User login
In 2015, more than 35,660 new cases of liver cancer and 24,550 liver cancer-related deaths are expected to occur in the U.S. About 80% of these cases will consist of hepatocellular carcinoma, (HCC).1 The incidence of HCC varies throughout the world: Incidence is as low as 5 in 100,000 individuals in North America and ranges up to > 20 in 100,000 individuals in sub-Saharan Africa and Eastern Asia.2 Nearly half of all cases of HCC are associated with hepatitis B virus (HBV), and another 25% are associated with hepatitis C virus (HCV). Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, flatoxin-contaminated food, diabetes, and obesity.3
Therapeutic options for advanced HCC are limited. The FDA approved sorafenib in 2008 for the treatment of unresectable HCC.4 According to the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with Stage C liver cancer may undergo a trial of sorafenib.4 National Comprehensive Cancer Network (NCCN) clinical guidelines for hepatobiliary cancers reserve sorafenib for patients with inoperable tumors, metastatic disease, or extensive liver tumor burden.5 Sorafenib is shown to inhibit multiple intracellular and cell surface kinases. Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis, and apoptosis.4 In the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial, median overall survival (OS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group.6 The predicted survival rates at 1 year were 44% in the sorafenib group and 33% in the placebo group.6The economic impact of oral chemotherapy on health care cannot be discounted. At about $50,000 to $100,000 per quality- adjusted life-year, the incremental cost-effectiveness ratio (ICER) of sorafenib over placebo was $62,473 per quality-adjusted life-year in 2007.7The purpose of this retrospective chart review was to evaluate sorafenib for efficacy and safety in a veteran population. Veterans have poorer health and more medical conditions compared with nonveterans.8 Furthermore, in the VHA, about 170,000 veterans have HCV.9 The rate of progression from HCV to HCC is about 3% to 5% annually. More than half of those diagnosed with HCC are late stage, and unfortunately, the 5-year OS rate for patients with liver cancer is 9% and 4% for those patients who are diagnosed at regional and distant stages of the disease.1 As the practice of oncology grows, it is necessary for pharmacists to be involved in the selection of chemotherapeutic agents in order to provide optimal pharmaceutical care.10
Related: VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinoma
Methods
A retrospective chart review was conducted to identify patients who were prescribed sorafenib from November 1, 2007, to September 30, 2011, at the VA Greater Los Angeles Healthcare System (VAGLAHS). Inclusion criteria included patients who had a diagnosis of advanced HCC, who were initiated and managed by a VAGLAHS provider and who were eligible for a 1-year safety evaluation period. The study was approved by the VAGLAHS institutional review board.
Baseline demographic, clinical, laboratory, and medication data were collected. Demographic, clinical, laboratory, and medication data were obtained from CPRS (Computerized Patient Record System) and VistA (Veterans Health Information Systems and Technology Architecture). Data were collected on secured servers and saved on encrypted files. The master list was destroyed once the records control schedule was finalized. No identifiers were collected on the data collection sheet.
Standard practice at VAGLAHS is to monitor European Cooperative Oncology Group Performance Status (ECOG-PS), Child-Pugh class, and alpha-fetoprotein (AFP) at initiation and every 3 months and to obtain laboratory data at initiation and every month before each medication refill. Patients were seen in the Oncology Clinic periodically at the provider’s discretion. The time of drug discontinuation and the reason for drug discontinuation were recorded. Time of death at any point was collected to measure OS.
It was determined that a total sample size of 42 patients would be insufficient to achieve 80% power to demonstrate any hypothesized effects. However, the Fisher exact test was used to calculate P values for simple comparison. Patient demographics and clinical characteristics were reported as total numbers and frequencies when applicable. Survival rate was measured from the time of sorafenib initiation to 1 year after therapy initiation. Overall survival was measured from the time of sorafenib initiation to time of death. Duration of therapy was measured from the time of sorafenib initiation to time of discontinuation, either by provider or by patient.
Results
There were 83 patients who were prescribed sorafenib between November 1, 2007, and September 30, 2011. Of the 83 patients, 27 patients were ineligible for a 1-year follow-up period, 9 patients were diagnosed with non-HCC, 3 were initiated or managed by providers outside the institution, and 2 were not started on therapy. In all, 42 patients met inclusion criteria and had received at least 1 dose of sorafenib. The primary etiologies for HCC were history of alcohol abuse, HCV, and HBV. The primary risk factors were obesity, smoking, and diabetes. Many patients presented with multiple etiologies and risk factors. Ten patients (23.8%) had moderate-to-severe hepatic impairment (Child-Pugh class B or C). Baseline characteristics of these patients are listed in Table 1.
Efficacy
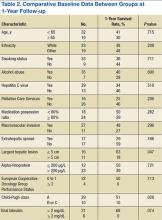
The median OS was 5.9 months and ranged from 21 days to 60 months. There were 17 patients who survived at the 1-year follow-up, including 1 patient who survived 363 days after treatment initiation, yielding an OS rate of 40.5%. Table 2 presents 1-year survival rates with respect to select baseline data. Baseline factors found to be negligible were age, smoking, alcohol abuse, obesity, presence of HCV, medication possession ratio (MPR), prior treatment, macrovascular invasion, and AFP. Neither initial dose regimen, final dose regimen achieved, or average dose correlated with the survival rate at the 1-year follow-up.
Factors possibly associated with a higher probability of survival were baseline ECOG-PS score and baseline Child-Pugh class (Table 2). Patients with an ECOG-PS score of 0 or 1 had a higher survival rate at 1 year than did patients with an ECOG-PS score of ≥ 2 (50% vs 0%, respectively; P = .113). Patients with Child-Pugh class B or C had a lower survival rate at 1 year than did patients with Child-Pugh class A (51% vs 10%, respectively; P = .028). Other indicators were size of largest hepatic lesion ≤ 5 cm, total bilirubin ≤ 2 mg/dL, concurrent treatment, almost exclusively embolization, and treatment after sorafenib discontinuation, such as another oral chemotherapeutic agent or embolization.
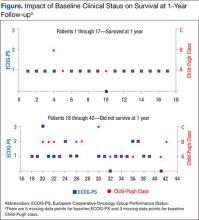
The 17 patients who survived at 1 year were reviewed to see if they shared characteristics that indicated a higher probability of survival. The figure shows the baseline ECOG-PS score and the Child-Pugh class the patients who did and did not survive at the 1-year follow-up. In the first group, all patient possessed an ECOG-PS score of 0 or 1, and only 1 patient presented with Child-Pugh class B or C. In contrast, in the group who did not survive at the 1-year follow-up, there were 4 patients with ECOG-PS scores of > 1 and 9 patients who presented with Child-Pugh class B or C. The mean AFP level of this group was < 200 µg/mL, and only 4 patients were followed by Palliative Care Services. The average normalized MPR of this group was 71.9% compared with 85.3% for those who did not survive at the 1-year follow-up.
In patients who experienced at least 1 adverse event (AE), 16 survived, whereas only 1 who did not experience an AE survived (45.7% vs 14.3%, respectively; P = .210). Thirteen patients who experienced ≥ 3 AEs survived at 1 year; and only 3 patients who reported < 3 AEs survived at 1 year (61.9% vs 14.0%, respectively; P = .011). However, when the number of AEs was normalized to duration of treatment per patient, the median frequency of AEs for all patients was 0.61 AEs per month treated. The difference in survival rates grew smaller and less significant between patients who had a frequency of AEs lower than the median compared with those with a higher ratio (52.4% vs 28.6%, respectively; P = .208). Patients affected by AEs in the first 30 days and 90 days of treatment had a survival rate at the 1-year follow-up of 42.4% and 30.2%, respectively. Patients who experienced dermatologic AEs had a higher survival rate than those who did not have dermatologic AEs (60.0% vs 29.6%, respectively; P = .099). This correlation was not found with 2 other classes of AEs, gastrointestinal (50.0% vs 27.8%; P = .208) or neurologic (64.0% vs 41.2%; P = .209).
