User login
THE CASE
A 75-year-old man with a history of osteoarthritis presented to our clinic with worsening weakness over the previous month. His signs and symptoms included profound fatigue, subjective fevers, a 10-pound weight loss, ankle swelling, myalgias in his legs and back, shortness of breath, and a persistent cough. The patient was otherwise previously healthy.
The patient’s heart and lung exams were normal. Initial outpatient labs showed significantly elevated inflammatory markers, with an erythrocyte sedimentation rate (ESR) of 102 mm/h (normal range for men ≥ 50 years, 0-20 mm/h) and a C-reactive protein (CRP) level of 11.1 mg/L (normal range, < 3 mg/L). The patient also had an elevated white blood cell count of 12,000/mcL (normal range, 4500-11,000/mcL). His hemoglobin was low (11 g/dL; normal range, 13.5-17.5 g/dL) and so was his albumin level (2.9 g/dL; normal range, 3.4-5.4 g/dL). The results of his prostate-specific antigen and brain natriuretic peptide tests were both normal. The results of a computed tomography scan of his thorax, abdomen, and pelvis were negative for malignancy.
The patient returned to our clinic 3 days later with severe weakness, which inhibited him from walking. He complained of a severe spasmodic pain between his shoulder blades. He denied joint stiffness, headaches, vision changes, or jaw claudication. The patient’s son had noted an overall increase in his father’s baseline heart rate, with readings increasing from the 50 beats/min range to the 70 beats/min range; this raised concern for a catecholamine-secreting tumor. There was also concern for occult infection and malignancy, or an autoimmune