User login
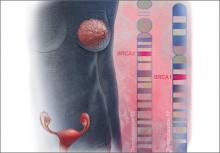
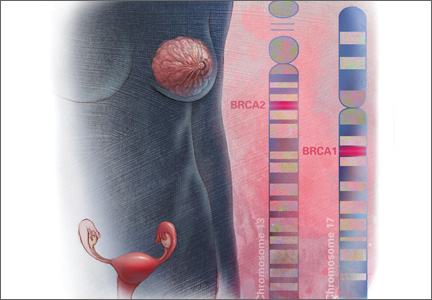
Angelina Jolie’s mother and maternal grandmother died of ovarian cancer; her mother’s sister died of breast cancer. After testing positive for a clinically significant BRCA mutation, Ms. Jolie became a “previvor,” a woman who is currently healthy but at very high risk for developing a life-threatening breast or ovarian malignancy. Advances in genetic testing will result in many more women being identified as BRCA previvors. These women face many daunting decisions concerning options for risk-reducing breast and pelvic surgery. We gynecologists need to be prepared to help shepherd them through this process.
Which of our patients should be tested for BRCA mutations?
Experts have provided complex guidance on who should be tested for a BRCA mutation.1 These recommendations include testing women with a personal history of:
• epithelial ovarian cancer
• fallopian tube cancer, or
• primary peritoneal cancer.
Women with a personal history of breast cancer at an early age also should consider being tested. If women with breast or ovarian cancer, or both, test positive for BRCA1 or BRCA2, then living family members can be offered testing.
Healthy women with a family history of breast and/or ovarian cancer also may benefit from genetic testing. My clinical experience is that, unless the family history is as dramatic as that of Ms. Jolie, it requires considerable time and expertise to assemble a valid extended-family history and provide an estimate of risk. This task may be best completed by a genetic counselor. A simple, Web-based BRCA risk-assessment tool is provided by Myriad Genetics.2
A clinically significant BRCA mutation is detected. What is your patient’s risk?
Unfortunately, women with BRCA mutations are at very high risk for breast and ovarian cancer.
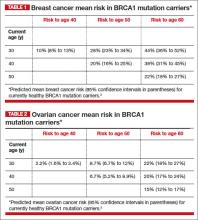
BRCA1. For patients with a BRCA1 mutation, the mean cumulative cancer risk to age 70 years is approximately 57% for breast cancer (95% confidence interval [CI], 47% to 66%) and 40% for ovarian cancer (95% CI, 35% to 46%).3
BRCA2. For patients with a BRCA2 mutation, the mean cumulative cancer risk to age 70 years is 49% for breast cancer (95% CI, 40% to 57%) and 18% for ovarian cancer (95% CI,13% to 23%). Tables 1 and 2 provide an approximation of the risk of developing breast or ovarian cancer for BRCA1 previvors aged 30, 40, and 50. The breast and ovarian cancer risk for BRCA2 previvors (not shown) is less than that for BRCA1 previvors.
Options for risk-reducing surgery
Risk-reducing breast and pelvic surgery markedly lessens the risk of developing cancer in the breast and ovary, decreasing the risk of mortality not only from breast cancer and ovarian cancer but from all causes.4
How many women choose risk-reducing surgery, and why? In a cohort of 306 healthy BRCA mutation carriers in the Netherlands, 10 years of follow-up revealed that 75% underwent risk-reducing pelvic surgery, and 50% underwent risk-reducing mastectomy.5 The age of the woman and her desire to preserve child-bearing strongly influenced the decision to undergo prophylactic surgery and the timing of the surgery.
Following risk-reducing surgery, most women are satisfied with their choice. In one follow-up study, the authors reported that women who had difficulty arriving at a decision were less satisfied with their choice to have risk-reducing surgery than women who arrived at their decision with confidence.6
Risk-reducing breast surgery: What’s involved?
As can be seen in Tables 1 and 2, the risk of your 30-year-old patient with a BRCA1 mutation developing breast cancer throughout her 30s is much greater than her risk of developing ovarian cancer. For this patient, bilateral total mastectomy is the standard risk-reducing procedure because subcutaneous mastectomy may leave islands of glandular tissue that may serve as a nidus for cancer development. However, both nipple-sparing and skin-sparing mastectomy have been reported to be successful in most cases, and may be favored by many women.8,9 Following risk-reducing breast surgery, most women can immediately begin multistage breast reconstruction.