The median overall time to discontinuation was 3.4 months. The main reasons cited for discontinuing sorafenib at 1 year included symptomatic progression (52.4%), radiographic progression (23.8%), severe AEs (16.7%), and mild-to-moderate AEs (11.9%). There was overlap, as 15 patients discontinued treatment for multiple reasons. For the 22 patients who discontinued medication due to symptomatic progression at 1 year, the median time to discontinuation was 3.8 months. For the 10 patients who discontinued medication due to radiographic progression at 1 year, median time to discontinuation was 5.6 months. Seven patients (16.7%) were still on therapy at 1 year.The study considered the impact of potential dose adjustments on survival rate and safety. The authors compared patients’ prescribed dose with the recommended dose based on the package insert and monthly laboratory values if recorded. The prescribed dose was recorded as appropriate dose, below dose, above dose, or indeterminate due to the lack of current laboratory values. Patients who survived at the 1-year follow-up had a composition of 26%, 21%, 10%, and 43%, respectively. These results were similar to those of patients who did not survive at the 1-year follow-up, 29%, 12%, 30%, and 29%, respectively.
Based on medication refill history and VA acquisition cost, the total prescription drug cost of treating 42 patients with sorafenib was $388,370.40. The total number of days survived for these patients was 16,607 days, which equates to $8,535.87 per year lived.
Safety
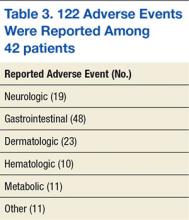
Of the 42 patients, 35 patients experienced ≥ 1 AE for a total of 122 AEs reported. The median number of AEs per patient was 2.5. The median time to the first AE was 21 days and ranged from 3 to 244 days. In the first 30 days of treatment, 23 patients (54.7%) reported 47 AEs (39.5%). In the first 90 days of treatment, 33 patients (78.6%) reported 88 AEs (73.9%). Common AEs in both instances were diarrhea, fatigue, erythematous plantar-palmar rash, and nausea (Table 3).
The predominant classes of AEs were GI (39.3%), dermatologic (18.9%), and neurologic (15.6%). Erythematous palmar-plantar rash, also known as hand-foot syndrome, has been noted as a potential dose-limiting sorafenib AE if the rash is recurrent or severe. One patient experienced recurrent grade-2 rashes, and sorafenib was immediately discontinued after an attempt to lower the dose. There were 8 patients who reported serious AEs, and 5 were hospitalized. One patient continued therapy despite GI hemorrhage. The other 4 patients discontinued therapy on hospitalization and were seen for intracranial hemorrhage, GI perforation, acute renal failure, and acute liver failure. In the first 3 cases, sorafenib could not be ruled out as the primary cause of death. None of these patients presented with comorbidities, such as hypertension, which predisposed them to AEs.
Overall, 38 patients ended therapy at the recommended regimen of 400 mg twice daily, and the average total daily dose was 619 mg, just below 80% of the recommended daily dose. Reasons for not achieving 400 mg twice daily included slow titration, AEs, and dose adjustments for compromised renal and hepatic function such as dialysis. Patients who had an ECOG-PS score of 0 or 1 or Child-Pugh class A reported ≥ 3 AEs, but when normalized to duration of treatment, no difference was observed. No correlations were found for average dose, creatinine clearance, aspartate aminotransferase, platelets, total bilirubin, or weight and number or frequency of AEs.
In regard to potential dose adjustments, the doses (400 mg twice daily, 600 mg daily [400 mg + 200 mg in 2 doses], 200 mg twice daily, and 200 mg daily) did not correlate well with AEs. Patients who had < 3 AEs presented with the breakdown 23%, 16%, 22%, and 38%, similar to patients who had ≥ 3 AEs—30%, 19%, 14%, and 37%. Likewise, patients who had a frequency of AEs lower than the median presented with the breakdown 22%, 22%, 15%, and 40% compared with patients who had more AEs than the median—37%, 9%, 23%, and 31%.
Related: Hepatocellular Carcinoma: To Biopsy or Not?
Discussion
Sorafenib is the only oral oncology medication approved by the FDA for treatment of unresectable HCC.3 Prior to sorafenib, the AASLD recommendation was supportive care for patients presenting with BCLC-Stage C liver cancer. However, guidelines changed when SHARP showed that sorafenib provided a survival benefit with a tolerable AE profile. The survival benefit of sorafenib has been replicated in a few large, multicenter trials. In Asia, Cheng and colleagues saw improved median OS of 6.5 months for sorafenib compared with 4.2 months with placebo, and in Italy, Iavarone and colleagues showed a median OS of 10.5 months without a placebo comparator.11,12
In the veteran population for this study, the OS rate of 40.5% was similar to the rate reported in the SHARP study, although the patients’ median OS fell short of the time described in SHARP and other trials. The medical complexities involved in treating veterans may explain this difference. The veteran population is heterogeneous with diverse ethnic backgrounds, several comorbidities, and varying degrees of organ dysfunction. The authors compared survival rates of different subgroups to test the hypothesis that the probability of survival while on therapy should not depend on demographics or medical history. However, in this study, patients with minimal impact from HCC, such as mild hepatic impairment and high-functional status, demonstrated higher survival rates at 1-year follow-up than did those without significant compromise.Although the high prevalence of HCV and alcohol abuse in the veteran population has resulted in a high incidence of hepatic dysfunction, this study suggests that these factors are independent of survival if liver function or integrity has not been compromised.9
Some researchers have hypothesized that clinical toxicities from tyrosine kinase inhibitors may correlate with survival.13 The authors noticed that the presentation of dermatologic AEs may reflect improved survival. In this study, patients who experienced ≥ 1 AE and ≥ 3 AEs had survival rates at the 1-year follow-up of 45.7% and 61.9%, respectively. Moreover, patients affected by AEs in the first 90 days of treatment had a survival rate at the 1-year follow-up of 42.4%.
Caution is advised when drawing conclusions from the number of AEs or when they appear, because this may falsely favor correlation. Patients who survive longer have additional time to report an AE. Therefore, the authors also looked at the ratio of AEs over time per patient to consider the number of AEs per duration of treatment and saw that there was little difference in survival rate in this regard. When considering patients affected by AEs only in the first 30 days of treatment, the survival rate at the 1-year follow-up fell to 30.2%.
A more likely factor for the survival of the 17 patients who were alive at the 1-year follow-up was their overall health relative to the rest of the study group. Overall health may indicate survival independent of sorafenib. The group of 17 who survived at the 1-year follow-up reflected a population that was different from the rest of the study population. The subset was generally healthier with better ECOG-PS scores and Child-Pugh classes, was not followed by Palliative Care Services, and had a mean AFP level under the threshold for diagnosis of HCC in patients who present with hepatic lesions and elevated AFP.14 This subset’s MPR, a surrogate marker for adherence, was less than the accepted threshold in clinical practice for oral medications.15Evaluating the patient’s dose regimen was expected to reveal a relationship between dosing and clinical outcomes, such as low survival rates with low doses or more AEs with high doses. However, the authors were not able to establish this link. In fact, the median time to discontinuation of 3.4 months for the study group, or duration of treatment, was much shorter than the median OS of 5.9 months.
These findings were consistent with Cabibbo and colleagues, who conducted a meta-analysis of survival rates for untreated patients and found that impaired performance status and Child-Pugh class B or C were independently associated with shorter survival.16 The SHARP study and Cheng and colleagues also attempted to exclude patients who were not Child-Pugh class A in their studies, which suggests a negligible correlation between sorafenib and survival time and a close relationship between baseline clinical status and survival.
