User login
Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
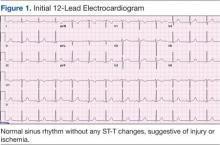
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
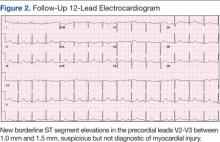
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
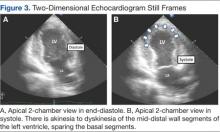
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
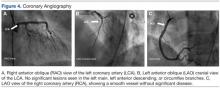
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
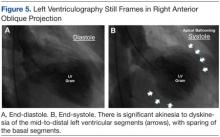
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
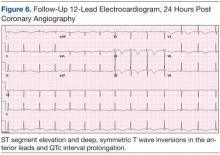
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
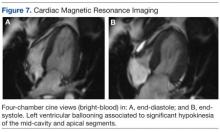
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).
The patient was treated with supportive care, psychotropic therapy, angiotensin-converting enzyme inhibitor (ACE-I), and beta blocker therapy. Within 9 days, NT-proBNP levels normalized (from peak 8,834 pg/mL to 191.5 pg/mL).
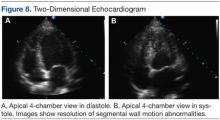
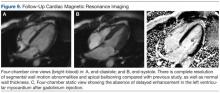
Six weeks later, an echocardiogram confirmed resolution of wall motion abnormalities (Figure 8). Follow-up cardiac MRI showed complete resolution of segmental wall motion abnormalities and the apical ballooning, normal wall thickness, and absent delayed enhancement (Figure 9). These findings further supported the diagnosis of ABS and excluded MI and myocarditis.
Discussion
What is striking about takotsubo cardiomyopathy is that the clinical presentation resembles an AMI. Several studies have reported that 1.7% to 2.2% of patients who had suspected acute coronary syndrome were subsequently diagnosed with takotsubo cardiomyopathy.6-8 Nearly 90% of reported cases involved postmenopausal women, and this may be related to loss of the cardioprotective effect of estrogen.5,9
A preceding stressful emotional or physical event is usually identified in about two-thirds of the patients with ABS.9 Most common emotional triggers are death of a relative or friend, broken relationships, assaults, and rapes, among others. Physical triggers include severe sepsis, shock, acute respiratory failure, seizures, and intracranial bleeds. Sometimes a specific trigger cannot be identified from the history, but the absence of an emotional or physical trigger does not exclude the diagnosis.
Although the exact pathogenesis of ABS remains unclear, it is likely that multiple factors are involved. Some of the suggested mechanisms are high levels of catecholamines, multivessel epicardial spasm, or coronary microvascular dysfunction.4 The catecholamine hypothesis has been supported by the finding that several patients with pheochromocytoma and subarachnoid hemorrhage also present with high levels of catecholamine and a cardiomyopathy resembling ABS. Furthermore, ABS has been reported in patients on catecholamine infusions and those treated with agents that inhibit reuptake of catecholamines.5
The presence of multivessel coronary spasm was suggested by early small studies in Japan, but more recent case series have not validated this hypothesis.5 The microvascular dysfunction hypothesis is supported by the presence of myocardial ischemia, diagnosed by ECG changes and elevated troponins, in the absence of significant coronary disease. However, it remains unclear whether this is a primary mechanism or a manifestation of a primary process.4 Microvascular dysfunction may be more likely related to impairment of myocardial relaxation with extramural coronary compression.
Signs and symptoms of ABS mimic those of AMI, with angina-like chest pain as the main presenting symptom in about 50% of cases.10 Other symptoms include dyspnea and less commonly, syncope or sudden cardiac death. Decompensated left heart failure occurs in 50% of patients, with severe hemodynamic compromise and cardiogenic shock not being uncommon. Other complications that may occur are tachyarrhythmias (atrial or ventricular) and ventricular thromboembolism.4
Common ECG changes in ABS include precordial ST segment elevations, symmetric T wave inversions, and nonspecific T wave changes.4,10 QT interval prolongation may be seen during the first days. Transient pathologic Q waves may be seen at presentation or afterward. These ECG changes tend to revert after weeks or months of presentation.