Ms. Jolie underwent a “nipple delay” surgery 2 weeks before her mastectomy. This procedure involves lifting a portion of the nipple skin off the underlying breast to stimulate the nipple to start drawing its blood flow from the surrounding skin rather than the underlying tissue. In addition, during the nipple delay procedure a biopsy of the tissue from beneath the nipple is performed to ensure that no cancer is present.
Risk-reducing pelvic surgery: Essential steps
The standard operation to reduce the risk of ovarian cancer in BRCA mutation carriers is a bilateral salpingo-oophorectomy (BSO). Additional steps should be performed to assess for distant disease, including:
• careful assessment of all peritoneal surfaces
• pelvic washing for cytology
• omental biopsy
• cytologic smear of the diaphragm.
The fallopian tube should be resected in its entirety, but cornual resection is not necessary. Care should be taken to remove the entire ovary and avoid leaving small remnants of the ovary on the proximal vascular pedicles. Consideration should be given to performing a hysterectomy at the time of risk-reducing BSO. If the uterus is removed, estrogen-only hormone therapy (HT) can be prescribed. Estrogen-only HT causes fewer adverse events than combination estrogen-progestin therapy.
Two-stage pelvic surgery approach
Recent research indicates that high-grade serous tumors caused by BRCA mutations often originate in the distal half of the fallopian tube and then progress to the ovary. Serous tumors also may arise from the ovary or peritoneum.10 Building on this finding (that in BRCA previvors serous tumors often begin in the distal fallopian tube), some experts now recommend that a two-stage risk-reducing pelvic surgery option be offered to women with BRCA mutations. No large clinical trials have been reported using the two-stage approach to risk-reducing pelvic surgery, but published case series suggest that it is a plausible approach.
Stage 1: Ovary preservation. In Stage 1 surgery, the fallopian tubes are removed through a laparoscopic approach, but the ovaries are preserved. Some experts also recommend removal of the fallopian tube–peritoneum–ovarian junction.11,12 Stage 1 surgery reduces the risk of developing a cancer that originates in the fallopian tube and preserves ovarian estradiol and progesterone secretion, thereby avoiding premature menopause.
Stage 2: Ovary removal. Years later, as late as age 50, Stage 2 surgery is performed, and the ovaries are removed laparoscopically.
In the Stage 2 operation, consideration should be given to performing a hysterectomy. Since this second surgery will make the woman menopausal, concomitant hysterectomy would permit estrogen-only HT.
It should be noted that, for BRCA previvors, removing the ovaries at an early age is associated with a reduced breast cancer risk. The two-stage surgical approach does not offer this advantage of breast cancer risk reduction.
Appropriate gyn follow-up after risk-reducing BsO
Consider HT or estrogen-progestin contraceptives. For young, premenopausal women who undergo risk-reducing BSO, HT or estrogen-progestin contraceptives will help to reduce the risk of hot flashes, sleep disturbance, and symptoms of vaginal dryness. Although there is a theoretical concern that estrogen and progestin treatment may increase the risk of breast cancer, many experts are comfortable with prescribing estrogen and progestins to young BRCA carriers following risk-reducing BSO.
If your young, premenopausal patient underwent risk-reducing BSO but declines HT, a bone density test should be obtained within 1 or 2 years of surgery; you can offer nonhormonal treatment for her menopausal symptoms.
After undergoing risk-reducing BSO, patients should be prescribed weight-bearing exercise, vitamin D 600 U daily, and calcium supplements. In addition, because of a 5% risk of developing a peritoneal cancer, provide an annual gynecologic exam with CA-125 measurement and pelvic sonography every 6 months.13
Appropriate surveillance for women who forego risk-reducing surgery
This surveillance strategy may be helpful in detecting cancer at an early stage:
1. Beginning at age 25: Perform clinical breast exam once or twice per year
2. Beginning at age 25, or based on individualized assessment influenced by the earliest age of onset of cancer in the family: Alternate breast magnetic resonance imaging and mammography every 6 months
3. Beginning at age 35, or 10 years before the earliest age of onset of ovarian cancer in the family: Perform pelvic examination, CA-125 measurement, and pelvic sonography every 6 months. In women with BRCA mutations, estrogen-progestin contraceptive use may reduce the risk of ovarian cancer without increasing the risk of breast cancer.7
Her genetics can put your patient at risk. You are in a position to help protect her.