The authors determined that prior treatment, including locoregional therapy, was not a factor in predicting survival. This observation is confirmed by the results of a phase 3 study that looked at sorafenib as adjuvant treatment for patients who had no detectable disease after surgical resection or local ablation.17 The trial did not meet its primary endpoint of improved recurrence-free survival. However, the authors observed in this study that 4 patients who underwent resection of the liver before sorafenib had a mean OS of 2.9 years. One patient, who was alive at the time of the study conclusion, received only 22 days of sorafenib treatment and survived for 4.9 years after sorafenib discontinuation. Patients who received concurrent or postsorafenib treatment had higher survival rates.
The cost of treatment in this study was found to be $8,535.87 per year lived. Although formal quality of life assessments were not captured, medication was discontinued at the first sign of disease progression or AE as determined by the provider or patient. When the cost of treatment was adjusted to account for median OS time and VA drug acquisition costs, estimated at average wholesale price minus 40%, the cost of treatment was within the threshold of $50,000-$100,000 per quality-adjusted life-year.7,18Of the 42 patients in this study, 28.6% discontinued therapy due to AEs, compared with 32% observed in the SHARP study. Common GI, dermatologic, and CNS AEs were comparable between the 2 studies. Serious AEs included intracranial hemorrhage, GI hemorrhage, GI perforation, acute liver failure, and acute renal failure; 3 of these events led to death. About 12% of patients experienced bleeding, regardless of severity, compared with the 18% seen in SHARP, despite no prior history of hemorrhage or GI perforation.5 The authors did not find any clinical factors at baseline that predisposed patients to AEs. It was also difficult to distinguish between drug-related AEs and general disease progression.
Although the authors did not find a relationship between dose or dose adjustments and the number or frequency of AEs, there were serious adverse outcomes in this study that were also rare complications observed in SHARP. The decision to start sorafenib should not be taken lightly.
Related: Diagnostic Dilemma of Hepatocellular Carcinoma Presenting as Hepatic Angiomyolipoma
Limitations
This retrospective review had several limitations. In SHARP and other large, multicenter trials, patients were continued on therapy until they experienced both symptomatic and radiographic progression. In this study, patients were discontinued at the first sign of progression, either symptomatic or radiographic or both. Had all patients remained on therapy until symptomatic and radiographic signs of progression were observed, there could have been a better correlation between duration of treatment and OS, symptomatic progression, or radiographic progression. The authors acknowledge, however, that there is diminishing benefit of administering chemotherapy when there are known and potentially serious AEs.
The data for this study were limited due to a small sample size, and it was not powered to evaluate for statistically significant characteristics between the patients who survived at the 1-year follow-up and the patients who did not survive at the 1-year follow-up. This information would be useful to identify potential prognostic factors and guide providers in sorafenib management. Finally, a long-term safety profile could not be established, as patients were evaluated for a 1-year period.
Ultimately, HCC is a multifactorial disease, and it is difficult to account for all potential confounding factors. Additional research, including studies comparing sunitinib or a control group to sorafenib, may provide further insight.
Conclusions
In light of these results, the authors believe that sorafenib may be considered for veterans with unresectable HCC and who are contraindicated for alternative treatments. One-year survival rates were similar to those seen in previous studies. However, there was no clear association between the duration of treatment and OS, and although the medication was well tolerated, there were also serious AEs. It is prudent to continually assess the need for therapy throughout the treatment period.
Pharmacists have a critical role in care for oncology patients, from the integration of certified clinical pharmacist practitioners into hematology-oncology clinics to patient monitoring through oral oncology pharmacy programs.19,20 These programs have been shown to improve patient outcomes and decrease overall health care use and may benefit the veteran population.
In this study, a veteran population achieved a survival rate at the 1-year follow-up similar to that found in SHARP: 40.5% vs 44%. However, OS was markedly shorter: 5.9 months vs 10.7 months. Patients with minimal impact from HCC, such as mild hepatic impairment and high functional status, demonstrated higher survival rates at the 1-year follow-up than did those with significant compromise. Thirty-five patients experienced ≥1 AE, most observed within the first 90 days of treatment, and for 3 patients, sorafenib could not be ruled out as the cause of death.
Sorafenib remains a viable therapeutic option for veterans with advanced HCC. However, it is uncertain how much benefit sorafenib affords to the veteran population, especially with the associated risks.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.
3. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
4. Nexavar [package insert]. Emeryville, CA: Bayer HealthCare Pharmaceuticals, Inc; 2009.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 2. 2015. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed October 13, 2015.
6. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
7. Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746.
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. National Viral Hepatitis Program. VHA Directive 1300.01. U.S. Department of Veterans Affairs Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1586. Updated February 22, 2013. Accessed October 13, 2015.
10. Patterson CJ. Best practices in specialty pharmacy management. J Manag Care Pharm. 2013;19(1):42-48.
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
12. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-2063.
13. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011;105(12):1811-1813.
14. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
15. Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999;5(1):47-51.
16. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283.
17. Bayer HealthCare. Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM). ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT00692770. Updated May 28, 2015. Accessed October 21, 2015.
18. Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods, 2009 Update (Version 2.0). J Manag Care Pharm. 2009;15(suppl 6-a):S3-S57.
19. Valgus JM, Faso A, Gregory KM, et al. Integration of a clinical pharmacist into the hematology-oncology clinics at an academic medical center. Am J Health Syst Pharm. 2011;68(7):613-619.
20. Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4(4):165-174.
In 2015, more than 35,660 new cases of liver cancer and 24,550 liver cancer-related deaths are expected to occur in the U.S. About 80% of these cases will consist of hepatocellular carcinoma, (HCC).1 The incidence of HCC varies throughout the world: Incidence is as low as 5 in 100,000 individuals in North America and ranges up to > 20 in 100,000 individuals in sub-Saharan Africa and Eastern Asia.2 Nearly half of all cases of HCC are associated with hepatitis B virus (HBV), and another 25% are associated with hepatitis C virus (HCV). Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, flatoxin-contaminated food, diabetes, and obesity.3
Therapeutic options for advanced HCC are limited. The FDA approved sorafenib in 2008 for the treatment of unresectable HCC.4 According to the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with Stage C liver cancer may undergo a trial of sorafenib.4 National Comprehensive Cancer Network (NCCN) clinical guidelines for hepatobiliary cancers reserve sorafenib for patients with inoperable tumors, metastatic disease, or extensive liver tumor burden.5 Sorafenib is shown to inhibit multiple intracellular and cell surface kinases. Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis, and apoptosis.4 In the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial, median overall survival (OS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group.6 The predicted survival rates at 1 year were 44% in the sorafenib group and 33% in the placebo group.6The economic impact of oral chemotherapy on health care cannot be discounted. At about $50,000 to $100,000 per quality- adjusted life-year, the incremental cost-effectiveness ratio (ICER) of sorafenib over placebo was $62,473 per quality-adjusted life-year in 2007.7The purpose of this retrospective chart review was to evaluate sorafenib for efficacy and safety in a veteran population. Veterans have poorer health and more medical conditions compared with nonveterans.8 Furthermore, in the VHA, about 170,000 veterans have HCV.9 The rate of progression from HCV to HCC is about 3% to 5% annually. More than half of those diagnosed with HCC are late stage, and unfortunately, the 5-year OS rate for patients with liver cancer is 9% and 4% for those patients who are diagnosed at regional and distant stages of the disease.1 As the practice of oncology grows, it is necessary for pharmacists to be involved in the selection of chemotherapeutic agents in order to provide optimal pharmaceutical care.10
Related: VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinoma
Methods
A retrospective chart review was conducted to identify patients who were prescribed sorafenib from November 1, 2007, to September 30, 2011, at the VA Greater Los Angeles Healthcare System (VAGLAHS). Inclusion criteria included patients who had a diagnosis of advanced HCC, who were initiated and managed by a VAGLAHS provider and who were eligible for a 1-year safety evaluation period. The study was approved by the VAGLAHS institutional review board.