process, such as polymyalgia rheumatica. Due to his extreme weakness, the patient was directly admitted to the hospital for further work-up.
THE DIAGNOSIS
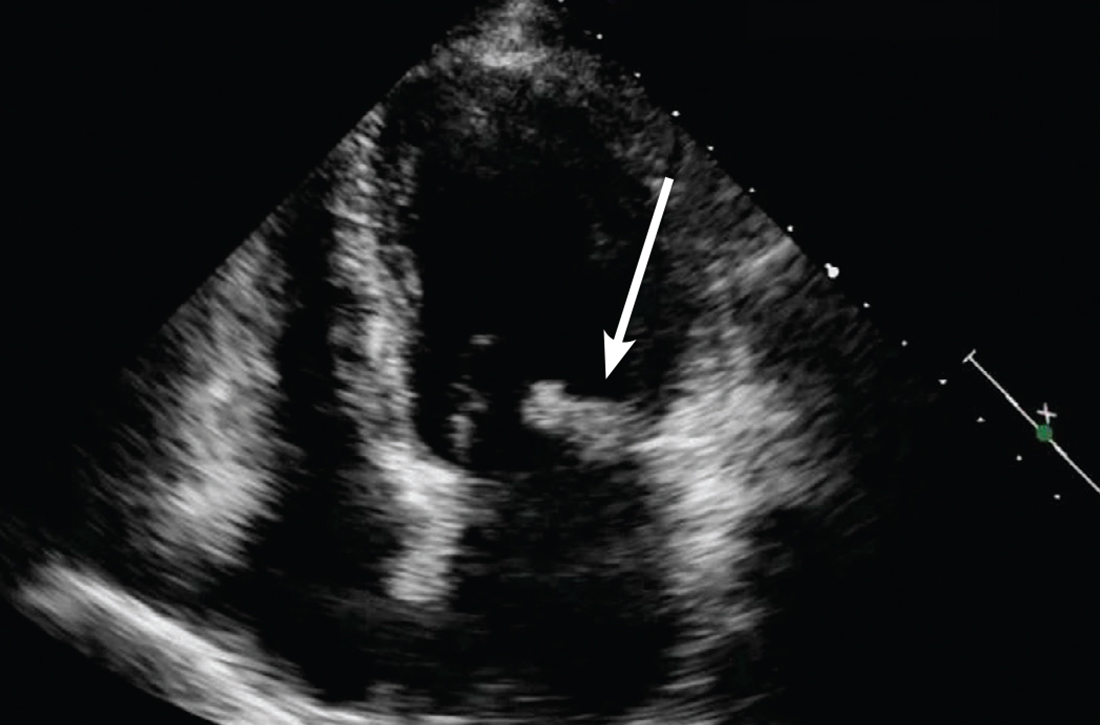
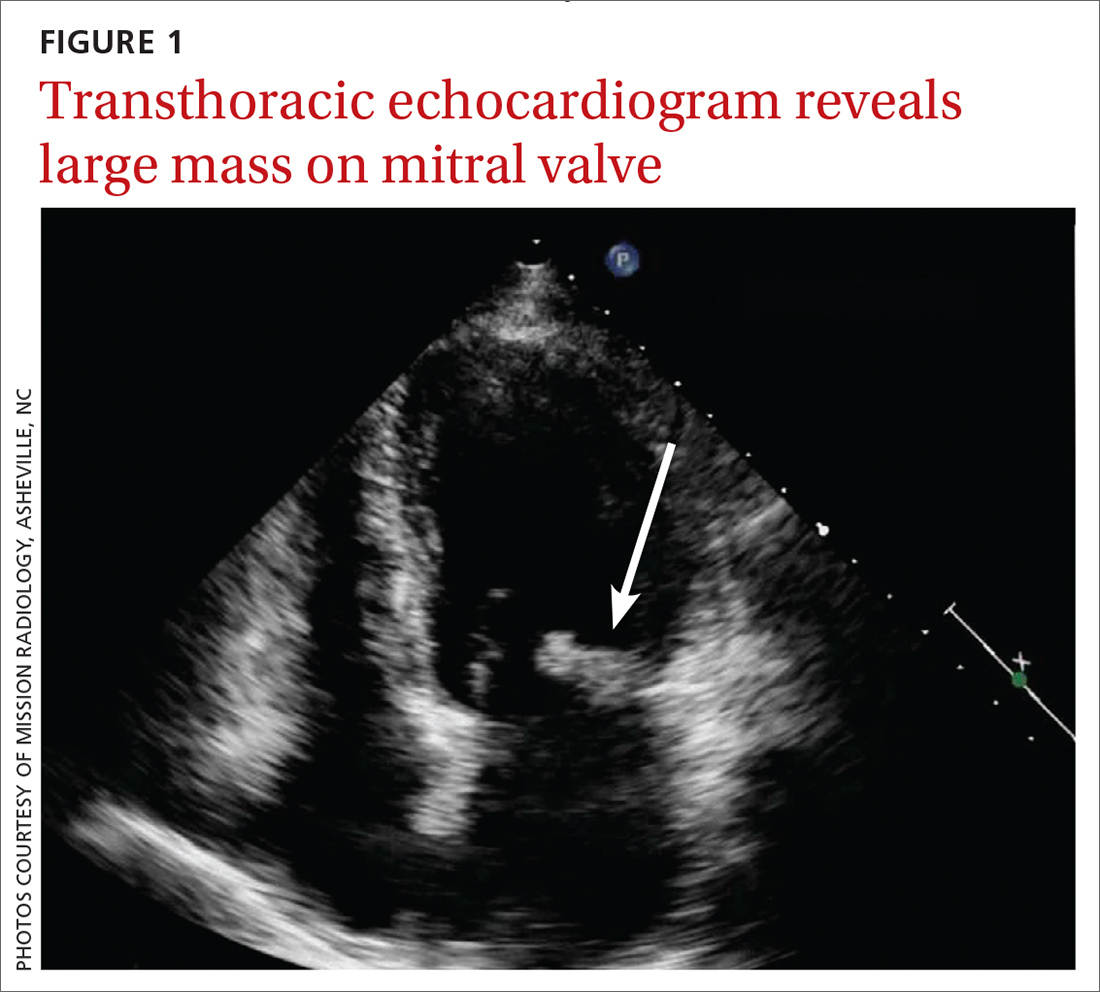
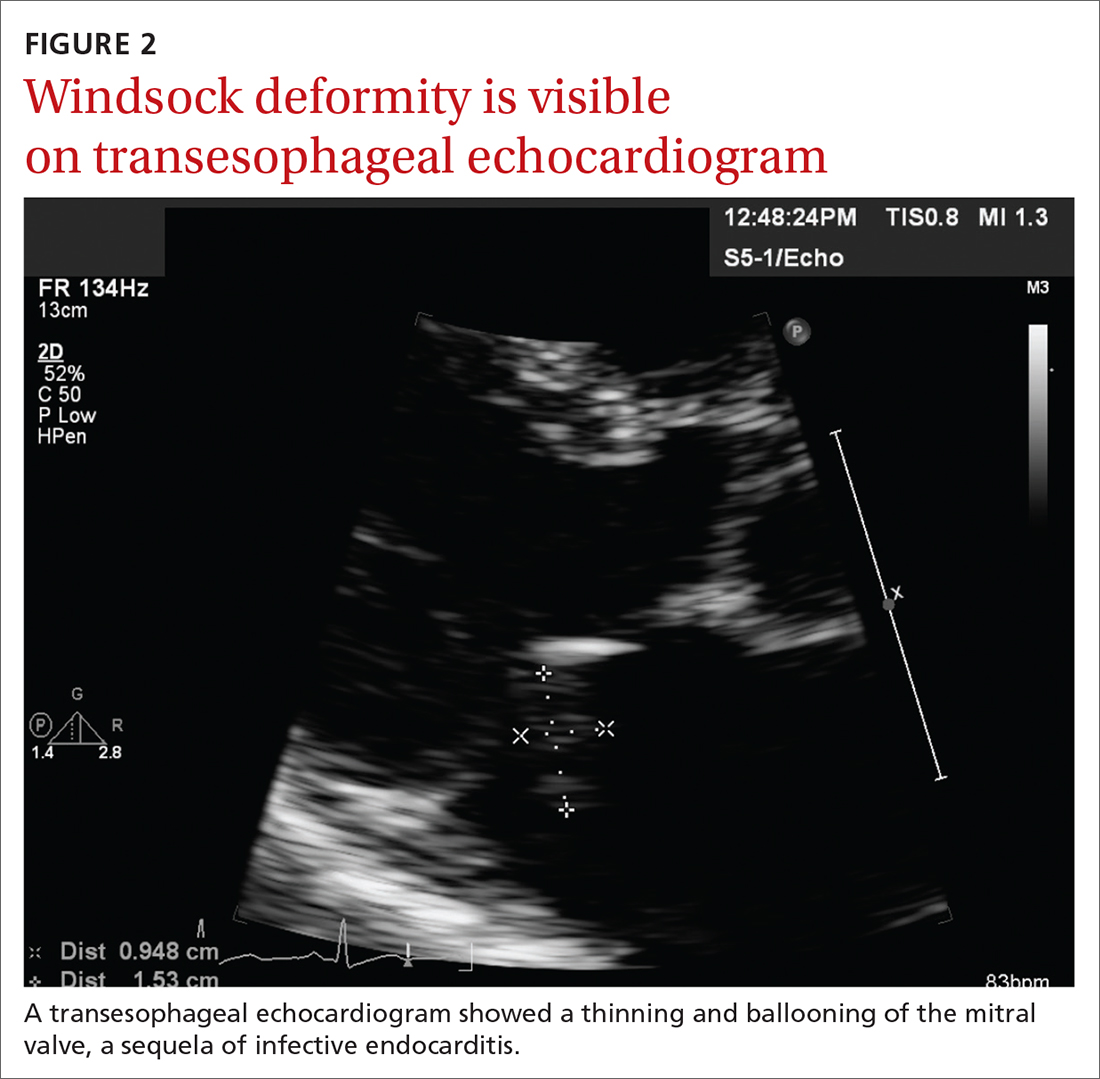
Concern for a smoldering infection prompted an order for a transthoracic echocardiogram. Images revealed a large mass on the mitral valve (FIGURE 1). Blood cultures quickly grew Streptococcus sanguinis. Additional work-up with a transesophageal echocardiogram (TEE) showed a “windsock” deformity (thinning and ballooning of the mitral valve), a known sequela of infective endocarditis (FIGURE 2).1 Further history obtained after the TEE revealed the patient had had a routine dental cleaning the month before his symptoms began. A murmur was then also detected.