Imagine prematurely losing your mother, mother’s sister, and maternal grandmother to breast and ovarian cancers. Each funeral for a young relative resurrects memories of previous painful losses. The children and adults worry, “Who will be the next to die?” The early identification of families with BRCA mutations offers the hope of options to reduce premature death, preserving the quality of life and keeping families intact, so they can gather together for enjoyable holidays, not funerals.
Tell us what you think, at [email protected]. Please include your name and city and state.
1. National Comprehensive Cancer Network. Ge-netic/Familial High-Risk Assessment: Breast and Ovarian. Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed August 20, 2013.
2. Myriad Genetic Laboratories. BRCA risk calculator. http://www.myriadpro.com/brca-risk-calcu lator/calc.html. Accessed August 20, 2013.
3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333.
4. Domchek SM, Friebel TM, Singer CF, et al. As-sociation of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975.
5. Skytte AB, Gerdes AM, Andresen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin Genet. 2010;77(4):342–349.
6. Westin SN, Sun CC, Lu KH, et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer. 2011;117(12):2659–2667.
7. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 2010;46(12):2275–2284.
8. Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32.
9. Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714.
10. Yates MS, Meyer LA, Deavers MT, et al. Microscopic and early-stage ovarian cancers in BRCA1/2 mutation carriers: Building a model for early BRCA-associated tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):463–470.
11. Leblanc E, Narducci F, Farre I, et al. Radical fimbriectomy: a reasonable temporary risk-reducing surgery for selected women with a germ line mutation of BRCA 1 or 2 genes? Rationale and preliminary development. Gynecol Oncol. 2011;121(3):472–476.
12. Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
13. Chapman JS, Powell CB, McLennan J, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–343.
Angelina Jolie’s mother and maternal grandmother died of ovarian cancer; her mother’s sister died of breast cancer. After testing positive for a clinically significant BRCA mutation, Ms. Jolie became a “previvor,” a woman who is currently healthy but at very high risk for developing a life-threatening breast or ovarian malignancy. Advances in genetic testing will result in many more women being identified as BRCA previvors. These women face many daunting decisions concerning options for risk-reducing breast and pelvic surgery. We gynecologists need to be prepared to help shepherd them through this process.
Which of our patients should be tested for BRCA mutations?
Experts have provided complex guidance on who should be tested for a BRCA mutation.1 These recommendations include testing women with a personal history of:
• epithelial ovarian cancer
• fallopian tube cancer, or
• primary peritoneal cancer.
Women with a personal history of breast cancer at an early age also should consider being tested. If women with breast or ovarian cancer, or both, test positive for BRCA1 or BRCA2, then living family members can be offered testing.
Healthy women with a family history of breast and/or ovarian cancer also may benefit from genetic testing. My clinical experience is that, unless the family history is as dramatic as that of Ms. Jolie, it requires considerable time and expertise to assemble a valid extended-family history and provide an estimate of risk. This task may be best completed by a genetic counselor. A simple, Web-based BRCA risk-assessment tool is provided by Myriad Genetics.2
A clinically significant BRCA mutation is detected. What is your patient’s risk?
Unfortunately, women with BRCA mutations are at very high risk for breast and ovarian cancer.
BRCA1. For patients with a BRCA1 mutation, the mean cumulative cancer risk to age 70 years is approximately 57% for breast cancer (95% confidence interval [CI], 47% to 66%) and 40% for ovarian cancer (95% CI, 35% to 46%).3
BRCA2. For patients with a BRCA2 mutation, the mean cumulative cancer risk to age 70 years is 49% for breast cancer (95% CI, 40% to 57%) and 18% for ovarian cancer (95% CI,13% to 23%). Tables 1 and 2 provide an approximation of the risk of developing breast or ovarian cancer for BRCA1 previvors aged 30, 40, and 50. The breast and ovarian cancer risk for BRCA2 previvors (not shown) is less than that for BRCA1 previvors.