Baseline demographic, clinical, laboratory, and medication data were collected. Demographic, clinical, laboratory, and medication data were obtained from CPRS (Computerized Patient Record System) and VistA (Veterans Health Information Systems and Technology Architecture). Data were collected on secured servers and saved on encrypted files. The master list was destroyed once the records control schedule was finalized. No identifiers were collected on the data collection sheet.
Standard practice at VAGLAHS is to monitor European Cooperative Oncology Group Performance Status (ECOG-PS), Child-Pugh class, and alpha-fetoprotein (AFP) at initiation and every 3 months and to obtain laboratory data at initiation and every month before each medication refill. Patients were seen in the Oncology Clinic periodically at the provider’s discretion. The time of drug discontinuation and the reason for drug discontinuation were recorded. Time of death at any point was collected to measure OS.
It was determined that a total sample size of 42 patients would be insufficient to achieve 80% power to demonstrate any hypothesized effects. However, the Fisher exact test was used to calculate P values for simple comparison. Patient demographics and clinical characteristics were reported as total numbers and frequencies when applicable. Survival rate was measured from the time of sorafenib initiation to 1 year after therapy initiation. Overall survival was measured from the time of sorafenib initiation to time of death. Duration of therapy was measured from the time of sorafenib initiation to time of discontinuation, either by provider or by patient.
Results
There were 83 patients who were prescribed sorafenib between November 1, 2007, and September 30, 2011. Of the 83 patients, 27 patients were ineligible for a 1-year follow-up period, 9 patients were diagnosed with non-HCC, 3 were initiated or managed by providers outside the institution, and 2 were not started on therapy. In all, 42 patients met inclusion criteria and had received at least 1 dose of sorafenib. The primary etiologies for HCC were history of alcohol abuse, HCV, and HBV. The primary risk factors were obesity, smoking, and diabetes. Many patients presented with multiple etiologies and risk factors. Ten patients (23.8%) had moderate-to-severe hepatic impairment (Child-Pugh class B or C). Baseline characteristics of these patients are listed in Table 1.
Efficacy
The median OS was 5.9 months and ranged from 21 days to 60 months. There were 17 patients who survived at the 1-year follow-up, including 1 patient who survived 363 days after treatment initiation, yielding an OS rate of 40.5%. Table 2 presents 1-year survival rates with respect to select baseline data. Baseline factors found to be negligible were age, smoking, alcohol abuse, obesity, presence of HCV, medication possession ratio (MPR), prior treatment, macrovascular invasion, and AFP. Neither initial dose regimen, final dose regimen achieved, or average dose correlated with the survival rate at the 1-year follow-up.
Factors possibly associated with a higher probability of survival were baseline ECOG-PS score and baseline Child-Pugh class (Table 2). Patients with an ECOG-PS score of 0 or 1 had a higher survival rate at 1 year than did patients with an ECOG-PS score of ≥ 2 (50% vs 0%, respectively; P = .113). Patients with Child-Pugh class B or C had a lower survival rate at 1 year than did patients with Child-Pugh class A (51% vs 10%, respectively; P = .028). Other indicators were size of largest hepatic lesion ≤ 5 cm, total bilirubin ≤ 2 mg/dL, concurrent treatment, almost exclusively embolization, and treatment after sorafenib discontinuation, such as another oral chemotherapeutic agent or embolization.
The 17 patients who survived at 1 year were reviewed to see if they shared characteristics that indicated a higher probability of survival. The figure shows the baseline ECOG-PS score and the Child-Pugh class the patients who did and did not survive at the 1-year follow-up. In the first group, all patient possessed an ECOG-PS score of 0 or 1, and only 1 patient presented with Child-Pugh class B or C. In contrast, in the group who did not survive at the 1-year follow-up, there were 4 patients with ECOG-PS scores of > 1 and 9 patients who presented with Child-Pugh class B or C. The mean AFP level of this group was < 200 µg/mL, and only 4 patients were followed by Palliative Care Services. The average normalized MPR of this group was 71.9% compared with 85.3% for those who did not survive at the 1-year follow-up.
In patients who experienced at least 1 adverse event (AE), 16 survived, whereas only 1 who did not experience an AE survived (45.7% vs 14.3%, respectively; P = .210). Thirteen patients who experienced ≥ 3 AEs survived at 1 year; and only 3 patients who reported < 3 AEs survived at 1 year (61.9% vs 14.0%, respectively; P = .011). However, when the number of AEs was normalized to duration of treatment per patient, the median frequency of AEs for all patients was 0.61 AEs per month treated. The difference in survival rates grew smaller and less significant between patients who had a frequency of AEs lower than the median compared with those with a higher ratio (52.4% vs 28.6%, respectively; P = .208). Patients affected by AEs in the first 30 days and 90 days of treatment had a survival rate at the 1-year follow-up of 42.4% and 30.2%, respectively. Patients who experienced dermatologic AEs had a higher survival rate than those who did not have dermatologic AEs (60.0% vs 29.6%, respectively; P = .099). This correlation was not found with 2 other classes of AEs, gastrointestinal (50.0% vs 27.8%; P = .208) or neurologic (64.0% vs 41.2%; P = .209).
The median overall time to discontinuation was 3.4 months. The main reasons cited for discontinuing sorafenib at 1 year included symptomatic progression (52.4%), radiographic progression (23.8%), severe AEs (16.7%), and mild-to-moderate AEs (11.9%). There was overlap, as 15 patients discontinued treatment for multiple reasons. For the 22 patients who discontinued medication due to symptomatic progression at 1 year, the median time to discontinuation was 3.8 months. For the 10 patients who discontinued medication due to radiographic progression at 1 year, median time to discontinuation was 5.6 months. Seven patients (16.7%) were still on therapy at 1 year.The study considered the impact of potential dose adjustments on survival rate and safety. The authors compared patients’ prescribed dose with the recommended dose based on the package insert and monthly laboratory values if recorded. The prescribed dose was recorded as appropriate dose, below dose, above dose, or indeterminate due to the lack of current laboratory values. Patients who survived at the 1-year follow-up had a composition of 26%, 21%, 10%, and 43%, respectively. These results were similar to those of patients who did not survive at the 1-year follow-up, 29%, 12%, 30%, and 29%, respectively.
Based on medication refill history and VA acquisition cost, the total prescription drug cost of treating 42 patients with sorafenib was $388,370.40. The total number of days survived for these patients was 16,607 days, which equates to $8,535.87 per year lived.
Safety
Of the 42 patients, 35 patients experienced ≥ 1 AE for a total of 122 AEs reported. The median number of AEs per patient was 2.5. The median time to the first AE was 21 days and ranged from 3 to 244 days. In the first 30 days of treatment, 23 patients (54.7%) reported 47 AEs (39.5%). In the first 90 days of treatment, 33 patients (78.6%) reported 88 AEs (73.9%). Common AEs in both instances were diarrhea, fatigue, erythematous plantar-palmar rash, and nausea (Table 3).
The predominant classes of AEs were GI (39.3%), dermatologic (18.9%), and neurologic (15.6%). Erythematous palmar-plantar rash, also known as hand-foot syndrome, has been noted as a potential dose-limiting sorafenib AE if the rash is recurrent or severe. One patient experienced recurrent grade-2 rashes, and sorafenib was immediately discontinued after an attempt to lower the dose. There were 8 patients who reported serious AEs, and 5 were hospitalized. One patient continued therapy despite GI hemorrhage. The other 4 patients discontinued therapy on hospitalization and were seen for intracranial hemorrhage, GI perforation, acute renal failure, and acute liver failure. In the first 3 cases, sorafenib could not be ruled out as the primary cause of death. None of these patients presented with comorbidities, such as hypertension, which predisposed them to AEs.
