 0.59 for those taking COX-2 inhibitors, but no reduced risk for the subgroup taking nonselective NSAIDs (P = .02, .04, and .37, respectively).
“While NSAIDs are known to be associated with an increased risk of CVE in the general population, whether the anti-inflammatory effects of NSAIDs reduce or modify the CVE risk in AS is controversial,” Dr. Karmacharya and his collaborators wrote. In this context, the meta-analysis provides a useful perspective for rheumatologists who care for AS patients, Dr. Karmacharya said: “I think it’s important, because most of these patients are on NSAIDs long-term.”
However, all of the studies included in the meta-analysis were observational, with no randomized, controlled trials meeting inclusion criteria. Also, some analyses presented in the poster involved as few as two studies, so findings should be interpreted with caution, he added. “We don’t have a lot of studies included in the analysis. ... so we need more data for sure, but I think what data we have so far look reassuring.”
Dr. Karmacharya reported that he had no conflicts of interest, and reported no outside sources of funding.
REPORTING FROM SPARTAN 2019
Key clinical point: Individuals with ankylosing spondylitis (AS) who took cyclooxygenase 2 (COX-2) inhibitors had a reduced risk of cardiovascular events, compared with AS patients who were not using COX-2 inhibitors.
Major finding: Individuals with AS taking COX-2 inhibitors had a risk ratio of 0.48 for cardiovascular events (95% CI, 0.33-0.70; P = .001).
Study details: Systematic review and meta-analysis of nine observational studies that variably examined the association between NSAID use and tumor necrosis factor inhibitor use and cardiovascular events among individuals with AS.
Disclosures: The authors reported no conflicts of interest and no outside sources of funding.
Source: Karmacharya P. et al. SPARTAN 2019.
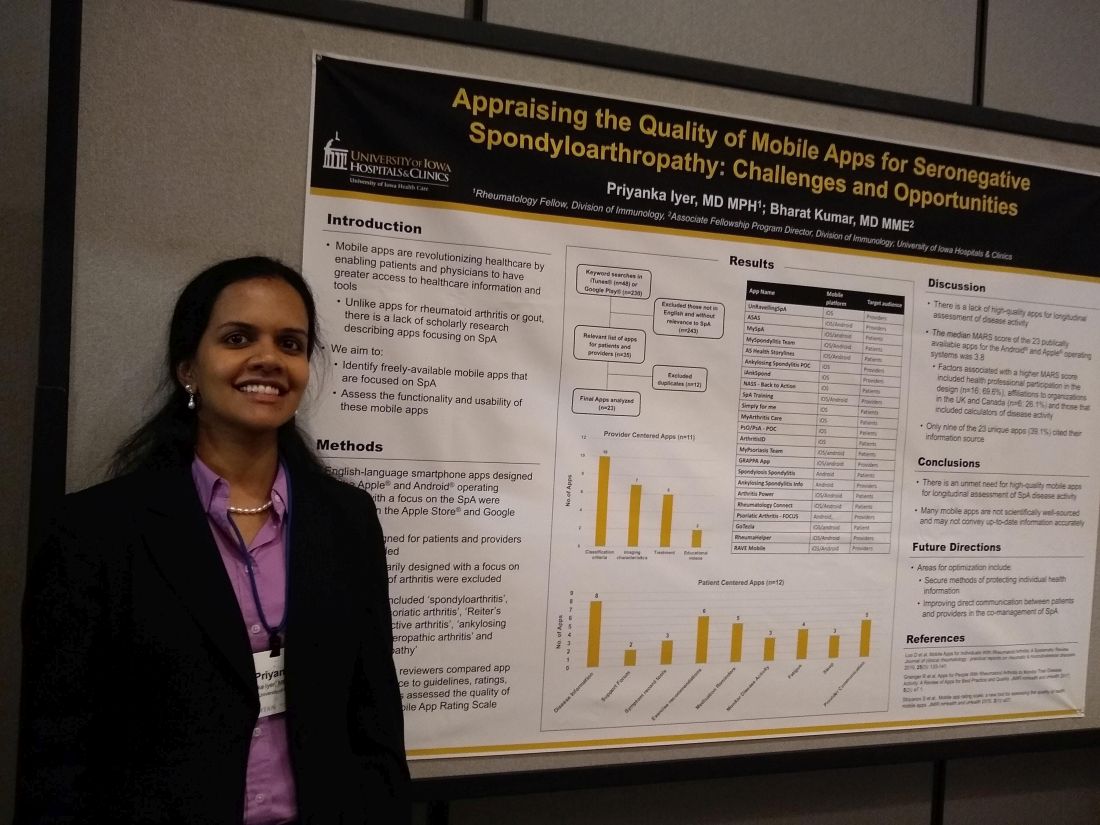
Mobile SpA apps abound, but there’s room for quality improvement
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
REPORTING FROM SPARTAN 2019