User login
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
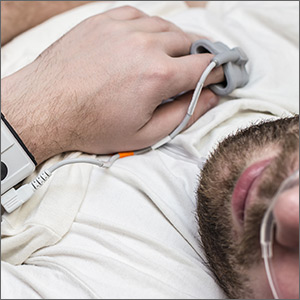
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
PRACTICE CHANGER
Consider ordering home respiratory polygraphy vs laboratory sleep studies for patients suspected of having obstructive sleep apnea.1
Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
STRENGTH OF RECOMMENDATION
B: Based on a multicenter, noninferiority randomized controlled trial and cost analysis study.