Elevation of cardiac biomarkers is usually present in laboratory data. Levels peak at 24 hours, and the degree of elevation is usually less than that seen in patients with an AMI.10 Most important, the degree of cardiac biomarker elevation is disproportionately low for the extent of involved coronary territory and left ventricular dysfunction. Other laboratory tests that are frequently altered are the BNP and pro-BNP levels, which are usually elevated due to transient left ventricular dysfunction. C-reactive protein elevates in most patients and indicates the presence of an acute inflammatory response.
Early coronary angiography should be performed in all patients with ABS to rule out the presence of a significant obstructive coronary lesion. Patients with ABS often have luminal irregularities or normal coronary vessels. However, concomitant obstructive coronary lesions may be found, especially in elderly patients.
The hallmark of ABS is a characteristic transient contractility abnormality of the left ventricle causing ballooning of the apex, which can be detected on left ventricular angiography or echocardiography. There are 3 distinct variants of ABS, according to the left ventricular myocardial wall segments involved.10 The classic form of takotsubo is characterized by hypokinesis, dyskinesis, or akinesis of the middle and apical segments of the left ventricle. The basal segment is usually spared and may be hyperdynamic. In the midventricular or apical sparing variant, the wall motion abnormalities are restricted to the midventricular segments, and apical contraction is preserved. This case resembles the atypical variant, because the midventricular segments were affected, whereas apical and basal regions were preserved. A rare variant of takotsubo exists with hypokinesis or akinesis of the base and preserved apical function.
Besides ABS and AMI, an important entity to consider in the differential diagnosis of transient wall motion abnormalities is regional myocarditis. Viral titers are helpful in excluding this condition. Furthermore, prolonged recovery is more commonly seen in myocarditis compared with ABS. Imaging studies are particularly helpful in this scenario.
Cardiac MRI demonstrates the wall motion abnormalities or apical ballooning typical for this condition and can differentiate ABS from myocarditis or MI. It is known that delayed myocardial enhancement is seen with myocardial fibrosis. Typically in ischemic cardiomyopathy, there is wall thinning with associated delayed enhancement that extends from the subendocardium to the epicardium (from 0%-90% of wall thickness) of a particular vascular territory. In myocarditis, the enhancement is usually seen in the involved intramyocardial (mesocardium) region, and the pattern is patchy. In ABS, the delayed enhancement is absent, because there is no fibrosis in the area of regional wall motion abnormalities, and wall thickness is usually normal.9,10
No evidence-based guidelines for treating ABS are currently available. Most patients are initially treated with antiplatelets/anticoagulant therapy, nitrates, and diuretics if the patient presents with heart failure. Patients should be admitted to an intensive care unit for close cardiac monitoring. Once ABS is diagnosed and significant coronary stenosis is excluded, patients should receive standard supportive care and optimal neurohormonal therapy. This should include beta blocker or combined alpha/beta blocker agents, an ACE-I or angiotensin receptor blocker, and diuretics if appropriate. Once left ventricular function (LVF) is recovered, therapy with inhibitors of the renin-angiotensin system may be discontinued, but patients should remain on long-term alpha or beta blocker therapy, because the sympathetic blockade provided by these agents may prevent recurrences of this disease.10
Prognosis is generally favorable, and most patients recover to normal LVF over weeks to months. It is important to assess the LVF 4 to 6 weeks after the patient is discharged to confirm the diagnosis of ABS. Recurrence may occur in up to 9% of cases.10 Long-term mortality is similar compared with the age-matched general population.
Conclusion
Apical ballooning syndrome is a relatively novel cardiomyopathy that has gained important attention among the cardiovascular community, mostly because its clinical presentation mimics that of an acute coronary syndrome. Awareness of this entity will result in a more focused diagnosis and appropriate treatment. Managing both cardiac and emotional components of this disease will have a permanent impact in the reversibility and secondary prevention of this cardiomyopathy.
Acknowledgments
Special thanks to the Radiology Service at the VA Caribbean Healthcare System, in particular Dr. Frances Aulet for interpretation of the cardiac MRI results and assistance with MRI images.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases [in Japanese]. J Cardiol. 1991;21(2):203-214.
2. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;9(4):311-316.
3. Afonso L, Bachour K, Awad K, Sandidge G. Takotsubo cardiomyopathy: Pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Eur J Echocardiogr. 2008;9(6):849-854.
4. Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: A review of the current literature. Postgrad Med J. 2007;83(978):261-264.
5. Hare J. The dilated, restrictive and infiltrative cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2012:1562-1580.
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343-346.
7. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—Comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115-122.
8. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417.
9. Lange R, Hills D. Chemical cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: Saunders; 2014:1609-1611.
10. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27(13):1523-1529.
Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).
The patient was treated with supportive care, psychotropic therapy, angiotensin-converting enzyme inhibitor (ACE-I), and beta blocker therapy. Within 9 days, NT-proBNP levels normalized (from peak 8,834 pg/mL to 191.5 pg/mL).
Six weeks later, an echocardiogram confirmed resolution of wall motion abnormalities (Figure 8). Follow-up cardiac MRI showed complete resolution of segmental wall motion abnormalities and the apical ballooning, normal wall thickness, and absent delayed enhancement (Figure 9). These findings further supported the diagnosis of ABS and excluded MI and myocarditis.
Discussion
What is striking about takotsubo cardiomyopathy is that the clinical presentation resembles an AMI. Several studies have reported that 1.7% to 2.2% of patients who had suspected acute coronary syndrome were subsequently diagnosed with takotsubo cardiomyopathy.6-8 Nearly 90% of reported cases involved postmenopausal women, and this may be related to loss of the cardioprotective effect of estrogen.5,9
A preceding stressful emotional or physical event is usually identified in about two-thirds of the patients with ABS.9 Most common emotional triggers are death of a relative or friend, broken relationships, assaults, and rapes, among others. Physical triggers include severe sepsis, shock, acute respiratory failure, seizures, and intracranial bleeds. Sometimes a specific trigger cannot be identified from the history, but the absence of an emotional or physical trigger does not exclude the diagnosis.
Although the exact pathogenesis of ABS remains unclear, it is likely that multiple factors are involved. Some of the suggested mechanisms are high levels of catecholamines, multivessel epicardial spasm, or coronary microvascular dysfunction.4 The catecholamine hypothesis has been supported by the finding that several patients with pheochromocytoma and subarachnoid hemorrhage also present with high levels of catecholamine and a cardiomyopathy resembling ABS. Furthermore, ABS has been reported in patients on catecholamine infusions and those treated with agents that inhibit reuptake of catecholamines.5
The presence of multivessel coronary spasm was suggested by early small studies in Japan, but more recent case series have not validated this hypothesis.5 The microvascular dysfunction hypothesis is supported by the presence of myocardial ischemia, diagnosed by ECG changes and elevated troponins, in the absence of significant coronary disease. However, it remains unclear whether this is a primary mechanism or a manifestation of a primary process.4 Microvascular dysfunction may be more likely related to impairment of myocardial relaxation with extramural coronary compression.
Signs and symptoms of ABS mimic those of AMI, with angina-like chest pain as the main presenting symptom in about 50% of cases.10 Other symptoms include dyspnea and less commonly, syncope or sudden cardiac death. Decompensated left heart failure occurs in 50% of patients, with severe hemodynamic compromise and cardiogenic shock not being uncommon. Other complications that may occur are tachyarrhythmias (atrial or ventricular) and ventricular thromboembolism.4
Common ECG changes in ABS include precordial ST segment elevations, symmetric T wave inversions, and nonspecific T wave changes.4,10 QT interval prolongation may be seen during the first days. Transient pathologic Q waves may be seen at presentation or afterward. These ECG changes tend to revert after weeks or months of presentation.
Elevation of cardiac biomarkers is usually present in laboratory data. Levels peak at 24 hours, and the degree of elevation is usually less than that seen in patients with an AMI.10 Most important, the degree of cardiac biomarker elevation is disproportionately low for the extent of involved coronary territory and left ventricular dysfunction. Other laboratory tests that are frequently altered are the BNP and pro-BNP levels, which are usually elevated due to transient left ventricular dysfunction. C-reactive protein elevates in most patients and indicates the presence of an acute inflammatory response.