DISCUSSION
Infective endocarditis (IE) is uncommon and difficult to diagnose; it has a high early-mortality rate of 30%.2 TEE is the recommended imaging study for IE, because it is more sensitive than a transthoracic echocardiogram for identifying vegetations on the valves and it is more cost effective.3
The modified Duke Criteria provide guidance for diagnosis of endocarditis. Major criteria focus on positive blood cultures and evidence of endocardial involvement. Minor criteria include predisposing heart conditions, intravenous drug use (IVDU), fever, and vascular and immunologic phenomena. As many as 90% of patients have a fever and often experience weight loss.4 Murmurs are auscultated in up to 85% of patients, and embolic features are present in up to 25% of patients at the time of diagnosis.4 In the developed world, Janeway lesions, Osler nodes, and splinter hemorrhages are increasingly rare, as patients usually present earlier in the disease course.4 While ESR and CRP are generally elevated in cases of IE, they are not part of the Duke Criteria.4
A closer look at risk factors
In 2007, guidelines for the prevention, treatment, and management of endocarditis were given significant categorical revision by the American Heart Association for the first time in 50 years.5 Recommendations for antibiotic prophylaxis prior to dental procedures became more restrictive, to include only 4 groups of high-risk patients: those with prosthetic cardiac valves, those with a history of IE, those with congenital heart disease, and cardiac transplant recipients.4 The rationale for these restrictions included the small risk for anaphylaxis and potential increase in risk for bacterial resistance associated with antibiotic prophylaxis.4 A review published in 2021 noted no increase in the frequency of, nor the morbidity and mortality from, viridans group streptococcal IE since the guideline updates.5
Continue to: There is an emerging consensus...
There is an emerging consensus that poor oral hygiene and gingival bleeding after tooth brushing promote a chronic low-grade bacteremia that may be more strongly associated with IE than an isolated dental extraction.6 Poor dental hygiene, defined as dental plaque and calculus, is especially common in the elderly, who are known to let their dental hygiene lapse.6 In our patient’s case, his generally poor oral hygiene was more likely the cause of his IE than his routine dental cleaning.
Other risk factors include IV drug use. At our tertiary care hospital in western North Carolina, 48% of patients with endocarditis had an additional diagnosis of opiate or narcotic dependence (Ryan Tilton, PharmD, email communication, June 7, 2018). Interestingly, though, only 16% of patients in North America with endocarditis were found to be currently using IV drugs.7
Our patient was treated with IV antibiotics for 4 weeks and underwent rehabilitation at a skilled nursing facility. Four weeks after diagnosis, he underwent an endoscopic porcine mitral valve replacement. Two months after that, he returned to his previously active lifestyle and began riding his stationary bike. The patient also began taking a daily aspirin. Consistent with current guidelines, he now gets antibiotic prophylaxis prior to dental procedures.
THE TAKEAWAY
This patient, without any history of IVDU or cardiac valvular abnormalities, presented with symptoms classic for a developing malignancy or possible rheumatologic condition. Subacute IE may manifest similarly, with vague symptoms such as myalgias, fatigue, chills, and/or anemia. In non-drug users, suspicion for endocarditis should be highest in men older than age 60. Also, it’s important to auscultate for a new heart murmur. In our patient’s case, no murmur was auscultated until after his TEE. JFP
CORRESPONDENCE
Ginger Poulton, MD, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
1. Paruchuru PK, Adluri K, Patel RL. Windsock deformity of the mitral valve—a late presentation of endocarditis. Eur J Cardiothorac Surg. 2002;21:88. doi: 10.1016/s1010-7940(01)01038-7
2. Toyoda N, Chikwe J, Itagaki S, et al. Trends in infective endocarditis in California and New York State, 1998-2013. JAMA. 2017;317:1652-1660. doi: 10.1001/jama.2017.4287
3. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486. doi: 10.1161/CIR.0000000000000296
4. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. doi: 10.1093/eurheartj/ehv319
5. Wilson, WR, Gewitz, M, Lockhart PB et al. Prevention of Viridans Group Streptococcal Infective Endocarditis. A Scientific Statement from the American Heart Association. Circulation. 2021; 143e963-e978.
6. Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238-1244. doi: 10.14219/jada.archive.2009.0046
7. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473. doi: 10.1001/archinternmed.2008.603
THE CASE
A 75-year-old man with a history of osteoarthritis presented to our clinic with worsening weakness over the previous month. His signs and symptoms included profound fatigue, subjective fevers, a 10-pound weight loss, ankle swelling, myalgias in his legs and back, shortness of breath, and a persistent cough. The patient was otherwise previously healthy.