Options for risk-reducing surgery
Risk-reducing breast and pelvic surgery markedly lessens the risk of developing cancer in the breast and ovary, decreasing the risk of mortality not only from breast cancer and ovarian cancer but from all causes.4
How many women choose risk-reducing surgery, and why? In a cohort of 306 healthy BRCA mutation carriers in the Netherlands, 10 years of follow-up revealed that 75% underwent risk-reducing pelvic surgery, and 50% underwent risk-reducing mastectomy.5 The age of the woman and her desire to preserve child-bearing strongly influenced the decision to undergo prophylactic surgery and the timing of the surgery.
Following risk-reducing surgery, most women are satisfied with their choice. In one follow-up study, the authors reported that women who had difficulty arriving at a decision were less satisfied with their choice to have risk-reducing surgery than women who arrived at their decision with confidence.6
Risk-reducing breast surgery: What’s involved?
As can be seen in Tables 1 and 2, the risk of your 30-year-old patient with a BRCA1 mutation developing breast cancer throughout her 30s is much greater than her risk of developing ovarian cancer. For this patient, bilateral total mastectomy is the standard risk-reducing procedure because subcutaneous mastectomy may leave islands of glandular tissue that may serve as a nidus for cancer development. However, both nipple-sparing and skin-sparing mastectomy have been reported to be successful in most cases, and may be favored by many women.8,9 Following risk-reducing breast surgery, most women can immediately begin multistage breast reconstruction.
Ms. Jolie underwent a “nipple delay” surgery 2 weeks before her mastectomy. This procedure involves lifting a portion of the nipple skin off the underlying breast to stimulate the nipple to start drawing its blood flow from the surrounding skin rather than the underlying tissue. In addition, during the nipple delay procedure a biopsy of the tissue from beneath the nipple is performed to ensure that no cancer is present.
Risk-reducing pelvic surgery: Essential steps
The standard operation to reduce the risk of ovarian cancer in BRCA mutation carriers is a bilateral salpingo-oophorectomy (BSO). Additional steps should be performed to assess for distant disease, including:
• careful assessment of all peritoneal surfaces
• pelvic washing for cytology
• omental biopsy
• cytologic smear of the diaphragm.
The fallopian tube should be resected in its entirety, but cornual resection is not necessary. Care should be taken to remove the entire ovary and avoid leaving small remnants of the ovary on the proximal vascular pedicles. Consideration should be given to performing a hysterectomy at the time of risk-reducing BSO. If the uterus is removed, estrogen-only hormone therapy (HT) can be prescribed. Estrogen-only HT causes fewer adverse events than combination estrogen-progestin therapy.
Two-stage pelvic surgery approach
Recent research indicates that high-grade serous tumors caused by BRCA mutations often originate in the distal half of the fallopian tube and then progress to the ovary. Serous tumors also may arise from the ovary or peritoneum.10 Building on this finding (that in BRCA previvors serous tumors often begin in the distal fallopian tube), some experts now recommend that a two-stage risk-reducing pelvic surgery option be offered to women with BRCA mutations. No large clinical trials have been reported using the two-stage approach to risk-reducing pelvic surgery, but published case series suggest that it is a plausible approach.
Stage 1: Ovary preservation. In Stage 1 surgery, the fallopian tubes are removed through a laparoscopic approach, but the ovaries are preserved. Some experts also recommend removal of the fallopian tube–peritoneum–ovarian junction.11,12 Stage 1 surgery reduces the risk of developing a cancer that originates in the fallopian tube and preserves ovarian estradiol and progesterone secretion, thereby avoiding premature menopause.
Stage 2: Ovary removal. Years later, as late as age 50, Stage 2 surgery is performed, and the ovaries are removed laparoscopically.
In the Stage 2 operation, consideration should be given to performing a hysterectomy. Since this second surgery will make the woman menopausal, concomitant hysterectomy would permit estrogen-only HT.
It should be noted that, for BRCA previvors, removing the ovaries at an early age is associated with a reduced breast cancer risk. The two-stage surgical approach does not offer this advantage of breast cancer risk reduction.
Appropriate gyn follow-up after risk-reducing BsO
Consider HT or estrogen-progestin contraceptives. For young, premenopausal women who undergo risk-reducing BSO, HT or estrogen-progestin contraceptives will help to reduce the risk of hot flashes, sleep disturbance, and symptoms of vaginal dryness. Although there is a theoretical concern that estrogen and progestin treatment may increase the risk of breast cancer, many experts are comfortable with prescribing estrogen and progestins to young BRCA carriers following risk-reducing BSO.