Early coronary angiography should be performed in all patients with ABS to rule out the presence of a significant obstructive coronary lesion. Patients with ABS often have luminal irregularities or normal coronary vessels. However, concomitant obstructive coronary lesions may be found, especially in elderly patients.
The hallmark of ABS is a characteristic transient contractility abnormality of the left ventricle causing ballooning of the apex, which can be detected on left ventricular angiography or echocardiography. There are 3 distinct variants of ABS, according to the left ventricular myocardial wall segments involved.10 The classic form of takotsubo is characterized by hypokinesis, dyskinesis, or akinesis of the middle and apical segments of the left ventricle. The basal segment is usually spared and may be hyperdynamic. In the midventricular or apical sparing variant, the wall motion abnormalities are restricted to the midventricular segments, and apical contraction is preserved. This case resembles the atypical variant, because the midventricular segments were affected, whereas apical and basal regions were preserved. A rare variant of takotsubo exists with hypokinesis or akinesis of the base and preserved apical function.
Besides ABS and AMI, an important entity to consider in the differential diagnosis of transient wall motion abnormalities is regional myocarditis. Viral titers are helpful in excluding this condition. Furthermore, prolonged recovery is more commonly seen in myocarditis compared with ABS. Imaging studies are particularly helpful in this scenario.
Cardiac MRI demonstrates the wall motion abnormalities or apical ballooning typical for this condition and can differentiate ABS from myocarditis or MI. It is known that delayed myocardial enhancement is seen with myocardial fibrosis. Typically in ischemic cardiomyopathy, there is wall thinning with associated delayed enhancement that extends from the subendocardium to the epicardium (from 0%-90% of wall thickness) of a particular vascular territory. In myocarditis, the enhancement is usually seen in the involved intramyocardial (mesocardium) region, and the pattern is patchy. In ABS, the delayed enhancement is absent, because there is no fibrosis in the area of regional wall motion abnormalities, and wall thickness is usually normal.9,10
No evidence-based guidelines for treating ABS are currently available. Most patients are initially treated with antiplatelets/anticoagulant therapy, nitrates, and diuretics if the patient presents with heart failure. Patients should be admitted to an intensive care unit for close cardiac monitoring. Once ABS is diagnosed and significant coronary stenosis is excluded, patients should receive standard supportive care and optimal neurohormonal therapy. This should include beta blocker or combined alpha/beta blocker agents, an ACE-I or angiotensin receptor blocker, and diuretics if appropriate. Once left ventricular function (LVF) is recovered, therapy with inhibitors of the renin-angiotensin system may be discontinued, but patients should remain on long-term alpha or beta blocker therapy, because the sympathetic blockade provided by these agents may prevent recurrences of this disease.10
Prognosis is generally favorable, and most patients recover to normal LVF over weeks to months. It is important to assess the LVF 4 to 6 weeks after the patient is discharged to confirm the diagnosis of ABS. Recurrence may occur in up to 9% of cases.10 Long-term mortality is similar compared with the age-matched general population.
Conclusion
Apical ballooning syndrome is a relatively novel cardiomyopathy that has gained important attention among the cardiovascular community, mostly because its clinical presentation mimics that of an acute coronary syndrome. Awareness of this entity will result in a more focused diagnosis and appropriate treatment. Managing both cardiac and emotional components of this disease will have a permanent impact in the reversibility and secondary prevention of this cardiomyopathy.
Acknowledgments
Special thanks to the Radiology Service at the VA Caribbean Healthcare System, in particular Dr. Frances Aulet for interpretation of the cardiac MRI results and assistance with MRI images.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).
The patient was treated with supportive care, psychotropic therapy, angiotensin-converting enzyme inhibitor (ACE-I), and beta blocker therapy. Within 9 days, NT-proBNP levels normalized (from peak 8,834 pg/mL to 191.5 pg/mL).