The patient’s heart and lung exams were normal. Initial outpatient labs showed significantly elevated inflammatory markers, with an erythrocyte sedimentation rate (ESR) of 102 mm/h (normal range for men ≥ 50 years, 0-20 mm/h) and a C-reactive protein (CRP) level of 11.1 mg/L (normal range, < 3 mg/L). The patient also had an elevated white blood cell count of 12,000/mcL (normal range, 4500-11,000/mcL). His hemoglobin was low (11 g/dL; normal range, 13.5-17.5 g/dL) and so was his albumin level (2.9 g/dL; normal range, 3.4-5.4 g/dL). The results of his prostate-specific antigen and brain natriuretic peptide tests were both normal. The results of a computed tomography scan of his thorax, abdomen, and pelvis were negative for malignancy.
The patient returned to our clinic 3 days later with severe weakness, which inhibited him from walking. He complained of a severe spasmodic pain between his shoulder blades. He denied joint stiffness, headaches, vision changes, or jaw claudication. The patient’s son had noted an overall increase in his father’s baseline heart rate, with readings increasing from the 50 beats/min range to the 70 beats/min range; this raised concern for a catecholamine-secreting tumor. There was also concern for occult infection and malignancy, or an autoimmune process, such as polymyalgia rheumatica. Due to his extreme weakness, the patient was directly admitted to the hospital for further work-up.
THE DIAGNOSIS
Concern for a smoldering infection prompted an order for a transthoracic echocardiogram. Images revealed a large mass on the mitral valve (FIGURE 1). Blood cultures quickly grew Streptococcus sanguinis. Additional work-up with a transesophageal echocardiogram (TEE) showed a “windsock” deformity (thinning and ballooning of the mitral valve), a known sequela of infective endocarditis (FIGURE 2).1 Further history obtained after the TEE revealed the patient had had a routine dental cleaning the month before his symptoms began. A murmur was then also detected.

DISCUSSION
Infective endocarditis (IE) is uncommon and difficult to diagnose; it has a high early-mortality rate of 30%.2 TEE is the recommended imaging study for IE, because it is more sensitive than a transthoracic echocardiogram for identifying vegetations on the valves and it is more cost effective.3

The modified Duke Criteria provide guidance for diagnosis of endocarditis. Major criteria focus on positive blood cultures and evidence of endocardial involvement. Minor criteria include predisposing heart conditions, intravenous drug use (IVDU), fever, and vascular and immunologic phenomena. As many as 90% of patients have a fever and often experience weight loss.4 Murmurs are auscultated in up to 85% of patients, and embolic features are present in up to 25% of patients at the time of diagnosis.4 In the developed world, Janeway lesions, Osler nodes, and splinter hemorrhages are increasingly rare, as patients usually present earlier in the disease course.4 While ESR and CRP are generally elevated in cases of IE, they are not part of the Duke Criteria.4
A closer look at risk factors
In 2007, guidelines for the prevention, treatment, and management of endocarditis were given significant categorical revision by the American Heart Association for the first time in 50 years.5 Recommendations for antibiotic prophylaxis prior to dental procedures became more restrictive, to include only 4 groups of high-risk patients: those with prosthetic cardiac valves, those with a history of IE, those with congenital heart disease, and cardiac transplant recipients.4 The rationale for these restrictions included the small risk for anaphylaxis and potential increase in risk for bacterial resistance associated with antibiotic prophylaxis.4 A review published in 2021 noted no increase in the frequency of, nor the morbidity and mortality from, viridans group streptococcal IE since the guideline updates.5
Continue to: There is an emerging consensus...
There is an emerging consensus that poor oral hygiene and gingival bleeding after tooth brushing promote a chronic low-grade bacteremia that may be more strongly associated with IE than an isolated dental extraction.6 Poor dental hygiene, defined as dental plaque and calculus, is especially common in the elderly, who are known to let their dental hygiene lapse.6 In our patient’s case, his generally poor oral hygiene was more likely the cause of his IE than his routine dental cleaning.