If your young, premenopausal patient underwent risk-reducing BSO but declines HT, a bone density test should be obtained within 1 or 2 years of surgery; you can offer nonhormonal treatment for her menopausal symptoms.
After undergoing risk-reducing BSO, patients should be prescribed weight-bearing exercise, vitamin D 600 U daily, and calcium supplements. In addition, because of a 5% risk of developing a peritoneal cancer, provide an annual gynecologic exam with CA-125 measurement and pelvic sonography every 6 months.13
Appropriate surveillance for women who forego risk-reducing surgery
This surveillance strategy may be helpful in detecting cancer at an early stage:
1. Beginning at age 25: Perform clinical breast exam once or twice per year
2. Beginning at age 25, or based on individualized assessment influenced by the earliest age of onset of cancer in the family: Alternate breast magnetic resonance imaging and mammography every 6 months
3. Beginning at age 35, or 10 years before the earliest age of onset of ovarian cancer in the family: Perform pelvic examination, CA-125 measurement, and pelvic sonography every 6 months. In women with BRCA mutations, estrogen-progestin contraceptive use may reduce the risk of ovarian cancer without increasing the risk of breast cancer.7
Her genetics can put your patient at risk. You are in a position to help protect her.
Imagine prematurely losing your mother, mother’s sister, and maternal grandmother to breast and ovarian cancers. Each funeral for a young relative resurrects memories of previous painful losses. The children and adults worry, “Who will be the next to die?” The early identification of families with BRCA mutations offers the hope of options to reduce premature death, preserving the quality of life and keeping families intact, so they can gather together for enjoyable holidays, not funerals.
Tell us what you think, at [email protected]. Please include your name and city and state.
Angelina Jolie’s mother and maternal grandmother died of ovarian cancer; her mother’s sister died of breast cancer. After testing positive for a clinically significant BRCA mutation, Ms. Jolie became a “previvor,” a woman who is currently healthy but at very high risk for developing a life-threatening breast or ovarian malignancy. Advances in genetic testing will result in many more women being identified as BRCA previvors. These women face many daunting decisions concerning options for risk-reducing breast and pelvic surgery. We gynecologists need to be prepared to help shepherd them through this process.
Which of our patients should be tested for BRCA mutations?
Experts have provided complex guidance on who should be tested for a BRCA mutation.1 These recommendations include testing women with a personal history of:
• epithelial ovarian cancer
• fallopian tube cancer, or
• primary peritoneal cancer.
Women with a personal history of breast cancer at an early age also should consider being tested. If women with breast or ovarian cancer, or both, test positive for BRCA1 or BRCA2, then living family members can be offered testing.
Healthy women with a family history of breast and/or ovarian cancer also may benefit from genetic testing. My clinical experience is that, unless the family history is as dramatic as that of Ms. Jolie, it requires considerable time and expertise to assemble a valid extended-family history and provide an estimate of risk. This task may be best completed by a genetic counselor. A simple, Web-based BRCA risk-assessment tool is provided by Myriad Genetics.2
A clinically significant BRCA mutation is detected. What is your patient’s risk?
Unfortunately, women with BRCA mutations are at very high risk for breast and ovarian cancer.
BRCA1. For patients with a BRCA1 mutation, the mean cumulative cancer risk to age 70 years is approximately 57% for breast cancer (95% confidence interval [CI], 47% to 66%) and 40% for ovarian cancer (95% CI, 35% to 46%).3
BRCA2. For patients with a BRCA2 mutation, the mean cumulative cancer risk to age 70 years is 49% for breast cancer (95% CI, 40% to 57%) and 18% for ovarian cancer (95% CI,13% to 23%). Tables 1 and 2 provide an approximation of the risk of developing breast or ovarian cancer for BRCA1 previvors aged 30, 40, and 50. The breast and ovarian cancer risk for BRCA2 previvors (not shown) is less than that for BRCA1 previvors.