Six weeks later, an echocardiogram confirmed resolution of wall motion abnormalities (Figure 8). Follow-up cardiac MRI showed complete resolution of segmental wall motion abnormalities and the apical ballooning, normal wall thickness, and absent delayed enhancement (Figure 9). These findings further supported the diagnosis of ABS and excluded MI and myocarditis.
Discussion
What is striking about takotsubo cardiomyopathy is that the clinical presentation resembles an AMI. Several studies have reported that 1.7% to 2.2% of patients who had suspected acute coronary syndrome were subsequently diagnosed with takotsubo cardiomyopathy.6-8 Nearly 90% of reported cases involved postmenopausal women, and this may be related to loss of the cardioprotective effect of estrogen.5,9
A preceding stressful emotional or physical event is usually identified in about two-thirds of the patients with ABS.9 Most common emotional triggers are death of a relative or friend, broken relationships, assaults, and rapes, among others. Physical triggers include severe sepsis, shock, acute respiratory failure, seizures, and intracranial bleeds. Sometimes a specific trigger cannot be identified from the history, but the absence of an emotional or physical trigger does not exclude the diagnosis.
Although the exact pathogenesis of ABS remains unclear, it is likely that multiple factors are involved. Some of the suggested mechanisms are high levels of catecholamines, multivessel epicardial spasm, or coronary microvascular dysfunction.4 The catecholamine hypothesis has been supported by the finding that several patients with pheochromocytoma and subarachnoid hemorrhage also present with high levels of catecholamine and a cardiomyopathy resembling ABS. Furthermore, ABS has been reported in patients on catecholamine infusions and those treated with agents that inhibit reuptake of catecholamines.5
The presence of multivessel coronary spasm was suggested by early small studies in Japan, but more recent case series have not validated this hypothesis.5 The microvascular dysfunction hypothesis is supported by the presence of myocardial ischemia, diagnosed by ECG changes and elevated troponins, in the absence of significant coronary disease. However, it remains unclear whether this is a primary mechanism or a manifestation of a primary process.4 Microvascular dysfunction may be more likely related to impairment of myocardial relaxation with extramural coronary compression.
Signs and symptoms of ABS mimic those of AMI, with angina-like chest pain as the main presenting symptom in about 50% of cases.10 Other symptoms include dyspnea and less commonly, syncope or sudden cardiac death. Decompensated left heart failure occurs in 50% of patients, with severe hemodynamic compromise and cardiogenic shock not being uncommon. Other complications that may occur are tachyarrhythmias (atrial or ventricular) and ventricular thromboembolism.4
Common ECG changes in ABS include precordial ST segment elevations, symmetric T wave inversions, and nonspecific T wave changes.4,10 QT interval prolongation may be seen during the first days. Transient pathologic Q waves may be seen at presentation or afterward. These ECG changes tend to revert after weeks or months of presentation.
Elevation of cardiac biomarkers is usually present in laboratory data. Levels peak at 24 hours, and the degree of elevation is usually less than that seen in patients with an AMI.10 Most important, the degree of cardiac biomarker elevation is disproportionately low for the extent of involved coronary territory and left ventricular dysfunction. Other laboratory tests that are frequently altered are the BNP and pro-BNP levels, which are usually elevated due to transient left ventricular dysfunction. C-reactive protein elevates in most patients and indicates the presence of an acute inflammatory response.
Early coronary angiography should be performed in all patients with ABS to rule out the presence of a significant obstructive coronary lesion. Patients with ABS often have luminal irregularities or normal coronary vessels. However, concomitant obstructive coronary lesions may be found, especially in elderly patients.
The hallmark of ABS is a characteristic transient contractility abnormality of the left ventricle causing ballooning of the apex, which can be detected on left ventricular angiography or echocardiography. There are 3 distinct variants of ABS, according to the left ventricular myocardial wall segments involved.10 The classic form of takotsubo is characterized by hypokinesis, dyskinesis, or akinesis of the middle and apical segments of the left ventricle. The basal segment is usually spared and may be hyperdynamic. In the midventricular or apical sparing variant, the wall motion abnormalities are restricted to the midventricular segments, and apical contraction is preserved. This case resembles the atypical variant, because the midventricular segments were affected, whereas apical and basal regions were preserved. A rare variant of takotsubo exists with hypokinesis or akinesis of the base and preserved apical function.
