User login
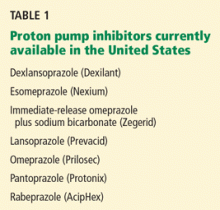
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
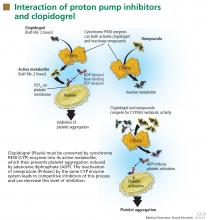
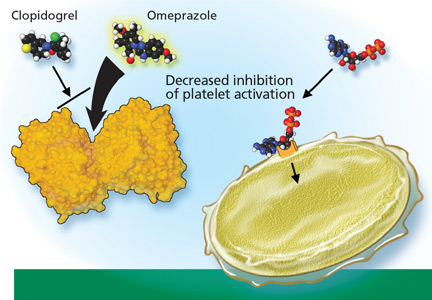
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
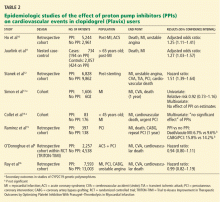
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
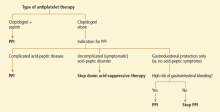
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
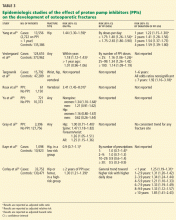
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
KEY POINTS
- The US Food and Drug Administration has issued alerts that PPIs may increase the rate of osteoporosis-related fractures and may decrease the effectiveness of clopidogrel (Plavix) for preventing serious cardiovascular events.
- Other concerns include increased rates of pneumonia, Clostridium difficile infection, and other infections.
- A prudent approach to managing these concerns in day-to-day practice is required: PPIs, like any other drugs, should be prescribed only if indicated.