Options for risk-reducing surgery
Risk-reducing breast and pelvic surgery markedly lessens the risk of developing cancer in the breast and ovary, decreasing the risk of mortality not only from breast cancer and ovarian cancer but from all causes.4
How many women choose risk-reducing surgery, and why? In a cohort of 306 healthy BRCA mutation carriers in the Netherlands, 10 years of follow-up revealed that 75% underwent risk-reducing pelvic surgery, and 50% underwent risk-reducing mastectomy.5 The age of the woman and her desire to preserve child-bearing strongly influenced the decision to undergo prophylactic surgery and the timing of the surgery.
Following risk-reducing surgery, most women are satisfied with their choice. In one follow-up study, the authors reported that women who had difficulty arriving at a decision were less satisfied with their choice to have risk-reducing surgery than women who arrived at their decision with confidence.6
Risk-reducing breast surgery: What’s involved?
As can be seen in Tables 1 and 2, the risk of your 30-year-old patient with a BRCA1 mutation developing breast cancer throughout her 30s is much greater than her risk of developing ovarian cancer. For this patient, bilateral total mastectomy is the standard risk-reducing procedure because subcutaneous mastectomy may leave islands of glandular tissue that may serve as a nidus for cancer development. However, both nipple-sparing and skin-sparing mastectomy have been reported to be successful in most cases, and may be favored by many women.8,9 Following risk-reducing breast surgery, most women can immediately begin multistage breast reconstruction.
Ms. Jolie underwent a “nipple delay” surgery 2 weeks before her mastectomy. This procedure involves lifting a portion of the nipple skin off the underlying breast to stimulate the nipple to start drawing its blood flow from the surrounding skin rather than the underlying tissue. In addition, during the nipple delay procedure a biopsy of the tissue from beneath the nipple is performed to ensure that no cancer is present.
Risk-reducing pelvic surgery: Essential steps
The standard operation to reduce the risk of ovarian cancer in BRCA mutation carriers is a bilateral salpingo-oophorectomy (BSO). Additional steps should be performed to assess for distant disease, including:
• careful assessment of all peritoneal surfaces
• pelvic washing for cytology
• omental biopsy
• cytologic smear of the diaphragm.
The fallopian tube should be resected in its entirety, but cornual resection is not necessary. Care should be taken to remove the entire ovary and avoid leaving small remnants of the ovary on the proximal vascular pedicles. Consideration should be given to performing a hysterectomy at the time of risk-reducing BSO. If the uterus is removed, estrogen-only hormone therapy (HT) can be prescribed. Estrogen-only HT causes fewer adverse events than combination estrogen-progestin therapy.
Two-stage pelvic surgery approach
Recent research indicates that high-grade serous tumors caused by BRCA mutations often originate in the distal half of the fallopian tube and then progress to the ovary. Serous tumors also may arise from the ovary or peritoneum.10 Building on this finding (that in BRCA previvors serous tumors often begin in the distal fallopian tube), some experts now recommend that a two-stage risk-reducing pelvic surgery option be offered to women with BRCA mutations. No large clinical trials have been reported using the two-stage approach to risk-reducing pelvic surgery, but published case series suggest that it is a plausible approach.
Stage 1: Ovary preservation. In Stage 1 surgery, the fallopian tubes are removed through a laparoscopic approach, but the ovaries are preserved. Some experts also recommend removal of the fallopian tube–peritoneum–ovarian junction.11,12 Stage 1 surgery reduces the risk of developing a cancer that originates in the fallopian tube and preserves ovarian estradiol and progesterone secretion, thereby avoiding premature menopause.
Stage 2: Ovary removal. Years later, as late as age 50, Stage 2 surgery is performed, and the ovaries are removed laparoscopically.
In the Stage 2 operation, consideration should be given to performing a hysterectomy. Since this second surgery will make the woman menopausal, concomitant hysterectomy would permit estrogen-only HT.
It should be noted that, for BRCA previvors, removing the ovaries at an early age is associated with a reduced breast cancer risk. The two-stage surgical approach does not offer this advantage of breast cancer risk reduction.
Appropriate gyn follow-up after risk-reducing BsO
Consider HT or estrogen-progestin contraceptives. For young, premenopausal women who undergo risk-reducing BSO, HT or estrogen-progestin contraceptives will help to reduce the risk of hot flashes, sleep disturbance, and symptoms of vaginal dryness. Although there is a theoretical concern that estrogen and progestin treatment may increase the risk of breast cancer, many experts are comfortable with prescribing estrogen and progestins to young BRCA carriers following risk-reducing BSO.