Overall, 38 patients ended therapy at the recommended regimen of 400 mg twice daily, and the average total daily dose was 619 mg, just below 80% of the recommended daily dose. Reasons for not achieving 400 mg twice daily included slow titration, AEs, and dose adjustments for compromised renal and hepatic function such as dialysis. Patients who had an ECOG-PS score of 0 or 1 or Child-Pugh class A reported ≥ 3 AEs, but when normalized to duration of treatment, no difference was observed. No correlations were found for average dose, creatinine clearance, aspartate aminotransferase, platelets, total bilirubin, or weight and number or frequency of AEs.
In regard to potential dose adjustments, the doses (400 mg twice daily, 600 mg daily [400 mg + 200 mg in 2 doses], 200 mg twice daily, and 200 mg daily) did not correlate well with AEs. Patients who had < 3 AEs presented with the breakdown 23%, 16%, 22%, and 38%, similar to patients who had ≥ 3 AEs—30%, 19%, 14%, and 37%. Likewise, patients who had a frequency of AEs lower than the median presented with the breakdown 22%, 22%, 15%, and 40% compared with patients who had more AEs than the median—37%, 9%, 23%, and 31%.
Related: Hepatocellular Carcinoma: To Biopsy or Not?
Discussion
Sorafenib is the only oral oncology medication approved by the FDA for treatment of unresectable HCC.3 Prior to sorafenib, the AASLD recommendation was supportive care for patients presenting with BCLC-Stage C liver cancer. However, guidelines changed when SHARP showed that sorafenib provided a survival benefit with a tolerable AE profile. The survival benefit of sorafenib has been replicated in a few large, multicenter trials. In Asia, Cheng and colleagues saw improved median OS of 6.5 months for sorafenib compared with 4.2 months with placebo, and in Italy, Iavarone and colleagues showed a median OS of 10.5 months without a placebo comparator.11,12
In the veteran population for this study, the OS rate of 40.5% was similar to the rate reported in the SHARP study, although the patients’ median OS fell short of the time described in SHARP and other trials. The medical complexities involved in treating veterans may explain this difference. The veteran population is heterogeneous with diverse ethnic backgrounds, several comorbidities, and varying degrees of organ dysfunction. The authors compared survival rates of different subgroups to test the hypothesis that the probability of survival while on therapy should not depend on demographics or medical history. However, in this study, patients with minimal impact from HCC, such as mild hepatic impairment and high-functional status, demonstrated higher survival rates at 1-year follow-up than did those without significant compromise.Although the high prevalence of HCV and alcohol abuse in the veteran population has resulted in a high incidence of hepatic dysfunction, this study suggests that these factors are independent of survival if liver function or integrity has not been compromised.9
Some researchers have hypothesized that clinical toxicities from tyrosine kinase inhibitors may correlate with survival.13 The authors noticed that the presentation of dermatologic AEs may reflect improved survival. In this study, patients who experienced ≥ 1 AE and ≥ 3 AEs had survival rates at the 1-year follow-up of 45.7% and 61.9%, respectively. Moreover, patients affected by AEs in the first 90 days of treatment had a survival rate at the 1-year follow-up of 42.4%.
Caution is advised when drawing conclusions from the number of AEs or when they appear, because this may falsely favor correlation. Patients who survive longer have additional time to report an AE. Therefore, the authors also looked at the ratio of AEs over time per patient to consider the number of AEs per duration of treatment and saw that there was little difference in survival rate in this regard. When considering patients affected by AEs only in the first 30 days of treatment, the survival rate at the 1-year follow-up fell to 30.2%.
A more likely factor for the survival of the 17 patients who were alive at the 1-year follow-up was their overall health relative to the rest of the study group. Overall health may indicate survival independent of sorafenib. The group of 17 who survived at the 1-year follow-up reflected a population that was different from the rest of the study population. The subset was generally healthier with better ECOG-PS scores and Child-Pugh classes, was not followed by Palliative Care Services, and had a mean AFP level under the threshold for diagnosis of HCC in patients who present with hepatic lesions and elevated AFP.14 This subset’s MPR, a surrogate marker for adherence, was less than the accepted threshold in clinical practice for oral medications.15Evaluating the patient’s dose regimen was expected to reveal a relationship between dosing and clinical outcomes, such as low survival rates with low doses or more AEs with high doses. However, the authors were not able to establish this link. In fact, the median time to discontinuation of 3.4 months for the study group, or duration of treatment, was much shorter than the median OS of 5.9 months.
These findings were consistent with Cabibbo and colleagues, who conducted a meta-analysis of survival rates for untreated patients and found that impaired performance status and Child-Pugh class B or C were independently associated with shorter survival.16 The SHARP study and Cheng and colleagues also attempted to exclude patients who were not Child-Pugh class A in their studies, which suggests a negligible correlation between sorafenib and survival time and a close relationship between baseline clinical status and survival.
The authors determined that prior treatment, including locoregional therapy, was not a factor in predicting survival. This observation is confirmed by the results of a phase 3 study that looked at sorafenib as adjuvant treatment for patients who had no detectable disease after surgical resection or local ablation.17 The trial did not meet its primary endpoint of improved recurrence-free survival. However, the authors observed in this study that 4 patients who underwent resection of the liver before sorafenib had a mean OS of 2.9 years. One patient, who was alive at the time of the study conclusion, received only 22 days of sorafenib treatment and survived for 4.9 years after sorafenib discontinuation. Patients who received concurrent or postsorafenib treatment had higher survival rates.
The cost of treatment in this study was found to be $8,535.87 per year lived. Although formal quality of life assessments were not captured, medication was discontinued at the first sign of disease progression or AE as determined by the provider or patient. When the cost of treatment was adjusted to account for median OS time and VA drug acquisition costs, estimated at average wholesale price minus 40%, the cost of treatment was within the threshold of $50,000-$100,000 per quality-adjusted life-year.7,18Of the 42 patients in this study, 28.6% discontinued therapy due to AEs, compared with 32% observed in the SHARP study. Common GI, dermatologic, and CNS AEs were comparable between the 2 studies. Serious AEs included intracranial hemorrhage, GI hemorrhage, GI perforation, acute liver failure, and acute renal failure; 3 of these events led to death. About 12% of patients experienced bleeding, regardless of severity, compared with the 18% seen in SHARP, despite no prior history of hemorrhage or GI perforation.5 The authors did not find any clinical factors at baseline that predisposed patients to AEs. It was also difficult to distinguish between drug-related AEs and general disease progression.
Although the authors did not find a relationship between dose or dose adjustments and the number or frequency of AEs, there were serious adverse outcomes in this study that were also rare complications observed in SHARP. The decision to start sorafenib should not be taken lightly.
Related: Diagnostic Dilemma of Hepatocellular Carcinoma Presenting as Hepatic Angiomyolipoma
Limitations
This retrospective review had several limitations. In SHARP and other large, multicenter trials, patients were continued on therapy until they experienced both symptomatic and radiographic progression. In this study, patients were discontinued at the first sign of progression, either symptomatic or radiographic or both. Had all patients remained on therapy until symptomatic and radiographic signs of progression were observed, there could have been a better correlation between duration of treatment and OS, symptomatic progression, or radiographic progression. The authors acknowledge, however, that there is diminishing benefit of administering chemotherapy when there are known and potentially serious AEs.
The data for this study were limited due to a small sample size, and it was not powered to evaluate for statistically significant characteristics between the patients who survived at the 1-year follow-up and the patients who did not survive at the 1-year follow-up. This information would be useful to identify potential prognostic factors and guide providers in sorafenib management. Finally, a long-term safety profile could not be established, as patients were evaluated for a 1-year period.