Other risk factors include IV drug use. At our tertiary care hospital in western North Carolina, 48% of patients with endocarditis had an additional diagnosis of opiate or narcotic dependence (Ryan Tilton, PharmD, email communication, June 7, 2018). Interestingly, though, only 16% of patients in North America with endocarditis were found to be currently using IV drugs.7
Our patient was treated with IV antibiotics for 4 weeks and underwent rehabilitation at a skilled nursing facility. Four weeks after diagnosis, he underwent an endoscopic porcine mitral valve replacement. Two months after that, he returned to his previously active lifestyle and began riding his stationary bike. The patient also began taking a daily aspirin. Consistent with current guidelines, he now gets antibiotic prophylaxis prior to dental procedures.
THE TAKEAWAY
This patient, without any history of IVDU or cardiac valvular abnormalities, presented with symptoms classic for a developing malignancy or possible rheumatologic condition. Subacute IE may manifest similarly, with vague symptoms such as myalgias, fatigue, chills, and/or anemia. In non-drug users, suspicion for endocarditis should be highest in men older than age 60. Also, it’s important to auscultate for a new heart murmur. In our patient’s case, no murmur was auscultated until after his TEE. JFP
CORRESPONDENCE
Ginger Poulton, MD, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
THE CASE
A 75-year-old man with a history of osteoarthritis presented to our clinic with worsening weakness over the previous month. His signs and symptoms included profound fatigue, subjective fevers, a 10-pound weight loss, ankle swelling, myalgias in his legs and back, shortness of breath, and a persistent cough. The patient was otherwise previously healthy.
The patient’s heart and lung exams were normal. Initial outpatient labs showed significantly elevated inflammatory markers, with an erythrocyte sedimentation rate (ESR) of 102 mm/h (normal range for men ≥ 50 years, 0-20 mm/h) and a C-reactive protein (CRP) level of 11.1 mg/L (normal range, < 3 mg/L). The patient also had an elevated white blood cell count of 12,000/mcL (normal range, 4500-11,000/mcL). His hemoglobin was low (11 g/dL; normal range, 13.5-17.5 g/dL) and so was his albumin level (2.9 g/dL; normal range, 3.4-5.4 g/dL). The results of his prostate-specific antigen and brain natriuretic peptide tests were both normal. The results of a computed tomography scan of his thorax, abdomen, and pelvis were negative for malignancy.
The patient returned to our clinic 3 days later with severe weakness, which inhibited him from walking. He complained of a severe spasmodic pain between his shoulder blades. He denied joint stiffness, headaches, vision changes, or jaw claudication. The patient’s son had noted an overall increase in his father’s baseline heart rate, with readings increasing from the 50 beats/min range to the 70 beats/min range; this raised concern for a catecholamine-secreting tumor. There was also concern for occult infection and malignancy, or an autoimmune process, such as polymyalgia rheumatica. Due to his extreme weakness, the patient was directly admitted to the hospital for further work-up.
THE DIAGNOSIS
Concern for a smoldering infection prompted an order for a transthoracic echocardiogram. Images revealed a large mass on the mitral valve (FIGURE 1). Blood cultures quickly grew Streptococcus sanguinis. Additional work-up with a transesophageal echocardiogram (TEE) showed a “windsock” deformity (thinning and ballooning of the mitral valve), a known sequela of infective endocarditis (FIGURE 2).1 Further history obtained after the TEE revealed the patient had had a routine dental cleaning the month before his symptoms began. A murmur was then also detected.

DISCUSSION
Infective endocarditis (IE) is uncommon and difficult to diagnose; it has a high early-mortality rate of 30%.2 TEE is the recommended imaging study for IE, because it is more sensitive than a transthoracic echocardiogram for identifying vegetations on the valves and it is more cost effective.3

The modified Duke Criteria provide guidance for diagnosis of endocarditis. Major criteria focus on positive blood cultures and evidence of endocardial involvement. Minor criteria include predisposing heart conditions, intravenous drug use (IVDU), fever, and vascular and immunologic phenomena. As many as 90% of patients have a fever and often experience weight loss.4 Murmurs are auscultated in up to 85% of patients, and embolic features are present in up to 25% of patients at the time of diagnosis.4 In the developed world, Janeway lesions, Osler nodes, and splinter hemorrhages are increasingly rare, as patients usually present earlier in the disease course.4 While ESR and CRP are generally elevated in cases of IE, they are not part of the Duke Criteria.4
A closer look at risk factors
In 2007, guidelines for the prevention, treatment, and management of endocarditis were given significant categorical revision by the American Heart Association for the first time in 50 years.5 Recommendations for antibiotic prophylaxis prior to dental procedures became more restrictive, to include only 4 groups of high-risk patients: those with prosthetic cardiac valves, those with a history of IE, those with congenital heart disease, and cardiac transplant recipients.4 The rationale for these restrictions included the small risk for anaphylaxis and potential increase in risk for bacterial resistance associated with antibiotic prophylaxis.4 A review published in 2021 noted no increase in the frequency of, nor the morbidity and mortality from, viridans group streptococcal IE since the guideline updates.5
Continue to: There is an emerging consensus...