Besides ABS and AMI, an important entity to consider in the differential diagnosis of transient wall motion abnormalities is regional myocarditis. Viral titers are helpful in excluding this condition. Furthermore, prolonged recovery is more commonly seen in myocarditis compared with ABS. Imaging studies are particularly helpful in this scenario.
Cardiac MRI demonstrates the wall motion abnormalities or apical ballooning typical for this condition and can differentiate ABS from myocarditis or MI. It is known that delayed myocardial enhancement is seen with myocardial fibrosis. Typically in ischemic cardiomyopathy, there is wall thinning with associated delayed enhancement that extends from the subendocardium to the epicardium (from 0%-90% of wall thickness) of a particular vascular territory. In myocarditis, the enhancement is usually seen in the involved intramyocardial (mesocardium) region, and the pattern is patchy. In ABS, the delayed enhancement is absent, because there is no fibrosis in the area of regional wall motion abnormalities, and wall thickness is usually normal.9,10
No evidence-based guidelines for treating ABS are currently available. Most patients are initially treated with antiplatelets/anticoagulant therapy, nitrates, and diuretics if the patient presents with heart failure. Patients should be admitted to an intensive care unit for close cardiac monitoring. Once ABS is diagnosed and significant coronary stenosis is excluded, patients should receive standard supportive care and optimal neurohormonal therapy. This should include beta blocker or combined alpha/beta blocker agents, an ACE-I or angiotensin receptor blocker, and diuretics if appropriate. Once left ventricular function (LVF) is recovered, therapy with inhibitors of the renin-angiotensin system may be discontinued, but patients should remain on long-term alpha or beta blocker therapy, because the sympathetic blockade provided by these agents may prevent recurrences of this disease.10
Prognosis is generally favorable, and most patients recover to normal LVF over weeks to months. It is important to assess the LVF 4 to 6 weeks after the patient is discharged to confirm the diagnosis of ABS. Recurrence may occur in up to 9% of cases.10 Long-term mortality is similar compared with the age-matched general population.
Conclusion
Apical ballooning syndrome is a relatively novel cardiomyopathy that has gained important attention among the cardiovascular community, mostly because its clinical presentation mimics that of an acute coronary syndrome. Awareness of this entity will result in a more focused diagnosis and appropriate treatment. Managing both cardiac and emotional components of this disease will have a permanent impact in the reversibility and secondary prevention of this cardiomyopathy.
Acknowledgments
Special thanks to the Radiology Service at the VA Caribbean Healthcare System, in particular Dr. Frances Aulet for interpretation of the cardiac MRI results and assistance with MRI images.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases [in Japanese]. J Cardiol. 1991;21(2):203-214.
2. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;9(4):311-316.
3. Afonso L, Bachour K, Awad K, Sandidge G. Takotsubo cardiomyopathy: Pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Eur J Echocardiogr. 2008;9(6):849-854.
4. Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: A review of the current literature. Postgrad Med J. 2007;83(978):261-264.
5. Hare J. The dilated, restrictive and infiltrative cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2012:1562-1580.
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343-346.
7. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—Comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115-122.
8. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417.
9. Lange R, Hills D. Chemical cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: Saunders; 2014:1609-1611.
10. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27(13):1523-1529.
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases [in Japanese]. J Cardiol. 1991;21(2):203-214.
2. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;9(4):311-316.
3. Afonso L, Bachour K, Awad K, Sandidge G. Takotsubo cardiomyopathy: Pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Eur J Echocardiogr. 2008;9(6):849-854.
4. Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: A review of the current literature. Postgrad Med J. 2007;83(978):261-264.
5. Hare J. The dilated, restrictive and infiltrative cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2012:1562-1580.
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343-346.
7. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—Comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115-122.
8. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417.
9. Lange R, Hills D. Chemical cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: Saunders; 2014:1609-1611.
10. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27(13):1523-1529.