Ultimately, HCC is a multifactorial disease, and it is difficult to account for all potential confounding factors. Additional research, including studies comparing sunitinib or a control group to sorafenib, may provide further insight.
Conclusions
In light of these results, the authors believe that sorafenib may be considered for veterans with unresectable HCC and who are contraindicated for alternative treatments. One-year survival rates were similar to those seen in previous studies. However, there was no clear association between the duration of treatment and OS, and although the medication was well tolerated, there were also serious AEs. It is prudent to continually assess the need for therapy throughout the treatment period.
Pharmacists have a critical role in care for oncology patients, from the integration of certified clinical pharmacist practitioners into hematology-oncology clinics to patient monitoring through oral oncology pharmacy programs.19,20 These programs have been shown to improve patient outcomes and decrease overall health care use and may benefit the veteran population.
In this study, a veteran population achieved a survival rate at the 1-year follow-up similar to that found in SHARP: 40.5% vs 44%. However, OS was markedly shorter: 5.9 months vs 10.7 months. Patients with minimal impact from HCC, such as mild hepatic impairment and high functional status, demonstrated higher survival rates at the 1-year follow-up than did those with significant compromise. Thirty-five patients experienced ≥1 AE, most observed within the first 90 days of treatment, and for 3 patients, sorafenib could not be ruled out as the cause of death.
Sorafenib remains a viable therapeutic option for veterans with advanced HCC. However, it is uncertain how much benefit sorafenib affords to the veteran population, especially with the associated risks.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
In 2015, more than 35,660 new cases of liver cancer and 24,550 liver cancer-related deaths are expected to occur in the U.S. About 80% of these cases will consist of hepatocellular carcinoma, (HCC).1 The incidence of HCC varies throughout the world: Incidence is as low as 5 in 100,000 individuals in North America and ranges up to > 20 in 100,000 individuals in sub-Saharan Africa and Eastern Asia.2 Nearly half of all cases of HCC are associated with hepatitis B virus (HBV), and another 25% are associated with hepatitis C virus (HCV). Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, flatoxin-contaminated food, diabetes, and obesity.3
Therapeutic options for advanced HCC are limited. The FDA approved sorafenib in 2008 for the treatment of unresectable HCC.4 According to the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with Stage C liver cancer may undergo a trial of sorafenib.4 National Comprehensive Cancer Network (NCCN) clinical guidelines for hepatobiliary cancers reserve sorafenib for patients with inoperable tumors, metastatic disease, or extensive liver tumor burden.5 Sorafenib is shown to inhibit multiple intracellular and cell surface kinases. Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis, and apoptosis.4 In the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial, median overall survival (OS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group.6 The predicted survival rates at 1 year were 44% in the sorafenib group and 33% in the placebo group.6The economic impact of oral chemotherapy on health care cannot be discounted. At about $50,000 to $100,000 per quality- adjusted life-year, the incremental cost-effectiveness ratio (ICER) of sorafenib over placebo was $62,473 per quality-adjusted life-year in 2007.7The purpose of this retrospective chart review was to evaluate sorafenib for efficacy and safety in a veteran population. Veterans have poorer health and more medical conditions compared with nonveterans.8 Furthermore, in the VHA, about 170,000 veterans have HCV.9 The rate of progression from HCV to HCC is about 3% to 5% annually. More than half of those diagnosed with HCC are late stage, and unfortunately, the 5-year OS rate for patients with liver cancer is 9% and 4% for those patients who are diagnosed at regional and distant stages of the disease.1 As the practice of oncology grows, it is necessary for pharmacists to be involved in the selection of chemotherapeutic agents in order to provide optimal pharmaceutical care.10
Related: VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinoma
Methods
A retrospective chart review was conducted to identify patients who were prescribed sorafenib from November 1, 2007, to September 30, 2011, at the VA Greater Los Angeles Healthcare System (VAGLAHS). Inclusion criteria included patients who had a diagnosis of advanced HCC, who were initiated and managed by a VAGLAHS provider and who were eligible for a 1-year safety evaluation period. The study was approved by the VAGLAHS institutional review board.
Baseline demographic, clinical, laboratory, and medication data were collected. Demographic, clinical, laboratory, and medication data were obtained from CPRS (Computerized Patient Record System) and VistA (Veterans Health Information Systems and Technology Architecture). Data were collected on secured servers and saved on encrypted files. The master list was destroyed once the records control schedule was finalized. No identifiers were collected on the data collection sheet.
Standard practice at VAGLAHS is to monitor European Cooperative Oncology Group Performance Status (ECOG-PS), Child-Pugh class, and alpha-fetoprotein (AFP) at initiation and every 3 months and to obtain laboratory data at initiation and every month before each medication refill. Patients were seen in the Oncology Clinic periodically at the provider’s discretion. The time of drug discontinuation and the reason for drug discontinuation were recorded. Time of death at any point was collected to measure OS.
It was determined that a total sample size of 42 patients would be insufficient to achieve 80% power to demonstrate any hypothesized effects. However, the Fisher exact test was used to calculate P values for simple comparison. Patient demographics and clinical characteristics were reported as total numbers and frequencies when applicable. Survival rate was measured from the time of sorafenib initiation to 1 year after therapy initiation. Overall survival was measured from the time of sorafenib initiation to time of death. Duration of therapy was measured from the time of sorafenib initiation to time of discontinuation, either by provider or by patient.
Results
There were 83 patients who were prescribed sorafenib between November 1, 2007, and September 30, 2011. Of the 83 patients, 27 patients were ineligible for a 1-year follow-up period, 9 patients were diagnosed with non-HCC, 3 were initiated or managed by providers outside the institution, and 2 were not started on therapy. In all, 42 patients met inclusion criteria and had received at least 1 dose of sorafenib. The primary etiologies for HCC were history of alcohol abuse, HCV, and HBV. The primary risk factors were obesity, smoking, and diabetes. Many patients presented with multiple etiologies and risk factors. Ten patients (23.8%) had moderate-to-severe hepatic impairment (Child-Pugh class B or C). Baseline characteristics of these patients are listed in Table 1.
Efficacy
The median OS was 5.9 months and ranged from 21 days to 60 months. There were 17 patients who survived at the 1-year follow-up, including 1 patient who survived 363 days after treatment initiation, yielding an OS rate of 40.5%. Table 2 presents 1-year survival rates with respect to select baseline data. Baseline factors found to be negligible were age, smoking, alcohol abuse, obesity, presence of HCV, medication possession ratio (MPR), prior treatment, macrovascular invasion, and AFP. Neither initial dose regimen, final dose regimen achieved, or average dose correlated with the survival rate at the 1-year follow-up.
Factors possibly associated with a higher probability of survival were baseline ECOG-PS score and baseline Child-Pugh class (Table 2). Patients with an ECOG-PS score of 0 or 1 had a higher survival rate at 1 year than did patients with an ECOG-PS score of ≥ 2 (50% vs 0%, respectively; P = .113). Patients with Child-Pugh class B or C had a lower survival rate at 1 year than did patients with Child-Pugh class A (51% vs 10%, respectively; P = .028). Other indicators were size of largest hepatic lesion ≤ 5 cm, total bilirubin ≤ 2 mg/dL, concurrent treatment, almost exclusively embolization, and treatment after sorafenib discontinuation, such as another oral chemotherapeutic agent or embolization.