There is an emerging consensus that poor oral hygiene and gingival bleeding after tooth brushing promote a chronic low-grade bacteremia that may be more strongly associated with IE than an isolated dental extraction.6 Poor dental hygiene, defined as dental plaque and calculus, is especially common in the elderly, who are known to let their dental hygiene lapse.6 In our patient’s case, his generally poor oral hygiene was more likely the cause of his IE than his routine dental cleaning.
Other risk factors include IV drug use. At our tertiary care hospital in western North Carolina, 48% of patients with endocarditis had an additional diagnosis of opiate or narcotic dependence (Ryan Tilton, PharmD, email communication, June 7, 2018). Interestingly, though, only 16% of patients in North America with endocarditis were found to be currently using IV drugs.7
Our patient was treated with IV antibiotics for 4 weeks and underwent rehabilitation at a skilled nursing facility. Four weeks after diagnosis, he underwent an endoscopic porcine mitral valve replacement. Two months after that, he returned to his previously active lifestyle and began riding his stationary bike. The patient also began taking a daily aspirin. Consistent with current guidelines, he now gets antibiotic prophylaxis prior to dental procedures.
THE TAKEAWAY
This patient, without any history of IVDU or cardiac valvular abnormalities, presented with symptoms classic for a developing malignancy or possible rheumatologic condition. Subacute IE may manifest similarly, with vague symptoms such as myalgias, fatigue, chills, and/or anemia. In non-drug users, suspicion for endocarditis should be highest in men older than age 60. Also, it’s important to auscultate for a new heart murmur. In our patient’s case, no murmur was auscultated until after his TEE. JFP
CORRESPONDENCE
Ginger Poulton, MD, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
1. Paruchuru PK, Adluri K, Patel RL. Windsock deformity of the mitral valve—a late presentation of endocarditis. Eur J Cardiothorac Surg. 2002;21:88. doi: 10.1016/s1010-7940(01)01038-7
2. Toyoda N, Chikwe J, Itagaki S, et al. Trends in infective endocarditis in California and New York State, 1998-2013. JAMA. 2017;317:1652-1660. doi: 10.1001/jama.2017.4287
3. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486. doi: 10.1161/CIR.0000000000000296
4. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. doi: 10.1093/eurheartj/ehv319
5. Wilson, WR, Gewitz, M, Lockhart PB et al. Prevention of Viridans Group Streptococcal Infective Endocarditis. A Scientific Statement from the American Heart Association. Circulation. 2021; 143e963-e978.
6. Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238-1244. doi: 10.14219/jada.archive.2009.0046
7. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473. doi: 10.1001/archinternmed.2008.603
1. Paruchuru PK, Adluri K, Patel RL. Windsock deformity of the mitral valve—a late presentation of endocarditis. Eur J Cardiothorac Surg. 2002;21:88. doi: 10.1016/s1010-7940(01)01038-7
2. Toyoda N, Chikwe J, Itagaki S, et al. Trends in infective endocarditis in California and New York State, 1998-2013. JAMA. 2017;317:1652-1660. doi: 10.1001/jama.2017.4287
3. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486. doi: 10.1161/CIR.0000000000000296
4. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. doi: 10.1093/eurheartj/ehv319
5. Wilson, WR, Gewitz, M, Lockhart PB et al. Prevention of Viridans Group Streptococcal Infective Endocarditis. A Scientific Statement from the American Heart Association. Circulation. 2021; 143e963-e978.
6. Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238-1244. doi: 10.14219/jada.archive.2009.0046
7. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473. doi: 10.1001/archinternmed.2008.603