If your young, premenopausal patient underwent risk-reducing BSO but declines HT, a bone density test should be obtained within 1 or 2 years of surgery; you can offer nonhormonal treatment for her menopausal symptoms.
After undergoing risk-reducing BSO, patients should be prescribed weight-bearing exercise, vitamin D 600 U daily, and calcium supplements. In addition, because of a 5% risk of developing a peritoneal cancer, provide an annual gynecologic exam with CA-125 measurement and pelvic sonography every 6 months.13
Appropriate surveillance for women who forego risk-reducing surgery
This surveillance strategy may be helpful in detecting cancer at an early stage:
1. Beginning at age 25: Perform clinical breast exam once or twice per year
2. Beginning at age 25, or based on individualized assessment influenced by the earliest age of onset of cancer in the family: Alternate breast magnetic resonance imaging and mammography every 6 months
3. Beginning at age 35, or 10 years before the earliest age of onset of ovarian cancer in the family: Perform pelvic examination, CA-125 measurement, and pelvic sonography every 6 months. In women with BRCA mutations, estrogen-progestin contraceptive use may reduce the risk of ovarian cancer without increasing the risk of breast cancer.7
Her genetics can put your patient at risk. You are in a position to help protect her.
Imagine prematurely losing your mother, mother’s sister, and maternal grandmother to breast and ovarian cancers. Each funeral for a young relative resurrects memories of previous painful losses. The children and adults worry, “Who will be the next to die?” The early identification of families with BRCA mutations offers the hope of options to reduce premature death, preserving the quality of life and keeping families intact, so they can gather together for enjoyable holidays, not funerals.
Tell us what you think, at [email protected]. Please include your name and city and state.
1. National Comprehensive Cancer Network. Ge-netic/Familial High-Risk Assessment: Breast and Ovarian. Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed August 20, 2013.
2. Myriad Genetic Laboratories. BRCA risk calculator. http://www.myriadpro.com/brca-risk-calcu lator/calc.html. Accessed August 20, 2013.
3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333.
4. Domchek SM, Friebel TM, Singer CF, et al. As-sociation of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975.
5. Skytte AB, Gerdes AM, Andresen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin Genet. 2010;77(4):342–349.
6. Westin SN, Sun CC, Lu KH, et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer. 2011;117(12):2659–2667.
7. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 2010;46(12):2275–2284.
8. Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32.
9. Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714.
10. Yates MS, Meyer LA, Deavers MT, et al. Microscopic and early-stage ovarian cancers in BRCA1/2 mutation carriers: Building a model for early BRCA-associated tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):463–470.
11. Leblanc E, Narducci F, Farre I, et al. Radical fimbriectomy: a reasonable temporary risk-reducing surgery for selected women with a germ line mutation of BRCA 1 or 2 genes? Rationale and preliminary development. Gynecol Oncol. 2011;121(3):472–476.
12. Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
13. Chapman JS, Powell CB, McLennan J, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–343.
1. National Comprehensive Cancer Network. Ge-netic/Familial High-Risk Assessment: Breast and Ovarian. Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed August 20, 2013.
2. Myriad Genetic Laboratories. BRCA risk calculator. http://www.myriadpro.com/brca-risk-calcu lator/calc.html. Accessed August 20, 2013.
3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333.
4. Domchek SM, Friebel TM, Singer CF, et al. As-sociation of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975.
5. Skytte AB, Gerdes AM, Andresen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin Genet. 2010;77(4):342–349.
6. Westin SN, Sun CC, Lu KH, et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer. 2011;117(12):2659–2667.
7. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 2010;46(12):2275–2284.
8. Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32.
9. Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714.
10. Yates MS, Meyer LA, Deavers MT, et al. Microscopic and early-stage ovarian cancers in BRCA1/2 mutation carriers: Building a model for early BRCA-associated tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):463–470.
11. Leblanc E, Narducci F, Farre I, et al. Radical fimbriectomy: a reasonable temporary risk-reducing surgery for selected women with a germ line mutation of BRCA 1 or 2 genes? Rationale and preliminary development. Gynecol Oncol. 2011;121(3):472–476.
12. Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
13. Chapman JS, Powell CB, McLennan J, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–343.