The 17 patients who survived at 1 year were reviewed to see if they shared characteristics that indicated a higher probability of survival. The figure shows the baseline ECOG-PS score and the Child-Pugh class the patients who did and did not survive at the 1-year follow-up. In the first group, all patient possessed an ECOG-PS score of 0 or 1, and only 1 patient presented with Child-Pugh class B or C. In contrast, in the group who did not survive at the 1-year follow-up, there were 4 patients with ECOG-PS scores of > 1 and 9 patients who presented with Child-Pugh class B or C. The mean AFP level of this group was < 200 µg/mL, and only 4 patients were followed by Palliative Care Services. The average normalized MPR of this group was 71.9% compared with 85.3% for those who did not survive at the 1-year follow-up.
In patients who experienced at least 1 adverse event (AE), 16 survived, whereas only 1 who did not experience an AE survived (45.7% vs 14.3%, respectively; P = .210). Thirteen patients who experienced ≥ 3 AEs survived at 1 year; and only 3 patients who reported < 3 AEs survived at 1 year (61.9% vs 14.0%, respectively; P = .011). However, when the number of AEs was normalized to duration of treatment per patient, the median frequency of AEs for all patients was 0.61 AEs per month treated. The difference in survival rates grew smaller and less significant between patients who had a frequency of AEs lower than the median compared with those with a higher ratio (52.4% vs 28.6%, respectively; P = .208). Patients affected by AEs in the first 30 days and 90 days of treatment had a survival rate at the 1-year follow-up of 42.4% and 30.2%, respectively. Patients who experienced dermatologic AEs had a higher survival rate than those who did not have dermatologic AEs (60.0% vs 29.6%, respectively; P = .099). This correlation was not found with 2 other classes of AEs, gastrointestinal (50.0% vs 27.8%; P = .208) or neurologic (64.0% vs 41.2%; P = .209).
The median overall time to discontinuation was 3.4 months. The main reasons cited for discontinuing sorafenib at 1 year included symptomatic progression (52.4%), radiographic progression (23.8%), severe AEs (16.7%), and mild-to-moderate AEs (11.9%). There was overlap, as 15 patients discontinued treatment for multiple reasons. For the 22 patients who discontinued medication due to symptomatic progression at 1 year, the median time to discontinuation was 3.8 months. For the 10 patients who discontinued medication due to radiographic progression at 1 year, median time to discontinuation was 5.6 months. Seven patients (16.7%) were still on therapy at 1 year.The study considered the impact of potential dose adjustments on survival rate and safety. The authors compared patients’ prescribed dose with the recommended dose based on the package insert and monthly laboratory values if recorded. The prescribed dose was recorded as appropriate dose, below dose, above dose, or indeterminate due to the lack of current laboratory values. Patients who survived at the 1-year follow-up had a composition of 26%, 21%, 10%, and 43%, respectively. These results were similar to those of patients who did not survive at the 1-year follow-up, 29%, 12%, 30%, and 29%, respectively.
Based on medication refill history and VA acquisition cost, the total prescription drug cost of treating 42 patients with sorafenib was $388,370.40. The total number of days survived for these patients was 16,607 days, which equates to $8,535.87 per year lived.
Safety
Of the 42 patients, 35 patients experienced ≥ 1 AE for a total of 122 AEs reported. The median number of AEs per patient was 2.5. The median time to the first AE was 21 days and ranged from 3 to 244 days. In the first 30 days of treatment, 23 patients (54.7%) reported 47 AEs (39.5%). In the first 90 days of treatment, 33 patients (78.6%) reported 88 AEs (73.9%). Common AEs in both instances were diarrhea, fatigue, erythematous plantar-palmar rash, and nausea (Table 3).
The predominant classes of AEs were GI (39.3%), dermatologic (18.9%), and neurologic (15.6%). Erythematous palmar-plantar rash, also known as hand-foot syndrome, has been noted as a potential dose-limiting sorafenib AE if the rash is recurrent or severe. One patient experienced recurrent grade-2 rashes, and sorafenib was immediately discontinued after an attempt to lower the dose. There were 8 patients who reported serious AEs, and 5 were hospitalized. One patient continued therapy despite GI hemorrhage. The other 4 patients discontinued therapy on hospitalization and were seen for intracranial hemorrhage, GI perforation, acute renal failure, and acute liver failure. In the first 3 cases, sorafenib could not be ruled out as the primary cause of death. None of these patients presented with comorbidities, such as hypertension, which predisposed them to AEs.
Overall, 38 patients ended therapy at the recommended regimen of 400 mg twice daily, and the average total daily dose was 619 mg, just below 80% of the recommended daily dose. Reasons for not achieving 400 mg twice daily included slow titration, AEs, and dose adjustments for compromised renal and hepatic function such as dialysis. Patients who had an ECOG-PS score of 0 or 1 or Child-Pugh class A reported ≥ 3 AEs, but when normalized to duration of treatment, no difference was observed. No correlations were found for average dose, creatinine clearance, aspartate aminotransferase, platelets, total bilirubin, or weight and number or frequency of AEs.
In regard to potential dose adjustments, the doses (400 mg twice daily, 600 mg daily [400 mg + 200 mg in 2 doses], 200 mg twice daily, and 200 mg daily) did not correlate well with AEs. Patients who had < 3 AEs presented with the breakdown 23%, 16%, 22%, and 38%, similar to patients who had ≥ 3 AEs—30%, 19%, 14%, and 37%. Likewise, patients who had a frequency of AEs lower than the median presented with the breakdown 22%, 22%, 15%, and 40% compared with patients who had more AEs than the median—37%, 9%, 23%, and 31%.
Related: Hepatocellular Carcinoma: To Biopsy or Not?
Discussion
Sorafenib is the only oral oncology medication approved by the FDA for treatment of unresectable HCC.3 Prior to sorafenib, the AASLD recommendation was supportive care for patients presenting with BCLC-Stage C liver cancer. However, guidelines changed when SHARP showed that sorafenib provided a survival benefit with a tolerable AE profile. The survival benefit of sorafenib has been replicated in a few large, multicenter trials. In Asia, Cheng and colleagues saw improved median OS of 6.5 months for sorafenib compared with 4.2 months with placebo, and in Italy, Iavarone and colleagues showed a median OS of 10.5 months without a placebo comparator.11,12
In the veteran population for this study, the OS rate of 40.5% was similar to the rate reported in the SHARP study, although the patients’ median OS fell short of the time described in SHARP and other trials. The medical complexities involved in treating veterans may explain this difference. The veteran population is heterogeneous with diverse ethnic backgrounds, several comorbidities, and varying degrees of organ dysfunction. The authors compared survival rates of different subgroups to test the hypothesis that the probability of survival while on therapy should not depend on demographics or medical history. However, in this study, patients with minimal impact from HCC, such as mild hepatic impairment and high-functional status, demonstrated higher survival rates at 1-year follow-up than did those without significant compromise.Although the high prevalence of HCV and alcohol abuse in the veteran population has resulted in a high incidence of hepatic dysfunction, this study suggests that these factors are independent of survival if liver function or integrity has not been compromised.9
Some researchers have hypothesized that clinical toxicities from tyrosine kinase inhibitors may correlate with survival.13 The authors noticed that the presentation of dermatologic AEs may reflect improved survival. In this study, patients who experienced ≥ 1 AE and ≥ 3 AEs had survival rates at the 1-year follow-up of 45.7% and 61.9%, respectively. Moreover, patients affected by AEs in the first 90 days of treatment had a survival rate at the 1-year follow-up of 42.4%.
Caution is advised when drawing conclusions from the number of AEs or when they appear, because this may falsely favor correlation. Patients who survive longer have additional time to report an AE. Therefore, the authors also looked at the ratio of AEs over time per patient to consider the number of AEs per duration of treatment and saw that there was little difference in survival rate in this regard. When considering patients affected by AEs only in the first 30 days of treatment, the survival rate at the 1-year follow-up fell to 30.2%.
A more likely factor for the survival of the 17 patients who were alive at the 1-year follow-up was their overall health relative to the rest of the study group. Overall health may indicate survival independent of sorafenib. The group of 17 who survived at the 1-year follow-up reflected a population that was different from the rest of the study population. The subset was generally healthier with better ECOG-PS scores and Child-Pugh classes, was not followed by Palliative Care Services, and had a mean AFP level under the threshold for diagnosis of HCC in patients who present with hepatic lesions and elevated AFP.14 This subset’s MPR, a surrogate marker for adherence, was less than the accepted threshold in clinical practice for oral medications.15Evaluating the patient’s dose regimen was expected to reveal a relationship between dosing and clinical outcomes, such as low survival rates with low doses or more AEs with high doses. However, the authors were not able to establish this link. In fact, the median time to discontinuation of 3.4 months for the study group, or duration of treatment, was much shorter than the median OS of 5.9 months.
These findings were consistent with Cabibbo and colleagues, who conducted a meta-analysis of survival rates for untreated patients and found that impaired performance status and Child-Pugh class B or C were independently associated with shorter survival.16 The SHARP study and Cheng and colleagues also attempted to exclude patients who were not Child-Pugh class A in their studies, which suggests a negligible correlation between sorafenib and survival time and a close relationship between baseline clinical status and survival.
The authors determined that prior treatment, including locoregional therapy, was not a factor in predicting survival. This observation is confirmed by the results of a phase 3 study that looked at sorafenib as adjuvant treatment for patients who had no detectable disease after surgical resection or local ablation.17 The trial did not meet its primary endpoint of improved recurrence-free survival. However, the authors observed in this study that 4 patients who underwent resection of the liver before sorafenib had a mean OS of 2.9 years. One patient, who was alive at the time of the study conclusion, received only 22 days of sorafenib treatment and survived for 4.9 years after sorafenib discontinuation. Patients who received concurrent or postsorafenib treatment had higher survival rates.
The cost of treatment in this study was found to be $8,535.87 per year lived. Although formal quality of life assessments were not captured, medication was discontinued at the first sign of disease progression or AE as determined by the provider or patient. When the cost of treatment was adjusted to account for median OS time and VA drug acquisition costs, estimated at average wholesale price minus 40%, the cost of treatment was within the threshold of $50,000-$100,000 per quality-adjusted life-year.7,18Of the 42 patients in this study, 28.6% discontinued therapy due to AEs, compared with 32% observed in the SHARP study. Common GI, dermatologic, and CNS AEs were comparable between the 2 studies. Serious AEs included intracranial hemorrhage, GI hemorrhage, GI perforation, acute liver failure, and acute renal failure; 3 of these events led to death. About 12% of patients experienced bleeding, regardless of severity, compared with the 18% seen in SHARP, despite no prior history of hemorrhage or GI perforation.5 The authors did not find any clinical factors at baseline that predisposed patients to AEs. It was also difficult to distinguish between drug-related AEs and general disease progression.
Although the authors did not find a relationship between dose or dose adjustments and the number or frequency of AEs, there were serious adverse outcomes in this study that were also rare complications observed in SHARP. The decision to start sorafenib should not be taken lightly.
Related: Diagnostic Dilemma of Hepatocellular Carcinoma Presenting as Hepatic Angiomyolipoma
Limitations
This retrospective review had several limitations. In SHARP and other large, multicenter trials, patients were continued on therapy until they experienced both symptomatic and radiographic progression. In this study, patients were discontinued at the first sign of progression, either symptomatic or radiographic or both. Had all patients remained on therapy until symptomatic and radiographic signs of progression were observed, there could have been a better correlation between duration of treatment and OS, symptomatic progression, or radiographic progression. The authors acknowledge, however, that there is diminishing benefit of administering chemotherapy when there are known and potentially serious AEs.
The data for this study were limited due to a small sample size, and it was not powered to evaluate for statistically significant characteristics between the patients who survived at the 1-year follow-up and the patients who did not survive at the 1-year follow-up. This information would be useful to identify potential prognostic factors and guide providers in sorafenib management. Finally, a long-term safety profile could not be established, as patients were evaluated for a 1-year period.
Ultimately, HCC is a multifactorial disease, and it is difficult to account for all potential confounding factors. Additional research, including studies comparing sunitinib or a control group to sorafenib, may provide further insight.
Conclusions
In light of these results, the authors believe that sorafenib may be considered for veterans with unresectable HCC and who are contraindicated for alternative treatments. One-year survival rates were similar to those seen in previous studies. However, there was no clear association between the duration of treatment and OS, and although the medication was well tolerated, there were also serious AEs. It is prudent to continually assess the need for therapy throughout the treatment period.
Pharmacists have a critical role in care for oncology patients, from the integration of certified clinical pharmacist practitioners into hematology-oncology clinics to patient monitoring through oral oncology pharmacy programs.19,20 These programs have been shown to improve patient outcomes and decrease overall health care use and may benefit the veteran population.
In this study, a veteran population achieved a survival rate at the 1-year follow-up similar to that found in SHARP: 40.5% vs 44%. However, OS was markedly shorter: 5.9 months vs 10.7 months. Patients with minimal impact from HCC, such as mild hepatic impairment and high functional status, demonstrated higher survival rates at the 1-year follow-up than did those with significant compromise. Thirty-five patients experienced ≥1 AE, most observed within the first 90 days of treatment, and for 3 patients, sorafenib could not be ruled out as the cause of death.
Sorafenib remains a viable therapeutic option for veterans with advanced HCC. However, it is uncertain how much benefit sorafenib affords to the veteran population, especially with the associated risks.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.
3. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
4. Nexavar [package insert]. Emeryville, CA: Bayer HealthCare Pharmaceuticals, Inc; 2009.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 2. 2015. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed October 13, 2015.
6. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
7. Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746.
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. National Viral Hepatitis Program. VHA Directive 1300.01. U.S. Department of Veterans Affairs Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1586. Updated February 22, 2013. Accessed October 13, 2015.
10. Patterson CJ. Best practices in specialty pharmacy management. J Manag Care Pharm. 2013;19(1):42-48.
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
12. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-2063.
13. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011;105(12):1811-1813.
14. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
15. Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999;5(1):47-51.
16. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283.
17. Bayer HealthCare. Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM). ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT00692770. Updated May 28, 2015. Accessed October 21, 2015.
18. Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods, 2009 Update (Version 2.0). J Manag Care Pharm. 2009;15(suppl 6-a):S3-S57.
19. Valgus JM, Faso A, Gregory KM, et al. Integration of a clinical pharmacist into the hematology-oncology clinics at an academic medical center. Am J Health Syst Pharm. 2011;68(7):613-619.
20. Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4(4):165-174.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.
3. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
4. Nexavar [package insert]. Emeryville, CA: Bayer HealthCare Pharmaceuticals, Inc; 2009.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 2. 2015. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed October 13, 2015.
6. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
7. Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746.
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. National Viral Hepatitis Program. VHA Directive 1300.01. U.S. Department of Veterans Affairs Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1586. Updated February 22, 2013. Accessed October 13, 2015.
10. Patterson CJ. Best practices in specialty pharmacy management. J Manag Care Pharm. 2013;19(1):42-48.
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
12. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-2063.
13. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011;105(12):1811-1813.
14. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
15. Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999;5(1):47-51.
16. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283.
17. Bayer HealthCare. Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM). ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT00692770. Updated May 28, 2015. Accessed October 21, 2015.
18. Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods, 2009 Update (Version 2.0). J Manag Care Pharm. 2009;15(suppl 6-a):S3-S57.
19. Valgus JM, Faso A, Gregory KM, et al. Integration of a clinical pharmacist into the hematology-oncology clinics at an academic medical center. Am J Health Syst Pharm. 2011;68(7):613-619.
20. Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4(4):165-174.